Antibody Selection for Cancer Target Validation of FSH-Receptor in Immunohistochemical Settings
Abstract
:1. Introduction
2. Results
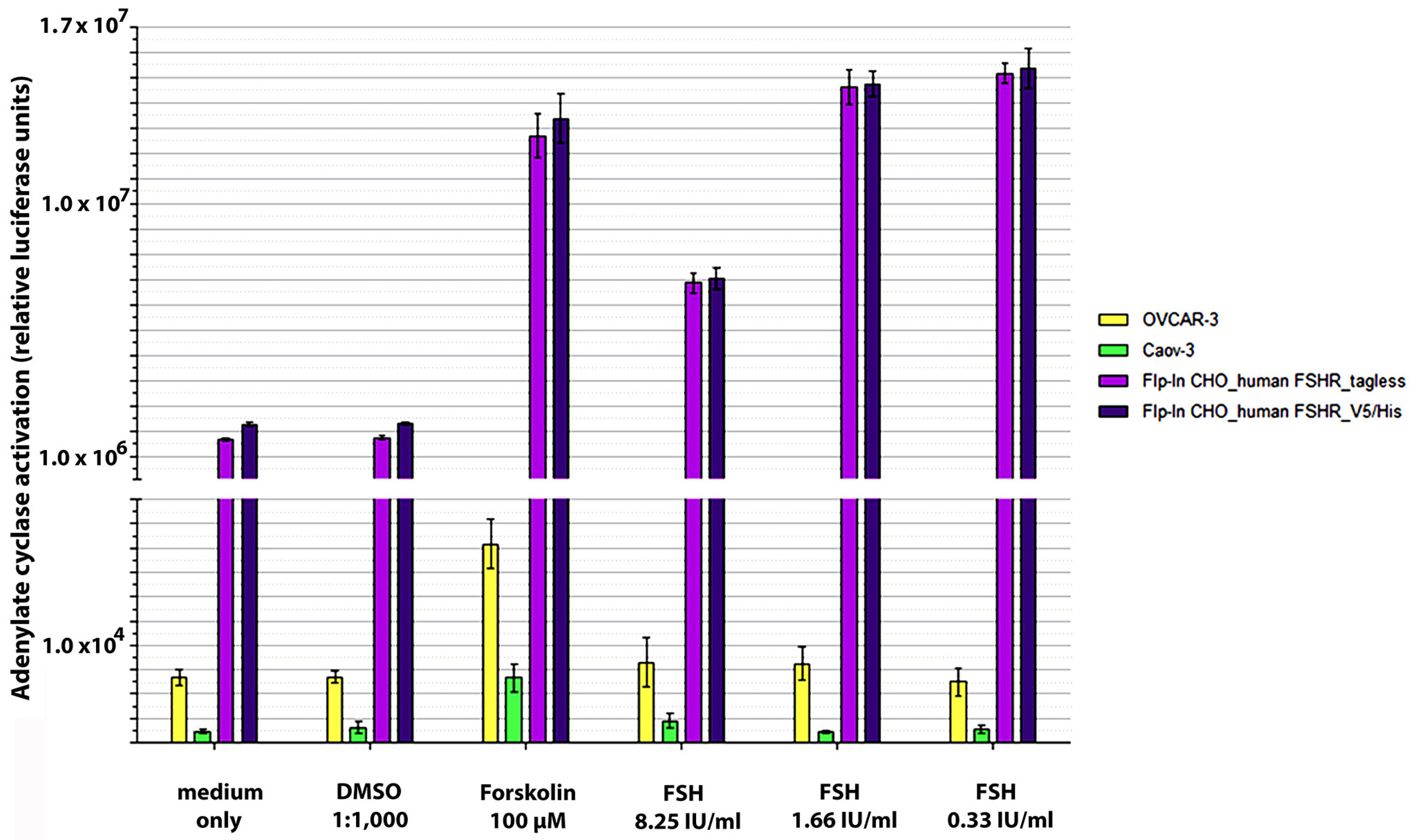
2.1. Generation of Cell Lines and VLPs for Antibody and FSHR Target Validation
2.2. Validation of Anti-FSHR Antibodies
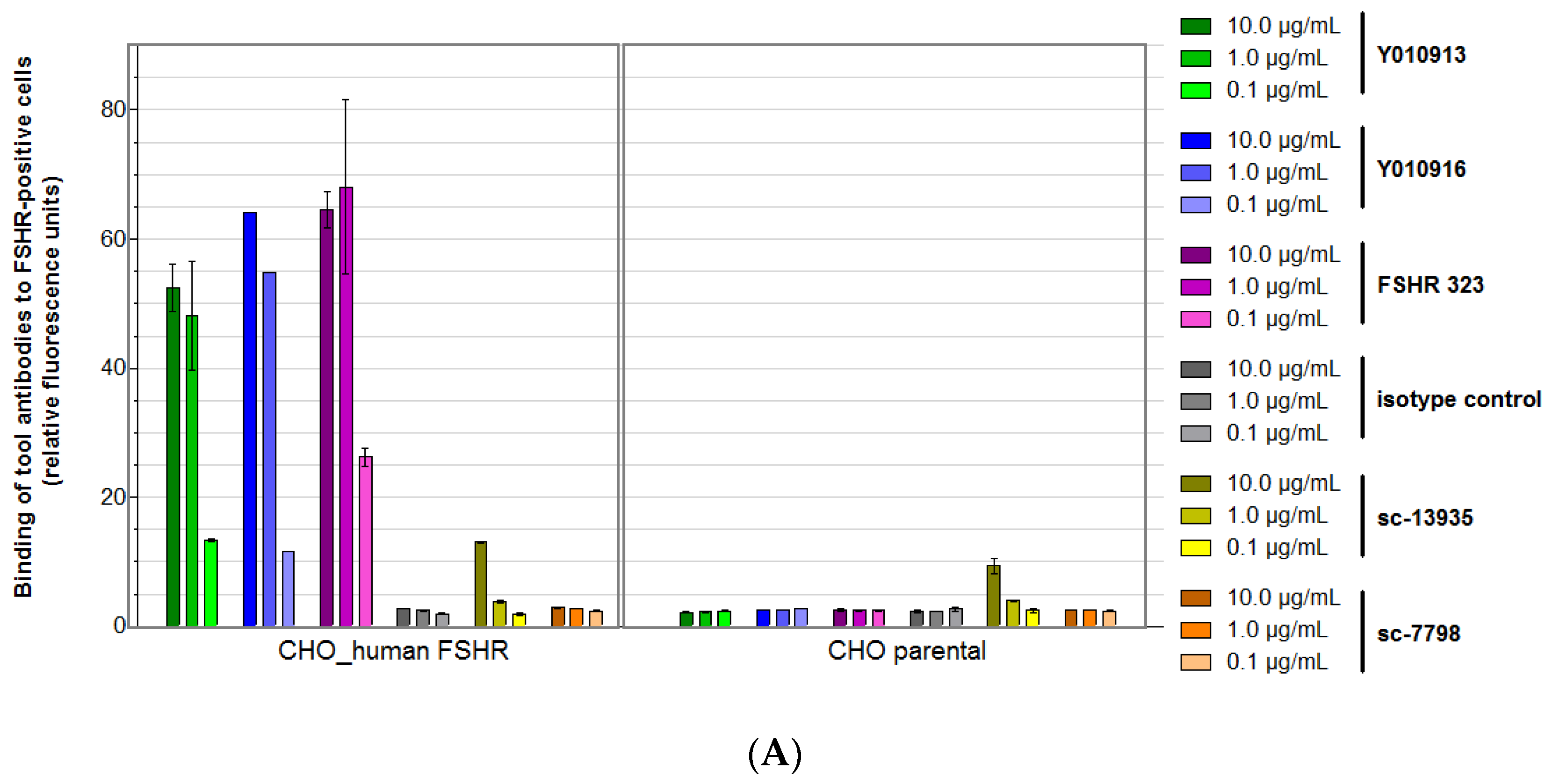
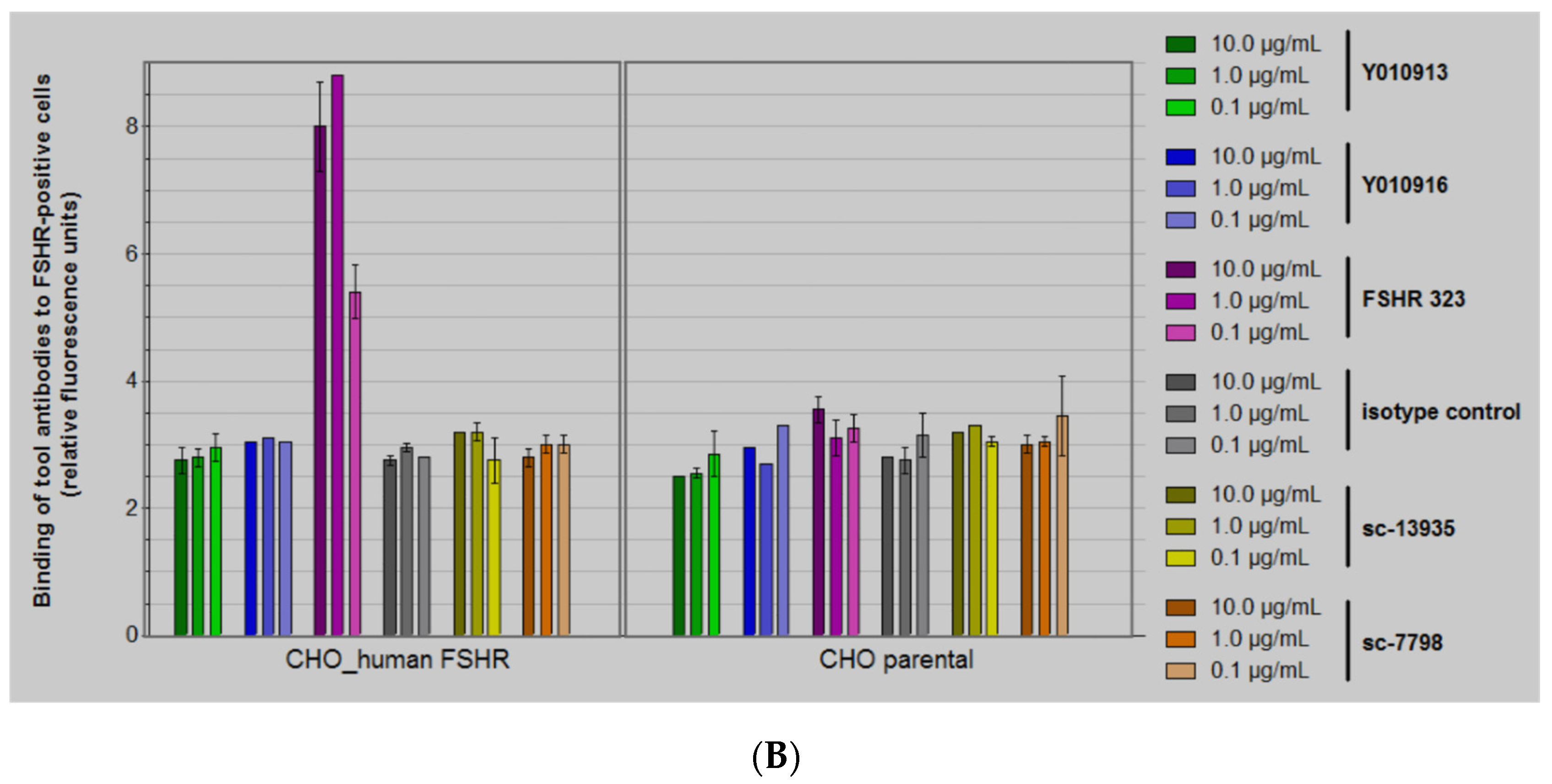
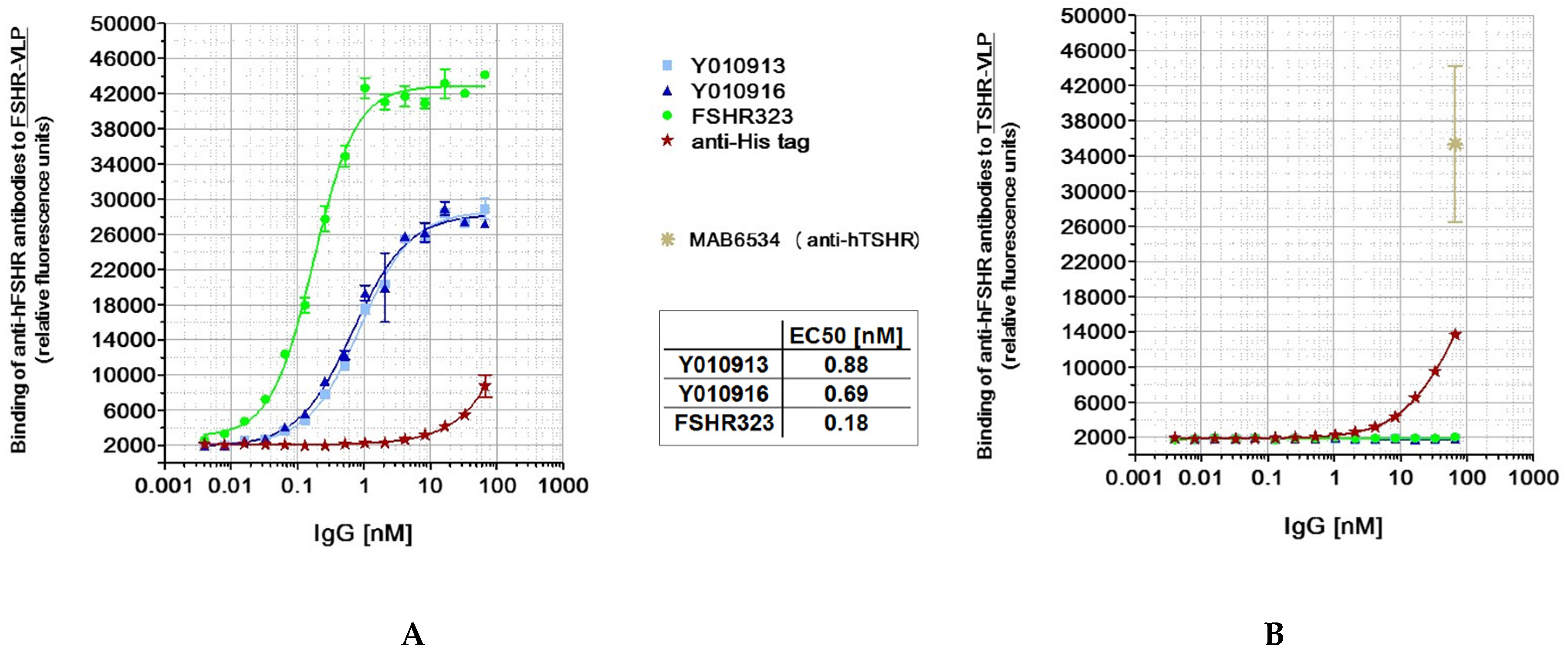
2.2.1. Cell Binding Experiments
2.2.2. Thorough Specificity Assessment
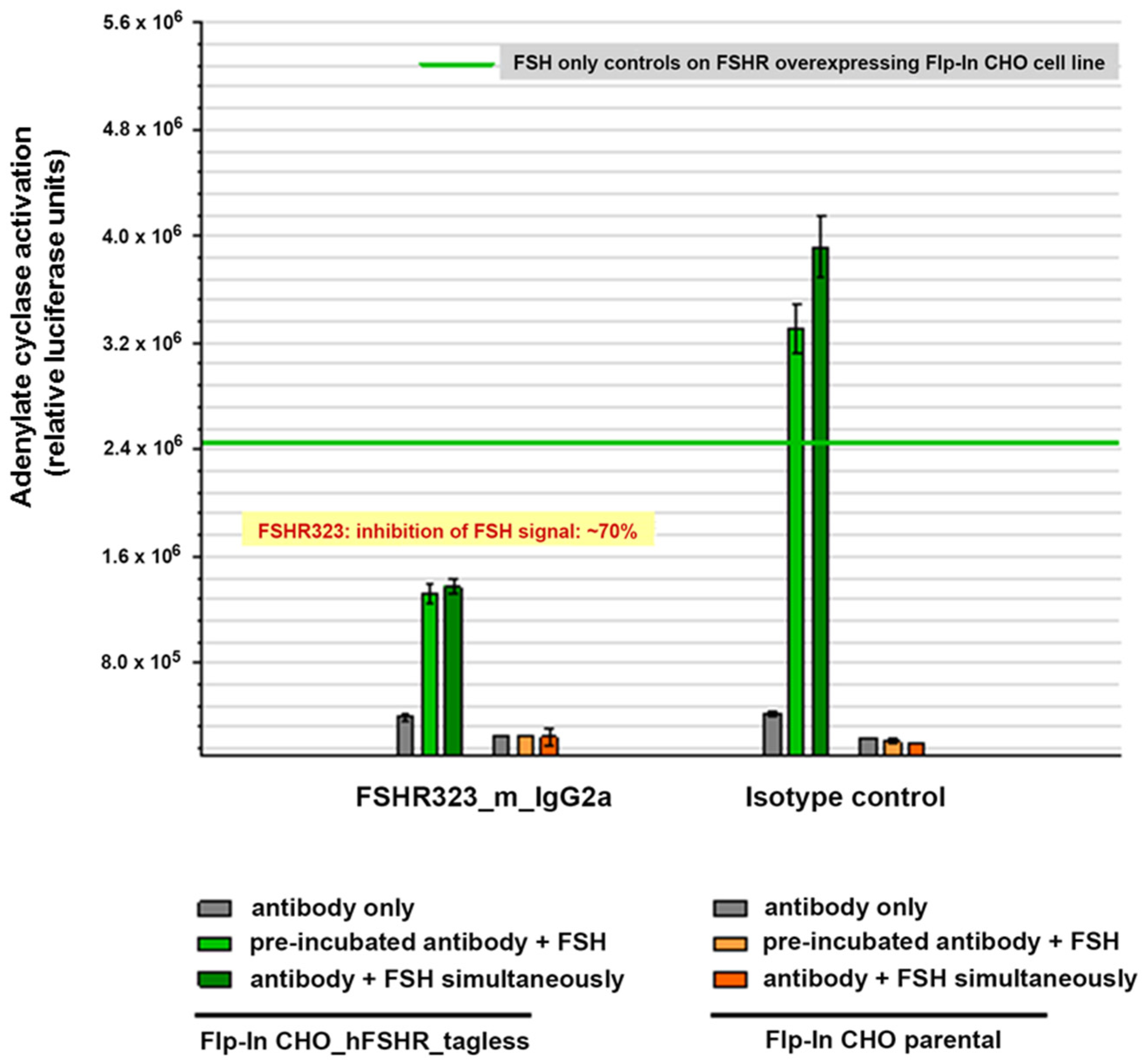
2.2.3. Competition of Antibodies with FSH for Binding to the FSHR
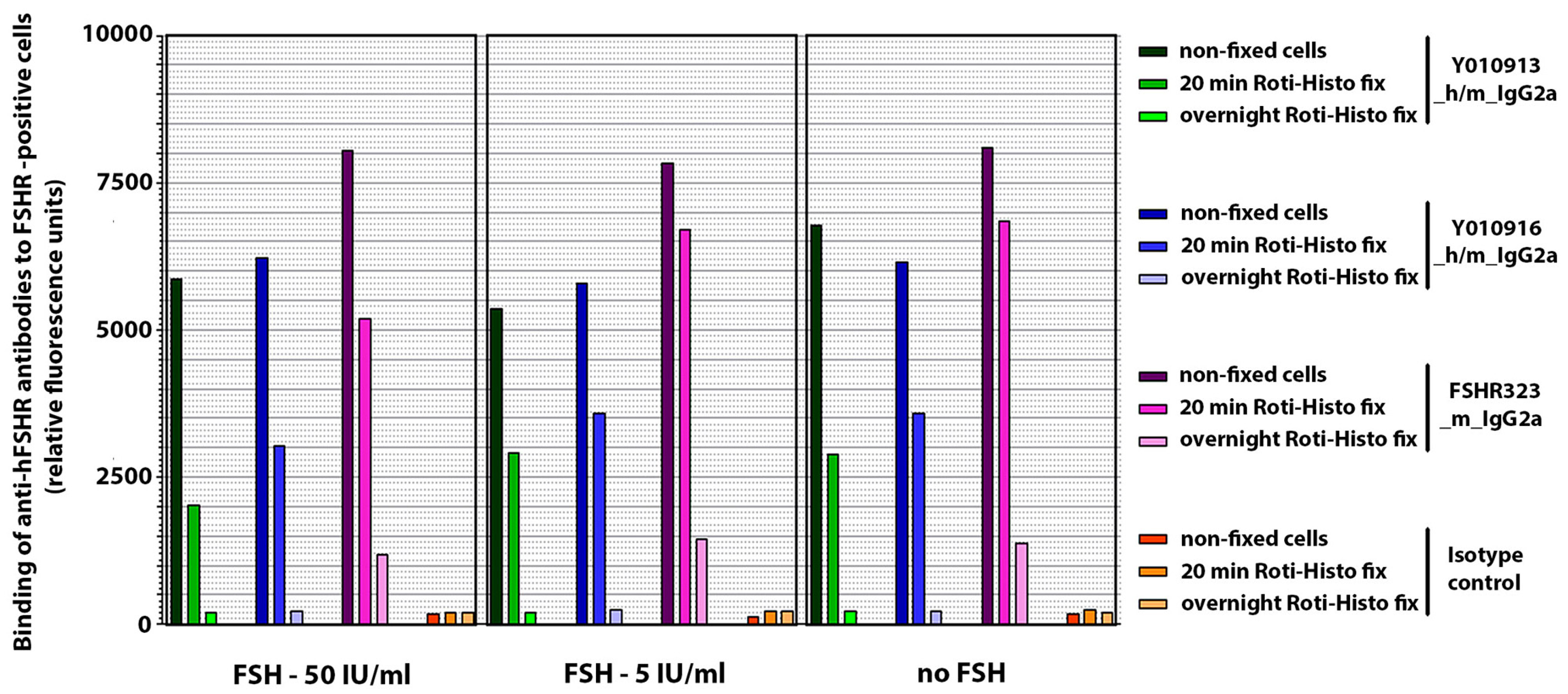
2.2.4. IHC Pre-Experiments Analyzing Antibody Binding to Cells after Fixation
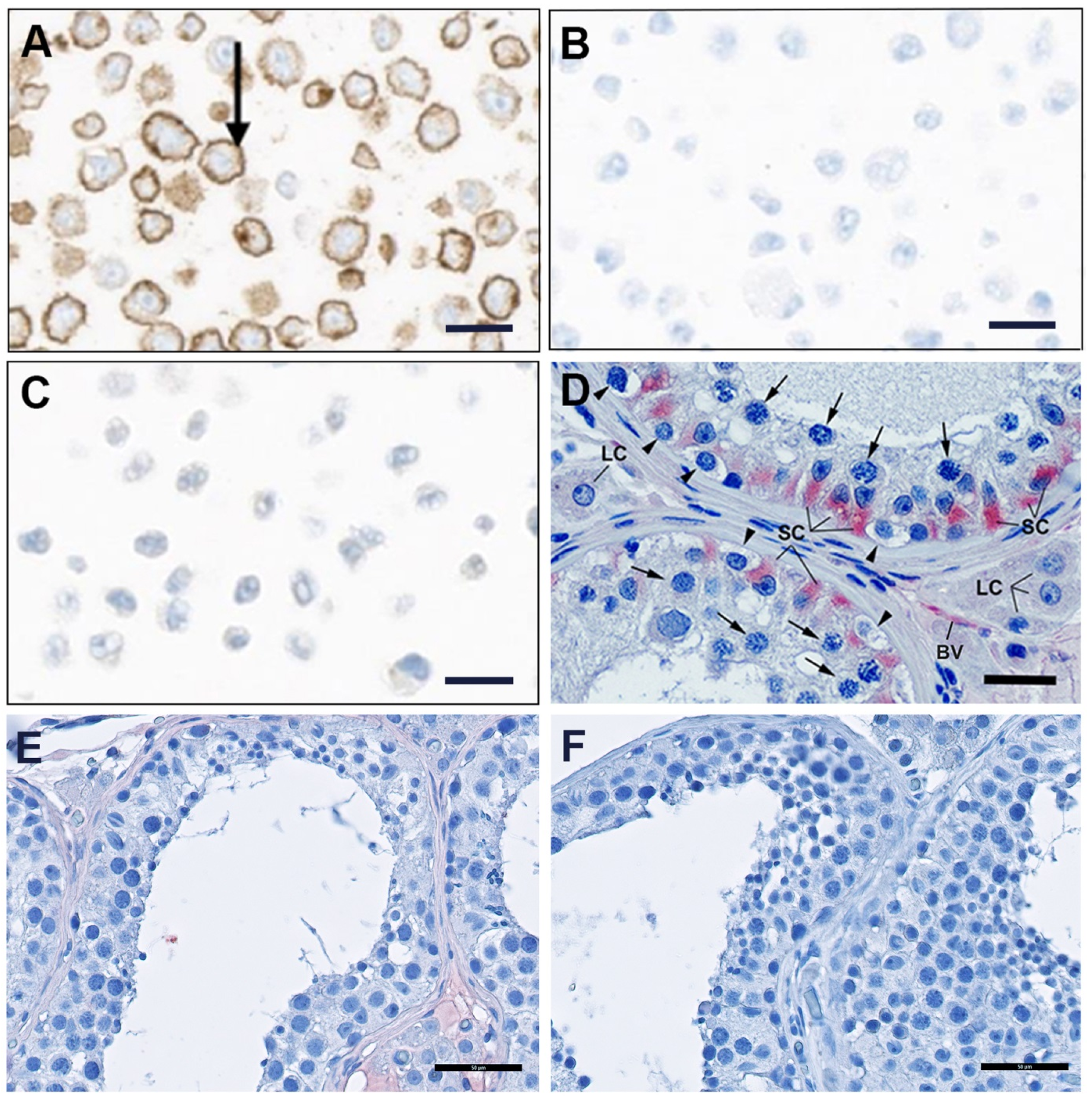
2.2.5. Immunohistochemical Detection of FSHR in Human Testis
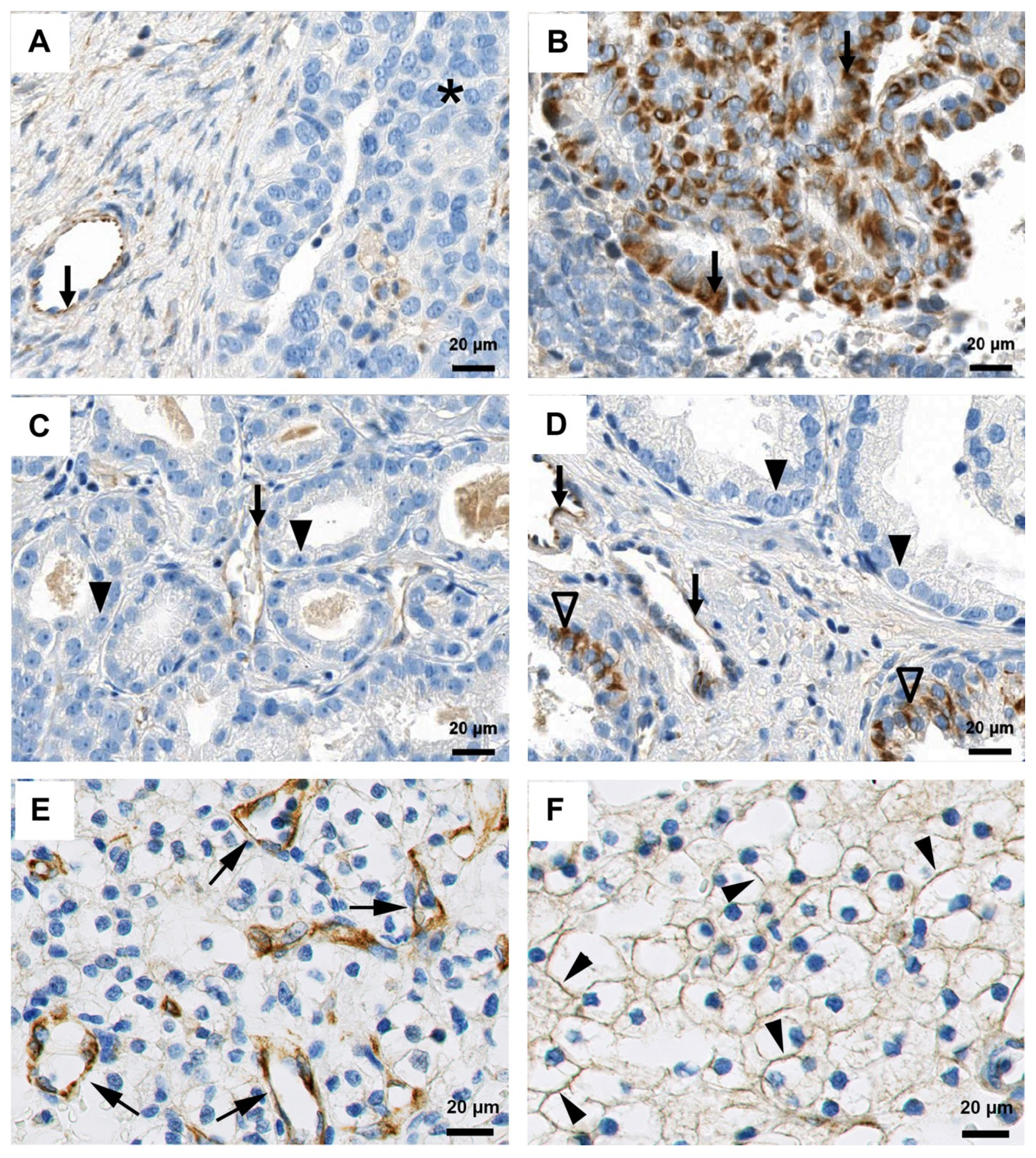
2.3. Characterization of FSHR as a Target for Human Cancer
3. Discussion
4. Material and Methods
4.1. Human Tissue Specimens
4.2. Cell Lines and Cell Culture
4.3. Construction of Expression Vectors Encoding hFSHR
4.4. Generation of Potentially Therapeutic Antibodies against Human Follicle Stimulating Hormone Receptor
4.5. Generation of Cells Stably Expressing FSHR
4.6. Generation of VLPs Containing Full-Length hFSHR, hTSHR, or hLHR
4.7. Antibodies
4.8. ELISA Assays
4.9. Flow Cytometry
4.10. Fixation
Fixation in Presence of FSH
4.11. FSH-Mediated Stimulation of Adenylate Cyclase
4.12. Immunocytochemistry and Immunohistochemistry
4.13. Histopathological Evaluation
4.14. Specificity Analysis Using Protein Panel Profiling Assay
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Macklon, N.S.; Fauser, B.C. Follicle development during the normal menstrual cycle. Maturitas 1998, 30, 181–188. [Google Scholar] [CrossRef]
- Plant, T.M.; Marshall, G.R. The functional significance of FSH in spermatogenesis and the control of its secretion in male primates. Endocr. Rev. 2001, 22, 764–786. [Google Scholar] [CrossRef] [PubMed]
- Sprengel, R.; Braun, T.; Nikolics, K.; Segaloff, D.L.; Seeburg, P.H. The testicular receptor for follicle stimulating hormone: Structure and functional expression of cloned cDNA. Mol. Endocrinol. 1990, 4, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.B.; Wang, H.; Segaloff, D.L. Extracellular domain of lutropin/choriogonadotropin receptor expressed in transfected cells binds choriogonadotropin with high affinity. J. Biol. Chem. 1990, 265, 21411–21414. [Google Scholar] [PubMed]
- Nagayama, Y.; Wadsworth, H.L.; Chazenbalk, G.D.; Russo, D.; Seto, P.; Rapoport, B. Thyrotropin-luteinizing hormone/chorionic gonadotropin receptor extracellular domain chimeras as probes for thyrotropin receptor function. Proc. Natl. Acad. Sci. USA 1991, 88, 902–905. [Google Scholar] [CrossRef] [PubMed]
- Vannier, B.; Loosfelt, H.; Meduri, G.; Pichon, C.; Milgrom, E. Anti-human FSH receptor monoclonal antibodies: Immunochemical and immunocytochemical characterization of the receptor. Biochemistry 1996, 35, 1358–1366. [Google Scholar] [CrossRef] [PubMed]
- Vu Hai, M.T.; Lescop, P.; Loosfelt, H.; Ghinea, N. Receptor-mediated transcytosis of follicle-stimulating hormone through the rat testicular microvasculature. Biol. Cell 2004, 96, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Stilley, J.A.W.; Christensen, D.E.; Dahlem, K.B.; Guan, R.; Santillan, D.A.; England, S.K.; Al-Hendy, A.; Kirby, P.A.; Segaloff, D.L. FSH Receptor (FSHR) Expression in human extragonadal reproductive tissues and the developing placenta, and the impact of its deletion on pregnancy in mice. Biol. Reprod. 2014, 91, 74. [Google Scholar] [CrossRef] [PubMed]
- Dirnhofer, S.; Berger, C.; Hermann, M.; Steiner, G.; Madersbacher, S.; Berger, P. Coexpression of gonadotropic hormones and their corresponding FSH- and LH/CG-receptors in the human prostate. Prostate 1998, 35, 212–220. [Google Scholar] [CrossRef]
- Ben-Josef, E.; Yang, S.Y.; Ji, T.H.; Bidart, J.M.; Garde, S.V.; Chopra, D.P.; Porter, A.T.; Tang, D.G. Hormone-refractory prostate cancer cells express functional follicle-stimulating hormone receptor (FSHR). J. Urol. 1999, 161, 970–976. [Google Scholar] [CrossRef]
- Mariani, S.; Salvatori, L.; Basciani, S.; Arizzi, M.; Franco, G.; Petrangeli, E.; Spera, G.; Gnessi, L. Expression and cellular localization of follicle-stimulating hormone receptor in normal human prostate, benign prostatic hyperplasia and prostate cancer. J. Urol. 2006, 175, 2072–2077. [Google Scholar] [CrossRef]
- Zheng, W.; Lu, J.J.; Luo, F.; Zheng, Y.; Feng, Y.; Felix, J.C.; Lauchlan, S.C.; Pike, M.C. Ovarian epithelial tumor growth promotion by follicle-stimulating hormone and inhibition of the effect by luteinizing hormone. Gynecol. Oncol. 2000, 76, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Rushdi, S.; Zumpe, E.T.; Mamers, P.; Healy, D.L.; Jobling, T.; Burger, H.G.; Fuller, P.J. FSH-regulated gene expression profiles in ovarian tumours and normal ovaries. Mol. Hum. Reprod. 2002, 8, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lin, L.; Parkash, V.; Schwartz, P.E.; Lauchlan, S.C.; Zheng, W. Quantitative analysis of follicle-stimulating hormone receptor in ovarian epithelial tumors: A novel approach to explain the field effect of ovarian cancer development in secondary mullerian systems. Int. J. Cancer 2003, 103, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Radu, A.; Pichon, C.; Camparo, P.; Antoine, M.; Allory, Y.; Couvelard, A.; Fromont, G.; Hai, M.T.; Ghinea, N. Expression of follicle-stimulating hormone receptor in tumor blood vessels. N. Engl. J. Med. 2010, 363, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Yan, Y.; Shi, S.; Graves, S.A.; Krasteva, L.K.; Nickles, R.J.; Yang, M.; Cai, W. PET of follicle-stimulating hormone receptor: Broad applicability to cancer imaging. Mol. Pharm. 2015, 12, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Urbanska, K.; Stashwick, C.; Poussin, M.; Powell, D.J., Jr. Follicle-stimulating hormone receptor as a target in the redirected T-cell therapy for cancer. Cancer Immunol. Res. 2015, 3, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Perales-Puchalt, A.; Svoronos, N.; Rutkowski, M.R.; Allegrezza, M.J.; Tesone, A.J.; Payne, K.K.; Wickramasinghe, J.; Nguyen, J.M.; O'Brien, S.W.; Gumireddy, K.; et al. Follicle-stimulating hormone receptor is expressed by most ovarian cancer subtypes and is a safe and effective immunotherapeutic target. Clin. Cancer Res. 2016, 23, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Lenhard, M.; Lennerová, T.; Ditsch, N.; Kahlert, S.; Friese, K.; Mayr, D.; Jeschke, U. Opposed roles of follicle-stimulating hormone and luteinizing hormone receptors in ovarian cancer survival. Histopathology 2011, 58, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Siraj, M.A.; Pichon, C.; Radu, A.; Ghinea, N. Endothelial follicle stimulating hormone receptor in primary kidney cancer correlates with subsequent response to sunitinib. J. Cell. Mol. Med. 2012, 16, 2010–2016. [Google Scholar] [CrossRef] [PubMed]
- Planeix, F.; Siraj, M.A.; Bidard, F.C.; Robin, B.; Pichon, C.; Sastre-Garau, X.; Antoine, M.; Ghinea, N. Endothelial follicle-stimulating hormone receptor expression in invasive breast cancer and vascular remodeling at tumor periphery. J. Exp. Clin. Cancer Res. 2015, 34, 12. [Google Scholar] [CrossRef] [PubMed]
- Ghinea, N.; Robin, B.; Planeix, F. Endothelial FSH receptor expression and vascular remodeling in hormone-refractory prostate cancer. J. Clin. Oncol. 2015, 33, e16035. [Google Scholar]
- Pawlikovski, M.; Pisarek, H.; Kubiak, R.; Jaranovska, M.; Stępień, H. Immunohistochemical detection of FSH receptors in pituitary adenomas and adrenal tumors. Folia Histochem. Cytobiol. 2012, 50, 325–330. [Google Scholar] [CrossRef]
- Pawlikowski, M.; Winczyk, K.; Stępień, H. Immunohistochemical detection of follicle stimulating hormone receptor (FSHR) in neuroendocrine tumours. Endocrynol. Pol. 2013, 64, 268–271. [Google Scholar] [CrossRef]
- Pawlikowski, M.; Jaranovska, M.; Pisarek, H.; Kubiak, R.; Fuss-Chmielevska, J.; Winczyk, K. Ectopic expression of follicle-stimulating hormone receptors in thyroid tumors. Arch. Med. Sci. 2015, 11, 1314–1317. [Google Scholar] [CrossRef] [PubMed]
- Peterson, V.M.; Castro, C.M.; Chung, J.; Miller, N.C.; Ullal, A.V.; Castano, M.D.; Penson, R.T.; Lee, H.; Birrer, M.J.; Weissleder, R.; et al. Ascites analysis by a microfluidic chip allows tumor-cell profiling. Proc. Natl. Acad. Sci. USA 2013, 110, E4978–E4986. [Google Scholar] [CrossRef] [PubMed]
- Sardella, C.; Russo, D.; Raggi, F.; Lombardi, M.; Urbani, C.; Brogioni, S.; Boggi, U.; Funel, N.; Chifenti, B.; Campani, D.; et al. Ectopic expression of FSH receptor isoforms in neoplastic but not in endothelial cells from pancreatic neuroendocrine tumours. J. Endocrinol. Invest. 2013, 36, 174–179. [Google Scholar] [PubMed]
- Sanchez, A.M.; Flamini, M.I.; Russo, E.; Casarosa, E.; Pacini, S.; Petrini, M.; Genazzani, A.R.; Simoncini, T. LH and FSH promote migration and invasion properties of a breast cancer cell line through regulatory actions on the actin cytoskeleton. Mol. Cell. Endocrinol. 2016, 437, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Renner, M.; Goeppert, B.; Siraj, M.A.; Radu, A.; Penzel, R.; Wardelmann, E.; Lehner, B.; Ulrich, A.; Stenzinger, A.; Warth, A.; et al. Follicle-stimulating hormone receptor expression in soft tissue sarcomas. Histopathology 2013, 63, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Siraj, A.; Desestret, V.; Antoine, M.; Fromont, G.; Huerre, M.; Sanson, M.; Camparo, P.; Pichon, C.; Planeix, F.; Gonin, J.; et al. Expression of follicle-stimulating hormone receptor by the vascular endothelium in tumor metastases. BMC Cancer 2013, 13, 246. [Google Scholar] [CrossRef] [PubMed]
- Modi, D.A.; Sunoqrot, S.; Bugno, J.; Lantvit, D.D.; Hong, S.; Burdette, J.E. Targeting of follicle-stimulating hormone peptide-conjugated dendrimers to ovarian cancer cells. Nanoscale 2014, 6, 2812–2820. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Pan, D.; Zhu, C.; Xu, Q.; Wang, L.; Chen, F.; Yang, R.; Luo, S.; Yang, M.; Yan, Y. Pilot study of a novel 18F-labeled FSHR probe for tumor imaging. Mol. Imaging Biol. 2014, 16, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Frese, K.; Eisenmann, M.; Ostendorp, R.; Brocks, B.; Pabst, S. An automated immunoassay for early specificity profiling of antibodies. mAbs 2013, 5, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Meduri, G.; Charnaux, N.; Loosfelt, H.; Jolivet, A.; Spyratos, F.; Brailly, S.; Milgrom, E. Luteinizing hormone/human chorionic gonadotropin receptors in breast cancer. Cancer Res. 1997, 57, 857–864. [Google Scholar] [PubMed]
- Gyftaki, R.; Liacos, C.; Politi, E.; Liontos, M.; Saltiki, K.; Papageorgiou, T.; Thomakos, N.; Haidopoulos, D.; Rodolakis, A.; Alevizaki, M.; et al. Differential transcriptional and protein expression of thyroid-stimulating hormone receptor in ovarian carcinomas. Int. J. Gynecol. Cancer 2014, 24, 851–856. [Google Scholar] [PubMed]
- D’Agostino, M.; Sponziello, M.; Puppin, C.; Celano, M.; Maggisano, V.; Baldan, F.; Biffoni, M.; Bulotta, S.; Durante, C.; Filetti, S.; et al. Different expression of TSH receptor and NIS genes in thyroid cancer: Role of epigenetics. J. Mol. Endocrinol. 2014, 52, 121–131. [Google Scholar]
- Mertens-Walker, I.; Baxter, R.C.; Marsh, D.J. Gonadotropin signaling in epithelial ovarian cancer. Cancer Lett. 2012, 324, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Rzepka-Gorska, I.; Chudecka-Glaz, A.; Kosmowska, B. FSH and LH serum/tumor fluid ratios and malignant tumors of the ovary. Endocrine-Related Cancer 2004, 11, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.C.; Oskay-Ozcelik, G.; Bühling, K.J.; Köpstein, U.; Mentze, M.; Lichtenegger, W.; Sehouli, J. Prognostic value of serum and ascites levels of estradiol, FSH, LH and prolactin in ovarian cancer. Anticancer Res. 2009, 29, 1575–1578. [Google Scholar] [PubMed]
- Fritschy, J.-M. Is my antibody-staining specific? How to deal with pitfalls of immunohistochemistry. Eur. J. Neuorosci. 2008, 28, 2365–2370. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, A.R.; Plückthun, A. Getting to reproducible antibodies: The rationale for sequenced recombinant characterized reagents. Protein. Eng. Des. Sel. 2015, 28, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Tiller, T.; Schuster, I.; Deppe, D.; Siegers, K.; Strohner, R.; Herrmann, T.; Berenguer, M.; Poujol, D.; Stehel, Y.; Stark, Y.; et al. A fully synthetic human Fab antibody library based on fixed VH/VL framework pairings with favorable biophysical properties. mAbs 2013, 5, 445–470. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Dias, J.A.; He, X. Structural biology of glycoprotein hormones and their receptors: Insights to signaling. Mol. Cell. Endocrinol. 2014, 382, 424–451. [Google Scholar] [CrossRef] [PubMed]
| Antibody | Specificity | Description | Isotype | Application (Acc. to Supplier) | Antigen | Supplier |
|---|---|---|---|---|---|---|
| sc-7798 | anti-hFSHR | Goat polyclonal | WB, ELISA,IF, IP | Peptide map-ping near the N-terminus of hFSHR | Santa Cruz | |
| sc-13935 | anti-hFSHR | Rabbit polyclonal | WB, ELISA,IF, IP | aa1-190 | Santa Cruz | |
| FSHR323 | anti-hFSHR | Mouse monoclonal | IgG2a | WB, ELISA,IF, IP, ICC, IHC | Obtained from INSERM | |
| Y010913 | anti-hFSHR | Human/mouse monoclonal | IgG2a | Ylanthia® antibody | ||
| Y010916 | anti-hFSHR | Human/mouse monoclonal | IgG2a | Ylanthia® antibody | ||
| Isotype control | Irrelevant antigen | Human/mouse monoclonal | IgG2a | irrelevant non-human protein | HuCAL® antibody |
| Staining Instrument | Discovery XT |
|---|---|
| Fixation | 4% Paraformaldehyde |
| Epitope retrieval | CC1 Mild (Cell conditioning solution 1, EDTA buffer); 30 min, 100 °C |
| Blocking | Normal goat serum diluted (Dispenser OPTION 4), 1:50, 8 min |
| Dilution buffer | DCS antibody diluent |
| Primary antibody | FSHR323, 2 µg/mL, 1 h, 37 °C |
| Secondary antibody | OmniMap DAB /AEC Kit |
| Counterstain | Hematoxylin II, 8 min; Bluing Reagent, 4 min |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moeker, N.; Peters, S.; Rauchenberger, R.; Ghinea, N.; Kunz, C. Antibody Selection for Cancer Target Validation of FSH-Receptor in Immunohistochemical Settings. Antibodies 2017, 6, 15. https://doi.org/10.3390/antib6040015
Moeker N, Peters S, Rauchenberger R, Ghinea N, Kunz C. Antibody Selection for Cancer Target Validation of FSH-Receptor in Immunohistochemical Settings. Antibodies. 2017; 6(4):15. https://doi.org/10.3390/antib6040015
Chicago/Turabian StyleMoeker, Nina, Solveig Peters, Robert Rauchenberger, Nicolae Ghinea, and Christian Kunz. 2017. "Antibody Selection for Cancer Target Validation of FSH-Receptor in Immunohistochemical Settings" Antibodies 6, no. 4: 15. https://doi.org/10.3390/antib6040015
APA StyleMoeker, N., Peters, S., Rauchenberger, R., Ghinea, N., & Kunz, C. (2017). Antibody Selection for Cancer Target Validation of FSH-Receptor in Immunohistochemical Settings. Antibodies, 6(4), 15. https://doi.org/10.3390/antib6040015




