Extraction of Cellulose Nano-Whiskers Using Ionic Liquid-Assisted Ultra-Sonication: Optimization and Mathematical Modelling Using Box–Behnken Design
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method
2.2.1. Microwave Pre-Treatment Using KOH
2.2.2. Peroxide Bleaching
2.2.3. Extraction of CNWs Using Ultrasonication
2.2.4. Experimental Design and Optimization
2.2.5. Characterizations
3. Results and Discussion
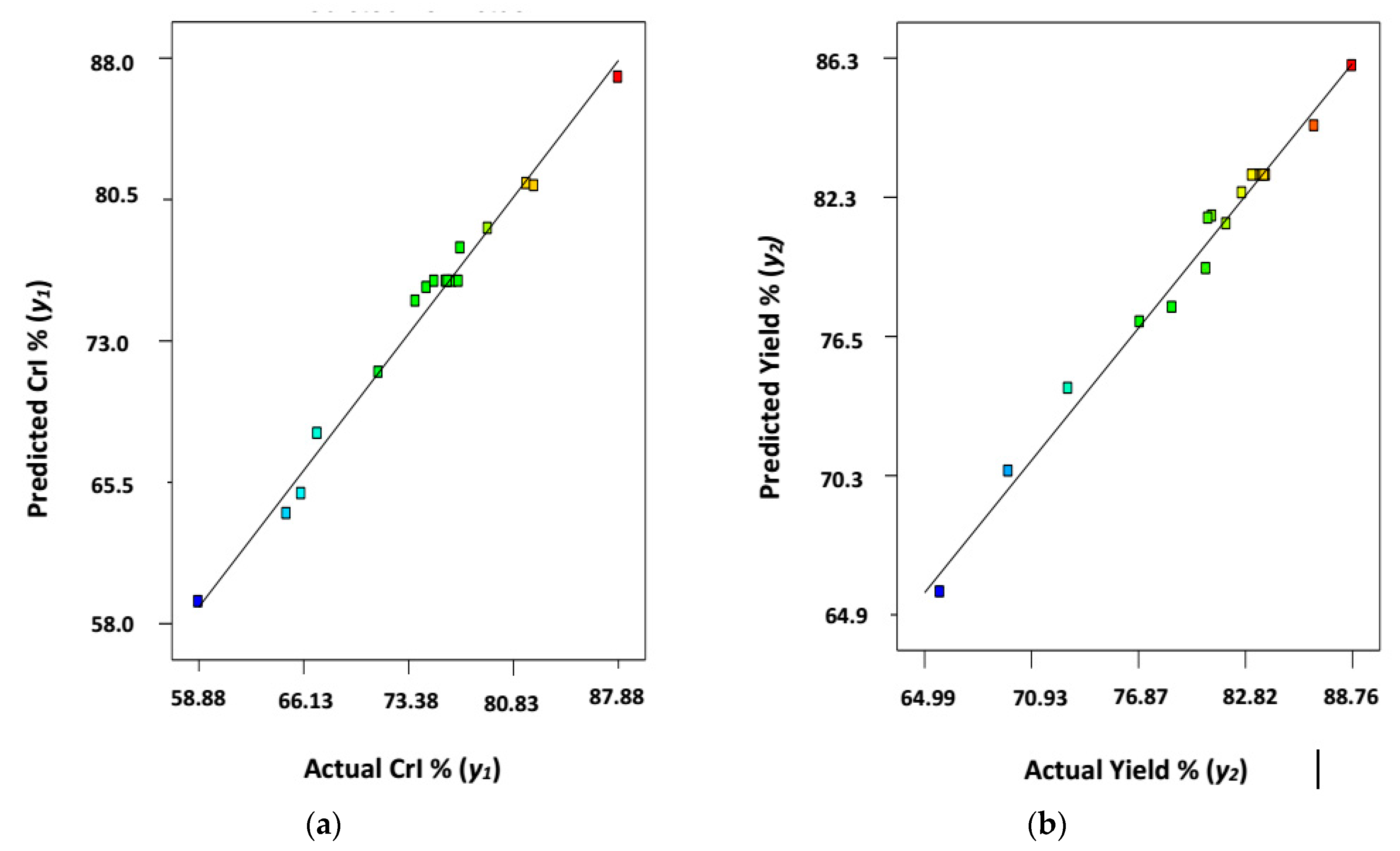
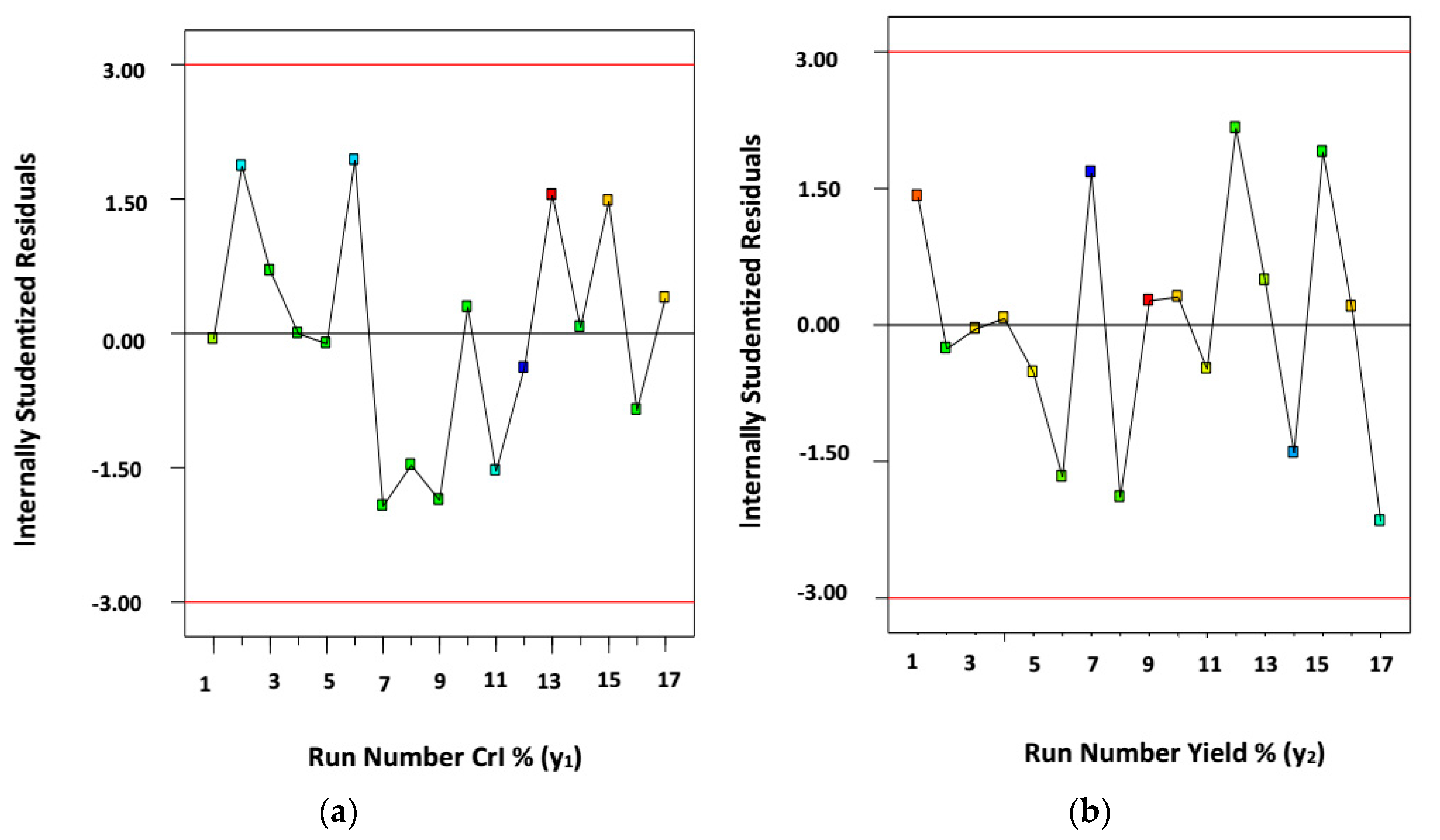
3.1. Mathematical Modeling and Statistical Analysis
3.2. Analysis of Variance (ANOVA) Test and Statistical Analysis
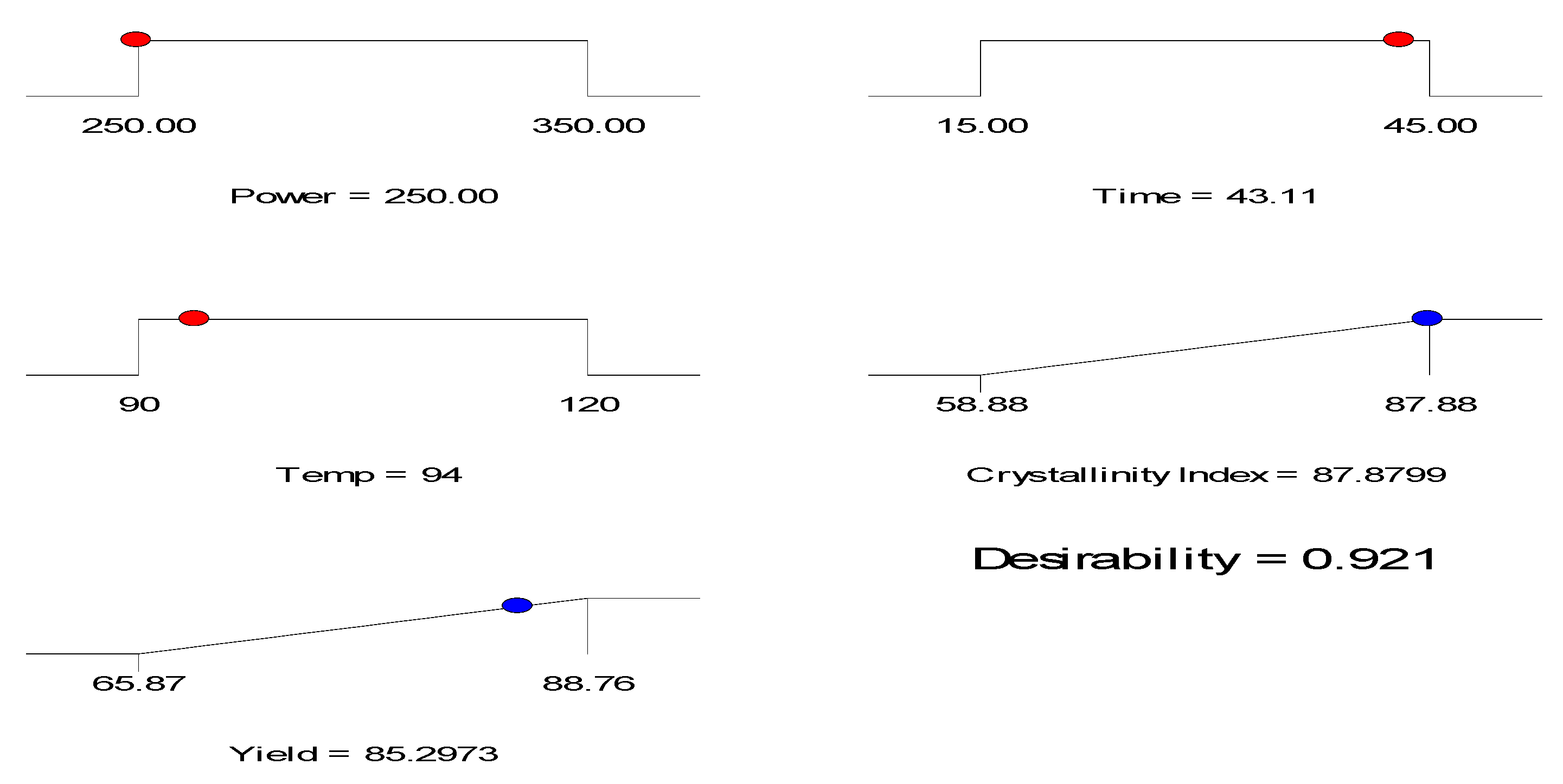
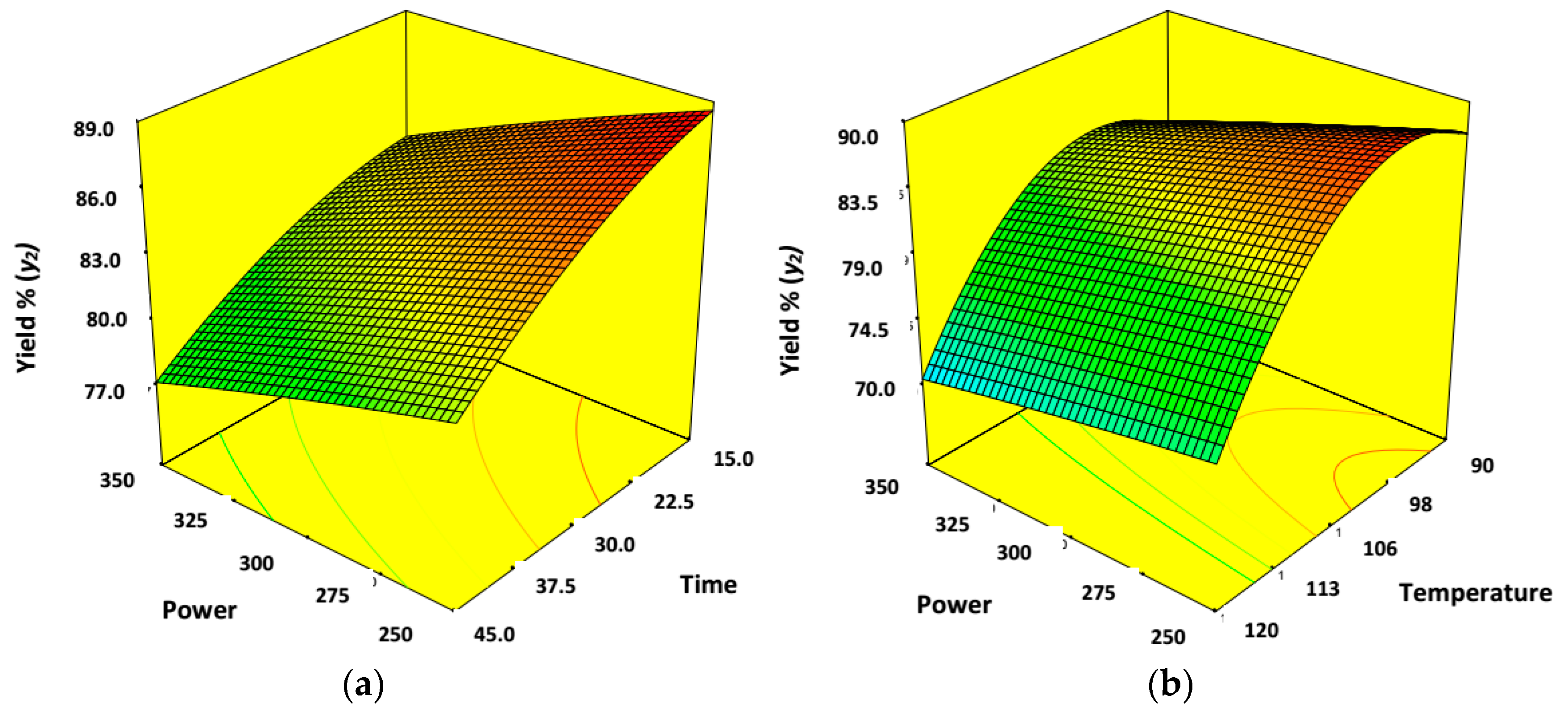
3.3. Process Variables Optimization
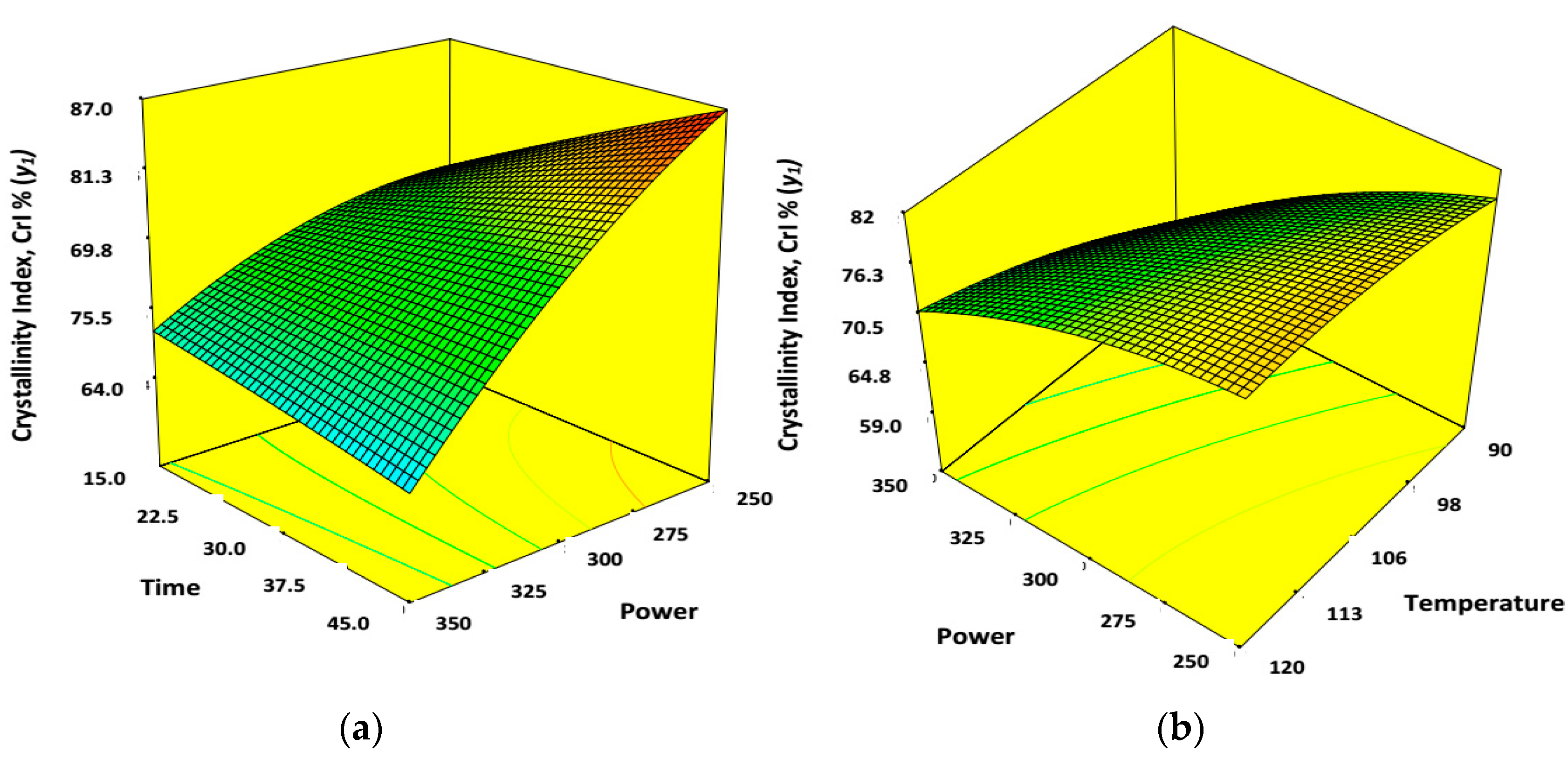
3.4. Effect of Process Variables for Synthesis of Cellulose Nano-Whiskers (CNWs)
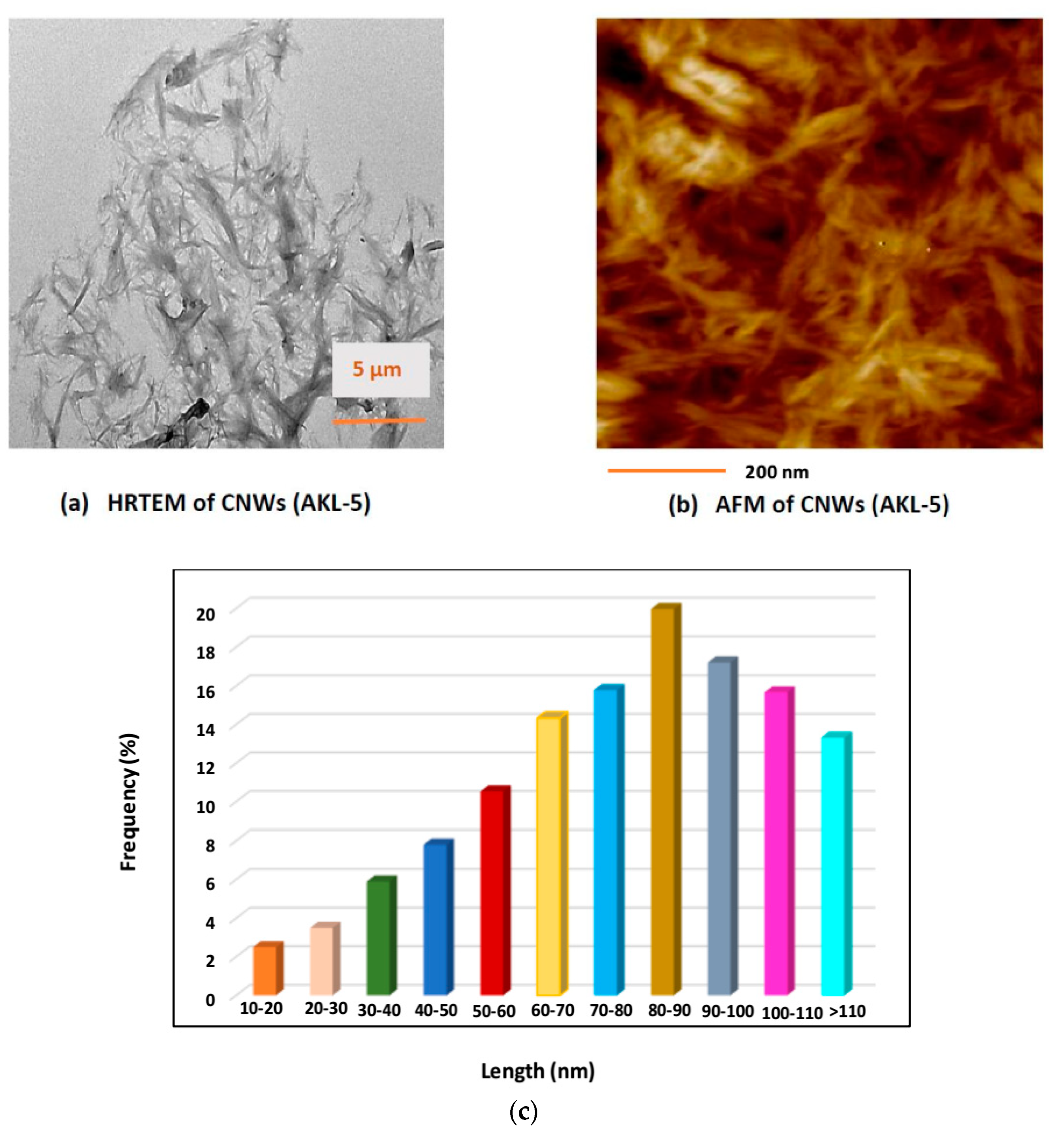
3.5. Characterizations
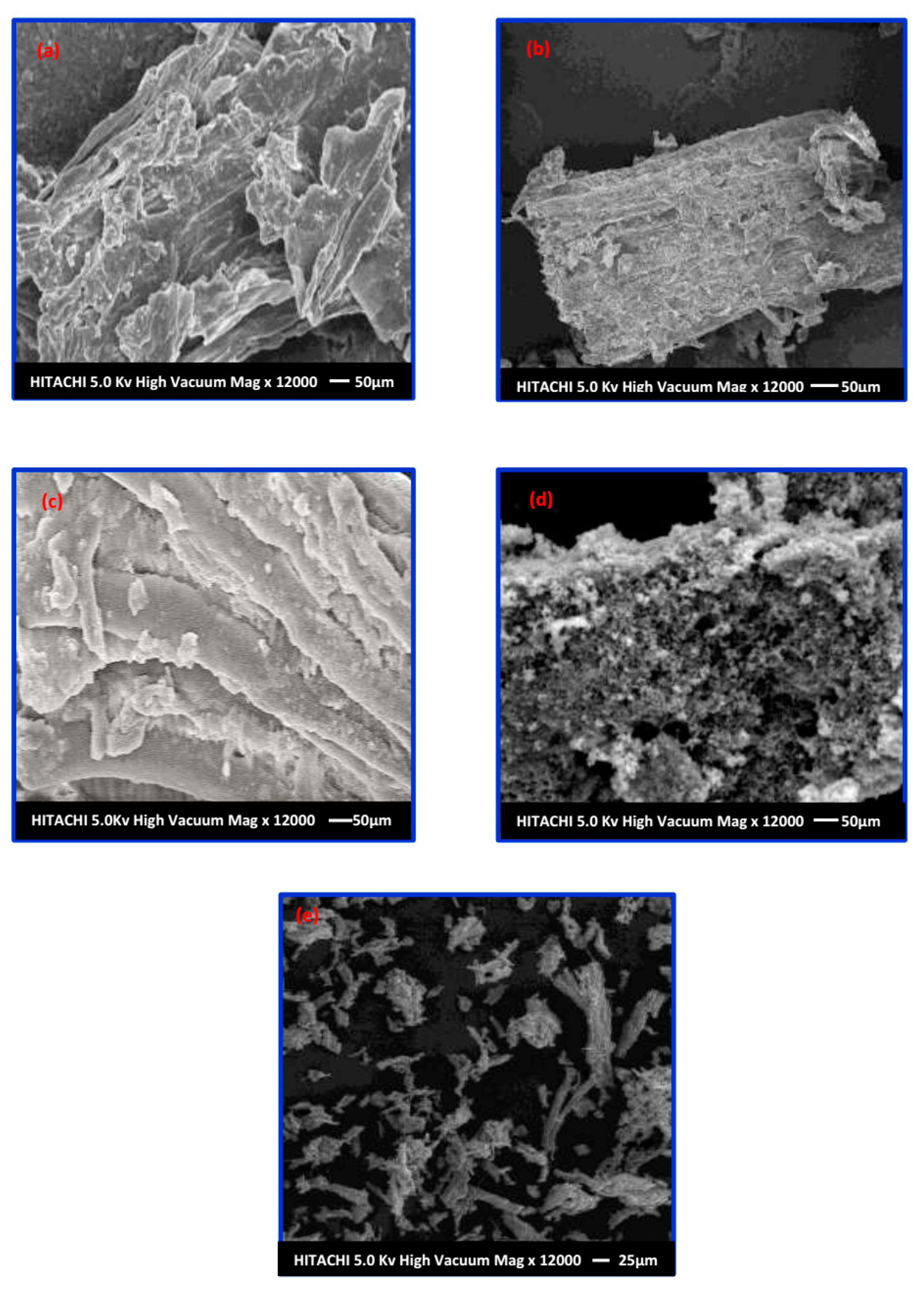
3.5.1. Surface Morphological Studies
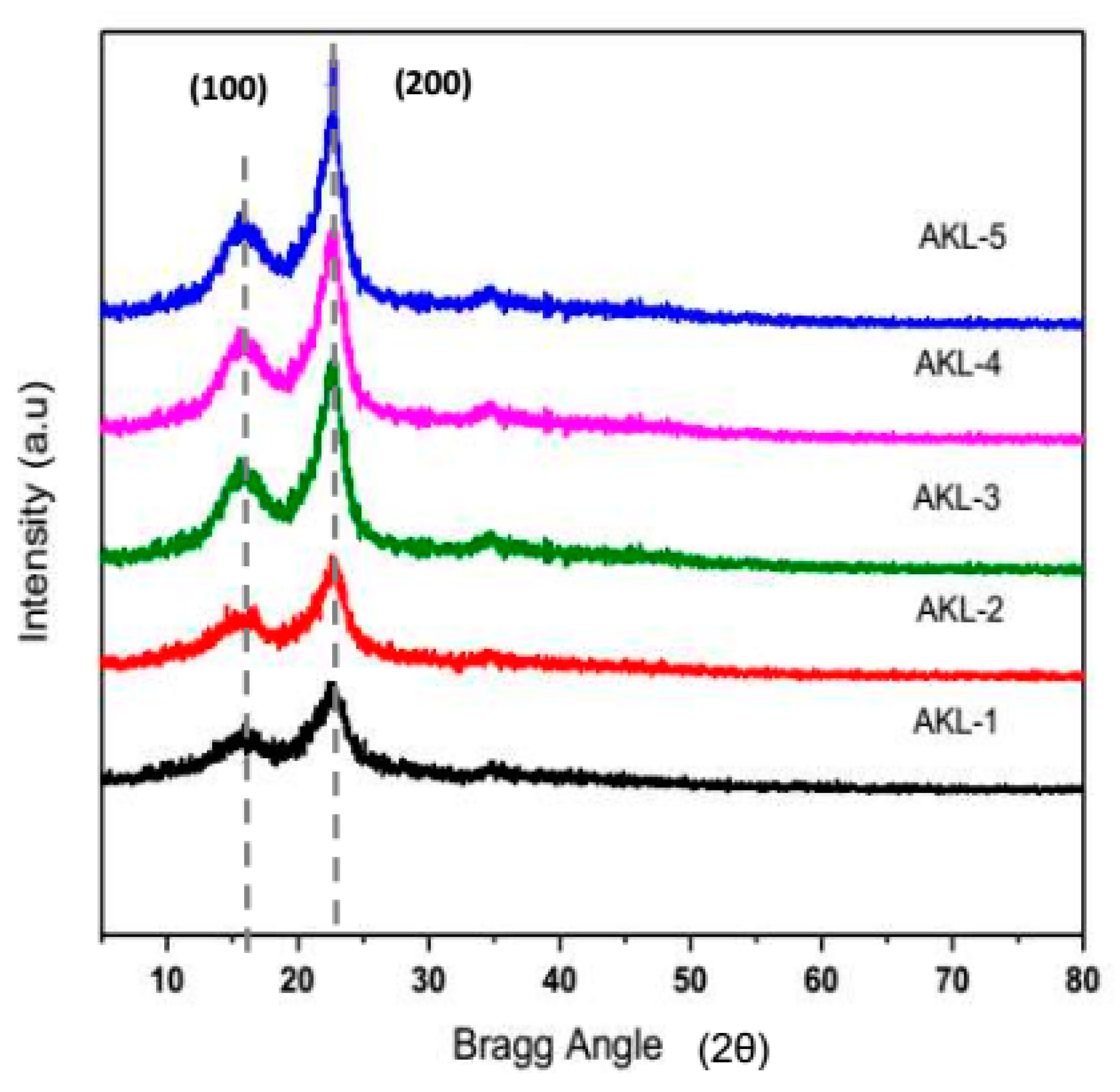
3.5.2. XRD Analysis
3.5.3. Thermogravimetric Analysis
3.5.4. Surface Functional Groups Analysis
3.5.5. Surface Charge Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abd Hamid, S.B.; Chowdhury, Z.Z.; Karim, M.Z. Catalytic Extraction of Microcrystalline Cellulose (MCC) from Elaeis guineensis using Central Composite Design (CCD). BioResources 2014, 9, 7403–7426. [Google Scholar] [CrossRef]
- Ma, Y.; Xia, Q.; Liu, Y.; Chen, Y.; Liu, S.; Wang, Q.; Liu, Y.; Li, J.; Yu, H. Production of Nanocellulose Using Hydrated Deep Eutectic Solvent Combined with Ultrasonic Treatment. ACS Omega 2019, 4, 8539–8547. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, L.; Sreekala, M.S.; Thomas, S. Novel processing parameters for the extraction of cellulose nanofibers (CNF) from environmentally benign pineapple leaf fibres (PALF): Structure-property relationships. Int. J. Biol. Macromol. 2019, 131, 858–870. [Google Scholar] [CrossRef] [PubMed]
- Vanitjinda, G.; Nimchua, T.; Sukyai, P. Effect of xylanase-assisted pretreatment on the properties of cellulose and regenerated cellulose films from sugarcane bagasse. Int. J. Biol. Macromol. 2019, 122, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Mangalam, A.P.; Simonsen, J.; Benight, A.S. Cellulose/DNA hybrid nanomaterial. Biomacromolecules 2009, 10, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Mendez, J.D.; Weder, C. Synthesis, electrical properties, and nanocomposites of poly (3, 4-ethylenedioxythiophene) nanorods. Polym. Chem. 2010, 1, 137–1244. [Google Scholar] [CrossRef][Green Version]
- Zhou, C.; Wu, Q.; Yue, Y.; Zhang, Q. Application of rod-shaped cellulose nanocrystals in polyacrylamide hydrogels. J. Colloid Interface Sci. 2011, 353, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yu, H.; Liu, Y.; Chen, P.; Zhang, M.; Hai, Y. Individualization of Cellulose Nanofibers from Wood using High Intensity Ultrasonication Combined with Chemical Pretreatments. Carbohydr. Polym. 2011, 83, 1804–1811. [Google Scholar] [CrossRef]
- Khalil, H.P.S.A.; Davoudpour, Y.; Nazrul Islam, M.; Mustapha, A.; Sudesh, K.; Dungani, R.; Jawaid, M. Production and Modification of Nanofibrillated Cellulose using Various Mechanical Processes: A Review. Carbohydr. Polym. 2014, 99, 649–665. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y.-L. Chemically and Mechanically Isolated Nanocellulose and Their Self-Assembled Structures. Carbohydr. Polym. 2013, 95, 32–40. [Google Scholar] [CrossRef]
- Meng, X.; Pu, Y.; Yoo, C.G.; Li, M.; Bali, G.; Park, D.Y.; Gjersing, E.; Davis, M.F.; Muchero, W.; Tuskan, G.A.; et al. An In-Depth Understanding of Biomass Recalcitrance using Natural Poplar Variants as the Feedstock. ChemSusChem 2017, 10, 39–150. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, Y.; Yoo, C.G.; Yang, X.; Lin, X.; Ralph, J.; Pan, X. An Uncondensed Lignin Depolymerized in the Solid State and Isolated from Lignocellulosic Biomass: A Mechanistic Study. Green Chem. 2018, 20, 4224–4235. [Google Scholar] [CrossRef]
- Xu, J.; Hou, H.; Liu, B.; Hu, J. The integration of different pretreatments and ionic liquid processing of eucalyptus: hemicellulosic products and regenerated cellulose fibers. Ind. Crop. Prod. 2017, 101, 11–20. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, X.; Hao, M.; Huang, C.; Xue, Z.; Mu, T. Preparation and characterization of regenerated cellulose from ionic liquid using different methods. Carbohydr. Polym. 2015, 117, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, J.; Wang, K.; Qian, X.; Sun, R. Highly thermostable, flexible, and conductive films prepared from cellulose, graphite, and polypyrrole nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 15641–15648. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Heck, B.; Reiter, G.; Laborie, M.P. Cellulose nanocrystals’ production in near theoretical yields by 1-butyl-3-methylimidazolium hydrogen sulfate ([Bmim]HSO4)–mediated hydrolysis. Carbohydr. Polym. 2015, 117, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Han, Y.; Lu, C.; Zhang, X.; Yuan, G. Acidic ionic liquid as “quasi-homogeneous” catalyst for controllable synthesis of cellulose acetate. Carbohydr. Polym. 2014, 113, 83–90. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, S.; Wang, J.; Zhao, M. Effects of plasticization conditions on the structures and properties of cellulose packaging films from ionic liquid [BMIM] Cl. J. Appl. Polym. Sci. 2012, 125, 704–709. [Google Scholar] [CrossRef]
- Chen, W.S.; Yu, H.P.; Liu, Y.X. Preparation of millimetre- long cellulose I nanofibers with diameters of 30–80 nm from bamboo fibers. Carbohydr. Polym. 2011, 86, 453–461. [Google Scholar] [CrossRef]
- Chen, W.S.; Yu, H.P.; Liu, Y.X.; Hai, Y.F.; Zhang, M.X.; Chen, P. Isolation and characterization of cellulose nanofibers from four plant cellulose fibers using a chemical-ultrasonic process. Cellulose 2011, 18, 433–442. [Google Scholar] [CrossRef]
- Cheng, Q.Z.; Wang, S.Q.; Han, Q.Y. Novel process for isolating fibrils from cellulose fibres by high-intensity ultra-sonication. II. Fibril characterization. J. Appl. Polym. Sci. 2010, 115, 2756–2762. [Google Scholar] [CrossRef]
- Cheng, Q.; Wang, S.Q.; Rials, T.G. Poly (vinyl alcohol) nanocomposites reinforced with cellulose fibrils isolated by high intensity ultrasonication. Compos Part A. Appl. Sci. 2009, 40, 218–224. [Google Scholar] [CrossRef]
- Wang, S.Q.; Cheng, Q.Z. A novel process to isolate fibrils from cellulose fibers by high intensity ultrasonication part 1: process optimization. J. Appl. Polym. Sci. 2011, 113, 1270–1275. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of Lignocellulosic materials for Ethanol Production: A Review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Chowdhury, Z.; Zain, S.; Khan, R.; Ahmad, A.; Islam, M.; Arami-Niya, A. Application of central composite design for preparation of Kenaf fiber based activated carbon for adsorption of manganese (II) ion. Int. J. Phys. Sci. 2011, 6, 7191–7202. [Google Scholar] [CrossRef]
- Chowdhury, Z.Z.; Zain, S.M.; Khan, R.A.; Arami-Niya, A.; Khalid, K. Process variables optimization for preparation and characterization of novel adsorbent from Lignocellulosic Waste. Bioresources 2012, 7, 3732–3754. [Google Scholar]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Belin, A.; Levin, D.B. Biomass Pretreatment: Fundamental towards Application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef]
- Mazlita, Y. Catalytic Synthesis of Nanocellulose from Oil Palm Empty Fruit Bunch Fiber. Master’s Thesis, University of Malaya, Kuala Lumpur, Malaysia, 2016. [Google Scholar]
- Karim, Md. Z.; Chowdhury, Z.Z.; Abd Hamid, S.B.; Ali, E.M. Statistical Optimization for Acid Hydrolysis of Microcrystalline Cellulose and Its Physiochemical Characterization by Using Metal Ion Catalyst. Materials 2014, 7, 6982–6999. [Google Scholar] [CrossRef]
- Li, W.; Wang, R.; Liu, S. Nanocrystalliline cellulose prepared from softwood craft pulp via ultrasonic assisted acid hydrolysis. Bioresources 2011, 6, 4271–4281. [Google Scholar]
- Abraham, E.; Deepa, B.; Pothan, L.A.; Jacob, M.; Thomas, S.; Cvelbar, U.; Anand, R. Extraction of Nanocellulosic fibers from lignocellulosic fibers: A novel approach. Carbohydr. Polym. 2011, 86, 1468–1475. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y.L. Controlled defibrillation of rice straw cellulose nanofibrills into highly crystalline fibrous materials. RSC Adv. 2013, 3, 12366–12375. [Google Scholar] [CrossRef]
- Holmes, J.; Lassi, U. Ionic Liquids in Pretreatment of Lignocellulosic Biomass in Ionic Liquids Application and Perspectives; Intech Open: London, UK, 2011; pp. 545–560. ISBN 978-953-307-248-7. [Google Scholar]
- Han, J.; Zhou, C.; French, A.D.; Han, G.; Wu, Q. Characterizations of cellulose II nanoparticles regenerated from 1-butyl-3methylimidazolium chloride. Carbohydr. Polym. 2013, 94, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Moon, R.J.; Martini, A.; Narin, J.; Simonsen, J.; Youngblood, J. Cellulose nanoparticles review: Structure, Properties and Nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Tischer, P.C.S.F.; Sierakowski, M.R.; Westfahl, H.; Tischer, C.A. Nanostructural Reorganization of Bacterial Cellulose by Ultrasonic Treatment. Biomacromolecules 2010, 11, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Abd Hamid, S.B.; Chowdhury, Z.Z.; Karim, M.Z.; Ali, M.E. Catalytic Isolation and Physicochemical Propertiesof Nanocrystalline Cellulose(NCC) using HCl-FeCl3System Combined with Ultrasonication. BioResources 2016, 11, 3840–3855. [Google Scholar] [CrossRef]
- Tang, A.M.; Zhang, H.; Chen, G.; Xie, G.; Liang, W.Z. Influence of ultrasound treatment on accessibility and Region-selective oxidation reactivity of cellulose. Ultrason. Sonochem. 2005, 12, 467–472. [Google Scholar]
- Chowdhury, Z.Z.; Abd Hamid, S.B. 'Preparation and Characterization of Nanocrystalline Cellulose Using Ultrasonication Combined with a Microwave-Assisted Pretreatment Process. BioResources 2016, 11, 3397–3415. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Sugiyama, J.; Chanzy, H.; Langan, P. Crystal structure and hydrogen bonding system in cellulose I from synchrotron X-ray and neuron fiber diffraction. J. Am. Chem. Soc. 2003, 125, 14300–14306. [Google Scholar] [CrossRef]
- Lu, Q.; Lin, W.; Tang, L.; Wang, S.; Chen, X.; Huang, B. A mechanochemical approach to manufacturing bamboo cellulose nanocrystals. J. Mater. Sci. 2015, 50, 611–619. [Google Scholar] [CrossRef]
- Deepa, B.; Eldho, A.; Nerieda, C.; Miran, M.; Mathew, A.P.; Oksman, K.; Faria, M.; Thomas, S.; Pothan, L.A. Utilization of various lignocellulosic biomass for the production of nanocellulose: A comparative study. Cellulose 2015, 22, 1075–1090. [Google Scholar] [CrossRef]
- Li, M.; Wang, L.; Li, D.; Cheng, Y.; Adhikari, B. Preparation and characterizations of cellulose nanofiber from depectinized sugar beet pulp. Carbohydr. Polym. 2014, 102, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Ind. Crops Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Chirayil, C.J.; Mathew, L.; Thomas, S. Review of recent research in nano cellulose preparation from different lignocellulosic fibers. Rev. Adv. Mater. Sci. 2014, 37, 20–28. [Google Scholar]
- Mandal, A.; Chakrabarty, D. Isolation of nanocellulose from waste sugarcane bagasse (SCB) and its characterization. Carbohydr. Polym. 2011, 86, 1291–1299. [Google Scholar] [CrossRef]
- Maiti, S.; Jayaramudu, J.; Das, K.; Reddy, S.M.; Sadiku, R.; Ray, S.S.; Liu, D. Preparation and characterization of nano-cellulose with new shape from different precursor. Carbohydr. Polym. 2013, 98, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Li, H.; Zhang, J.; Fu, L.; Liu, M.; Fu, J.; Ren, P. Dissolution of cellulose in phosphate-based ionic liquids. Carbohydr. Polym. 2012, 87, 1490–1494. [Google Scholar] [CrossRef]
- Guo, J.; Guo, X.; Wang, S.; Yin, Y. Effects of ultrasonic treatment during acid hydrolysis on the yield, particle size and structure of cellulose nanocrystals. Carbohydr. Polym. 2016, 135, 248–255. [Google Scholar] [CrossRef]
| Factor | Input Parameters | Units | Low Actual | High Actual | Low Coded | High Coded | Output Responses |
| x1 | Power | Watt | 250 | 350 | −1 | +1 | |
| x2 | Time | Minutes | 15 | 45 | −1 | +1 | y1 = Crystallinity Index (%) |
| x3 | Temperature | °C | 90 | 120 | −1 | +1 | y2 = Yield of CNWs (%) |
| Std. Order | Run | Point Type | Power (watt) | Time (Minutes) | Temperature (°C) | Crystallinity Index (CrI) (%) | Yield (%) |
|---|---|---|---|---|---|---|---|
| 14 | 3 | Center | 300.00 | 30.00 | 105.00 | 76.87 | 83.66 |
| 16 | 4 | Center | 300.00 | 30.00 | 105.00 | 76.11 | 83.77 |
| 15 | 5 | Center | 300.00 | 30.00 | 105.00 | 75.99 | 83.21 |
| 13 | 10 | Center | 300.00 | 30.00 | 105.00 | 76.43 | 83.99 |
| 17 | 16 | Center | 300.00 | 30.00 | 105.00 | 75.18 | 83.89 |
| 5 | 1 | IBFact | 250.00 | 30.00 | 90.00 | 78.88 | 86.66 |
| 4 | 2 | IBFact | 350.00 | 45.00 | 105.00 | 65.99 | 76.98 |
| 9 | 6 | IBFact | 250.00 | 15.00 | 90.00 | 64.97 | 80.99 |
| 12 | 7 | IBFact | 350.00 | 45.00 | 120.00 | 73.89 | 65.87 |
| 10 | 8 | IBFact | 350.00 | 45.00 | 90.00 | 76.99 | 80.77 |
| 1 | 9 | IBFact | 250.00 | 15.00 | 105.00 | 74.65 | 88.76 |
| 2 | 11 | IBFact | 350.00 | 15.00 | 105.00 | 67.11 | 82.65 |
| 6 | 12 | IBFact | 350.00 | 30.00 | 90.00 | 58.88 | 80.65 |
| 3 | 13 | IBFact | 250.00 | 45.00 | 105.00 | 87.88 | 81.78 |
| 8 | 14 | IBFact | 350.00 | 30.00 | 120.00 | 71.33 | 69.67 |
| 11 | 15 | IBFact | 250.00 | 15.00 | 120.00 | 82.08 | 78.77 |
| 7 | 17 | IBFact | 300.00 | 30.00 | 120.00 | 81.55 | 72.99 |
| Statistical Variables | Crystallinity Index (CrI%) | CNWs Yield% |
|---|---|---|
| y1 | y2 | |
| Standard Deviation, SD% | 1.22 | 1.06 |
| Correlation Coefficient, R2 | 0.987 | 0.986 |
| Adjusted R2 | 0.970 | 0.968 |
| Mean | 74.40 | 80.30 |
| Coefficient of Variation, CV | 1.64 | 1.31 |
| Adequate Precision | 29.82 | 29.20 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | Prob > F | Comments |
|---|---|---|---|---|---|---|
| Model | 787.93 | 9 | 87.55 | 59.15 | <0.0001 | Significant |
| x1 | 444.77 | 1 | 444.77 | 300.49 | <0.0001 | |
| x2 | 31.76 | 1 | 31.76 | 21.46 | 0.0024 | |
| x3 | 106.07 | 1 | 106.07 | 71.66 | <0.0001 | |
| x1x2 | 51.48 | 1 | 51.48 | 34.78 | 0.0006 | |
| x1x3 | 23.91 | 1 | 23.91 | 16.16 | 0.0051 | |
| x2x3 | 102.11 | 1 | 102.11 | 68.99 | <0.0001 | |
| x12 | 17.10 | 1 | 17.10 | 11.56 | 0.0115 | |
| x22 | 0.16 | 1 | 0.16 | 0.11 | 0.7543 | |
| x33 | 8.74 | 1 | 8.74 | 5.90 | 0.0454 | |
| Residuals | 10.36 | 7 | 1.48 | |||
| Lack of Fit | 8.80 | 3 | 2.93 | 7.53 | 0.0039 | Not Significant |
| Pure Error | 1.56 | 4 | 0.39 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | Prob > F | Comments |
|---|---|---|---|---|---|---|
| Model | 559.08 | 9 | 62.12 | 55.79 | <0.0001 | Significant |
| x1 | 51.21 | 1 | 51.21 | 45.99 | 0.0003 | |
| x2 | 83.01 | 1 | 83.01 | 74.55 | <0.0001 | |
| x3 | 218.09 | 1 | 218.09 | 195.87 | <0.0001 | |
| x1x2 | 0.43 | 1 | 0.43 | 0.39 | 0.5544 | |
| x1x3 | 1.81 | 1 | 1.81 | 1.62 | 0.2431 | |
| x2x3 | 40.20 | 1 | 40.20 | 36.10 | 0.0005 | |
| x12 | 0.76 | 1 | 0.76 | 0.068 | 0.8012 | |
| x22 | 4.44 | 1 | 4.44 | 3.99 | 0.0860 | |
| x33 | 155.49 | 1 | 155.49 | 139.65 | <0.0001 | |
| Residuals | 7.79 | 7 | 1.11 | |||
| Lack of Fit | 7.43 | 3 | 2.48 | 27.00 | 0.0041 | Not Significant |
| Pure Error | 0.37 | 4 | 0.09 |
| Ultrasonication Power | Time | Temperature | Crystallinity Index of CNWs (%) (y1) | Yield of CNWs (%) (y2) | ||||
|---|---|---|---|---|---|---|---|---|
| (Watt) | (Minutes) | (°C) | Predicted | Experimental | Error | Predicted | Experimental | Error |
| 200 | 43.11 | 94 | 87.88 | 86.46 | 1.61 | 85.29 | 84.18 | 1.30 |
| Sample | Crystallinity Index (%) | Crystallites Sizes (nm) |
|---|---|---|
| AKL-1 | 51.43 | 12.34 |
| AKL-2 | 52.22 | 10.34 |
| AKL-3 | 64.77 | 7.54 |
| AKL-4 | 71.32 | 4.24 |
| AKL-5 | 86.46 | 3.43 |
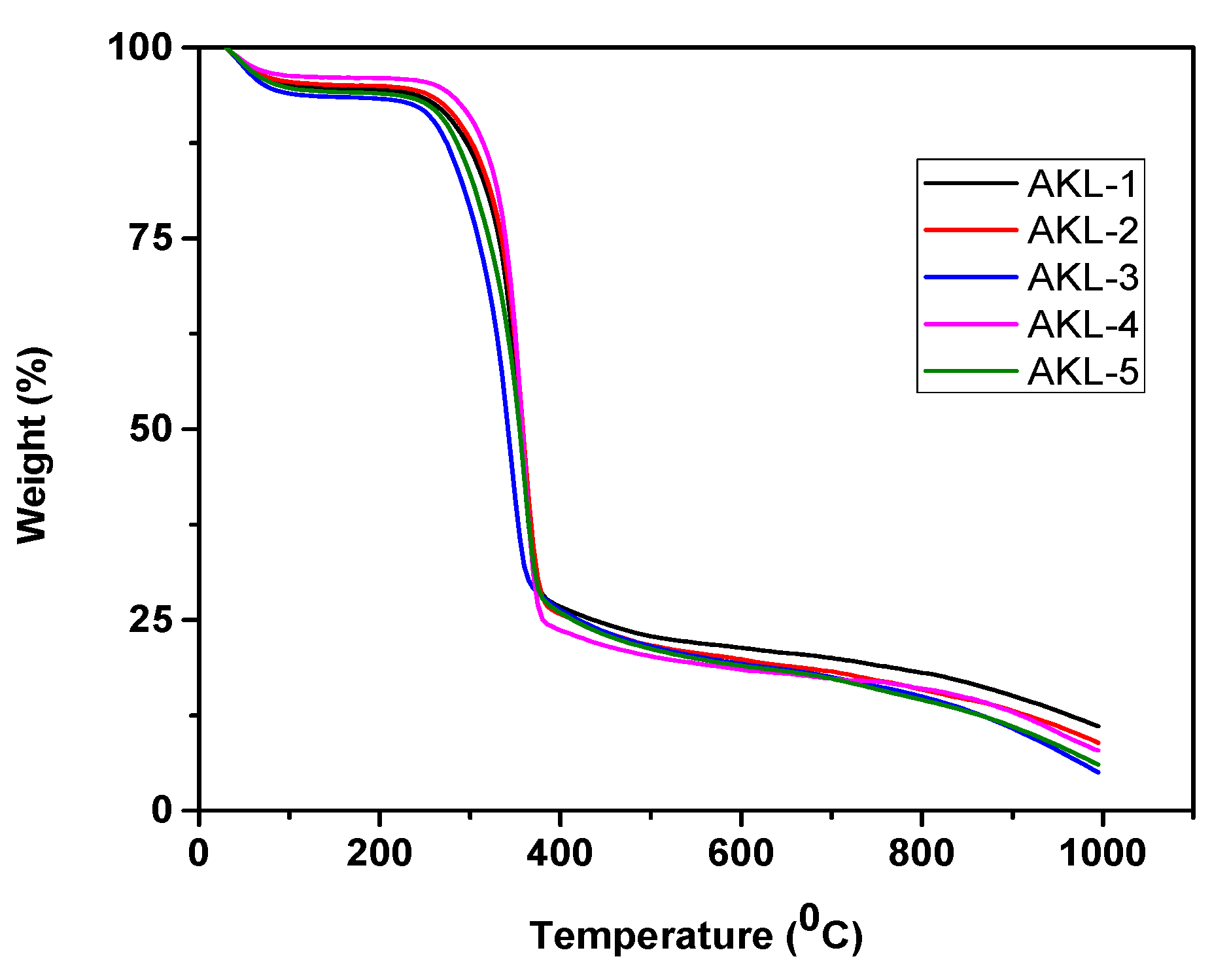
| Sample | Step-1 | Char Residues (%) | DTGmax (°C) | |
|---|---|---|---|---|
| Temperature (°C) | Weight Loss (%) | |||
| AKL-1 | 100-120 | 3.5 | 19.76 | 362.01 |
| AKL-2 | 100-120 | 3.1 | 17.22 | 360.56 |
| AKL-3 | 100-120 | 2.8 | 15.89 | 359.70 |
| AKL-4 | 100-120 | 2.3 | 14.44 | 356.87 |
| AKL-5 | 100-120 | 1.9 | 12.99 | 351.09 |
| Wave Number (cm−1)/Frequency Level | Peak Assignment |
|---|---|
| 3200–3400 | –OH Stretching |
| 2800–2900 | –CH Stretching |
| 1640–1650 | –OH bending for moisture adsorbed |
| 1400–1450 | CH2 Bending |
| 1020–1054 | C–O–C pyranose ring stretching |
| 1150–1200 | Asymmetric vibration of C–O–C bond |
| 890–900 | Glycocidic β linkages of cellulosic chain |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chowdhury, Z.Z.; Chandran, R.R.R.; Jahan, A.; Khalid, K.; Rahman, M.M.; Al-Amin, M.; Akbarzadeh, O.; Badruddin, I.A.; Khan, T.M.Y.; Kamangar, S.; et al. Extraction of Cellulose Nano-Whiskers Using Ionic Liquid-Assisted Ultra-Sonication: Optimization and Mathematical Modelling Using Box–Behnken Design. Symmetry 2019, 11, 1148. https://doi.org/10.3390/sym11091148
Chowdhury ZZ, Chandran RRR, Jahan A, Khalid K, Rahman MM, Al-Amin M, Akbarzadeh O, Badruddin IA, Khan TMY, Kamangar S, et al. Extraction of Cellulose Nano-Whiskers Using Ionic Liquid-Assisted Ultra-Sonication: Optimization and Mathematical Modelling Using Box–Behnken Design. Symmetry. 2019; 11(9):1148. https://doi.org/10.3390/sym11091148
Chicago/Turabian StyleChowdhury, Zaira Zaman, R. Reevenishaa Ravi Chandran, Afrin Jahan, Khalisanni Khalid, Md Mahfujur Rahman, Md Al-Amin, Omid Akbarzadeh, Irfan Anjum Badruddin, T. M. Yunus Khan, Sarfaraz Kamangar, and et al. 2019. "Extraction of Cellulose Nano-Whiskers Using Ionic Liquid-Assisted Ultra-Sonication: Optimization and Mathematical Modelling Using Box–Behnken Design" Symmetry 11, no. 9: 1148. https://doi.org/10.3390/sym11091148
APA StyleChowdhury, Z. Z., Chandran, R. R. R., Jahan, A., Khalid, K., Rahman, M. M., Al-Amin, M., Akbarzadeh, O., Badruddin, I. A., Khan, T. M. Y., Kamangar, S., Hamizi, N. A. B., Wahab, Y. A., Johan, R. B., & Adebisi, G. A. (2019). Extraction of Cellulose Nano-Whiskers Using Ionic Liquid-Assisted Ultra-Sonication: Optimization and Mathematical Modelling Using Box–Behnken Design. Symmetry, 11(9), 1148. https://doi.org/10.3390/sym11091148







