Asymmetric Contributions of the Fronto-Parietal Network to Emotional Conflict in the Word–Face Interference Task
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Word–Face Interference Task
2.3. Online Transcranial Direct Current Stimulation
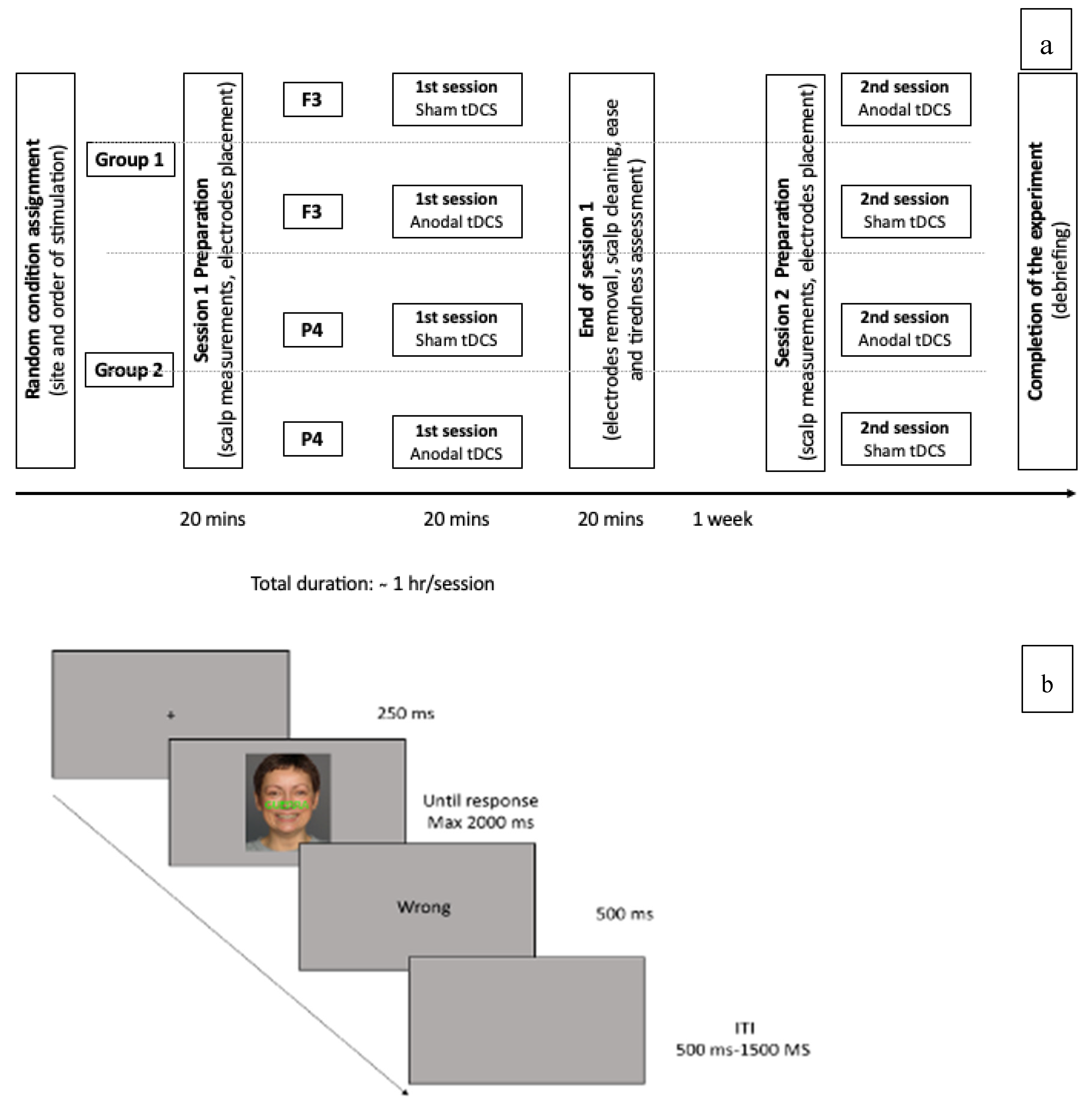
2.4. Procedure
2.5. Experimental Design
2.6. Data Analyses
3. Results
Overall Results
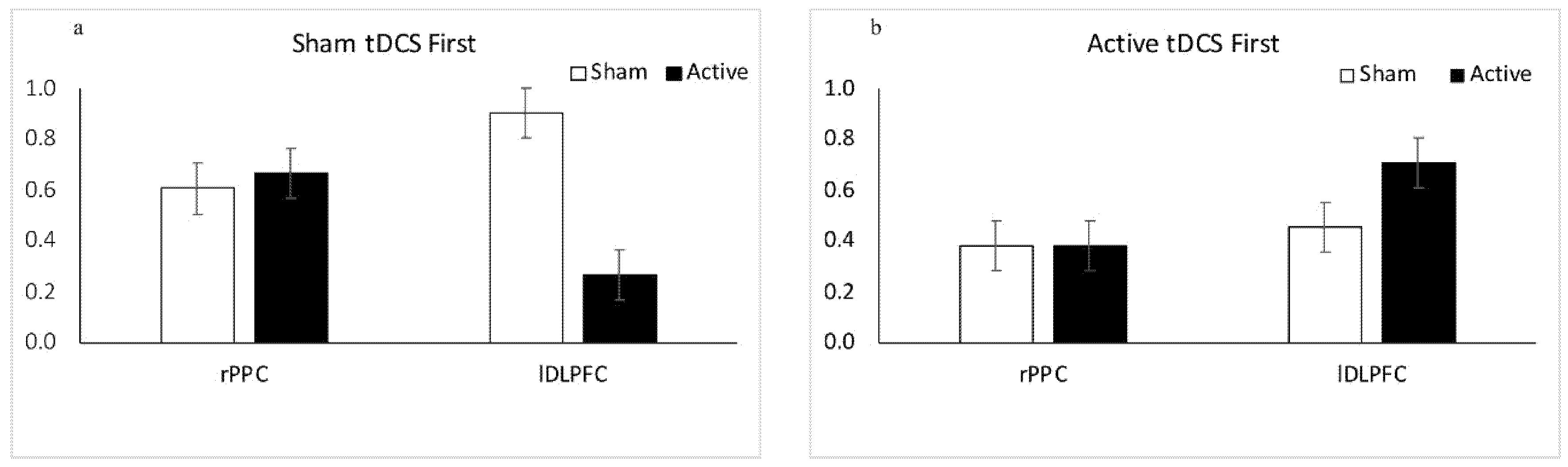
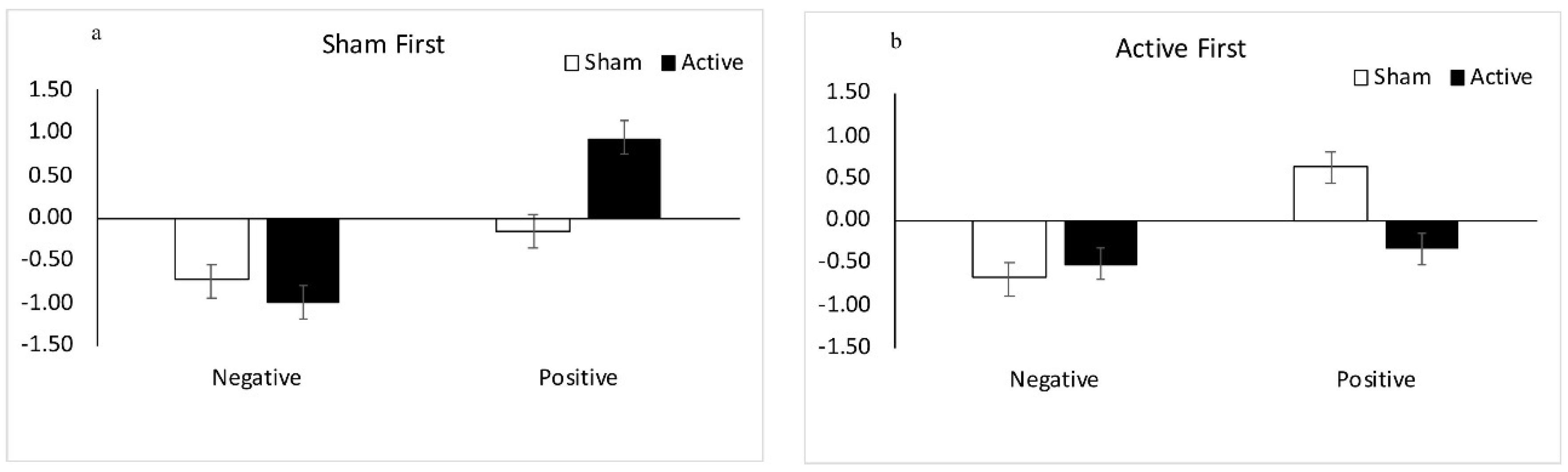
3.1.1. Results: Sham tDCS First
3.1.2. Results: Active tDCS First
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Corbetta, M.; Patel, G.; Shulman, G.L. The Reorienting System of the Human Brain: From Environment to Theory of Mind. Neuron 2008, 58, 306–324. [Google Scholar] [CrossRef] [PubMed]
- Theeuwes, J. Endogenous and Exogenous Control of Visual Selection. Perception 1994, 23, 429–440. [Google Scholar] [CrossRef]
- Corbetta, M.; Shulman, G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002, 3, 201–215. [Google Scholar] [CrossRef] [PubMed]
- DeSimone, R.; Duncan, J. Neural Mechanisms of Selective Visual Attention. Annu. Rev. Neurosci. 1995, 18, 193–222. [Google Scholar] [CrossRef]
- Corbetta, M.; Miezin, F.M.; Shulman, G.L.; Petersen, S.E. A PET study of visuospatial attention. J. Neurosci. 1993, 13, 1202–1226. [Google Scholar] [CrossRef]
- Friedman-Hill, S.R.; Robertson, L.C.; DeSimone, R.; Ungerleider, L.G. Posterior parietal cortex and the filtering of distractors. Proc. Natl. Acad. Sci. USA 2003, 100, 4263–4268. [Google Scholar] [CrossRef]
- Balan, P.F.; Gottlieb, J. Integration of Exogenous Input into a Dynamic Salience Map Revealed by Perturbing Attention. J. Neurosci. 2006, 26, 9239–9249. [Google Scholar] [CrossRef]
- Bendiksby, M.S.; Platt, M.L. Neural correlates of reward and attention in macaque area LIP. Neuropsychologia 2006, 44, 2411–2420. [Google Scholar] [CrossRef]
- Fecteau, J.H.; Munoz, D.P. Salience, relevance, and firing: A priority map for target selection. Trends Cogn. Sci. 2006, 10, 382–390. [Google Scholar] [CrossRef]
- Geng, J.J.; Mangun, G.R. Anterior Intraparietal Sulcus is Sensitive to Bottom-Up Attention Driven by Stimulus Salience. J. Cogn. Neurosci. 2009, 21, 1584–1601. [Google Scholar] [CrossRef]
- Zénon, A.; Filali, N.; Duhamel, J.-R.; Olivier, E. Salience Representation in the Parietal and Frontal Cortex. J. Cogn. Neurosci. 2010, 22, 918–930. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.J.; Bradley, M.M. Emotion and the motivational brain. Biol. Psychol. 2010, 84, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Pourtois, G.; Schettino, A.; Vuilleumier, P. Brain mechanisms for emotional influences on perception and attention: What is magic and what is not. Biol. Psychol. 2013, 92, 492–512. [Google Scholar] [CrossRef] [PubMed]
- Vuilleumier, P. How brains beware: Neural mechanisms of emotional attention. Trends Cogn. Sci. 2005, 9, 585–594. [Google Scholar] [CrossRef]
- Pessoa, L. How do emotion and motivation direct executive control? Trends Cogn. Sci. 2009, 13, 160–166. [Google Scholar] [CrossRef]
- Mohanty, A.; Gitelman, D.R.; Small, D.M.; Mesulam, M.M. The Spatial Attention Network Interacts with Limbic and Monoaminergic Systems to Modulate Motivation-Induced Attention Shifts. Cereb. Cortex 2008, 18, 2604–2613. [Google Scholar] [CrossRef]
- Troiani, V.; Price, E.T.; Schultz, R.T. Unseen fearful faces promote amygdala guidance of attention. Soc. Cogn. Affect. Neurosci. 2012, 9, 133–140. [Google Scholar] [CrossRef][Green Version]
- Troiani, V.P.; Schultz, R.T. Amygdala, pulvinar, and inferior parietal cortex contribute to early processing of faces without awareness. Front. Hum. Neurosci. 2013, 7, 241. [Google Scholar] [CrossRef] [PubMed]
- De Gelder, B.; Van Honk, J.; Tamietto, M. Emotion in the brain: Of low roads, high roads and roads less travelled. Nat. Rev. Neurosci. 2011, 12, 425. [Google Scholar] [CrossRef] [PubMed]
- Sabatinelli, D.; Fortune, E.E.; Li, Q.; Siddiqui, A.; Krafft, C.; Oliver, W.T.; Beck, S.; Jeffries, J. Emotional perception: Meta-analyses of face and natural scene processing. NeuroImage 2011, 54, 2524–2533. [Google Scholar] [CrossRef] [PubMed]
- Barber, A.D.; Carter, C.S. Cognitive Control Involved in Overcoming Prepotent Response Tendencies and Switching Between Tasks. Cereb. Cortex 2004, 15, 899–912. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, A.W. Dissociating the Role of the Dorsolateral Prefrontal and Anterior Cingulate Cortex in Cognitive Control. Science 2000, 288, 1835–1838. [Google Scholar] [CrossRef] [PubMed]
- Etkin, A.; Egner, T.; Peraza, D.M.; Kandel, E.R.; Hirsch, J. Resolving Emotional Conflict: A Role for the Rostral Anterior Cingulate Cortex in Modulating Activity in the Amygdala. Neuron 2006, 51, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Egner, T.; Etkin, A.; Gale, S.; Hirsch, J. Dissociable Neural Systems Resolve Conflict from Emotional versus Nonemotional Distracters. Cereb. Cortex 2007, 18, 1475–1484. [Google Scholar] [CrossRef]
- Dolcos, F.; LaBar, K.S.; Cabeza, R. Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaluation and subsequent memory: An event-related fMRI study. NeuroImage 2004, 23, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Grimm, S.; Weigand, A.; Kazzer, P.; Jacobs, A.M.; Bajbouj, M. Neural mechanisms underlying the integration of emotion and working memory. NeuroImage 2012, 61, 1188–1194. [Google Scholar] [CrossRef]
- Phan, K.L.; Fitzgerald, D.A.; Nathan, P.J.; Moore, G.J.; Uhde, T.W.; Tancer, M.E. Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biol. Psychiatry 2005, 57, 210–219. [Google Scholar] [CrossRef]
- Herrington, J.D.; Mohanty, A.; Koven, N.S.; Fisher, J.E.; Stewart, J.L.; Banich, M.T.; Webb, A.G.; Miller, G.A.; Heller, W. Emotion-Modulated Performance and Activity in Left Dorsolateral Prefrontal Cortex. Emotion 2005, 5, 200–207. [Google Scholar] [CrossRef]
- Mak, A.K.; Hu, Z.-G.; Zhang, J.X.; Xiao, Z.-W.; Lee, T.M.C. Neural correlates of regulation of positive and negative emotions: An fMRI study. Neurosci. Lett. 2009, 457, 101–106. [Google Scholar] [CrossRef]
- Viinikainen, M.; Jääskeläinen, I.P.; Alexandrov, Y.; Balk, M.H.; Autti, T.; Sams, M. Nonlinear relationship between emotional valence and brain activity: Evidence of separate negative and positive valence dimensions. Hum. Brain Mapp. 2009, 31, 1030–1040. [Google Scholar] [CrossRef]
- Lindquist, K.A.; Satpute, A.B.; Wager, T.D.; Weber, J.; Barrett, L.F. The Brain Basis of Positive and Negative Affect: Evidence from a Meta-Analysis of the Human Neuroimaging Literature. Cereb. Cortex 2015, 26, 1910–1922. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.J. Affect, cognition, and hemispheric specialization. In Emotions, Cognition and Behavior; Izard, C.E., Kagan, J., Zajonc, R.B., Eds.; Cambridge University Press: New York, NY, USA, 1984. [Google Scholar]
- Killgore, W.D.S.; Yurgelun-Todd, D.A. The right-hemisphere and valence hypotheses: Could they both be right (and sometimes left)? Soc. Cogn. Affect. Neurosci. 2007, 2, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Carver, C.S.; Harmon-Jones, E. Anger is an approach-related affect: Evidence and implications. Psychol. Bull. 2009, 135, 183–204. [Google Scholar] [CrossRef]
- Sparing, R.; Thimm, M.; Hesse, M.D.; Küst, J.; Karbe, H.; Fink, G.R. Bidirectional alterations of interhemispheric parietal balance by non-invasive cortical stimulation. Brain 2009, 132 Pt 11, 3011–3020. [Google Scholar] [CrossRef]
- Bolognini, N.; Fregni, F.; Casati, C.; Olgiati, E.; Vallar, G. Brain polarization of parietal cortex augments training-induced improvement of visual exploratory and attentional skills. Brain Res. 2010, 1349, 76–89. [Google Scholar] [CrossRef]
- Grèzes, J.; Pichon, S.; De Gelder, B. Perceiving fear in dynamic body expressions. NeuroImage 2007, 35, 959–967. [Google Scholar] [CrossRef]
- Kitada, R.; Johnsrude, I.S.; Kochiyama, T.; Lederman, S.J. Brain networks involved in haptic and visual identification of facial expressions of emotion: An fMRI study. NeuroImage 2010, 49, 1677–1689. [Google Scholar] [CrossRef]
- Sarkheil, P.; Goebel, R.; Schneider, F.; Mathiak, K. Emotion unfolded by motion: A role for parietal lobe in decoding dynamic facial expressions. Soc. Cogn. Affect. Neurosci. 2012, 8, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Zachariou, V.; Nikas, C.V.; Safiullah, Z.N.; Gotts, S.J.; Ungerleider, L.G. Spatial Mechanisms within the Dorsal Visual Pathway Contribute to the Configural Processing of Faces. Cereb. Cortex 2017, 27, 4124–4138. [Google Scholar] [CrossRef][Green Version]
- Pitcher, D. Facial Expression Recognition Takes Longer in the Posterior Superior Temporal Sulcus than in the Occipital Face Area. J. Neurosci. 2014, 34, 9173–9177. [Google Scholar] [CrossRef]
- Pitcher, D.; Pilkington, A.; Rauth, L.; Baker, C.; Kravitz, D.J.; Ungerleider, L.G. The Human Posterior Superior Temporal Sulcus Samples Visual Space Differently From Other Face-Selective Regions. Cereb. Cortex 2019, 30, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Sliwinska, M.W.; Pitcher, D. TMS demonstrates that both right and left superior temporal sulci are important for facial expression recognition. NeuroImage 2018, 183, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Vanderhasselt, M.-A.; De Raedt, R.; Baeken, C.; Leyman, L.; D’Haenen, H. The influence of rTMS over the left dorsolateral prefrontal cortex on Stroop task performance. Exp. Brain Res. 2006, 169, 279–282. [Google Scholar] [CrossRef]
- Bermpohl, F.; Fregni, F.; Boggio, P.S.; Thut, G.; Northoff, G.; Otachi, P.T.M.; Rigonatti, S.P.; Marcolin, M.A.; Pascual-Leone, A. Left prefrontal repetitive transcranial magnetic stimulation impairs performance in affective go/no-go task. NeuroReport 2005, 16, 615–619. [Google Scholar] [CrossRef][Green Version]
- De Raedt, R.; Vanderhasselt, M.-A.; Baeken, C. Neurostimulation as an intervention for treatment resistant depression: From research on mechanisms towards targeted neurocognitive strategies. Clin. Psychol. Rev. 2015, 41, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Clarke, P.J.; Browning, M.; Hammond, G.; Notebaert, L.; MacLeod, C. The Causal Role of the Dorsolateral Prefrontal Cortex in the Modification of Attentional Bias: Evidence from Transcranial Direct Current Stimulation. Biol. Psychiatry 2014, 76, 946–952. [Google Scholar] [CrossRef]
- Heeren, A.; Baeken, C.; Vanderhasselt, M.-A.; Philippot, P.; De Raedt, R. Impact of Anodal and Cathodal Transcranial Direct Current Stimulation over the Left Dorsolateral Prefrontal Cortex during Attention Bias Modification: An Eye-Tracking Study. PLoS ONE 2015, 10, e0124182. [Google Scholar] [CrossRef]
- Sanchez-Lopez, A.; Vanderhasselt, M.-A.; Allaert, J.; Baeken, C.; De Raedt, R. Neurocognitive mechanisms behind emotional attention: Inverse effects of anodal tDCS over the left and right DLPFC on gaze disengagement from emotional faces. Cogn. Affect. Behav. Neurosci. 2018, 18, 485–494. [Google Scholar] [CrossRef]
- Wolkenstein, L.; Plewnia, C. Amelioration of Cognitive Control in Depression by Transcranial Direct Current Stimulation. Biol. Psychiatry 2013, 73, 646–651. [Google Scholar] [CrossRef]
- Vanderhasselt, M.-A.; De Raedt, R.; Brunoni, A.R.; Campanhã, C.; Baeken, C.; Remue, J.; Boggio, P.S. tDCS over the Left Prefrontal Cortex Enhances Cognitive Control for Positive Affective Stimuli. PLoS ONE 2013, 8, e62219. [Google Scholar] [CrossRef]
- Stenberg, G.; Wiking, S.; Dahl, M. Judging Words at Face Value: Interference in a Word Processing Task Reveals Automatic Processing of Affective Facial Expressions. Cogn. Emot. 1998, 12, 755–782. [Google Scholar] [CrossRef]
- Pecchinenda, A.; Heil, M. Role of working memory load on selective attention to affectively valent information. Eur. J. Cogn. Psychol. 2007, 19, 898–909. [Google Scholar] [CrossRef]
- Pecchinenda, A.; Ferlazzo, F.; Lavidor, M. Modulation of selective attention by polarity-specific tDCS effects. Neuropsychologia 2015, 68. [Google Scholar] [CrossRef] [PubMed]
- Stroop, J.R. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1935, 18, 643–662. [Google Scholar] [CrossRef]
- Beall, P.M.; Herbert, A.M. The face wins: Stronger automatic processing of affect in facial expressions than words in a modified Stroop task. Cogn. Emot. 2008, 22, 1613–1642. [Google Scholar] [CrossRef]
- Kuehne, M.; Schmidt, K.; Heinze, H.-J.; Zaehle, T. Modulation of Emotional Conflict Processing by High-Definition Transcranial Direct Current Stimulation (HD-TDCS). Front. Behav. Neurosci. 2019, 13, 224. [Google Scholar] [CrossRef]
- Zhu, X.-R.; Zhang, H.-J.; Wu, T.; Luo, W.; Luo, Y.-J. Emotional conflict occurs at an early stage: Evidence from the emotional face–word Stroop task. Neurosci. Lett. 2010, 478. [Google Scholar] [CrossRef]
- Batabyal, T.; Muthukrishnan, S.-P.; Sharma, R.; Tayade, P.; Kaur, S. Neural substrates of emotional interference: A quantitative EEG study. Neurosci. Lett. 2018, 685, 1–6. [Google Scholar] [CrossRef]
- Banich, M.; Smolker, H.R.; Snyder, H.R.; Lewis-Peacock, J.A.; Godinez, D.A.; Wager, T.D.; Hankin, B.L. Turning down the heat: Neural mechanisms of cognitive control for inhibiting task-irrelevant emotional information during adolescence. Neuropsychologia 2019, 125, 93–108. [Google Scholar] [CrossRef]
- Miller, E.K.; Cohen, J.D. An Integrative Theory of Prefrontal Cortex Function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef]
- Banich, M. Executive Function. Curr. Dir. Psychol. Sci. 2009, 18, 89–94. [Google Scholar] [CrossRef]
- Banich, M.T.; Milham, M.P.; Atchley, R.; Cohen, N.J.; Webb, A.; Wszalek, T.; Kramer, A.F.; Liang, Z.-P.; Wright, A.; Shenker, J.; et al. fMRI Studies of Stroop Tasks Reveal Unique Roles of Anterior and Posterior Brain Systems in Attentional Selection. J. Cogn. Neurosci. 2000, 12, 988–1000. [Google Scholar] [CrossRef]
- Kanske, P.; Kotz, S.A. Effortful control, depression, and anxiety correlate with the influence of emotion on executive attentional control. Biol. Psychol. 2012, 91, 88–95. [Google Scholar] [CrossRef][Green Version]
- Opitz, A.; Paulus, W.; Will, S.; Antunes, A.; Thielscher, A. Determinants of the electric field during transcranial direct current stimulation. NeuroImage 2015, 109, 140–150. [Google Scholar] [CrossRef]
- Campbell, J.I.D.; Thompson, V. More power to you: Simple power calculations for treatment effects with one degree of freedom. Behav. Res. Methods Instrum. Comput. 2002, 34, 332–337. [Google Scholar] [CrossRef][Green Version]
- Agusti, A.I.; Satorres, E.; Pitarque, A.; Meléndez, J.C. An emotional Stroop task with faces and words. A comparison of young and older adults. Conscious. Cogn. 2017, 53, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Ebner, N.C.; Riediger, M.; Lindenberger, U. FACES—A database of facial expressions in young, middle-aged, and older women and men: Development and validation. Behav. Res. Methods 2010, 42, 351–362. [Google Scholar] [CrossRef]
- Montefinese, M.; Ambrosini, E.; Fairfield, B.; Emammarella, N. The adaptation of the Affective Norms for English Words (ANEW) for Italian. Behav. Res. Methods 2013, 46, 887–903. [Google Scholar] [CrossRef] [PubMed]
- Bradley, M.M.; Lang, P.J. Affective Norms for English Words (ANEW): Instruction Manual and Affective Ratings; University of Florida, Center for Research in Psychophysiology: Gainesville, FL, USA, 1999. [Google Scholar]
- Peressotti, F.; Pesciarelli, F.; Job, R. Le associazioni verbali PD-DPSS: Norme per 294 parole. G. Ital. Psicol. 2002, 1, 153–172. [Google Scholar] [CrossRef]
- Nelson, D.L.; McEvoy, C.L.; Schreiber, T.A. The University of South Florida free association, rhyme, and word fragment norms. Behav. Res. Methods Instrum. Comput. 2004, 36, 402–407. [Google Scholar] [CrossRef]
- Flöel, A.; Rösser, N.; Michka, O.; Knecht, S.; Breitenstein, C. Noninvasive Brain Stimulation Improves Language Learning. J. Cogn. Neurosci. 2008, 20, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Cohen, L.G.; Wassermann, E.M.; Priori, A.; Lang, N.; Antal, A.; Paulus, W.; Hummel, F.; Boggio, P.S.; Fregni, F.; et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008, 1, 206–223. [Google Scholar] [CrossRef] [PubMed]
- Shilo, G.; Lavidor, M. Non-linear effects of cathodal transcranial direct current stimulation (tDCS) of the primary motor cortex on implicit motor learning. Exp. Brain Res. 2019, 237, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Azarpaikan, A.; Torbati, H.R.T.; Sohrabi, M.; Boostani, R.; Ghoshoni, M. Timing-Dependent Priming Effects of Anodal tDCS on Two-Hand Coordination. J. Psychophysiol. 2019, 1–11. [Google Scholar] [CrossRef]
- Stagg, C.; Jayaram, G.; Pastor, D.; Kincses, Z.; Matthews, P.; Johansen-Berg, H. Polarity and timing-dependent effects of transcranial direct current stimulation in explicit motor learning. Neuropsychologia 2011, 49, 800–804. [Google Scholar] [CrossRef]
- Luna, F.G.; Román-Caballero, R.; Barttfeld, P.; Lupiáñez, J.; Martín-Arévalo, E. A High-Definition tDCS and EEG study on attention and vigilance: Brain stimulation mitigates the executive but not the arousal vigilance decrement. Neuropsychologia 2020, 142, 107447. [Google Scholar] [CrossRef]
- Petrucci, M.; Pecchinenda, A. The role of cognitive control mechanisms in selective attention towards emotional stimuli. Cogn. Emot. 2016, 31, 1480–1492. [Google Scholar] [CrossRef]
- Harmon-Jones, E.; Gable, P.A.; Peterson, C.K. The role of asymmetric frontal cortical activity in emotion-related phenomena: A review and update. Biol. Psychol. 2010, 84, 451–462. [Google Scholar] [CrossRef]
- Jacoby, N.; Lavidor, M. Null tDCS Effects in a Sustained Attention Task: The Modulating Role of Learning. Front. Psychol. 2018, 9, 476. [Google Scholar] [CrossRef]
- Hammer, A.; Mohammadi, B.; Schmicker, M.; Saliger, S.; Münte, T.F. Errorless and errorful learning modulated by transcranial direct current stimulation. BMC Neurosci. 2011, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Zaehle, T.; Sandmann, P.; Thorne, J.D.; Jäncke, L.; Herrmann, C.S. Transcranial direct current stimulation of the prefrontal cortex modulates working memory performance: Combined behavioural and electrophysiological evidence. BMC Neurosci. 2011, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, C.M.; Macdonald, P.A. Interdimensional interference in the Stroop effect: Uncovering the cognitive and neural anatomy of attention. Trends Cogn. Sci. 2000, 4, 383–391. [Google Scholar] [CrossRef]
- Öhman, A.; Mineka, S. Fears, phobias, and preparedness: Toward an evolved module of fear and fear learning. Psychol. Rev. 2001, 108, 483–522. [Google Scholar] [CrossRef] [PubMed]
- Loftus, A.M.; Yalcin, O.; Baughman, F.; Vanman, E.J.; Hagger, M.S. The impact of transcranial direct current stimulation on inhibitory control in young adults. Brain Behav. 2015, 5, e00332. [Google Scholar] [CrossRef] [PubMed]
- Frings, C.; Brinkmann, T.; Friehs, M.A.; Van Lipzig, T. Single session tDCS over the left DLPFC disrupts interference processing. Brain Cogn. 2018, 120, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Imani, M.; Ghoreishi, S.F. Bayesian Optimization Objective-Based Experimental Design. In Proceedings of the 2020 American Control Conference (ACC), Denver, CO, USA, 1–3 July 2020; Institute of Electrical and Electronics Engineers (IEEE): Piscatvey, NJ, USA, 2020. [Google Scholar]
- Tsai, M.-H.; Hsia, C.-Y.; Wu, S.K.; Chen, T.-L. An Individual Specific Electroencephalography Signal Pattern Verification Model Based on Machine Learning and Convolutional Neural Network. J. Adv. Comput. Netw. 2020, 8, 1–9. [Google Scholar] [CrossRef]
- Bălan, O.; Moise, G.; Moldoveanu, A.; Leordeanu, M.; Moldoveanu, F. Fear Level Classification Based on Emotional Dimensions and Machine Learning Techniques. Sensors 2019, 19, 1738. [Google Scholar] [CrossRef]
- Chiew, K.S.; Braver, T.S. Neural Circuitry of Emotional and Cognitive Conflict Revealed through Facial Expressions. PLoS ONE 2011, 6, e17635. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luca, F.D.; Petrucci, M.; Monachesi, B.; Lavidor, M.; Pecchinenda, A. Asymmetric Contributions of the Fronto-Parietal Network to Emotional Conflict in the Word–Face Interference Task. Symmetry 2020, 12, 1701. https://doi.org/10.3390/sym12101701
Luca FD, Petrucci M, Monachesi B, Lavidor M, Pecchinenda A. Asymmetric Contributions of the Fronto-Parietal Network to Emotional Conflict in the Word–Face Interference Task. Symmetry. 2020; 12(10):1701. https://doi.org/10.3390/sym12101701
Chicago/Turabian StyleLuca, Francesca De, Manuel Petrucci, Bianca Monachesi, Michal Lavidor, and Anna Pecchinenda. 2020. "Asymmetric Contributions of the Fronto-Parietal Network to Emotional Conflict in the Word–Face Interference Task" Symmetry 12, no. 10: 1701. https://doi.org/10.3390/sym12101701
APA StyleLuca, F. D., Petrucci, M., Monachesi, B., Lavidor, M., & Pecchinenda, A. (2020). Asymmetric Contributions of the Fronto-Parietal Network to Emotional Conflict in the Word–Face Interference Task. Symmetry, 12(10), 1701. https://doi.org/10.3390/sym12101701





