Production of Single-Chain Variable-Fragments against Carbohydrate Antigens
Abstract
:1. Introduction
2. Phage Display Technology in Combination with the Use of Structurally Defined Carbohydrate Probes
2.1. Isolation and Characterization of Anti-Trimannose Antibodies by Phage Display Technology Using Neoglycolipids as Probes
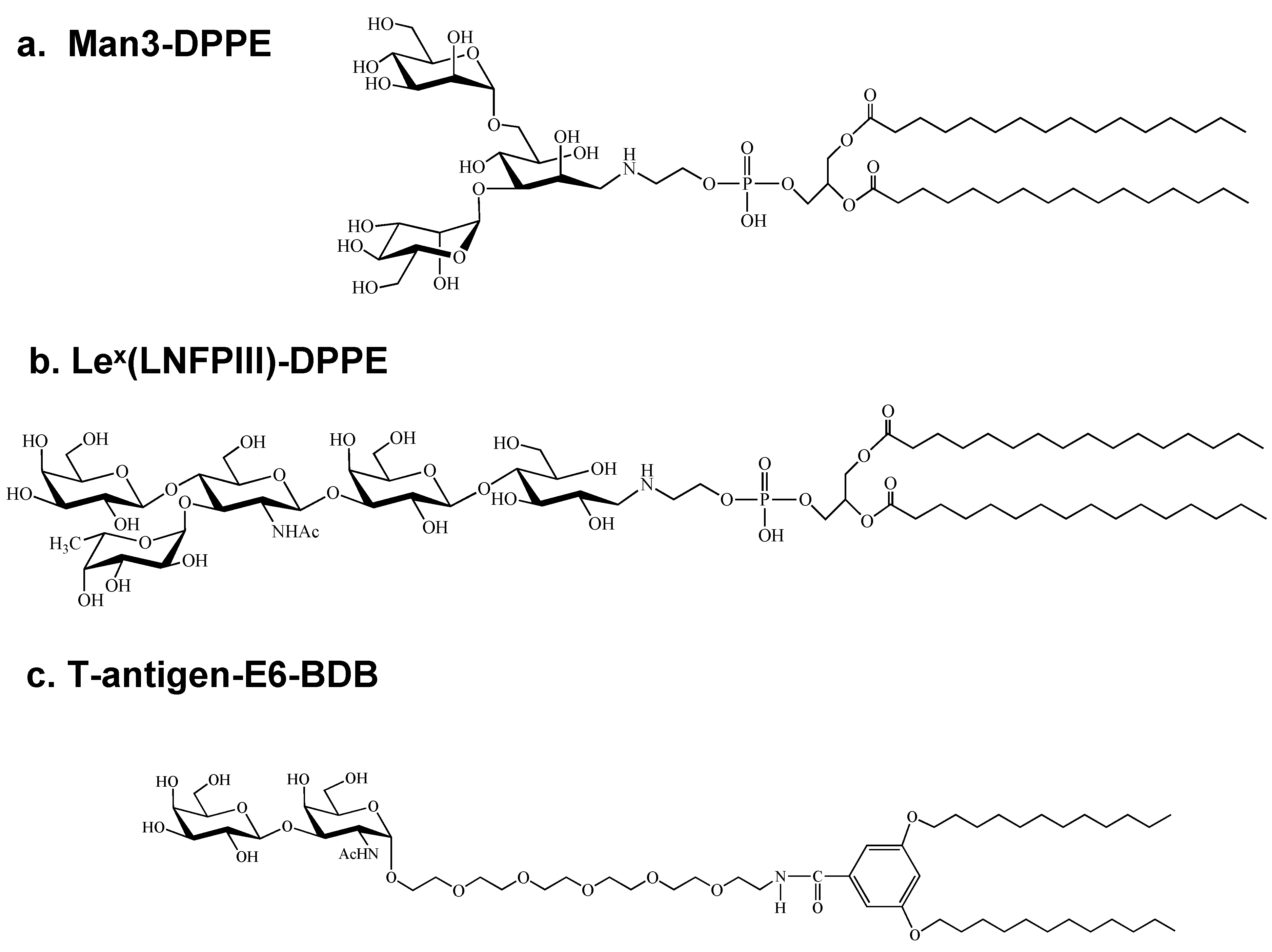
2.2. Purification and Characterization of Anti-Carbohydrate Antibodies Expressed in Mammalian Cells
| No. | Carbohydrate Probes Used | Positive Clones/Total Clones Screened (S/N Ratio) | Phage Clones with scFv Gene | Phage Clones with scFv DNA Sequence Confirmed | Phage Clones Analyzed for scFv Proteins |
|---|---|---|---|---|---|
| 1 | Mannotriose (Man3)-DPPE | 26/672 (>2) | 25 | 15 | 5A3, 1A4, 1G4, 5C10 |
| 2 | LeX(LNFPIII)-DPPE | 100/1,470 (>4) | 5 | 3 | 1F12, 3F1 |
| 3 | T-antigen E6-BDB | 25/96 (>4) | 22 | 11 | 1E6, 1E8, 1E10, 1G11 |
| 4 | Tn- antigen E6-BDB | 0/928 | NA | NA | NA |
| 5 | Tn3-peptide | 8/96 (>2) | 8 | 6 | 4E10, 4G2 |
| Clone (Probe Used for Screening) | Antibody Constructs Expressed | Cells Used for Expression | Outcome | Reference |
|---|---|---|---|---|
| 1A4, 1G4 (Man3-DPPE) | scFv-Fc/pEE12.4 | NS0 | No clone | [16] |
| 1A4, 1G4 (Man3-DPPE) | scFv-Fc/pCI-neo | CHO | No clone | [16] |
| 5A3 (Man3-DPPE) | scFv-Fc/pEE12.4 | NS0 | One clone | [16] |
| 1F12 (LNFPIII-DPPE) | scFv-Fc/pEE12.4 | NS0 | One clone | [20] |
| 3F1 (LNFPIII-DPPE) | scFv-Fc/pEE12.4 | NS0 | No clone | [20] |
| 1G11 (T-antigen E6-BDB) | scFv/phagemid | E. coli TOP10F' | Soluble | [24] |
| 4E10, 4G2 (Tn3-peptide) | scFv/phagemid | E. coli TOP10F' | Soluble | [25] |
| 5A3, 5C10 | GST-scFv/pGX-4T-1 | E. coli BL21 | Soluble | [16] |
| 1A4, 1G4, 5A3 (Man3-DPPE) | scFv/pET22 | E. coli BL21 | Inclusion bodies | [26] |
| 1E6, 1E8 (T-antigen E6-BDB) | scFv/pET22 | E. coli BL21 | Inclusion bodies | [27] |
| 1E8, 1E6 (T-antigen E6-BDB) | scFv/pMT/Bip/V5-HIS | Drosophila S2 | Soluble | [28] |
3. Production and Characterization of Anti-T- and Tn-Antigen Antibodies
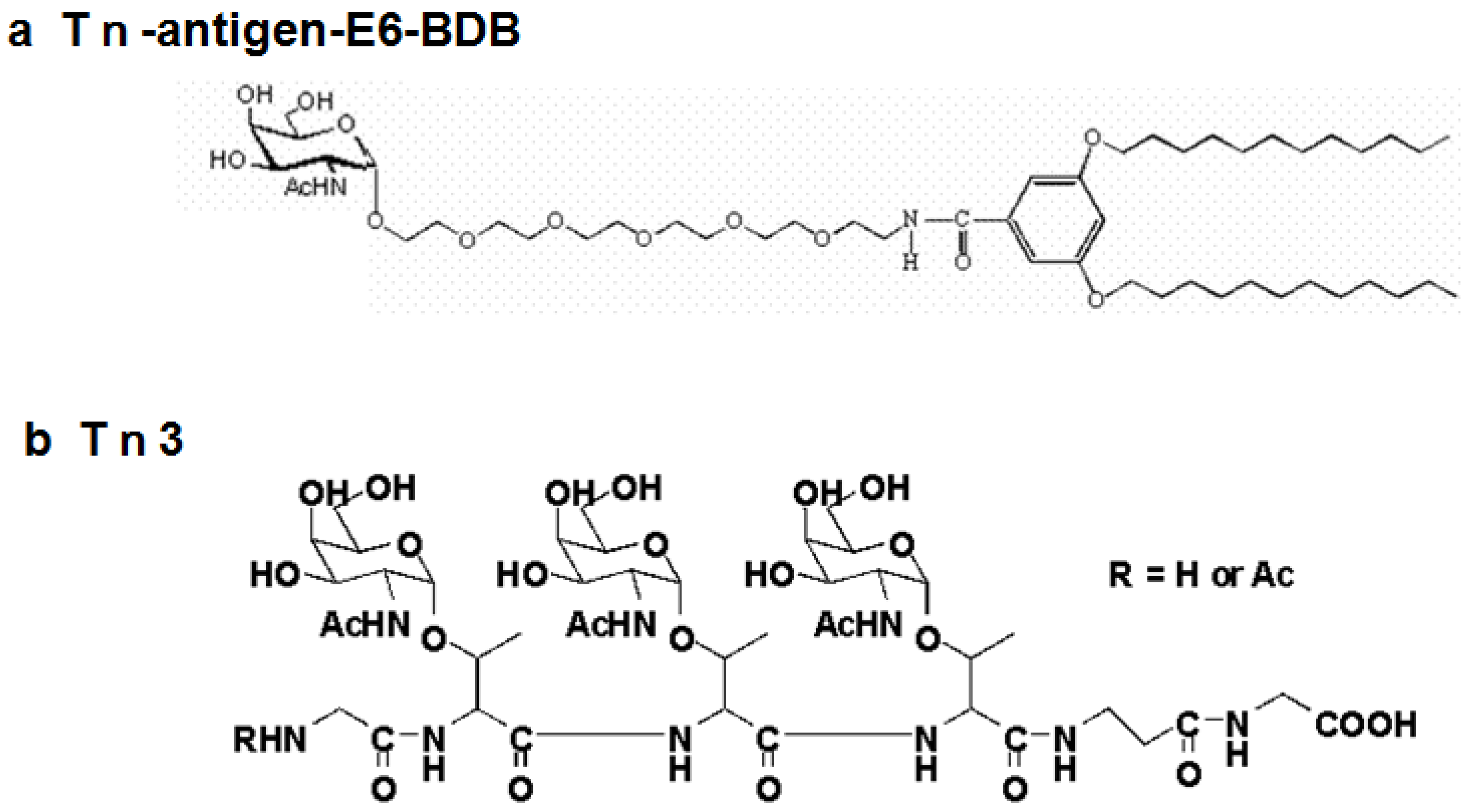
4. Purification and Characterization of Anti-Carbohydrate Antibodies Expressed in E. coli Cells
4.1. Preparation of Soluble scFv Proteins from Phagemid-Infected E. coli Cells
4.2. Purification and Characterization of scFv Proteins as Fusion Proteins
4.3. Expression of scFv Proteins in E. coli by An Inducible T7 Expression System
5. Purification and Characterization of Anti-Carbohydrate Antibodies Expressed in Drosophila S2 Cells
6. Conclusion
Acknowledgments
Conflicts of Interest
References
- Vos, Q.; Lees, A.; Wu, Z.Q.; Snapper, C.M.; Mond, J.J. B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunol. Rev. 2000, 176, 154–570. [Google Scholar] [CrossRef]
- Heimburg-Molinaro, J.; Lum, M.; Vijay, G.; Jain, M.; Almogren, A.; Rittenhouse-Olson, K. Cancer vaccines and carbohydrate epitopes. Vaccine 2011, 29, 8802–8826. [Google Scholar] [CrossRef]
- Cunto-Amesty, G.; Dam, T.K.; Luo, P.; Monzavi-Karbassi, B.; Brewer, C.F.; Van Cott, T.C.; Kieber-Emmons, T. Directing the immune response to carbohydrate antigens. J. Biol. Chem. 2001, 276, 30490–30498. [Google Scholar] [CrossRef]
- Hakomori, S. Tumor-associated carbohydrate antigens defining tumor malignancy: Basis for development of anti-cancer vaccines. Adv. Exp. Med. Biol. 2001, 491, 369–402. [Google Scholar] [CrossRef]
- Marks, J.D.; Hoogenboom, H.R.; Bonnet, T.P.; McCafferty, J.; Griffiths, A.D.; Winter, G. By-passing immunization. Human antibodies from V-gene libraries displayed on phage. J. Mol. Biol. 1991, 222, 581–597. [Google Scholar] [CrossRef]
- Deng, S.; MacKenzie, C.R.; Sadowaka, J.; Michniewicz, J.; Young, N.M.; Bundle, D.R.; Narang, S.A. Selection of antibody single-chain variable fragments with improved carbohydrate binding by phage display. J. Biol. Chem. 1994, 269, 9533–9538. [Google Scholar]
- MacKenzie, C.R.; Hirama, T.; Deng, S.; Bundle, D.R.; Narang, S.A.; Young, N.M. Analysis by surface plasmon resonance of the influence of valence on the ligand binding affinity and kinetics of an anti-carbohydrate antibody. J. Biol. Chem. 1996, 271, 1527–1533. [Google Scholar]
- Springer, G.F. Immunoreactive T and Tn epitopes in cancer diagnosis, prognosis, and immunotherapy. J. Mol. Med. 1997, 75, 594–602. [Google Scholar]
- Van Kuppevelt, T.H.; Dennissen, M.A.B.A.; van Venrooij, W.J.; Hoet, R.M.A.; Veerkamp, J.H. Generation and application of type-specific anti-heparan sulfate antibodies using phage display technology. Further evidence for heparan sulfate heterogeneity in the kidney. J. Biol. Chem. 1998, 273, 12960–12966. [Google Scholar]
- Mao, S.; Gao, C.; Lo, C.-H.L.; Wirsching, P.; Wong, C.-H.; Janda, K.D. Phage-display library selection of high-affinity human single-chain antibodies to tumor-associated carbohydrate antigens sialyl Lewisx and Lewisx. Proc. Natl. Acad. Sci. USA 1999, 96, 6953–6958. [Google Scholar] [CrossRef]
- Lee, K.J.; Mao, S.; Sun, C.; Gao, C.; Blixt, O.; Arrues, S.; Hom, L.G.; Kaufmann, G.F.; Hoffman, T.Z.; Coyle, A.R.; et al. Phage-display selection of a human single-chain fv antibody highly specific for melanoma and breast cancer cells using a chemoenzymatically synthesized G(M3)-carbohydrate antigen. J. Am. Chem. Soc. 2002, 124, 12439–12446. [Google Scholar] [CrossRef]
- Ravn, P.; Danielczyk, A.; Jensen, K.B.; Kristensen, P.; Christensen, P.A.; Larsen, M.; Karsten, U.; Goletz, S. Multivalent scFv display of phagemid repertoires for the selection of carbohydrate-specific antibodies and its application to the Thomsen-Friedenreich antigen. J. Mol. Biol. 2004, 343, 985–996. [Google Scholar] [CrossRef]
- Stoll, M.S.; Mizuochi, T.; Childs, R.A.; Feizi, T. Improved procedure for the construction of neoglycolipids having antigenic and lectin-binding activities, from reducing oligosaccharides. J. Biochem. 1988, 256, 661–665. [Google Scholar]
- Shimizu, Y.; Nakata, M.; Matsunuma, J.; Mizuochi, T. Simultaneous quantification of components of neoglycolipid-coated liposomes using high-performance liquid chromatography with evaporative light scattering detection. J. Chromatogr. B. Biomed. Sci. Appl. 2001, 754, 127–133. [Google Scholar] [CrossRef]
- Sakai, K.; Shimizu, Y.; Chiba, T.; Matsumoto-Takasaki, A.; Kusada, Y.; Zhang, W.; Nakata, M.; Kojima, N.; Toma, K.; Takayanagi, A.; et al. Isolation and characterization of phage-displayed single chain antibodies recognizing non-reducing terminal mannose residues. 1. A new strategy for generation of anti-carbohydrate antibodies. Biochemistry 2007, 46, 253–262. [Google Scholar]
- Zhang, W.; Matsumoto-Takasaki, A.; Kusada, Y.; Sakaue, H.; Sakai, K.; Nakata, M.; Fujita-Yamaguchi, Y. Isolation and characterization of phage-displayed single chain antibodies recognizing non-reducing terminal mannose residues. 2. Expression, purification, and characterization of recombinant single chain antibodies. Biochemistry 2007, 46, 263–270. [Google Scholar]
- Vaughan, T.J.; Williams, A.J.; Pritchard, K.; Osbourn, J.K.; Pope, A.R.; Earnshaw, J.C.; McCafferty, J.; Hodits, R.A.; Wilton, J.; Johnson, K.S. Human antibodies with subnanomolar affinities isolated from a large non-immunized phage display library. Nat. Biotechnol. 1996, 14, 309–314. [Google Scholar] [CrossRef]
- Sblattero, D.; Bradbury, A. A definitive set of oligonucleotide primers for amplifying human V regions. Immunotechnology 1998, 3, 271–278. [Google Scholar] [CrossRef]
- Sheets, M.D.; Amersdorfer, P.; Finnern, R.; Sargent, P.; Lindovist, E.; Schier, R.; Hemingsen, G.; Wong, C.; Gerhart, J.C.; Marks, J.D. Efficient construction of a large nonimmune phage antibody library: The production of high-affinity human single-chain antibodies to protein antigens. Proc. Natl. Acad. Sci. USA 1998, 95, 6157–6162. [Google Scholar] [CrossRef]
- Yuasa, N.; Zhang, W.; Goto, T.; Sakaue, H.; Matsumoto-Takasaki, A.; Kimura, M.; Ohshima, H.; Tsuchida, Y.; Koizumi, T.; Sakai, K.; et al. Production of anti-carbohydrate antibodies by phage-display technologies: Potential impairment of cell growth as a result of endogenous expression. J. Biol. Chem. 2010, 285, 30587–30597. [Google Scholar] [CrossRef]
- Li, S.-L.; Liang, S.-J.; Guo, N.; Wu, A.M.; Fujita-Yamaguchi, Y. Single chain antibodies against human insulin-like growth factor-I receptor: Expression, purification, and effect on tumor growth. Cancer Immunol. Immunother. 2000, 49, 243–252. [Google Scholar]
- Guo, N.; Ye, J.-J.; Liang, S.-J.; Mineo, M.; Li, S.L.; Giannini, S.; Plymate, S.R.; Sike, R.A.; Fujita-Yamaguchi, Y. The role of insulin-like growth factor-II in cancer growth and progression evidenced by the use of ribozymes and prostate cancer progression models. Growth Horm. IGF Res. 2003, 13, 44–53. [Google Scholar] [CrossRef]
- Sachdev, D.; Li, S.-L; Hartell, J.S.; Fujita-Yamaguchi, Y.; Miller, J.S.; Yee, D. A chimeric humanized single chain antibody against the type I insulin-like growth factor receptor renders breast cancer cells refractory to the mitogenic effects of insulin-like growth factor-I. Cancer Res. 2003, 63, 627–635. [Google Scholar]
- Matsumoto-Takasaki, A.; Horie, J.; Sakai, K.; Furui, Y.; Sato, R.; Kawakami, H.; Toma, K.; Takayanagi, A.; Shimizu, N.; Fujita-Yamaguchi, Y. Isolation and characterization of anti-T-antigen single chain antibodies from a phage library. Biosci. Trends. 2007, 3, 87–95. [Google Scholar]
- Sakai, K.; Yuasa, N.; Tsukamoto, K.; Takasaki-Matsumoto, A.; Yajima, Y.; Sato, R.; Kawakami, H.; Mizuno, M.; Takayanagi, A.; Shimizu, N.; et al. Isolation and characterization of antibodies against three consecutive Tn-antigen clusters from a phage library displaying human single-chain variable fragments. J. Biochem. 2010, 147, 809–817. [Google Scholar]
- Matsumoto-Takasaki, A.; Yuasa, N.; Katagiri, D.; Koyama, T.; Sakai, K.; Zamri, N.; Phung, S.; Chen, S.; Nakada, H.; Nakata, M.; et al. Characterization of three different single chain antibodies recognizing nonreducing terminal mannose residues expressed in Escherichia coli by an inducible T7 expression system. J. Biochem. 2011, 150, 439–450. [Google Scholar]
- Yuasa, N.; Koyama, T.; Fujita-Yamaguchi, Y. Purification and refolding of anti-T-antigen single chain antibodies (scFvs) expressed in E. coli as inclusion bodies. Biosci. Trends 2014, 8, 24–31. [Google Scholar]
- Yuasa, N.; Koyama, T.; Subedi, G.P.; Yamaguchi, Y.; Matsushita, M.; Fujita-Yamaguchi, Y. Expression and structural characterization of anti-T-antigen single chain antibodies (scFvs) and analysis of their binding to T-antigen by surface plasmon resonance and NMR spectroscopy. J. Biochem. 2013, 154, 521–529. [Google Scholar]
- Numata, Y.Y.; Nakada, H.; Fukui, S.; Kitagawa, H.; Ozaki, H.; Inoue, K.; Kawasaki, M.; Funakoshi, T.; Yamashina, I. A monoclonal antibody directed to Tn antigen. Biochem. Biophys. Res. Commun. 1990, 170, 981–985. [Google Scholar] [CrossRef]
- Pancino, G.F.; Osinaga, E.; Vorauher, W.; Kakouche, A.; Mistro, D.; Charpin, C.; Roseto, A. Production of a monoclonal antibody as immunohistochemical marker on paraffin embedded tissues using a new immunization method. Hybridoma 1990, 9, 389–395. [Google Scholar] [CrossRef]
- Osinaga, E.; Bay, S.; Tello, D.; Babino, A.; Pritsch, O.; Assemat, K.; Cantacuzene, D.; Nakada, H.; Alzari, P. Analysis of the fine specificity of Tn-binding proteins using synthetic glycopeptide epitopes and a biosensor based on surface plasmon resonance spectroscopy. FEBS Lett. 2000, 469, 24–28. [Google Scholar]
- Schietinger, A.; Philip, M.; Yoshida, B.A.; Azadi, P.; Liu, H.; Meredith, S.C.; Schreiber, H. A mutant chaperone converts a wild-type protein into a tumor-specific antigen. Science 2006, 314, 304–308. [Google Scholar] [CrossRef]
- Matsumoto-Takasaki, A.; Hanashima, S.; Aoki, A.; Yuasa, N.; Ogawa, H.; Sato, R.; Kawakami, H.; Mizuno, M.; Nakada, H.; Yamaguchi, Y.; et al. Surface plasmon resonance and NMR analyses of anti Tn-antigen MLS128 monoclonal antibody binding to two or three consecutive Tn-antigen cluster. J. Biochem. 2012, 151, 273–282. [Google Scholar] [CrossRef]
- Yuasa, N.; Ogawa, H.; Koizumi, T.; Tsukamoto, K.; Matsumoto-Takasaki, A.; Asanuma, H.; Nakada, H.; Fujita-Yamaguchi, Y. Construction and expression of anti-Tn-antigen-specific single chain antibody genes from hybridoma producing MLS128 monoclonal antibody. J. Biochem. 2012, 151, 371–381. [Google Scholar]
- Subedi, G.P.; Satoh, T.; Hanashima, S.; Ikeda, A.; Nakada, H.; Sato, R.; Mizuno, M.; Yuasa, N.; Fujita-Yamaguchi, Y.; Yamaguchi, Y. Overproduction of anti-Tn antibody MLS128 single-chain Fv fragment in Escherichia coli cytoplasm using a novel pCold-PDI vector. Protein Expr. Puri. 2012, 82, 197–204. [Google Scholar] [CrossRef]
- Fujita-Yamaguchi, Y. Renewed interest in basic and applied research involving monoclonal antibodies against an oncofetal Tn-antigen. J. Biochem. 2013, 152, 103–105. [Google Scholar]
- Umetsu, M.; Tsumoto, K.; Hara, M.; Ashish, K.; Goda, S.; Adschiri, T.; Kumagai, I. How additives influence the refolding of immunoglobulin-folded proteins in a stepwise dialysis system. Spectroscopic evidence for highly efficient refolding of a single-chain Flv fragment. J. Biol. Chem. 2003, 278, 8979–8987. [Google Scholar]
- Gilmartin, A.A.; Lamp, B.; Rümenapf, T.; Persson, M.A.; Rey, F.A.; Krey, T. High-level secretion of recombinant monomeric murine and human single-chain Fv antibodies from Drosophila S2 cells. Protein Eng. Des. Sel. 2012, 25, 59–66. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fujita-Yamaguchi, Y. Production of Single-Chain Variable-Fragments against Carbohydrate Antigens. Antibodies 2014, 3, 155-168. https://doi.org/10.3390/antib3010155
Fujita-Yamaguchi Y. Production of Single-Chain Variable-Fragments against Carbohydrate Antigens. Antibodies. 2014; 3(1):155-168. https://doi.org/10.3390/antib3010155
Chicago/Turabian StyleFujita-Yamaguchi, Yoko. 2014. "Production of Single-Chain Variable-Fragments against Carbohydrate Antigens" Antibodies 3, no. 1: 155-168. https://doi.org/10.3390/antib3010155
APA StyleFujita-Yamaguchi, Y. (2014). Production of Single-Chain Variable-Fragments against Carbohydrate Antigens. Antibodies, 3(1), 155-168. https://doi.org/10.3390/antib3010155




