Abstract
The toxic transformation products of hydrazines are of great concern. These products’ properties combined with their formation mechanisms are needed to assess their potential environmental and human impacts. In this study, the gas-phase reaction of hydrazine (N2H4), monomethyldrazine (MMH) and unsymmetrical dimethyhydrazine (UDMH) with O3 have been studied at varying reactant ratios, both in the presence and absence of a radical trap. Gas chromatography-mass spectroscopy (GC-MS) has been implied to follow reactant consumption and product formation. Apart from the reported products detected by Fourier transform infrared spectroscopy (FT-IR), the newly found compounds (hydrazones, formamides, dimethylamine, 1,1,4,4-tetramethyl-1,2-tetrazene,dimethylamino-acetonitrile, N2, H2O, et al.) are identified by GC-MS. The relative yields of the organic products vary considerably at different O3/MMH or UDMH ratios. UDMH and MMH are confirmed as high potential precursors of N-nitrosodimethylamine (NDMA). The presence of hydroxyl radicals (HO·) hinders NDMA formation in MMH-O3 system. Meanwhile, it increases NDMA formation in UDMH-O3 system. The suggested reaction mechanisms which account for the observed products are discussed.
1. Introduction
Hydrazine (N2H4) and its alkyl derivatives, monomethyldrazine (MMH) and unsymmetrical dimethylhydrazine (UDMH) have been the main high-energy fuels for rocket launching for decades. Considering these compounds are used in large quantity every year, the releases to the atmosphere stemming from storage, transportation and filling cannot be ignored [1]. The hydrazines themselves are hazards to humans. The American Conference of Governmental Industrial Hygienist (ACGIH) has recommended that the threshold limit values (TLVs) for N2H4, MMH, and UDMH be lowered from 100, 200 and 500 ppb, respectively, to 10 ppb in air [2,3]. A major concern is that the toxicity of considerable amounts of the huge list of transformation products are equal to or greater than that of the hydrazines themselves [4,5]. N-nitrosodimethylamine (NDMA) can be taken as an example, whose theoretical cancer risk level is 0.7 ng/m3 in air [6].
In order to explore the atmospheric fate of these chemicals, lots of studies have been done to obtain chemical properties and formation mechanism of transformation products. Since the hydrazines cannot be photolyzed in the solar actinic region (λ > 290 nm), their likely reaction pathways in the atmosphere are reaction with ozone and hydroxyl radical. In the 1980’s, The rate constants for the reactions of hydroxyl radical, ozone with all three of the hydrazines have been measured by the Statewide Air pollution Reasearch Center (SAPRC) of the University of California at Riverside [7]. The results show that all three of the hydrazines could be rapidly consumed by reactions with ozone as well as by reactions with hydroxyl radicals [8]. The specific ozonation products observed in this laboratory are H2O2 and N2O from N2H4; methyl hydroperoxide, methyldiazene, diazomethane, HCHO, CH3OH from MMH; NDMA in large yields from UDMH [9]. The reaction of UDMH with ozone is of great interest because it leads to a high yields of NDMA [10,11,12]. The NDMA formation mechanism from UDMH ozonation has been investigated by theoretical calculations [13,14]. The NDMA formation pathways include hydrogen atom abstraction and direct oxygen atom addition [15]. The results also indicate that HO· plays a key role in promoting NDMA formation. However, the experiment shows that the radical traps increase the NDMA formation [11]. Furthermore, various analytical techniques such as high performance liquid chromatography-mass spectroscopy (HPLC-MS) [16,17,18], gas chromatography-mass spectroscopy (GC-MS) [3,19,20], and solid phase micro-extraction (SPME-GC/MS) [21] have been used for the identification and quantitative determination of these transformation products, and more than 30 oxidative products have been identified. However, the information about the products of the hydrazines reaction with ozone is scarce, and the HO· influence on NDMA formation mechanism is still in discussion. In order to find more products and more deeply understand the mechanism of the hydrazines reaction with ozone, GC-MS is utilized to follow reactant consumption and product formation. The reactions of N2H4, MMH, and UDMH with O3 are investigated at varying reactant ratios, both in the presence and absence of radical traps. Meanwhile, the information of the highest occupied molecular orbital (HOMO) has been found to account for the possible attack sites of N2H4, MMH, UDMH by ozone. Moreover, the formation mechanisms of the major detected products have been discussed.
2. Experimental Section
2.1. Reaction Chamber and GC-MS System
The experiments were carried out using a chamber (internal volume of 500 mL, cylinder). The detailed schematic diagram of this apparatus is provided in Figure 1.
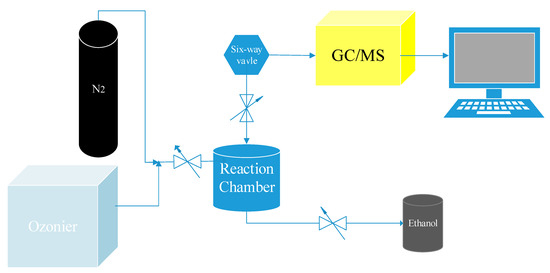
Figure 1.
Schematic diagram of the main experimental apparatus.
2.2. Materials
Anhydrous hydrazine (stated purity 97+%, Henan Liming Chemical Reagent Co., Zhengzhou, China), methylhydrazine (98%, Henan Liming Chemical Reagent Co., China), and unsymmetrical dimethylhydrazine (99+%, Henan Liming Chemical Reagent Co., China) were commercial available. Ozone was produced in a laboratory ozonizer with oxygen source (Model FL-810ET, Shenzhen Feili Electric Technology Co., Shenzhen, China).
2.3. Products Analysis
Before each runs, the reaction chamber was flushed with the pure N2. The blanked sample was analyzed to make sure that the gas in the reaction chamber was pure. Then the reaction chamber (500 mL, cylinder) was evacuated. The samples (N2H4 10 μL, MMH 10 μL, UDMH 10 μL) were injected into it. After 5 min, the chamber was flushed with different concentration ozone until the pressure reaches 1.8 bar pressure. Then the reacted samples were detected by the GC-MS through the six-way valve on line. To further investigate the intermediate reaction in the presence of O3, the chamber was flushed with O3 after the experiments of each sample. Each operation was repeated three times. The reaction chamber was thoroughly flushed with the pure N2, and the exhaust gas was washed with ethanol solution to remove the toxic gas (NDMA, etc.). Each sample was detected through GC-MS (7890A-5795C, Agilent, Santa Clara, CA, USA), based on the calibrated internal standards. Separation was performed on a DB-225S (30 m × 250 μm × 0.25 μm) capillary column. The conditions of the GC-MS were as follows: helium as the carrier gas at a rate of 1.5 mL/min, the temperature of the injector 35 °С, column from 35 °С to 150 °С with a rate of 10 °С/min; the split ratio 1:1.
3. Results and Discussion
3.1. The Reactions between HH with Ozone
Figure 2 shows the final products of N2H4 during ozonation detected by GC-MS. The major products are listed in Table 1. The products detected in the reaction of O3 with hydrazine are H2O, N2 and N2O. Compared to the product observed by FT-IR, H2O and N2 are newly discovered products and the N2O is relatively minor too. NOx (NO or NO2) is also not detected. Based on the products, we can propose that the oxidation of NH2 group is initiated by hydrogen atom abstraction rather than by direct oxygen atom addition. Therefore, the ultimate fates of N2H4 reaction with ozone are N2 and H2O. The release of N2H4 will probably have little effect on the levels of NOx, nitrates in the atmosphere.
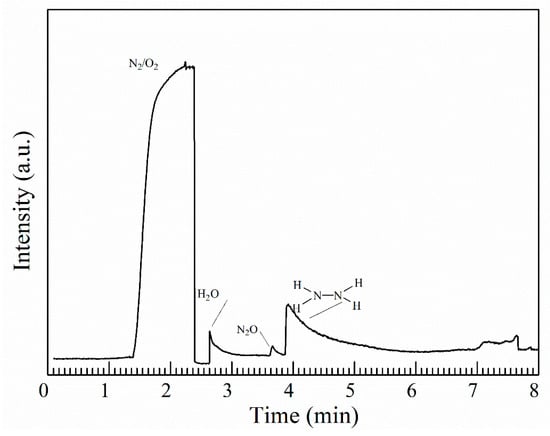
Figure 2.
Gas chromatogram of the ozonation products of hydrazine.

Table 1.
Products of the reaction of hydrazine with ozone.
3.2. The Reactions between MMH with Ozone
3.2.1. Products
Figure 3 shows that the final products are detected at vary O3/MMH ratios. The detailed information of all the products is listed in Table 2. As shown in Table 2, the mixture of products in the MMH + O3 system detected by GC-MS is more complex than that observed by FT-IR. The number of the various oxidation products reaches 16.
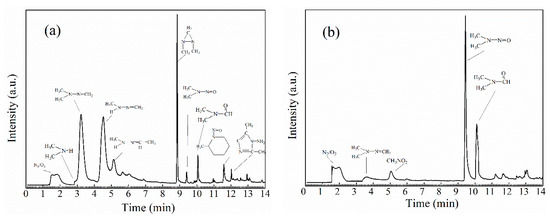
Figure 3.
Gas chromatogram of the ozonation products of methylhydrazine: (a) O3/MMH ≈ 1:1; (b) O3/MMH ≈ 10:1.

Table 2.
Products of the reaction of hydrazine with ozone.
The products are different in the excess of O3 and of MMH. In the excess of MMH, the major observed products are FDMH, FMH, DDZ, with minor NDMA and DMF. Meanwhile, in the excess of ozone, the major products are NDMA and DMF, with minor yields of CH3NO2. The NDMA and DMF are formed in 58% and 17.95% yields within 5 min. From the observed products, the dominant reaction mechanism is H-abstraction in the excess of MMH, which can explain the high yields of hydrazones. Otherwise, in the excess of ozone, the main reaction mechanism includes not only H-abstraction but also O-addition, which account for NDMA and DMF formation.
Furthermore, the observed products (CH3COOH, CH3OH and HCHO) by FT-IR yields [22] in the presence of excess O3 accounted for 92% of the initial carbon in MMH. However, these products are not detected by GC-MS in our experimental results, which probably because that these compounds are difficult to be separated from O2. At least 95% of the initial nitrogen in MMH could not be observed by FT-IR. Whereas, the dimethyl-compounds with nitrogen are detected by GC-MS which account for the initial nitrogen.
3.2.2. The Variation of the Major Products Conversion Rates during Ozonation
The reaction between MMH and ozone is too fast to be measured. In order to investigate the major products variation during ozonation, each run is flushed with ozone in each 15 min. The results are shown in Figure 4.
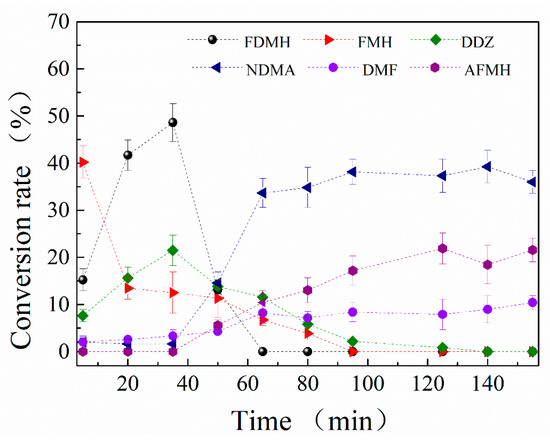
Figure 4.
The curves of change in relative conversion rate of the products in MMH + O3 system (flushed with ozone continuously).
Within 60 min, the yields of FDMH, FMH and DDZ increase at the early stage and then decrease. Hence, the FDMH, FMH and DDZ are proposed to be the intermediates during the ozonation process. Moreover, the amounts of NDMA, DMF and AFMH increase during the ozonation process. When the FDMH decreases sharply within 40–60 min, the NDMA increases sharply. So the FDMH may convert to the NDMA during ozonation. This is consistent with the result that relatively high molar NDMA yields 95% during FDMH ozonation in aqueous [23].
In conclusion, the FMH and DDZ may convert to DMF and AFMH. The ultimate products of MMH reaction with ozone are NDMA, DMF and AFMH.
3.2.3. Influence of Radical Trap (Propylene Alcohol) on NDMA Formation
The influence of radical trap on the formation of NDMA, as a result of ozone reaction with MMH, is presented in Figure 5, which shows that the radical trap decreases NDMA formation and increases DMF formation significantly. The yields of NDMA occupy 58% in the absence of the radical trap and 30.7% in the presence of radical trap. This means that the radicals play a significant role in promoting the formation of NDMA in the MMH-O3 system.
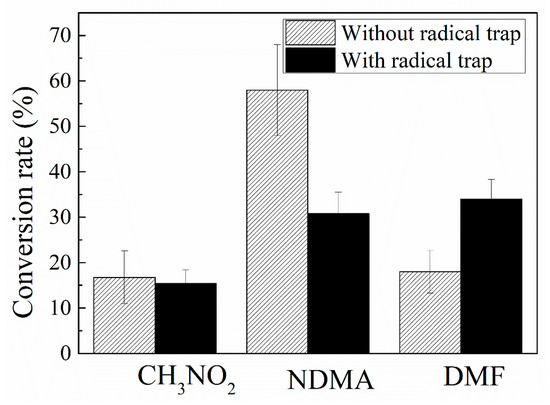
Figure 5.
Influence of radical trap on NDMA formation (O3/UDMH ratio ≈ 10:1).
3.3. The Reactions between UDMH with Ozone
3.3.1. Products
The runs are conducted in different O3/UDMH ratios (See Figure 6). The observed reaction rate of UDMH with O3 is as fast as that of MMH with O3. The relative yields of the organic products vary considerably at different O3/UDMH ratios. The details of the total products observed by GC-MS are listed in Table 3.
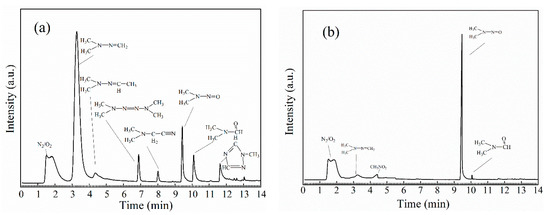
Figure 6.
Gas chromatogram of the ozonation products of methylhydrazine: (a) O3/UDMH ≈ 1:1; (b) O3/UDMH ≈ 10:1.

Table 3.
Products of the reaction of 1,1-dimethyldrazine with ozone.
The major products observed in the UDMH-O3 system are FDMH and NDMA, with minor yields TMT, DMF, MT. In the excess of UDMH, the major product is FDMH, while, in the excess of ozone, the major product is NDMA, whose molar conversion rate reaches 88.1%. The large yields of FDMH is identified in the excess of UDMH by GC-MS, which may be the unidentified product with its strongest absorption [9] at ~976 cm−1. The high yields of NDMA formation is consistent with that of EC Tuzon’s study [7].
3.3.2. The Variation of the Major Products Conversion Rates during Ozonation
Similar to the MMH + O3 system, the experiment is first conducted with low ozone concentration. After each sample, the chamber was injected with ozone. The results are shown in Figure 7.
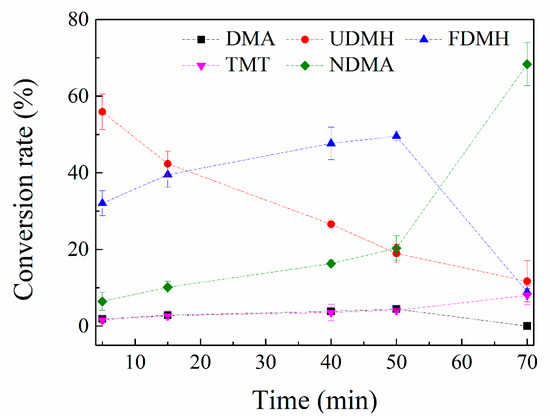
Figure 7.
The curves of change in relative conversion rate of the products in MMH + O3 system (flushed with ozone continuously).
With the consumption of UDMH, the main products FDMH, NDMA, DMA and TMT are generating. The concentration of FDMH (47.5%) increases quickly at first 10 min and reaches to a peak at 40 min through a 20 min terrace, after which it decreases and is consumed over 50 min. NDMA generates slowly at the first 40 min and then increases faster. The sharp increase of NDMA may be due to the FDMH consuming. The concentrations of TMT and DMA are almost unchanged with the ozone injection, indicating that they are in low reactivity with ozone. The molar NDMA conversion rate of UDMH is 57.4% after 70 min.
3.3.3. Influence of Radical Trap (Propylene Alcohol) on NDMA Formation
The influence of radical trap on the formation of NDMA, as a result of ozone reaction with UDMH, is presented in Figure 8.
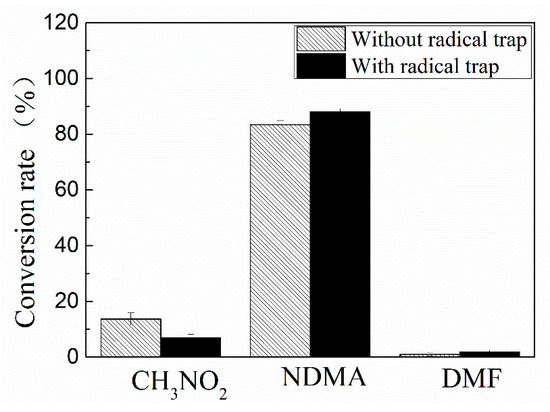
Figure 8.
Influence of radical trap on the major products formation (O3/UDMH ratio 5:1).
The influence of radical trap on NDMA formation from UDMH is opposite to that from MMH. The radical traps increase NDMA formation. The yields of the other products are suppressed by the presence of the radical trap.
3.4. Mechanism of the Reactions between Hydrazines with Ozone
3.4.1. Frontier Molecular Orbitals
The frontier molecular orbitals [24] are often used to reveal the relationship between the electronic and geometric structures and to obtain qualitative information about the optical and electrical properties of molecules. The charges are computed by finding the electron population of each atom (Multiwfn) [25]. The result is that the energy gaps between the lowest unoccupied molecular orbital (LUMO) of O3 and the highest occupied molecular orbital (HOMO) of H2H4, MMH, UDMH are smaller than that between the LUMO of HH, MMH, UDMH and the HOMO of O3, respectively. Thus, the reactions occur through the electron transfer from the HOMO of H2H4, MMH and UDMH to the LUMO of O3, suggesting an electrophilic attack of O3 to the hydrazines. The plots of energy levels, HOMO and LUMO are presented in Figure 9.
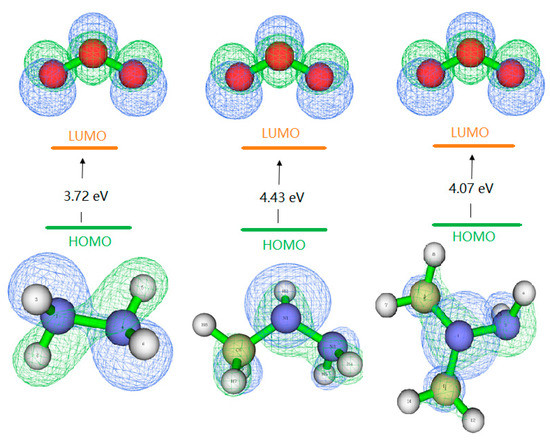
Figure 9.
Frontier molecular orbital energies levels and the electronic distribution of hydrazines (HOMO) and ozone (LUMO) (energy unit is eV).
To evaluate the reactive site, we calculated the atom contribution by Hirshfeld method [26]. For H2H4, the contributions of N1 and N2 atom are similar, 40.65%. For MMH, the contributions of N1, N3, C6 are 59.6%, 10.1%, 8.7%, respectively. This indicates that the reactions based on attack of a electrophilic are favored on N1 atom. For UDMH, the contributions of N1, N2, C3, and C4 are 55.1%, 8.6%, 7.7%, 7.4%, respectively. Even though the N1 makes the highest contribution, it is difficult to attack due to spaces steric effect. The more the contribution to the HOMO, the easier it is to be oxidized. Therefore, the most reactive site may be the N-H site.
Overall, the possible initial step proceeds via H-abstraction of methyl group or amino group. In addition, the H-abstraction of N-H bond is prior to that of C-H bond.
3.4.2. The Products Formation Mechanism Initialed by H-Abstraction of N-H Bond
Based on the frontier molecular orbitals results, the reactions between H2H4, MMH and UDMH with ozone are suggested to be initialed by H-abstraction. The reaction of ozone with N-H bond is preference to the reaction of ozone with C-H bond. The oxidation of N-H bond in UDMH-O3 system leads to NDMA formation. The NDMA formation is mainly through H-abstraction and O-addition. The detail NDMA formation mechanism is as follow:
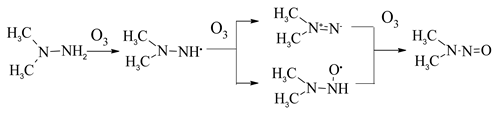

In the MMH-O3 system, the formation way of methyl radicals is probably an important reaction pathway [27,28]. The atom contribution results indicate that the possible reaction site is at N1-H bond. The H-abstraction of N1-H bond leads to the H3C-N=N formation, which cleave to CH3 and N2 (2). With the subsequent reactions (2), the methyl radicals formed which account for the high yields of the observed dimethylamine groups.
It is noticeable that MMH has also high potential for NDMA formation. The main reason is the methyl radical (CH3·) formation in MMH-O3 system. The methyl process can promote more stable compounds with dimethylamine groups formation which finally generate NDMA, so that the products in MMH-O3 system are similar to those in the UDMH-O3 system.
3.4.3. The Products Formation Mechanism Initialed by H-Abstraction of C-H Bond
The observed non-negligible yields of HCHO indicate that the reactions initialed by H-abstraction of N-H bond cannot be the only process. The formation of these products can be attributed to the occurrence of an alternate reaction route initialed by H-abstraction of C-H bond.
Many researches [29,30,31] report that the atmospheric element of the -CH3 oxidation is HCHO, which is detected by FT-IR in MMH-O3 and UDMH-O3 system. The HCHO formation pathway is:
As soon as HCHO formed [32,33], MMH and UDMH would immediately react with HCHO, finally lead to the hydrazones formation. This can explain for the high yields of hydrazones observed by GC-MS.
Furthermore, UDMH removes one methyl group to form MMH. MMH after removing one methyl group converts to N2H4. In the N2H4-O3 system, the major products are H2O2, N2 and H2O observed in experiments. The major ultimate fate of nitrogen in the hydrazines-O3 system is N2. Hence, the demethylation process is an important step for the conversion of UDMH and MMH to N2.
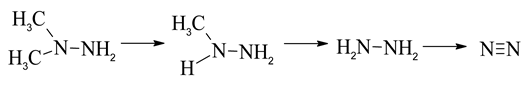
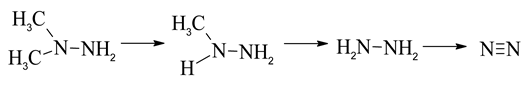
Moreover, the formation pathway of another main products (DMF) would include N-N bond cleavage step. Based on the Sun’s reports, the C·H2NHNH2 radical dissociates quickly to the products H2C=NH + NH2 with a low barrier of 12.0 kcal/mol [34,35].
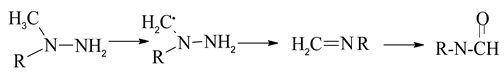
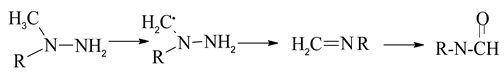
Thus, H2C=NH is an important intermediate for DMF formation. DMF formation proceeds via H-abstraction of C-H bond.
3.4.4. Influence of Radicals on NDMA Mechanism
The heterolytic cleavage pathways explain the significant HO· formation during ozonation of MMH and UDMH [36,37].
In the presence of excess ozone, the radical trap would increase the NDMA yields from UDMH, and decrease the NDMA yields from MMH. This may indicate that HO· plays a key role in destruction of the C-H and N-H bonds.
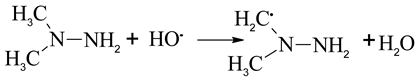
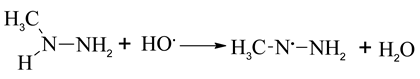
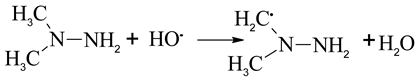
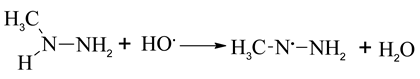
The difference between UDMH and MMH is the (CH3)2N group and (CH3)HN group. As shown in (5), UDMH could convert to NDMA directly by the oxidation the amino groups, with high yields (88%). The reaction between HO· and the C-H bonds of UDMH leads to HCHO and other products (amines and CH3NO2 et al.) formation. Meanwhile, for MMH, the reaction between HO· and N-H bond of MMH can promote the methyl radical formation, finally leads to the increase of NDMA yields.
4. Conclusions
The products of N2H4, MMH and UDMH reaction with ozone are detected by GC-MS. The major products in the presence of excess hydrazines or excess ozone, the radical influence on NDMA formation are investigated. The reaction mechanisms which account for the major products (especially NDMA) are discussed. More specific conclusions are follows:
Apart from the products (NDMA, N2O et al.) detected by FT-IR, the new compounds (hydrazones, formamides, dimethylamine, 1,1,4,4-tetramethyl-1,2-tetrazene, dimethylamino-acetonitrile, N2, H2O, et al.) are identified by GC-MS. And the relative yields of the organic products vary considerably depending on the O3/MMH or UDMH ratios.
The ozonation of UDMH yields high amounts of NDMA (>50%). It is noticeable that MMH also has potential for NDMA formation in the presence of excess ozone.
Radicals play an important role in NDMA formation. It can hinder NDMA formation from UDMH and promote NDMA formation from MMH. It may be due to that HO· makes many contributions to destructing C-H and N-H bonds.
An analysis of the energy gap between the frontier molecular orbitals of the reactants indicates that the oxidations of the hydrazines as well as N-H and C-H bonds are initiated by hydrogen atom abstraction. The oxidation of N-H bond is the most favorable.
The major ultimate fate of nitrogen (N2H4, MMH, UDMH) may be N2 through the demethylation process. However, the reaction of ozone with N-H bonds is preference to that with C-H bonds. NDMA and methyl radical formation proceeds via H-abstraction from N-H bonds. The N2, formaldehyde, amines, formamide and hydrazones formation proceeds via H-abstraction from C-H bonds.
Author Contributions
Formal Analysis, D.H.; Investigation, D.H.; Data Curation, Y.Y.; Writing-Original Draft Preparation, D.H.; Supervision, X.G., X.L. and X.W.; Funding Acquisition, Z.X.
Acknowledgments
Authors thank for Mis Yang for the English checking.
Funding
This research was financially supported by China Postdoctoral Science Foundation (grant No. 2016M600084).
Conflicts of Interest
The authors declare no competing financial interests.
References
- Catoire, L.; Chaumeix, N.; Paillard, C. Journal of Propulsion and Power-20(1):87-PDF (AIAA). J. Propuls. Power 2004, 20, 87–92. [Google Scholar] [CrossRef]
- Collins, G.E.; Rosepehrsson, S.L. Fluorescent detection of hydrazine, monomethylhydrazine, and 1,1-dimethylhydrazine by derivatization with aromatic dicarbaldehydes. Analyst 1994, 119, 1907–1913. [Google Scholar] [CrossRef]
- Holtzclaw, J.R.; Rose, S.L.; Wyatt, J.R.; Rounbehler, D.P.; Fine, D.H. Simultaneous determination of hydrazine, methylhydrazine, and 1,1-dimethylhydrazine in air by derivatization/gas chromatography. Anal. Chem. 1984, 56, 2952–2956. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, L.; Kenessov, B.N.; Batyrbekova, S.Y. A QSAR/QSTR study on the human health impact of the rocket fuel 1,1-dimethyl hydrazine and its transformation products Multicriteria hazard ranking based on partial order methodologies. Environ. Toxicol. Pharmacol. 2009, 27, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Lars, C.; Kenessov, B.N.; Ye, B.S. A QSAR/QSTR Study on the Environmental Health Impact by the Rocket Fuel 1,1-Dimethyl Hydrazine and its Transformation Products. Environ. Health Insights 2008, 1, 11. [Google Scholar]
- Hong, Y.; Kim, K.H.; Sang, B.I.; Kim, H. Simple quantification method for N-nitrosamines in atmospheric particulates based on facile pretreatment and GC-MS/MS. Environ. Pollut. 2017, 226, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Pitts, J.N., Jr.; Tuazon, E.C.; Carter, W.P.L.; Winer, A.M.; Harris, G.W. Atmospheric Chemistry of Hydrazines: Gas Phase Kinetics and Mechanistic Studies; California Univ Riverside Statewide Air Pollution Research Center: Riverside, CA, USA, 1980.
- Coleman, D.J.; Judeikis, H.S.; Lang, V. Gas-Phase Rate Constant Measurements for Reactions of Ozone with Hydrazines; Aerospace Corp el Segundo ca el Segundo Technical Operations: El Segundo, CA, USA, 1996. [Google Scholar]
- Tuazon, E.C.; Carter, W.P.L.; Winer, A.M.; Pitts, J.N. Reactions of hydrazines with ozone under simulated atmospheric conditions. Environ. Sci. Technol. 1981, 15, 823–828. [Google Scholar] [CrossRef]
- Marti, E.J.; Pisarenko, A.N.; Peller, J.R.; Dickenson, E.R. N-nitrosodimethylamine (NDMA) formation from the ozonation of model compounds. Water Res. 2015, 72, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Lee, W.; Na, S.; Shin, J.; Lee, Y. N-nitrosodimethylamine (NDMA) formation during ozonation of N,N-dimethylhydrazine compounds: Reaction kinetics, mechanisms, and implications for NDMA formation control. Water Res. 2016, 105, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Tanaka, H. Formation of N-nitrosamines by chloramination or ozonation of amines listed in Pollutant Release and Transfer Registers (PRTRs). Chemosphere 2014, 95, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.D.; Zhong, R. Comparison of N-nitrosodimethylamine formation mechanisms from dimethylamine during chloramination and ozonation: A computational study. J. Hazard. Mater. 2017, 321, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yu, G.; Chen, J.; Wang, B.; Huang, J.; Deng, S. Unveiling formation mechanism of carcinogenic N-nitrosodimethylamine in ozonation of dimethylamine: A density functional theoretical investigation. J. Hazard. Mater. 2014, 279, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Ge, P.; Chen, J.; Xie, H.; Luo, Y. The degradation mechanism of sulfamethoxazole under ozonation: A DFT study. Environ. Sci. Process Impacts 2017, 19, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Rodin, I.A.; Smirnov, R.S.; Smolenkov, A.D.; Shpigun, O.A. Transformation of unsymmetrical dimethylhydrazine in soils. Eurasian Soil Sci. 2012, 45, 386–391. [Google Scholar] [CrossRef]
- Smolenkov, A.D.; Smirnov, R.S.; Rodin, I.A.; Tataurova, O.G.; Shpigun, O.A. Effect of sample preparation conditions on the determination of the total concentrations of unsymmetrical dimethylhydrazine in soils. J. Anal. Chem. 2012, 67, 6–13. [Google Scholar] [CrossRef]
- Kenessov, B.; Alimzhanova, M.; Sailaukhanuly, Y.; Baimatova, N.; Abilev, M.; Batyrbekova, S.; Carlsen, L.; Tulegenov, A.; Nauryzbayev, M. Transformation products of 1,1-dimethylhydrazine and their distribution in soils of fall places of rocket carriers in Central Kazakhstan. Sci. Total Environ. 2012, 427–428, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Rodin, I.A.; Anan’Eva, I.A.; Smolenkov, A.D.; Shpigun, O.A. Determination of the products of the oxidative transformation of unsymmetrical dimethylhydrazine in soils by liquid chromatography/mass spectrometry. J. Anal. Chem. 2010, 65, 1405–1410. [Google Scholar] [CrossRef]
- Ul’yanovskii, N.V.; Kosyakov, D.S.; Pokryshkin, S.A.; Bogolitsyn, K.G. Determination of transformation products of 1,1-dimethylhydrazine by gas chromatography–tandem mass spectrometry. J. Anal. Chem. 2015, 70, 1553–1560. [Google Scholar] [CrossRef]
- Kosyakov, D.S.; Ul’Yanovskii, N.V.; Pokryshkin, S.A.; Lakhmanov, D.E.; Shpigun, O.A. Rapid determination of 1,1-dimethylhydrazine transformation products in soil by accelerated solvent extraction coupled with gas chromatography–tandem mass spectrometry. Int. J. Environ. Anal. Chem. 2015, 95, 1321–1337. [Google Scholar] [CrossRef]
- Tuazon, E.C.; Carter, W.P.L.; Brown, R.V.; Atkinson, R.; Winer, A.M. Atmospheric Reaction Mechanisms of Amine Fuels; Atmospheric Reaction Mechanisms of Amine Fuels; California Univ Riverside Air Pollution Research Center: Riverside, CA, USA, 1982. [Google Scholar]
- Kosaka, K.; Fukui, K.; Kayanuma, Y.; Asami, M.; Akiba, M. N-Nitrosodimethylamine Formation from Hydrazine Compounds on Ozonation. Ozone Sci. Eng. 2014, 36, 215–220. [Google Scholar] [CrossRef]
- Wang, W.Y.; Kan, Y.H.; Wang, L.; Sun, S.L.; Qiu, Y.Q. Large Nonlinear Optical Responses of Dimers Bearing a Donor and Acceptor: Long, Intradimer Multicenter Bonding. J. Phys. Chem. C 2014, 118, 28746–28756. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Bultinck, P. The Hirshfeld-I method: Atoms in molecules and chemical bonding perspective. Acta Crystallogr. 2011, 67, C85–C86. [Google Scholar] [CrossRef]
- Lambert, C.E.; Shank, R.C. Role of formaldehyde hydrazone and catalase in hydrazine-induced methylation of DNA guanine. Carcinogenesis 1988, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.F.; Augusto, O. Formation of methyl radicals during the catalase-mediated oxidation of formaldehyde hydrazone. Carcinogenesis 1991, 12, 1351–1353. [Google Scholar] [CrossRef] [PubMed]
- Martínezavilés, M.; And, R.R.; Francisco, J.S. Atmospheric Oxidation Mechanism of Bromoethane. J. Phys. Chem. A 2007, 111, 11652. [Google Scholar] [CrossRef] [PubMed]
- Kagiya, T.; Yokoyama, N.; Takemoto, K. Radiation-Induced Degradation of Poly (Ethylene Oxide) in the Atmosphere of Chlorine Compounds (Special Issue on Physical, Chemical and Biological Effects of Gamma Radiation, XVI). Bull. Inst. Chem. Res. 1976, 54, 15–22. [Google Scholar]
- Carlier, P.; Hannachi, H.; Mouvier, G. The chemistry of carbonyl compounds in the atmosphere—A review. Atmos. Environ. 1986, 20, 2079–2099. [Google Scholar] [CrossRef]
- Sisler, H.H.; Mathur, M.A.; Jain, S.R.; Greengard, R. Studies of the chloramination of dimethylamine and 1,1-dimethylhydrazine. Ind. Eng. Chem. Prod. Res. Dev. 1981, 20, 181–185. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, R. Density functional theory study on OH-initiated atmospheric oxidation of m-xylene. J. Phys. Chem. A 2008, 112, 4314–4323. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Law, C.K. Thermochemical and kinetic analysis of the thermal decomposition of monomethylhydrazine: An elementary reaction mechanism. J. Phys. Chem. A 2007, 111, 3748. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Catoire, L.; Law, C.K. Thermal decomposition of monomethylhydrazine: Shock tube experiments and kinetic modeling. Int. J. Chem. Kinetics 2009, 41, 176–186. [Google Scholar] [CrossRef]
- Nelander, B.; Engdahl, A.; Svensson, T. The HOOO radical. A matrix isolation study. Chem. Phys. Lett. 2000, 332, 403–408. [Google Scholar] [CrossRef]
- Varner, M.E.; Harding, M.E.; Vázquez, J.; Gauss, J.; Stanton, J.F. Dissociation energy of the HOOO radical. J. Phys. Chem. A 2009, 113, 11238–11241. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).