Hand Preference in Rhinopithecus roxellana Infants: Is It Influenced by Familial Inheritance?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Species
2.2. Data Collection
2.3. Data Analyses
3. Results
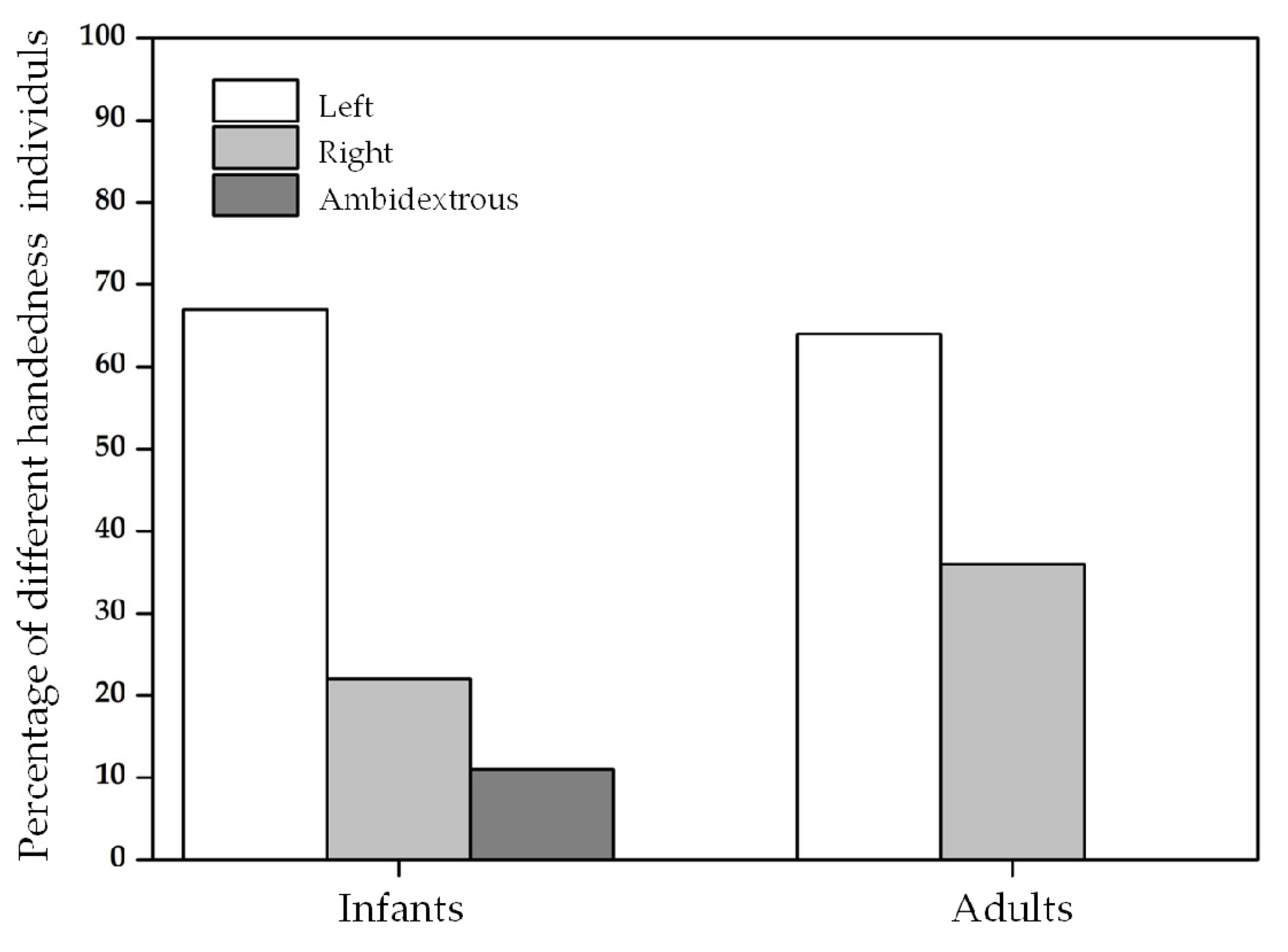
3.1. Hand Preference in Infants
3.2. Hand Preference in Adults
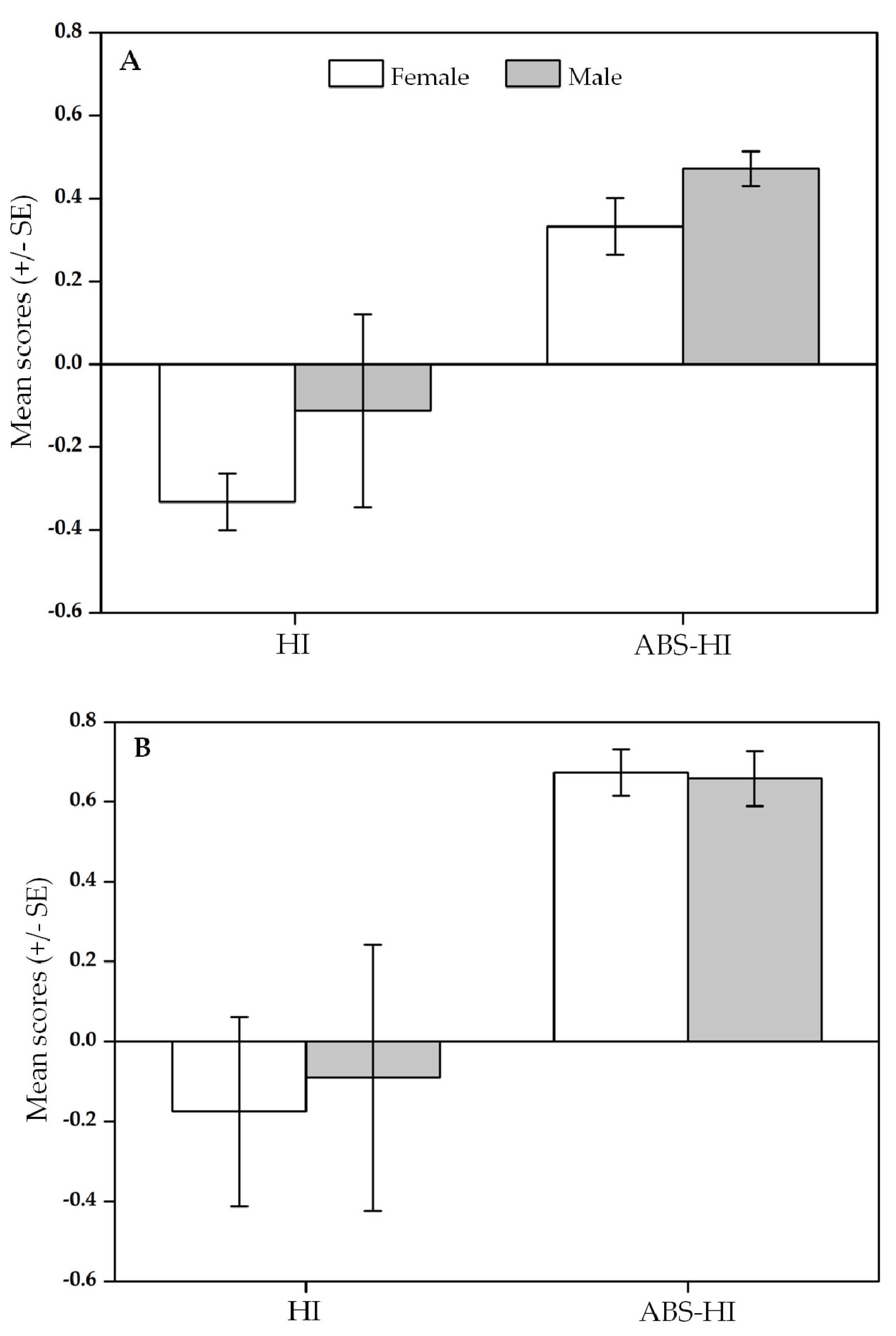
3.3. Relationship between Infants and Their Parents
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ocklenburg, S.; Beste, C.; Güntürkün, O. Handedness: A neurogenetic shift of perspective. Neurosci. Biobehav. Rev. 2013, 37, 2788–2793. [Google Scholar] [CrossRef] [PubMed]
- Perelle, I.B.; Ehrman, L. On the other hand. Behav. Genet. 2005, 35, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Annett, M. Handedness and Brain Asymmetry: The Right Shift Theory; Psychology Press: Hove, UK, 2002. [Google Scholar]
- Hopkins, W.D.; Bard, K.A. The ontogeny of lateralized behavior in nonhuman primates with special reference to chimpanzees (Pan troglodytes). In Primate Laterality: Current Behavioral Evidence of Primate Asymmetries; Ward, J.P., Hopkins, W.D., Eds.; Springer: New York, NY, USA, 1993; pp. 251–265. [Google Scholar]
- Olulade, O.A.; Seydell-Greenwald, A.; Chambers, C.E.; Turkeltaub, P.E.; Dromerick, A.W.; Berl, M.M.; Gaillard, W.D.; Newport, E.L. The neural basis of language development: Changes in lateralization over age. Proc. Natl. Acad. Sci. USA 2020, 117, 23477–23483. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.M.; Marcinowski, E.C.; Babik, I.; Michel, G.F. The influence of a hand preference for acquiring objects on the development of a hand preference for unimanual manipulation from 6 to 14 months. Infant Behav. Dev. 2015, 39, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.M.; Marcinowski, E.C.; Michel, G.F. The development of neuromotor skills and hand preference during infancy. Dev. Psychobiol. 2017, 60, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ferre, C.L.; Babik, I.; Michel, G.F. Development of infant prehension handedness: A longitudinal analysis during the 6- to 14-month age period. Infant Behav. Dev. 2010, 33, 492–502. [Google Scholar] [CrossRef]
- Michel, G.F.; Babik, I.; Sheu, C.F.; Campbell, J.M. Latent classes in the developmental trajectories of infant handedness. Dev. Psychobiol. 2014, 50, 349–359. [Google Scholar] [CrossRef]
- Marcori, A.J.; Okazaki, V.H.A. A historical, systematic review of handedness origins. Laterality 2020, 25, 87–108. [Google Scholar] [CrossRef]
- Carter-Saltzman, L. Biological and sociocultural effects on handedness: Comparison between biological and adoptive parents. Science 1980, 209, 1263–1265. [Google Scholar] [CrossRef]
- Curt, F.; De Agostini, M.; Maccario, J.; Dellatolas, G. Parental hand preference and manual functional asymmetry in preschool children. Behav. Genet. 1995, 25, 525–536. [Google Scholar] [CrossRef]
- McManus, I.C.; Bryden, M.P. The genetics of handedness, cerebral dominance, and lateralization. In Handbook of Neuropsychology; Boller, F., Rafman, J., Eds.; Elsevier: Amsterdam, The Netherlands, 1992; Volume 6, pp. 115–143. [Google Scholar]
- Laland, K.N. Exploring gene–culture interactions: Insights from handedness, sexual selection and niche-construction case studies. Philos. Trans. R. Soc. 2008, 363, 3577–3589. [Google Scholar] [CrossRef] [PubMed]
- Porac, C.; Coren, S. Life-span age trends in the perception of the mueller-lyer: Additional evidence for the existence of two illusions. Can. J. Psychol. 1981, 35, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Wells, D.L.; Millsopp, S. The ontogenesis of lateralized behavior in the domestic cat, Felis silvestris catus. J. Comp. Psychol. 2012, 126, 23–30. [Google Scholar] [CrossRef]
- Giljov, A.; Karenina, K.; Ingram, J.; Malashichev, Y. Early expression of manual lateralization in bipedal marsupials. J. Comp. Psychol. 2017, 131, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Hook, M.A.; Rogers, L.J. Development of hand preferences in marmosets (Callithrix jacchus) and effects of aging. J. Comp. Psychol. 2000, 114, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.D. The Evolution of Hemispheric Specialization in Primates; Academic Press: San Diego, CA, USA, 2007. [Google Scholar]
- Meguerditchian, A.; Donnot, J.; Molesti, S.; Francioly, R.; Vauclair, J. Sex difference in squirrel monkeys’ handedness for unimanual and bimanual tasks. Anim. Behav. 2012, 83, 635–643. [Google Scholar] [CrossRef]
- Westergaard, G.C.; Suomi, S.J. Hand preference in capuchin monkeys varies with age. Primates 1993, 34, 295–299. [Google Scholar] [CrossRef]
- Westergaard, G.C.; Suomi, S.J. Lateral bias in capuchin monkeys (Cebus apella): Concordance between parents and offspring. Dev. Psychobiol. 1997, 31, 143–147. [Google Scholar] [CrossRef]
- Ward, J.P.; Milliken, G.W.; Dodson, D.L.; Stafford, D.K.; Wallace, M. Handedness as a function of sex and age in a large population of Lemur. J. Comp. Psychol. 1990, 104, 167–173. [Google Scholar] [CrossRef]
- Matoba, M.; Masataka, N.; Tanioka, Y. Cross-generational continuity of hand-use preferences in marmosets. Behaviour 1991, 117, 281–286. [Google Scholar]
- Westergaard, G.C.; Lussier, I.D.; Higley, J.D. Between-species variation in the development of hand preference among macaques. Neuropsychologia 2001, 39, 1373–1378. [Google Scholar] [CrossRef]
- Brooker, R.J.; Lehman, R.A.W.; Heimbuch, R.C.; Kidd, K.K. Hand usage in a colony of bonnett monkeys, Macaca radiata. Behav. Genet. 1981, 11, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.W.; Byrne, J.M. Hand preferences in the skilled gathering tasks of mountain gorillas (Gorilla g. berengei). Cortex 1991, 27, 521–536. [Google Scholar] [CrossRef]
- Hopkins, W.D. Heritability of hand preference in chimpanzees (Pan troglodytes): Evidence from a partial interspecies cross-fostering study. J. Comp. Psychol. 1999, 113, 307–313. [Google Scholar] [CrossRef]
- Fu, W.W.; Wang, X.W.; Wang, C.L.; Zhao, H.T.; Ren, Y.; Li, B.G. Effects of age, sex and manual task on hand preference in wild Rhinopithecus roxellana. Zool. Res. 2019, 40, 129–138. [Google Scholar]
- Liang, B.; Zhang, S.Y. Hand preference in Sichuan snub-nosed monkeys (Rhinopithecus roxellana). Acta Theriol. Sin. 1998, 18, 107–111. [Google Scholar]
- Zhao, D.P.; Ji, W.H.; Watanabe, K.; Li, B.G. Hand preference during unimanual and bimanual reaching actions in Sichuan snub-nosed monkeys (Rhinopithecus roxellana). Am. J. Primatol. 2008, 70, 500–504. [Google Scholar] [CrossRef]
- Zhao, D.P.; Gao, X.; Li, B.G. Hand preference for spontaneously unimanual and bimanual coordinated tasks in wild Sichuan snub-nosed monkeys: Implication for hemispheric specialization. Behav. Brain Res. 2010, 208, 85–89. [Google Scholar] [CrossRef]
- Zhao, D.P.; Hopkins, W.D.; Li, B.G. Handedness in nature: First evidence on manual laterality on bimanual coordinated tube task in wild primates. Am. J. Phys. Anthropol. 2012, 148, 36–44. [Google Scholar] [CrossRef]
- Zhang, P.; Watanabe, K.; Li, B.G.; Tan, C.L. Social organization of Sichuan snub-nosed monkeys (Rhinopithecus roxellana) in the Qinling Mountains, Central China. Primates 2006, 47, 374–382. [Google Scholar] [CrossRef]
- Li, B.G.; Zhao, D.P. Copulation behavior within one-male groups of wild Rhinopithecus roxellana in the Qinling Mountains of China. Primates 2007, 48, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Altmann, J. Observational Study of Behavior: Sampling Methods. Behaviour 1974, 49, 227–267. [Google Scholar] [CrossRef] [PubMed]
- Westergaard, G.C.; Byrne, G.; Suomi, S.J. Early lateral bias in tufted capuchins (Cebus apella). Dev. Psychobiol. 1998, 32, 45–50. [Google Scholar] [CrossRef]
- Hopkins, W.D.; Bales, S.A.; Bennett, A.J. Heritability of hand preference in chimpanzees (Pan). Intern. J. Neuroence. 1994, 74, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Meunier, H.; Vauclair, J. Hand preferences on unimanual and bimanual tasks in white-face capuchins (Cebus capucinus). Am. J. Primatol. 2007, 69, 1064–1069. [Google Scholar] [CrossRef]
- Li, B.G.; Chen, C.; Ji, W.H.; Ren, B.P. Seasonal home range changes of the Sichuan snub-nosed monkey (Rhinopithecus roxellana) in the Qinling Mountains of China. Folia. Primatol. 2000, 71, 375–386. [Google Scholar] [CrossRef]
- Teixeira, L.A. Categories of manual asymmetry and their variation with advancing age. Cortex 2008, 44, 707–716. [Google Scholar] [CrossRef]
- McGrew, W.C.; Marchant, L.F. On the other hand: Current issues in and meta-analysis of the behavioral laterality of hand function in non-human primates. Am. J. Phys. Anthropol. 1997, 40, 201–232. [Google Scholar] [CrossRef]
- Meguerditchian, A.; Calcutt, S.E.; Lonsdorf, E.V.; Ross, S.R.; Hopkins, W.D. Brief communication: Captive gorillas are right-handed for bimanual feeding. Am. J. Phys. Anthropol. 2010, 141, 638–645. [Google Scholar] [CrossRef]
- Corp, N.; Byrne, R.W. Sex difference in chimpanzee handedness. Am. J. Phys. Anthropol. 2004, 123, 62–68. [Google Scholar] [CrossRef]
- Rogers, L.J.; Kaplan, G. Hand preferences and other lateral biases in rehabilitated orangutans, Pongo pygmaeus pygmaeus. Anim. Behav. 1996, 51, 13–25. [Google Scholar] [CrossRef]
- Schweitzer, C.; Bec, P.; Blois-Heulin, C. Does the complexity of the task influence manual laterality in de Brazza’s monkeys (Cercopithecus neglectus)? Ethology 2007, 113, 983–994. [Google Scholar] [CrossRef]
- Phillips, K.A.; Sherwood, C.C. Cerebral petalias and their relationship to handedness in capuchin monkeys (Cebus apella). Neuropsychologia 2007, 45, 2398–2401. [Google Scholar] [CrossRef] [PubMed]


| Number | Sex | L/R | HI | z-Score | Handedness |
|---|---|---|---|---|---|
| 1 | Female | 34/13 | −0.45 | −3.06 | Left |
| 2 | Female | 24/10 | −0.41 | −2.40 | Left |
| 3 | Male | 7/24 | 0.55 | 3.05 | Right |
| 4 | Female | 24/12 | −0.33 | −2.00 | Left |
| 5 | Male | 25/7 | −0.56 | −3.18 | Left |
| 6 | Female | 20/15 | −0.14 | −0.85 | Ambidextrous |
| 7 | Male | 11/23 | 0.35 | 2.06 | Right |
| 8 | Male | 28/12 | −0.40 | −2.53 | Left |
| 9 | Male | 30/10 | −0.50 | −3.16 | Left |
| Number | Mother | Father | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Name | L/R | HI | z-Score | Handedness | Name | L/R | HI | z-Score | Handedness | |
| 1 | QB | 28/6 | −0.65 | −3.77 | Left | HD | 46/5 | −0.80 | −5.74 | Left |
| 2 | MB | 36/8 | −0.64 | −4.22 | Left | |||||
| 3 | FB | 36/17 | −0.36 | −2.61 | Left | |||||
| 4 | XF | 32/4 | −0.78 | −4.67 | Left | DX | 7/81 | 0.84 | 7.89 | Right |
| 5 | PP | 41/7 | −0.71 | −4.91 | Left | |||||
| 6 | KD | 2/38 | 0.90 | 5.69 | Right | YQ | 6/22 | 0.57 | 3.02 | Right |
| 7 | CY | 32/6 | −0.68 | −4.22 | Left | |||||
| 8 | YK | 3/42 | 0.87 | 5.81 | Right | BD | 44/15 | −0.49 | −3.78 | Left |
| 9 | LS | 19/53 | 0.47 | 4.01 | Right | XL | 64/17 | −0.58 | −5.22 | Left |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, W.-W.; Ren, Y.; Wang, C.-L.; Wang, X.-W.; Li, B.-G. Hand Preference in Rhinopithecus roxellana Infants: Is It Influenced by Familial Inheritance? Symmetry 2020, 12, 1905. https://doi.org/10.3390/sym12111905
Fu W-W, Ren Y, Wang C-L, Wang X-W, Li B-G. Hand Preference in Rhinopithecus roxellana Infants: Is It Influenced by Familial Inheritance? Symmetry. 2020; 12(11):1905. https://doi.org/10.3390/sym12111905
Chicago/Turabian StyleFu, Wei-Wei, Yi Ren, Cheng-Liang Wang, Xiao-Wei Wang, and Bao-Guo Li. 2020. "Hand Preference in Rhinopithecus roxellana Infants: Is It Influenced by Familial Inheritance?" Symmetry 12, no. 11: 1905. https://doi.org/10.3390/sym12111905
APA StyleFu, W.-W., Ren, Y., Wang, C.-L., Wang, X.-W., & Li, B.-G. (2020). Hand Preference in Rhinopithecus roxellana Infants: Is It Influenced by Familial Inheritance? Symmetry, 12(11), 1905. https://doi.org/10.3390/sym12111905





