Reverse Signaling Contributes to Control of Chronic Inflammation by Anti-TNF Therapeutics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Culture Stimulation
2.3. Bone Marrow-Derived Mouse Macrophages
2.4. Quantitative Reverse Transcriptase Polymerase Chain Reaction (QRT-PCR)
| A | ||
|---|---|---|
| Target name | Forward (5'→3') | Reverse (3'→5') |
| GAPDH | CAGTCAGCCGCATCTTCTTTTG | CGCCCAATACGACCAAATCC |
| TNF | GCCTCTTCTCCTTCCTGATCG | GCTTGAGGGTTTGCTACAACAT |
| CKIP-1 | ACCTGCAACCGACGATTCTT | CATTCCATGAAGTCAGCGATATGT |
| IL-1β | CCACCTCCAGGGACAGGATA | TTTGGGATCTACACTCTCCAGC |
| IL-6 | GGATTCAATGAGGAGACTTGCC | CTGGCATTTGTGGTTGGGTC |
| IL-8 | ACTCCAAACCTTTCCACCCC | TTCTCAGCCCTCTTCAAAAACTTC |
| IL-10 | GGCACCCAGTCTGAGAACAG | GGCAACCCAGGTAACCCTTAAA |
| CXCL9 | AGTGCAAGGAACCCCAGTAG | TCACATCTGCTGAATCTGGGTT |
| ICAM1 | TGTGACCAGCCCAAGTTGTT | TGGAGTCCAGTACACGGTGA |
| TGF-β | ACCAACTATTGCTTCAGCTCCA | CTTGCTGTACTGCGTGTCCA |
| TNFAIP3 | GCTGAAAACGAACGGTGACGG | AGAGACTCCAGTTGCCAGCGG |
| B | ||
| GAPDH | CTGGAAAGCTGTGGCGTGAT | ATACTTGGCAGGTTTCTCCAGG |
| TNF | TAGCCCACGTCGTAGCAAAC | GCAGCCTTGTCCCTTGAAGA |
| CKIP-1 | GGATGAGGTCACCGTTGAGG | CGGAAGCTCTCAGCACAAGA |
| CXCL10 | TCATCCTGCTGGGTCTGAGT | TCATCGTGGCAATGATCTCAACA |
| IL-1β | AACCTTTGACCTGGGCTGTC | CAGAGGATGGGCTCTTCTTCAA |
2.5. Data Representation and Statistical Analysis
3. Results
3.1. Infliximab Stimulation Attenuates the LPS-Induced Gene Expression of Pro-Inflammatory Molecules in THP-1 Cells
3.2. The Effect of Infliximab Does Not Depend on Its Fc Fragment
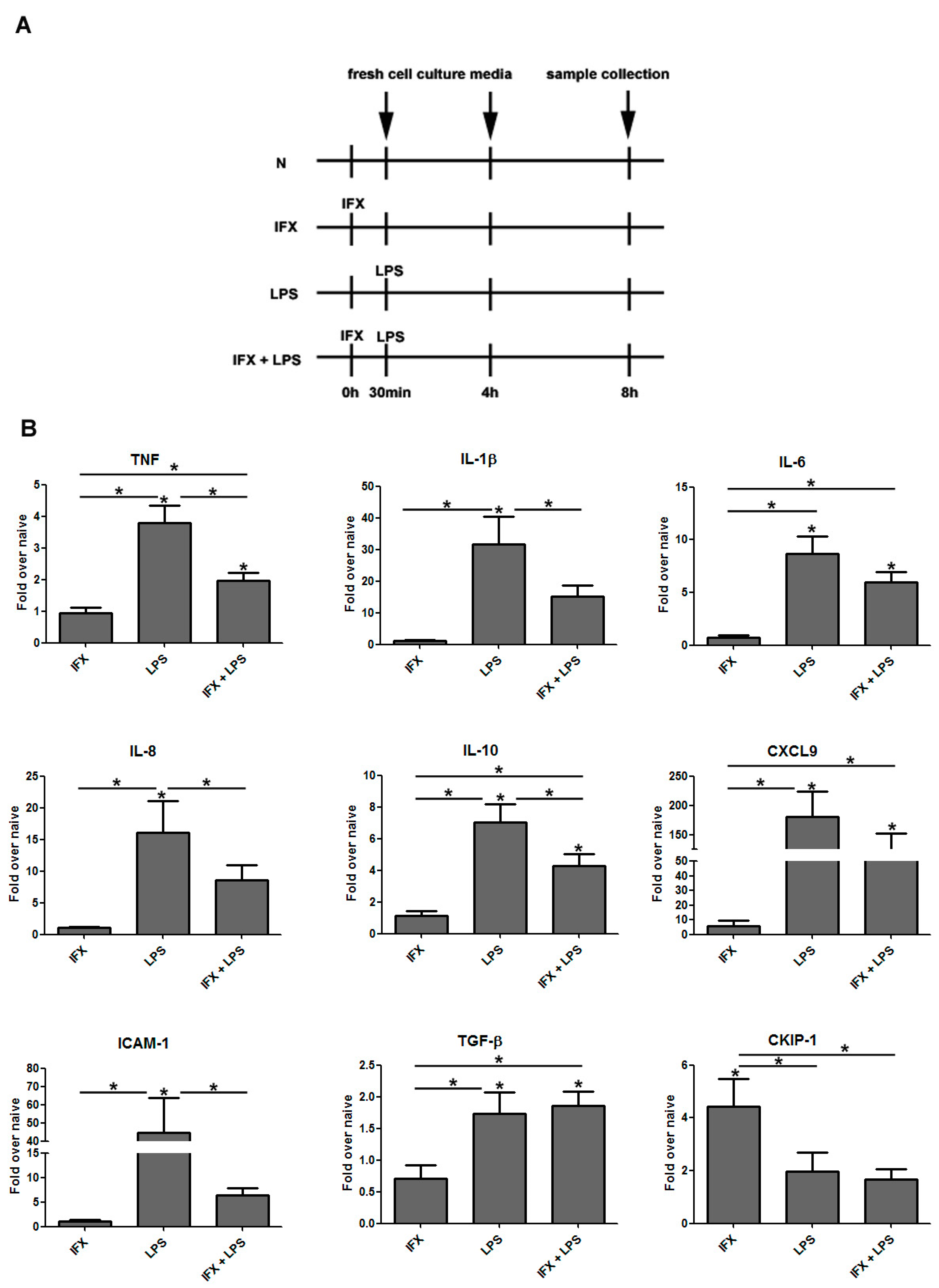
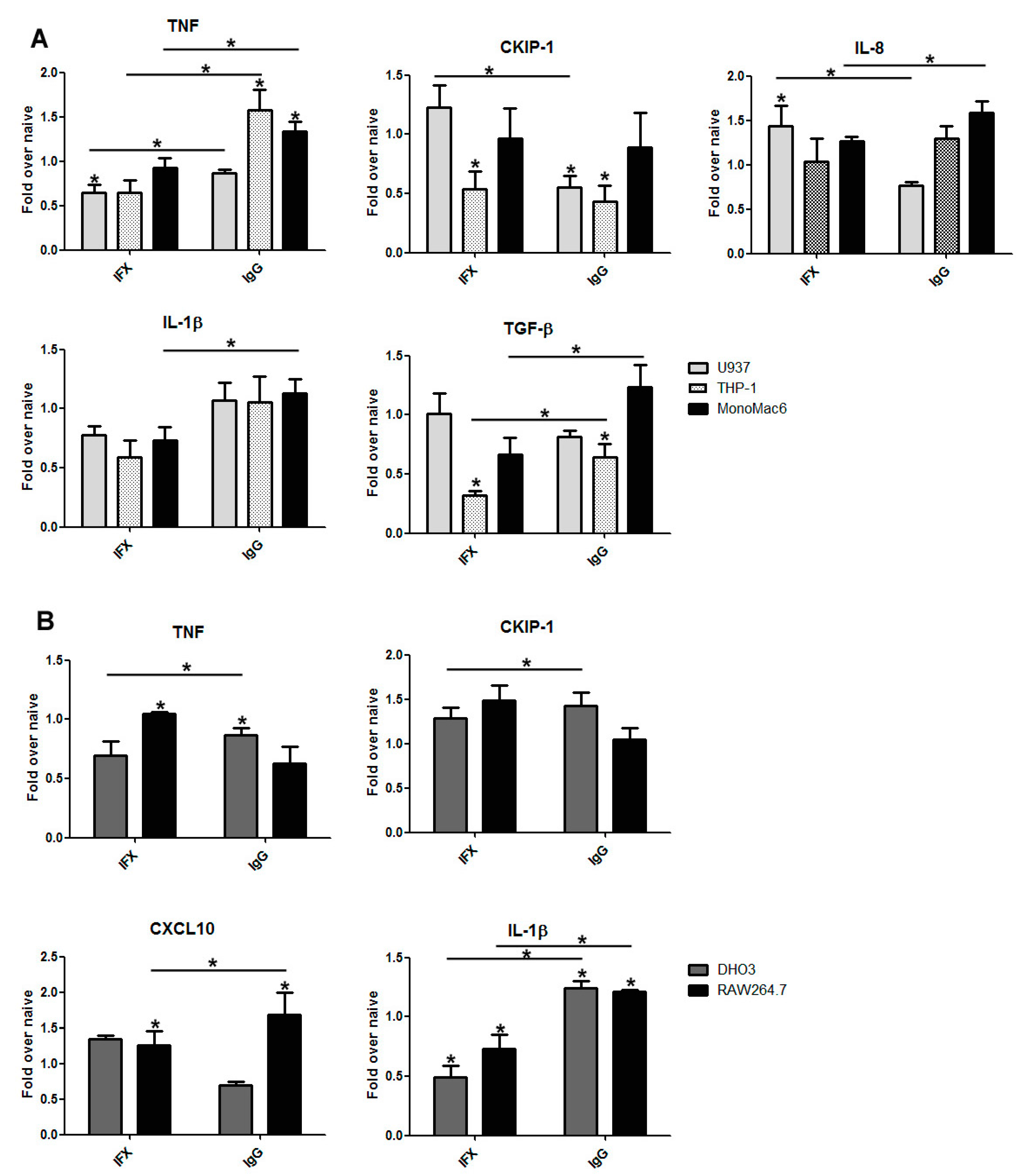
3.3. The Induction of Reverse Signaling by Monoclonal Antibodies Modulates the Expression Pattern of Cytokines and Chemokines in a Time-Dependent Manner
3.4. Certolizumab Pegol Decreases the Relative Gene Expression of Inflammation-Related Effector Molecules in a Time-Dependent Manner under Inflammatory Conditions
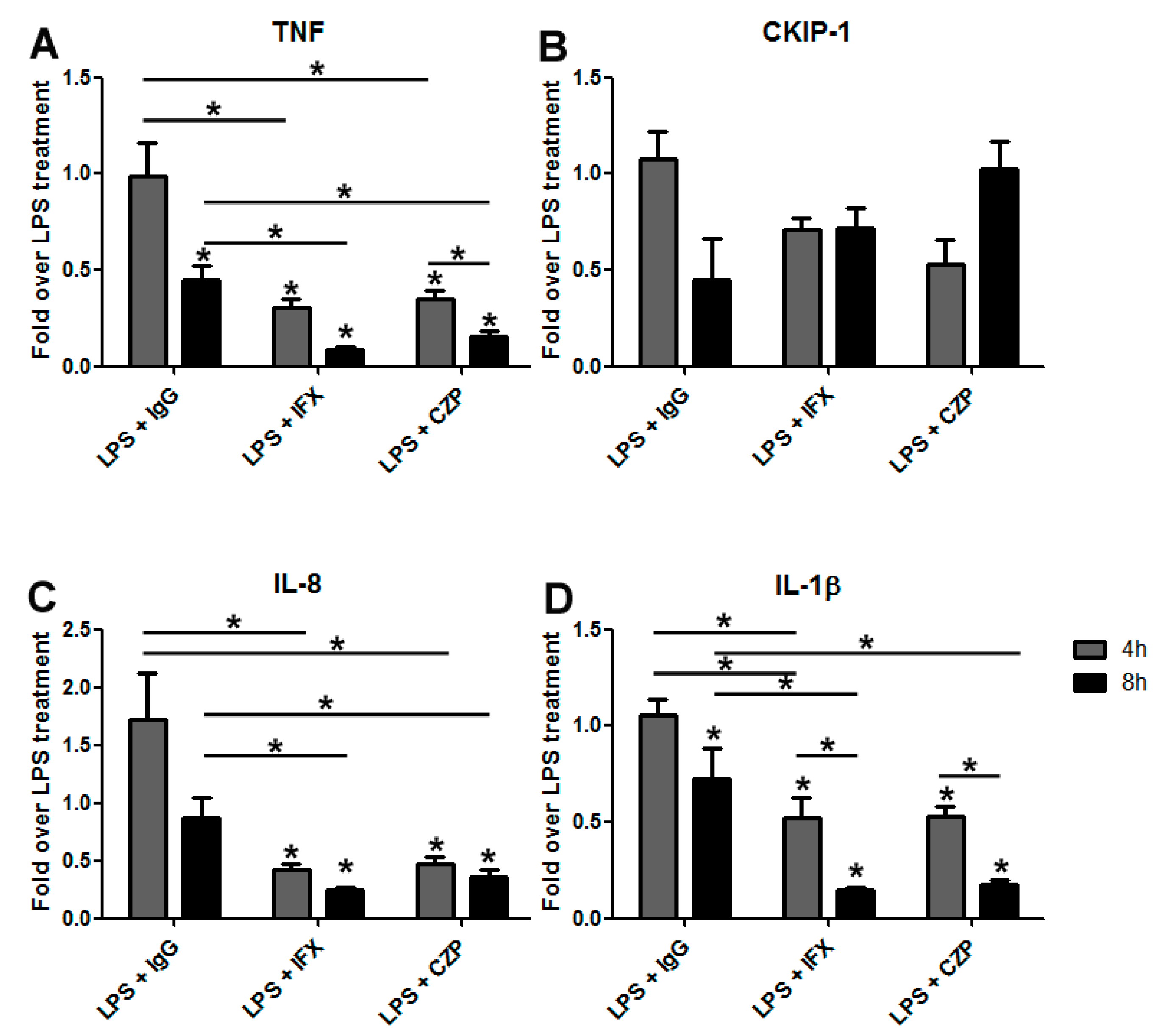
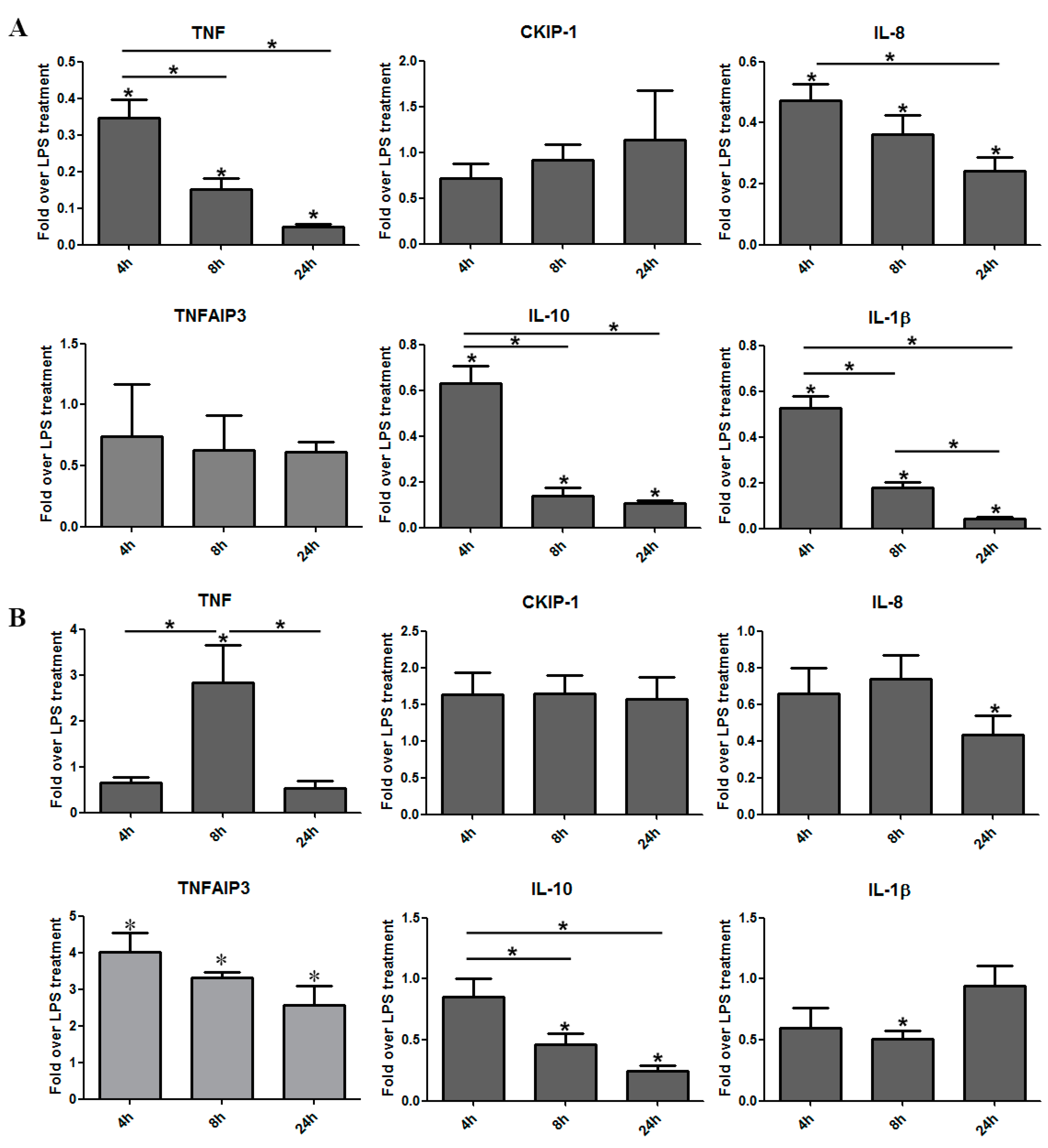
3.5. The Regulatory Effect of IFX Treatment on Activated Bone Marrow-Derived Mouse Macrophages
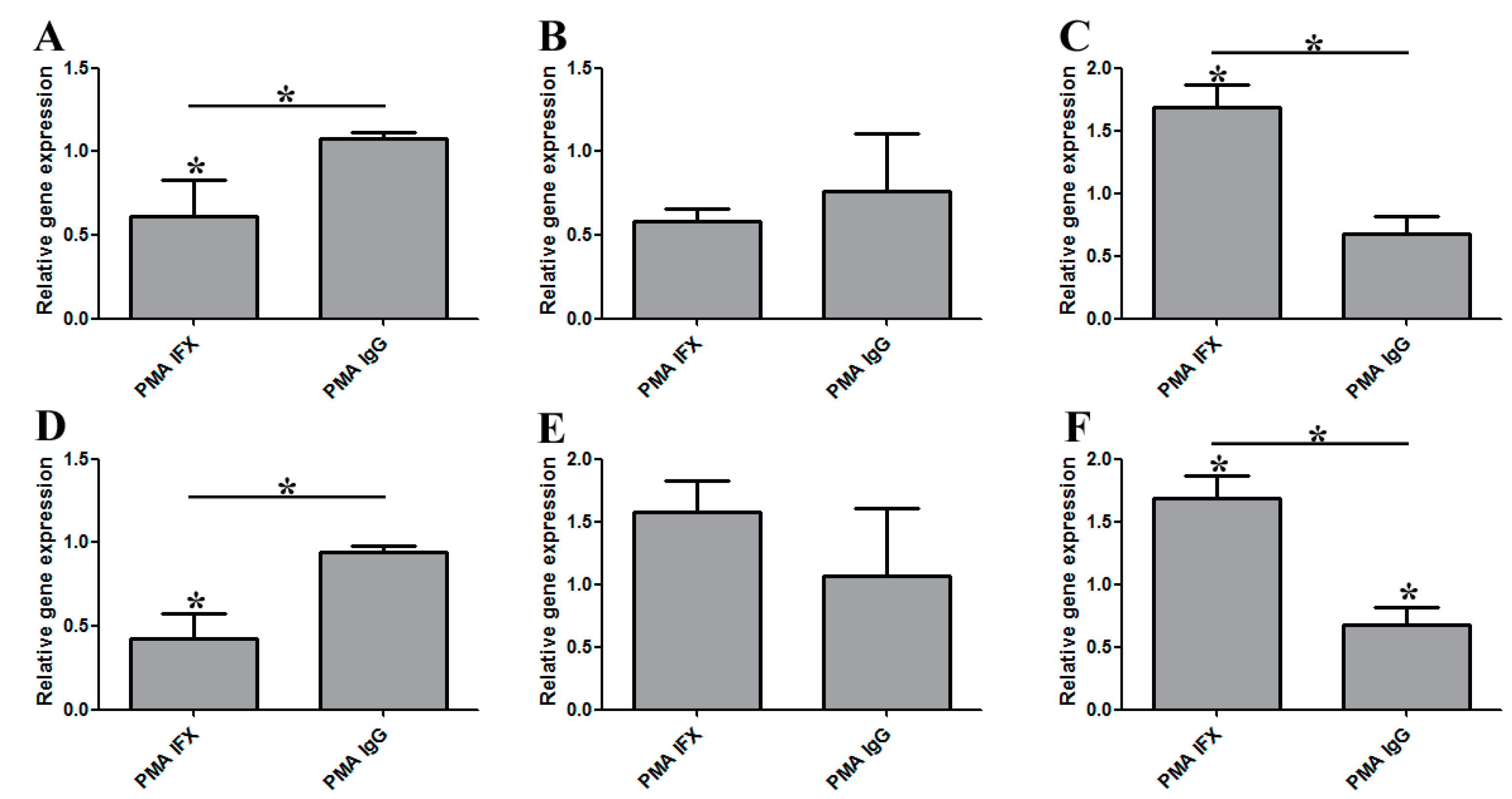
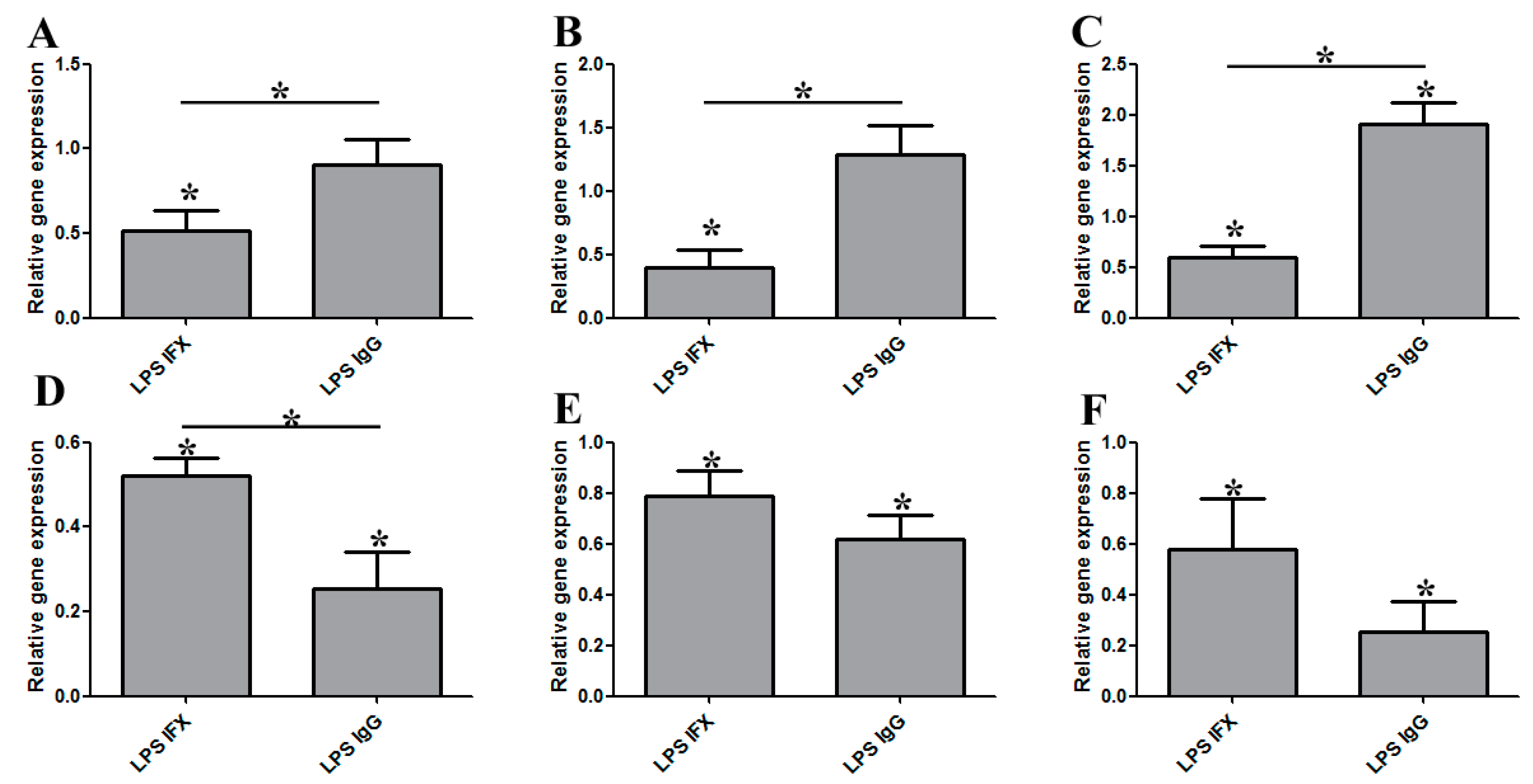
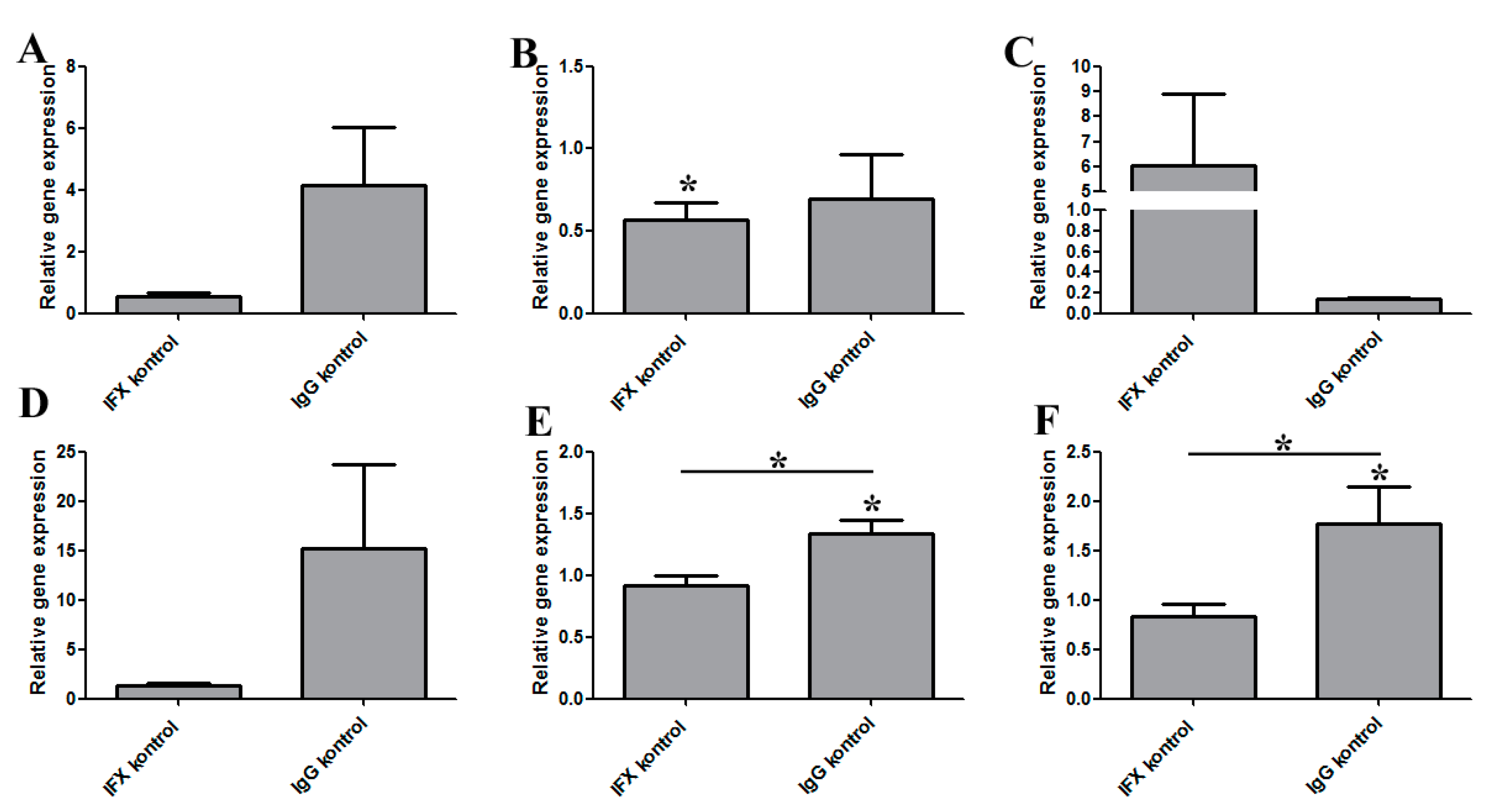
4. Discussion
5. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Ramadori, G.; Van Damme, J.; Rieder, H. Meyer zum Buschenfelde, K.H. Interleukin 6, the third mediator of acute-phase reaction, modulates hepatic protein synthesis in human and mouse. Comparison with interleukin 1 beta and tumor necrosis factor-alpha. Eur. J. Immunol. 1988, 18, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctot, K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Matera, M.G.; Calzetta, L.; Cazzola, M. TNF-alpha inhibitors in asthma and COPD: We must not throw the baby out with the bath water. Pulm. Pharmacol. Ther. 2010, 23, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Kalman, J.; Mayer, L.; Fillit, H.M.; Packer, M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N. Engl. J. Med. 1990, 323, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Nickoloff, B.J.; Xin, H.; Nestle, F.O.; Qin, J.Z. The cytokine and chemokine network in psoriasis. Clin. Dermatol. 2007, 25, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, M.; Maini, R.N. Anti-TNF alpha therapy of rheumatoid arthritis: What have we learned? Annu. Rev. Immunol. 2001, 19, 163–196. [Google Scholar] [CrossRef] [PubMed]
- Chey, W.Y. Infliximab for patients with refractory ulcerative colitis. Inflamm. Bowel Dis. 2001, 7 (Suppl. 1), S30–S33. [Google Scholar] [CrossRef] [PubMed]
- Knight, D.M.; Trinh, H.; Le, J.; Siegel, S.; Shealy, D.; McDonough, M.; Scallon, B.; Moore, M.A.; Vilcek, J.; Daddona, P.; et al. Construction and initial characterization of a mouse-human chimeric anti-TNF antibody. Mol. Immunol. 1993, 30, 1443–1453. [Google Scholar] [CrossRef]
- Nightingale, S.L. From the Food and Drug Administration. JAMA 1998, 280, 1128. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.; Armstrong, A.W. Anti-drug antibodies in psoriasis: A critical evaluation of clinical significance and impact on treatment response. Expert Rev. Clin. Immunol. 2013, 9, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, T.; Mitoma, H.; Harashima, S.; Tsukamoto, H.; Shimoda, T. Transmembrane TNF-alpha: Structure, function and interaction with anti-TNF agents. Rheumatology (Oxford) 2010, 49, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Kane, S.V.; Acquah, L.A. Placental transport of immunoglobulins: a clinical review for gastroenterologists who prescribe therapeutic monoclonal antibodies to women during conception and pregnancy. Am. J. Gastroenterol. 2009, 104, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, U.; Wolf, D.C.; Dubinsky, M.; Cortot, A.; Lee, S.D.; Siegel, C.A.; Ullman, T.; Glover, S.; Valentine, J.F.; Rubin, D.T.; et al. Placental transfer of anti-tumor necrosis factor agents in pregnant patients with inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2013, 11, 286–292, quiz e224. [Google Scholar] [CrossRef] [PubMed]
- Domonkos, A.; Udvardy, A.; Laszlo, L.; Nagy, T.; Duda, E. Receptor-like properties of the 26 kDa transmembrane form of TNF. Eur. Cytokine Netw. 2001, 12, 411–419. [Google Scholar] [PubMed]
- Eissner, G.; Kirchner, S.; Lindner, H.; Kolch, W.; Janosch, P.; Grell, M.; Scheurich, P.; Andreesen, R.; Holler, E. Reverse signaling through transmembrane TNF confers resistance to lipopolysaccharide in human monocytes and macrophages. J. Immunol. 2000, 164, 6193–6198. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, K.; Zvara, A.; Lipp, A.M.; Nimmervoll, B.; Sonnleitner, A.; Balogi, Z.; Duda, E. Casein kinase 2-interacting protein-1, an inflammatory signaling molecule interferes with TNF reverse signaling in human model cells. Immunol. Lett. 2013, 152, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Harashima, S.; Horiuchi, T.; Hatta, N.; Morita, C.; Higuchi, M.; Sawabe, T.; Tsukamoto, H.; Tahira, T.; Hayashi, K.; Fujita, S.; et al. Outside-to-inside signal through the membrane TNF-alpha induces E-selectin (CD62E) expression on activated human CD4+ T cells. J. Immunol. 2001, 166, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Mitoma, H.; Horiuchi, T.; Hatta, N.; Tsukamoto, H.; Harashima, S.; Kikuchi, Y.; Otsuka, J.; Okamura, S.; Fujita, S.; Harada, M. Infliximab induces potent anti-inflammatory responses by outside-to-inside signals through transmembrane TNF-alpha. Gastroenterology 2005, 128, 376–392. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Fejer, G.; Kusz, E.; Biological Research Centre of the Hungarian Academy of Sciences, Szeged, Hungary. Unpublished work. 2006.
- de Waal Malefyt, R.; Abrams, J.; Bennett, B.; Figdor, C.G.; de Vries, J.E. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 1991, 174, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, D.F.; Bond, M.W.; Mosmann, T.R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J. Exp. Med. 1989, 170, 2081–2095. [Google Scholar] [CrossRef] [PubMed]
- Alanara, T.; Karstila, K.; Moilanen, T.; Silvennoinen, O.; Isomaki, P. Expression of IL-10 family cytokines in rheumatoid arthritis: Elevated levels of IL-19 in the joints. Scand. J. Rheumatol. 2010, 39, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.S.; Cheng, G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, S.; Holler, E.; Haffner, S.; Andreesen, R.; Eissner, G. Effect of different tumor necrosis factor (TNF) reactive agents on reverse signaling of membrane integrated TNF in monocytes. Cytokine 2004, 28, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Huang, C.; Ma, T.T.; Bian, E.B.; He, Y.; Zhang, L.; Li, J. SOCS1 hypermethylation mediated by DNMT1 is associated with lipopolysaccharide-induced inflammatory cytokines in macrophages. Toxicol. Lett. 2014, 225, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Chan, F.K.; Chun, H.J.; Zheng, L.; Siegel, R.M.; Bui, K.L.; Lenardo, M.J. A domain in TNF receptors that mediates ligand-independent receptor assembly and signaling. Science 2000, 288, 2351–2354. [Google Scholar] [CrossRef] [PubMed]
- Kisiswa, L.; Osorio, C.; Erice, C.; Vizard, T.; Wyatt, S.; Davies, A.M. TNFalpha reverse signaling promotes sympathetic axon growth and target innervation. Nat. Neurosci. 2013, 16, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.A.; Heffner, D.L.; Kim, S.; Espy, S.M.; Spano, A.J.; Cleland, C.L.; Deppmann, C.D. TNF-alpha/TNFR1 signaling is required for the development and function of primary nociceptors. Neuron 2014, 82, 587–602. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Gupta, S.C.; Kim, J.H. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood 2012, 119, 651–665. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Kim, E.J.; Suk, K.; Lee, W.H. Stimulation of FasL induces production of proinflammatory mediators through activation of mitogen-activated protein kinases and nuclear factor-kappaB in THP-1 cells. Inflammation 2012, 35, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.T.; Kim, W.J.; Lee, S.M.; Lee, M.Y.; Park, S.B.; Lee, S.H.; Kim, I.S.; Suk, K.; Choi, B.K.; Choi, E.M.; et al. Reverse signaling through BAFF differentially regulates the expression of inflammatory mediators and cytoskeletal movements in THP-1 cells. Immunol. Cell Biol. 2010, 88, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Koh, L.K.; Jiang, D.; Schwarz, H. CD137 ligand reverse signaling skews hematopoiesis towards myelopoiesis during aging. Aging (Albany NY) 2013, 5, 643–652. [Google Scholar] [PubMed]
- Bae, E.M.; Kim, W.J.; Suk, K.; Kang, Y.M.; Park, J.E.; Kim, W.Y.; Choi, E.M.; Choi, B.K.; Kwon, B.S.; Lee, W.H. Reverse signaling initiated from GITRL induces NF-kappaB activation through ERK in the inflammatory activation of macrophages. Mol. Immunol. 2008, 45, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Brito-Zeron, P.; Munoz, S.; Soto, M.J. A systematic review of the off-label use of biological therapies in systemic autoimmune diseases. Medicine (Baltimore) 2008, 87, 345–364. [Google Scholar] [CrossRef] [PubMed]
- Fejer, G.; Szalay, K.; Gyory, I.; Fejes, M.; Kusz, E.; Nedieanu, S.; Pali, T.; Schmidt, T.; Siklodi, B.; Lazar, G., Jr.; et al. Adenovirus infection dramatically augments lipopolysaccharide-induced TNF production and sensitizes to lethal shock. J. Immunol. 2005, 175, 1498–1506. [Google Scholar] [CrossRef]
- Kirchner, S.; Boldt, S.; Kolch, W.; Haffner, S.; Kazak, S.; Janosch, P.; Holler, E.; Andreesen, R.; Eissner, G. LPS resistance in monocytic cells caused by reverse signaling through transmembrane TNF (mTNF) is mediated by the MAPK/ERK pathway. J. Leukoc. Biol. 2004, 75, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Filkor, K.; Hegedus, Z.; Szasz, A.; Tubak, V.; Kemeny, L.; Kondorosi, E.; Nagy, I. Genome wide transcriptome analysis of dendritic cells identifies genes with altered expression in psoriasis. PLoS One 2013, 8, e7343. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.Y.; Choi, E.K.; Lee, S.W.; Jung, K.O.; Seo, S.K.; Choi, I.W.; Park, S.G.; Choi, I. Programmed death-1 receptor negatively regulates LPS-mediated IL-12 production and differentiation of murine macrophage RAW264.7 cells. Immunol. Lett. 2009, 127, 39–47. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sipos, O.; Török, A.; Kalic, T.; Duda, E.; Filkor, K. Reverse Signaling Contributes to Control of Chronic Inflammation by Anti-TNF Therapeutics. Antibodies 2015, 4, 123-140. https://doi.org/10.3390/antib4020123
Sipos O, Török A, Kalic T, Duda E, Filkor K. Reverse Signaling Contributes to Control of Chronic Inflammation by Anti-TNF Therapeutics. Antibodies. 2015; 4(2):123-140. https://doi.org/10.3390/antib4020123
Chicago/Turabian StyleSipos, Orsolya, Annamária Török, Tanja Kalic, Ernő Duda, and Kata Filkor. 2015. "Reverse Signaling Contributes to Control of Chronic Inflammation by Anti-TNF Therapeutics" Antibodies 4, no. 2: 123-140. https://doi.org/10.3390/antib4020123
APA StyleSipos, O., Török, A., Kalic, T., Duda, E., & Filkor, K. (2015). Reverse Signaling Contributes to Control of Chronic Inflammation by Anti-TNF Therapeutics. Antibodies, 4(2), 123-140. https://doi.org/10.3390/antib4020123





