From Science to Practice: A Review of Laterality Research on Ungulate Livestock
Abstract
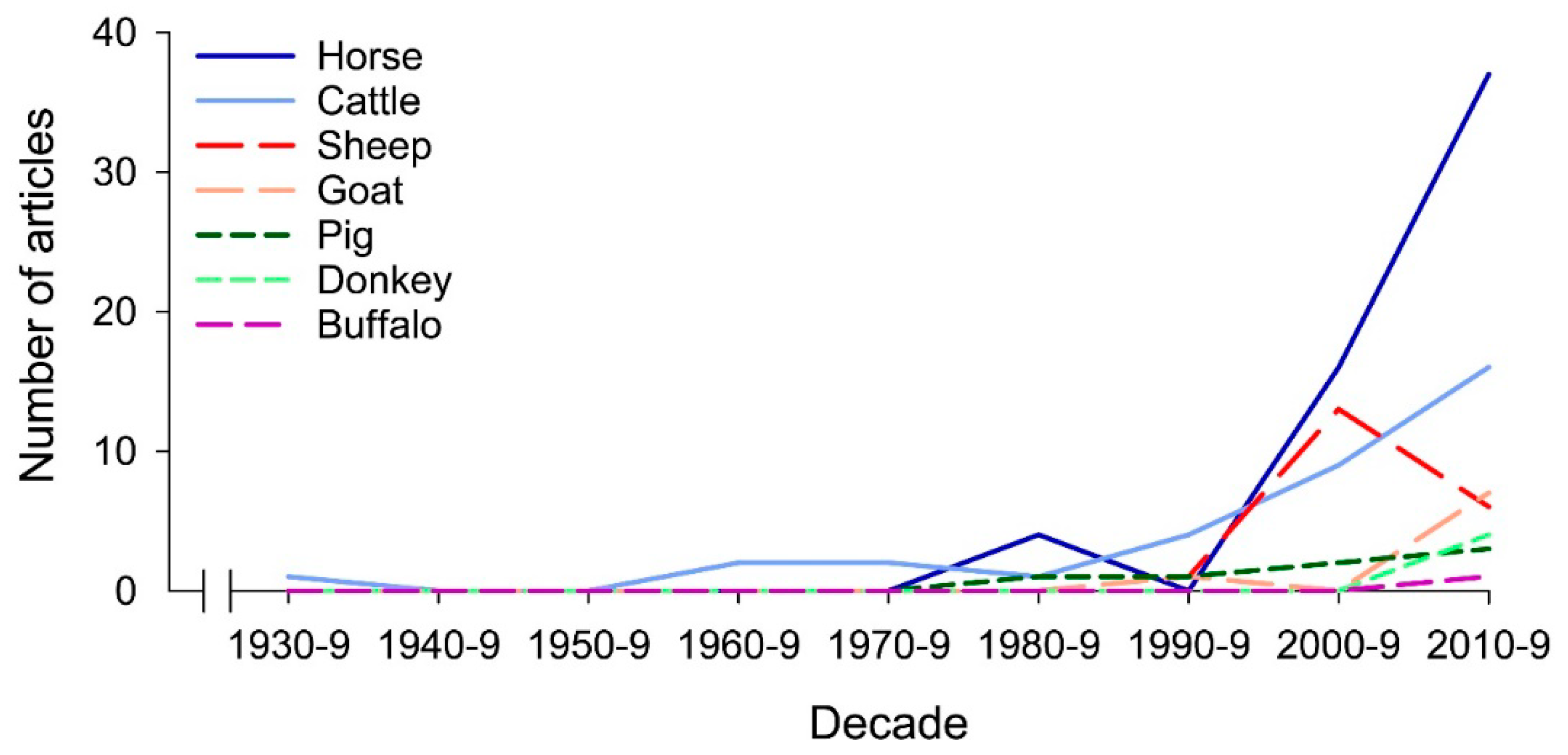
1. Introduction
2. Approach
3. Why Study Functional Laterality in Ungulate Livestock?
4. Insight Gained from Laterality Research on Ungulate Livestock
4.1. Motor Laterality
4.2. Cognitive Performance and Strategies
4.3. Emotional Processing
4.4. Development of Laterality and Sex Differences
4.5. Personality
4.6. Health, Stress and Welfare
4.7. Production and Performance
5. Future Directions
6. Summary and Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Vallortigara, G.; Rogers, L.J.; Bisazza, A. Possible evolutionary origins of cognitive brain lateralization. Brain Res. Rev. 1999, 30, 164–175. [Google Scholar] [CrossRef]
- Rogers, L.J.; Vallortigara, G. When and Why Did brains break symmetry? Symmetry 2015, 7, 2181–2194. [Google Scholar] [CrossRef]
- Vallortigara, G.; Rogers, L.J. Survival with an asymmetrical brain: Advantages and disadvantages of cerebral lateralization. Behav. Brain Sci. 2005, 28, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Ghirlanda, S.; Vallortigara, G. The evolution of brain lateralization: A game-theoretical analysis of population structure. Proc. R. Soc. B 2004, 271, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Halpern, M.E.; Güntürkün, O.; Hopkins, W.D.; Rogers, L.J. Lateralization of the vertebrate brain: Taking the side of model systems. J. Neurosci. 2005, 9, 10351–10357. [Google Scholar] [CrossRef] [PubMed]
- Meguerditchian, A.; Vauclair, J.; Hopkins, W.D. On the origins of human handedness and language: A comparative review of hand preferences for bimanual coordinated actions and gestural communication in nonhuman primates. Dev. Psychobiol. 2013, 55, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.J. Light experience and asymmetry of brain function in chickens. Nature 1982, 297, 223–225. [Google Scholar] [CrossRef]
- Chiandetti, C.; Gallusi, J.; Andrew, R.J.; Vallortigara, G. Early-light embryonic stimulation suggests a second route, via gene activation, to cerebral lateralization in vertebrates. Sci. Rep. 2013, 3, 2701. [Google Scholar] [CrossRef]
- Rogers, L.J. Asymmetry of brain and behavior in animals: Its development, function, and human relevance. Genesis 2014, 52, 555–571. [Google Scholar] [CrossRef]
- Williams, J. Laterality: Implications for equine management and performance. Vet. Nurse 2014, 2, 434–441. [Google Scholar] [CrossRef]
- Von Keyserlingk, M.A.G.; Weary, D.M. A 100-Year Review: Animal welfare in the Journal of Dairy Science-The first 100 years. J. Dairy Sci. 2017, 100, 10432–10444. [Google Scholar] [CrossRef] [PubMed]
- Veissier, I.; Miele, M. Animal welfare: Towards transdisciplinarity–the European experience. Anim. Prod. Sci. 2014, 54, 1119–1129. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data (accessed on 15 July 2019).
- Austin, N.P.; Rogers, L.J. Lateralization of agonistic and vigilance responses inPrzewalski horses (Equus przewalskii). Appl. Anim. Behav. Sci. 2014, 151, 43–50. [Google Scholar] [CrossRef]
- Karenina, K.; Giljov, A.; Malashichev, Y. Lateralization of mother-infant interactions in wild horses. Behav. Process. 2018, 148, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Arave, C.W.; Lamb, R.C.; Arambel, M.J.; Purcell, D.; Walters, J.L. Behaviour and maze learning ability of dairy calves as influenced by housing, sex and sire. Appl. Anim. Behav. Sci. 1992, 33, 149–163. [Google Scholar] [CrossRef]
- Murphy, J.; Hall, C.; Arkins, S. What Horses and Humans See: A Comparative Review. Int. J. Zool. 2009, 2009, 721798. [Google Scholar] [CrossRef]
- Piggins, D.; Phillips, C.J.C. The eye of the domesticated sheep with implications for vision. Anim. Sci. 1996, 62, 301–308. [Google Scholar] [CrossRef]
- Cummings, J.F.; Lahunta, A. An experimental study of the retinal projections in the horse and sheep. Ann. N. Y. Acad. Sci. 1969, 167, 293–318. [Google Scholar] [CrossRef]
- Herron, M.A.; Martin, J.E.; Joyce, J.R. Quantitative study of decussating optic axons in pony, cow, sheep, and pig. Am. J. Vet. Res. 1978, 39, 1137–1139. [Google Scholar]
- Reefmann, N.; Bütikofer Kaszàs, F.; Wechsler, B.; Gygax, L. Ear and tail postures as indicators of emotional valence in sheep. Appl. Anim. Behav. Sci. 2009, 118, 199–207. [Google Scholar] [CrossRef]
- Goursot, C.; Düpjan, S.; Tuchscherer, A.; Puppe, B.; Leliveld, L.M.C. Behavioural lateralization in domestic pigs (Sus scrofa)—Variations between motor functions and individuals. Laterality 2018, 23, 576–598. [Google Scholar] [CrossRef] [PubMed]
- Stolba, A.; Wood-Gush, D.G.M. The behavior of pigs in a semi-natural environment. Anim. Prod. 1989, 48, 419–425. [Google Scholar]
- Proctor, H.S.; Carder, G. Can ear postures reliably measure the positive emotional state of cows? Appl. Anim. Behav. Sci. 2014, 161, 20–27. [Google Scholar] [CrossRef]
- Reimert, I.; Bolhuis, J.E.; Kemp, B.; Rodenburg, T.B. Indicators of positive and negative emotions and emotional contagion in pigs. Physiol. Behav. 2013, 109, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Briefer, E.F.; Tettamanti, F.; McElligott, A.G. Emotions in goats: Mapping physiological, behavioral and vocal profiles. Anim. Behav. 2015, 99, 131–143. [Google Scholar] [CrossRef]
- Wathan, J.; Proops, L.; Grounds, K.; Mccomb, K. Horses discriminate between facial expressions of conspecifics. Sci. Rep. 2016, 6, 38322. [Google Scholar] [CrossRef]
- Halley, A.C. Minimal variation in eutherian brain growth rates during fetal neurogenesis. Proc. R. Soc. B 2017, 284, 20170219. [Google Scholar] [CrossRef] [PubMed]
- Ernst, L.; Darschnik, S.; Roos, J.; González, M.; Christa, G.; Beemelmans, C.; Beemelmans, C.; Engelhardt, M.; Meyer, G.; Wahle, P. Fast prenatal development of the NPY neuron system in the neocortex of the European wild boar, Sus scrofa. Brain Struct. Funct. 2018, 223, 3855–3873. [Google Scholar] [CrossRef]
- Keerthipriya, P.; Tewari, R.; Vidya, T.N.C. Lateralization in trunk and forefoot movements in a population of free-ranging Asian elephants (Elephas maximus). J. Comp. Psychol. 2015, 129, 377–387. [Google Scholar] [CrossRef]
- Price, E.O. Behavioral aspects of animal domestication. Q. Rev. Biol. 1984, 59, 1–32. [Google Scholar] [CrossRef]
- Kaiser, S.; Hennessy, M.B.; Sachser, N. Domestication affects the structure, development and stability of biobehavioral profiles. Front. Zool. 2015, 12 (Suppl. 1), S19. [Google Scholar] [CrossRef]
- Ratcliffe, V.F.; Reby, D. Orienting asymmetries in dogs’ responses to different communicatory components of human speech. Curr. Biol. 2014, 24, 2908–2912. [Google Scholar] [CrossRef] [PubMed]
- Siniscalchi, M.; d’Ingeo, S.; Fornelli, S.; Quaranta, A. Lateralized behavior and cardiac activity of dogs in response to human emotional vocalizations. Sci. Rep. 2018, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Nawroth, C.; Albuquerque, N.; Savalli, C.; Single, M.-S.; McElligott, A.G. Goats prefer positive human emotional facial expressions. R. Soc. Open Sci. 2018, 5, 180491. [Google Scholar] [CrossRef] [PubMed]
- Bensoussan, S.; Tigeot, R.; Lemasson, A.; Meunier-salaün, M.-C.; Tallet, C. Domestic piglets (Sus scrofa domestica) are attentive to human voice and able to discriminate some prosodic features. Appl. Anim. Behav. Sci. 2019, 210, 38–45. [Google Scholar] [CrossRef]
- Proops, L.; McComb, K. Attributing attention: The use of human-given cues by domestic horses (Equus caballus). Anim. Cogn. 2010, 13, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.; Nordquist, R.E.; van der Staay, F.J. A review of behavioral methods to study emotion and mood in pigs, Sus scrofa. Appl. Anim. Behav. Sci. 2014, 159, 9–28. [Google Scholar] [CrossRef]
- Forkman, B.; Boissy, A.; Meunier-Salaün, M.C.; Canali, E.; Jones, R.B. A critical review of fear tests used on cattle, pigs, sheep, poultry and horses. Physiol. Behav. 2007, 92, 340–374. [Google Scholar] [CrossRef]
- Finkemeier, M.-A.; Langbein, J.; Puppe, B. Personality Research in Mammalian Farm Animals: Concepts, Measures, and Relationship to Welfare. Front. Vet. Sci. 2018, 5, 1–15. [Google Scholar] [CrossRef]
- Nawroth, C.; Langbein, J.; Coulon, M.; Gabor, V.; Oesterwind, S.; Benz-Schwarzburg, J.; von Borell, E. Farm Animal Cognition—Linking Behavior, Welfare and Ethics. Front. Vet. Sci. 2019, 6, 1–16. [Google Scholar] [CrossRef]
- Lind, N.M.; Moustgaard, A.; Jelsing, J.; Vajta, G.; Cumming, P.; Hansen, A.K. The use of pigs in neuroscience: Modeling brain disorders. Neurosci. Biobehav. Rev. 2007, 31, 728–751. [Google Scholar] [CrossRef] [PubMed]
- Tate, A.J.; Fischer, H.; Leigh, A.E.; Kendrick, K.M. Behavioural and neurophysiological evidence for face identity and face emotion processing in animals. Philos. Trans. R. Soc. B 2006, 361, 2155–2172. [Google Scholar] [CrossRef] [PubMed]
- Leliveld, L.M.C.; Langbein, J.; Puppe, B. The emergence of emotional lateralization: Evidence in non-human vertebrates and implications for farm animals. Appl. Anim. Behav. Sci. 2013, 145, 1–14. [Google Scholar] [CrossRef]
- Rogers, L.J. Relevance of brain and behavioral lateralization to animal welfare. Appl. Anim. Behav. Sci. 2010, 127, 1–11. [Google Scholar] [CrossRef]
- Morgante, M.; Vallortigara, G. Animal welfare: Neuro-cognitive approaches. Ital. J. Anim. Sci. 2009, 8, 255–264. [Google Scholar] [CrossRef]
- Ocklenburg, S.; Korte, S.M.; Peterburs, J.; Wolf, O.T.; Güntürkün, O. Stress and laterality-The comparative perspective. Physiol. Behav. 2016, 164, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Sumner, R.C.; Paerton, A.; Nowicky, A.V.; Kishore, U.; Gidron, Y. Hemispheric lateralisation and immune function: A systematic review of human research. J. Neuroimmunol. 2011, 240–241, 1–12. [Google Scholar] [CrossRef]
- Colborne, G.R.; Heaps, L.A.; Franklin, S.H. Horizontal moment around the hoofʼs center of pressure during walking in a straight line. Equine Vet. J. 2009, 41, 242–246. [Google Scholar] [CrossRef]
- Deuel, N.R.; Lawrence, L.M. Laterality in the gallop gait of horses. J. Biomech. 1987, 20, 645–649. [Google Scholar] [CrossRef]
- Oosterlinck, M.; Hardeman, L.C.; van der Meij, B.R.; Veraa, S.; van der Kolk, J.H.; Wijnberg, I.D.; Pille, F.; Back, W. Pressure plate analysis of toe-heel and medio-lateral hoof balance at the walk and trot in sound sport horses. Vet. J. 2013, 198 (Suppl.1), 9–13. [Google Scholar] [CrossRef]
- Starke, S.D.; Raistrick, K.J.; May, S.A.; Pfau, T. The effect of trotting speed on the evaluation of subtle lameness in horses. Vet. J. 2013, 197, 245–252. [Google Scholar] [CrossRef]
- Williams, D.E.; Norris, B.J. Laterality in stride pattern preferences in racehorses. Anim. Behav. 2007, 74, 941–950. [Google Scholar] [CrossRef]
- Drevemo, S.; Fredricson, I.; Hjertén, G.; McMiken, D. Early development of gait asymmetries in trotting Standardbred colts. Equine Vet. J. 1987, 19, 189–191. [Google Scholar] [CrossRef]
- McGreevy, P.D.; Rogers, L.J. Motor and sensory laterality in thoroughbred horses. Appl. Anim. Behav. Sci. 2005, 92, 337–352. [Google Scholar] [CrossRef]
- Warren-Smith, A.; McGreevy, P. The use of pedometers to estimate motor laterality in grazing horses. J. Vet. Behav. Clin. Appl. Res. 2010, 5, 177–179. [Google Scholar] [CrossRef]
- McGreevy, P.D.; Thomson, P.C. Differences in motor laterality between breeds of performance horse. Appl. Anim. Behav. Sci. 2006, 99, 183–190. [Google Scholar] [CrossRef]
- Wells, A.E.D.; Blache, D. Horses do not exhibit motor bias when their balance is challenged. Animal 2008, 2, 1645–1650. [Google Scholar] [CrossRef]
- Siniscalchi, M.; Padalino, B.; Lusito, R.; Quaranta, A. Is the left forelimb preference indicative of a stressful situation in horses? Behav. Process. 2014, 107, 61–67. [Google Scholar] [CrossRef]
- Colborne, G.R.; Routh, J.E.; Weir, K.R.; McKendry, J.E.; Busschers, E. Associations between hoof shape and the position of the frontal plane ground reaction force vector in walking horses. N. Z. Vet. J. 2016, 64, 76–81. [Google Scholar] [CrossRef]
- Cully, P.; Nielsen, B.; Lancaster, B.; Martin, J.; McGreevy, P. The laterality of the gallop gait in Thoroughbred racehorses. PLoS ONE 2018, 13, e0198545. [Google Scholar] [CrossRef]
- Drevemo, S.; Fredricson, I.; Dalin, G.; Bjorne, K. Equine locomotion: 2. The analysis of coordination between limbs of trotting Standardbreds. Equine Vet. J. 1980, 12, 66–70. [Google Scholar] [CrossRef]
- Murphy, J.; Arkins, S. Facial hair whorls (trichoglyphs) and the incidence of motor laterality in the horse. Behav. Process. 2008, 79, 7–12. [Google Scholar] [CrossRef]
- Murphy, J.; Sutherland, A.; Arkins, S. Idiosyncratic motor laterality in the horse. Appl. Anim. Behav. Sci. 2005, 91, 297–310. [Google Scholar] [CrossRef]
- Oosterlinck, M.; Pille, F.; Back, W.; Dewulf, J.; Gasthuys, F. Use of a stand-alone pressure plate for the objective evaluation of forelimb symmetry in sound ponies at walk and trot. Vet. J. 2010, 183, 305–309. [Google Scholar] [CrossRef]
- Weishaupt, M.A.; Wiestner, T.; Hogg, H.P.; Jordan, P.; Auer, J.A. Vertical ground reaction force-time histories of sound Warmblood horses trotting on a treadmill. Vet. J. 2004, 168, 304–311. [Google Scholar] [CrossRef]
- Austin, N.P.; Rogers, L.J. Limb preferences and lateralization of aggression, reactivity and vigilance in feral horses, Equus caballus. Anim. Behav. 2012, 83, 239–247. [Google Scholar] [CrossRef]
- Austin, N.P.; Rogers, L.J. Asymmetry of flight and escape turning responses in horses. Laterality. 2007, 12, 464–474. [Google Scholar] [CrossRef]
- Komárková, M.; Bartošová, J. Lateralized suckling in domestic horses (Equus caballus). Anim. Cogn. 2013, 16, 343–349. [Google Scholar] [CrossRef]
- Whishaw, I.Q. Absence of population asymmetry in the American Quarter Horse (Equus ferus caballus) performing skilled left and right manoeuvres in reining competition. Laterality 2015, 20, 604–617. [Google Scholar] [CrossRef]
- Grant, R.J.; Colenbrander, V.F.; Albright, J.L. Effect of particle size of forage and rumen cannulation upon chewing activity and laterality in dairy cows. J. Dairy Sci. 1990, 73, 3158–3164. [Google Scholar] [CrossRef]
- Hixson, C.L.; Krawczel, P.D.; Caldwell, J.M.; Miller-Cushon, E.K. Behavioral changes in group-housed dairy calves infected with Mannheimia haemolytica. J. Dairy Sci. 2018, 101, 10351–10360. [Google Scholar] [CrossRef]
- Wagnon, K.A.; Rollins, W.C. Bovine laterality. J. Anim. Sci. 1972, 35, 486–488. [Google Scholar] [CrossRef]
- Eberhart, N.L.; Storer, J.M.; Caldwell, M.; Saxton, A.M.; Krawczel, P.D. Behavioral and physiologic changes in Holstein steers experimentally infected with Mannheimia haemolytica. Am. J. Vet. Res. 2017, 78, 1056–1064. [Google Scholar] [CrossRef]
- Boa, J.; Giller, P.S. Observations on the changes in behavioral activities of dairy cows prior to and after parturition. Ir. Vet. J. 1991, 44, 43–47. [Google Scholar]
- Eberhart, N.L.; Krawczel, P.D. The effect of hock injury laterality and lameness on lying behaviors and lying laterality in holstein dairy cows. Animals 2017, 7, 86. [Google Scholar] [CrossRef]
- Forsberg, A.M.; Pettersson, G.; Ljungberg, T.; Svennersten-Sjaunja, K. A brief note about cow lying behavior-Do cows choose left and right lying side equally? Appl. Anim. Behav. Sci. 2008, 114, 32–36. [Google Scholar] [CrossRef]
- Phillips, C.J.C.; Llewellyn, S.; Claudia, A. Laterality in bovine behavior in an extensive partially suckled herd and an intensive dairy herd. J. Dairy Sci. 2003, 86, 3167–3173. [Google Scholar] [CrossRef]
- Tucker, C.B.; Cox, N.R.; Weary, D.M.; Špinka, M. Laterality of lying behavior in dairy cattle. Appl. Anim. Behav. Sci. 2009, 120, 125–131. [Google Scholar] [CrossRef]
- Uhrbrock, R.S. Bovine laterality. J. Genet. Psychol. Res. Theory. Hum. Dev. 1969, 115, 77–79. [Google Scholar] [CrossRef]
- Hopster, H.; Van Der Werf, J.T.N.; Blokhuis, H.J. Side preference of dairy cows in the milking parlor and its effects on behavior and heart rate during milking. Appl. Anim. Behav. Sci. 1998, 55, 213–229. [Google Scholar] [CrossRef]
- Fahim, A.D.; Kamboj, M.L.; Bhakat, M.; Mohanty, T.K.; Gupta, R. Preference of side and standing in relationship with milking characteristics and temperament score of crossbred dairy cows in an 8 × 2 herringbone milking parlor. Turkish J. Vet. Anim. Sci. 2018, 42, 49–54. [Google Scholar] [CrossRef]
- Paranhos da Costa, M.J.P.; Broom, D.M. Consistency of side choice in the milking parlor by holstein-friesian cows and its relationship with their reactivity and milk yield. Appl. Anim. Behav. Sci. 2001, 70, 177–186. [Google Scholar] [CrossRef]
- Versace, E.; Morgante, M.; Pulina, G.; Vallortigara, G. Behavioural lateralization in sheep (Ovis aries). Behav. Brain Res. 2007, 184, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Barnard, S.; Matthews, L.; Messori, S.; Podaliri-Vulpiani, M.; Ferri, N. Laterality as an indicator of emotional stress in ewes and lambs during a separation test. Anim. Cogn. 2016, 19, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Navarro, G.; Santurtun, E.; Phillips, C.J.C. Effects of simulated sea motion on stepping behavior in sheep. Appl. Anim. Behav. Sci. 2017, 188, 17–25. [Google Scholar] [CrossRef]
- Anderson, D.M.; Murray, L.W. Sheep laterality. Laterality 2013, 18, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Lane, A.; Phillips, C. A note on behavioral laterality in neonatal lambs. Appl. Anim. Behav. Sci. 2004, 86, 161–167. [Google Scholar] [CrossRef]
- Hosoi, E.; Swift, D.M.; Rittenhouse, L.R.; Richards, R.W. Comparative foraging strategies of sheep and goats in a T-maze apparatus. Appl. Anim. Behav. Sci. 1995, 44, 37–45. [Google Scholar] [CrossRef]
- Baruzzi, C.; Nawroth, C.; McElligott, A.; Baciadonna, L. Motor asymmetry in goats during a stepping task. Laterality 2018, 23, 599–609. [Google Scholar] [CrossRef]
- Nawroth, C.; Baciadonna, L.; McElligott, A.G. Goats learn socially from humans in a spatial problem-solving task. Anim. Behav. 2016, 121, 123–129. [Google Scholar] [CrossRef]
- Špinka, M.; Stěhulová, I.; Zachařová, J.; Maletínská, J.; Illmann, G. Nursing behavior and nursing vocalisations in domestic sows: Repeatability and relationship with maternal investment. Behaviour 2002, 139, 1077–1097. [Google Scholar]
- Zucca, P.; Cerri, F.; Carluccio, A.; Baciadonna, L. Space availability influence laterality in donkeys (Equus asinus). Behav. Process. 2011, 88, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Meij, H.S.; Meij, J.C.P. Functional asymmetry in the motor system of the horse. S. Afr. J. Sci. 1980, 76, 552–556. [Google Scholar]
- Haussler, K.K.; Erb, H.N. Mechanical nociceptive thresholds in the axial skeleton of horses. Equine Vet. J. 2006, 38, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Larose, C.; Richard-Yris, M.A.; Hausberger, M.; Rogers, L.J. Laterality of horses associated with emotionality in novel situations. Laterality 2006, 11, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Van Heel, M.C.V.; Kroekenstoel, A.M.; van Dierendonck, M.C.; van Weeren, P.R.; Back, W. Uneven feet in a foal may develop as a consequence of lateral grazing behavior induced by conformational traits. Equine Vet. J. 2006, 38, 646–651. [Google Scholar] [CrossRef]
- De Boyer Des Roches, A.; Richard-Yris, M.-A.; Henry, S.; EzzaouÏa, M.; Hausberger, M. Laterality and emotions: Visual laterality in the domestic horse (Equus caballus) differs with objects’ emotional value. Physiol. Behav. 2008, 94, 487–490. [Google Scholar] [CrossRef]
- Haussler, K.K.; Behre, T.H.; Hill, A.E. Mechanical nociceptive thresholds within the pastern region of Tennessee Walking Horses. Equine Vet. J. 2008, 40, 455–459. [Google Scholar] [CrossRef]
- Basile, M.; Boivin, S.; Boutin, A.; Blois-Heulin, C.; Hausberger, M.; Lemasson, A. Socially dependent auditory laterality in domestic horses (Equus caballus). Anim. Cogn. 2009, 12, 611–619. [Google Scholar] [CrossRef]
- Thorpe, C.T.; Marlin, D.J.; Franklin, S.H.; Colborne, G.R. Transverse and dorso-ventral changes in thoracic dimension during equine locomotion. Vet. J. 2009, 179, 370–377. [Google Scholar] [CrossRef]
- Farmer, K.; Krueger, K.; Byrne, R.W. Visual laterality in the domestic horse (Equus caballus) interacting with humans. Anim. Cogn. 2010, 13, 229–238. [Google Scholar] [CrossRef]
- Van Heel, M.C.V.; van Dierendonck, M.C.; Kroekenstoel, A.M.; Back, W. Lateralised motor behavior leads to increased unevenness in front feet and asymmetry in athletic performance in young mature Warmblood horses. Equine Vet. J. 2010, 42, 444–450. [Google Scholar] [CrossRef]
- Baragli, P.; Vitale, V.; Paoletti, E.; Sighieri, C.; Reddon, A.R. Detour behavior in horses (Equus caballus). J. Ethol. 2011, 29, 227–234. [Google Scholar] [CrossRef]
- De Boyer Des Roches, A.; Durier, V.; Richard-Yris, M.-A.; Blois-Heulin, C.; Ezzaouïa, M.; Hausberger, M.; Henry, S. Differential outcomes of unilateral interferences at birth. Biol. Lett. 2011, 7, 177–180. [Google Scholar] [CrossRef]
- Heaps, L.A.; Franklin, S.H.; Colborne, G.R. Horizontal moment around the hoof center of pressure during walking on right and left circles. Equine Vet. J. 2011, 43, 190–195. [Google Scholar] [CrossRef]
- Sankey, C.; Henry, S.; Clouard, C.; Richard-Yris, M.A.; Hausberger, M. Asymmetry of behavioral responses to a human approach in young naive vs. trained horses. Physiol. Behav. 2011, 104, 464–468. [Google Scholar] [CrossRef]
- König von Borstel, U.; Keil, J. Horses’ behavior and heart rate in a preference test for shorter and longer riding bouts. J. Vet. Behav. Clin. Appl. Res. 2012, 7, 362–374. [Google Scholar] [CrossRef]
- Pfau, T.; Stubbs, N.C.; Kaiser, L.J.; Brown, L.E.; Clayton, H.M. Effect of trotting speed and circle radius on movement symmetry in horses during lunging on a soft surface. Am. J. Vet. Res. 2012, 73, 1890–1899. [Google Scholar] [CrossRef]
- Proops, L.; McComb, K. Cross-modal individual recognition in domestic horses (Equus caballus) extends to familiar humans. Proc. R. Soc. B 2012, 279, 3131–3138. [Google Scholar] [CrossRef]
- Lucidi, P.; Bacco, G.; Sticco, M.; Mazzoleni, G.; Benvenuti, M.; Bernabò, N.; Trentini, R. Assessment of motor laterality in foals and young horses (Equus caballus) through an analysis of derailment at trot. Physiol. Behav. 2013, 109, 8–13. [Google Scholar] [CrossRef]
- Brocklehurst, C.; Weller, R.; Pfau, T. Effect of turn direction on body lean angle in the horse in trot and canter. Vet. J. 2014, 199, 258–262. [Google Scholar] [CrossRef]
- Ahrendt, L.P.; Labouriau, R.; Malmkvist, J.; Nicol, C.J.; Christensen, J.W. Development of a standard test to assess negative reinforcement learning in horses. Appl. Anim. Behav. Sci. 2015, 169, 38–42. [Google Scholar] [CrossRef]
- Siniscalchi, M.; Padalino, B.; Aubé, L.; Quaranta, A. Right-nostril use during sniffing at arousing stimuli produces higher cardiac activity in jumper horses. Laterality 2015, 20, 483–500. [Google Scholar] [CrossRef]
- Lopes, M.A.F.; Dearo, A.C.O.; Lee, A.; Reed, S.K.; Kramer, J.; Pai, P.F.; Yonezawa, Y.; Maki, H.; Morgan, T.L.; Wilson, D.A.; et al. An attempt to detect lameness in galloping horses by use of body-mounted inertial sensors. Am. J. Vet. Res. 2016, 77, 1121–1131. [Google Scholar] [CrossRef]
- Shivley, C.; Grandin, T.; Deesing, M. Behavioral laterality and facial hair whorls in horses. J. Equine Vet. Sci. 2016, 44, 62–66. [Google Scholar] [CrossRef]
- Smith, A.V.; Proops, L.; Grounds, K.; Wathan, J.; McComb, K. Functionally relevant responses to human facial expressions of emotion in the domestic horse (Equus caballus). Biol. Lett. 2016, 12, 20150907. [Google Scholar] [CrossRef]
- Van de Water, E.; Oosterlinck, M.; Pille, F. The effect of perineural anaesthesia and handler position on limb loading and hoof balance of the vertical ground reaction force in sound horses. Equine Vet. J. 2016, 48, 608–612. [Google Scholar] [CrossRef]
- Baragli, P.; Vitale, V.; Sighieri, C.; Lanata, A.; Palagi, E.; Reddon, A.R. Consistency and flexibility in solving spatial tasks: Different horses show different cognitive styles. Sci. Rep. 2017, 7, 16557. [Google Scholar] [CrossRef]
- Byström, A.; Egenvall, A.; Roepstorff, L.; Rhodin, M.; Bragança, F.S.; Hernlund, E.; van Weeren, R.; Weishaupt, M.A.; Clayton, H.M. Biomechanical findings in horses showing asymmetrical vertical excursions of the withers at walk. PLoS ONE 2018, 13, e0204548. [Google Scholar] [CrossRef]
- Farmer, K.; Krüger, K.; Byrne, R.W.; Marr, I. Sensory laterality in affiliative interactions in domestic horses and ponies (Equus caballus). Anim. Cogn. 2018, 21, 631–637. [Google Scholar] [CrossRef]
- Karenina, K.; Giljov, A.; Ingam, J.; Rowntree, V.J.; Malashichev, Y. Lateralisation of mother-infant interactions in a diverse range of mammal species. Nat. Ecol. Evol. 2017, 1, 30. [Google Scholar] [CrossRef]
- Marr, I.; Farmer, K.; Krüger, K. Evidence for Right-Sided Horses Being More Optimistic than Left-Sided Horses. Animals 2018, 8, 219. [Google Scholar] [CrossRef]
- Rochais, C.; Sébilleau, M.; Menoret, M.; Oger, M.; Henry, S.; Hausberger, M. Attentional state and brain processes: State-dependent lateralization of EEG profiles in horses. Sci. Rep. 2018, 8, 10153. [Google Scholar] [CrossRef]
- Smith, A.V.; Proops, L.; Grounds, K.; Wathan, J.; Scott, S.K.; McComb, K. Domestic horses (Equus caballus) discriminate between negative and positive human nonverbal vocalisations. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Squibb, K.; Griffin, K.; Favier, R.; Ijichi, C. Poker Face: Discrepancies in behavior and affective states in horses during stressful handling procedures. Appl. Anim. Behav. Sci. 2018, 202, 34–38. [Google Scholar] [CrossRef]
- Esch, L.; Wöhr, C.; Erhard, M.; Krüger, K. Horses’ (Equus caballus) laterality, stress hormones, and task related behavior in innovative problem-solving. Animals 2019, 9, 265. [Google Scholar] [CrossRef]
- Fenner, K.; Freire, R.; Mclean, A.; Mcgreevy, P. Behavioral, demographic, and management in fl uences on equine responses to negative reinforcement. J. Vet. Behav. Clin. Appl. Res. 2019, 29, 11–17. [Google Scholar] [CrossRef]
- Bryan, C.S.; Taylor, G.E. The relation of certain physical factors to infection with streptococcic mastitis. N. Am. Vet. 1938, 19, 26–30. [Google Scholar]
- Ewbank, R. A Possible correlation in one herd between certain aspects of the lying behavior of tied-up dairy cows. Vet. Rec. 1966, 78, 299–303. [Google Scholar] [CrossRef]
- Gadbury, J.C. Some preliminary field observations on the order of entry of cows into herringbone parlors. Appl. Anim. Ethol. 1975, 1, 275–281. [Google Scholar] [CrossRef]
- Arave, C.W.; Walters, J.L. Factors affecting lying behavior and stall utilization of dairy cattle. Appl. Anim. Ethol. 1980, 6, 369–376. [Google Scholar] [CrossRef]
- Wilson, L.L.; Terosky, T.L.; Stull, C.L.; Stricklin, W.R. Effects of individual housing design and size on behavior and stress indicators of special-fed Holstein veal calves. J. Anim. Sci. 1999, 77, 1341–1347. [Google Scholar] [CrossRef]
- Prelle, I.; Phillips, C.J.C.; Paranhos Da Costa, M.J.; Vandenberghe, N.C.; Broom, D.M. Are cows that consistently enter the same side of a two-sided milking parlor more fearful of novel situations or more competitive? Appl. Anim. Behav. Sci. 2004, 87, 193–203. [Google Scholar] [CrossRef]
- Rizhova, L.Y.; Kokorina, E.P. Behavioural asymmetry is involved in regulation of autonomic processes: Left side presentation of food improves reproduction and lactation in cows. Behav. Brain Res. 2005, 161, 75–81. [Google Scholar] [CrossRef]
- Kikkers, B.H.; Ózsvári, L.; Van Eerdenburg, F.J.C.M.; Bajcsy, Á.C.; Szenci, O. The influence of laterality on mastitis incidence in dairy cattle-preliminary study. Acta Vet. Hung. 2006, 54, 161–171. [Google Scholar] [CrossRef]
- Kilgour, R.J.; Melville, G.J.; Greenwood, P.L. Individual differences in the reaction of beef cattle to situations involving social isolation, close proximity of humans, restraint and novelty. Appl. Anim. Behav. Sci. 2006, 99, 21–40. [Google Scholar] [CrossRef]
- Grasso, F.; De Rosa, G.; Napolitano, F.; Di Francia, A.; Bordi, A. Entrance order and side preference of dairy cows in the milking parlor. Ital. J. Anim. Sci. 2007, 6, 187–194. [Google Scholar] [CrossRef]
- Ledgerwood, D.N.; Winckler, C.; Tucker, C.B. Evaluation of data loggers, sampling intervals, and editing techniques for measuring the lying behavior of dairy cattle. J. Dairy Sci. 2010, 93, 5129–5139. [Google Scholar] [CrossRef]
- Robins, A.; Phillips, C. Lateralised visual processing in domestic cattle herds responding to novel and familiar stimuli. Laterality. 2010, 15, 514–534. [Google Scholar] [CrossRef]
- Medrano-Galarza, C.; Gibbons, J.; Wagner, S.; de Passillé, A.M.; Rushen, J. Behavioral changes in dairy cows with mastitis. J. Dairy Sci. 2012, 95, 6994–7002. [Google Scholar] [CrossRef]
- Yunta, C.; Guasch, I.; Bach, A. Short communication: Lying behavior of lactating dairy cows is influenced by lameness especially around feeding time. J. Dairy Sci. 2012, 95, 6546–6549. [Google Scholar] [CrossRef]
- Gibbons, J.; Medrano-Galarza, C.; Marie de Passillé, A.; Rushen, J. Lying laterality and the effect of IceTag data loggers on lying behavior of dairy cows. Appl. Anim. Behav. Sci. 2012, 136, 104–107. [Google Scholar] [CrossRef]
- Phillips, C.J.C.; Oevermans, H.; Syrett, K.L.; Jespersen, A.Y.; Pearce, G.P. Lateralization of behavior in dairy cows in response to conspecifics and novel persons. J. Dairy Sci. 2015, 98, 2389–2400. [Google Scholar] [CrossRef]
- Miguel-Pacheco, G.G.; Thomas, H.J.; Kaler, J.; Craigon, J.; Huxley, J.N. Effects of lameness treatment for claw horn lesions on lying behavior in dairy cows. Appl. Anim. Behav. Sci. 2016, 179, 11–16. [Google Scholar] [CrossRef]
- Večeřa, M.; Falta, D.; Filipčík, R.; Chládek, G.; Lategan, F. The effect of low and high cowshed temperatures on the behavior and milk performance of Czech fleckvieh cows. Ann. Anim. Sci. 2016, 16, 1153–1162. [Google Scholar] [CrossRef]
- Broucek, J.; Uhrincat, M.; Mihina, S.; Soch, M.; Mrekajova, A.; Hanus, A. Dairy Cows Produce Less Milk and Modify Their Behaviour during the Transition between Tie-Stall to Free-Stall. Animals 2017, 7, 16. [Google Scholar] [CrossRef]
- Kappel, S.; Mendl, M.T.; Barrett, D.C.; Murrell, J.C.; Whay, H.R. Lateralized behavior as indicator of affective state in dairy cows. PLoS ONE 2017, 12, e0184933. [Google Scholar] [CrossRef]
- Goma, A.A.; Pearce, G.P.; Uddin, J.; Rimon, E.; Davies, H.; Phillips, C.J.C. A forced lateralisation test for dairy cows and its relation to their behavior. Appl. Anim. Behav. Sci. 2018, 207, 8–19. [Google Scholar]
- Robins, A.; Goma, A.A.; Ouine, L.; Phillips, C.J.C. The eyes have it: Lateralized coping strategies in cattle herds responding to human approach. Anim. Cogn. 2018, 21, 685–702. [Google Scholar] [CrossRef]
- Broad, K.D.; Mimmack, M.L.; Kendrick, K.M. Is right hemisphere specialization for face discrimination specific to humans? Eur. J. Neurosci. 2000, 12, 731–741. [Google Scholar] [CrossRef]
- Peirce, J.W.; Leigh, A.E.; Kendrick, K.M. Configurational coding, familiarity and the right hemisphere advantage for face recognition in sheep. Neuropsychologia 2000, 38, 475–483. [Google Scholar] [CrossRef]
- Peirce, J.W.; Leigh, A.E.; da Costa, A.P.C.; Kendrick, K.M. Human face recognition in sheep: Lack of configurational coding and right hemisphere advantage. Behav. Process. 2001, 55, 13–26. [Google Scholar] [CrossRef]
- Peirce, J.W.; Kendrick, K.M. Functional asymmetry in sheep temporal cortex. Neuroreport 2002, 13, 2395–2399. [Google Scholar] [CrossRef]
- Da Costa, A.P.; Leigh, A.E.; Man, M.-S.; Kendrick, K.M. Face pictures reduce behavioral, autonomic, endocrine and neural indices of stress and fear in sheep. Proc. R. Soc. B 2004, 271, 2077–2084. [Google Scholar] [CrossRef]
- Erhard, H.W.; Boissy, A.; Rae, M.T.; Rhind, S.M. Effects of prenatal undernutrition on emotional reactivity and cognitive flexibility in adult sheep. Behav. Brain. Res. 2004, 151, 25–35. [Google Scholar] [CrossRef]
- Morgante, M.; Gianesella, M.; Stelletta, C.; Versace, E.; Cannizzo, C.; Ravarotto, L.; Vallortigara, G. Short-term adaptive response in strongly versus weakly lateralized dairy ewes. Ital. J. Anim. Sci. 2007, 6 (Suppl. 1), 567–569. [Google Scholar] [CrossRef]
- Ge, T.; Kendrick, K.M.; Feng, J. A novel extended granger causal model approach demonstrates brain hemispheric differences during face recognition learning. PLoS Comput. Biol. 2009, 5, e1000570. [Google Scholar] [CrossRef]
- Hernandez, C.E.; Harding, J.E.; Oliver, M.H.; Bloomfield, F.H.; Held, S.D.E.; Matthews, L.R. Effects of litter size, sex and periconceptional ewe nutrition on side preference and cognitive flexibility in the offspring. Behav. Brain. Res. 2009, 204, 82–87. [Google Scholar] [CrossRef]
- Simitzis, P.E.; Charismiadou, M.A.; Kotsampasi, B.; Papadomichelakis, G.; Christopoulou, E.P.; Papavlasopoulou, E.K.; Deligeorgis, S.G. Influence of maternal undernutrition on the behavior of juvenile lambs. Appl. Anim. Behav. Sci. 2009, 116, 191–197. [Google Scholar] [CrossRef]
- Morgante, M.; Gianesella, M.; Versace, E.; Contalbrigo, L.; Casella, S.; Cannizzo, C.; Piccione, G.; Stelletta, C. Preliminary study on metabolic profile of pregnant and non-pregnant ewes with high or low degree of behavioral lateralization. Anim. Sci. J. 2010, 81, 722–730. [Google Scholar] [CrossRef]
- Simitzis, P.; Petrou, M.; Demiris, N.; Deligeorgis, S. Effect of pre-weaning temporary isolation within different age periods on the early post-weaning behavior of juvenile lambs. Appl. Anim. Behav. Sci. 2012, 141, 43–48. [Google Scholar] [CrossRef]
- Raoult, C.M.C.; Gygax, L. Valence and intensity of video stimuli of dogs and conspecifics in sheep: Approach-avoidance, operant response, and attention. Animals 2018, 8, 121. [Google Scholar] [CrossRef] [PubMed]
- Langbein, J. Investigations on training, recall and reversal learning of a Y-maze by dwarf goats (Capra hircus): The impact of lateralisation. Behav. Process. 2012, 89, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Gygax, L.; Reefmann, N.; Wolf, M.; Langbein, J. Prefrontal cortex activity, sympatho-vagal reaction and behavior distinguish between situations of feed reward and frustration in dwarf goats. Behav. Brain. Res. 2013, 239, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Madan, A.K.; Rastogi, S.K.; Das, A.K.; Korde, J.P.; Singh, I.; Singh, G.K. Electroencephalographic electrode montage in goats: Topographical, radiological, and physiological assessment. Turkish J. Vet. Anim. Sci. 2017, 41, 265–272. [Google Scholar] [CrossRef]
- Langbein, J. Motor self-regulation in goats (Capra aegagrus hircus) in a detour-reaching task. PeerJ 2018, 6, e5139. [Google Scholar] [CrossRef] [PubMed]
- Baciadonna, L.; Nawroth, C.; Briefer, E.F.; McElligott, A.G. Perceptual lateralization of vocal stimuli in goats. Curr. Zool. 2018, 65, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Newberry, R.C.; Wood-Gush, D.G.M. The suckling behavior of domestic pigs in a semi-natural environment. Behaviour 1984, 95, 11–25. [Google Scholar] [CrossRef]
- Loijens, L.W.S.; Schouten, W.G.P.; Wiepkema, P.R.; Wiegant, V.M. Brain opioid receptor density relates to stereotypies in chronically stressed pigs. Stress 1999, 3, 17–26. [Google Scholar] [CrossRef]
- Van der beek, E.M.; Wiegant, V.M.; Schouten, W.G.P.; van Eerdenburg, F.J.C.M.; Loijens, L.W.S.; van der Plas, C.; Benning, M.A.; de Vries, H.; de Kloet, E.R.; Lucassen, P.J. Neuronal number, volume, and apoptosis of the left dentate gyrus of chronically stressed pigs correlate negatively with basal saliva cortisol levels. Hippocampus 2004, 14, 688–700. [Google Scholar] [CrossRef]
- Camerlink, I.; Menneson, S.; Turner, S.P.; Farish, M.; Arnott, G. Lateralization influences contest behavior in domestic pigs. Sci. Rep. 2018, 8, 12116. [Google Scholar] [CrossRef]
- Goursot, C.; Düpjan, S.; Kanitz, E.; Tuchscherer, A.; Puppe, B.; Leliveld, L.M.C. Assessing animal individuality: Links between personality and laterality in pigs. Curr. Zool. 2018, zoy071, 1–11. [Google Scholar] [CrossRef]
- Grint, N.J.; Beths, T.; Yvorchuk, K.; Taylor, P.M.; Dixon, M.; Whay, H.R.; Murrell, J.C. The influence of various confounding factors on mechanical nociceptive thresholds in the donkey. Vet. Anaesth. Analg. 2014, 41, 421–429. [Google Scholar] [CrossRef]
- Grint, N.J.; Whay, H.R.; Beths, T.; Yvorchuk, K.; Murrell, J.C. Challenges of thermal nociceptive threshold testing in the donkey. Vet. Anaesth. Analg. 2015, 42, 205–214. [Google Scholar] [CrossRef]
- Gonzalez-De Cara, C.A.; Perez-Ecija, A.; Aguilera-Aguilera, R.; Rodero-Serrano, E.; Mendoza, F.J. Temperament test for donkeys to be used in assisted therapy. Appl. Anim. Behav. Sci. 2017, 186, 64–71. [Google Scholar] [CrossRef]
- Polikarpus, A.; Grasso, F.; Pacelli, C.; Napolitano, F.; De Rosa, G. Milking behavior of buffalo cows: Entrance order and side preference in the milking parlor. J. Dairy Res. 2014, 81, 24–29. [Google Scholar] [CrossRef]
- Corballis, M.C. The evolution and genetics of cerebral asymmetry. Philos. Trans. R. Soc. B 2009, 364, 867–879. [Google Scholar] [CrossRef]
- Ströckens, F.; Güntürkün, O.; Ocklenburg, S. Laterality: Asymmetries of Body, Brain and Cognition. Laterality 2013, 18, 536–575. [Google Scholar] [CrossRef]
- Fagot, J.; Vauclair, J. Manual laterality in nonhuman primates: A distinction between handedness and manual specialization. Psychol. Bull. 1991, 109, 76–89. [Google Scholar] [CrossRef]
- Siniscalchi, M.; Lusito, R.; Vallortigara, G.; Quaranta, A. Seeing left-or right-asymmetric tail wagging produces different emotional responses in dogs. Curr. Biol. 2013, 23, 2279–2282. [Google Scholar] [CrossRef]
- Kiley-Worthington, M. The Tail Movements of Ungulates, Canids and Felids With Particular Reference To Their Causation and Function as Displays. Behaviour 1976, 56, 69–114. [Google Scholar] [CrossRef]
- Rogers, L.J. Hand and paw preferences in relation to the lateralized brain. Philos. Trans. R. Soc. B 2009, 364, 943–954. [Google Scholar] [CrossRef]
- Gonzalez, C.L.R.; van Rootselaar, N.A.; Gibb, R.L. Sensorimotor lateralization scaffolds cognitive specialization. Prog. Brain. Res. 2018, 238, 405–433. [Google Scholar]
- Ocklenburg, S.; Ströckens, F.; Güntürkün, O. Lateralisation of conspecific vocalisation in non-human vertebrates. Laterality 2013, 18, 1–31. [Google Scholar] [CrossRef]
- Rosa Salva, O.; Regolin, L.; Mascalzoni, E.; Valortigara, G. Cerebral and behavioral asymmetries in animal social recogntion. Comp. Cogn. Behav. Rev. 2012, 7, 110–138. [Google Scholar] [CrossRef]
- Kendrick, K.M. Brain asymmetries for face recognition and emotion control in sheep. Cortex 2006, 42, 96–98. [Google Scholar] [CrossRef]
- Vogel, J.J.; Bowers, C.A.; Vogel, D.S. Cerebral lateralization of spatial abilities: A meta-analysis. Brain Cogn. 2003, 52, 197–204. [Google Scholar] [CrossRef]
- Güntürkün, O.; Ocklenburg, S. Ontogenesis of Lateralization. Neuron 2017, 94, 249–263. [Google Scholar] [CrossRef]
- Oleksiaka, A.; Postma, A.; van der Hamc, I.J.M.; Klink, P.C.; van Wezel, R.J.A. A review of lateralization of spatial functioning in nonhuman primates. Brain Res. Rev. 2011, 67, 56–72. [Google Scholar] [CrossRef]
- Vallortigara, G. The Cognitive Chicken: Visual and Spatial Cognition in a Nonmammalian Brain. In Comparative Cognition: Experimental Explorations of Animal Intelligence; Wasserman, E.A., Zentall, T.R., Eds.; Oxford University Press: New York, NY, USA, 2006; pp. 53–70. [Google Scholar]
- Silberman, E.K.; Weingartner, H. Hemispheric lateralisation of functions related to emotion. Brain Cogn. 1987, 5, 322–353. [Google Scholar] [CrossRef]
- Siniscalchi, M.; Sasso, R.; Pepe, A.M.; Dimatteo, S.; Vallortigara, G.; Quaranta, A. Sniffing with the right nostril: Lateralization of response to odour stimuli by dogs. Anim. Behav. 2011, 82, 399–404. [Google Scholar] [CrossRef]
- Prieur, J.; Lemasson, A.; Barbu, S.; Blois-Heulin, C. History, development and current advances concerning the evolutionary roots of human right-handedness and language: Brain lateralisation and manual laterality in non-human primates. Ethology 2019, 125, 1–28. [Google Scholar] [CrossRef]
- Schmitz, J.; Metz, G.A.S.; Güntürkün, O.; Ocklenburg, S. Beyond the genome—Towards an epigenetic understanding of handedness ontogenesis. Prog. Neurobiol. 2017, 159, 69–89. [Google Scholar] [CrossRef]
- Sommer, I.E.; Aleman, A.; Somers, M.; Boks, M.P.; Kahna, R.S. Sex differences in handedness, asymmetry of the Planum Temporale and functional language lateralization. Brain Res. 2008, 1206, 76–88. [Google Scholar] [CrossRef]
- Pfannkuche, K.A.; Bouma, A.; Groothuis, T.G.G. Does testosterone affect lateralization of brain and behavior? A meta-analysis in humans and other animal species. Philos. Trans. R. Soc. B 2009, 364, 929–942. [Google Scholar] [CrossRef]
- Geschwind, N.; Galaburda, A.M. Cerebral Lateralization. Arch. Neurol. 1985, 42, 634–654. [Google Scholar] [CrossRef]
- Tops, M.; Quirin, M.; Boksem, M.A.S.; Koole, S.L. Large-scale neural networks and the lateralization of motivation and emotion. Int. J. Psychophysiol. 2017, 119, 41–49. [Google Scholar] [CrossRef]
- Hardie, S.M.; Wright, L.; Clark, L. Handedness and social anxiety: Using Bryden’s research as a catalyst to explore the influence of familial sinistrality and degree of handedness. Laterality 2016, 21, 329–347. [Google Scholar] [CrossRef]
- Cameron, R.; Rogers, L.J. Hand preference of the common marmoset (Callithrix jacchus): Problem solving and responses in a novel setting. J. Comp. Psychol. 1999, 113, 149–157. [Google Scholar] [CrossRef]
- Braccini, S.N.; Caine, N.G. Hand preference predicts reactions to novel foods and predators in marmosets (Callithrix geoffroyi). J. Comp. Psychol. 2009, 123, 18–25. [Google Scholar] [CrossRef]
- Gordon, D.J.; Rogers, L.J. Differences in social and vocal behavior between left- and right-handed common marmosets Callithrix jacchus. J. Comp. Psychol. 2010, 124, 402–411. [Google Scholar] [CrossRef]
- Riederer, P.; Jellinger, K.A.; Kolber, P.; Hipp, G.; Sian-Hülsmann, J.; Krüger, R. Lateralisation in Parkinson disease. Cell Tissue Res. 2018, 373, 297–312. [Google Scholar] [CrossRef]
- Ocklenburg, S.; Güntürkün, O.; Hugdahl, K.; Hirnstein, M. Laterality and mental disorders in the postgenomic age-A closer look at schizophrenia and language lateralization. Neurosci. Biobehav. Rev. 2015, 59, 100–110. [Google Scholar] [CrossRef]
- Hecht, D. Depression and the hyperactive right-hemisphere. Neurosci. Res. 2010, 68, 77–87. [Google Scholar] [CrossRef]
- Herbert, M.R.; Ziegler, D.A.; Deutsch, C.K.; O’Brien, L.M.; Kennedy, D.N.; Filipek, P.A.; Bakardjiev, A.I.; Hodgson, J.; Takeoka, M.; Makris, N.; et al. Brain asymmetries in autism and developmental language disorder: A nested whole-brain analysis. Brain 2005, 128, 213–226. [Google Scholar] [CrossRef]
- Cerqueira, J.J.; Almeida, O.F.X.; Sousa, N. The stressed prefrontal cortex. Left? Right! Brain Behav. Immun. 2008, 22, 630–638. [Google Scholar] [CrossRef]
- Veissier, I.; Boissy, A. Stress and welfare: Two complementary concepts that are intrinsically related to the animal’s point of view. Physiol. Behav. 2007, 92, 429–433. [Google Scholar] [CrossRef]
- Warren, J.M. Handedness and laterality in humans and other animals. Physiol. Psychol. 1980, 8, 351–359. [Google Scholar] [CrossRef]
- Ruby, M.B.; Heine, S.J. Too close to home. Factors predicting meat avoidance. Appetite 2012, 59, 47–52. [Google Scholar] [CrossRef]
- Schwartzkopf-Genswein, K.S.; Faucitano, L.; Dadgar, S.; Shand, P.; González, L.A.; Crowe, T.G. Road transport of cattle, swine and poultry in North America and its impact on animal welfare, carcass and meat quality: A review. Meat Sci. 2012, 92, 227–243. [Google Scholar] [CrossRef]
- Rauw, W.; Kanis, E.; Noordhuizen-Stassen, E.; Grommers, F. Undesirable side effects of selection for high production efficiency in farm animals: A review. Livest. Prod. Sci. 1998, 56, 15–33. [Google Scholar] [CrossRef]
- Hopkins, W.D. Comparing human and nonhuman primate handedness: Challenges and a modest proposal for consensus. Dev. Psychobiol. 2013, 55, 621–636. [Google Scholar] [CrossRef]
| Directional Biases | Individual Biases | |||
|---|---|---|---|---|
| Significant | Non-Significant | Significant | Non-Significant | |
| Horse | Locomotion: [49,50,51,52,53], [54] 1 Standing: [55,56], [57] 3, [58] 4 Stepping down: [59] Loading on truck: [59] | Locomotion: [58,59,60,61,62,63,64,65,66], [54] 2 Standing: [67], [57] 5, [58] 6 Stepping up: [59] Unloading from truck: [59] Obstacle avoidance: [64] Rolling: [64] Stretching: [55] Turning during flight: [68] Suckling: [69] Competition maneuvers: [70] | Locomotion: [53,61,63,64] Standing: [56] Obstacle avoidance: [64] Rolling: [64] | |
| Cattle | Lying: [71], [72] 7, [73] 8, [74] 9 | Lying: [75,76,77,78,79,80], [72] 10, [73] 11, [74] 12 Parlor entry: [78,81,82,83] Feeding: [78] Rumination: [78] Locomotion: [78] Tail swishing: [78] Side of track: [78] | Parlor entry: [78] 13, [81] 14 Lying: [78] 13 Feeding: [78] 13 Rumination: [78] 13 | Parlor entry: [78] 15, [81] 14 Lying: [78] 15 Feeding: [78] 15 Rumination: [78] 15 Locomotion: [78] Tail swishing: [78] Side of track: [78] |
| Sheep | Obstacle avoidance: [84] 16, [85] 17 Foot movements during transport: [86] Side preference in maze: [87] | Obstacle avoidance: [84] 18, [85] 19 Stepping up: [84] Rumination: [84] Locomotion: [88] Lying: [88] Tail posture: [88] | Obstacle avoidance: [85] Side preference in maze: [89] | |
| Goat | Side preference in maze: [89] | Stepping down: [90] | Side preference in maze: [89] | Detour direction: [91] Stepping down: [90] |
| Pig | Lying during nursing: [92] 20 Tail posture: [22] | Lying during nursing: [92] 21 Manipulation with snout: [22] | Lying during nursing: [92] 22 Tail posture: [22] Manipulation with snout: [22] | Lying during nursing: [92] 23 Stepping up: [22] Stepping down: [22] |
| Donkey | Standing: [93] 24 | Standing: [93] 25 | Standing: [93] 24 | |
| Article | N | Modality | Function | T1 | C | E | S | A | Pe | H | Pr |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Horse | |||||||||||
| [62] | 10 | Motor | Stepping pattern during trot | Y | - | - | - | - | - | - | - |
| [94] | 30 | Motor | Sidedness while being ridden | N | - | - | - | - | - | - | Y |
| [50] | 4 | Motor | Leading limb during gallop | Y | - | - | - | - | - | - | - |
| [54] | 10 | Motor | Stepping pattern during trot | Y | - | - | - | Y | - | - | - |
| [66] | 30 | Motor | Asymmetrical locomotion on a treadmill | Y | - | - | - | - | - | - | - |
| [55] | 106 & 157 | Motor & Olfactory | 1. Forelimb while grazing 2. Hind leg stretching 3. Sniffing stallion feces | Y | - | - | N | Y | N | - | N |
| [64] | 40 | Motor/Visual | 1. Forelimb while starting locomotion 2. Obstacle avoidance 3. Obstacle avoidance when ridden 4. Rolling direction | Y | - | - | Y | - | - | - | - |
| [57] | 186 | Motor | Forelimb while grazing | Y | - | - | - | Y | - | - | - |
| [95] | 36 | Touch | Mechanical nociception | N | - | - | - | - | - | N | - |
| [96] | 65 | Visual/Olfactory | Eye/nostril use while inspecting a novel object | N | - | Y | N | Y | - | - | - |
| [97] | 24 | Motor | Forelimb while grazing | N | - | - | - | - | - | Y | Y |
| [68] | 30 | Visual & Motor | 1. Response to novel object from the side 2. Turning during flight | Y | - | Y | N | - | - | - | - |
| [53] | 9362 | Motor | 1. Forelimb while starting to gallop 2. Forelimb at the start of a race 3. Stride pattern during gallop | Y | - | - | N | - | - | - | - |
| [98] | 38 | Visual/Olfactory | Eye/nostril use while inspecting objects | N | - | Y | - | - | - | - | - |
| [99] | 25 | Touch | Mechanical nociception | N | - | - | - | - | - | N | - |
| [63] | 219 | Motor | Sidedness while being ridden | Y | - | - | Y | - | - | - | - |
| [58] | 30 | Motor | 1. Cantering direction 2. Forelimb while grazing | Y | - | - | - | - | - | - | - |
| [100] | 12 | Auditory | Ear and head orientation towards conspecific whinnies | N | Y | - | - | - | - | - | - |
| [49] | 9 | Motor | Hoof’s center of pressure during walking | Y | - | - | - | - | - | - | - |
| [101] | 4 | Motor | Asymmetrical changes in thoracic shape during locomotion | N | - | - | - | - | - | - | - |
| [102] | 55 | Visual/Motor | Entrance in arena with/without a human in the middle | N | Y | - | N | - | - | - | - |
| [65] | 5 | Motor | Forelimb loading during locomotion | Y | - | - | - | - | - | - | - |
| [103] | 17 | Motor | Forelimb while: 1. Grazing 2. Starting canter 3. Jumping | N | - | - | - | - | - | Y | Y |
| [56] | 6 | Motor | Leg movements during grazing | Y | - | - | - | - | - | - | - |
| [104] | 10 | Motor/Visual | Detouring a symmetrical/ asymmetrical barrier | N | - | - | - | - | - | - | - |
| [105] | 28 | Touch/Visual | Emotional reactivity to humans after unilateral tactile stimulation | N | - | Y | - | - | - | Y | - |
| [106] | 6 | Motor | Hoof’s center of pressure on left and right circles | N | - | - | - | - | - | - | - |
| [107] | 45 | Visual/Touch | Response to approaching human from the side | N | - | Y | - | Y | - | - | - |
| [67] | 24–66 | Visual & Motor | 1. Eye use during agonistic interactions 2. Head turn bias during vigilance and reactivity 3. Forelimb while grazing | Y | - | Y | N | Y | - | - | - |
| [108] | 14 | Motor/Visual | Side preference in Y maze | N | - | - | - | N | - | - | - |
| [109] | 11 | Motor | Body lean angle during turning | N | - | - | - | - | - | - | - |
| [110] | 72 | Visual/Auditory | 1. Cross-modal discrimination between owner and stranger 2. Cross-modal recognition of familiar humans 3. Eye use to view humans 4. Head turn response to human voices | N | Y | - | - | - | - | - | - |
| [111] | 46 | Motor | Derailment during locomotion in a circle | N | - | - | N | Y | - | - | - |
| [69] | 79 | Visual/Motor | Suckling side | Y | - | N | N | Y | - | - | - |
| [51] | 7 | Motor | Hoof balance during locomotion | Y | - | - | - | - | - | - | - |
| [52] | 11 | Motor | Asymmetrical locomotion in a circle | Y | - | - | - | - | - | Y | - |
| [112] | 20 | Motor | Body lean angle during turning | N | - | - | - | - | - | - | - |
| [59] | 14 | Motor | Forelimb while: 1. Starting locomotion 2. Stepping up 3. Stepping down 4. Loading on truck 5. Unloading from truck | Y | - | Y | N | N | - | - | - |
| [113] | 24 | Touch | Response to pressure on side of body | N | - | - | - | - | - | - | Y |
| [60] | 26 | Motor | Asymmetrical locomotion | Y | - | - | - | - | - | - | - |
| [114] | 12 | Olfactory | Nostril use while smelling different odors | N | - | Y | N | - | - | - | - |
| [70] | 482 | Motor | Maneuvers during competition | Y | - | - | - | - | - | - | - |
| [115] | 12 | Motor | Leading limb during galloping | N | - | - | - | - | - | N | - |
| [116] | 19 | Visual/Motor | Turning during flight | N | - | N | N | N | - | - | - |
| [117] | 28 | Visual | Eye use while viewing pictures of human faces | N | - | Y | - | - | - | - | - |
| [118] | 6 | Visual/Touch | Side of handler during trotting | N | - | - | - | - | - | - | Y |
| [119] | 26 | Motor/visual | Detouring a symmetrical/asymmetrical barrier | N | Y | - | - | - | Y | - | - |
| [120] | 7 | Motor | Asymmetrical locomotion | N | - | - | - | - | - | - | - |
| [61] | 2095 | Motor | Forelimb while starting to gallop | Y | - | - | Y | N | - | - | N |
| [121] | 31 | Visual | Eye use during affiliative behaviors | N | - | Y | - | - | N | - | - |
| [122] | 8–27 pairs | Visual | Lateral preferences of mother-infant pairs during: 1. Slow travelling 2. Resting 3. Approach to suckle without detour 3. Approach to suckle with detour 4. Fleeing | N | Y | Y | N | N | - | - | - |
| [123] | 17 | Motor & Visual/Olfactory/Auditory | 1. Relaxed forelimb position 2. Forelimb while starting locomotion 3. Forelimb while investigating box 4. Eye/nostril/ear use while inspecting novel object | N | - | - | - | - | Y | - | - |
| [124] | 12 | Visual/Brain activity | Attention to laser light | N | Y | - | - | - | - | - | - |
| [125] | 28 | Auditory | Ear movements while hearing human emotional vocalizations | N | Y | Y | - | - | - | - | - |
| [126] | 46 | Brain activity | Eye temperature after novel handling test | N | - | - | - | - | N | - | - |
| [127] | 16 | Visual & Motor | 1. Eye use while approaching a novel feeder 2. Forelimb while grazing | N | - | - | - | - | N | - | - |
| [128] | 96 | Visual/Touch | Side of trainer while learning new task | N | Y | - | - | - | - | - | Y |
| Cattle | |||||||||||
| [129] | ~70 | Motor | Lying side preference | N | - | - | - | - | - | - | - |
| [130] | 73 | Motor | Lying side preference | N | - | - | - | - | - | Y | - |
| [80] | 388 | Motor | Lying side preference | Y | - | - | - | - | - | - | - |
| [73] | 35 | Motor | Lying side preference | Y | - | - | - | - | - | - | - |
| [131] | 217 | Motor | Side in milking parlor | N | - | - | - | - | - | - | - |
| [132] | 77 | Motor | Lying side preference | N | - | - | - | Y | - | - | Y |
| [71] | 6 | Motor | Lying side preference | Y | - | - | - | - | - | - | - |
| [75] | 44 | Motor | Lying side preference | Y | - | - | - | - | - | - | Y |
| [81] | 89 | Motor | Side in milking parlor | Y | - | - | - | N | Y | Y | Y |
| [133] | 108 | Motor | Lying side preference | N | - | - | - | - | - | - | - |
| [83] | 60–90 | Motor | Side in milking parlor | Y | - | - | - | - | - | N | N |
| [78] | 182 | Motor | 1. Tongue protrusion direction [feeding] 2. Jaw movement direction [rumination] 3. Forelimb while starting locomotion 4. Lying side preference 5. Tail swishing direction 6. Side of track 7. Side in milking parlor | Y | - | - | - | - | - | Y | - |
| [134] | 24 | Motor | Side in milking parlor | N | - | - | - | - | Y | - | - |
| [135] | max. 400 | Visual | Arrival of food from the side | N | - | - | - | Y | - | - | Y |
| [136] | 227 | Motor | Lying side preference | N | - | - | - | - | - | Y | - |
| [137] | 94 | Motor/Visual | Obstacle avoidance | N | - | - | - | - | Y | - | - |
| [138] | 146 | Motor | Side in milking parlor | N | - | - | - | - | - | - | - |
| [77] | 248–250 | Motor | Lying side preference | Y | - | - | - | N | - | - | Y |
| [79] | 186 | Motor | Lying side preference | Y | - | - | - | - | - | - | Y |
| [139] | 12 | Motor | Lying side preference | N | - | - | - | - | - | - | - |
| [140] | 124 | Visual | Eye use to observe an approaching human | N | - | Y | - | N | - | - | - |
| [141] | 38 | Motor | Lying side preference | N | - | - | - | - | - | Y | - |
| [142] | ~1290 | Motor | Lying side preference | N | - | - | - | N | - | N | - |
| [143] | 40 | Motor | Lying side preference | N | - | - | - | - | - | - | - |
| [144] | 233 | Visual/Motor | 1. Eye use in agonistic interactions 2. Passing a familiar/unfamiliar person 3. Side while walking through a track | N | Y | Y | - | Y | Y | - | Y |
| [145] | 78 | Motor | Lying side preference | N | - | - | - | - | - | N | Y |
| [146] | ~98 | Motor | Lying side preference | N | - | - | - | - | - | - | Y |
| [147] | 41 | Motor | Lying side preference | N | - | - | - | Y | - | - | Y |
| [76] | 195 | Motor | Lying side preference | Y | - | - | - | N | - | N | - |
| [74] | 12 | Motor | Lying side preference | Y | - | - | - | - | - | Y | - |
| [148] | 216 | Visual/Olfactory | 1. Observing bilaterally placed novel objects 2. Nostril use for sniffing novel objects | N | - | Y | - | - | - | N | - |
| [82] | 72 | Motor | Side in milking parlor | Y | - | - | - | - | Y | - | Y |
| [149] | 202 | Visual/Motor | 1. Passing a novel person in a laneway 2. Side in milking parlor 3. Hanging tail | N | Y | Y | - | - | Y | - | Y |
| [72] | 24 | Motor | Lying side preference | Y | - | - | - | - | - | Y | Y |
| [150] | ~4900 | Visual | Response to approach of familiar looking/ masked human from the side | N | Y | Y | - | - | - | - | - |
| Sheep | |||||||||||
| [89] | 8 | Motor | Side preference in T-maze | Y | - | - | - | - | - | - | - |
| [151] | 20 | Visual/Brain activity | Discrimination of sheep vs. human faces | N | Y | - | - | - | - | - | - |
| [152] | 10 | Visual | Effect of eye on face recognition | N | Y | - | - | - | - | - | |
| [153] | 10 | Visual | Effect of eye use on human face recognition | N | N | - | - | - | - | - | - |
| [154] | 6 | Visual/Brain activity | Face recognition | N | Y | - | - | - | - | - | - |
| [155] | 20 | Visual/Brain activity | Viewing pictures of faces | N | - | Y | - | - | - | - | - |
| [156] | 32 | Motor | Side preference in T-maze | N | - | - | Y | - | - | Y | - |
| [88] | 54 | Motor | 1. Forelimb while starting locomotion 2. Tail movement direction during suckling 3. Lying side preference | Y | - | - | N | - | - | - | - |
| [157] | 57 | Motor | 1. Rotation around own axis 2. Obstacle avoidance 3. Forelimb in front of obstacle | N | - | - | - | - | - | Y | - |
| [84] | 77 | Visual & Motor | 1. Obstacle avoidance to join flock mate/mother 2. Forelimb while stepping up 3. Jaw movement direction [rumination] | Y | - | Y | N | Y | - | - | - |
| [158] | 3 | Visual/Brain activity | Face recognition learning | N | Y | - | - | - | - | - | - |
| [159] | 87 | Motor | Side preference in T-maze | N | - | - | Y | Y | - | Y | - |
| [21] | 19 | Motor | Ear postures | N | - | Y | - | - | - | - | - |
| [160] | 34 | Motor/Visual | Side of entrance in arena with novel & familiar objects | N | - | - | Y | - | - | Y | - |
| [161] | 57 | Visual & Motor | 1. Obstacle avoidance to join flock mate/mother 2. Forelimb while stepping up 3. Jaw movement direction [rumination] | N | - | - | - | - | - | Y | - |
| [162] | 27 | Motor/Visual | Side of entrance in Y-maze with novel & familiar objects | N | - | - | - | N | - | Y | - |
| [87] | 309 | Motor | Side preference in T-maze | Y | - | - | - | N | - | - | N |
| [85] | 86 | Visual/Motor | Obstacle avoidance to join flock mate/mother | Y | - | Y | N | Y | Y | - | - |
| [86] | 4 | Motor & Visual | Steps during sea motion | Y | - | Y | - | - | - | Y | - |
| [163] | 33 | Visual/Motor | Eye use and ear postures while viewing videos of dogs and sheep | N | - | N | - | - | - | - | - |
| Goat | |||||||||||
| [89] | 11 | Motor | Side preference in T-maze | Y | - | - | - | - | - | - | - |
| [164] | 29 | Visual & Motor | 1. Effect of side of maze during maze learning 2. Forelimb while starting locomotion | N | Y | - | - | - | - | - | - |
| [165] | 8 | Brain activity | Brain activity during food expectation fulfilment and frustration | N | - | Y | - | - | - | - | - |
| [91] | 42 | Motor/Visual | Detour to access food | Y | - | - | - | - | - | - | - |
| [166] | 7 | Brain activity | Brain activity during resting | N | Y | Y | - | - | - | - | - |
| [90] | 30 | Motor | Forelimb while stepping down | Y | - | - | N | N | - | - | - |
| [167] | 20 | Motor/Visual | Side to access food inside transparent cylinder | N | - | - | - | - | N | - | - |
| [168] | 18 | Auditory | Head turn to conspecfic/heterospecific calls | N | N | N | - | - | - | - | - |
| Domestic pig | |||||||||||
| [169] | 5 | Motor | Lying during nursing | N | - | - | - | - | - | - | - |
| [170] | 32 | Brain activity | Brain morphology after tethering | N | - | - | - | - | - | Y | - |
| [92] | 11 | Motor | Lying during nursing | Y | - | - | - | - | - | - | - |
| [171] | 11 | Brain activity | Brain morphology after tethering | N | - | - | - | - | - | Y | - |
| [22] | 76 | Motor | 1. Side of snout used to open a flap door 2. Tail curling direction 3. Forelimb while stepping up 4. Forelimb while stepping down | Y | - | - | - | - | - | - | - |
| [172] | 104 | Visual | Eye use in agonistic interactions | N | - | N | Y | - | - | Y | - |
| [173] | 76 | Motor | 1. Side of snout used to open a flap door 2. Tail curling direction | N | - | - | - | - | Y | - | - |
| Donkey | |||||||||||
| [93] | 19 | Motor | Forelimb while standing | Y | - | - | Y | Y | - | Y | - |
| [174] | 16 | Touch | Mechanical nociception | N | - | - | - | - | - | N | - |
| [175] | 16 | Touch | Thermal nociception | N | - | - | - | - | - | N | - |
| [176] | 36 | Touch | Response to tactile stimulation | N | - | - | - | - | - | N | - |
| Buffalo | |||||||||||
| [177] | 112 | Motor | Side in milking parlor | N | - | - | - | - | - | - | - |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leliveld, L.M.C. From Science to Practice: A Review of Laterality Research on Ungulate Livestock. Symmetry 2019, 11, 1157. https://doi.org/10.3390/sym11091157
Leliveld LMC. From Science to Practice: A Review of Laterality Research on Ungulate Livestock. Symmetry. 2019; 11(9):1157. https://doi.org/10.3390/sym11091157
Chicago/Turabian StyleLeliveld, Lisette M. C. 2019. "From Science to Practice: A Review of Laterality Research on Ungulate Livestock" Symmetry 11, no. 9: 1157. https://doi.org/10.3390/sym11091157
APA StyleLeliveld, L. M. C. (2019). From Science to Practice: A Review of Laterality Research on Ungulate Livestock. Symmetry, 11(9), 1157. https://doi.org/10.3390/sym11091157




