Interactions of Aβ1-42 Peptide and Its Three Fragments (Aβ8-12, Aβ8-13, and Aβ5-16) with Selected Nonsteroidal Drugs and Compounds of Natural Origin
Abstract
1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Fluorescence Spectroscopy
2.3. Molecular Dynamics (MD)
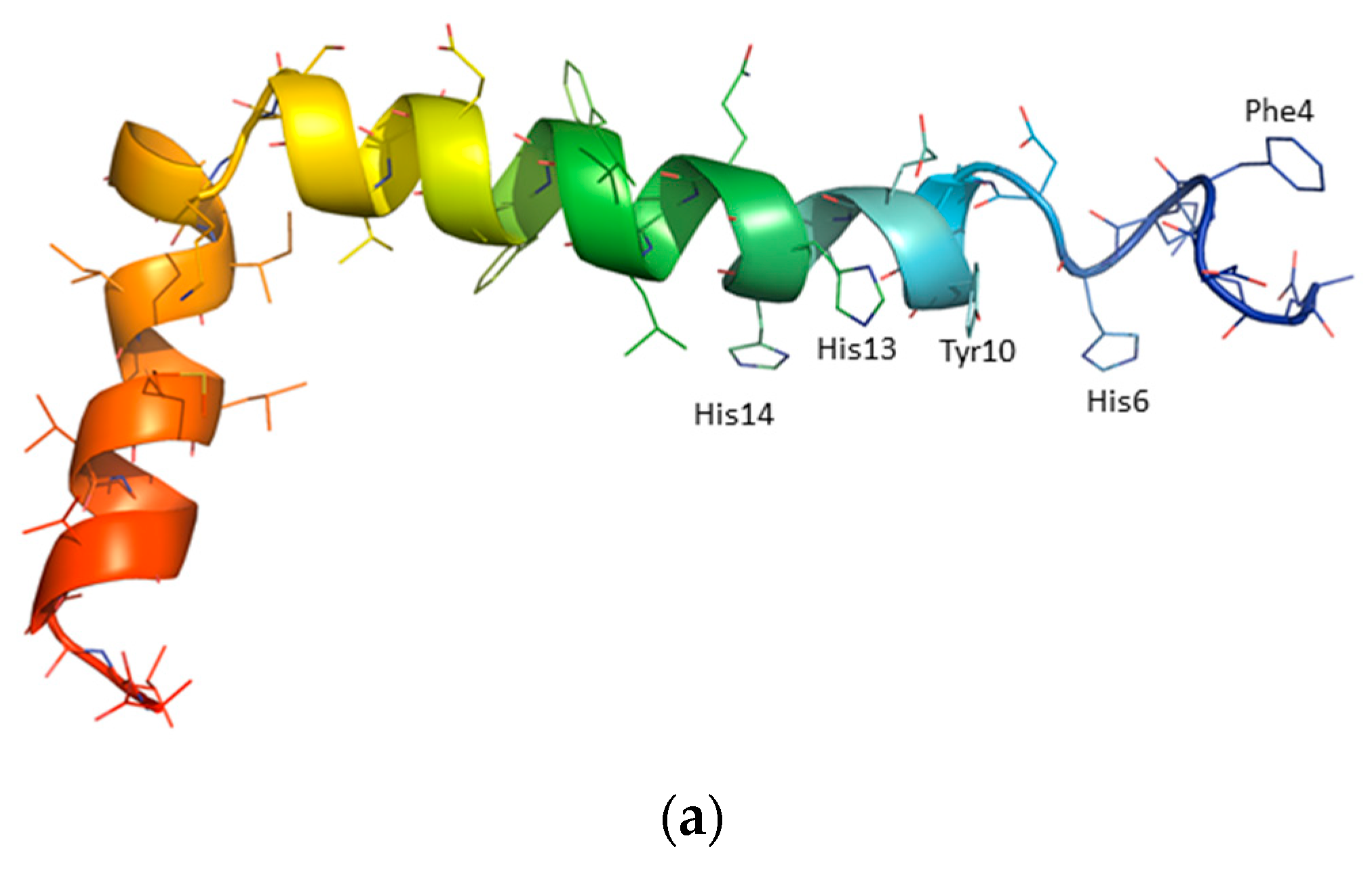
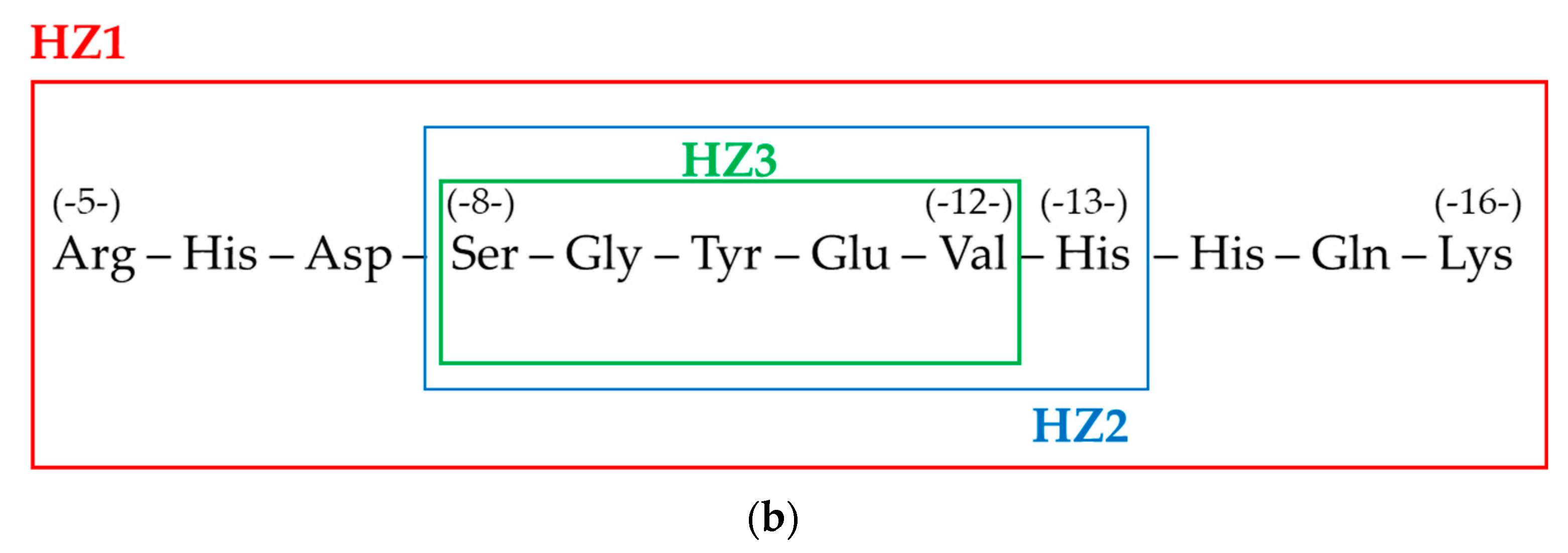
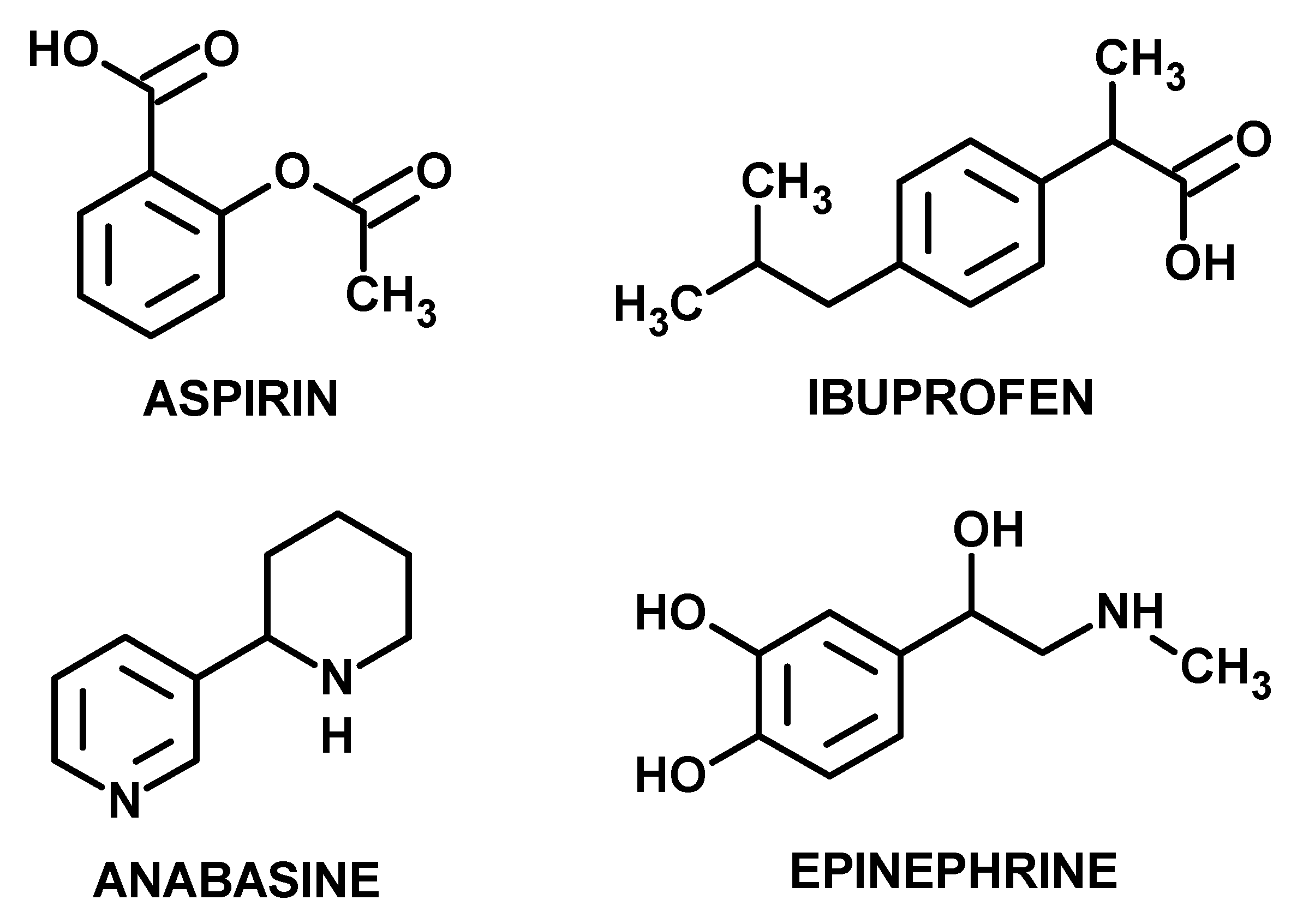
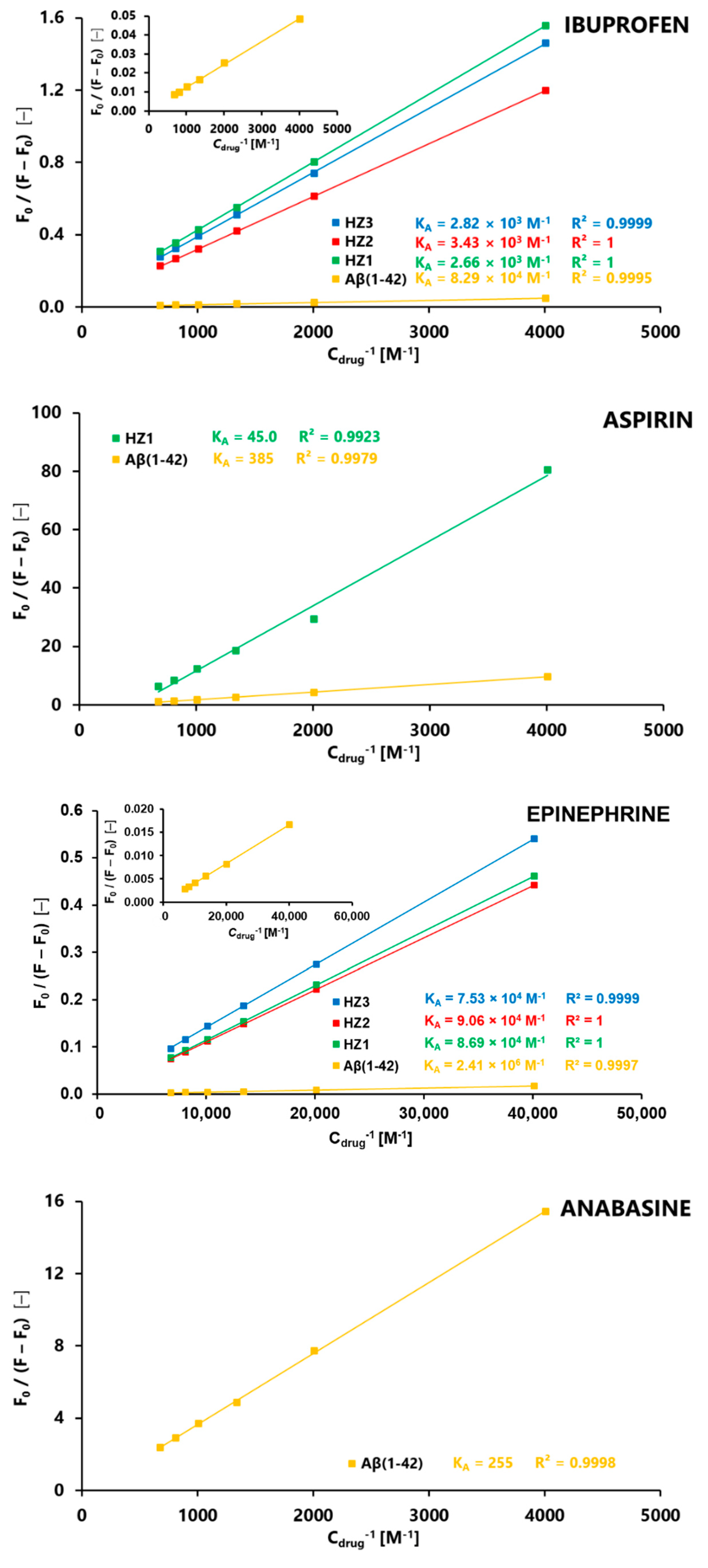
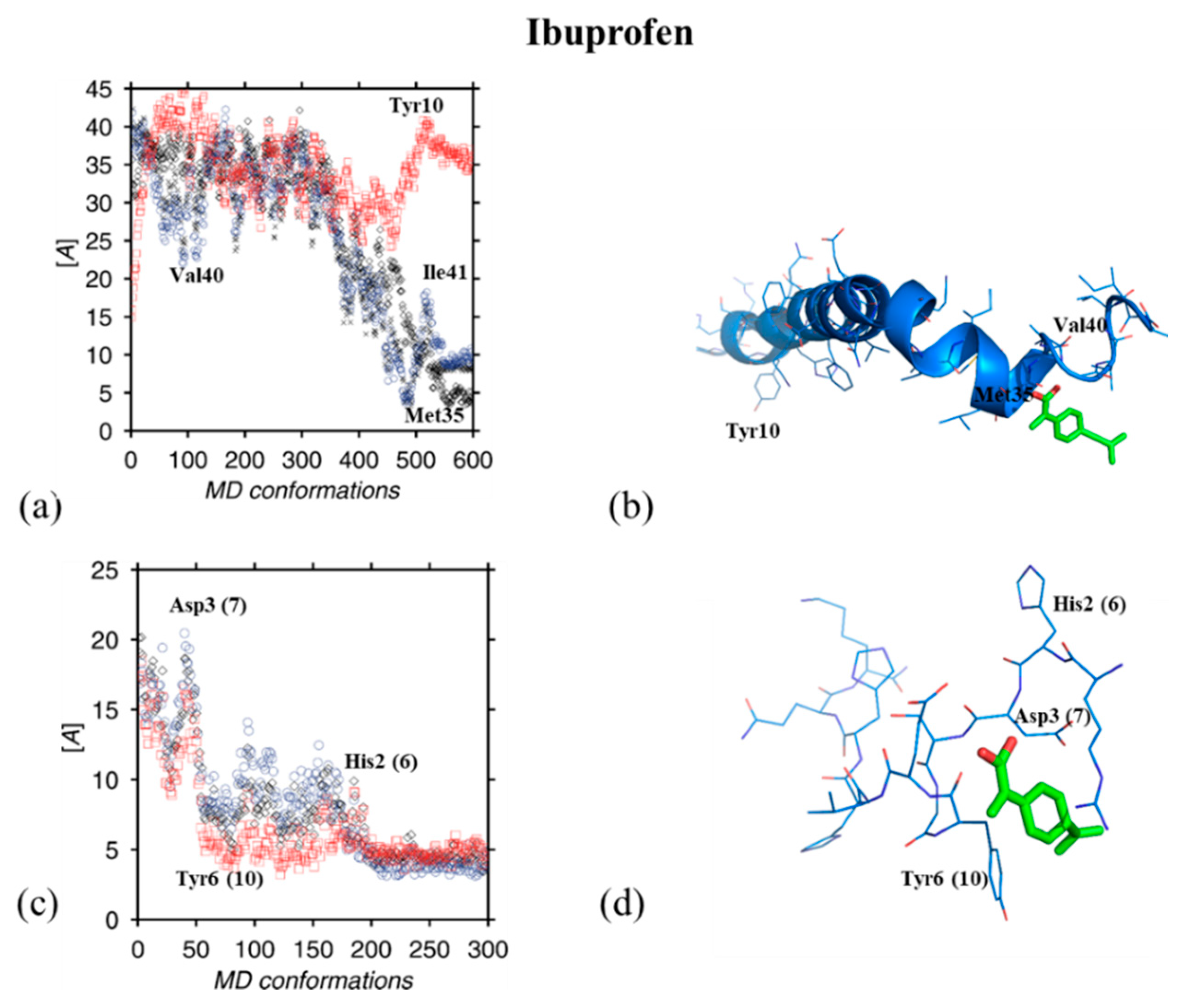
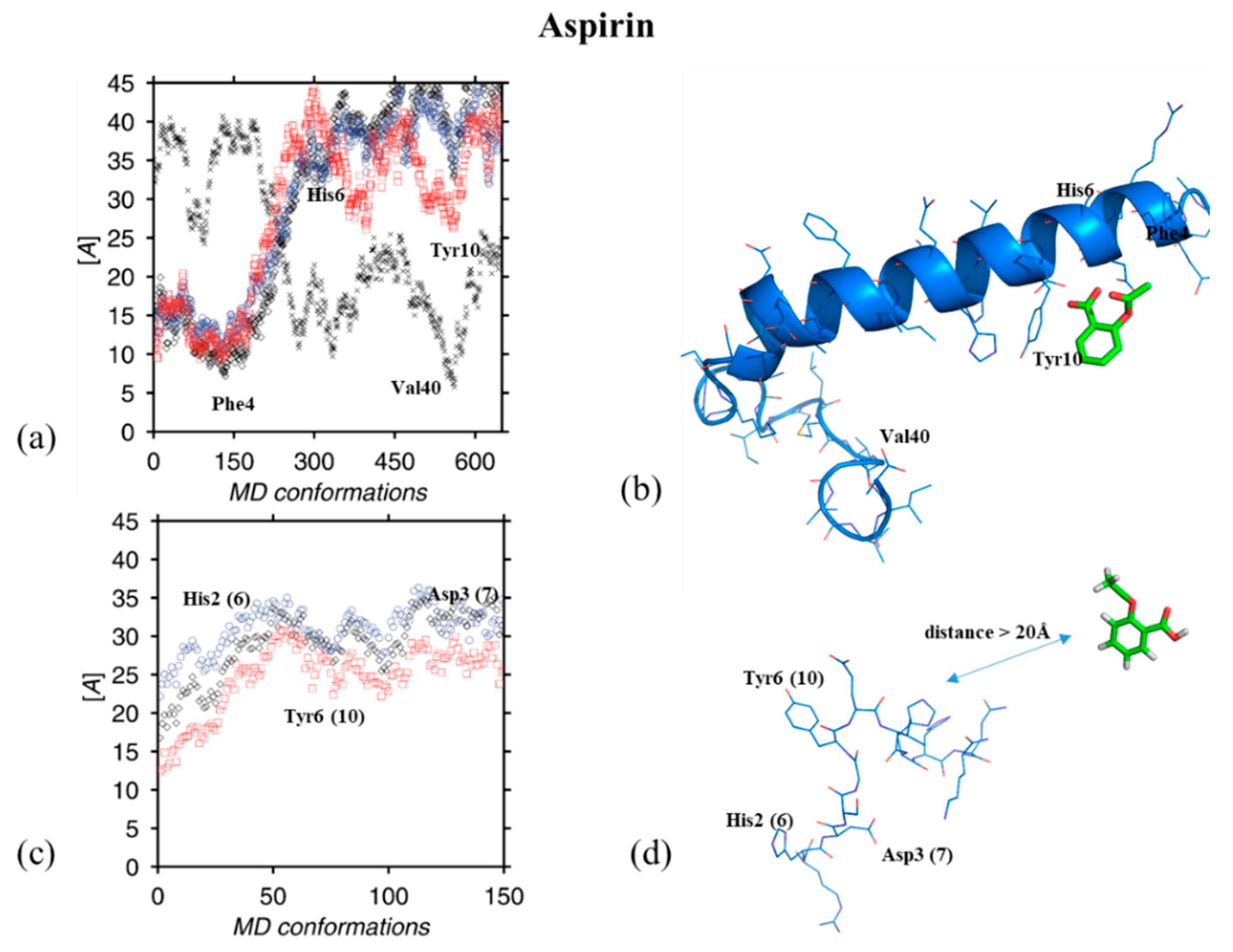
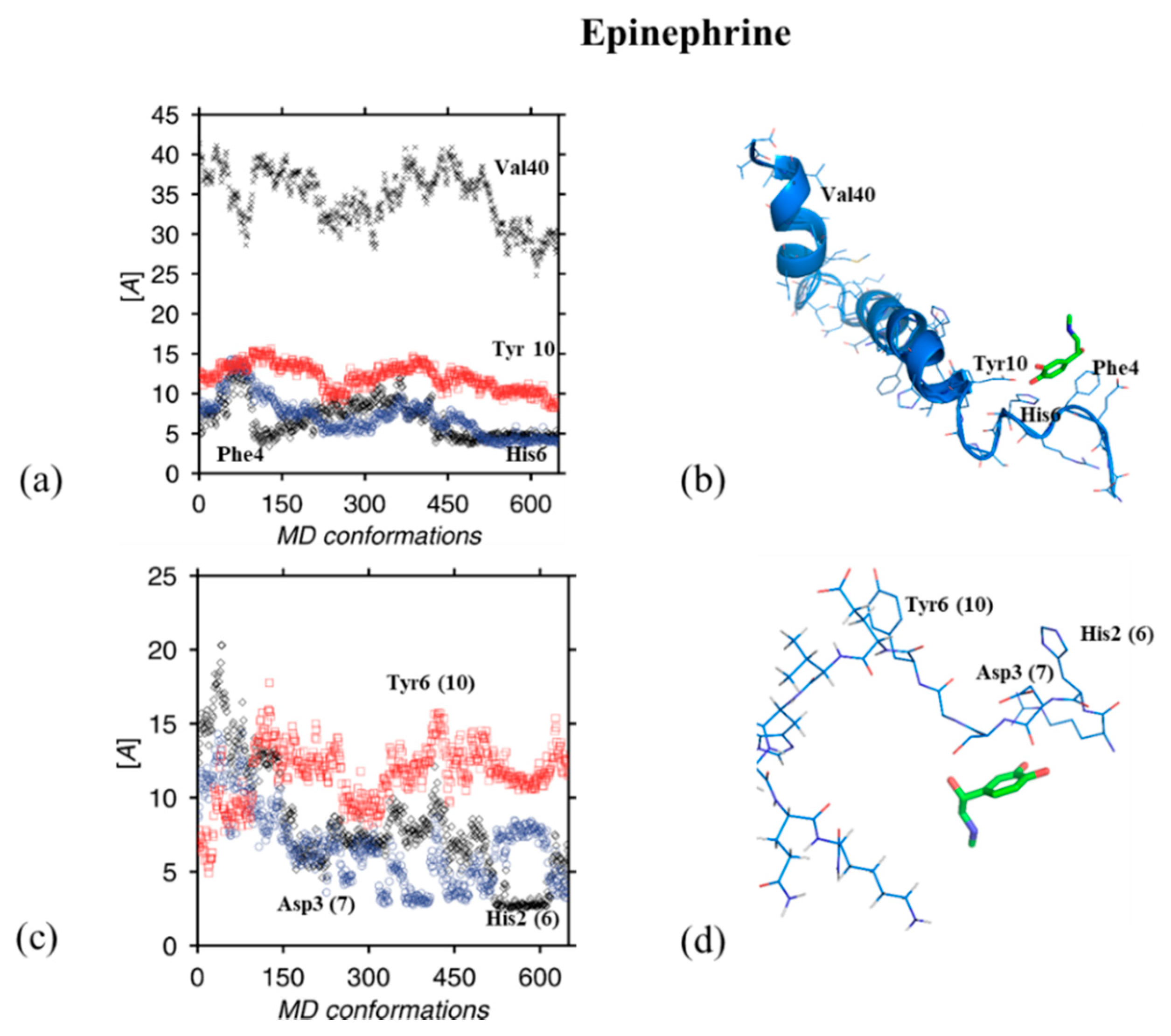
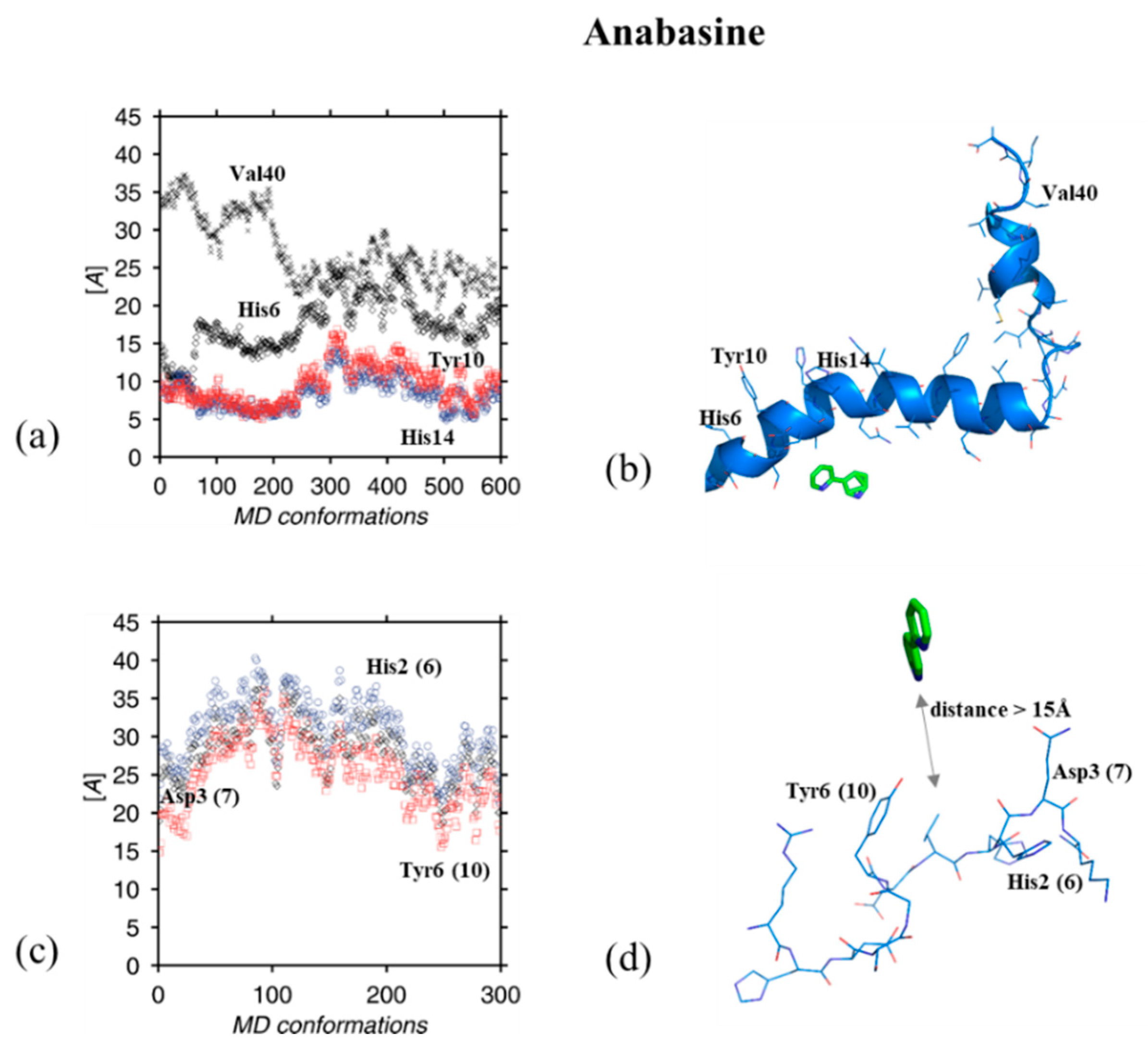
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Minati, L.; Edginton, T.; Grazia Bruzzone, M.; Giaccone, G. Reviews: Current concepts in Alzheimer’s disease: A multidisciplinary review. Am. J. Alzheimer’s Dis. Other Dement. 2009, 24, 95–121. [Google Scholar] [CrossRef]
- Wärmländer, S.; Tiiman, A.; Abelein, A.; Luo, J.; Jarvet, J.; Söderberg, K.L.; Danielsson, J.; Gräslund, A. Biophysical studies of the amyloid β-peptide: Interactions with metal ions and small molecules. ChemBioChem 2013, 14, 1692–1704. [Google Scholar] [CrossRef] [PubMed]
- Hiremathad, A. A review: Natural compounds as anti-Alzheimer’s disease agents. Curr. Nutr. Food Sci. 2017, 13, 247–254. [Google Scholar] [CrossRef]
- Perez, A.; Li, T.; Hernandez, S.; Zhang, R.; Cao, C. The rationale of using coffee and melatonin as an alternative treatment for Alzheimer’s disease. J. Alzheimer’s Dis. Parkinsonism 2016, 6, 2161-0460. [Google Scholar] [CrossRef]
- Zhao, D.; Simon, J.E.; Wu, Q. A critical review on grape polyphenols for neuroprotection: Strategies to enhance bioefficacy. Crit. Rev. Food Sci. Nutr. 2020, 60, 597–625. [Google Scholar] [CrossRef]
- Abd El Wahab, M.G.; Ali, S.S.; Ayuob, N.N. The role of musk in relieving the neurodegenerative changes induced after exposure to chronic stress. Am. J. Alzheimer’s Dis. Other Dement. 2018, 33, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.T.; Uddin, M.S.; Zaman, S.; Begum, Y.; Ashraf, G.M.; Bin-Jumah, M.N.; Bungau, S.G.; Mousa, S.A.; Abdel-Daim, M.M. Molecular mechanisms of metal toxicity in the pathogenesis of Alzheimer’s disease. Mol. Neurobiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Żamojć, K.; Zdrowowicz, M.; Rudnicki-Velasquez, P.B.; Krzymiński, K.; Zaborowski, B.; Niedziałkowski, P.; Jacewicz, D.; Chmurzyński, L. The development of 1,3-diphenylisobenzofuran as a highly selective probe for the detection and quantitative determination of hydrogen peroxide. Free Radic. Res. 2017, 51, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Żamojć, K.; Jacewicz, D.; Zdrowowicz, M.; Chmurzyński, L. Kinetics of the reaction between 1,3-diphenylisobenzofuran and nitrogen dioxide studied by steady-state fluorescence. Res. Chem. Intermed. 2013, 39, 3023–3031. [Google Scholar] [CrossRef]
- Hong, Y.; Zhi, S.; Sheng, C. Current advances in the treatment of Alzheimer’s disease: Focused on considerations targeting Aβ and tau. Transl. Neurodegener. 2012, 1, 21. [Google Scholar] [CrossRef]
- Ali, M.M.; Ghouri, R.G.; Ans, A.H.; Akbar, A.; Toheed, A. Recommendations for anti-inflammatory treatments in Alzheimer’s disease: A comprehensive review of the literature. Cureus 2019, 11, e4620. [Google Scholar] [CrossRef] [PubMed]
- Fendrick, A.M.; Greenberg, B.P. A review of the benefits and risks of nonsteroidal anti-inflammatory drugs in the management of mild-to-moderate osteoarthritis. Osteopath. Med. Prim. Care 2009, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Suleyman, H.; Demircan, B.; Karagoz, Y. Anti-inflammatory and side effects of cyclo-oxygenase inhibitors. Pharmacol. Rep. 2007, 59, 247–258. [Google Scholar]
- Akiyama, H.; Barger, S.; Barnum, S.; Bradt, B.; Bauer, J.; Cole, G.M.; Cooper, N.R.; Eikelenboom, P.; Emmerling, M.; Fiebich, B.L.; et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging 2000, 21, 383–421. [Google Scholar] [CrossRef]
- Jaturapatporn, D.; Isaac, M.G.E.K.N.; McCleery, J.; Tabet, N. Aspirin, steroidal and non-steroidal anti-inflammatory drugs for the treatment of Alzheimer’s disease. Cochrane Database Syst. Rev. 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Etminan, M.; Gill, S.; Samii, A. Effect of non-steroidal anti-inflammatory drugs on risk of Alzheimer’s disease: Systematic review and meta-analysis of observational studies. BMJ 2003, 327, 128. [Google Scholar] [CrossRef]
- Budimir, A. Metal ions, Alzheimer’s disease and chelation therapy. Acta Pharm. 2011, 61, 1–14. [Google Scholar] [CrossRef]
- Chandra, S.; Jana, M.; Pahan, K. Aspirin induces lysosomal biogenesis and attenuates amyloid plaque pathology in a mouse model of Alzheimer’s disease via PPARα. J. Neurosci. 2018, 38, 6682–6699. [Google Scholar] [CrossRef]
- Pasqualetti, P.; Bonomini, C.; Dal Forno, G.; Paulon, L.; Sinforiani, E.; Marra, C.; Zanetti, O.; Rossini, P.M. A randomized controlled study on effects of ibuprofen on cognitive progression of Alzheimer’s disease. Aging Clin. Exp. Res. 2009, 21, 102–110. [Google Scholar] [CrossRef]
- Sardiello, M.; Palmieri, M.; di Ronza, A.; Medina, D.L.; Valenza, M.; Gennarino, V.A.; Di Malta, C.; Donaudy, F.; Embrione, V.; Polishchuk, R.S.; et al. A gene network regulating lysosomal biogenesis and function. Science 2009, 325, 473–477. [Google Scholar] [CrossRef]
- Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M.D.C. Natural compounds for Alzheimer’s disease therapy: A systematic review of preclinical and clinical studies. Int. J. Mol. Sci. 2019, 20, 2313. [Google Scholar] [CrossRef] [PubMed]
- Swan, G.E.; Lessov-Schlaggar, C.N. The effects of tobacco smoke and nicotine on cognition and the brain. Neuropsychol. Rev. 2007, 17, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.A.; Huckerby, T.N.; Gibson, G.L.; Fullwood, N.J.; Turnbull, S.; Tabner, B.J.; El-Agnaf, O.M.A.; Allsop, D. Both the d-(+) and l-(−) enantiomers of nicotine inhibit Aβ aggregation and cytotoxicity. Biochemistry 2004, 43, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, I.; Radecka, H.; Burza, A.; Radecki, J.; Kaliszan, M.; Kaliszan, R. Association constants of pyridine and piperidine alkaloids to amyloid ß peptide determined by electrochemical impedance spectroscopy. Curr. Alzheimer Res. 2010, 7, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.D.; Hao, I.; Burke, D.A.; Cauley, M.; Hall, B.J.; Rezvani, A.H. Effects of tobacco smoke constituents, anabasine and anatabine, on memory and attention in female rats. J. Psychopharmacol. 2014, 28, 915–922. [Google Scholar] [CrossRef]
- Rodgman, A.; Perfetti, T.A. The Chemical Components of Tobacco and Tobacco Smoke; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Buckingham, S.D.; Jones, A.K.; Brown, L.A.; Sattelle, D.B. Nicotinic acetylcholine receptor signaling: Roles in Alzheimer’s disease and amyloid neuroprotection. Pharmacol. Rev. 2009, 61, 39–61. [Google Scholar] [CrossRef]
- Daly, J.W. Nicotinic agonists, antagonists, and modulators from natural sources. Cell. Mol. Neurobiol. 2005, 25, 513–552. [Google Scholar] [CrossRef]
- Pradel, K.; Blasiak, T.; Solecki, W.B. Adrenergic receptor agonists’ modulation of dopaminergic and non-dopaminergic neurons in the ventral tegmental area. Neuroscience 2018, 375, 119–134. [Google Scholar] [CrossRef]
- Raskind, M.A.; Wilkinson, C.W.; Peskind, E.R. Aging and Alzheimer’s disease. Horm. Brain Behav. 2002, 5, 637–664. [Google Scholar]
- Oliveira, A.; Martinho, R.; Serrão, P.; Moreira-Rodrigues, M. Epinephrine released during traumatic events may strengthen contextual fear memory through increased hippocampus mRNA expression of Nr4a transcription factors. Front. Mol. Neurosci. 2018, 11, 334. [Google Scholar] [CrossRef]
- Makowska, J.; Żamojć, K.; Wyrzykowski, D.; Żmudzińska, W.; Uber, D.; Wierzbicka, M.; Wiczk, W.; Chmurzyński, L. Probing the binding of Cu2+ ions to a fragment of the Aβ(1–42) polypeptide using fluorescence spectroscopy, isothermal titration calorimetry and molecular dynamics simulations. Biophys. Chem. 2016, 216, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Hureau, C.; Dorlet, P. Coordination of redox active metal ions to the amyloid precursor protein and to amyloid-β peptides involved in Alzheimer disease. Part 2: Dependence of Cu(II) binding sites with Aβ sequences. Coord. Chem. Rev. 2012, 256, 2175–2187. [Google Scholar] [CrossRef]
- Kim, D.; Kim, N.H.; Kim, S.H. 34 GHz pulsed ENDOR characterization of the copper coordination of an amyloid β peptide relevant to Alzheimer’s disease. Angew. Chem. Int. Ed. 2013, 52, 1139–1142. [Google Scholar] [CrossRef] [PubMed]
- Crescenzi, O.; Tomaselli, S.; Guerrini, R.; Salvadori, S.; D’Ursi, A.M.; Temussi, P.A.; Picone, D. Solution structure of the Alzheimer amyloid β-peptide (1–42) in an apolar microenvironment: Similarity with a virus fusion domain. Eur. J. Biochem. 2002, 269, 5642–5648. [Google Scholar] [CrossRef]
- Uber, D.; Wyrzykowski, D.; Tiberi, C.; Sabatino, G.; Żmudzińska, W.; Chmurzyński, L.; Papini, A.M.; Makowska, J. Conformation-dependent affinity of Cu (II) ions peptide complexes derived from the human Pin1 protein. J. Therm. Anal. Calorim. 2017, 127, 1431–1443. [Google Scholar] [CrossRef]
- Żamojć, K.; Kamrowski, D.; Zdrowowicz, M.; Wyrzykowski, D.; Wiczk, W.; Chmurzyński, L.; Makowska, J. A pentapeptide with tyrosine moiety as fluorescent chemosensor for selective nanomolar-level detection of copper(II) ions. Int. J. Mol. Sci. 2020, 21, 743. [Google Scholar] [CrossRef]
- Żamojć, K.; Zdrowowicz, M.; Hać, A.; Witwicki, M.; Rudnicki-Velasquez, P.B.; Wyrzykowski, D.; Wiczk, W.; Chmurzyński, L. Dihydroxy-substituted coumarins as fluorescent probes for nanomolar-level detection of the 4-amino-TEMPO spin label. Int. J. Mol. Sci. 2019, 20, 3802. [Google Scholar] [CrossRef]
- Makowska, J.; Żamojć, K.; Wyrzykowski, D.; Wiczk, W.; Chmurzyński, L. Copper (II) complexation by fragment of central part of FBP28 protein from Mus musculus. Biophys. Chem. 2018, 241, 55–60. [Google Scholar] [CrossRef]
- Case, D.A.; Berryman, J.; Betz, R.M.; Cerutti, D.S.; Cheatham, T.E., III; Darden, T.A.; Walker, R.C.; Onufriev, A.; Izadi, S.; Wu, X.; et al. Amber 2015 Reference Manual; University of California: San Francisco, CA, USA, 2015. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żamojć, K.; Streńska, K.; Wyrzykowski, D.; Chmurzyński, L.; Makowska, J. Interactions of Aβ1-42 Peptide and Its Three Fragments (Aβ8-12, Aβ8-13, and Aβ5-16) with Selected Nonsteroidal Drugs and Compounds of Natural Origin. Symmetry 2020, 12, 1579. https://doi.org/10.3390/sym12101579
Żamojć K, Streńska K, Wyrzykowski D, Chmurzyński L, Makowska J. Interactions of Aβ1-42 Peptide and Its Three Fragments (Aβ8-12, Aβ8-13, and Aβ5-16) with Selected Nonsteroidal Drugs and Compounds of Natural Origin. Symmetry. 2020; 12(10):1579. https://doi.org/10.3390/sym12101579
Chicago/Turabian StyleŻamojć, Krzysztof, Karolina Streńska, Dariusz Wyrzykowski, Lech Chmurzyński, and Joanna Makowska. 2020. "Interactions of Aβ1-42 Peptide and Its Three Fragments (Aβ8-12, Aβ8-13, and Aβ5-16) with Selected Nonsteroidal Drugs and Compounds of Natural Origin" Symmetry 12, no. 10: 1579. https://doi.org/10.3390/sym12101579
APA StyleŻamojć, K., Streńska, K., Wyrzykowski, D., Chmurzyński, L., & Makowska, J. (2020). Interactions of Aβ1-42 Peptide and Its Three Fragments (Aβ8-12, Aβ8-13, and Aβ5-16) with Selected Nonsteroidal Drugs and Compounds of Natural Origin. Symmetry, 12(10), 1579. https://doi.org/10.3390/sym12101579





