Use of Bone Marrow Aspirate Concentrate (BMAC) Associated with Hyperbaric Oxygenation Therapy in Maxillary Appositional Bone Reconstruction. A Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
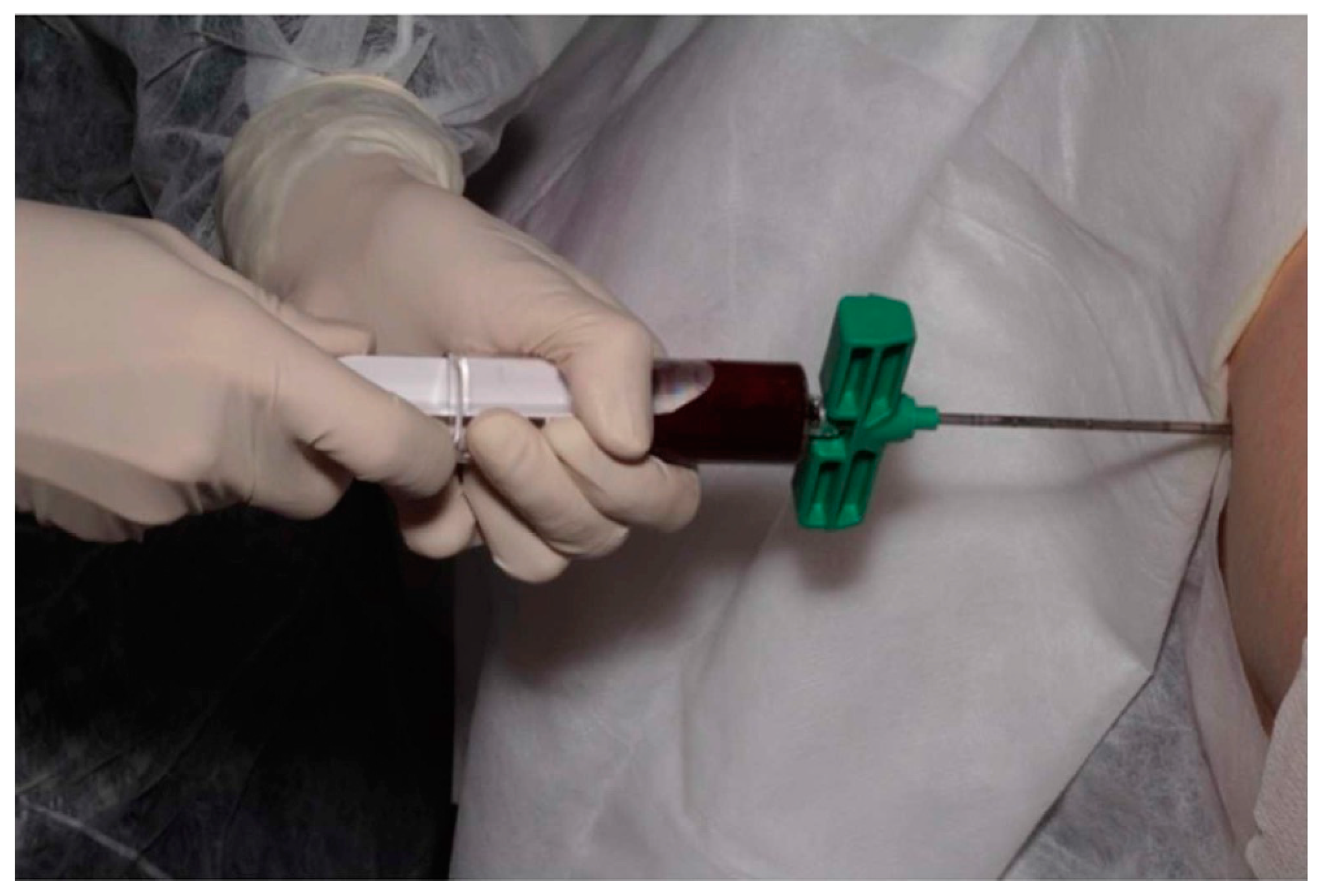
2.1. Bone Marrow Aspirate Concentrate (BMAC)
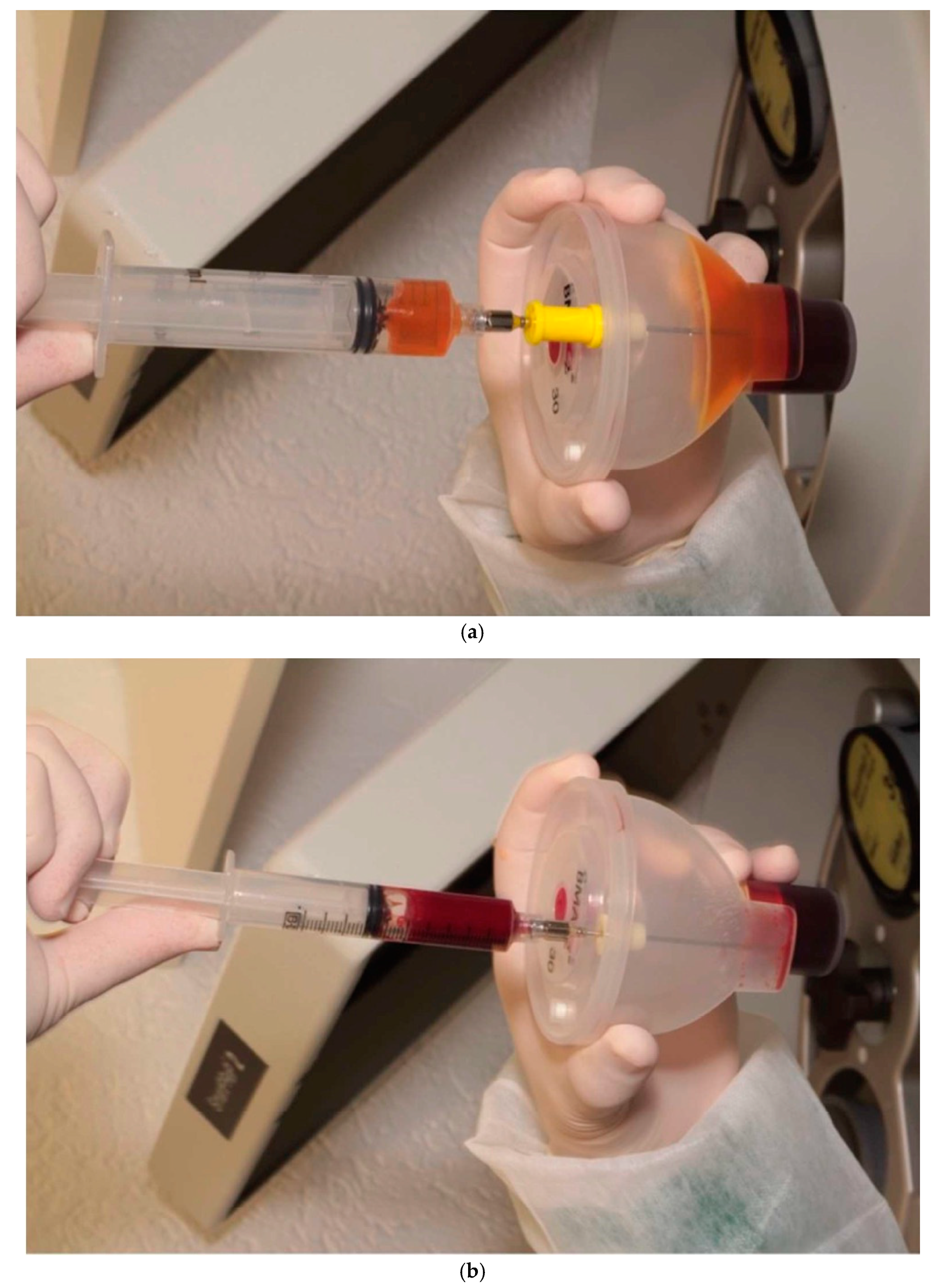
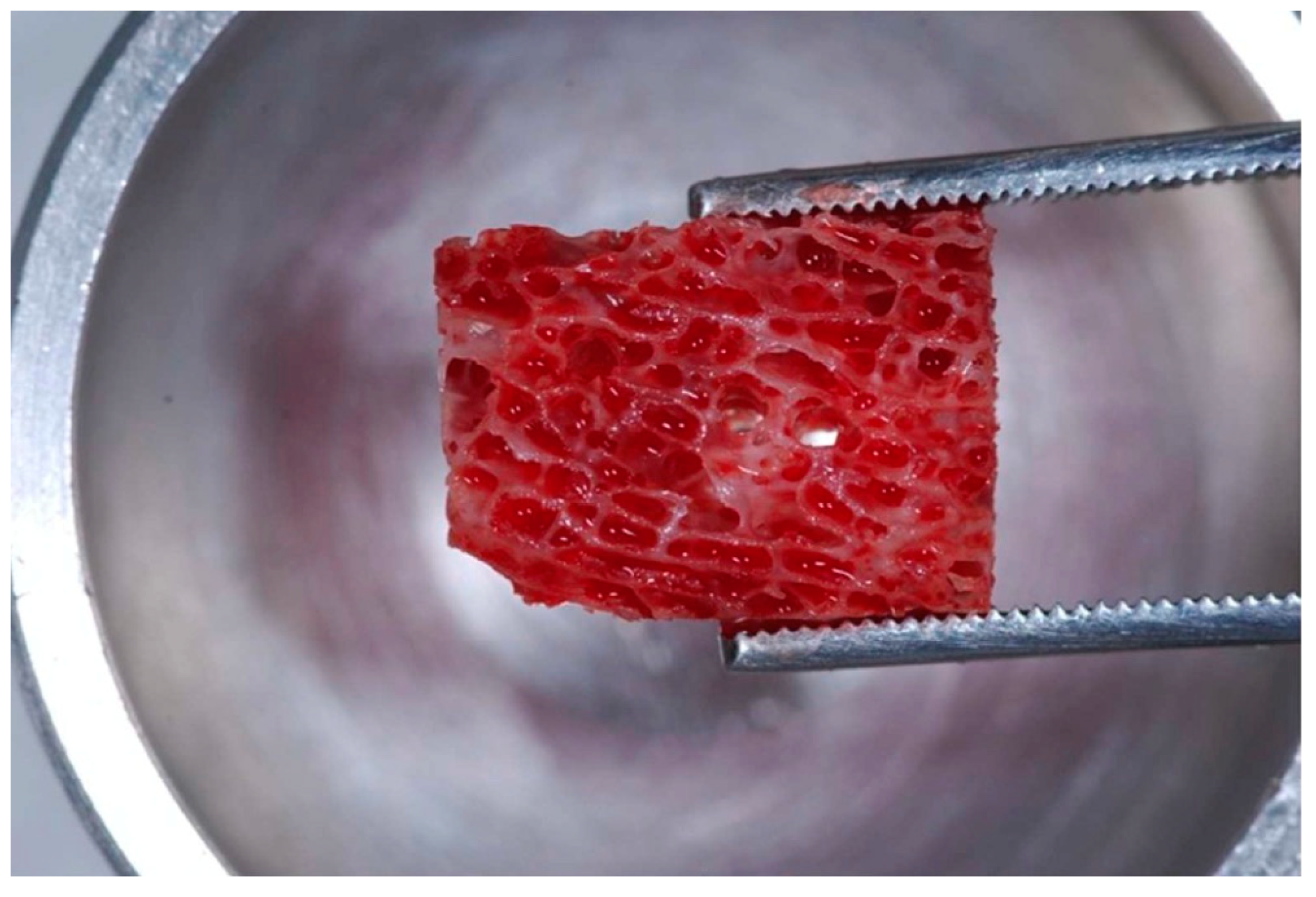
2.2. Association of the Bone Marrow Aspirate Concentrate to the Xenograft Blocks
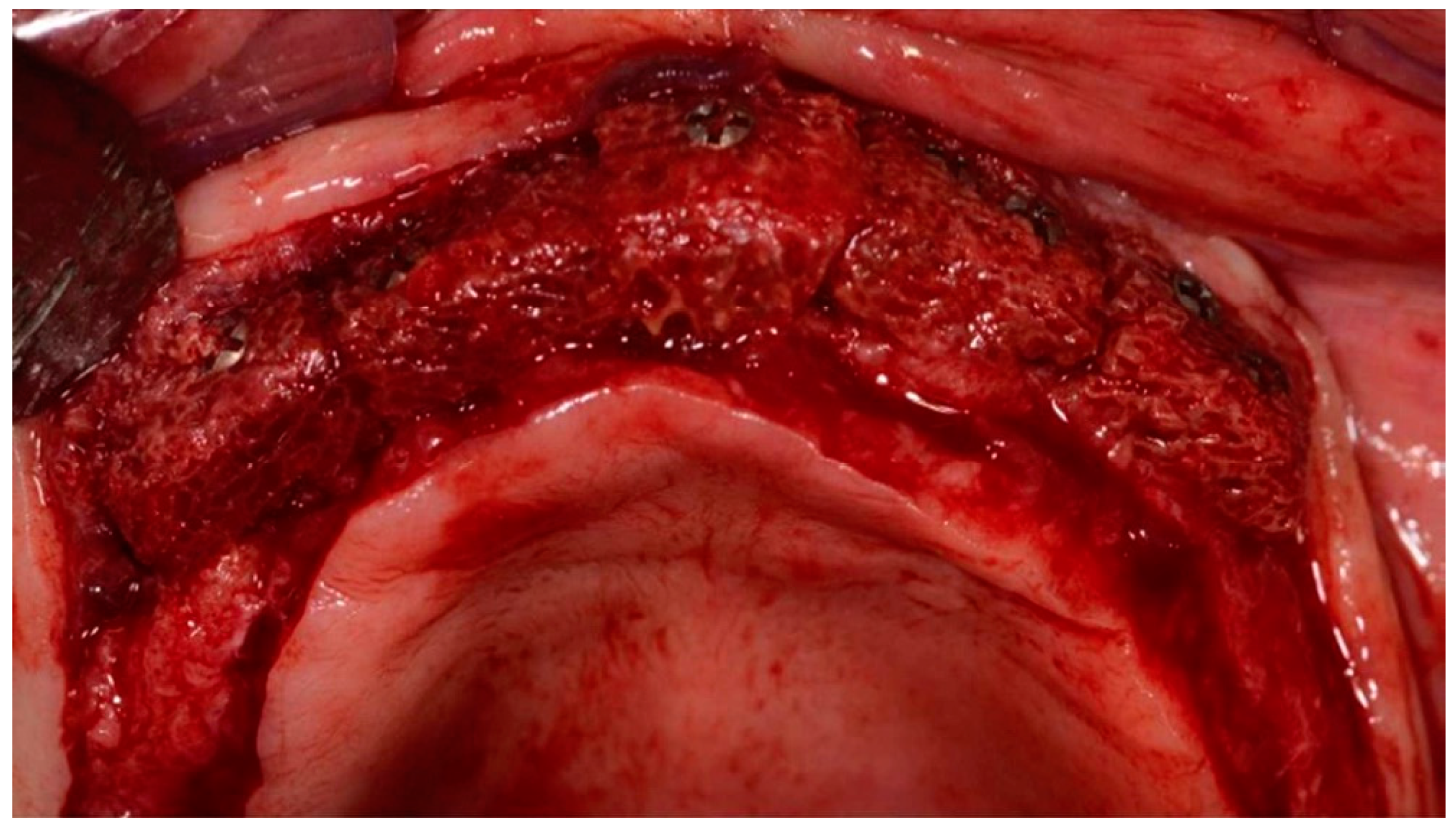
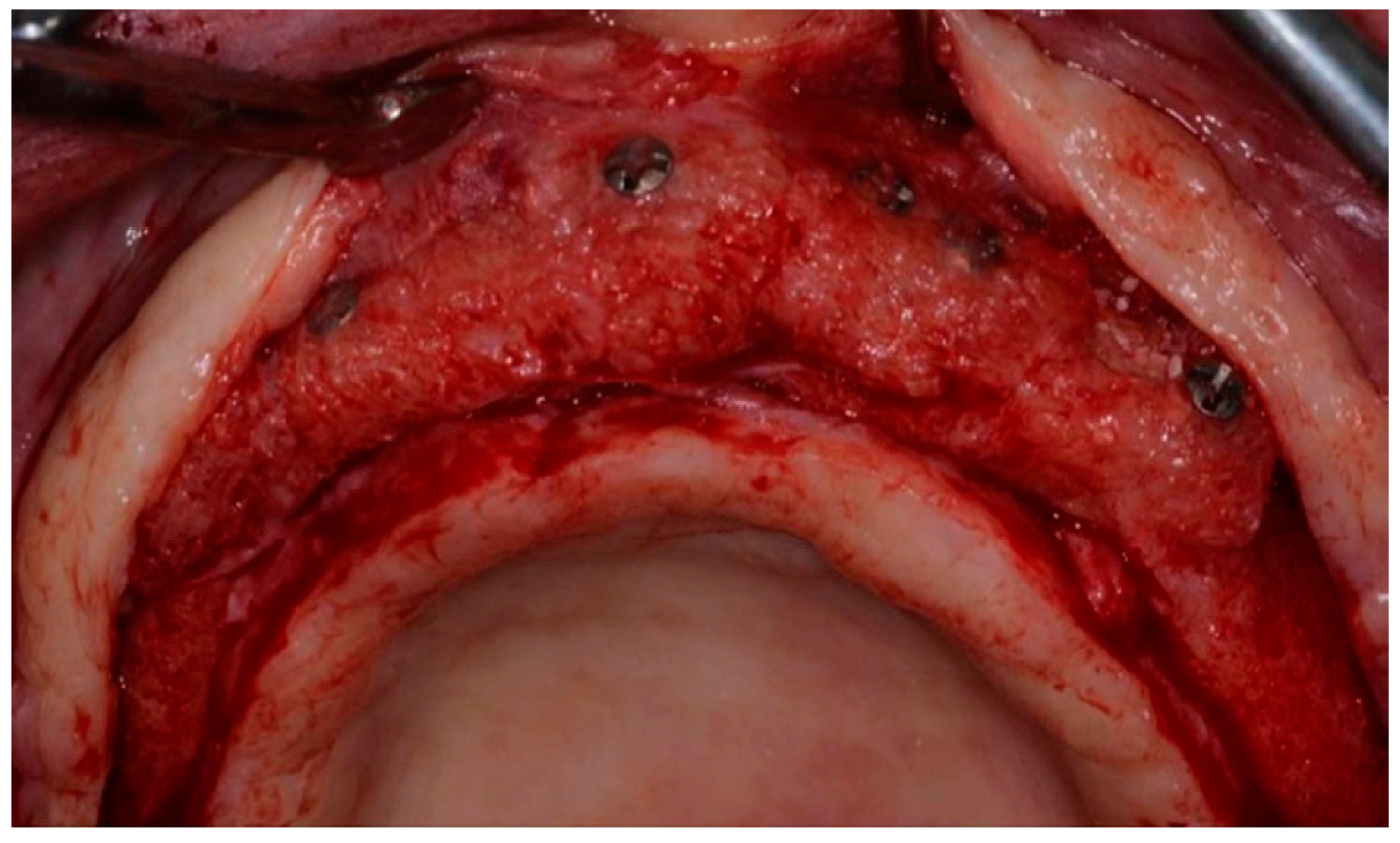
2.3. Surgical Technique for Bone Grafting
2.4. Hyberbaric Oxygenation (HBO)
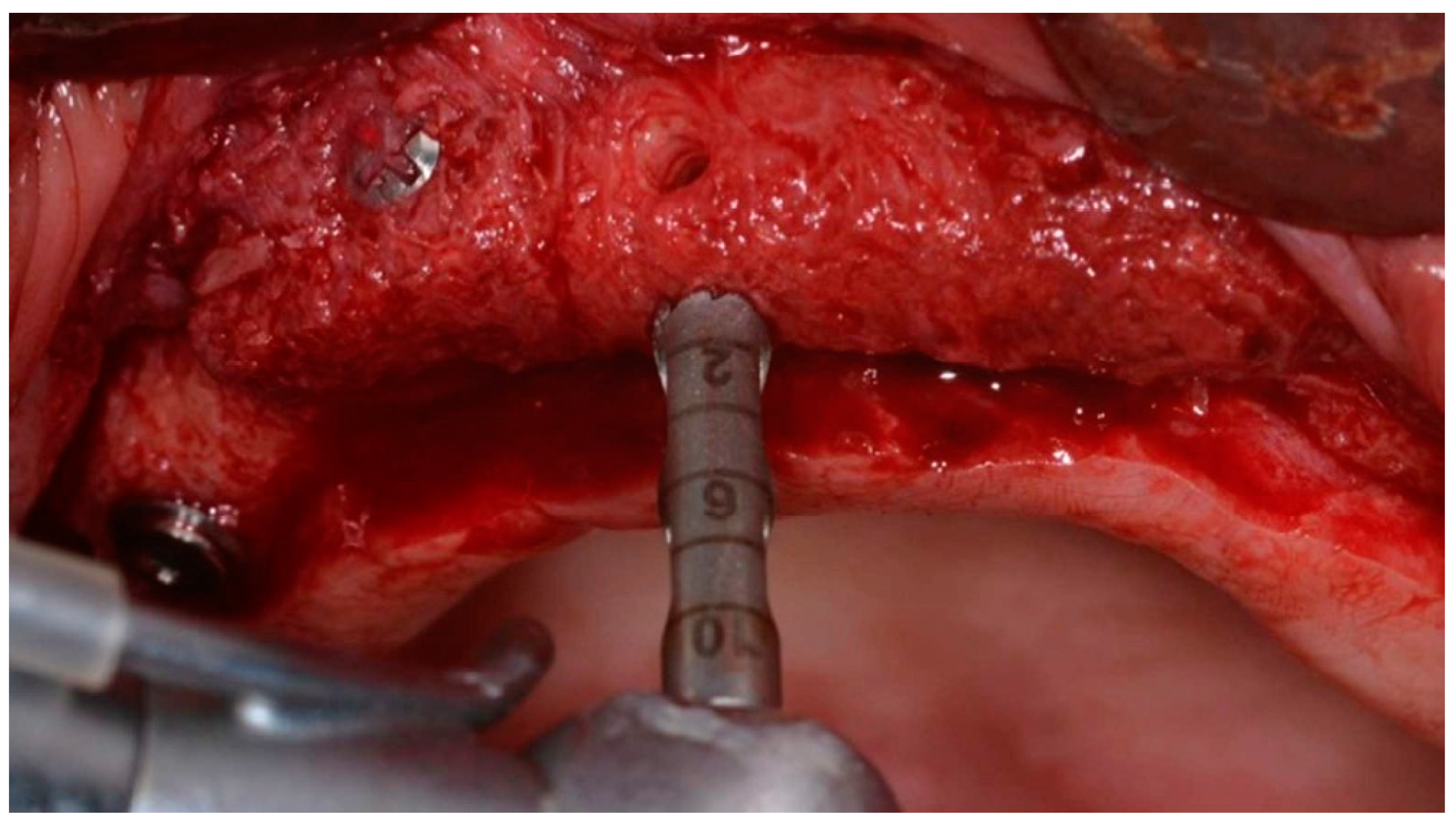
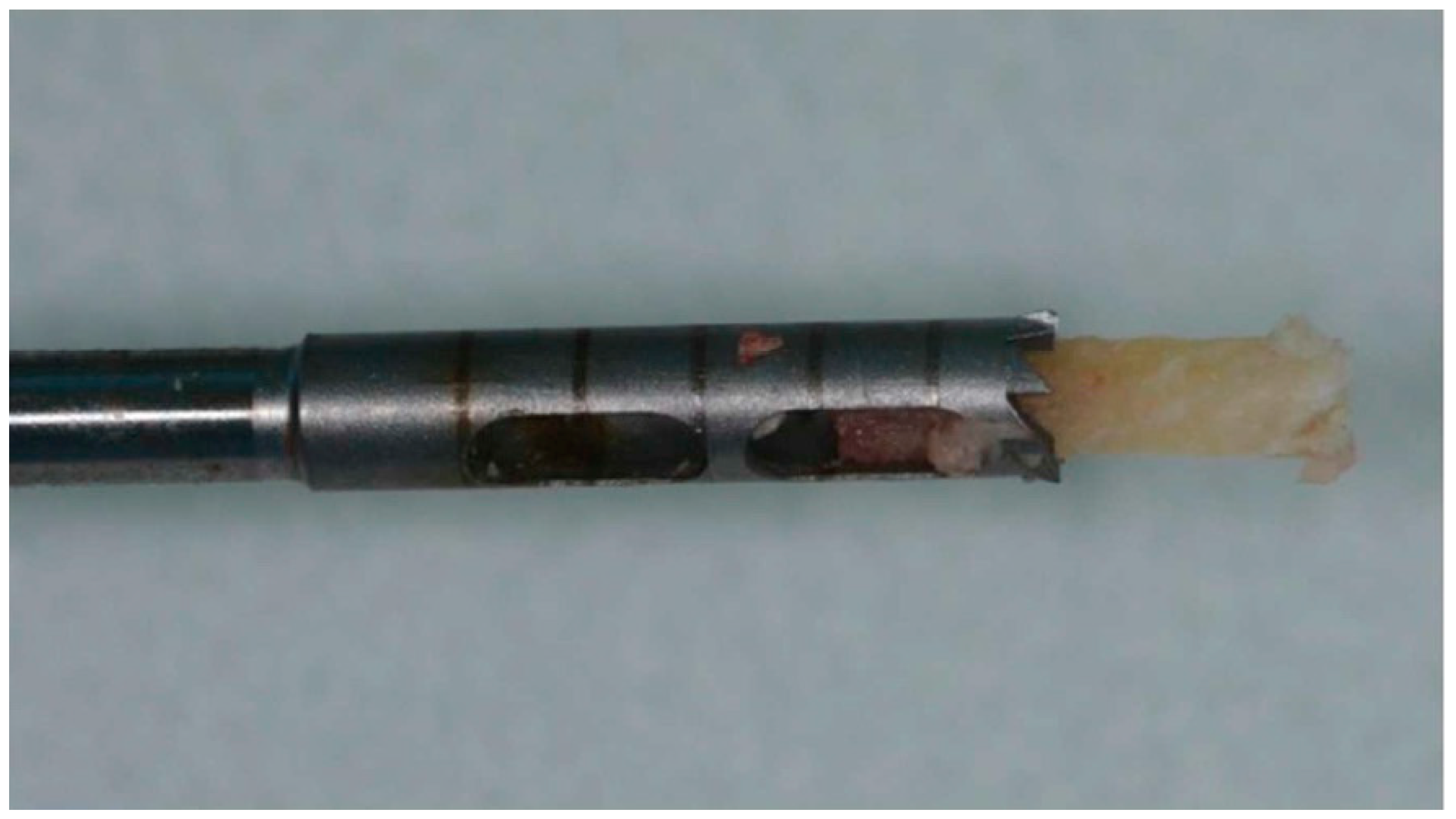
2.5. Bone Biopsies
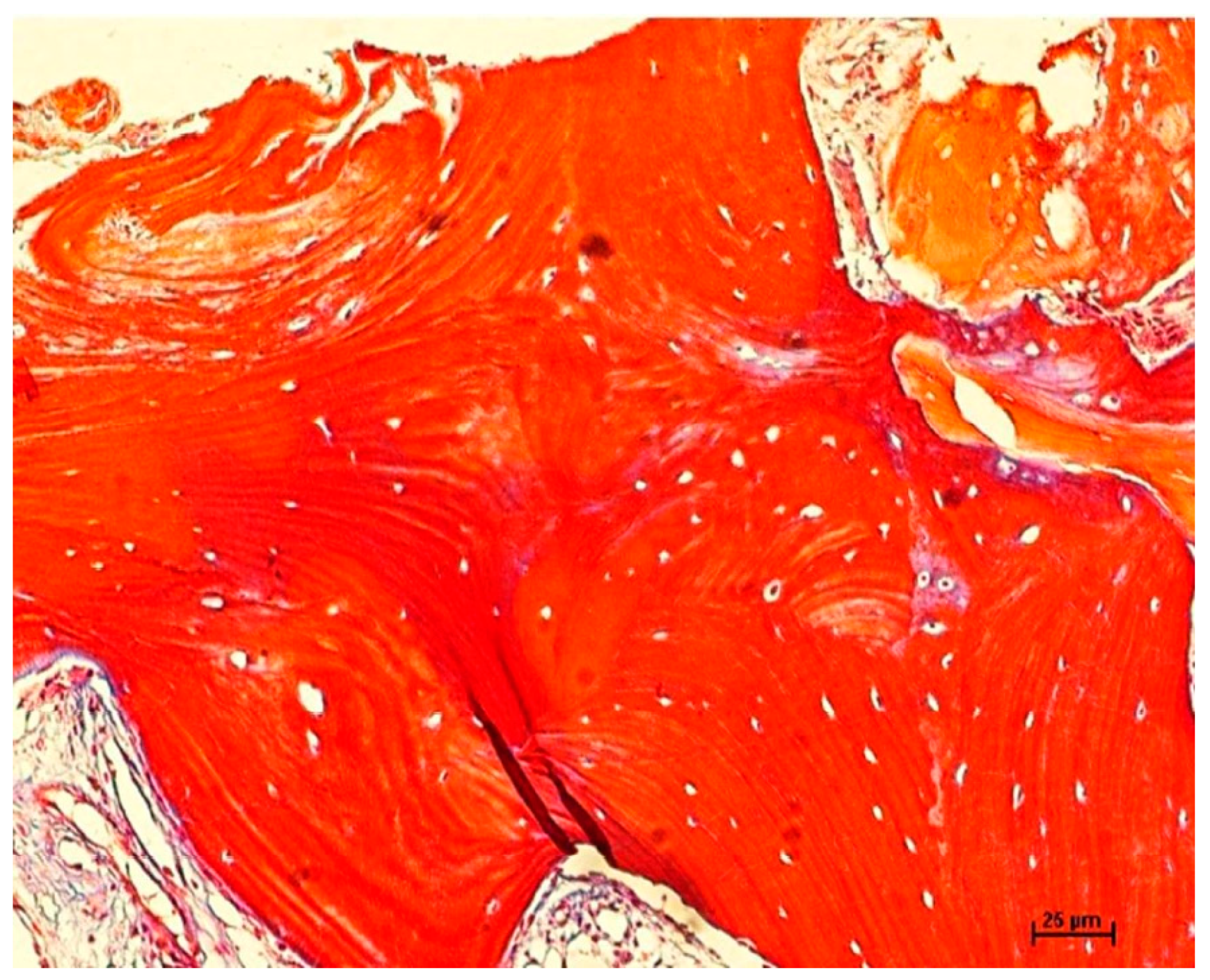
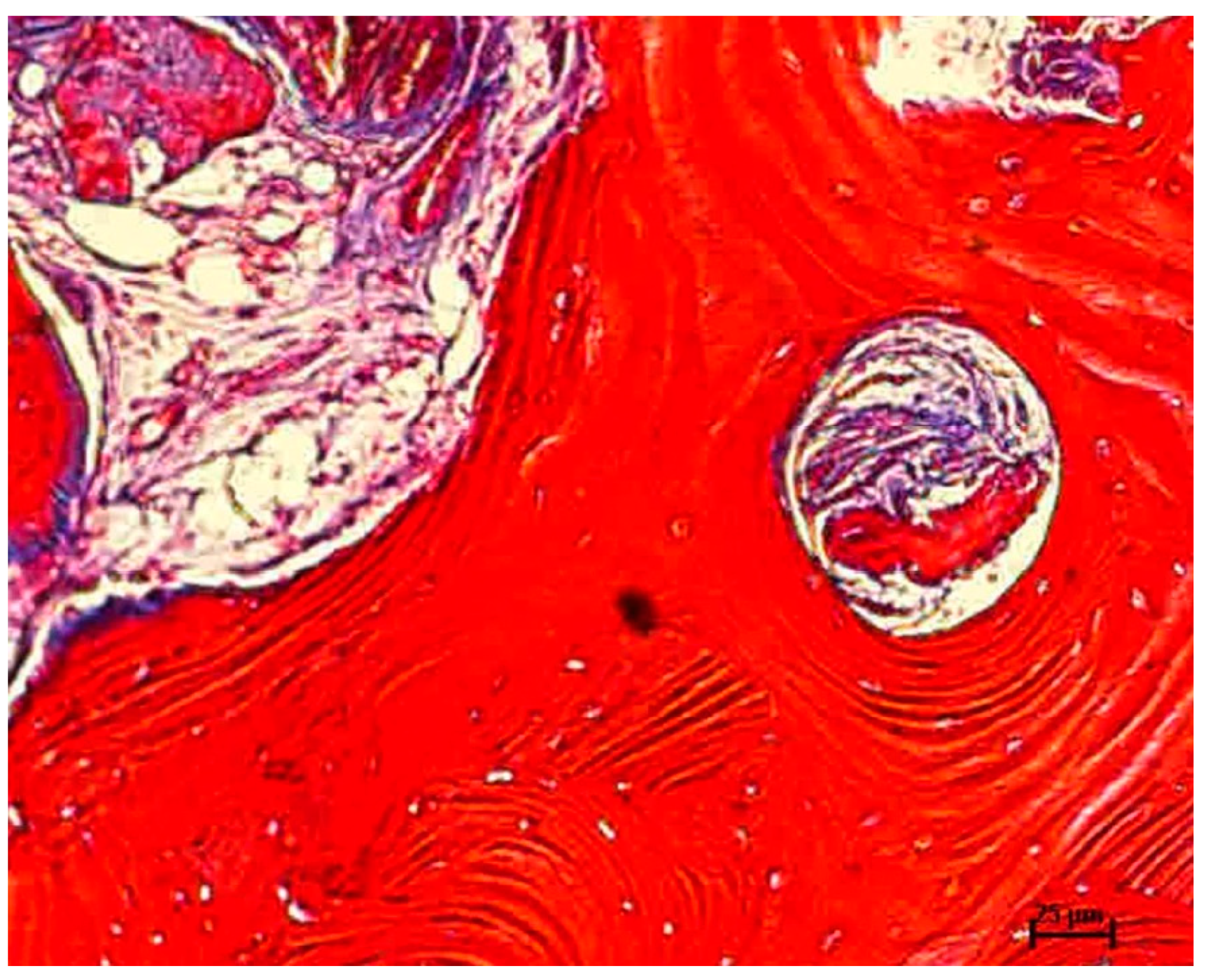
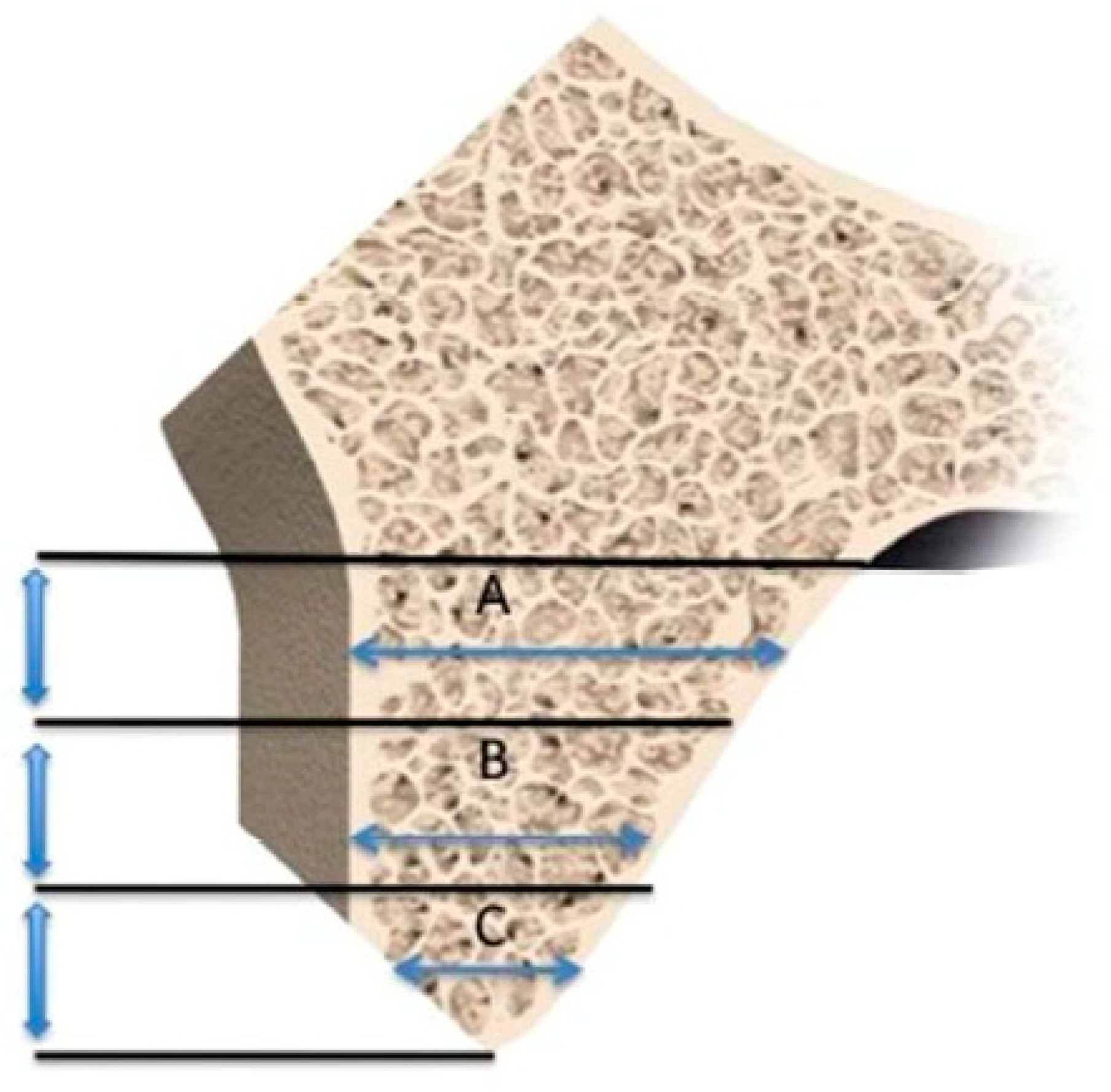
2.6. Histomorphometric Evaluation
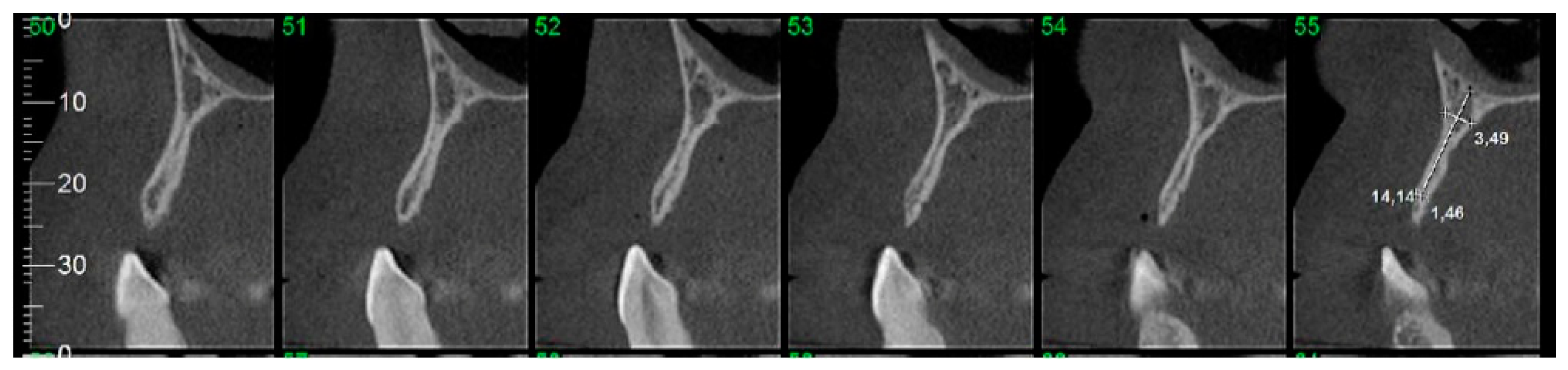
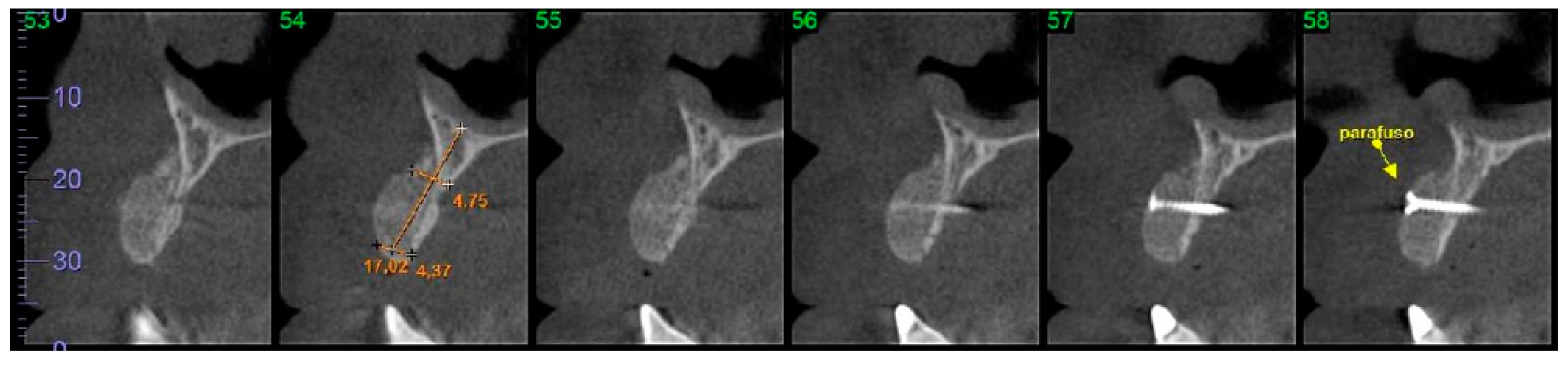
2.7. Computed Tomographic (CT) Evaluation
2.8. Randomization Procedure
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable Materials for Bone Repair and Tissue Engineering Applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef] [PubMed]
- Pistilli, R. Case of severe bone atrophy of the posterior maxilla rehabilitated with blocks of equine origin bone: Histological results. Implant Dent. 2013, 22, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Quarto, R.; Mastrogiacomo, M.; Cancedda, R.; Kutepov, S.K.; Muchechev, V.; Lavroukov, A.; Kon, E.; Maracacci, M. Repair of large bone defects with the use of autologous bone marrow stromal cells. N. Engl. J. Med. 2001, 344, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Lemoli, R.M.; Bertolini, F.; Cancedda, R.; De Luca, M.; Del Santo, A.; Ferrari, G.; Ferrari, S.; Martino, G.; Mavilio, F.; Tura, S. Stem cell plasticity: Time for a reappraisal. Haematologica 2005, 90, 360–381. [Google Scholar] [PubMed]
- Sefat, F.; Raja, T.I.; Zafar, M.S.; Khurshid, Z.; Najeeb, S.; Zohaib, S.; Ahmadi, E.D.; Rahmati, M.; Mozafari, M. Chapter 3—Nanoengineered Biomaterials for Cartilage Repair. Nanoeng. Biomater. Regener. Med. 2019, 39–71. [Google Scholar] [CrossRef]
- Kale, S.; Long, M.W. Osteopoiesis the early development of bone cells. Crit. Rev. Eukaryot. Gene Expr. 2000, 10, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Furuya, K.; Hanada, K. Progressive development of the osteoblast phenotype during differentiation of osteoprogenitor cells derived from fetal rat calvaria: Model for in vitro bone formation. Biol. Pharm. Bull. 2002, 25, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Kaigler, D.; Krebsbach, P.H.; Polverini, P.J.; Mooney, D.J. Role of vascular endothelial growth factor in bone marrow stromal cell modulation of endothelial cells. Tissue Eng. 2003, 9, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Lucarelli, E.; Fini, M.; Beccheroni, A.; Giavaresi, G.; Di Bella, C.; Aldini, N.N.; Guzzardella, G.; Martini, L.; Cenacchi, A.; Di Maggio, N.; et al. Stromal stem cells and platelet-rich plasma improve bone allograft integration. Clin. Orthop. Relat. Res. 2005, 435, 62–68. [Google Scholar] [CrossRef]
- Jung, Y.; Song, J.; Shiozawa, Y.; Wang, J.; Wang, Z.; Williams, B.; Havens, A.; Schneider, A.; Ge, C.; Franceschi, R.T.; et al. Hematopoietic Stem Cells Regulate Mesenchymal Stromal Cell Induction into Osteoblasts Thereby Participating in the Formation of the Stem Cell Niche. Stem Cells 2008, 26, 2042–2051. [Google Scholar] [CrossRef] [PubMed]
- Kevy, S.V.; Jacobson, M.S. Comparison of Methods for Point of Care Preparation of Autologous Platelet Gel. J. Extra Corpor. Technol. 2004, 36, 28–35. [Google Scholar] [PubMed]
- Hernigou, P.; Desroches, A.; Queinnec, S.; Flouzat Lachaniette, C.H.; Poignard, A.; Allain, J.; Chevallier, N.; Rouard, H. Morbidity of graft harvesting versus bone marrow aspiration in cell regenerative therapy. Int. Orthop. 2014, 38, 1855–1860. [Google Scholar] [CrossRef] [PubMed]
- Cicciu, M. Real opportunity for the present and a forward step for the future of bone tissue engineering. J. Craniofac. Surg. 2017, 28, 592–593. [Google Scholar] [CrossRef] [PubMed]
- García-Covarrubias, L.; Cuauhtémoc Sánchez-Rodríguez, E. Hyperbaric oxygenation therapy, basic concepts. Gac. Med. Mex. 2000, 136, 45–56. [Google Scholar] [PubMed]
- Hong, P.; Boyd, D.; Beyea, S.D.; Bezuhly, M. Enhancement of bone consolidation in mandibular distraction osteogenesis: A contemporary review of experimental studies involving adjuvant therapies. J. Plast. Reconstr. Aesthet. Surg. 2013, 66, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Burchardt, H. The biology of bone graft repair. Clin. Orthop. Relat. Res. 1983, 174, 28–42. [Google Scholar] [CrossRef]
- Raghoebar, G.M.; Louwerse, C.; Kalk, W.W.; Vissink, A. Morbidity of chin bone harvesting. Clin. Oral Implants Res. 2001, 12, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Boniello, R.; Gasparini, G.; D’Amato, G.; Torroni, A.; Marianetti, T.M.; Foresta, E.; Azzuni, C.; Cervelli, D.; Pelo, S. Reconstruction of severe atrophic jaws with Fresh Frized Bone Allografts: Clinical histologic and histomorphometric evaluation. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 1411–1418. [Google Scholar] [PubMed]
- Sununliganon, L.; Peng, L.; Singhatanadgit, W.; Cheung, L.K. Osteogenic efficacy of bone marrow concentrate in rabbit maxillary sinus grafting. J. Craniomaxillofac. Surg. 2014, 42, 1753–1765. [Google Scholar] [CrossRef] [PubMed]
- Pelegrine, A.A.; Aloise, A.C.; Zimmermann, A.; Mello e Oliveira, R.; Ferreira, L.M. Repair of critical-size bone defects using bone marrow stromal cells: A histomorphometric study in rabbit calvaria. Part I: Use of fresh bone marrow or bone marrow mononuclear fraction. Clin. Oral Implants Res. 2014, 25, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Pelegrine, A.A.; Teixeira, M.L.; Sperandio, M.; Almada, T.S.; Kahnberg, K.E.; Pasquali, P.J.; Aloise, A.C. Can bone marrow aspirate concentrate change the mineralization pattern of the anterior maxilla treated with xenografts? A preliminary study. Contemp. Clin. Dent. 2016, 7, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Nissan, J.; Mardinger, O.; Calderon, S.; Romanos, G.E.; Chaushu, G. Cancellous bone block allografts for the augmentation of the anterior atrophic maxilla. Clin. Implant Dent. Relat. Res. 2011, 13, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Pelegrine, A.A.; Sorgi da Costa, C.E.; Sendyk, W.R.; Gromatzky, A. The comparative analysis of homologous fresh frozen bone and autologous bone graft, associated or not with autologous bone marrow, in rabbit calvaria: A clinical and histomorphometric study. Cell Tissue Bank. 2011, 12, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.A.; Aloise, A.C.; Orosz, J.E.; de Mello e Oliveira, R.; Carvalho, P.; Pelegrine, A.A. Double Centrifugation Versus Single Centrifugation of Bone Marrow Aspirate Concentrate in Sinus Floor Elevation: A Pilot Study. Int. J. Oral Maxillofac. Implants 2016, 31, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Soltan, M.; Smiler, D.; Choi, J. Bone marrow: Orchestrated cells, cytokines, and growth factors for bone regeneration. Implant Dent. 2009, 18, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Rickert, D.; Sauerbier, S.; Nagursky, H.; Menne, D.; Vissink, A.; Raghoebar, G.M. Maxillary sinus floor elevation with bovine bone mineral combined with either autogenous bone or autogenous stem cells: A prospective randomized clinical trial. Clin. Oral Implants Res. 2011, 22, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Haapanemi, T.; Sirsjö, A.; Nylander, G.; Larsson, J. Hyperbaric oxygen treatment attenuates glutathione depletion and improves metabolic restitution in postischemic skeletal muscle. Free Radic. Res. 1995, 23, 91–101. [Google Scholar] [CrossRef]
- Korhonen, K.; Hirn, M.; Niinikoski, J. Hyperbaric oxygen in the treatment of Fournier’s gangrene. Eur. J. Surg. 1998, 164, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Mindrup, S.R.; Kealey, G.P.; Fallon, B. Hyperbaric oxygen for the treatment of Fournier’s gangrene. J. Urol. 2005, 173, 1975–1977. [Google Scholar] [CrossRef] [PubMed]
- Rose, D. Hyperbaric oxygen therapy for chronic refractory osteomyelitis. Am. Fam. Physician 2012, 86, 888. [Google Scholar] [PubMed]
- Delasotta, L.A.; Hanflik, A.; Bicking, G.; Mannella, W.J. Hyperbaric oxygen for osteomyelitis in a compromised host. Open Orthop. J. 2013, 7, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Goerger, E.; Honnorat, E.; Savini, H.; Coulange, M.; Bergmann, E.; Simon, F.; Seng, P.; Stein, A. Anti-infective therapy without antimicrobials: Apparent successful treatment of multidrug resistant osteomyelitis with hyperbaric oxygen therapy. IDCases 2016, 6, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Sato, S.; Yagi, H.; Ujiie, H.; Ezawa, S.; Ito, K. Correlation in the densities of augmented and existing bone in guided bone augmentation. Clin. Oral Implants Res. 2012, 23, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Pelegrine, A.A.; Romito, G.; Villar, C.C.; Macedo, L.G.S.; Teixeira, M.L.; Aloise, A.C.; Moy, P.K. Horizontal bone reconstruction on sites with different amounts of native bone: A retrospective study. Braz. Oral Res. 2018, 32, e21. [Google Scholar] [CrossRef] [PubMed]

| CG | G1 | G2 | |
|---|---|---|---|
| VMT | 36.58 ± 9.56a | 55.64 ± 2.83A | 55.30 ± 1.41A |
| NMT | 51.21 ± 11.54a | 39.76 ± 10.33A | 40.3 ± 11.48A |
| NVMT | 11.16 ± 2.37a | 3.65 ± 0.87A | 4.10 ± 0.41A |
| Effect Size | Non-Centrality Parameter (δ) | Power Achieved (%) | ||
|---|---|---|---|---|
| VMT | CG vs. G1 | 2.703 | 5.283 | 99.90 * |
| CG vs. G2 | 2.739 | 5.354 | 99.90 * | |
| G1 vs. G2 | 0.152 | 0.297 | 8.60 | |
| NMT | CG vs. G1 | 0.994 | 1.944 | 57.80 |
| CG vs. G2 | 0.947 | 1.852 | 54.40 | |
| G1 vs. G2 | 0.047 | 0.091 | 5.90 | |
| NVMT | CG vs. G1 | 4.206 | 8.221 | 100.00 * |
| CG vs. G2 | 3.954 | 7.729 | 100.00 * | |
| G1 vs. G2 | 0.517 | 1.010 | 24.60 |
| 4 Months | 8 Months | |
|---|---|---|
| CG | 4.17 ± 0.37ab | 4.01 ± 0.86 ab |
| G1 | 3.85 ± 0.50ab | 3.83 ± 0.27ab |
| G2 | 3.83 ± 0.73ab | 3.57 ± 0.49ab |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aloise, A.C.; Pasquali, P.; Sperandio, M.; Scavone de Macedo, L.G.; Lucchesi Teixeira, M.; Pelegrine, A.A.; Calvo-Guirado, J.L. Use of Bone Marrow Aspirate Concentrate (BMAC) Associated with Hyperbaric Oxygenation Therapy in Maxillary Appositional Bone Reconstruction. A Randomized Clinical Trial. Symmetry 2018, 10, 533. https://doi.org/10.3390/sym10100533
Aloise AC, Pasquali P, Sperandio M, Scavone de Macedo LG, Lucchesi Teixeira M, Pelegrine AA, Calvo-Guirado JL. Use of Bone Marrow Aspirate Concentrate (BMAC) Associated with Hyperbaric Oxygenation Therapy in Maxillary Appositional Bone Reconstruction. A Randomized Clinical Trial. Symmetry. 2018; 10(10):533. https://doi.org/10.3390/sym10100533
Chicago/Turabian StyleAloise, Antonio Carlos, Paulo Pasquali, Marcelo Sperandio, Luis Guilherme Scavone de Macedo, Marcelo Lucchesi Teixeira, André Antonio Pelegrine, and José Luis Calvo-Guirado. 2018. "Use of Bone Marrow Aspirate Concentrate (BMAC) Associated with Hyperbaric Oxygenation Therapy in Maxillary Appositional Bone Reconstruction. A Randomized Clinical Trial" Symmetry 10, no. 10: 533. https://doi.org/10.3390/sym10100533
APA StyleAloise, A. C., Pasquali, P., Sperandio, M., Scavone de Macedo, L. G., Lucchesi Teixeira, M., Pelegrine, A. A., & Calvo-Guirado, J. L. (2018). Use of Bone Marrow Aspirate Concentrate (BMAC) Associated with Hyperbaric Oxygenation Therapy in Maxillary Appositional Bone Reconstruction. A Randomized Clinical Trial. Symmetry, 10(10), 533. https://doi.org/10.3390/sym10100533






