Human Natural Antibodies to Mammalian Carbohydrate Antigens as Unsung Heroes Protecting against Past, Present, and Future Viral Infections
Abstract
1. Introduction
2. Anti-MCA Antibodies
2.1. Anti-Gal Antibody
2.2. Anti-Neu5Gc Antibody
2.3. Anti-Forssman Antibody
3. Biosynthesis of MCA on Enveloped Viruses
4. Anti-Gal Antibody Protection against Zoonosis
5. Anti-Gal Amplifies Immune Response to Viruses with α-Gal Epitopes
6. Increasing Anti-Gal Titers for Protecting Travelers against Zoonotic Viruses
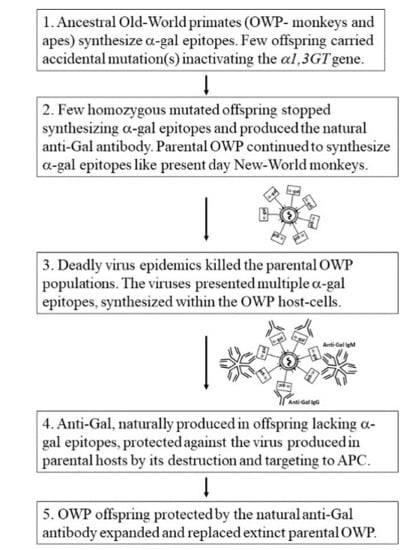
7. Anti-Gal Associated Prevention of Extinction among Ancestral Old-World Monkeys and Apes
8. Past Prevention of Hominin Extinction by the Natural Anti-Neu5Gc Antibody
9. The Natural Anti-Forssman Antibody and Mammalian Evolution
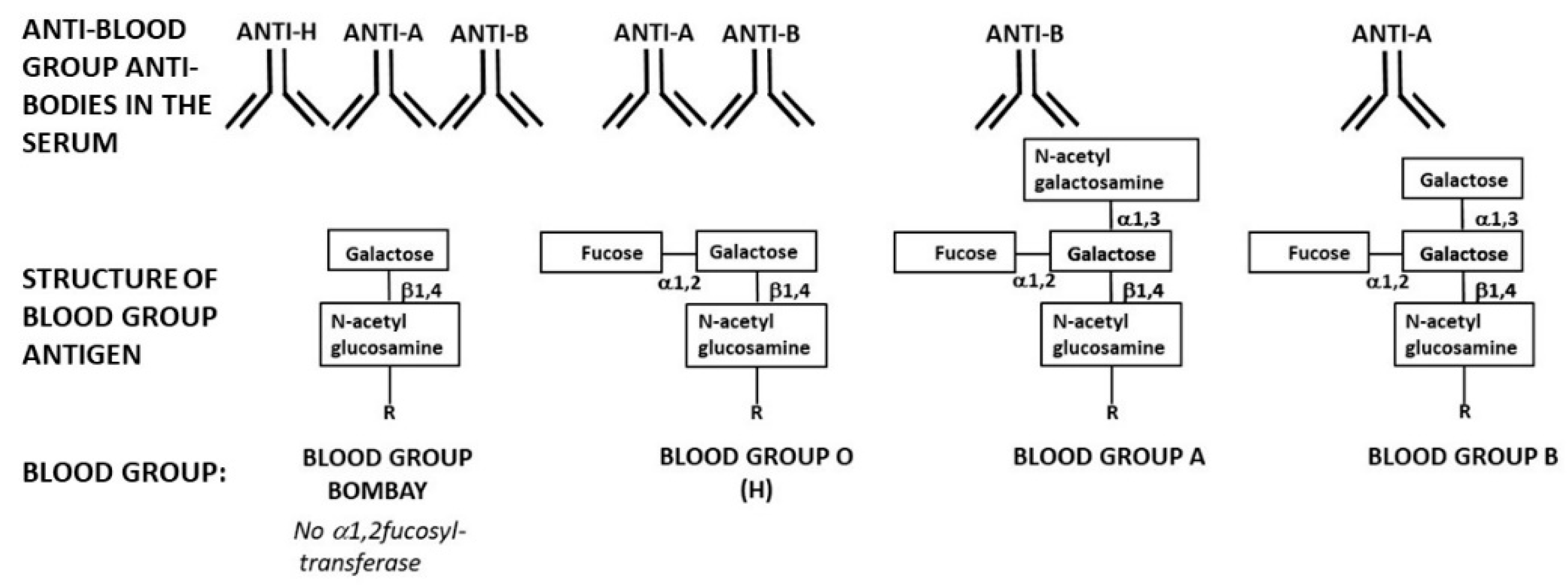
10. The Natural Anti-H Antibody of Blood-Group Bombay in Future Viral Epidemics
11. Conclusions
Funding
Conflicts of Interest
References
- Ochsenbein, A.F.; Fehr, T.; Lutz, C.; Suter, M.; Brombacher, F.; Hengartner, H.; Zinkernagel, R.M. Control of early viral and bacterial distribution and disease by natural antibodies. Science 1999, 286, 2156–2159. [Google Scholar] [CrossRef] [PubMed]
- Bovin, N. Natural antibodies to glycans. Biochemistry [Moscow] 2013, 78, 786–797. [Google Scholar] [CrossRef] [PubMed]
- Blixt, O.; Head, S.; Mondala, T.; Scanlan, C.; Huflejt, M.E.; Alvarez, R.; Bryan, M.C.; Fazio, F.; Calarese, D.; Stevens, J.; et al. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc. Natl. Acad. Sci. USA 2004, 101, 17033–17038. [Google Scholar] [CrossRef] [PubMed]
- Stowell, S.R.; Arthur, C.M.; McBride, R.; Berger, O.; Razi, N.; Heimburg-Molinaro, J.; Rodrigues, L.C.; Gourdine, J.-P.; Noll, A.J.; von Gunten, S.; et al. Microbial glycan microarrays define key features of host-microbial interactions. Nat. Chem. Biol. 2014, 10, 470–476. [Google Scholar] [CrossRef]
- Wiener, A.S. Origin of naturally occurring hemagglutinins and hemolysins; a review. J. Immunol. 1951, 66, 287–295. [Google Scholar]
- Springer, G.F. Blood-group and Forssman antigenic determinants shared between microbes and mammalian cells. Prog. Allergy 1971, 15, 9–77. [Google Scholar]
- Hooperm, L.V.; Macpherson, A.J. Immune adaptation that maintain homeostasis with intestinal microbiota. Nat. Rev. Immunol. 2010, 10, 159–169. [Google Scholar]
- Gerritsen, J.; Smidt, H.; Rijkers, G.T.; de Vos, W.M. Intestinal microbiota in human health and disease: The impact of probiotics. Genes Nutr. 2011, 6, 209–240. [Google Scholar] [CrossRef]
- Watkins, W.M. Biochemistry and Genetics of the ABO, Lewis, and P blood group systems. Adv. Hum. Genet. 1980, 10, 379–385. [Google Scholar]
- Galili, U.; Rachmilewitz, E.A.; Peleg, A.; Flechner, I. A unique natural human IgG antibody with anti-α-galactosyl specificity. J. Exp. Med. 1984, 160, 1519–1531. [Google Scholar] [CrossRef]
- Hamadeh, R.M.; Galili, U.; Zhou, P.; Griffiss, J.M. Human secretions contain IgA, IgG and IgM anti-Gal [anti-α-galactosyl] antibodies. Clin. Diagn. Lab. Immunol. 1995, 2, 125–131. [Google Scholar] [CrossRef] [PubMed]
- McMorrow, I.M.; Comrack, C.A.; Sachs, D.H.; DerSimonian, H. Heterogeneity of human anti-pig natural antibodies cross-reactive with the Gal[α1,3]Galactose epitope. Transplantation 1997, 64, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Parker, W.; Lin, S.S.; Yu, P.B.; Sood, A.; Nakamura, Y.C.; Song, A.; Platt, J.L. Naturally occurring anti-α-galactosyl antibodies: Relationship to xenoreactive anti- α-galactosyl antibodies. Glycobiology 1999, 9, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Galili, U.; Anaraki, F.; Thall, A.; Hill-Black, C.; Radic, M. One percent of circulating B lymphocytes are capable of producing the natural anti-Gal antibody. Blood 1993, 82, 2485–2493. [Google Scholar] [CrossRef]
- Wang, L.; Anaraki, F.; Henion, T.R.; Galili, U. Variations in activity of the human natural anti-Gal antibody in young and elderly populations. J. Gerontol. Med. Sci. 1995, 50A, M227–M233. [Google Scholar] [CrossRef]
- Galili, U.; Mandrell, R.E.; Hamadeh, R.M.; Shohet, S.B.; Griffiss, J.M. Interaction between human natural anti-α-galactosyl immunoglobulin G and bacteria of the human flora. Infect. Immun. 1988, 56, 1730–1737. [Google Scholar] [CrossRef]
- Posekany, K.J.; Pittman, H.K.; Bradfield, J.F.; Haisch, C.E.; Verbanac, K.M. Induction of cytolytic anti-Gal antibodies in α-1,3-galactosyltransferase gene knockout mice by oral inoculation with Escherichia coli O86:B7 bacteria. Infect. Immun. 2002, 70, 6215–6222. [Google Scholar] [CrossRef]
- Mañez, R.; Blanco, F.J.; Díaz, I.; Centeno, A.; Lopez-Pelaez, E.; Hermida, M.; Davies, H.F.; Katopodis, A. Removal of bowel aerobic gram-negative bacteria is more effective than immunosuppression with cyclophosphamide and steroids to decrease natural α-galactosyl IgG antibodies. Xenotransplantation 2001, 8, 15–23. [Google Scholar] [CrossRef]
- Galili, U.; Macher, B.A.; Buehler, J.; Shohet, S.B. Human natural anti-α-galactosyl IgG. II. The specific recognition of α[1,3]-linked galactose residues. J. Exp. Med. 1985, 162, 573–582. [Google Scholar] [CrossRef]
- Towbin, H.; Rosenfelder, G.; Wieslander, J.; Avila, J.L.; Rojas, M.; Szarfman, A.; Esser, K.; Nowack, H.; Timpl, R. Circulating antibodies to mouse laminin in Chagas disease, American cutaneous leishmaniasis, and normal individuals recognize terminal galactosyl [α1-3]-galactose epitopes. J. Exp. Med. 1987, 166, 419–432. [Google Scholar] [CrossRef]
- Teneberg, S.; Lönnroth, I.; Torres Lopez, J.F.; Galili, U.; Olwegard Halvarsson, M.; Angstrom, J.; Karlsson, K.A. Molecular mimicry in the recognition of glycosphingolipids by Galα3Galß4GlcNAcß-binding Clostridium difficile toxin A, human natural anti-α-galactosyl IgG and the monoclonal antibody Gal-13: Characterization of a binding-active human glycosphingolipid, non-identical with the animal receptor. Glycobiology 1996, 6, 599–609. [Google Scholar] [PubMed]
- Wang, L.; Radic, M.Z.; Galili, U. Human anti-Gal heavy chain genes: Preferential use of VH3 and the presence of somatic mutations. J. Immunol. 1995, 155, 1276–1285. [Google Scholar] [PubMed]
- Galili, U.; Buehler, J.; Shohet, S.B.; Macher, B.A. The human natural anti-Gal IgG. III. The subtlety of immune tolerance in man as demonstrated by crossreactivity between natural anti-Gal and anti-B antibodies. J. Exp. Med. 1987, 165, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Galili, U.; Clark, M.R.; Shohet, S.B.; Buehler, J.; Macher, B.A. Evolutionary relationship between the anti-Gal antibody and the Galα1-3Gal epitope in primates. Proc. Natl. Acad. Sci. USA 1987, 84, 1369–1373. [Google Scholar] [CrossRef] [PubMed]
- Galili, U.; Shohet, S.B.; Kobrin, E.; Stults, C.L.M.; Macher, B.A. Man, apes, and Old-World monkeys differ from other mammals in the expression of α-galactosyl epitopes on nucleated cells. J. Biol. Chem. 1988, 263, 17755–17762. [Google Scholar] [PubMed]
- Basu, M.; Basu, S. Enzymatic synthesis of blood group related pentaglycosyl ceramide by an α-galactosyltransferase. J. Biol. Chem. 1973, 248, 1700–1706. [Google Scholar]
- Larsen, R.D.; Rivera-Marrero, C.A.; Ernst, L.K.; Cummings, R.D.; Lowe, J.B. Frameshift and nonsense mutations in a human genomic sequence homologous to a murine UDP-Gal:β-D-Gal[1,4]-D-GlcNAc α[1,3]-galactosyltransferase cDNA. J. Biol. Chem. 1990, 265, 7055–7061. [Google Scholar]
- Galili, U.; Swanson, K. Gene sequences suggest inactivation of α1-3 galactosyltransferase in catarrhines after the divergence of apes from monkeys. Proc. Natl. Acad. Sci. USA 1991, 88, 7401–7404. [Google Scholar] [CrossRef]
- Koike, C.; Fung, J.J.; Geller, D.A.; Kannagi, R.; Libert, T.; Luppi, P.; Nakashima, I.; Profozich, J.; Rudert, W.; Sharma, S.B.; et al. Molecular basis of evolutionary loss of the α1,3-galactosyltransferase gene in higher primates. J. Biol. Chem. 2002, 277, 10114–10120. [Google Scholar] [CrossRef]
- Lanteri, M.; Giordanengo, V.; Vidal, F.; Gaudray, P.; Lefebvre, J.C. A complete α1,3-galactosyltransferase gene is present in the human genome and partially transcribed. Glycobiology 2002, 12, 785–792. [Google Scholar] [CrossRef]
- Teranishi, K.; Mañez, R.; Awwad, M.; Cooper, D.K. Anti-Galα 1-3Gal IgM and IgG antibody levels in sera of humans and old world non-human primates. Xenotransplantation 2002, 9, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Good, A.H.; Cooper, D.C.K.; Malcolm, A.J.; Ippolito, R.M.; Koren, E.; Neethling, F.A.; Ye, Y.; Zuhdi, N.; Lamontagne, L.R. Identification of carbohydrate structures which bind human anti-porcine antibodies: Implication for discordant xenografting in man. Transplant. Proc. 1992, 24, 559–562. [Google Scholar] [PubMed]
- Galili, U. Interaction of the natural anti-Gal antibody with α-galactosyl epitopes: A major obstacle for xenotransplantation in humans. Immunol. Today 1993, 14, 480–482. [Google Scholar] [CrossRef]
- Cooper, D.K.C.; Good, A.H.; Koren, E.; Oriol, R.; Malcolm, A.J.; Ippolito, R.M.; Neethling, F.A.; Ye, Y.; Romano, E.; Zuhdi, N. Identification of α-galactosyl and other carbohydrate epitopes that are bound by human anti-pig antibodies: Relevance to discordant xenografting in man. Transpl. Immunol. 1993, 1, 198–205. [Google Scholar] [CrossRef]
- Sandrin, M.S.; Vaughan, H.A.; Dabkowski, P.L.; McKenzie, I.F.C. Anti-pig IgM antibodies in human serum react predominantly with Gal (αl-3)Gal epitopes. Proc. Natl. Acad. Sci. USA 1993, 90, 11391–11395. [Google Scholar] [CrossRef] [PubMed]
- Collins, B.H.; Cotterell, A.H.; McCurry, K.R.; Alvarado, C.G.; Magee, J.C.; Parker, W.; Platt, J.L. Cardiac xenografts between primate species provide evidence for the importance of the α-galactosyl. determinant in hyperacute rejection. J. Immunol. 1995, 154, 5500–5510. [Google Scholar]
- Xu, Y.; Lorf, T.; Sablinski, T.; Gianello, P.; Bailin, M.; Monroy, R.; Kozlowski, T.; Awwad, M.; Cooper, D.K.; Sachs, D.H. Removal of anti-porcine natural antibodies from human and nonhuman primate plasma in vitro and in-vivo by a Galα1-3Galβ1-4βGlc-X immunoaffinity column. Transplantation 1998, 65, 172–179. [Google Scholar] [CrossRef]
- Shaw, L.; Schauer, R. The biosynthesis of N-glycoloylneuraminic acid occurs by hydroxylation of the CMP-glycoside of N-acetylneuraminic acid. Biol. Chem. Hoppe. Seyler 1988, 369, 477–486. [Google Scholar] [CrossRef]
- Muchmore, E.A.; Diaz, S.; Varki, A. A structural difference between the cell surfaces of humans and the great apes. Am. J. Phys. Anthropol. 1998, 107, 187–198. [Google Scholar] [CrossRef]
- Gagneux, P.; Varki, A. Evolutionary considerations in relating oligosaccharide diversity to biological function. Glycobiology 1999, 9, 747–755. [Google Scholar] [CrossRef]
- Varki, A. Colloquium paper: Uniquely human evolution of sialic acid genetics and biology. Proc. Natl. Acad. Sci. USA 2010, 107 (Suppl. S2), 8939–8946. [Google Scholar] [CrossRef]
- Chou, H.H.; Takematsu, H.; Diaz, S.; Iber, J.; Nickerson, E.; Wright, K.L.; Muchmore, E.A.; Nelson, D.L.; Warren, S.T.; Varki, A. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc. Natl. Acad. Sci. USA 1998, 95, 11751–11756. [Google Scholar] [CrossRef] [PubMed]
- Irie, A.; Koyama, S.; Kozutsumi, Y.; Kawasaki, T.; Suzuki, A. The molecular basis for the absence of N-glycolylneuraminic acid in humans. J. Biol. Chem. 1998, 273, 15866–15871. [Google Scholar] [CrossRef] [PubMed]
- Merrick, J.M.; Zadarlik, K.; Milgrom, F. Characterization of the Hanganutziu-Deicher [serum-sickness] antigen as gangliosides containing N-glycolylneuraminic acid. Int. Arch. Allergy Appl. Immunol. 1978, 57, 477–480. [Google Scholar] [CrossRef]
- Zhu, A.; Hurst, R. Anti-N-glycolylneuraminic acid antibodies identified in healthy human serum. Xenotransplantation 2002, 9, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Padler-Karavani, V.; Yu, H.; Cao, H.; Chokhawala, H.; Karp, F.; Varki, N.; Chen, X.; Varki, A. Diversity in specificity, abundance, and composition of anti-Neu5Gc antibodies in normal humans: Potential implications for disease. Glycobiology 2008, 18, 818–830. [Google Scholar] [CrossRef]
- Tahara, H.; Ide, K.; Basnet, N.B.; Tanaka, Y.; Matsuda, H.; Takematsu, H.; Kozutsumi, Y.; Ohdan, O. Immunological property of antibodies against N-glycolylneuraminic acid epitopes in cytidine monophospho-N-acetylneuraminic acid hydroxylase-deficient mice. J. Immunol. 2010, 184, 3269–3275. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.H.; Tangvoranuntakul, P.; Varki, A. Effects of natural human antibodies against a nonhuman sialic acid that metabolically incorporates into activated and malignant immune cells. J. Immunol. 2005, 175, 228–236. [Google Scholar] [CrossRef]
- Padler-Karavani, V.; Varki, A. Potential impact of the non-human sialic acid N-glycolylneuraminic acid on transplant rejection risk. Xenotransplantation 2011, 18, 1–5. [Google Scholar] [CrossRef]
- Young, W.W.; Hakomori, S.I.; Levine, P. Characterization of anti-Forssman [anti-Fs] antibodies in human sera: Their specificity and possible changes in patients with cancer. J. Immunol. 1979, 123, 92–96. [Google Scholar]
- Kijimoto-Ochiai, S.; Takahashi, W.; Makita, A. Anti-Forssman antibody in human sera: Properties and decreased level in cancer patients. Jpn. J. Exp. Med. 1981, 51, 149–155. [Google Scholar] [PubMed]
- Siddiqui, B.; Hakomori, S. A revised structure for the Forssman glycolipid hapten. J. Biol. Chem. 1971, 246, 5766–5769. [Google Scholar] [PubMed]
- Das, K.K.; Basu, M.; Basu, S.; Evans, C.H. Biosynthesis in vitro of a globoside containing a 2-acetamido-2-deoxy-beta-D-galactopyranosyl group [1-.3]- linked and Forssman glycolipid by two N-acetylgalactosaminyltransferases from chemically transformed guinea pig cells. Carbohydr. Res. 1986, 149, 119–135. [Google Scholar] [CrossRef]
- Haslam, D.B.; Baenzige, J.U. Expression cloning of Forssman glycolipid synthetase: A novel member of the histo-blood group ABO gene family. Proc. Natl. Acad. Sci. USA 1996, 93, 10697–10702. [Google Scholar] [CrossRef]
- Narasimhan, R.; Murray, R.K. Comparative study of the glycosphingolipids of chicken bursa of Fabricius and of chicken, rat and human thymus. Biochem. J. 1978, 173, 475–482. [Google Scholar] [CrossRef]
- Breimer, M.E.; Hansson, G.C.; Karlsson, K.A.; Leffler, H. Blood group type glycosphingolipids from the small intestine of different animals analyzed by mass spectrometry and thin-layer chromatography. A note on species diversity. J. Biochem. 1981, 90, 589–609. [Google Scholar] [CrossRef]
- Yamamoto, M.; Cid, E.; Yamamoto, F. Molecular genetic basis of the human Forssman glycolipid antigen negativity. Sci. Rep. 2012, 2, 975. [Google Scholar] [CrossRef]
- Jesus, C.; Hesse, C.; Rocha, C.; Osório, N.; Valado, A.; Caseiro, A.; Gabriel, A.; Svensson, L.; Moslemi, A.-L.; Abu Siba, W.; et al. Prevalence of antibodies to a new histo-blood system: The FORS system. Blood Transfus. 2018, 16, 178–183. [Google Scholar]
- Rini, J.M.; Esko, J.D. Glycosyltransferases and Glycan-Processing Enzymes [Chapter 6]. In Essentials of Glycobiology [Internet], 3rd ed.; Varki, A., Cummings, R.D., Esko, J.D., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK310274/ (accessed on 15 March 2019).
- Repik, P.M.; Strizki, M.; Galili, U. Differential host dependent expression of α-galactosyl epitopes on viral glycoproteins: A study of Eastern equine encephalitis virus as a model. J. Gen. Virol. 1994, 75, 1177–1181. [Google Scholar] [CrossRef]
- Galili, U.; Repik, P.M.; Anaraki, F.; Mozdzanowska, K.; Washko, G.; Gerhard, W. Enhancement of antigen presentation of influenza virus hemagglutinin by the natural anti-Gal antibody. Vaccine 1996, 14, 321–328. [Google Scholar] [CrossRef]
- Pipperger, L.; Koske, I.; Wild, N.; Müllauer, B.; Krenn, D.; Stoiber, H.; Wollmann, G.; Kimpel, J.; von Laer, D.; Bánki, Z. Xenoantigen-dependent complement-mediated neutralization of LCMV glycoprotein pseudotyped VSV in human serum. J. Virol. 2019, 93, e00567-19. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.F.; Larsen, R.D.; Mattox, S.; Lowe, J.B.; Cummings, R.D. Transfer and expression of a murine UDP-Gal:β-D-Gal-α1,3-galactosyltransferase gene in transfected Chinese hamster ovary cells. Competition reactions between the α1,3-galactosyltransferase and the endogenous α2,3-sialyltransferase. J. Biol. Chem. 1990, 265, 6225–6234. [Google Scholar] [PubMed]
- Taatjes, D.J.; Roth, J.; Weinstein, J.; Paulson, J.C.; Shaper, N.L.; Shaper, J.H. Distribution of galactosyl- and sialyltransferase: Reorganization of trans Golgi apparatus elements in hepatocytes in intact liver and cell culture. Eur. J. Cell Biol. 1987, 44, 187–194. [Google Scholar] [PubMed]
- Henion, T.R.; Gerhard, W.; Anaraki, F.; Galili, U. Synthesis of α-gal epitopes on influenza virus vaccines, by recombinant α1,3galactosyltransferase, enables the formation of immune complexes with the natural anti-Gal antibody. Vaccine 1997, 15, 1174–1182. [Google Scholar] [CrossRef]
- Rother, R.P.; Fodor, W.L.; Springhorn, J.P.; Birks, C.W.; Setter, E.; Sandrin, M.S.; Squinto, S.P.; Rollins, S.A. A novel mechanism of retrovirus inactivation in human serum mediated by anti-α-galactosyl natural antibody. J. Exp. Med. 1995, 182, 1345–1355. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Porter, C.D.; Strahan, K.M.; Preece, A.F.; Gustafsson, K.; Cosset, F.L.; Weiss, R.A.; Collins, M.K. Sensitization of cells and retroviruses to human serum by [α1-3] galactosyltransferase. Nature 1996, 379, 85–88. [Google Scholar] [CrossRef]
- Patience, C.; Takeuchi, Y.; Weiss, R.A. Infection of human cells by an endogenous retrovirus of pigs. Nat. Med. 1997, 3, 282–286. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Liong, S.H.; Bieniasz, P.D.; Jäger, U.; Porter, C.D.; Friedman, T.; McClure, M.O.; Weiss, R.A. Sensitization of rhabdo-, lenti-, and spumaviruses to human serum by galactosyl(α1-3)galactosylation. J. Virol. 1997, 71, 6174–6178. [Google Scholar] [CrossRef]
- Welsh, R.M.; O’Donnell, C.L.; Reed, D.J.; Rother, R.P. Evaluation of the Galα1-3Gal epitope as a host modification factor eliciting natural humoral immunity to enveloped viruses. J. Virol. 1998, 72, 4650–4656. [Google Scholar] [CrossRef]
- Hayashi, S.; Ogawa, S.; Takashima, Y.; Otsuka, H. The neutralization of pseudorabies virus by anti-α-galactocyl natural antibody in normal serum. Virus Res. 2004, 99, 1–7. [Google Scholar] [CrossRef]
- Preece, A.F.; Strahan, K.M.; Devitt, J.; Yamamoto, F.; Gustafsson, K. Expression of ABO or related antigenic carbohydrates on viral envelopes leads to neutralization in the presence of serum containing specific natural antibodies and complement. Blood 2002, 99, 2477–2482. [Google Scholar] [CrossRef] [PubMed]
- Dürrbach, A.; Baple, E.; Preece, A.F.; Charpentier, B.; Gustafsson, K. Virus recognition by specific natural antibodies and complement results in MHC I cross-presentation. J. Immunol. 2007, 37, 1254–1265. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.Y.; Jung, W.W.; Oh, Y.K.; Chun, T.; Park, H.Y.; Lee, H.T.; Han, I.K.; Yang, J.M.; Kim, Y.B. Natural protection from zoonosis by α-gal epitopes on virus particles in xenotransmission. Xenotransplantation 2007, 14, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Cheng, G.; Chui, C.H.; Lau, F.Y. ABO blood group and susceptibility to severe acute respiratory syndrome. JAMA 2005, 293, 1450–1451. [Google Scholar] [PubMed]
- Guillon, P.; Clement, M.; Sebille, V.; Rivain, J.-G.; Chou, C.-F.; Ruvoen-Clouet, N.; Le Pendu, J. Inhibition of the interaction between the SARS-CoV spike protein and its cellular receptor by anti-histo-blood group antibodies. Glycobiology 2008, 18, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Paradis, K.; Langford, G.; Long, Z.; Heneine, W.; Sandstrom, P.; Switzer, W.M.; Chapman, L.E.; Lockey, C.; Onions, D.; Otto, E. Search for cross-species transmission of porcine endogenous retrovirus in patients treated with living pig tissue. The XEN 111 Study Group. Science 1999, 285, 1236–1241. [Google Scholar] [CrossRef]
- Houston, W.E.; Kremer, R.J.; Crabbs, C.L.; Spertzel, R.O. Inactivated Venezuelan equine encephalomyelitis virus vaccine complexed with specific antibody: Enhanced primary immune response and altered pattern of antibody class elicited. J. Infect. Dis. 1977, 135, 600–610. [Google Scholar] [CrossRef]
- Villinger, F.; Mayne, A.E.; Bostik, P.; Mori, K.; Jensen, P.E.; Ahmed, R. Evidence for antibody-mediated enhancement of simian immunodeficiency virus [SIV] Gag antigen processing and cross presentation in SIV-infected rhesus macaques. J. Virol. 2003, 77, 10–24. [Google Scholar] [CrossRef]
- Wen, Y.M.; Qu, D.; Zhou, S.H. Antigen-antibody complex as therapeutic vaccine for viral hepatitis B. Int. Rev. Immunol. 1999, 18, 251–258. [Google Scholar] [CrossRef]
- Abdel-Motal, U.M.; Wigglesworth, K.; Galili, U. Mechanism for increased immunogenicity of vaccines that form in-vivo immune complexes with the natural anti-Gal antibody. Vaccine 2009, 27, 3072–3082. [Google Scholar] [CrossRef]
- Abdel-Motal, U.M.; Guay, H.M.; Wigglesworth, K.; Welsh, R.M.; Galili, U. Increased immunogenicity of influenza virus vaccine by anti-Gal mediated targeting to antigen presenting cells. J. Virol. 2007, 81, 9131–9141. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Motal, U.M.; Wang, S.; Lu, S.; Wigglesworth, K.; Galili, U. Increased Immunogenicity of Human Immunodeficiency Virus gp120 Engineered to Express Galα1-3Galβ1-4GlcNAc-R Epitopes. J. Virol. 2006, 80, 6943–6951. [Google Scholar] [CrossRef]
- Abdel-Motal, U.M.; Wang, S.; Awwad, S.; Lu, S.; Wigglesworth, K.; Galili, U. Increased immunogenicity of HIV-1 p24 and gp120 following immunization with gp120/p24 fusion protein vaccine expressing α-gal epitopes. Vaccine 2010, 28, 1758–1765. [Google Scholar] [CrossRef] [PubMed]
- Benatuil, L.; Kaye, J.; Rich, R.F.; Fishman, J.A.; Green, W.R.; Iacomini, J. The influence of natural antibody specificity on antigen immunogenicity. Eur. J. Immunol. 2005, 35, 2638–2647. [Google Scholar] [CrossRef] [PubMed]
- Galili, U.; Wigglesworth, K.; Abdel-Motal, U.M. Intratumoral injection of α-gal glycolipids induces xenograft-like destruction and conversion of lesions into endogenous vaccines. J. Immunol. 2007, 178, 4676–4687. [Google Scholar] [CrossRef]
- Buonomano, R.; Tinguely, C.; Rieben, R.; Mohacsi, P.J.; Nydegger, U.E. Quantitation and characterization of anti-Galα1-3Gal antibodies in sera of 200 healthy persons. Xenotransplantation 1999, 6, 173–180. [Google Scholar] [CrossRef]
- Maruyama, S.; Cantu, E., 3rd; DeMartino, C.; Wang, C.Y.; Chen, J.; Al-Mohanna, F.; Nakeeb, S.M.; D’Agati, V.; Pernis, B.; Galili, U.; et al. Interaction of baboon anti-α-galactosyl antibody with pig tissues. Am. J. Pathol. 1999, 155, 1635–1649. [Google Scholar] [CrossRef]
- Galili, U. The Natural Anti-Gal Antibody as Foe Turned Friend in Medicine; Elsevier, Academic Press: London, UK, 2018; p. 14. [Google Scholar]
- Galili, U.; Tibell, A.; Samuelsson, B.; Rydberg, L.; Groth, C.G. Increased anti-Gal activity in diabetic patients transplanted with fetal porcine islet cell clusters. Transplantation 1995, 59, 1549–1556. [Google Scholar] [CrossRef]
- Almeida, I.C.; Milani, S.R.; Groin, P.A.J.; Travassos, L.R. Complement-mediated lysis of Trypanosoma cruzi trypomastigotes by human anti-α-galactosyl antibodies. J. Immunol. 1991, 146, 2394–2400. [Google Scholar]
- Almeida, I.C.; Ferguson, M.A.; Schenkman, S.; Travassos, L.R. Lytic anti-α-galactosyl antibodies from patients with chronic Chagas’ disease recognize novel O-linked oligosaccharides on mucin-like glycosyl-phosphatidylinositol-anchored glycoproteins of Trypanosoma cruzi. Biochem. J. 1994, 304, 793–802. [Google Scholar] [CrossRef]
- Ramasamy, R.; Reese, R.T. Terminal galactose residues and the antigenicity of Plasmodium falciparum glycoproteins. J. Clin. Microbiol. 1987, 25, 2075–2079. [Google Scholar] [CrossRef]
- Ravindran, B.; Satapathy, A.K.; Das, M.K. Naturally-occurring anti-α-galactosyl antibodies in human Plasmodium falciparum infections: A possible role for autoantibodies in malaria. Immunol. Lett. 1988, 19, 137–141. [Google Scholar] [CrossRef]
- Ramasamy, R.; Rajakaruna, R. Association of malaria with inactivation of α1,3-galactosyl transferase in catarrhines. Biochim. Biophys. Acta 1997, 1360, 241–246. [Google Scholar] [CrossRef]
- Yilmaz, B.; Portugal, S.; Tran, T.M.; Gozzelino, R.; Ramos, S.; Gomes, J.; Regalado, A.; Cowan, P.J.; d’Apice, A.J.F.; Chong, A.S.; et al. Gut microbiota elicits a protective immune response against malaria transmission. Cell 2014, 159, 1277–1289. [Google Scholar] [CrossRef]
- Cabezas-Cruz, A.; Mateos-Hernández, L.; Alberdi, P.; Villar, M.; Riveau, G.; Hermann, E. Effect of blood type on anti-α-Gal immunity and the incidence of infectious diseases. Exp. Mol. Med. 2017, 10, e301. [Google Scholar] [CrossRef] [PubMed]
- Groth, C.G.; Korsgren, O.; Tibell, A.; Tollerman, J.; Möller, E.; Bolinder, J.; Ostman, J.; Reinholt, F.P.; Hellerström, C.; Andersson, A. Transplantation of fetal porcine pancreas to diabetic patients: Biochemical and histological evidence for graft survival. Lancet 1994, 344, 1402–1404. [Google Scholar] [CrossRef]
- Whalen, G.F.; Sullivan, M.; Piperdi, B.; Wasseff, W.; Galili, U. Cancer Immunotherapy by intratumoral injection of α-gal glycolipids. Anticancer Res. 2012, 32, 3861–3868. [Google Scholar]
- Konakci, K.Z.; Bohle, B.; Blumer, R.; Hoetzenecker, W.; Roth, G.; Moser, B.; Boltz-Nitulescu, G.; Gorlitzer, M.; Klepetko, W.; Wolner, E.; et al. α-gal on bioprostheses: Xenograft immune response in cardiac surgery. Eur. J. Clin. Investig. 2005, 35, 17–23. [Google Scholar] [CrossRef]
- Böer, U.; Buettner, F.F.R.; Schridde, A.; Klingenberg, M.; Sarikouch, S.; Haverich, A.; Wilhelmi, M. Antibody formation towards porcine tissue in patients implanted with crosslinked heart valves is directed to antigenic tissue proteins and αGal epitopes and is reduced in healthy vegetarian subjects. Xenotransplantation 2017, 24. [Google Scholar] [CrossRef]
- Stone, K.R.; Abdel-Motal, U.; Walgenbach, A.W.; Turek, T.J.; Galili, U. Replacement of human anterior cruciate ligament with pig ligament: A model for anti-non gal antibody response in long-term xenotransplantation. Transplantation 2007, 83, 211–219. [Google Scholar] [CrossRef]
- Feldmann, H.; Nichol, S.T.; Klenk, H.D.; Peters, C.J.; Sanchez, A. Characterization of filoviruses based on differences in structure and antigenicity of the virion glycoprotein. Virology 1994, 199, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Commins, S.P.; Platts-Mills, T.A. Tick bites and red meat allergy. Curr. Opin. Allergy Clin. Immunol. 2013, 13, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.; Schuyler, A.J.; Workman, L.; Gupta, M.; James, H.R.; Posthumus, J.; McGowan, E.C.; Commins, S.P.; Platts-Mills, T.A.E. Investigation into the α-Gal Syndrome: Characteristics of 261 Children and Adults Reporting Red Meat Allergy. J. Allergy. Clin. Immunol. Pract. 2019, 7, 2348–2358.e4. [Google Scholar] [CrossRef] [PubMed]
- Steiper, M.E.; Young, N.M.; Sukarna, T.Y. Genomic data support the hominoid slowdown and an early Oligocene estimate for the hominoid-cercopithecoid divergence. Proc. Natl. Acad. Sci. USA 2004, 101, 17021–17026. [Google Scholar] [CrossRef]
- Schrago, C.G.; Mello, B.; Soares, A.E. Combining fossil and molecular data to date the diversification of New World primates. J. Evol. Biol. 2013, 26, 2438–2446. [Google Scholar] [CrossRef]
- Larsen, R.D.; Rajan, V.P.; Ruff, M.M.; Kukowska-Latallo, J.; Cummings, R.D.; Lowe, J.B. Isolation of a cDNA encoding murine UDP galactose: ßD-galactosyl-1,4-N-acetyl-D-glucosaminide α1,3-galactosyltransferase: Expression cloning by gene transfer. Proc. Natl. Acad. Sci. USA 1989, 86, 8227–8231. [Google Scholar] [CrossRef]
- Joziasse, D.H.; Shaper, J.H.; Van den Eijnden, D.H.; Van Tunen, A.H.; Shaper, N.L. Bovine α1-3galactosyltransferase: Isolation and characterization of a cDNA clone. Identification of homologous sequences in human genomic DNA. J. Biol. Chem. 1989, 264, 14290–14297. [Google Scholar]
- Henion, T.R.; Macher, B.A.; Anaraki, F.; Galili, U. Defining the minimal size of catalytically active primate α1,3galactosyltransferase: Structure function studies on the recombinant truncated enzyme. Glycobiology 1994, 4, 193–201. [Google Scholar] [CrossRef]
- Miller, E.R.; Benefit, B.R.; McCrossin, M.L.; Plavcan, J.M.; Leakey, M.G.; El-Barkooky, A.N.; Hamdan, M.A.; Abdel Gawad, M.K.; Hassan, S.M.; Simons, E.L. Systematics of early and middle Miocene Old World monkeys. J. Hum. Evol. 2009, 57, 195–211. [Google Scholar] [CrossRef]
- Lewis, H. Catastrophic selection as a factor in speciation. Evolution 1962, 16, 257–271. [Google Scholar] [CrossRef]
- Lai, L.; Kolber-Simonds, D.; Park, K.W.; Cheong, H.T.; Greenstein, J.L.; Im, G.S.; Samuel, M.; Bonk, A.; Rieke, A.; Day, B.N.; et al. Production of α-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science 2002, 295, 1089–1092. [Google Scholar] [CrossRef] [PubMed]
- Phelps, C.J.; Koike, C.; Vaught, T.D.; Boone, J.; Wells, K.D.; Chen, S.H.; Ball, S.; Specht, S.M.; Polejaeva, I.A.; Monahan, J.A.; et al. Production of α1,3-galactosyltransferase-deficient pigs. Science 2003, 299, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Dor, F.J.; Tseng, Y.L.; Cheng, J.; Moran, K.; Sanderson, T.M.; Lancos, C.J.; Shimizu, A.; Yamada, K.; Awwad, A.; Sachs, D.H.; et al. α1,3-Galactosyltransferase gene-knockout miniature swine produce natural cytotoxic anti-Gal antibodies. Transplantation 2004, 78, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Walters, A.; Hara, H.; Long, C.; Yeh, P.; Ayares, D.; Cooper, D.K.; Bianchi, J. Anti-gal antibodies in α1,3-galactosyltransferase gene knockout pigs. Xenotransplantation 2012, 19, 305–310. [Google Scholar] [CrossRef]
- Galili, U. α1,3Galactosyltransferase knockout pigs produce the natural anti-Gal antibody and simulate the evolutionary appearance of this antibody in primates. Xenotransplantation 2013, 20, 267–276. [Google Scholar] [CrossRef]
- Bhende, Y.M.; Deshpande, C.K.; Bhatia, H.M.; Sanger, R.; Race, R.R.; Morgan, W.T.; Watkins, W.M. A new blood group character related to the ABO system. Lancet 1952, 1, 903–904. [Google Scholar]
- Bhatia, H.M.; Sathe, M.S. Incidence of Bombay Oh phenotype and weaker variants of A and B antigens in Bombay (India). Vox Sang. 1974, 27, 524–532. [Google Scholar] [CrossRef]
- Gerard, G.; Vitrac, D.; Le Pendu, J.; Muller, A.; Oriol, R. H-deficient blood groups (Bombay) of Réunion Island. Am. J. Hum. Genet. 1982, 34, 937–947. [Google Scholar]
- Mulet, C.; Cartron, J.P.; Badet, J.; Salmon, C. Activity of 2-α-Lfucosyltransferase in human sera and red cell membranes. Study of common ABH blood donors, rare ‘Bombay’ and ‘Parabombay’ individuals. FEBS Lett. 1977, 84, 74–78. [Google Scholar] [CrossRef]
- Le Pendu, J.; Clamagirand-Mulet, C.; Cartron, J.P.; Gerard, G.; Vitrac, D.; Oriol, R. H-deficient blood groups of Reunion Island. III. α-2-Lfucosyltransferase activity in sera of homozygous and heterozygous individuals. Am. J. Hum. Genet. 1983, 35, 497–507. [Google Scholar]
- Balgir, R.S. Detection of a rare blood group “Bombay (Oh) Phenotype” among the Kutia Kondh primitive tribe of Orissa, India. Int. J. Hum. Genet. 2005, 5, 193–198. [Google Scholar] [CrossRef]
- Altman, M.O.; Gagneux, P. Absence of Neu5Gc and Presence of Anti-Neu5Gc Antibodies in Humans-An Evolutionary Perspective. Front. Immunol. 2019, 10, 789. [Google Scholar] [CrossRef] [PubMed]
- Gerl, M.J.; Sampaio, J.L.; Urban, S.; Kalvodova, L.; Verbavatz, J.M.; Binnington, B.; Lindemann, D.; Lingwood, C.A.; Shevchenko, A.; Schroeder, C.; et al. Quantitative analysis of the lipidomes of the influenza virus envelope and MDCK cell apical membrane. J. Cell. Biol. 2012, 196, 213–221. [Google Scholar] [CrossRef]
- Campbell, C.T.; Gulley, J.; Oyelaran, O.; Hodge, J.W.; Schlom, J.; Gildersleeve, J.C. Humoral response to a viral glycan correlates with survival on PROSTVAC-VF. Proc. Natl. Acad. Sci. USA 2014, 111, E1749–E1758. [Google Scholar] [CrossRef]
- Yeh, P.; Ezzelarab, M.; Bovin, N.; Hara, H.; Long, C.; Tomiyama, K.; Sun, F.; Ayares, D.; Awwad, M.; Cooper, D.K. Investigation of potential carbohydrate antigen targets for human and baboon antibodies. Xenotransplantation 2010, 17, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Briles, E.B.; Tomasz, A. Physiological studies on the pneumococcal Forssman antigen: A choline-containing lipoteichoic acid. J. Gen. Microbiol. 1975, 86, 267–274. [Google Scholar] [CrossRef][Green Version]
- Ostrander, G.K.; Levery, S.B.; Hakomori, S.; Holmes, E.H. Isolation and characterization of the major acidic glycosphingolipids from the liver of the English sole (Parophrys vetulus). Presence of a novel ganglioside with a Forssman antigen determinant. J. Biol. Chem. 1988, 263, 3103–3110. [Google Scholar]
- Bouhours, D.; Liaigre, J.; Richard, C.; Oriol, R.; Bouhours, J.F. Forssman penta- and tetraglycosylceramide are xenoantigens of ostrich kidney and liver. Glycobiology 1999, 9, 875–886. [Google Scholar] [CrossRef]
- Kawsar, S.M.; Matsumoto, R.; Fujii, Y.; Yasumitsu, H.; Uchiyama, H.; Hosono, M.; Nitta, K.; Hamako, J.; Matsui, T.; Kojima, N.; et al. Glycan-binding profile and cell adhesion activity of American bullfrog [Rana catesbeiana] oocyte galectin-1. Protein Pept. Lett. 2009, 16, 677–684. [Google Scholar] [CrossRef]
- Larsen, R.D.; Ernst, L.K.; Nair, R.P.; Lowe, J.B. Molecular cloning, sequence, and expression of a human GDP-L-fucose:β-Dgalactoside 2-α-L-fucosyltransferase cDNA that can form the H blood group antigen. Proc. Natl. Acad. Sci. USA 1990, 87, 6674–6678. [Google Scholar] [CrossRef]
- Kelly, R.J.; Ernst, L.K.; Larsen, R.D.; Bryant, J.G.; Robinson, J.S.; Lowe, J.B. Molecular basis for H blood group deficiency in Bombay (Oh) and para-Bombay individuals. Proc. Natl. Acad. Sci. USA 1994, 91, 5843–5847. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, M.; Nishihara, S.; Shinya, N.; Kudo, T.; Iwasaki, H.; Seno, T. Wide variety of point mutations in the H gene of Bombay and para-Bombay individuals that inactivate H enzyme. Blood 1997, 90, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Koda, Y.; Soejima, M.; Johnson, P.H.; Smart, E.; Kimura, H. Missense mutation of FUT1 and deletion of FUT2 are responsible for Indian Bombay phenotype of ABO blood group system. Biochem. Biophys. Res. Commun. 1997, 238, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Wagner, F.F.; Flegel, W.A. Polymorphism of the h allele and the population frequency of sporadic nonfunctional alleles. Transfusion 1997, 37, 284–290. [Google Scholar] [CrossRef]
- Oriol, R.; Candelier, J.J.; Mollicone, R. Molecular genetics of H. Vox Sang. 2000, 78, 105–108. [Google Scholar]
- Storry, J.R.; Johannesson, J.S.; Poole, J.; Strindberg, J.; Rodrigues, M.J.; Yahalom, V.; Levene, C.; Fujita, C.; Castilho, L.; Hustinx, H.; et al. Identification of six new alleles at the FUT1 and FUT2 loci in ethnically diverse individual s with Bombay and ParaBombay phenotypes. Transfusion 2006, 46, 2149–2155. [Google Scholar] [CrossRef]
- Dipta, T.F.; Hossain, A.Z. The Bombay blood group: Are we out of risk? Mymensingh Med. J. 2011, 20, 536–540. [Google Scholar]
- Malhotra, S.; Dhawan, H.K.; Jain, A.; Sachdev, S.; Marwaha, N. Acute hemolytic transfusion reaction in a patient with Bombay phenotype: Implications for ABO grouping. Indian J. Hematol. Blood Transfus. 2014, 30, 108–110. [Google Scholar] [CrossRef]
- Agarwal, R.K.; Ankita, K.; Gowda, P.; Agarwal, S.; Sabnavis, A.G.; Panthangi, R.; Sedai, A.; Periyavan, S. Managing rare blood group requests—Bombay oh phenotype: Our experience with www.bombaybloodgroup.org. Blood Transfus. 2016, 14, 89–90. [Google Scholar]
- Seymour, R.M.; Allan, M.J.; Pomiankowski, A.; Gustafsson, K. Evolution of the human ABO polymorphism by two complementary selective pressures. Proc. Biol. Sci. 2004, 271, 1065–1072. [Google Scholar] [CrossRef]
- Neil, S.J.; McKnight, A.; Gustafsson, K.; Weiss, R.A. HIV-1 incorporates ABO histo-blood group antigens that sensitize virions to complement-mediated inactivation. Blood 2005, 105, 4693–4699. [Google Scholar] [CrossRef] [PubMed]
- Aho, K.; Pyhala, R.; Visakorpi, R. ABO associated genetic determinant in H1N1 influenza. Tissue Antigens 1980, 16, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Naikhin, A.N.; Katorgina, L.G.; Tsaritsyna, I.M.; Kim, T.N.; Reznik, V.N.; Trusov, N.V.; Denisov, G.M. Indicators of collective immunity to influenza depending on the blood group and sex of the population. Voprosy Virusologii 1989, 34, 419–423. [Google Scholar] [PubMed]

| Natural Ab | Carbohydrate Ag | Species Producing Ab | Species Synthesizing Ag |
|---|---|---|---|
| Anti-Gal | Galα1-3Galβ1-4GlcNAc-R (α-gal epitope) | Humans, apes, Old-World monkeys | Non-primate mammals, lemurs, New-World monkeys |
| Anti-Neu5Gc | Neu5Gc-R | Humans 1 | Apes, Old-World monkeys, most non-primate mammals |
| Anti-Forssman | GalNAcα1-3GalNAc-R (Forssman antigen) | Humans, monkeys, pig | Sheep, horse, dog, cat, mouse, hamster, guinea-pig, non-mammalian vertebrates |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galili, U. Human Natural Antibodies to Mammalian Carbohydrate Antigens as Unsung Heroes Protecting against Past, Present, and Future Viral Infections. Antibodies 2020, 9, 25. https://doi.org/10.3390/antib9020025
Galili U. Human Natural Antibodies to Mammalian Carbohydrate Antigens as Unsung Heroes Protecting against Past, Present, and Future Viral Infections. Antibodies. 2020; 9(2):25. https://doi.org/10.3390/antib9020025
Chicago/Turabian StyleGalili, Uri. 2020. "Human Natural Antibodies to Mammalian Carbohydrate Antigens as Unsung Heroes Protecting against Past, Present, and Future Viral Infections" Antibodies 9, no. 2: 25. https://doi.org/10.3390/antib9020025
APA StyleGalili, U. (2020). Human Natural Antibodies to Mammalian Carbohydrate Antigens as Unsung Heroes Protecting against Past, Present, and Future Viral Infections. Antibodies, 9(2), 25. https://doi.org/10.3390/antib9020025