Abstract
In this study, the chemical composition and biological activities of the essential oil (EO) extracts (from leaves and cones) of the Tunisian Thuja occidentalis were evaluated. The composition of the leaf EO extract was more complex than that of the cones. The major components of the leaf EO extract were α-Pinene (34.4%), cedrol (13.17%), and β-Phellandrene (8.04%), while the composition of the cone EO extract was characterized by the predominance of α-Pinene (58.55%) and 3-Carene (24.08%). All EO extracts showed much better antioxidant activity than Trolox against 2, 2′-diphenyl-1-picryl hydrazyl (DPPH) radical scavenging, but EOs extracted from leaves exhibited the highest total antioxidant activity. All EOs showed strong antibacterial and antifungal activities against nine tested foodborne microorganisms (Bacillus cereus American Type Culture Collection (ATCC) 1247, Listeria monocytogenes ATCC 7644, Staphylococcus aureus ATCC 29213, Aeromonas hydrophila EI, Escherichia coli ATCC 8739, Pseudomonas aeruginosa ATCC 27853, Salmonella typhimurium NCTC 6017, Aspergillus flavus (foodborne isolate), and Aspergillus niger CTM 10099. The highest antimicrobial activities by disk diffusion assay were recorded for the EOs extracted from leaves, while no difference in potency was marked between leaf and cone EO extracts by the agar dilution method. The most potent antimicrobial activity was recorded among fungi. This study confirms the strong antimicrobial and antioxidant potential of EO extracts from the Tunisian T. occidentalis (from the Sidi Bou Said site), highlighting its potential as a natural preservative against foodborne pathogens, particularly against E. coli and S. typhimurium.
1. Introduction
Thuja is a small genus of the Cupressaceae family that has five existing species, including Thuja occidentalis [1]. T. occidentalis is also known as American Arbor vitae or white cedar. It is indigenous to eastern North America and is grown in Europe as an ornamental tree [2]. Native Indians first identified the plant in the 16th century as a remedy for the treatment of weakness from scurvy (British Herbal Pharmacopoeia. Thuja, British Herbal Medicine Association, West Yorks, UK, 1983). In folk medicine, T. occidentalis has also been used to treat bronchial catarrhal, enuresis, cystitis, psoriasis, uterine carcinomas, amenorrhea, and rheumatism [3]. Moreover, its essential oils (EOs) isolated from the leaf have been used to treat fungus infections, cancer, moles, and parasitic worms [4].
In recent years, the interest in the antioxidant activity and antimicrobial activity of EOs, plant extracts, and/or other isolated natural compounds from higher plants has markedly increased [5,6]. In fact, both the antioxidant activity and antimicrobial activity of plants are directly related to their EO composition [5,7]. There is limited data on the antioxidant and antimicrobial activities of Thuja in genaral, as most studies have focused on Thuja orientalis. The limited number of reports on T. occidentalis are from origins other than Tunisia [8,9,10]. Since the EO composition varies based on geographical origin and edaphoclimatic conditions [8,9], we investigated the chemical composition of the EOs of the Tunisian T. occidentalis (leaves and cones). Moreover, the antioxidant, antibacterial, and antifungal activities of its EO extracts were also determined.
2. Materials and Methods
2.1. Sampling
Leaves and cones of T. occidentalis were handpicked in May 2017 from Sidi Bou Said (northeast of Tunis, situated at 36.87° north latitude, 10.34° east longitude, and 98-m elevation above sea level). Aerial parts of the plants were air-dried at room temperature for two weeks before EO extraction and further analyses.
2.2. Essential Oil Extraction
Volatile oils were extracted from 100 g of air-dried leaves by hydrodistillation for 3 h using a Clevenger-type apparatus. EO yields were estimated on the basis of the dry weight of plant material. Oils were dried over anhydrous sodium sulphate, filtered, and then stored at 4 °C until use.
2.3. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
The chemical composition of EOs was analyzed by GC–MS (Hewlett Packard 6890) coupled with an HP 5973 mass selective detector (Agilent technologies, Inc., 2850 Centerville Road Wilmington, DE 19808-1610, USA), set to scan from 20 to 550 m/z. GC–MS analysis was performed using a capillary column HP-5ms (5% phenyl methyl siloxane), 30 m (length) × 0.25 mm (diameter), 0.25 μm (film thickness). The column oven temperature was initially held at 40 °C, programmed to reach 250 °C at a rate increase of 10 °C/min, and then held at 250 °C for 10 min. The total run time was 75 min. The temperatures of the injector and detector were set at 250 and 310 °C, respectively. The carrier gas was helium at a working flow rate of 1.5 mL/min. The injection (1 µL) was carried out manually in the split mode at a 1:50 split ratio. The ionization energy was 70 eV. EOs were identified by comparing their retention time and mass spectra using the Wiley/NBS mass spectral library. Compound percentages were calculated using the peak area normalization [11].
2.4. Determination of Total Antioxidant Activity
The assay was based on the reduction of Mo (VI) to Mo (V) by the extract and subsequent formation of a green phosphate/Mo(V) complex at acid pH [12]. Briefly, an aliquot of 0.1 mL of sample solution was combined in a vial with 1mL of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate). The blank solution contained 4 mL of reagent solution and 1 mL of methanol. The vials were capped and incubated in a water bath at 95 °C for 90 min, then cooled to room temperature. The absorbance of each sample was measured at 695 nm against the blank. The total antioxidant activity of T. occidentalis EOs is expressed as Trolox equivalent.
2.5. 2, 2′-diphenyl-1-picryl hydrazyl (DPPH) Free Radical Scavenging Assay
The antioxidant activity percentage of each EO extract was assessed using the DPPH free radical scavenging assay [13]. The samples were reacted with the stable DPPH radical in a methanol solution. The reaction mixture consisted of different sample concentrations diluted in methanol (1, 10, and 100 µg/mL), to which was added 0.25 mL of DPPH radical solution (0.2 mM). When DPPH reacts with an antioxidant compound, which can donate hydrogen, it is reduced and causes changes in color (from deep violet to light yellow). After 30 min of incubation at room temperature, changes in absorbance at 517 nm, relative to a blank solution, were monitored.
The antioxidant activity percentage was calculated as follows [14]:
where A0 and A1 correspond to the absorbance of the control and the sample, respectively.
Antioxidant activity percentage (%) = [(A0 − A1)/A0] × 100
The concentration of EO that could scavenge 50% of DPPH (IC50) was also calculated.
2.6. Antimicrobial Activity
2.6.1. Microbial Strains
A panel of microorganisms was used that included three Gram-positive bacteria (Bacillus cereus American Type Culture Collection (ATCC) 1247, Listeria monocytogenes ATCC 7644, and Staphylococcus aureus ATCC 29213), four Gram-negative bacteria (Aeromonas hydrophila EI, Escherichia coli ATCC 8739, Pseudomonas aeruginosa ATCC 27853, and Salmonella typhimurium NCTC 6017), two fungi strains (Aspergillus flavus (foodborne isolate) and Aspergillus niger CTM 10099), and one yeast strain (Candida albicans ATCC 2091). The A. flavus strain used in this study was a foodborne fungi strain isolated, identified to the species level, and stored at the Pasteur Institute of Tunis (University of Carthage, BP 74, 13 Place Pasteur, Belvédère, 1002 Tunis, Tunisia) [15].
Bacterial strains were grown on trypto-casein-soy agar (TSA) plates and incubated at 37 °C for 24 h (except for A. hydrophila EI: grown at 30 °C for 24 h) [16]. Fungal strains were grown on potato dextrose agar (PDA) plates at 28 °C for 72 h. C. albicans was grown on a Sabouraud dextrose agar (SDA) plate at 30 °C for 48 h. All culture media plates were purchased from ThermoFisher Scientific.
2.6.2. Disk Diffusion Assay
The antibacterial and antifungal activities of the two evaluated EOs were determined by disk diffusion assay [17]. The inoculum was prepared by direct colony suspension [17]. Briefly, 100 μL of suspension containing 108 CFU/mL of bacteria cells, 106 CFU/mL of yeast, and 104 spores/mL of fungi were spread on Petri plates containing TSA, SDA, and PDA media, respectively. The paper disks (6 mm in diameter) (Sigma Aldrich, Saint-Louis, MO, USA) were separately impregnated with 15 μL of each essential oil and placed on the agar, which had previously been inoculated with the selected test microorganisms. Gentamicin (10 µg/disk) and amphotericin B (20 µg/disk) (Biolab Zrt, Budapest, Hungary) were used as positive controls for bacteria and fungi, respectively. Disks without samples were used as a negative control. Plates were kept at 4 °C for 1 h. The inoculated plates were incubated for 24 h for pathogenic bacterial strains, 48 h for yeast, and 72 h for fungi isolates at 37, 30, and 27 °C, respectively. Antimicrobial activity was assessed by measuring the diameter of the growth-inhibition zone in millimeters for the test organisms compared to the positive control.
2.6.3. Determination of minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
The MIC and MBC of EO extracts were determined for the selected test strains using the agar dilution method described by CLSI document Vet 01-A4 [17]. Tested concentrations were 0.03–400 µg/mL.
3. Results and Discussion
3.1. Essential Oil Yields
The hydrodistillation of T. occidentalis organs furnished odoriferous and very pale yellow colored EOs. The EO yield, based on dry weight, varied between cones (2.1%) and leaves (0.9%). The present data are in line with the literature, as Al Hafi et al. (2013) [18] and Vaičiulytė and Ložienė (2013) [19] reported that the EO yield in Juniperus excelsa and Cupressus arizonica, respectively, was higher in cones.
The recorded EO yield for the Tunisian T. occidentalis was relatively high compared to some other plants used industrially for their EOs: Artemisia absinthium (0.57%) and Artemisia pontica (0.31%) [20], rosa (0.1–0.35%), lavender (0.8–1.8%), menthe (0.5–1%), bitter orange (1%), and rosemary (1–2.5%) [21].
The reported EO yields from T. occidentalis were different from those reported in the literature for the same plant harvested from different locations (cones, Iran: 0.97%; leaves, northwestern part of Himalaya: 0.2%) [22,23]. This variation is explained by the difference in the geographical origin, climate, and edaphology, which impact the EO composition [8,10].
3.2. Chemical Composition of Essential Oils
The chemical composition of EOs collected from two organs (leaves and cones) of T. occidentalis was analyzed. Table 1 and Figure 1 summarize the identified compounds, their percentages, as well as their corresponding retention times. Qualitative and quantitative differences among the EOs collected from leaves and cones of T. occidentalis were noted. The number of EOs extracted from the leaves (n = 25) of T. occidentalis was higher than the EOs extracted from the cones (n = 13). Those EO components accounted for 100% and 99.98% of the EOs extracted from leaves and cones, respectively. The major constituents identified in EOs extracted from the leaves of T. occidentalis were α-Pinene (34.4%), cedrol (13.17%), β-Phellandrene (8.04%), and 3-Carene (7.32%) (Table 1). The major constituents identified in EOs extracted from the cones were α-Pinene (58.55%), followed by 3-Carene (24.08%) and terpinolene (3.73%) (Table 1).

Table 1.
Chemical composition of EO extracts from cones and leaves of Tunisian T. occidentalis.
Figure 1.
Essential oil (EO) extract chromatograms from cones (A) and leaves (B) of Tunisian Thuja occidentalis.
Different EO constituents have also been reported for T. occidentalis in the literature [8,24]. While the percentages and overall EO composition varies from the ones reported herein, some identified EO constituents are similar to those from different studies. For instance, EOs extracted from leaves of a Bulgarian T. occidentalis contained 70 components and was dominated by sabinyl acetate (16.55%), fenchone (12.87%), sabinene (12.14%), β-Thujone (9.48%), and α-Pinene (3.33%) [24], while the EOs extracted from Slovakian T. occidentalis were rich in α-Thujone, β-Thujone, and fenchone, accounting for more than 50% of all the oils (Swajdlenka et al. 1999) [8].
Moreover, similar EO components to those reported in our study but at different percentages, along with additional constituents, have been reported in the literature for T. orientalis) [22,23]. For example, the major components of EOs extracted from the leaves of Iranian T. orientalis were α-Pinene (21.9%), α-Cedrol (20.3%), ∆-3-Carene (10.5%), and limonene (7.2%) [22], while the major components of EOs from the leaves of T. orientalis harvested from the northwestern Himalaya were α-Pinene (29.2%), Δ-3-Carene (20.1%), α-Cedrol (9.8%), caryophyllene (7.5%), and limonene (5.4%) [23].
These variations in EO profiles may be attributed to the differences in geographical origin, genetic variability, environmental conditions, harvesting season, climate, soil composition, drying procedure, and organs [25,26].
3.3. Antioxidant Activities
All the tested EO extracts (leaves and cones) from the Tunisian T. occidentalis exhibited significant antioxidant activity, higher than that of Trolox (Figure 2). Our results agree with the known antioxidant potential of EO extracts from T. occidentalis [27,28,29,30,31]. The EOs extracted from leaves exhibited a higher total antioxidant activity than the EOs extracted from cones (Figure 2), which was consistent with their respective IC50 (200 µg/mL for leaf extracts, 150 µg/mL for cone extracts, and 100 µg/mL for Trolox). Marked IC50 values for EO extracts were comparable to those reported in the literature in various studies (reported IC50 range 124.11–202.45 µg/mL) [28,29,31]. The scavenging of DPPH radicals of EO extracts from cones and leaves were comparable to that of Trolox when tested at the same concentration (Figure 3). Similar to Ololade et al., the percentage inhibition of free radicals was dose dependent [30].
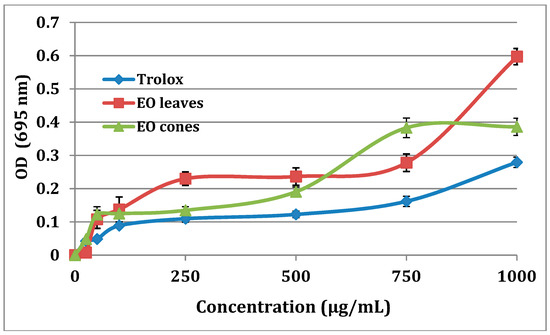
Figure 2.
Total antioxidant capacity of EO extracts from cones and leaves of Tunisian T. occidentalis.
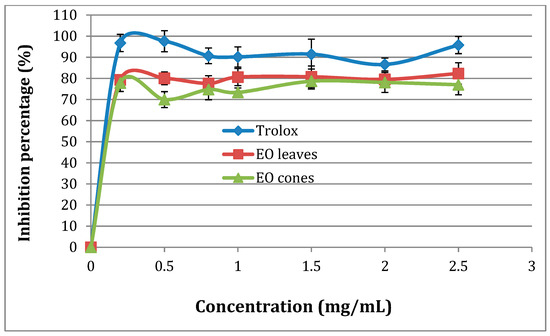
Figure 3.
Scavenging of 2,2′-diphenyl-1-picryl hydrazyl (DPPH) radicals of EO extracts from cones and leaves of Tunisian T. occidentalis.
3.4. Antimicrobial Activity
Both investigated EO extracts from cones and leaves of the Tunisian T. occidentalis exhibited antimicrobial activity against all tested microorganisms (MIC, MBC, and disk diffusion assays) (Table 2 and Table 3).

Table 2.
Test zone diameter of inhibition (mm) of EOs extracted from leaves and cones of Tunisian T. occidentalis against 10 different microorganisms.

Table 3.
The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) values (µg/mL) of Tunisian T. occidentalis essential oils extracted from leaves and cones against 10 microorganisms.
Considering test zone diameter of inhibition (mm) obtained by the disk diffusion assay, all EO extracts were less potent than the quality control antimicrobials used (gentamicin and amphotericin) (Table 2). In addition, EO extracts from leaves of Tunisian T. occidentalis were more potent than those extracted from its cones (Table 2). The most potent antimicrobial activity by the disk diffusion assay was noted for EO leaf extracts against the tested Gram-positive strains, with L. monocytogenes ATCC 7644 recording the highest test zone diameter of inhibition (16 mm) among tested strains (Table 2).
MICs (range 12.5–25 µg/mL) and MBCs (range 25–50 µg/mL) by the agar diffusion assay were recorded for all the tested strains (Table 3) and were in accordance with the disk diffusion assay data (Table 2). MIC and MBC data showed that EO extracts from leaves of Tunisian T. occidentalis were equipotent with those extracted from its cones, since the difference in both MIC and MBC values between EOs from cone extracts and EOs from leaf extracts was of twofold. Similarly, due to the twofold difference seen in MIC and MBC values among the tested Gram-positive and Gram-negative strains, no difference in potency for all the tested EOs (cones and leaves) extracted from the Tunisian T. occidentalis was recorded among tested Gram-positive and Gram-negative strains by the agar diffusion assay. In contrast, Khubeiz et al. (2016) [32] noted more potent antimicrobial activity among tested Gram-positive strains for EOs extracted from Syrian T. occidentalis. Similar to Khubeiz et al. (2016) [32], other studies also demonstrated that Gram-positive bacteria are more sensitive than Gram-negative bacteria to EOs extracted from various plants [33,34], which could be explained by the presence of the protective hydrophobic lipopolysaccharide in the outer membrane of Gram-negative bacteria [35,36].
Despite their comparable potency against the tested Gram-positive and Gram-negative bacteria, all the tested EOs (cones and leaves) extracted from the Tunisian T. occidentalis were most potent against fungi and yeast in our assay. However, EO extracts from leaves of Tunisian T. occidentalis were equipotent with those extracted from its cones against A. niger and C. albicans (MIC 6.25 µg/mL ± twofold dilution; MBC 12.5 µg/mL ± twofold dilution). The lowest MIC (3.12 µg/mL) and MBC (6.25 µg/mL) values recorded were for EO leaf extracts against A. niger (Table 3).
All the generated susceptibility data were in accordance with the literature, as the antimicrobial effect of EOs extracted from various Thuja, including T. occidentalis [37,38], T. orientalis [39], and T. plicata [10], has been reported against numerous microorganisms such as: S. aureus, Streptococcus spp., E. coli, and P. aeruginosa [37,38,39]; B. subtilis, C. diphtheriae, S. typhimurium, Shigella spp., and fungus strains [39]; C. albicans, C. tropicalis, and C. glabrata [10].
It is difficult to correlate the antimicrobial activity with a specific EO component without testing each component alone. In fact, the antimicrobial activity of the entire extract could be due to their major or minor component alone or due to a synergetic effect between two or more components. Nevertheless, biological activities have been demonstrated to be linked to the most abundant components [10,26,40]. We assume, pending further investigation, that the antimicrobial activity of EOs extracted from Tunisian T. occidentalis is mainly attributed to the presence of the major constituent α-Pinene (34.4% and 58.99% in leaf and cone extracts, respectively) (Table 1). The antimicrobial activity may also be partly due to the presence of 3-Carene (7.32% and 24.08% in leaf and cone extracts, respectively) and cedrol (13.17% in leaf extract) in the EO extracts (Table 1).
4. Conclusions
Our results revealed significant variations in the composition, yield, chemical profile, and antioxidant and antimicrobial potentials of EOs extracted from the leaves and cones of the Tunisian T. occidentalis. The highest antioxidant and antimicrobial activities (by the disk diffusion assay) were recorded for the EOs extracted from leaves. Considering the MIC and MBC susceptibility data (by the agar dilution method) alone, along with the acceptable the twofold difference in MIC and MBC values, the antimicrobial activities of EOs were comparable between leaf and cone extracts among the same tested microorganism. The most potent antimicrobial activity was recorded among fungi. Overall, the current data showed an interesting antimicrobial and antioxidant profile of EOs extracted from the Tunisian T. occidentalis (from the Sidi Bou Said locality, located northeast of Tunis), which could provide a benefit with the potential use of this plant and its EO extracts as a source of natural antioxidants and antimicrobials to preserve food. Similar studies should be conducted to verify whether the current findings apply to T. occidentalis from different localities in Tunis. Moreover, further investigation should be conducted to determine the active components of the EOs extracted of the Tunisian T. occidentalis, followed by more in vitro and in vivo experimentations, including toxicological tests.
Author Contributions
S.B. and C.A. contributed to this work equally; S.B.; C.A. and W.M. conceived and designed the experiments; S.B. and C.A. performed the experiments; W.D. and H.G. analyzed the data; C.J.; A.M. and A.C. contributed reagents/materials/analysis tools; S.B. and C.A. were involved in the writing—original draft of the study; and W.M.; C.S. and M.E.B. were involved in the writing—review and editing of the manuscript. W.M. was involved in the investigation and supervision.
Funding
This study was funded by the Tunisian Ministry of Higher Education and Scientific Research.
Acknowledgments
The authors would like to express their gratitude to Ahmed Slaheddine Masmoudi (ISBST-Tunisia) for technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Peng, D.; Wang, X.Q. Reticulate evolution in Thuja inferred from multiple gene sequences: Implications for the study of biogeographical disjunction between easren Asia and North America. Mol. Phylogenet. Evol. 2008, 47, 1190–1202. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.C.; Song, L.L.; Park, E.J. Bioactive constituents of Thuja occidentalis. J. Nat. Prod. 2000, 63, 1235–1238. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K. Contribution to anatomy of the central nervous system of the Japanese. Okajimas Folia Anatomica Japonica 1956, 28, 207–227. [Google Scholar] [CrossRef] [PubMed]
- Biswas, R.; Mandal, S.K.; Dutta, S.; Bhattacharyya, S.S.; Boujedaini, N.; Khuda-Bukhsh, A.R. Thujone-Rich Fraction of Thuja occidentalis Demonstrates Major Anti-Cancer Potentials: Evidences from In Vitro Studies on A375 Cells. Evid.-Based Complement. Altern. Med. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Mothana, R.A.; Alsaid, M.S.; Hasoon, S.S.; Al-Mosaiyb, N.M.; Al-Rehaily, A.J.; Al-Yahya, M.A. Antimicrobial and antioxidant activities and gas chromatography mass spectrometry (GC/MS) analysis of the essential oils of Ajuga bracteosa Wall. ex Benth. and Lavandula dentata L. growing wild in Yemen. J. Med. Plants Res. 2012, 6, 3066–3071. [Google Scholar]
- Zaouali, Y.; Chograni, H.; Trimech, R.; Boussaid, M. Changes in essential oil composition and phenolic fraction in Rosmarinus officinalis L. var. typicus Batt. organs during growth and incidence on the antioxidant activity. Ind. Crop. Prod. 2013, 43, 412–419. [Google Scholar]
- Mohammedi, Z.; Atik, F. Impact of solvent extraction type on total polyphenols Content and biological activity from Tamarix aphylla (L.) Karst. Int. J. Pharm. Biol. Sci. 2011, 2, 609–615. [Google Scholar]
- Svajdlenka, E.; Mártonfi, P.; Tomasko, I.; Grancai, D.; Nagy, M. Essential oil composition of Thuja occidentalis L. Samples from Slovakia. J. Essent. Oil Res. 1999, 11, 532–536. [Google Scholar] [CrossRef]
- Keita, S.M.; Vincent, C.; Schmidt, P.; Arnason, T. Insecticidal effects of Thuja occidentalis (Cupressaceae) essential oil on Callosobruchus maculatus [Coleoptera: Bruchidae]. Can. J. Plant Sci. 2001, 81, 173–177. [Google Scholar] [CrossRef]
- Tsiri, D.; Graikou, K.; Pobłocka-Oleh, L.; Krauze Baranowska, M.; Spyropoulos, C.; Chinou, I. Chemosystematic Value of the Essential Oil Composition of Thuja species Cultivated in Poland Antimicrobial Activity. Molecules 2009, 14, 4707–4715. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Co.: Carol Stream, IL, USA, 2009. [Google Scholar]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of complex: specific application to the determination of vit E. Anal. Biochem. 1999, 209, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Hatano, T.; Kagawa, H.; Yasuhara, T.; Okuda, T. Two new flavonoids and other constituents in licorice root: their relative astringency and radical scavenging effect. Chem. Pharm. Bull. 1988, 36, 2090–2097. [Google Scholar] [CrossRef] [PubMed]
- Mensor, L.L.; Menezes, F.S.; Leitão, G.G.; Reis, A.S.; dos Santos, T.C.; Coube, C.S.; Leitão, S.G. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Ghazghazi, H.; Aouadhi, C.; Weslati, M.; Trakhna, F.; Sebei, H.; Maaroufi, A.; Hasnaoui, B. Chemical composition and in vitro antimicrobial activities of Mentha pulegium leaves extracts against foodborne pathogens. J. Food Saf. 2013, 33, 239–246. [Google Scholar] [CrossRef]
- Statner, B.; Jones, M.J.; George, W.L. Effect of incubation temperature on growth and soluble protein profiles of motile Aeromonas strains. J. Clin. Microbiol. 1988, 26, 392–393. [Google Scholar] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; Approved Standard, 4th ed.; CLSI Document Vet 01-A4; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2013. [Google Scholar]
- Al Hafi, M.; Arnold, N.; Bouez, J.; Fabrice, C.; Antoine, A.; Beyrouthy, M. Chemical composition of the essential oils from (berries, leaves and twigs) of Juniperus excelsa M. Bieb. growing wild in Lebanon. J. Essent. Oil. Bear. Pl. 2013, 18, 901626. [Google Scholar] [CrossRef]
- Vaičiulytė, V.; Ložienė, K. Variation of chemical and morphological characters of leaves and unripe cones in Juniperus communis. Bot Lith. 2013, 19, 37–47. [Google Scholar]
- Derwich, E.; Benziane, Z.; Boukir, A. Chemical composition and antibacterial activity of leaves essential oil of Laurus nobilis from Morocco. Aust. J. Basic Appl. Sci. 2009, 3, 3818–3824. [Google Scholar]
- Edward, P.C.; Varro, E.T.; Lynn, R.B. Pharmacognosy, 6th ed.; Philadelphia Lea & Febiger: Philadelphia, PA, USA, 1987; pp. 184–187. [Google Scholar]
- Nickavara, B.; Amin, G.; Parhami, S. Volatile Constituents of the Fruit and Leaf Oils of Thuja orientalis L. Grown in Iran. Z. Naturforsch. 2003, 58c, 171–182. [Google Scholar] [CrossRef]
- Guleria, S.; Kumar, A.; Tiku, A.K. Chemical composition and fungitoxic activity of essential oil of Thuja orientalis L. grown in the north-western Himalaya. Z. Naturforsch. C 2008, 63, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Jirovetz, L.; Buchbauer, G.; Denkova, Z.; Slavchev, A.; Stoyanova, A.; Schmidt, E. Chemical composition, antimicrobial activities and odor descriptions of various Salvia sp. and Thuja sp. essential oils. Eur. Renal Nutr. 2006, 30, 152–159. [Google Scholar]
- Müller-Riebau, F.; Berger, B.; Yegen, O.; Cakir, C. Seasonal variations in the chemical compositions of essential oils of selected aromatic plants growing wild in Turkey. J. Agric. Food Chem. 1997, 45, 4821–5825. [Google Scholar] [CrossRef]
- Riahi, L.; Ghazghazi, H.; Ayari, B.; Aouadhi, C.; Klay, I.; Chograni, H.; Ameur, C.; Zoghlami, N. Effect of environmental conditions on chemical polymorphism and biological activities among Artemisia absinthium L. essential oil provenances grown in Tunisia. Ind. Crop. Prod. 2015, 66, 96–102. [Google Scholar] [CrossRef]
- Alves, L.D.S.; Figueirêdo, C.B.M.; Silva, C.C.A.R.; Marques, G.S.; Ferreira, P.A.; Soares, M.F.R.; Silva, R.M.F.; Rolim-neto, P.J. Thuja occidentalis L. (Cupressaceae): Review of botanical, phytochemical, pharmacological and toxicological aspects. Int. J. Pharm. Sci. Res. 2014, 5, 1163–1176. [Google Scholar]
- Yogesh, K.; Jamshed, A. Potential of thuja (Thuja occidentalis) cones and peach (Prunus persia) seeds in raw chicken ground meat during refrigerated (4 ± 1 °C) storage. J. Food Sci. Technol. 2014, 51, 1547–1553. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.K.; Batra, A. Antioxidant activities of Thuja occidentalis Linn. Asian J. Pharm. Clin. Res. 2009, 2, 73–76. [Google Scholar]
- Ololade, Z.S.; Fakankun, O.A.; Alao, F.O. Phytochemical and Therapeutic Studies of the Fruit essential oil of Thuja orientalis from Nigeria. Glob. J. Sci. Front. Res. B Chem. 2014, 1, 14. [Google Scholar]
- Das, S.; Rani, R. Antioxidant and gastroprotective properties of the fruits of Thuja occidentalis Linn. Asian J. Pharm. Clin. Res. 2013, 3, 80–87. [Google Scholar]
- Khubeiz, M.J.; Mansour, G.; Zahraa, B. Antibacterial and Phytochemical Investigation of Thuja orientalis (L.) Leaves Essential Oil from Syria. Int. J. Curr. Pharm. Rev. Res. 2016, 7, 243–247. [Google Scholar]
- Mahboubi, M.; Haghi, G. Antimicrobial activity and chemical composition of Mentha pulegium L. essential oil. J. Ethnopharmacol. 2008, 119, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Boukhebti, H.; Chaker, A.N.; Belhadj, H.; Sahli, F.; Ramdhani, M.; Laouer, H.; Harzallah, H. Chemical composition and antibacterial activity of Mentha pulegium L. and Mentha spicata L. essential oils. Pharm. Lett. 2011, 3, 267–275. [Google Scholar]
- Nikaido, H.; Vaara, M. Molecular basis of bacterial outer membrane permeability. Microbiol. Rev. 1985, 49, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Hogg, S. Essential Microbiology; John Wiley and Sons Ltd.: Chichester, UK, 2005. [Google Scholar]
- Sah, S.N.; Regmi, S.; Tamung, M.K. Antibacterial Effects of Thuja Leaves Extract. Int. J. Appl. Sci. Biotechnol. 2017, 5, 256–260. [Google Scholar] [CrossRef]
- Shah, W.A.; Qadir, M. Chemical composition, Antioxidant and Antibacterial activity of Thuja orientalis essential oil. World J. Pharm. Sci. 2013, 2, 56–60. [Google Scholar]
- Rakesh, K.J.; Garg, S.C. Antimicrobial activity of the essential oil of Thuja orientalis L. Anc. Sci. Life 1997, 16, 186–189. [Google Scholar]
- Elaissi, A.; Rouis, Z.; Mabrouk, S.; Bel Haj Salah, K.; Aouni, M.; Larbi Khouja, M.; Farhat, F.; Chemli, R.; Harzallah-Skhiri, F. Correlation Between Chemical Composition and Antibacterial Activity of Essential Oils from Fifteen Eucalyptus Species Growing in the Korbous and Jbel Abderrahman Arboreta (North East Tunisia). Molecules 2012, 17, 3044–3057. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).