The Immobilization of ChEMBL474807 Molecules Using Different Classes of Nanostructures
Abstract
1. Introduction
2. Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kunikata, T.; Tatefuji, T.; Aga, H.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Indirubin inhibits inflammatory reactions in delayed-type hypersensitivity. Eur. J. Pharmacol. 2000, 410, 93–100. [Google Scholar] [CrossRef]
- Kim, S.-A.; Kwon, S.-M.; Kim, J.-A.; Kang, K.W.; Yoon, J.-H.; Ahn, S.-G. 5′-Nitro-indirubinoxime, an indirubin derivative, suppresses metastatic ability of human head and neck cancer cells through the inhibition of Integrin β1/FAK/Akt signaling. Cancer Lett. 2011, 306, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.P.; Nowicki, M.O.; Liu, F.; Press, R.; Godlewski, J.; Abdel-Rasoul, M.; Kaur, B.; Fernandez, S.A.; Chiocca, E.A.; Lawler, S.E. Indirubins decrease glioma invasion by blocking migratory phenotypes in both the tumor and stromal endothelial cell compartments. Cancer Res. 2011, 71, 5374–5380. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.-K.; Kim, J.-K. Indirubin derivative E804 inhibits angiogenesis. BMC Cancer 2012, 12, 164. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Magnaudeix, A.; Wilson, C.M.; Yardin, C.; Terro, F. The new indirubin derivative inhibitors of glycogen synthase kinase-3, 6-BIDECO and 6-BIMYEO, prevent tau phosphorylation and apoptosis induced by the inhibition of protein phosphatase-2A by okadaic acid in cultured neurons. J. Neurosci. Res. 2011, 89, 1802–1811. [Google Scholar] [CrossRef]
- Czeleń, P. Molecular dynamics study on inhibition mechanism of CDK-2 and GSK-3β by CHEMBL272026 molecule. Struct. Chem. 2016, 27, 1807–1818. [Google Scholar] [CrossRef]
- Czeleń, P. Inhibition mechanism of CDK-2 and GSK-3β by a sulfamoylphenyl derivative of indoline—a molecular dynamics study. J. Mol. Model. 2017, 23, 230. [Google Scholar] [CrossRef]
- Marko, D.; Schätzle, S.; Friedel, A.; Genzlinger, A.; Zankl, H.; Meijer, L.; Eisenbrand, G. Inhibition of cyclin-dependent kinase 1 (CDK1) by indirubin derivatives in human tumour cells. Br. J. Cancer 2001, 84, 283–289. [Google Scholar] [CrossRef]
- Hoessel, R.; Leclerc, S.; Endicott, J.A.; Nobel, M.E.M.; Lawrie, A.; Tunnah, P.; Leost, M.; Damiens, E.; Marie, D.; Marko, D.; et al. Indirubin, the active constituent of a Chinese antileukaemia medicine, inhibits cyclin-dependent kinases. Nat. Cell Biol. 1999, 1, 60–67. [Google Scholar] [CrossRef]
- Xiao, Z.; Hao, Y.; Liu, B.; Qian, L. Indirubin and Meisoindigo in the Treatment of Chronic Myelogenous Leukemia in China. Leuk. Lymphoma 2002, 43, 1763–1768. [Google Scholar] [CrossRef]
- Lee, M.-Y.; Liu, Y.-W.; Chen, M.-H.; Wu, J.-Y.; Ho, H.-Y.; Wang, Q.-F.; Chuang, J.-J. Indirubin-3′-monoxime promotes autophagic and apoptotic death in JM1 human acute lymphoblastic leukemia cells and K562 human chronic myelogenous leukemia cells. Oncol. Rep. 2013, 29, 2072–2078. [Google Scholar] [CrossRef]
- Czeleń, P.; Szefler, B. Molecular dynamics study of the inhibitory effects of ChEMBL474807 on the enzymes GSK-3β and CDK-2. J. Mol. Model. 2015, 21, 74. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J.A. Drug delivery and nanoparticles:applications and hazards. Int. J. Nanomedicine 2008, 3, 133–149. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, L.; Sun, Y. Nanotechnology applied to overcome tumor drug resistance. J. Control. Release 2012, 162, 45–55. [Google Scholar] [CrossRef]
- Morgen, M.; Bloom, C.; Beyerinck, R.; Bello, A.; Song, W.; Wilkinson, K.; Steenwyk, R.; Shamblin, S. Polymeric Nanoparticles for Increased Oral Bioavailability and Rapid Absorption Using Celecoxib as a Model of a Low-Solubility, High-Permeability Drug. Pharm. Res. 2012, 29, 427–440. [Google Scholar] [CrossRef]
- Turov, V.V.; Chehun, V.F.; Barvinchenko, V.N.; Krupskaya, T.V.; Prylutskyy, Y.I.; Scharff, P.; Ritter, U. Low-temperature 1H-NMR spectroscopic study of doxorubicin influence on the hydrated properties of nanosilica modified by DNA. J. Mater. Sci. Mater. Med. 2011, 22, 525–532. [Google Scholar] [CrossRef]
- Schuetze, C.; Ritter, U.; Scharff, P.; Fernekorn, U.; Prylutska, S.; Bychko, A.; Rybalchenko, V.; Prylutskyy, Y. Interaction of N-fluorescein-5-isothiocyanate pyrrolidine-C60 with a bimolecular lipid model membrane. Mater. Sci. Eng. C 2011, 31, 1148–1150. [Google Scholar] [CrossRef]
- Evstigneev, M.P.; Buchelnikov, A.S.; Voronin, D.P.; Rubin, Y.V.; Belous, L.F.; Prylutskyy, Y.I.; Ritter, U. Complexation of C60 Fullerene with Aromatic Drugs. ChemPhysChem 2013, 14, 568–578. [Google Scholar] [CrossRef]
- Qiao, R.; Roberts, A.P.; Mount, A.S.; Klaine, S.J.; Ke, P.C. Translocation of C60 and Its Derivatives Across a Lipid Bilayer. Nano Lett. 2007, 7, 614–619. [Google Scholar] [CrossRef]
- Prylutska, S.; Bilyy, R.; Overchuk, M.; Bychko, A.; Andreichenko, K.; Stoika, R.; Rybalchenko, V.; Prylutskyy, Y.; Tsierkezos, N.G.; Ritter, U. Water-soluble pristine fullerenes C60 increase the specific conductivity and capacity of lipid model membrane and form the channels in cellular plasma membrane. J. Biomed. Nanotechnol. 2012, 8, 522–527. [Google Scholar] [CrossRef]
- Prylutska, S.V.; Grynyuk, I.I.; Grebinyk, S.M.; Matyshevska, O.P.; Prylutskyy, Y.I.; Ritter, U.; Siegmund, C.; Scharff, P. Comparative study of biological action of fullerenes C60 and carbon nanotubes in thymus cells. Materwiss. Werksttech. 2009, 40, 238–241. [Google Scholar] [CrossRef]
- Johnston, H.J.; Hutchison, G.R.; Christensen, F.M.; Aschberger, K.; Stone, V. The Biological Mechanisms and Physicochemical Characteristics Responsible for Driving Fullerene Toxicity. Toxicol. Sci. 2010, 114, 162–182. [Google Scholar] [CrossRef]
- Andrievsky, G.; Klochkov, V.; Derevyanchenko, L. Is the C60 Fullerene Molecule Toxic?! Fuller. Nanotub. Carbon Nanostructures 2005, 13, 363–376. [Google Scholar] [CrossRef]
- Satoh, M.; Takayanagi, I. Pharmacological studies on fullerene (C60), a novel carbon allotrope, and its derivatives. J. Pharmacol. Sci. 2006, 100, 513. [Google Scholar] [CrossRef]
- Yin, J.-J.; Lao, F.; Fu, P.P.; Wamer, W.G.; Zhao, Y.; Wang, P.C.; Qiu, Y.; Sun, B.; Xing, G.; Dong, J.; et al. The scavenging of reactive oxygen species and the potential for cell protection by functionalized fullerene materials. Biomaterials 2009, 30, 611–621. [Google Scholar] [CrossRef]
- Partha, R.; Conyers, J.L. Biomedical applications of functionalized fullerene-based nanomaterials. Int. J. Nanomedicine 2009, 4, 261–275. [Google Scholar]
- Echalier, A.; Hole, A.J.; Lolli, G.; Endicott, J.A.; Noble, M.E.M. An inhibitor’s-eye view of the ATP-binding site of CDKs in different regulatory states. ACS Chem. Biol. 2014, 9, 1251–1256. [Google Scholar] [CrossRef]
- Simon, J.; Flahaut, E.; Golzio, M. Overview of Carbon Nanotubes for Biomedical Applications. Materials 2019, 12, 624. [Google Scholar] [CrossRef]
- Lucente-Schultz, R.M.; Moore, V.C.; Leonard, A.D.; Price, B.K.; Kosynkin, D.V.; Lu, M.; Partha, R.; Conyers, J.L.; Tour, J.M. Antioxidant Single-Walled Carbon Nanotubes. J. Am. Chem. Soc. 2009, 131, 3934–3941. [Google Scholar] [CrossRef]
- Czeleń, P.; Szefler, B. The Immobilization of Oxindole Derivatives with Use of Cube Rhombellane Homeomorphs. Symmetry 2019, 11, 900. [Google Scholar] [CrossRef]
- Szefler, B.; Czeleń, P. Docking of Cisplatin on Fullerene Derivatives and Some Cube Rhombellane Functionalized Homeomorphs. Symmetry 2019, 11, 874. [Google Scholar] [CrossRef]
- Szefler, B.; Czeleń, P.; Diudea, M.V. Docking of indolizine derivatives on cube rhombellane functionalized homeomorphs. Stud. Univ. Babes-Bolyai Chem. 2018, 63, 7–18. [Google Scholar] [CrossRef]
- Diudea, M.V.; Lungu, C.N.; Nagy, C.L.; Diudea, M.V.; Lungu, C.N.; Nagy, C.L. Cube-Rhombellane Related Structures: A Drug Perspective. Molecules 2018, 23, 2533. [Google Scholar] [CrossRef]
- CHEMBL database release 24.1. 2018. Available online: https://www.ebi.ac.uk/chembl/ (accessed on 2 February 2014).
- Kim, K.-H.; Ko, D.K.; Kim, Y.-T.; Kim, N.H.; Paul, J.; Zhang, S.-Q.; Murray, C.B.; Acharya, R.; Kim, Y.H.; DeGrado, W.F.; et al. Protein-directed self-assembly of a fullerene crystal. Nat. Commun. 2016, 7, 11429. [Google Scholar] [CrossRef]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 5 May 2019).
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Bartashevich, E.V.; Potemkin, V.A.; Grishina, M.A.; Belik, A.V. A Method for Multiconformational Modeling of the Three-Dimensional Shape of a Molecule. J. Struct. Chem. 2002, 43, 1033–1039. [Google Scholar] [CrossRef]
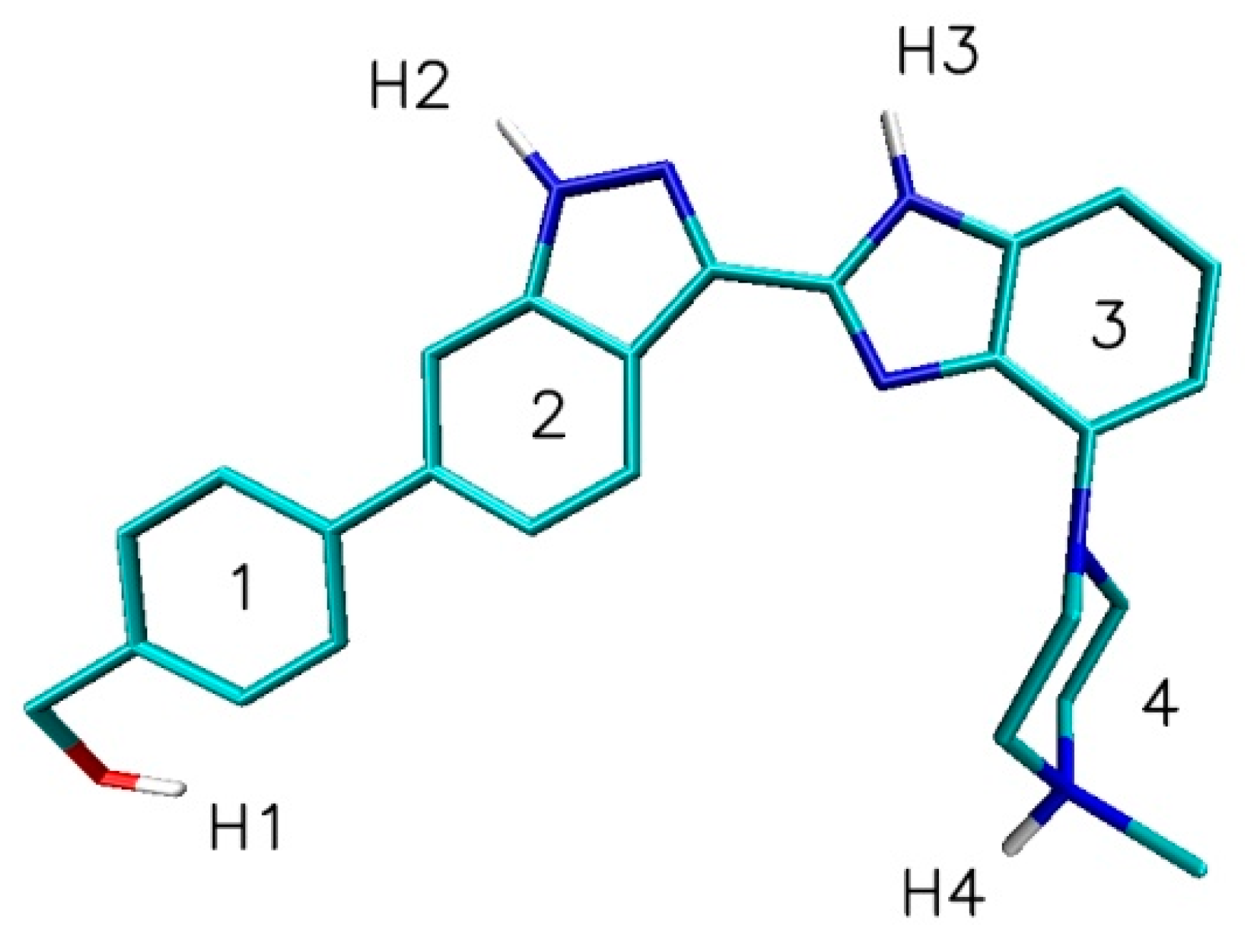
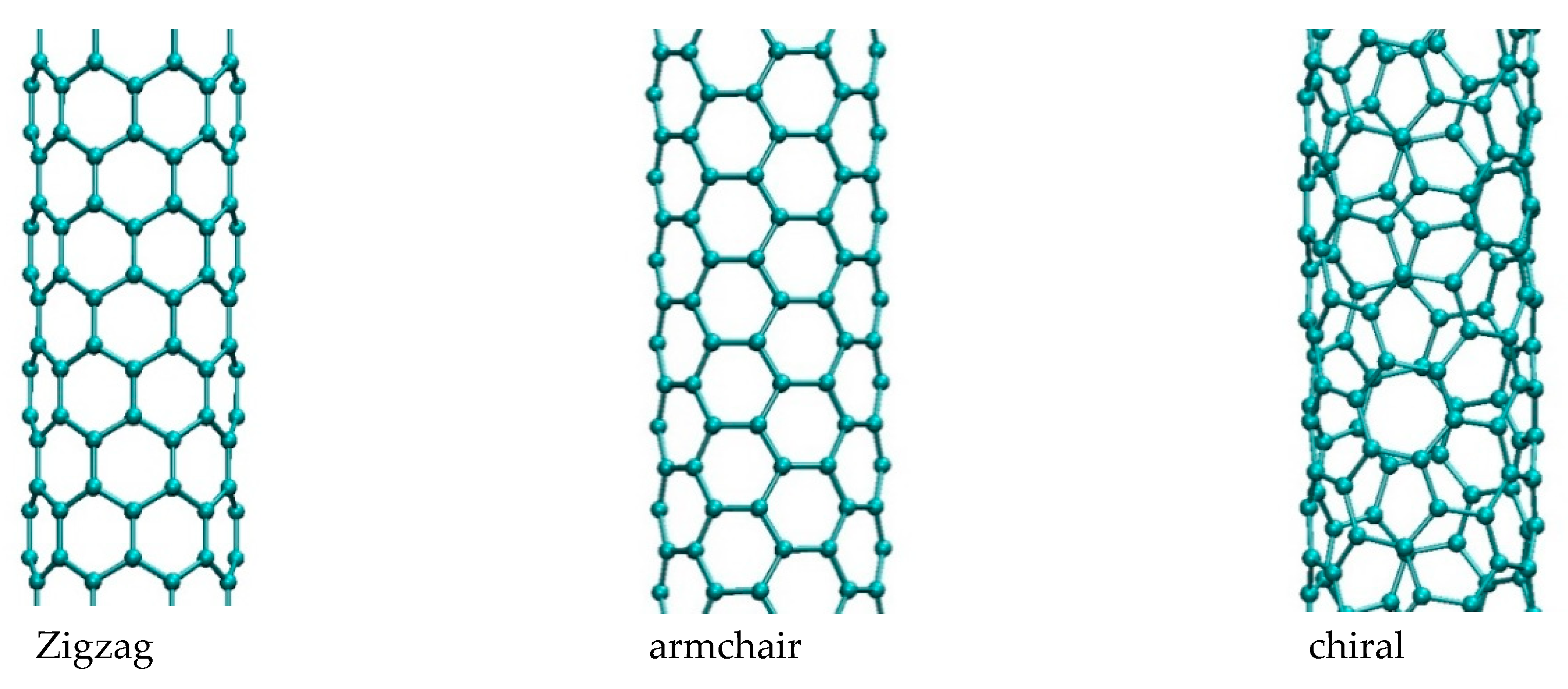
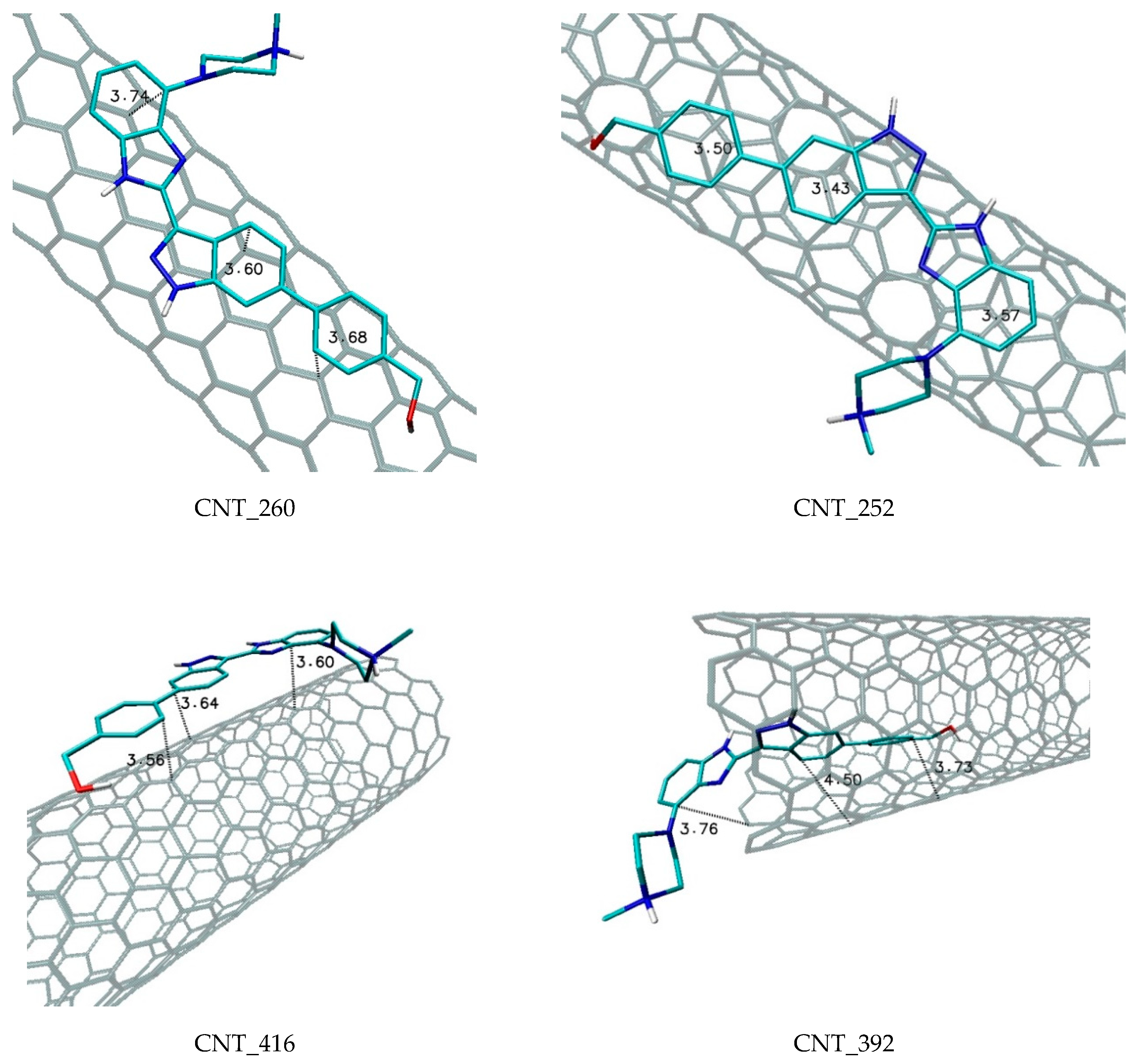
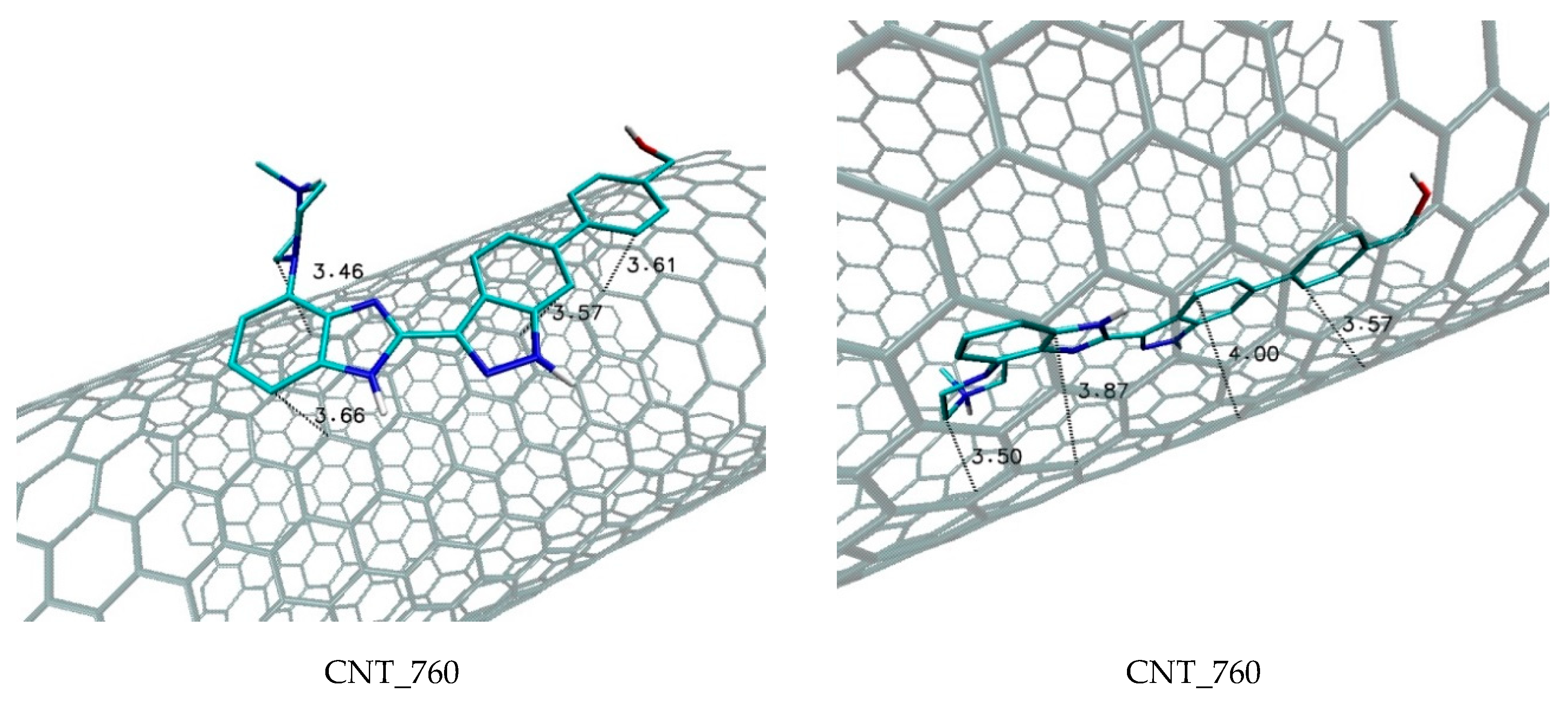
| Nanostructure Name | C Atoms | N chiral Index | M Chiral Index | L [nm] | Type | D [Å] | Diameter Class |
|---|---|---|---|---|---|---|---|
| CNT_260 | 260 | 5 | 5 | 3 | armchair | 6.25 | D1 |
| CNT_252 | 252 | 6 | 3 | 3 | chiral | 6.21 | D1 |
| CNT_256 | 256 | 8 | 0 | 3 | zigzag | 6.73 | D1 |
| CNT_416 | 416 | 8 | 8 | 3 | armchair | 10.83 | D2 |
| CNT_392 | 392 | 10 | 6 | 2 | chiral | 10.94 | D2 |
| CNT_448 | 448 | 14 | 0 | 3 | zigzag | 10.94 | D2 |
| CNT_676 | 676 | 13 | 13 | 3 | armchair | 17.47 | D3 |
| CNT_760 | 760 | 15 | 10 | 3 | chiral | 17.01 | D3 |
| CNT_704 | 704 | 22 | 0 | 3 | zigzag | 17.20 | D3 |
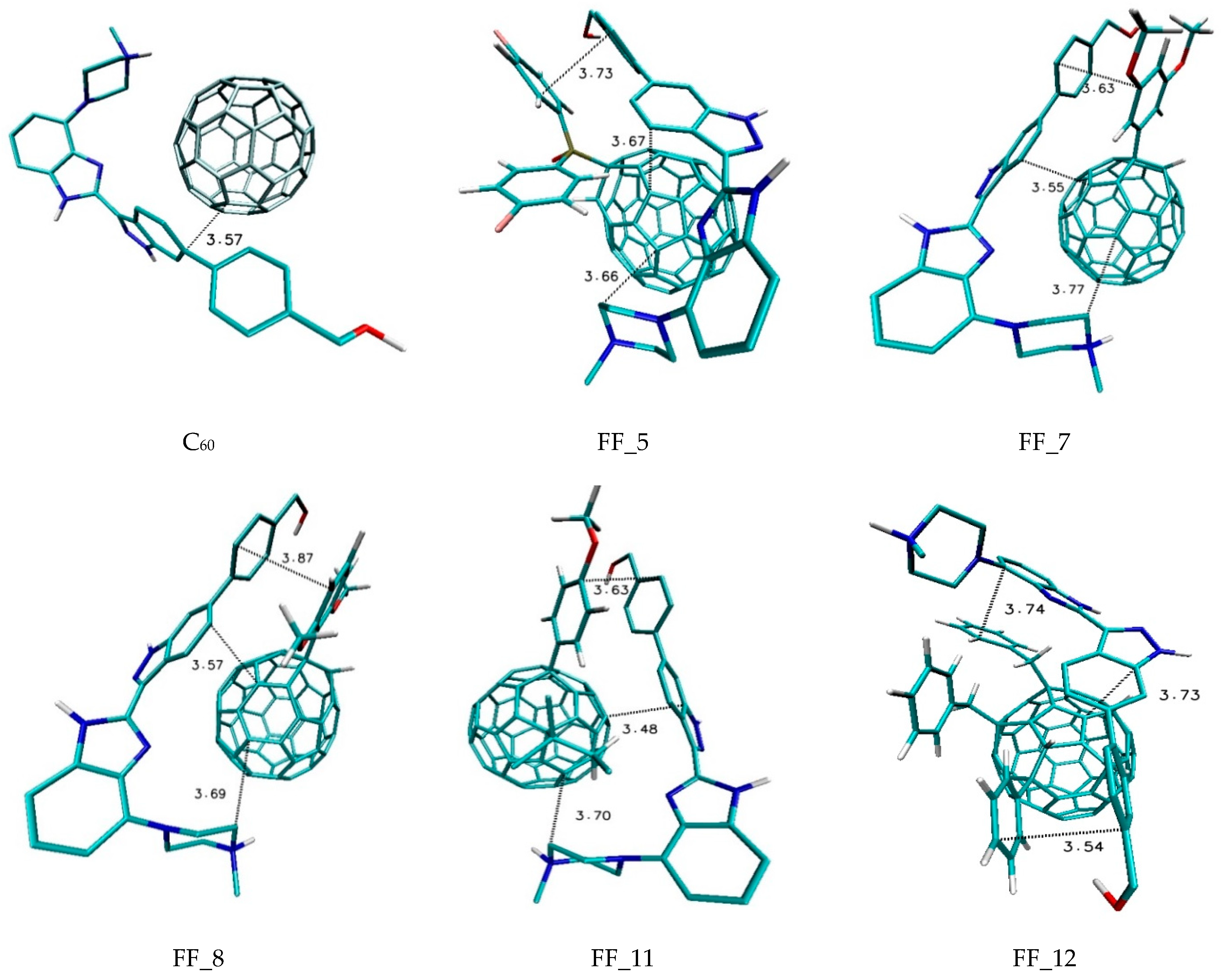
| Nanostructure Name | ΔG [kcal/mol] | Binding Constant [Kmax] | Difference of Kmax Relative to C60 [%] | |||
|---|---|---|---|---|---|---|
| MAX | MIN | AVERAGE | SD | |||
| FF_1 | −6.50 | −6.00 | −6.24 | 0.13 | 58,151.8 | 1388.7 |
| FF_2 | −6.10 | −5.80 | −5.96 | 0.09 | 29,604.6 | 657.9 |
| FF_3 | −6.50 | −6.10 | −6.31 | 0.11 | 58,151.8 | 1388.7 |
| FF_4 | −6.00 | −5.90 | −5.95 | 0.05 | 25,006.8 | 540.2 |
| FF_5 | −7.10 | −6.50 | −6.74 | 0.16 | 160,091.8 | 3998.5 |
| FF_6 | −6.10 | −5.70 | −5.85 | 0.13 | 29,604.6 | 657.9 |
| FF_7 | −6.80 | −6.40 | −6.57 | 0.10 | 96,486.4 | 2370.1 |
| FF_8 | −6.70 | −6.30 | −6.50 | 0.12 | 81,501.4 | 1986.5 |
| FF_9 | −6.60 | −6.40 | −6.48 | 0.07 | 68,843.7 | 1662.4 |
| FF_10 | −6.10 | −5.70 | −5.82 | 0.11 | 29,604.6 | 657.9 |
| FF_11 | −7.20 | −6.70 | −6.92 | 0.15 | 189,526.5 | 4752.0 |
| FF_12 | −8.00 | −7.40 | −7.66 | 0.18 | 731,270.0 | 18,621.0 |
| FF_13 | −5.90 | −5.80 | −5.83 | 0.05 | 21,123.1 | 440.8 |
| C60 | −4.90 | −4.90 | −4.90 | 0.00 | 3906.1 | 0.0 |
| Nanostructure Name | ΔG [kcal/mol] | Binding Constant [Kmax] | Type | |||
|---|---|---|---|---|---|---|
| MAX | MIN | AVERAGE | SD | |||
| CNT surface | ||||||
| CNT_260 | −10.8 | −10.6 | −10.7 | 0.06 | 8.25 × 107 | armchair |
| CNT_252 | −10.5 | −10.3 | −10.4 | 0.06 | 4.97 × 107. | chiral |
| CNT_256 | −10.4 | −10.3 | −10.4 | 0.05 | 4.20 × 107. | zigzag |
| CNT_416 | −11.6 | −11.4 | −11.5 | 0.06 | 3.18 × 108. | armchair |
| CNT_392 | −11.7 | −11.5 | −11.6 | 0.07 | 3.77 × 108 | chiral |
| CNT_448 | −11.6 | −11.5 | −11.6 | 0.05 | 3.18 × 108 | zigzag |
| CNT_676 | −12.6 | −12.4 | −12.5 | 0.05 | 1.72 × 109 | armchair |
| CNT_760 | −12.6 | −12.4 | −12.5 | 0.05 | 1.72 × 109 | chiral |
| CNT_704 | −12.5 | −12.4 | −12.4 | 0.05 | 1.45 × 109 | zigzag |
| CNT interior | ||||||
| CNT_260 | 134.9 | 142 | 138 | 2.6 | 1.31 × 10−99 | armchair |
| CNT_252 | 249.9 | 269 | 257 | 10.9 | 6.62 × 10−184 | chiral |
| CNT_256 | 232 | 271 | 257 | 21.7 | 8.74 × 10−171 | zigzag |
| CNT_416 | −19 | −18.9 | −18.9 | 0.02 | 8.46 × 1013 | armchair |
| CNT_392 | −19.4 | −19.2 | −19.3 | 0.09 | 1.66 × 1014 | chiral |
| CNT_448 | −19.1 | −19 | −19 | 0.05 | 1.00 × 1014 | zigzag |
| CNT_676 | −18.3 | −18.2 | −18.2 | 0.04 | 2.59 × 1013 | armchair |
| CNT_760 | −18.5 | −18.3 | −18.4 | 0.06 | 3.64 × 1013 | chiral |
| CNT_704 | −18.4 | −18.3 | −18.4 | 0.04 | 3.07 × 1013 | zigzag |
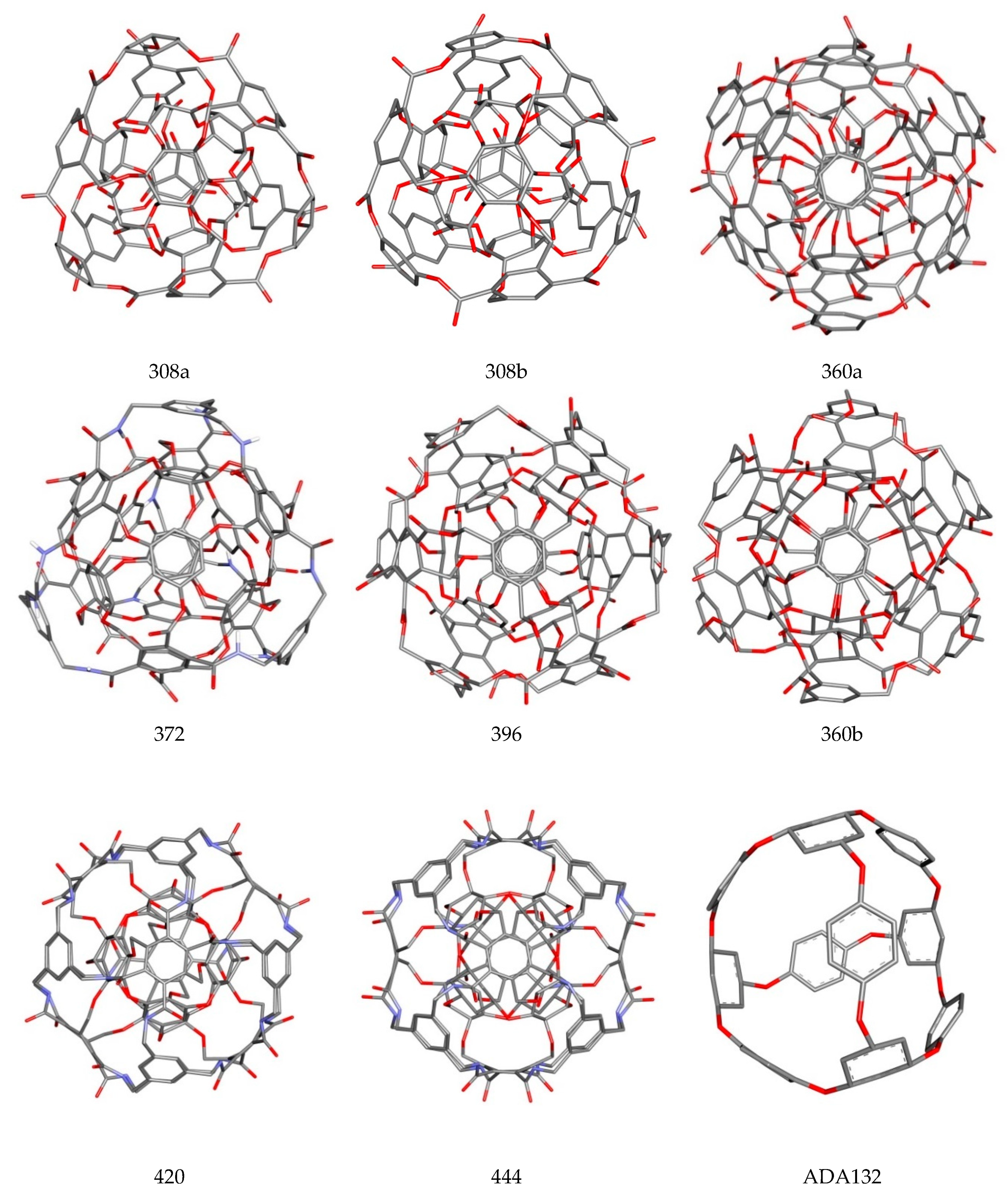
| Nanostructure Name | ΔG [kcal/mol] | Binding Constant [Kmax] | Difference of Kmax Relative to C60 [%] | |||
|---|---|---|---|---|---|---|
| MAX | MIN | AVERAGE | SD | |||
| 144_ex_ex | −3.40 | −3.30 | −3.32 | 0.04 | 310.6 | −92.0 |
| 144_in_ex | −3.80 | −3.80 | −3.80 | 0.00 | 610.2 | −84.4 |
| 156_ex_ex | −3.60 | −3.60 | −3.60 | 0.00 | 435.3 | −88.9 |
| 156_in_ex | −3.80 | −3.80 | −3.80 | 0.00 | 610.2 | −84.4 |
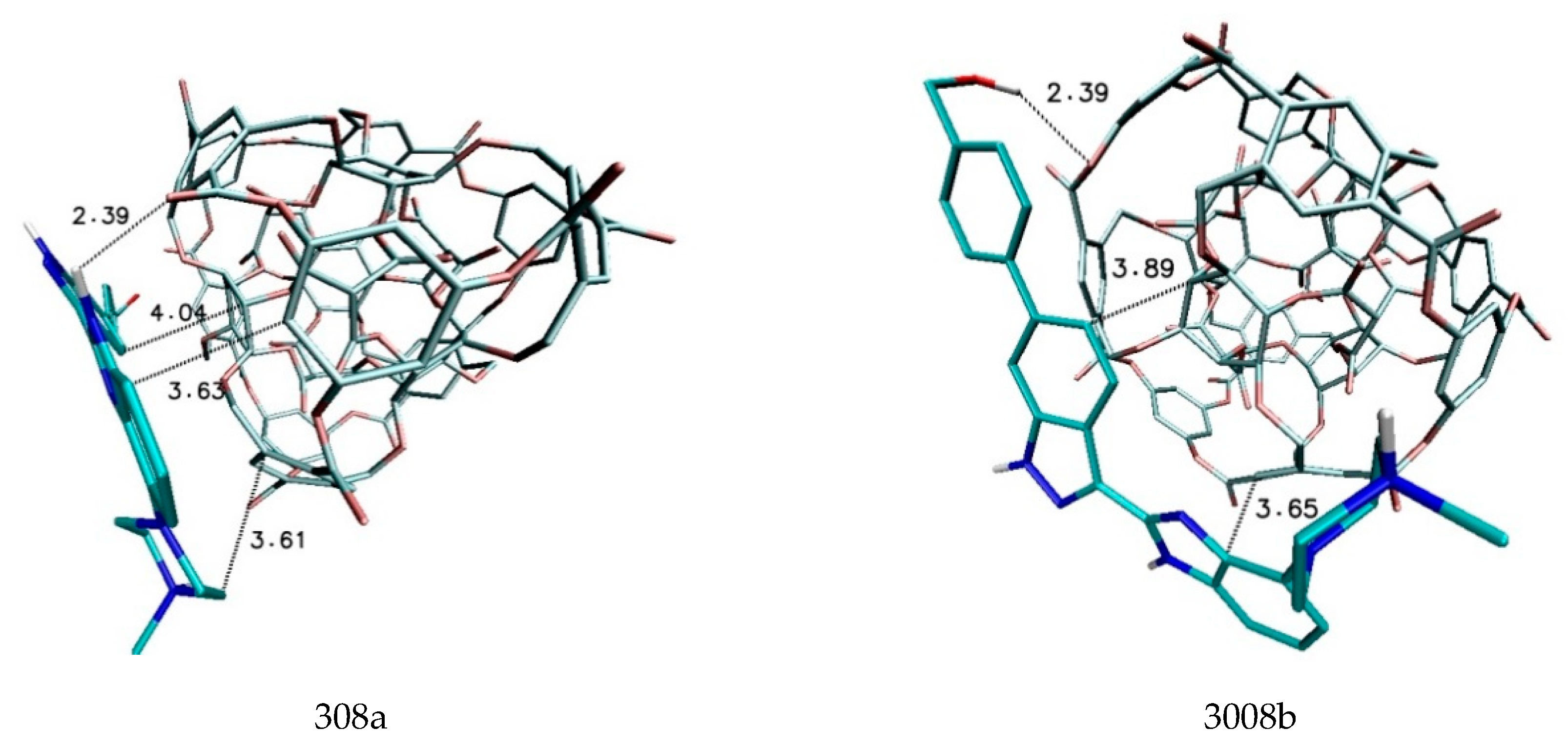
| 308a4 | −6.60 | −6.40 | −6.50 | 0.09 | 68,843.7 | 1662.4 |
| 308b4 | −6.50 | −6.40 | −6.41 | 0.03 | 58,151.8 | 1388.7 |
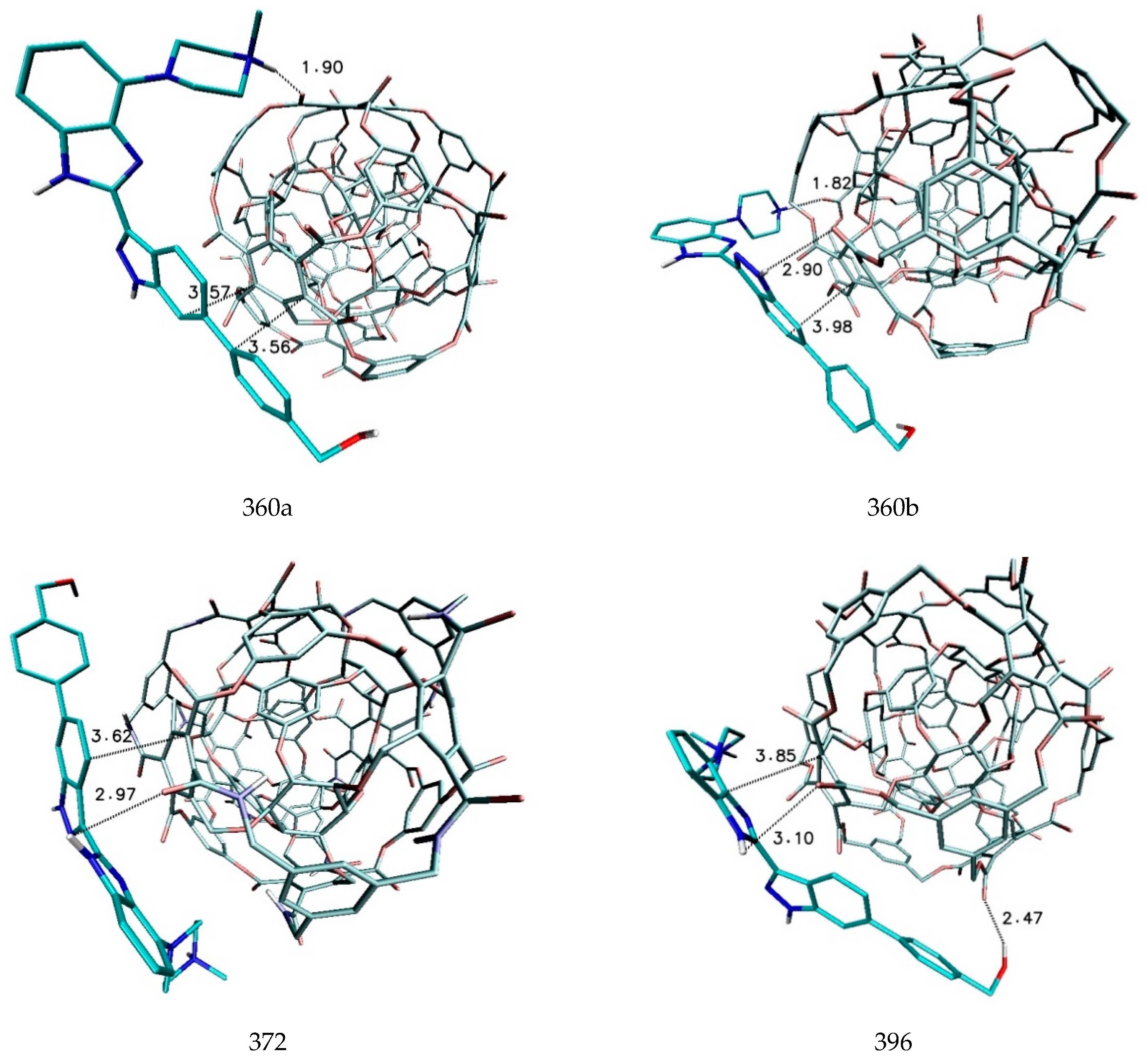
| 360a | −6.70 | −6.30 | −6.49 | 0.15 | 81,501.4 | 1986.5 |
| 360b | −6.00 | −5.90 | −5.98 | 0.04 | 25,006.8 | 540.2 |
| 372AB | −6.10 | −6.00 | −6.09 | 0.03 | 29,604.6 | 657.9 |
| 396 | −6.10 | −5.90 | −6.01 | 0.06 | 29,604.6 | 657.9 |
| 420 | −5.80 | −5.60 | −5.70 | 0.05 | 17,842.5 | 356.8 |
| 444 | −5.60 | −5.50 | −5.54 | 0.05 | 12,730.8 | 225.9 |
| 456 | −5.60 | −5.40 | −5.50 | 0.07 | 12,730.8 | 225.9 |
| ADA_132 | −5.80 | −5.70 | −5.72 | 0.04 | 17,842.5 | 356.8 |
| C60 | −4.90 | −4.90 | −4.90 | 0.00 | 3906.1 | 0.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czeleń, P.; Szefler, B. The Immobilization of ChEMBL474807 Molecules Using Different Classes of Nanostructures. Symmetry 2019, 11, 980. https://doi.org/10.3390/sym11080980
Czeleń P, Szefler B. The Immobilization of ChEMBL474807 Molecules Using Different Classes of Nanostructures. Symmetry. 2019; 11(8):980. https://doi.org/10.3390/sym11080980
Chicago/Turabian StyleCzeleń, Przemysław, and Beata Szefler. 2019. "The Immobilization of ChEMBL474807 Molecules Using Different Classes of Nanostructures" Symmetry 11, no. 8: 980. https://doi.org/10.3390/sym11080980
APA StyleCzeleń, P., & Szefler, B. (2019). The Immobilization of ChEMBL474807 Molecules Using Different Classes of Nanostructures. Symmetry, 11(8), 980. https://doi.org/10.3390/sym11080980






