The Face of Early Cognitive Decline? Shape and Asymmetry Predict Choice Reaction Time Independent of Age, Diet or Exercise
Abstract
:1. Introduction
1.1. Low Fluctuating Asymmetry: A Measure of Developmental Stability
1.2. Do Healthy Lifestyles Protect Against Epigenetic Stress?
1.2.1. Diet and Cognitive Performance
1.2.2. Exercise and Cognitive Performance
1.3. Current Study: Developmental Stability, Cognitive Performance and Controlling for Lifestyle and Background Factors
1.4. Hypotheses
2. Materials and Methods
2.1. Design
2.2. Participants
2.3. Materials
2.4. Procedure
2.5. Data Analytic Strategy
3. Results
3.1. Descriptive Statistics
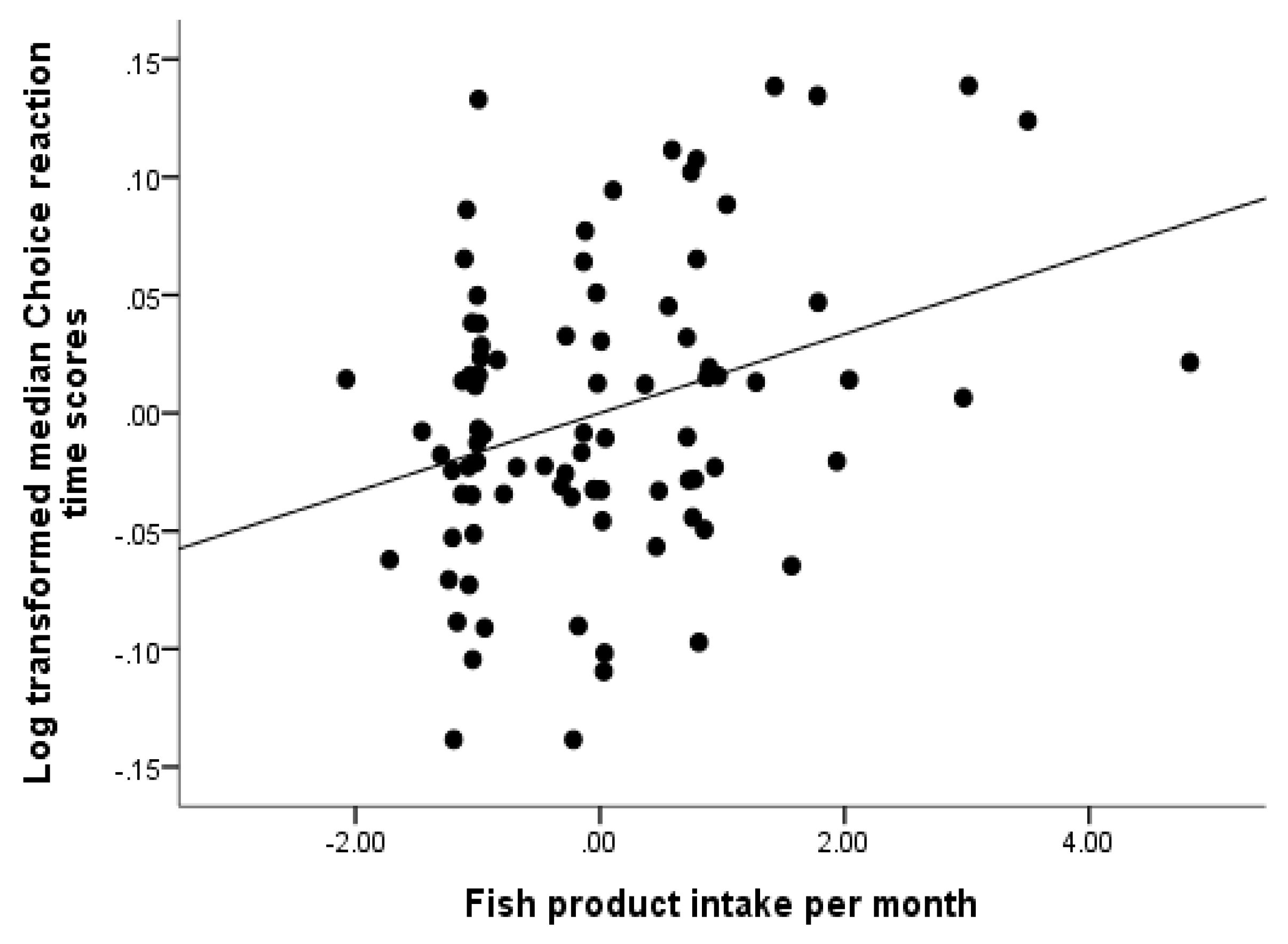
3.2. System Integrity Model: What is the Role of Diet and Exercise?
3.3. Does Face Shape and Symmetry Predict Cognitive Performance Above and Beyond Age, Sex, Diet and Exercise?
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Thompson, J.J.; Blair, M.R.; Henrey, A.J. Over the Hill at 24: Persistent age-related cognitive-motor decline in reaction times in an ecologically valid video game task begins in early adulthood. PLoS ONE 2014, 9, e94215. [Google Scholar] [CrossRef] [PubMed]
- Gale, C.R.; Batty, G.D.; Cooper, C.; Deary, I.J. Psychomotor coordination and intelligence in childhood and health in adulthood—Testing the system integrity hypothesis. Psychosom. Med. 2009, 71, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Penke, L.; Bates, T.C.; Gow, A.J.; Pattie, A.; Starr, J.M.; Jones, B.C.; Perrett, D.I.; Deary, I.J. Symmetric faces are a sign of successful cognitive aging. Evol. Hum. Behav. 2009, 30, 429–437. [Google Scholar] [CrossRef]
- Deary, I.J. Looking for “system integrity” in cognitive epidemiology. Gerontology 2012, 58, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Deary, I.J.; Der, G. Reaction time explains IQ’s association with death. Psychol. Sci. 2005, 16, 64–69. [Google Scholar] [CrossRef]
- Batty, G.D.; Deary, I.J.; Gottfredson, L.S. Premorbid (early life) IQ and later mortality risk: Systematic review. Ann. Epidemiol. 2007, 17, 278–288. [Google Scholar] [CrossRef]
- Van Valen, L. A study of fluctuating asymmetry. Evolution 1962, 16, 125–142. [Google Scholar] [CrossRef]
- Bates, T.C. Fluctuating asymmetry and intelligence. Intelligence 2007, 35, 41–46. [Google Scholar] [CrossRef]
- Van Dongen, S.; Gangestad, S.W. Human fluctuating asymmetry in relation to health and quality: A meta-analysis. Evol. Hum. Behav. 2011, 32, 380–398. [Google Scholar] [CrossRef]
- Thoma, R.J.; Yeo, R.A.; Gangestad, S.; Halgren, E.; Davis, J.; Paulson, K.M.; Lewine, J.D. Developmental instability and the neural dynamics of the speed–intelligence relationship. NeuroImage 2006, 32, 1456–1464. [Google Scholar] [CrossRef]
- Hope, D.; Bates, T.C.; Dykiert, D.; Der, G.; Deary, I.J. More symmetrical children have faster and more consistent choice reaction times. Dev. Psychol. 2015, 51, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Tucker-Drob, E.M. Global and domain-specific changes in cognition throughout adulthood. Dev. Psychol. 2011, 47, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Woods, D.L.; Wyma, J.M.; Yund, E.W.; Herron, T.J.; Reed, B. Age-related slowing of response selection and production in a visual choice reaction time task. Front. Hum. Neurosci. 2015, 9, 193. [Google Scholar] [PubMed]
- Zaninotto, P.; Batty, G.D.; Allerhand, M.; Deary, I.J. Cognitive function trajectories and their determinants in older people: 8 years of follow-up in the English Longitudinal Study of Ageing. J. Epidemiol. Community Health 2018, 72, 685–694. [Google Scholar] [CrossRef]
- Motes, M.A.; Spence, J.S.; Brier, M.R.; Chiang, H.-S.; Delarosa, B.L.; Eroh, J.; Maguire, M.J.; Mudar, R.A.; Tillman, G.D.; Kraut, M.A.; et al. Conjoint differences in inhibitory control and processing speed in childhood to older adult cohorts: Discriminant functions from a Go/No-Go task. Psychol. Aging 2018, 33, 1070–1078. [Google Scholar] [CrossRef]
- Waddington, C.H. The epigenotype. Endeavor 1942, 1, 18–20. [Google Scholar] [CrossRef]
- Deichmann, U. Epigenetics: The origins and evolution of a fashionable topic. Dev. Biol. 2016, 416, 249–254. [Google Scholar] [CrossRef]
- Brown, W.M. Exercise-associated DNA methylation change in skeletal muscle and the importance of imprinted genes: A bioinformatics meta-analysis. Br. J. Sp. Med. 2015, 49, 1567–1578. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Costello, P.M.; Lillycrop, K.A. The developmental environment, epigenetic biomarkers and long-term health. J. Dev. Orig. Health Dis. 2015, 6, 399–406. [Google Scholar] [CrossRef]
- Nöthling, J.; Malan-Müller, S.; Abrahams, N.; Hemmings, S.M.H.; Seedat, S. Epigenetic alterations associated with childhood trauma and adult mental health outcomes: A systematic review. World J. Biol. Psychol. 2019, 1–58. [Google Scholar] [CrossRef]
- Berson, A.; Nativio, R.; Berger, S.L.; Bonini, N.M. Epigenetic regulation in neurodegenerative diseases. Trends Neurosci. 2018, 41, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J.; Vijg, J. Does damage to DNA and other macromolecules play a role in aging? If so, how? J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2009, 64, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cai, G.; Chen, X. Dietary restriction delays the secretion of senescence associated secretory phenotype by reducing DNA damage response in the process of renal aging. Exp. Gerontol. 2018, 107, 4–10. [Google Scholar] [CrossRef]
- Zimmer, P.; Bloch, W. Physical exercise and epigenetic adaptations of the cardiovascular system. Herz 2015, 40, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Misuraca, R.; Miceli, S.; Teuscher, U. Three effective ways to nurture our brain: Physical activity, healthy nutrition, and music. A review. Europ. Psychol. 2017, 22, 101–120. [Google Scholar] [CrossRef]
- Canhada, S.; Castro, K.; Perry, I.S.; Luft, V.C. Omega-3 fatty acids’ supplementation in Alzheimer’s disease: A systematic review. Nutr. Neurosci. 2018, 21, 529–538. [Google Scholar] [CrossRef]
- Haast, R.A.; Kiliaan, A.J. Impact of fatty acids on brain circulation, structure and function. Prostaglandins Leukot. Essent. Fat. Acids 2015, 92, 3–14. [Google Scholar] [CrossRef]
- Gomez-Pinilla, F. Brain foods: The effects of nutrients on brain function. Nat. Rev. Neurosci. 2008, 9, 568–578. [Google Scholar] [CrossRef]
- McCann, J.C.; Ames, B.N. Is docosahexaenoic acid, an n−3 long-chain polyunsaturated fatty acid, required for development of normal brain function? An overview of evidence from cognitive and behavioral tests in humans and animals. Am. J. Clin. Nutr. 2005, 82, 281–295. [Google Scholar] [CrossRef]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Barnes, L.L.; Bennett, D.A.; Aggarwal, N.T. MIND diet slows cognitive decline with aging. Alzheimer’s Dement. 2015, 11, 1015–1022. [Google Scholar] [CrossRef] [Green Version]
- Morris, M.C.; Evans, D.A.; Tangney, C.C.; Bienias, J.L.; Wilson, R.S. Fish consumption and cognitive decline with age in a large community study. Archiv. Neurol. 2005, 62, 1849–1853. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Pinilla, F.; Tyagi, E. Diet and cognition: Interplay between cell metabolism and neuronal plasticity. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 726–733. [Google Scholar] [CrossRef]
- Mattson, M.P.; Gleichmann, M.; Cheng, A. Mitochondria in neuroplasticity and neurological disorders. Neuron 2008, 60, 748–766. [Google Scholar] [CrossRef]
- Agrawal, R.; Gomez-Pinilla, F. ‘Metabolic syndrome’ in the brain: Deficiency in omega-3 fatty acid exacerbates dysfunctions in insulin receptor signalling and cognition. J. Physiol. 2012, 590, 2485–2499. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.K.; Ha, C.H. The effect of exercise intensity on brain derived neurotrophic factor and memory in adolescents. Environ. Health Prev. Med. 2017, 22, 27. [Google Scholar] [CrossRef] [PubMed]
- Drigny, J.; Gremeaux, V.; Dupuy, O.; Gayda, M.; Bherer, L.; Juneau, M.; Nigam, A. Effect of interval training on cognitive functioning and cerebral oxygenation in obese patients: A pilot study. J. Rehabilit. Med. 2014, 46, 1050–1054. [Google Scholar] [CrossRef] [Green Version]
- Douris, P.C.; Handrakis, J.P.; Apergis, D.; Mangus, R.B.; Patel, R.; Limtao, J.; Platonova, S.; Gregorio, A.; Luty, E. The effects of aerobic exercise and gaming on cognitive performance. J. Hum. Kinet. 2018, 61, 73–83. [Google Scholar] [CrossRef]
- Davis, C.L.; Tomporowski, P.D.; McDowell, J.E.; Austin, B.P.; Miller, P.H.; Yanasak, N.E.; Allison, J.D.; Naglieri, J.A. Exercise improves executive function and achievement and alters brain activation in overweight children: A randomized, controlled trial. Health Psychol. 2011, 30, 91–98. [Google Scholar] [CrossRef]
- Busse, A.L.; Filho, W.J.; Magaldi, R.M.; Coelho, V.A.; Melo, A.C.; Betoni, R.A.; Santarém, J.M. Effects of resistance training exercise on cognitive performance in elderly individuals with memory impairment: Results of a controlled trial. Einstein 2008, 6, 402–407. Available online: https://www.researchgate.net/profile/Jose_Santarem/publication/26570915_Effects_of_resistance_training_exercise_on_cognitive_performance_in_elderly_individuals_with_memory_impairment_Results_of_a_controlled_trial/links/00b7d532c53a39ecf1000000/Effects-of-resistance-training-exercise-on-cognitive-performance-in-elderly-individuals-with-memory-impairment-Results-of-a-controlled-trial.pdf (accessed on 20 June 2019).
- Köbe, T.; Witte, A.; Schnelle, A.; Lesemann, A.; Fabian, S.; Tesky, V.A.; Pantel, J.; Flöel, A. Combined omega-3 fatty acids, aerobic exercise and cognitive stimulation prevents decline in gray matter volume of the frontal, parietal and cingulate cortex in patients with mild cognitive impairment. NeuroImage 2016, 131, 226–238. [Google Scholar] [CrossRef]
- Gomez-Pinilla, F. The combined effects of exercise and foods in preventing neurological and cognitive disorders. Prev. Med. 2011, 52, S75–S80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, K.N.; Kemper, S. Interventions to reduce cognitive decline in aging. J. Psychosoc. Nurs. Ment. Health Serv. 2010, 48, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Sleiman, S.F.; Henry, J.; Al-Haddad, R.; El Hayek, L.; Haidar, E.A.; Stringer, T.; Ulja, D.; Karuppagounder, S.S.; Holson, E.B.; Ratan, R.R.; et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. eLife 2016, 5, e15092. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.; Arida, R.M.; Gomez-Pinilla, F. Physical exercise as an epigenetic modulator of brain plasticity and cognition. Neurosci. Biobehav. Rev. 2017, 80, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.A.; Gavin, C.F.; White, J.A.; Parrish, R.R.; Honasoge, A.; Yancey, C.R.; Rivera, I.M.; Rubio, M.D.; Rumbaugh, G.; Sweatt, J.D. Cortical DNA methylation maintains remote memory. Nat. Neurosci. 2010, 13, 664–666. [Google Scholar] [CrossRef] [PubMed]
- Sadli, N.; Ackland, M.L.; De Mel, D.; Sinclair, A.J.; Suphioglu, C. Effects of zinc and DHA on the epigenetic regulation of human neuronal cells. Cell. Physiol. Biochem. 2012, 29, 87–98. [Google Scholar] [CrossRef]
- Cheatham, C.L.; Lupu, D.S.; Niculescu, M.D. Genetic and epigenetic transgenerational implications related to omega-3 fatty acids. Part II: Maternal FADS2 rs174575 genotype and DNA methylation predict toddler cognitive performance. Nutr. Res. 2015, 35, 948–955. [Google Scholar] [CrossRef]
- Kulkarni, A.; Dangat, K.; Kale, A.; Sable, P.; Chavan-Gautam, P.; Joshi, S. Effects of altered maternal folic acid, vitamin B12 and docosahexaenoic acid on placental global DNA methylation patterns in Wistar rats. PLoS ONE 2011, 6, e17706. [Google Scholar] [CrossRef]
- Townsend, G.; Richards, L.; Messer, L.B.; Hughes, T.; Pinkerton, S.; Seow, K.; Gotjamanos, T.; Gully, N.; Bockmann, M. Genetic and environmental influences on dentofacial structures and oral health: Studies of Australian twins and their families. Twin Res. Hum. Genet. 2006, 9, 727–732. [Google Scholar] [CrossRef]
- Okada, H.C.; Alleyne, B.; Varghai, K.; Kinder, K.; Guyuron, B. Facial changes caused by smoking: A comparison between smoking and nonsmoking identical twins. Plast. Reconstr. Surg. 2013, 132, 1085–1092. [Google Scholar] [CrossRef]
- Liu, M.T.; Iglesias, R.A.; Sekhon, S.S.; Li, Y.; Larson, K.; Totonchi, A.; Guyuron, B. Factors contributing to facial asymmetry in identical twins. Plast. Reconstr. Surg. 2014, 134, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Bruder, C.E.; Piotrowski, A.; Gijsbers, A.A.; Andersson, R.; Erickson, S.; De Ståhl, T.D.; Menzel, U.; Sandgren, J.; Von Tell, D.; Poplawski, A.; et al. Phenotypically concordant and discordant monozygotic twins display different DNA copy-number-variation profiles. Am. J. Hum. Genet. 2008, 82, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Coleman, S.R.; Grover, R. The anatomy of the aging face: Volume loss and changes in 3-dimensional topography. Aesthetic Surg. J. 2006, 26, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.B., Jr.; Katzel, E.B.; Koltz, P.F.; Yaremchuk, M.J.; Girotto, J.A.; Kahn, D.M.; Langstein, H.N. Aging of the facial skeleton: aesthetic implications and rejuvenation strategies. Plast. Reconstr. Surg. 2011, 127, 374–383. [Google Scholar] [CrossRef]
- Little, A.C.; Jones, B.C.; Debruine, L.M. Facial attractiveness: Evolutionary based research. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 1638–1659. [Google Scholar] [CrossRef]
- Waynforth, D. Fluctuating asymmetry and human male life-history traits in rural Belize. Proc. R. Soc. B Biol. Sci. 1998, 265, 1497–1501. [Google Scholar] [CrossRef]
- Thornhill, R.; Grammer, K. The body and face of woman: One ornament that signals quality? Evol. Hum. Behav. 1999, 20, 105–120. [Google Scholar] [CrossRef]
- Stephen, I.D.; Hiew, V.; Coetzee, V.; Tiddeman, B.P.; Perrett, D.I. Facial shape analysis identifies valid cues to aspects of physiological health in Caucasian, Asian, and African populations. Front. Psychol. 2017, 8, 1883. [Google Scholar] [CrossRef]
- Booth, T.; Royle, N.A.; Corley, J.; Gow, A.J.; Hernández, M.D.C.V.; Maniega, S.M.; Ritchie, S.J.; Bastina, M.E.; Starr, J.M.; Wardlaw, J.M.; et al. Association of allostatic load with brain structure and cognitive ability in later life. Neurobiol. Aging 2015, 36, 1390–1399. [Google Scholar] [CrossRef]
- Hagger-Johnson, G.; Deary, I.J.; Davies, C.A.; Weiss, A.; Batty, G.D. Reaction time and mortality from the major causes of death: The NHANES-III study. PLoS ONE 2014, 9, e82959. [Google Scholar] [CrossRef]
- Thorsdottir, I.; Tomasson, H.; Gunnarsdottir, I.; Gisladottir, E.; Kiely, M.; Parra, M.D.; Bandarra, N.M.; Schaafsma, G.; A Martinez, J.; Bandarra, N.; et al. Randomized trial of weight-loss-diets for young adults varying in fish and fish oil content. Int. J. Obes. 2007, 31, 1560–1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birgisdottir, B.; Kiely, M.; Martinez, J.; Thorsdottir, I. Validity of a food frequency questionnaire to assess intake of seafood in adults in three European countries. Food Control. 2008, 19, 648–653. [Google Scholar] [CrossRef]
- Thorsdottir, I.; Birgisdottir, B.; Kiely, M.; Martínez, J.; Bandarra, N.; Bandarra, N. Fish consumption among young overweight European adults and compliance to varying seafood content in four weight loss intervention diets. Public Health Nutr. 2009, 12, 592–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, T.; Bull, F. Development of the World Health Organization Global Physical Activity Questionnaire (GPAQ). J. Public Health 2006, 14, 66–70. [Google Scholar] [CrossRef]
- Herrmann, S.D.; Heumann, K.J.; Der Ananian, C.A.; Ainsworth, B.E. Validity and reliability of the Global Physical Activity Questionnaire (GPAQ). Meas. Phys. Educ. Exerc. Sci. 2013, 17, 221–235. [Google Scholar] [CrossRef]
- Jetté, M.; Sidney, K.; Blümchen, G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin. Cardiol. 1990, 13, 555–565. [Google Scholar] [CrossRef]
- Wiley, D.F.; Amenta, N.; Alcantara, D.A.; Ghosh, D.; Kil, Y.J..; Delson, E.; Rohlf, J.; St John, K.; Hamann, B. Evolutionary Morphing. In Proceedings of the VIS 05. IEEE Visualization, Minneapolis, MN, USA, 23–28 October 2005; pp. 431–438. [Google Scholar]
- Klingenberg, C.P. MorphoJ: An integrated software package for geometric morphometrics. Molec. Ecol. Res. 2011, 11, 353–357. [Google Scholar] [CrossRef]
- Mitteroecker, P.; Gunz, P. Advances in geometric morphometrics. Evol. Biol. 2009, 36, 235–247. [Google Scholar] [CrossRef]
- Klingenberg, C.P.; Barluenga, M.; Meyer, A. Shape analysis of symmetric structures: Quantifying variation among individuals and asymmetry. Evolutio 2002, 56, 1909–1920. [Google Scholar] [CrossRef]
- Simmons, L.W.; Rhodes, G.; Peters, M.; Koehler, N. Are human preferences for facial symmetry focused on signals of developmental instability? Behav. Ecol. 2004, 15, 864–871. [Google Scholar] [CrossRef]
- Windhager, S.; Bookstein, F.L.; Millesi, E.; Wallner, B.; Schaefer, K. Patterns of correlation of facial shape with physiological measurements are more integrated than patterns of correlation with ratings. Sci. Rep. 2017, 7, 45340. [Google Scholar] [CrossRef] [PubMed]
- Holzleitner, I.J.; Lee, A.J.; Hahn, A.; Kandrik, M.; Bovet, J.; Renoult, J.P.; Simmons, D.; Garrod, O.G.B.; Debruine, L.M.; Jones, B.C. Comparing theory-driven and data-driven attractiveness models using images of real women’s faces. PsyArXiv 2018. [Google Scholar] [CrossRef] [PubMed]
- Deary, I.J.; Liewald, D.; Nissan, J. A free, easy-to-use, computer-based simple and four-choice reaction time programme: The Deary-Liewald reaction time task. Behav. Res. Methods 2011, 43, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Woods, D.L.; Wyma, J.M.; Yund, E.W.; Herron, T.J.; Reed, B. Factors influencing the latency of simple reaction time. Front. Hum. Neurosci. 2015, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Ebner, N.C.; Luedicke, J.; Voelkle, M.C.; Riediger, M.; Lin, T.; Lindenberger, U. An adult developmental approach to perceived facial attractiveness and distinctiveness. Front. Psychol. 2018, 9, 561. [Google Scholar] [CrossRef] [PubMed]
- Kobyliansky, E.; Livshits, G. Age-dependent changes in morphometric and biochemical traits. Annu. Hum. Biol. 1989, 16, 237–247. [Google Scholar] [CrossRef]
- Whelan, R. Effective analysis of reaction time data. Psychol. Rec. 2008, 58, 475–482. [Google Scholar] [CrossRef]
- Tabachnick, B.G.; Fidell, L.S.; Ullman, J.B. Using Multivariate Statistics; Pearson: Hoboken, NJ, USA, 2013. [Google Scholar]
- Lu, P.H.; Lee, G.J.; Tishler, T.A.; Meghpara, M.; Thompson, P.M.; Bartzokis, G. Myelin breakdown mediates age-related slowing in cognitive processing speed in healthy elderly men. Brain Cogn. 2013, 81, 131–138. [Google Scholar] [CrossRef]
- Raz, N.; Lindenberger, U.; Rodrigue, K.M.; Kennedy, K.M.; Head, D.; Williamson, A.; Dahle, C.; Gerstorf, D.; Acker, J.D. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cereb. Cortex 2005, 15, 1676–1689. [Google Scholar] [CrossRef]
- Kuznetsova, K.A.; Maniega, S.M.; Ritchie, S.J.; Cox, S.R.; Storkey, A.J.; Starr, J.M.; Wardlaw, J.M.; Deary, I.J.; Bastin, M.E. Brain white matter structure and information processing speed in healthy older age. Brain Struct. Func. 2016, 221, 3223–3235. [Google Scholar] [CrossRef]
- Salthouse, T.A. The processing-speed theory of adult age differences in cognition. Psychol. Rev. 1996, 103, 403–428. [Google Scholar] [CrossRef] [PubMed]
- Seidler, R.D.; Bernard, J.A.; Burutolu, T.B.; Fling, B.W.; Gordon, M.T.; Gwin, J.T.; Kwak, Y.; Lipps, D.B. Motor control and aging: Links to age-related brain structural, functional, and biochemical effects. Neurosci. Biobehav. Rev. 2010, 34, 721–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedden, T.; Gabrieli, J.D.E. Insights into the ageing mind: A view from cognitive neuroscience. Nat. Rev. Neurosci. 2004, 5, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Salthouse, T.A. When does age-related cognitive decline begin? Neurobiol. Aging 2009, 30, 507–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colcombe, S.J.; Erickson, K.I.; Scalf, P.E.; Kim, J.S.; Prakash, R.; McAuley, E.; Elavsky, S.; Marquez, D.X.; Hu, L.; Kramer, A.F. Aerobic exercise training increases brain volume in aging humans. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 1166–1170. [Google Scholar] [CrossRef]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef] [Green Version]
- Voss, M.W.; Heo, S.; Prakash, R.S.; Erickson, K.I.; Alves, H.; Chaddock, L.; Szabo, A.N.; Mailey, E.L.; Wójcicki, T.R.; White, S.M.; et al. The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: Results of a one-year exercise intervention. Hum. Brain Mapp. 2013, 34, 2972–2985. [Google Scholar] [CrossRef]
- Rosano, C.; Venkatraman, V.K.; Guralnik, J.; Newman, A.B.; Glynn, N.W.; Launer, L.; Taylor, C.A.; Williamson, J.; Studenski, S.; Pahor, M.; et al. Psychomotor speed and functional brain MRI 2 years after completing a physical activity treatment. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2010, 65, 639–647. [Google Scholar] [CrossRef]
- Baranowski, T. Validity and reliability of self report measures of physical activity: An information-processing perspective. Res. Q. Exerc. Sport 1988, 59, 314–327. [Google Scholar] [CrossRef]
- Montoye, H.J.; Washburn, R.A. The assessment of physical activity by questionnaire. Am. J. Epidemiol. 1986, 123, 563–576. [Google Scholar]
- Warnecke, R.B.; Johnson, T.P.; Chávez, N.; Sudman, S.; O’Rourke, D.P.; Lacey, L.; Horm, J. Improving question wording in surveys of culturally diverse populations. Ann. Epidemiol. 1997, 7, 334–342. [Google Scholar] [CrossRef]
- Dowd, K.P.; Szeklicki, R.; Minetto, M.A.; Murphy, M.H.; Polito, A.; Ghigo, E.; Van Der Ploeg, H.; Ekelund, U.; Maciaszek, J.; Stemplewski, R.; et al. A systematic literature review of reviews on techniques for physical activity measurement in adults: A DEDIPAC study. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, W.; Sioen, I.; Pieniak, Z.; Van Camp, J.; De Henauw, S. Consumer perception versus scientific evidence about health benefits and safety risks from fish consumption. Public Health Nutr. 2005, 8, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, J.G. The possible role of long-chain, Omega-3 fatty acids in human brain phylogeny. Perspect. Biol. Med. 1996, 39, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Broadhurst, C.L.; Cunnane, S.C.; Crawford, M.A. Rift Valley lake fish and shellfish provided brain-specific nutrition for early Homo. Br. J. Nutr. 1998, 79, 3–21. [Google Scholar] [CrossRef]
- Heinrichs, S.C. Dietary ω-3 fatty acid supplementation for optimizing neuronal structure and function. Mol. Nutr. Food Res. 2010, 54, 447–456. [Google Scholar] [CrossRef]
- Zhang, W. Omega-3 polyunsaturated fatty acids in the brain: Metabolism and neuroprotection. Front. Biosci. 2011, 16, 2653. [Google Scholar] [CrossRef]
- Barnard, N.D.; Bunner, A.E.; Agarwal, U. Saturated and trans fats and dementia: A systematic review. Neurobiol. Aging 2014, 35, S65–S73. [Google Scholar] [CrossRef]
- Vaynman, S.; Gomez-Pinilla, F.; Gómez-Pinilla, F. Revenge of the “Sit”: How lifestyle impacts neuronal and cognitive health through molecular systems that interface energy metabolism with neuronal plasticity. J. Neurosci. Res. 2006, 84, 699–715. [Google Scholar] [CrossRef]
- Knecht, S.; Ellger, T.; Levine, J.A. Obesity in neurobiology. Prog. Neurobiol. 2008, 84, 85–103. [Google Scholar] [CrossRef]
- Howe, P.R.C.; Evans, H.M.; Kuszewski, J.C.; Wong, R.H.X. Effects of long chain Omega-3 polyunsaturated fatty acids on brain function in mildly hypertensive older adults. Nutrients 2018, 10, 1413. [Google Scholar] [CrossRef] [PubMed]
- Coley, N.; Cufi, M.-N.; Legrand, P.; Caubere, J.-P.; Weiner, M.; Vellas, B.; Guyonnet, S.; Carrié, I.; Brigitte, L.; Faisant, C.; et al. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): A randomised, placebo-controlled trial. Lancet Neurol. 2017, 16, 377–389. [Google Scholar]
- Wang, Y.; Hu, D.; Chen, W.; Xue, H.; Du, Y. Prenatal tobacco exposure modulated the association of genetic variants with diagnosed ADHD and its symptom domain in children: A community based case-control study. Sci. Rep. 2019, 9, 4274. [Google Scholar] [CrossRef] [PubMed]
- Chalk, T.E.; Brown, W.M. Exercise epigenetics and the fetal origins of disease. Epigenomics 2014, 6, 469–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Groot, R.; Ouwehand, C.; Jolles, J. Eating the right amount of fish: Inverted U-shape association between fish consumption and cognitive performance and academic achievement in Dutch adolescents. Prostaglandins, Leukot. Essent. Fat. Acids 2012, 86, 113–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.; Labban, J.; Gapin, J.; Etnier, J. The effects of acute exercise on cognitive performance: A meta-analysis. Brain Res. 2012, 1453, 87–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheib, J.E.; Gangestad, S.W.; Thornhill, R. Facial attractiveness, symmetry and cues of good genes. Proc. R. Soc. B Biol. Sci. 1999, 266, 1913–1917. [Google Scholar] [CrossRef] [Green Version]
- Linden, O.E.; He, J.K.; Morrison, C.S.; Sullivan, S.R.; Taylor, H.O.B. The relationship between age and facial asymmetry. Plast. Reconstr. Surg. 2018, 142, 1145–1152. [Google Scholar] [CrossRef]
- Gunn, D.A.; Rexbye, H.; Griffiths, C.E.M.; Murray, P.G.; Fereday, A.; Catt, S.D.; Tomlin, C.C.; Strongitharm, B.H.; Perrett, D.I.; Catt, M.; et al. Why some women look young for their age. PLoS ONE 2009, 4, e8021. [Google Scholar] [CrossRef]
- Ekrami, O.; Claes, P.; White, J.D.; Zaidi, A.A.; Shriver, M.D.; Van Dongen, S. Measuring asymmetry from high-density 3D surface scans: An application to human faces. PLoS ONE 2018, 13, e0207895. [Google Scholar] [CrossRef]
- Ritchie, S.J.; Tucker-Drob, E.M.; Cox, S.R.; Corley, J.; Dykiert, D.; Redmond, P.; Pattie, A.; Taylor, A.M.; Sibbett, R.; Starr, J.M.; et al. Predictors of ageing-related decline across multiple cognitive functions. Intelligence 2016, 59, 115–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
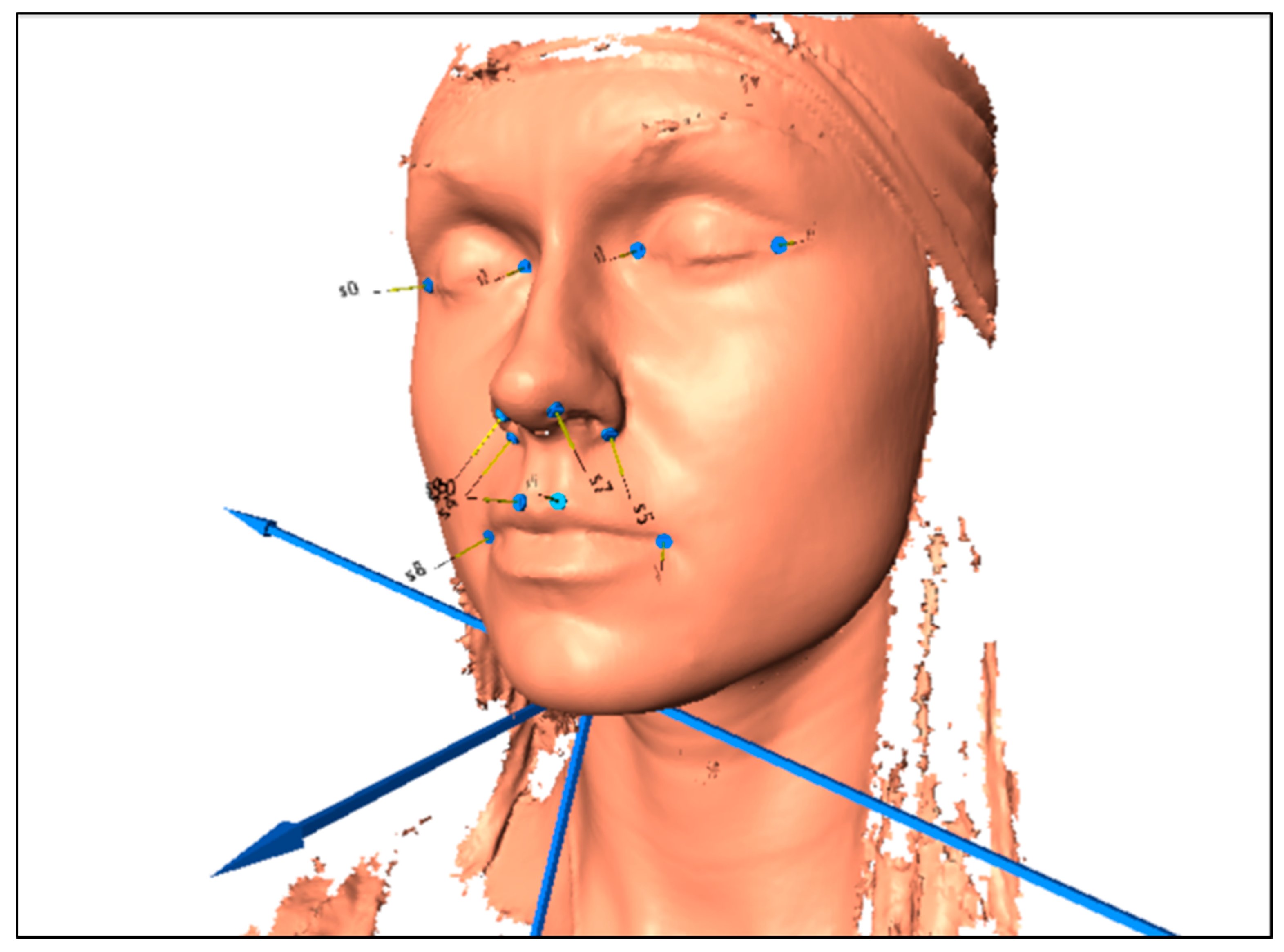
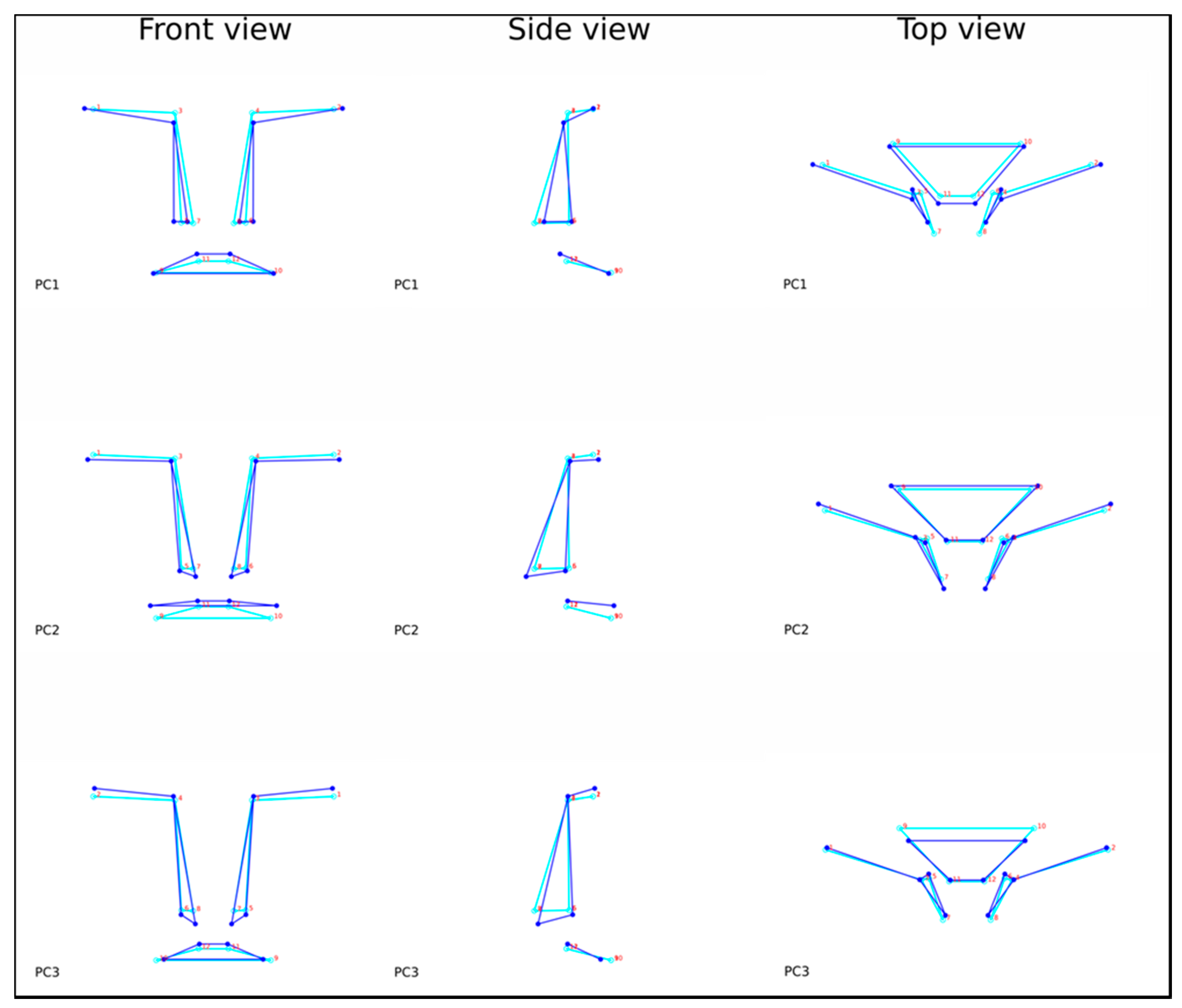
| Overall M (SD) | Male M (SD) | Female M (SD) | |
|---|---|---|---|
| Age * | 25.74 (9.26) | 23.73 (7.79) | 27.49 (10.13) |
| FFQ | 4.24 (1.26) | 4.43 (1.45) | 4.06 (1.05) |
| GPAQ | 6868.33(7296.45) | 7085.98 (5419.43) | 6678.47 (8664.36) |
| RT * | 288.74(44.99) | 278.24 (29.66) | 297.90(53.66) |
| CRT ** | 436.56 (76.08) | 413.82 (68.80) | 456.40 (77.26) |
| FA (log) | −4.84 (0.26) | −4.80 (0.24) | −4.86 (0.27) |
| DA | 0.0000(0.00003) | 0.0000(0.00003) | 0.0000(.00003) |
| PC 1 | −0.000011(0.0349747) | −0.002929(0.0380788) | 0.002534 (0.0322247) |
| PC 2 | 0.000003(0.0304065) | −0.000041(0.0319512) | 0.000041 (0.0293409) |
| PC 3 * | 0.000026(0.0260132) | 0.006657(0.0286105) | −0.005759(0.0222406) |
| FFQ | GPAQ | RT | CRT | FA | DA | PC1 | PC2 | PC3 | ||
|---|---|---|---|---|---|---|---|---|---|---|
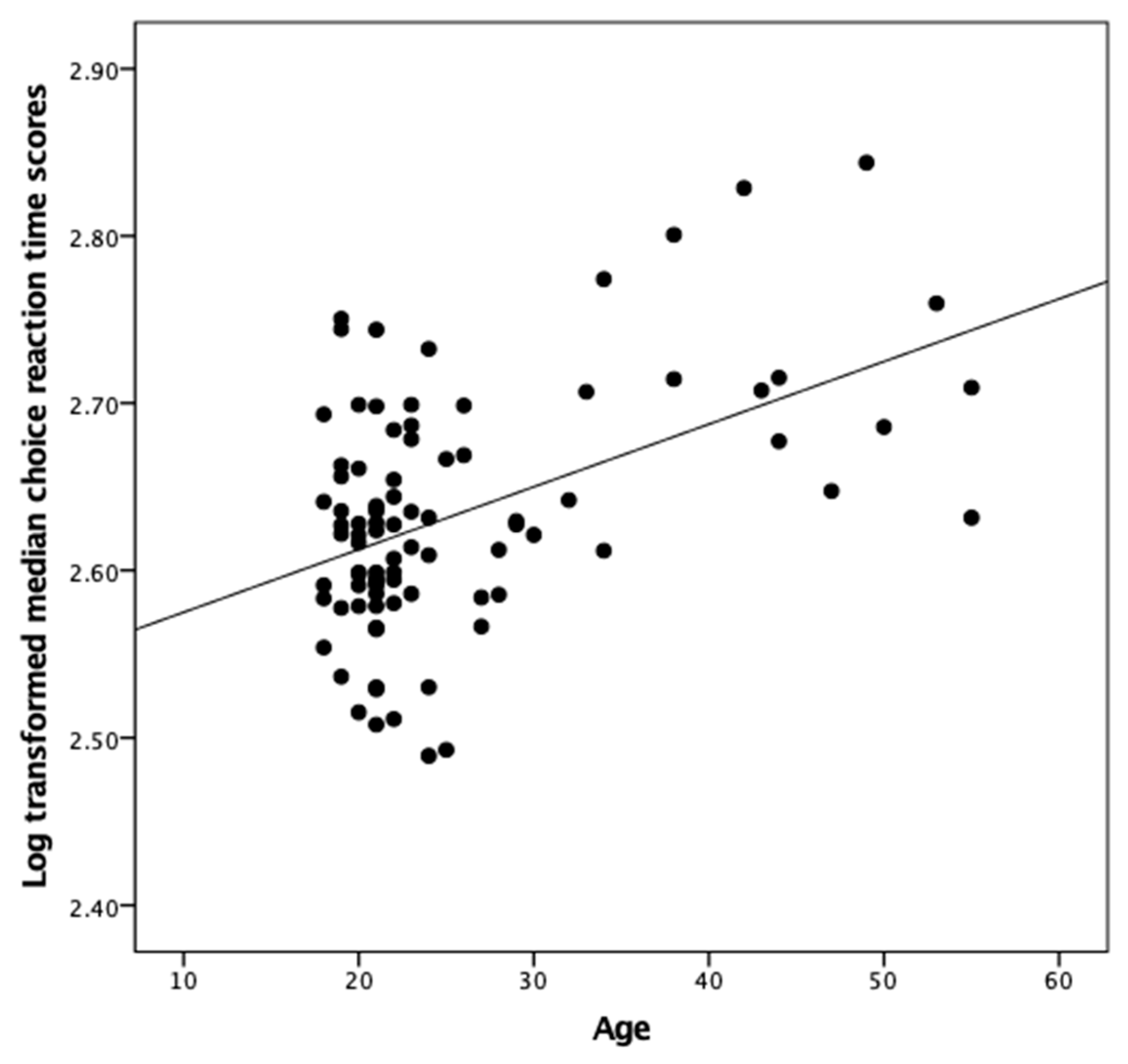
| Age | 0.20 | −0.08 | 0.17 | 0.48 ** | 0.07 | −0.03 | −0.16 | −0.01 | −0.48 ** | |
| FFQ | 0.10 | 0.29 ** | 0.38 ** | 0.05 | 0.01 | 0.05 | 0.007 | 0.07 | ||
| GPAQ | −0.07 | 0.05 | −0.11 | −0.17 | 0.20 * | 0.03 | 0.07 | |||
| RT | 0.47 ** | 0.16 | 0.14 | 0.01 | −0.08 | −0.20 | ||||
| CRT | 0.05 | 0.16 | 0.16 | −0.17 | −0.08 | |||||
| FA | 0.07 | −0.06 | 0.23 * | −0.03 | ||||||
| DA | 0.03 | −0.02 | −0.10 | |||||||
| PC1 | 0.00 | −0.00 | ||||||||
| PC2 | 0.00 | |||||||||
| B | SE B | β | |
|---|---|---|---|
| Constant | 2.47 | 0.03 | |
| Age | 0.003 | 0.001 | 0.43 ** |
| FFQ | 0.02 | 0.005 | 0.29 * |
| GPAQ | 5.68 | 0.00 | 0.06 |
| B | SE B | β | Partial r2 | |
|---|---|---|---|---|
| Lifestyle and background factors (F(3,84)=18.93, p < 0.001, Adjusted R2 = 0.38) | ||||
| Constant | 204.61 | 33.35 | ||
| Age | 3.08 | 0.73 | 0.38 ** | 0.24 |
| Sex | 39.19 | 13.32 | 0.26 ** | 0.12 |
| FFQ | 21.82 | 5.30 | 0.36 ** | 0.15 |
| Facial morphology factors (F(7,80)=12.31, p < 0.001, Adjusted R2 = 0.48) | ||||
| Constant | 169.12 | 33.13 | ||
| Facial asymmetry | 575,746.81 | 267,024.74 | 0.17 * | 0.06 |
| PC 1 | 456.66 | 174.51 | 0.21 * | 0.08 |
| PC 2 | −399.94 | 194.14 | −0.16 * | 0.05 |
| PC 3 | 588.60 | 266.44 | 0.20 * | 0.06 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, W.M.; Usacka, A. The Face of Early Cognitive Decline? Shape and Asymmetry Predict Choice Reaction Time Independent of Age, Diet or Exercise. Symmetry 2019, 11, 1364. https://doi.org/10.3390/sym11111364
Brown WM, Usacka A. The Face of Early Cognitive Decline? Shape and Asymmetry Predict Choice Reaction Time Independent of Age, Diet or Exercise. Symmetry. 2019; 11(11):1364. https://doi.org/10.3390/sym11111364
Chicago/Turabian StyleBrown, William M., and Agnese Usacka. 2019. "The Face of Early Cognitive Decline? Shape and Asymmetry Predict Choice Reaction Time Independent of Age, Diet or Exercise" Symmetry 11, no. 11: 1364. https://doi.org/10.3390/sym11111364






