Guidelines in the Preparation of Fully Synthetic, Human Single-Domain Antibody Phage Display Libraries
Abstract
1. Introduction
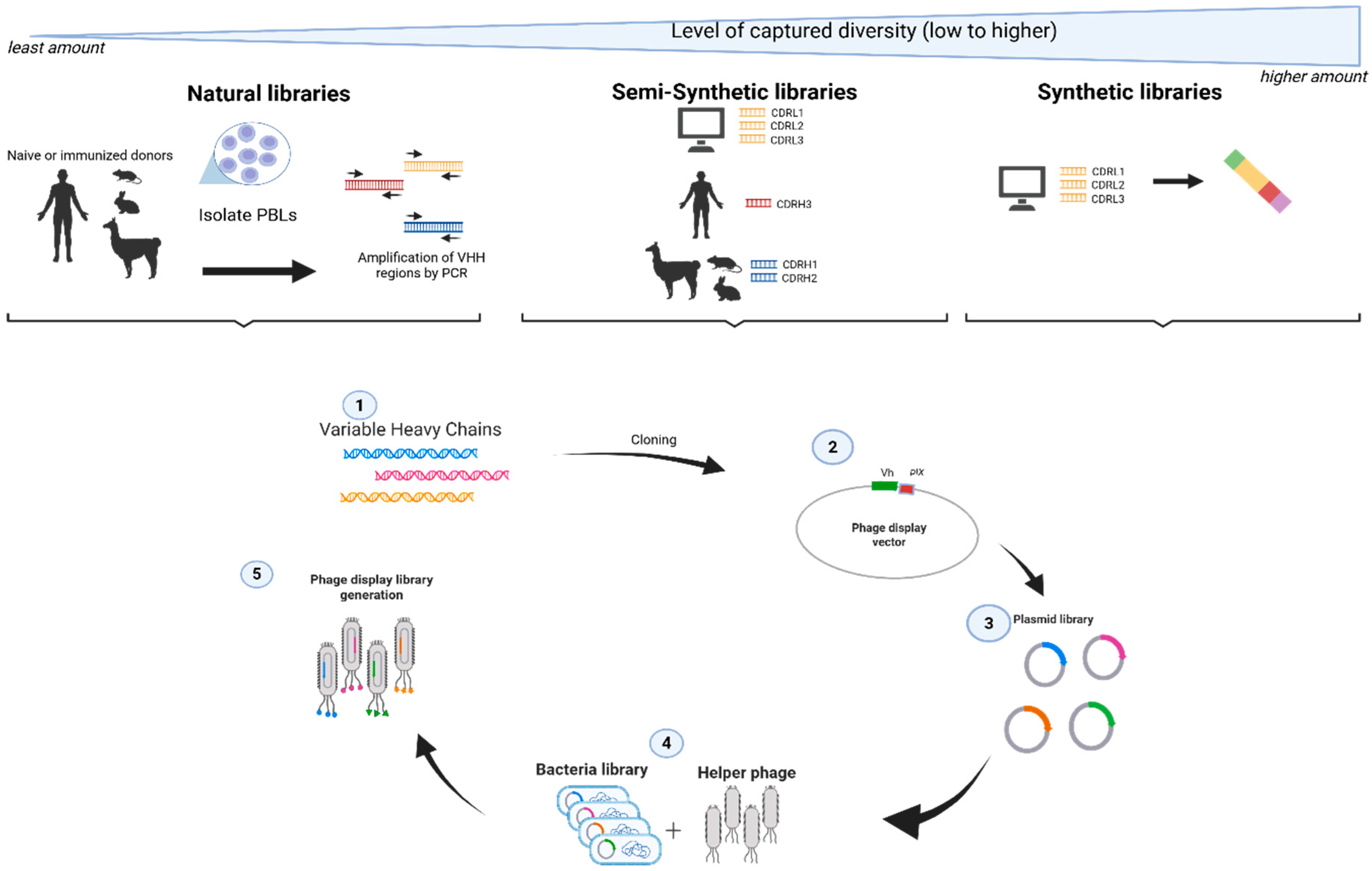
2. Materials and Methods
2.1. VHO Scaffold Phage Display Libraries
2.2. VHO Phage Biopanning to Generate Target Specific Candidates
2.3. Next Generation Sequencing and VHO Sequence Processing
2.4. VHO-Fc Expression Plasmid Construction via Automated Cloning
2.5. Transfections and Automated VHO-Fc Purifications
2.6. Biolayer Interferometry Kinetics
2.7. ELISA Binding
3. Results
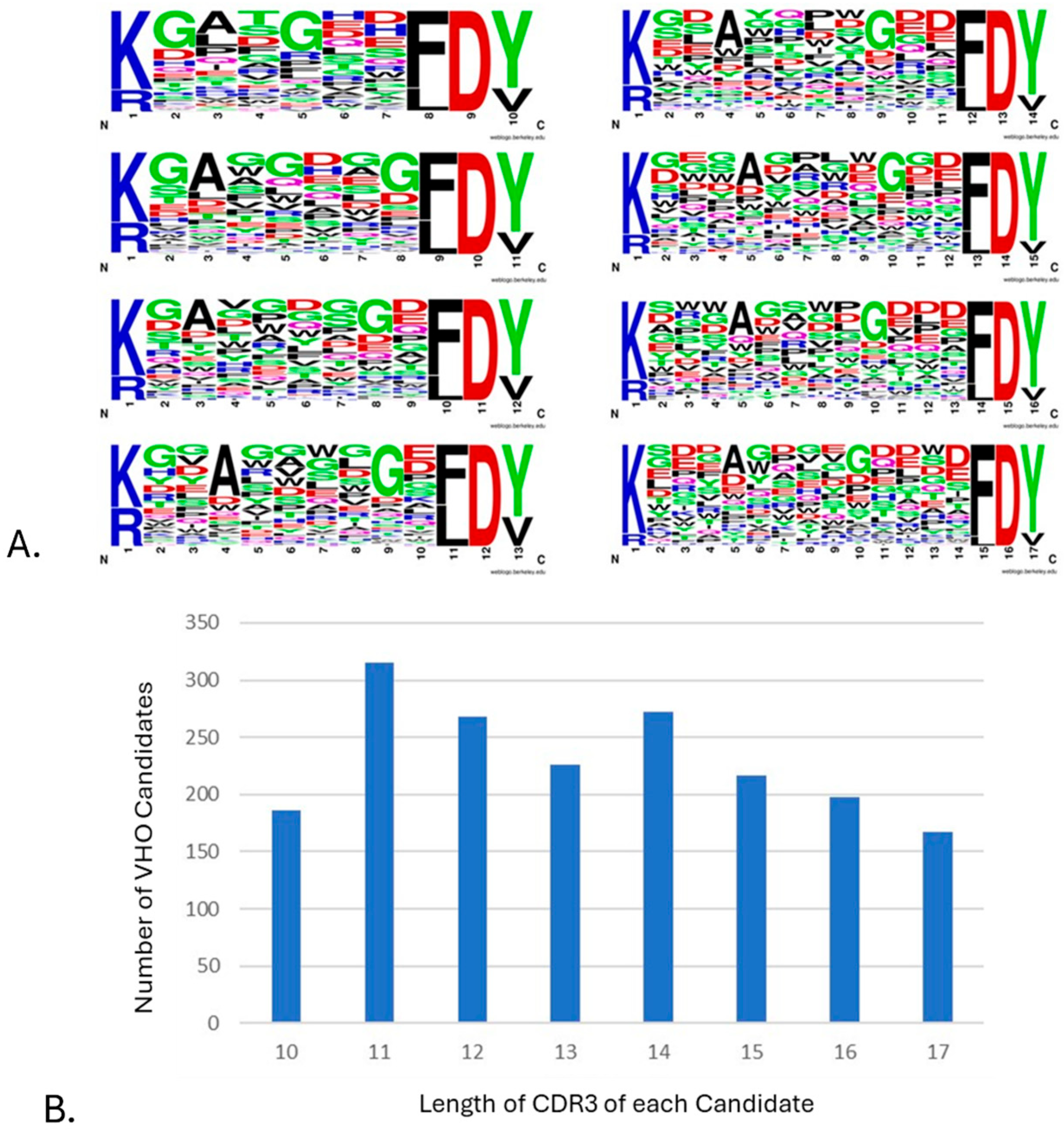
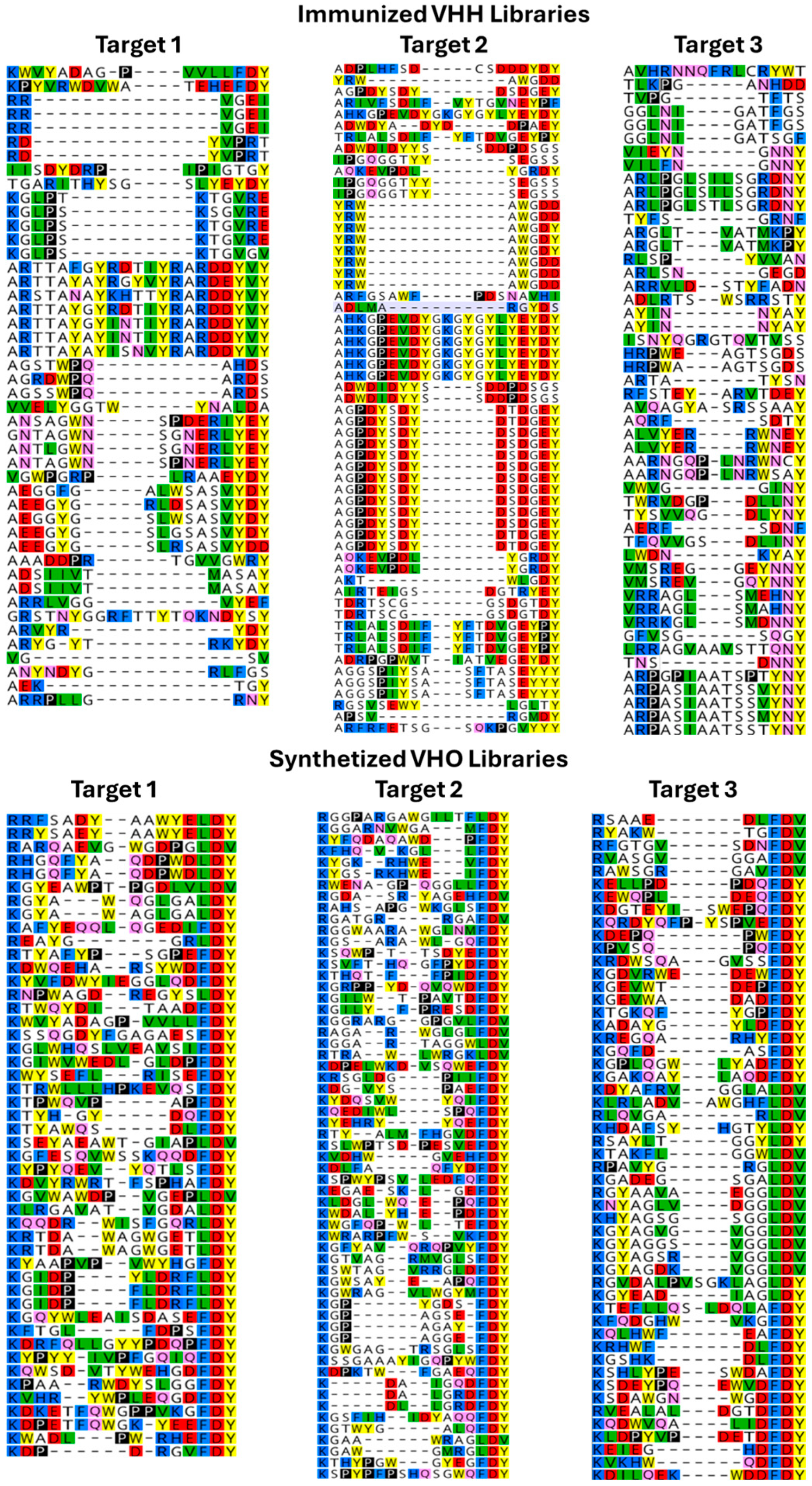
3.1. Library Design
3.2. Library QC
3.3. Biopanning Output (NGS Sequence)
3.4. Mammalian Expression of the Candidates
3.5. Candidate Activity Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Fiebig, D.; Bogen, J.P.; Carrara, S.C.; Deweid, L.; Zielonka, S.; Grzeschik, J.; Hock, B.; Kolmar, H. Streamlining the Transition From Yeast Surface Display of Antibody Fragment Immune Libraries to the Production as IgG Format in Mammalian Cells. Front. Bioeng. Biotechnol. 2022, 10, 794389. [Google Scholar] [CrossRef]
- Alfaleh, M.A.; Alsaab, H.O.; Mahmoud, A.B.; Alkayyal, A.A.; Jones, M.L.; Mahler, S.M.; Hashem, A.M. Phage Display Derived Monoclonal Antibodies: From Bench to Bedside. Front. Immunol. 2020, 11, 1986. [Google Scholar] [CrossRef]
- Nagano, K.; Tsutsumi, Y. Phage Display Technology as a Powerful Platform for Antibody Drug Discovery. Viruses 2021, 13, 178. [Google Scholar] [CrossRef]
- Banik, S.S.R.; Kushnir, N.; Doranz, B.J.; Chambers, R. Breaking barriers in antibody discovery: Harnessing divergent species for accessing difficult and conserved drug targets. MAbs 2023, 15, 2273018. [Google Scholar] [CrossRef] [PubMed]
- Cruz, E.; Kayser, V. Monoclonal antibody therapy of solid tumors: Clinical limitations and novel strategies to enhance treatment efficacy. Biologics 2019, 13, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Istomina, P.V.; Gorchakov, A.A.; Paoin, C.; Yamabhai, M. Phage display for discovery of anticancer antibodies. N. Biotechnol. 2024, 83, 205–218. [Google Scholar] [CrossRef]
- Mustafa, M.I.; Mohammed, A. Developing recombinant antibodies by phage display technology to neutralize viral infectious diseases. SLAS Discov. 2024, 29, 100140. [Google Scholar] [CrossRef]
- Lerner, R.A. Combinatorial antibody libraries: New advances, new immunological insights. Nat. Rev. Immunol. 2016, 16, 498–508. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory: New York, NY, USA, 1989. [Google Scholar]
- Richardson, S.M.; Nunley, P.W.; Yarrington, R.M.; Boeke, J.D.; Bader, J.S. GeneDesign 3.0 is an updated synthetic biology toolkit. Nucleic Acids Res. 2010, 38, 2603–2606. [Google Scholar] [CrossRef]
- Rossotti, M.A.; Bélanger, K.; Henry, K.A.; Tanha, J. Immunogenicity and humanization of single-domain antibodies. FEBS J. 2022, 289, 4304–4327. [Google Scholar] [CrossRef]
- Hülseweh, B.; Rülker, T.; Pelat, T.; Langermann, C.; Frenzel, A.; Schirrmann, T.; Dübel, S.; Thullier, P.; Hust, M. Human-like antibodies neutralizing Western equine encephalitis virus. MAbs 2014, 6, 718–727. [Google Scholar] [CrossRef]
- Frenzel, A.; Schirrmann, T.; Hust, M. Phage display-derived human antibodies in clinical development and therapy. MAbs 2016, 8, 1177–1194. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, Y.; He, Q.; Fu, J.; Wei, Q.; Lin, H.; Luo, Y.; Tu, Z. Sequence-based design and construction of synthetic nanobody library. Biotechnol. Bioeng. 2024, 121, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Honegger, A.; Malebranche, A.D.; Röthlisberger, D.; Plückthun, A. The influence of the framework core residues on the biophysical properties of immunoglobulin heavy chain variable domains. Protein Eng. Des. Sel. 2009, 22, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Laustsen, A.H.; Greiff, V.; Karatt-Vellatt, A.; Muyldermans, S.; Jenkins, T.P. Animal Immunization, in Vitro Display Technologies, and Machine Learning for Antibody Discovery. Trends Biotechnol. 2021, 39, 1263–1273. [Google Scholar] [CrossRef]
- Azevedo Reis Teixeira, A.; Erasmus, M.F.; D’Angelo, S.; Naranjo, L.; Ferrara, F.; Leal-Lopes, C.; Durrant, O.; Galmiche, C.; Morelli, A.; Scott-Tucker, A.; et al. Drug-like antibodies with high affinity, diversity and developability directly from next-generation antibody libraries. MAbs 2021, 13, 1980942. [Google Scholar] [CrossRef]
- Muyldermans, S. Nanobodies: Natural single-domain antibodies. Annu. Rev. Biochem. 2013, 82, 775–797. [Google Scholar] [CrossRef]
- Muyldermans, S. A guide to: Generation and design of nanobodies. FEBS J. 2021, 288, 2084–2102. [Google Scholar] [CrossRef]
- Ng, M.G.; Tan, H.Y.; Ng, P.Y.; Koh, R.Y.; Voon, K.G.L.; Chye, S.M. Cancer Antibody Engineering: Comparison of Mammalian, Yeast, Bacterial, Plants, Cell-free and Hybridoma Expression Systems. Curr. Pharm. Biotechnol. 2025, 26, 1797–1813. [Google Scholar] [CrossRef]
- Zhang, Y. Evolution of phage display libraries for therapeutic antibody discovery. MAbs 2023, 15, 2213793. [Google Scholar] [CrossRef]
- Mahdavi, S.Z.B.; Oroojalian, F.; Eyvazi, S.; Hejazi, M.; Baradaran, B.; Pouladi, N.; Tohidkia, M.R.; Mokhtarzadeh, A.; Muyldermans, S. An overview on display systems (phage, bacterial, and yeast display) for production of anticancer antibodies; advantages and disadvantages. Int. J. Biol. Macromol. 2022, 208, 421–442. [Google Scholar] [CrossRef] [PubMed]
- Ledsgaard, L.; Kilstrup, M.; Karatt-Vellatt, A.; McCafferty, J.; Laustsen, A.H. Basics of Antibody Phage Display Technology. Toxins 2018, 10, 236. [Google Scholar] [CrossRef] [PubMed]
- Glanville, J.; Zhai, W.; Berka, J.; Telman, D.; Huerta, G.; Mehta, G.R.; Ni, I.; Mei, L.; Sundar, P.D.; Day, G.M.R.; et al. Precise determination of the diversity of a combinatorial antibody library gives insight into the human immunoglobulin repertoire. Proc. Natl. Acad. Sci. USA 2009, 106, 20216–20221. [Google Scholar] [CrossRef] [PubMed]
- Tornetta, M.A.; Whitaker, B.P.; Cantwell, O.M.; Pisors, E.D.; Han, L.; MacWilliams, M.P.; Jiang, H.; Zhou, F.; Chiu, M.L. The process using a synthetic library that generates multiple diverse human single domain antibodies. Antib. Ther. 2024, 7, 283–294. [Google Scholar] [CrossRef]
- Hairul Bahara, N.H.; Chin, S.; Choong, Y.S.; Lim, T. Construction of a Semisynthetic Human VH Single-Domain Antibody Library and Selection of Domain Antibodies against-Crystalline of Mycobacterium tuberculosis. J. Biomol. Screen. 2015, 21, 35–43. [Google Scholar] [CrossRef]
- Pang, G.; Wang, R.; Yang, H.; Chai, M.; Gao, Y.; Chen, S.; Mao, T.; Du, L.; Lan, Y.; Li, S.; et al. A synthetic heavy chain variable domain antibody library (VHL) provides highly functional antibodies with favorable developability. Protein Sci. 2025, 34, e70090. [Google Scholar] [CrossRef]
- Sun, Z.; Li, W.; Mellors, J.W.; Orentas, R.; Dimitrov, D.S. Construction of a Large Size Human Immunoglobulin Heavy Chain Variable (VH) Domain Library, Isolation and Characterization of Novel Human Antibody VH Domains Targeting PD-L1 and CD22. Front. Immunol. 2022, 13, 869825. [Google Scholar] [CrossRef]
- Bracken, C.J.; Lim, S.A.; Solomon, P.; Rettko, N.J.; Nguyen, D.P.; Zha, B.S.; Schaefer, K.; Byrnes, J.R.; Zhou, J.; Lui, I.; et al. Bi-paratopic and multivalent VH domains block ACE2 binding and neutralize SARS-CoV-2. Nat. Chem. Biol. 2021, 17, 113–121. [Google Scholar] [CrossRef]
- Chen, W.; Zhu, Z.; Feng, Y.; Xiao, X.; Dimitrov, D.S. Construction of a large phage-displayed human antibody domain library with a scaffold based on a newly identified highly soluble, stable heavy chain variable domain. J. Mol. Biol. 2008, 382, 779–789. [Google Scholar] [CrossRef]
- Misson Mindrebo, L.E.; Mindrebo, J.T.; Tran, Q.; Wilkinson, M.C.; Smith, J.M.; Verma, M.; Casewell, N.R.; Lander, G.C.; Jardine, J.G. Importance of the Cysteine-Rich Domain of Snake Venom Prothrombin Activators: Insights Gained from Synthetic Neutralizing Antibodies. Toxins 2024, 16, 361. [Google Scholar] [CrossRef]
- Rouet, R.; Dudgeon, K.; Christ, D. Generation of human single domain antibody repertoires by Kunkel mutagenesis. Methods Mol. Biol. 2012, 907, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, C.; Xia, S.; Tian, X.; Kong, Y.; Wang, Z.; Gu, C.; Zhang, R.; Tu, C.; Xie, Y.; et al. Identification of Human Single-Domain Antibodies against SARS-CoV-2. Cell Host Microbe 2020, 27, 891–898.e895. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, I.; Egloff, P.; Hutter, C.A.; Arnold, F.M.; Stohler, P.; Bocquet, N.; Hug, M.N.; Huber, S.; Siegrist, M.; Hetemann, L.; et al. Synthetic single domain antibodies for the conformational trapping of membrane proteins. Elife 2018, 7, e34317. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Kumachi, S.; Matsunaga, Y.; Sato, M.; Wakabayashi-Nakao, K.; Masaki, H.; Yonehara, R.; Motohashi, M.; Nemoto, N.; Tsuchiya, M. Construction of a Humanized Artificial VHH Library Reproducing Structural Features of Camelid VHHs for Therapeutics. Antibodies 2022, 11, 10. [Google Scholar] [CrossRef]
- Sevy, A.M.; Chen, M.T.; Castor, M.; Sylvia, T.; Krishnamurthy, H.; Ishchenko, A.; Hsieh, C.M. Structure- and sequence-based design of synthetic single-domain antibody libraries. Protein Eng. Des. Sel. 2020, 33, gzaa028. [Google Scholar] [CrossRef]
- Moutel, S.; Bery, N.; Bernard, V.; Keller, L.; Lemesre, E.; de Marco, A.; Ligat, L.; Rain, J.-C.; Favre, G.; Olichon, A.; et al. NaLi-H1: A universal synthetic library of humanized nanobodies providing highly functional antibodies and intrabodies. eLife 2016, 5, e16228. [Google Scholar] [CrossRef]
- Nie, J.; Ma, X.; Hu, F.; Miao, H.; Feng, X.; Zhang, P.; Han, M.H.; You, F.; Yang, Y.; Zhang, W.; et al. Designing and constructing a phage display synthesized single domain antibodies library based on camel VHHs frame for screening and identifying humanized TNF-α-specific nanobody. Biomed. Pharmacother. 2021, 137, 111328. [Google Scholar] [CrossRef]
- Contreras, M.A.; Serrano-Rivero, Y.; González-Pose, A.; Salazar-Uribe, J.; Rubio-Carrasquilla, M.; Soares-Alves, M.; Parra, N.C.; Camacho-Casanova, F.; Sánchez-Ramos, O.; Moreno, E. Design and Construction of a Synthetic Nanobody Library: Testing Its Potential with a Single Selection Round Strategy. Molecules 2023, 28, 3708. [Google Scholar] [CrossRef]
- McMahon, C.; Baier, A.S.; Pascolutti, R.; Wegrecki, M.; Zheng, S.; Ong, J.X.; Erlandson, S.C.; Hilger, D.; Rasmussen, S.G.F.; Ring, A.M.; et al. Yeast surface display platform for rapid discovery of conformationally selective nanobodies. Nat. Struct. Mol. Biol. 2018, 25, 289–296. [Google Scholar] [CrossRef]
- Yan, J.; Li, G.; Hu, Y.; Ou, W.; Wan, Y. Construction of a synthetic phage-displayed Nanobody library with CDR3 regions randomized by trinucleotide cassettes for diagnostic applications. J. Transl. Med. 2014, 12, 343. [Google Scholar] [CrossRef]
- Chen, X.; Gentili, M.; Hacohen, N.; Regev, A. A cell-free nanobody engineering platform rapidly generates SARS-CoV-2 neutralizing nanobodies. Nat. Commun. 2021, 12, 5506. [Google Scholar] [CrossRef]
- Arras, P.; Yoo, H.B.; Pekar, L.; Clarke, T.; Friedrich, L.; Schröter, C.; Schanz, J.; Tonillo, J.; Siegmund, V.; Doerner, A.; et al. AI/ML combined with next-generation sequencing of VHH immune repertoires enables the rapid identification of de novo humanized and sequence-optimized single domain antibodies: A prospective case study. Front. Mol. Biosci. 2023, 10, 1249247. [Google Scholar] [CrossRef]
- Salhi, I.; Bessalah, S.; Snoun, D.; Khorchani, T.; Hammadi, M. Construction of a Nanobodies Phage Display Library From an Escherichia coli Immunized Dromedary. Iran. J. Biotechnol. 2020, 18, e2247. [Google Scholar] [CrossRef]
- Ganji, M.; Safarzadeh Kozani, P.; Rahbarizadeh, F. Characterization of novel CD19-specific VHHs isolated from a camelid immune library by phage display. J. Transl. Med. 2023, 21, 891. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, N.; Murakami, A.; Motohashi, M.; Nakayama, H.; Kondo, Y.; Ito, Y.; Azuma, T.; Kishimoto, H. An alpaca single-domain antibody (VHH) phage display library constructed by CDR shuffling provided high-affinity VHHs against desired protein antigens. Int. Immunol. 2022, 34, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Feng, Y.; Ma, X.; Li, J. A camel anti-lysozyme CDR3 only domain antibody selected from phage display VHH library acts as potent lysozyme inhibitor. Acta Biochim. Biophys. Sin. 2017, 49, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, F.; Lu, Y.; Hu, H.; Wang, J.; Guo, C.; Deng, Q.; Liao, C.; Wu, Q.; Hu, T.; et al. Highly potent multivalent VHH antibodies against Chikungunya isolated from an alpaca naïve phage display library. J. Nanobiotechnology 2022, 20, 231. [Google Scholar] [CrossRef]
- Xu, Y.; Xiong, L.; Li, Y.; Xiong, Y.; Tu, Z.; Fu, J.; Chen, B. Anti-idiotypic nanobody as citrinin mimotope from a naive alpaca heavy chain single domain antibody library. Anal. Bioanal. Chem. 2015, 407, 5333–5341. [Google Scholar] [CrossRef]
- Lefranc, M.P.; Giudicelli, V.; Ginestoux, C.; Bodmer, J.; Müller, W.; Bontrop, R.; Lemaitre, M.; Malik, A.; Barbié, V.; Chaume, D. IMGT, the international ImMunoGeneTics database. Nucleic Acids Res. 1999, 27, 209–212. [Google Scholar] [CrossRef]
- Dondelinger, M.; Filée, P.; Sauvage, E.; Quinting, B.; Muyldermans, S.; Galleni, M.; Vandevenne, M.S. Understanding the Significance and Implications of Antibody Numbering and Antigen-Binding Surface/Residue Definition. Front. Immunol. 2018, 9, 2278. [Google Scholar] [CrossRef]
- Shi, L.; Wheeler, J.C.; Sweet, R.W.; Lu, J.; Luo, J.; Tornetta, M.; Whitaker, B.; Reddy, R.; Brittingham, R.; Borozdina, L.; et al. De novo selection of high-affinity antibodies from synthetic fab libraries displayed on phage as pIX fusion proteins. J. Mol. Biol. 2010, 397, 385–396. [Google Scholar] [CrossRef]
- Kayushin, A.L.; Korosteleva, M.D.; Miroshnikov, A.I.; Kosch, W.; Zubov, D.; Piel, N. A convenient approach to the synthesis of trinucleotide phosphoramidites--synthons for the generation of oligonucleotide/peptide libraries. Nucleic Acids Res. 1996, 24, 3748–3755. [Google Scholar] [CrossRef]
- Kabat, E.A.; Wu, T.T. Identical V region amino acid sequences and segments of sequences in antibodies of different specificities. Relative contributions of VH and VL genes, minigenes, and complementarity-determining regions to binding of antibody-combining sites. J. Immunol. 1991, 147, 1709–1719. [Google Scholar] [CrossRef] [PubMed]
- Crooks, G.E.; Hon, G.; Chandonia, J.M.; Brenner, S.E. WebLogo: A Sequence Logo Generator. Available online: https://weblogo.berkeley.edu/ (accessed on 1 July 2024).
- Chaves, E.J.F.; Coêlho, D.F.; Cruz, C.H.B.; Moreira, E.G.; Simões, J.C.M.; Nascimento-Filho, M.J.; Lins, R.D. Structure-based computational design of antibody mimetics: Challenges and perspectives. FEBS Open Bio 2025, 15, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Baral, T.N.; MacKenzie, R.; Arbabi Ghahroudi, M. Single-domain antibodies and their utility. Curr. Protoc. Immunol. 2013, 103, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Arnaout, R.; Lee, W.; Cahill, P.; Honan, T.; Sparrow, T.; Weiand, M.; Nusbaum, C.; Rajewsky, K.; Koralov, S.B. High-resolution description of antibody heavy-chain repertoires in humans. PLoS ONE 2011, 6, e22365. [Google Scholar] [CrossRef] [PubMed]
- Tiller, T.; Schuster, I.; Deppe, D.; Siegers, K.; Strohner, R.; Herrmann, T.; Berenguer, M.; Poujol, D.; Stehle, J.; Stark, Y.; et al. A fully synthetic human Fab antibody library based on fixed VH/VL framework pairings with favorable biophysical properties. MAbs 2013, 5, 445–470. [Google Scholar] [CrossRef]
- He, B.; Shuning, L.; Mengxin, X.; Yunqi, H.; Kexin, L.; Yuanyuan, W.; Yong, M.; Yanmei, Z.; Xinyu, Y.; Lin, L.; et al. Comparative global B cell receptor repertoire difference induced by SARS-CoV-2 infection or vaccination via single-cell V(D)J sequencing. Emerg. Microbes Infect. 2022, 11, 2007–2020. [Google Scholar] [CrossRef]
- Masaki, Y.; Onishi, Y.; Seio, K. Quantification of synthetic errors during chemical synthesis of DNA and its suppression by non-canonical nucleosides. Sci. Rep. 2022, 12, 12095. [Google Scholar] [CrossRef]
- Prassler, J.; Thiel, S.; Pracht, C.; Polzer, A.; Peters, S.; Bauer, M.; Nörenberg, S.; Stark, Y.; Kölln, J.; Popp, A.; et al. HuCAL PLATINUM, a synthetic Fab library optimized for sequence diversity and superior performance in mammalian expression systems. J. Mol. Biol. 2011, 413, 261–278. [Google Scholar] [CrossRef]
- Hairul Bahara, N.H.; Tye, G.J.; Choong, Y.S.; Ong, E.B.; Ismail, A.; Lim, T.S. Phage display antibodies for diagnostic applications. Biologicals 2013, 41, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Sheets, M.D.; Amersdorfer, P.; Finnern, R.; Sargent, P.; Lindquist, E.; Schier, R.; Hemingsen, G.; Wong, C.; Gerhart, J.C.; Marks, J.D. Efficient construction of a large nonimmune phage antibody library: The production of high-affinity human single-chain antibodies to protein antigens. Proc. Natl. Acad. Sci. USA 1998, 95, 6157–6162. [Google Scholar] [CrossRef]
- Kirchhofer, A.; Helma, J.; Schmidthals, K.; Frauer, C.; Cui, S.; Karcher, A.; Pellis, M.; Muyldermans, S.; Casas-Delucchi, C.S.; Cardoso, M.C.; et al. Modulation of protein properties in living cells using nanobodies. Nat. Struct. Mol. Biol. 2010, 17, 133–138. [Google Scholar] [CrossRef]
- Jank, L.; Pinto-Espinoza, C.; Duan, Y.; Koch-Nolte, F.; Magnus, T.; Rissiek, B. Current Approaches and Future Perspectives for Nanobodies in Stroke Diagnostic and Therapy. Antibodies 2019, 8, 5. [Google Scholar] [CrossRef]
- Hoey, R.J.; Eom, H.; Horn, J.R. Structure and development of single domain antibodies as modules for therapeutics and diagnostics. Exp. Biol. Med. 2019, 244, 1568–1576. [Google Scholar] [CrossRef]

| Library Name | Source of sdAb | Scaffold | Diversified Regions | Library Size | CDR3 Diversity After Biopanning or Screening *** (%) | Number of Bins or Epitopes Per Target |
|---|---|---|---|---|---|---|
| Tornetta et al. [25] | Synthetic | Human VH3 | CDR1,2,3 | 2 × 1010 | >80 (n = 20) | Average of 10 |
| Bahara et al. [26] | Synthetic | Human VH3-23 | CDR1,2,3 | 7 × 109 | NA | NA |
| Pang et al. [27] | Synthetic | Human IGHV 3-23_04 | CDR3 | 2.6 × 1011 | 35–58 (n = 2) | At least 4 |
| Sun et al. [28] | Naïve | Human VH3-7, 3-30, 4-34 | CDR2,3 | 1.3 × 1011 | NA | NA |
| Bracken et al. [29] | Synthetic | Human 4D5 | CDR1,2,3 | 5 × 1010 | NA | At least 2 |
| Chen, W. et al. [30] | Synthetic | Human m0_VH3-23 | CDR1,2,3 | 3 × 1010 | NA | NA |
| Mindrebo et al. [31] + | Synthetic | Human VH3-23 # | CDR3 | 3 × 109 | NA | At least 4 |
| Rouet et al. [32] | Synthetic | Human VH3 | CDR1,2,3 | 3 × 109 | 15–90 (n = 4) | At least 4 |
| Wu et al. [33] | Synthetic | Human VH3-66.01 | CDR1,2,3 | NA | NA | At least 3 |
| Zimmerman et al. [34] | Synthetic | camel | CDR1,2,3 | 9 × 1012 * | 24–77 (n = 3) ** | At least 3 |
| Murakami et al. [35] | Synthetic | camel | CDR1,2,3 | 1 × 1013 * | 73–81 (n = 2) | NA |
| Sevy et al. [36] | Synthetic | Camel ˄˄ | CDR1,2,3 | 1 × 109 | 12–25 (n = 2) | NA |
| Moutel et al. [37] | Synthetic | Camel ˄˄ | CDR1,2,3 | 3 × 109 | 26–46 (n = 2) | NA |
| Nie et al. [38] | Synthetic | Alpaca ˄˄ | CDR3 | 2 × 109 | 5/17 | NA |
| Contreras et al. [39] | Synthetic | camel | CDR1,2,3 | 2 × 108 | 95–100 (n = 3) | NA |
| McMahon et al. [40] | Synthetic | camel | CDR1,2,3 | 5 × 108 | NA | NA |
| Yan et al. [41] | Synthetic | camel | CDR3 | 2 × 109 | 2–7 (n = 2) | NA |
| Chen, X. et al. [42] | Synthetic | camel | CDR1,2,3 | >1010 * | NA | NA |
| Arras et al. [43] | Immunization | Llama ˄˄ | CDR1,2,3 | NA | 41 | NA |
| Salhi et al. [44] | Immunization | camel | Vh | 1 × 109 | NA | NA |
| Ganji et al. [45] | Immunization | camel | Vh | 6 × 109 | NA | NA |
| Tsukahara et al. [46] | Immunization | alpaca | Vh | 2 × 1010 | NA | NA |
| Qiu et al. [47] | Immunization | camel | CDR3 | 3 × 105 | 63 | NA |
| Tsukahara et al. [46] | Naïve | alpaca | Vh | 2 × 1010 | NA | NA |
| Li et al. [48] | Naïve | alpaca | Vh | 2 × 109 | 17 | At least 2 |
| Xu et al. [49] | Naïve | alpaca | Vh | 3 × 109 | 37 | At least 2 |
| Library NGS QC | Selected VHO Uniqueness | ||||
|---|---|---|---|---|---|
| Library Design | Raw MiSeq Reads | Merged 2 × 300 Reads | Correct Merged Sequences * | Searching only with CDR3 | Searching the full VHO |
| 1 | 5,560,747 | 5,320,435 (95.7%) | 3,647,173 (68.6%) | 1 | 0 |
| 2 | 4,882,291 | 4,654,140 (95.3%) | 1,697,155 (36.5%) | 25 | 2 |
| Library NGS QC | Selected VHO Uniqueness | ||||
| Library Design | Raw NextSeq Reads | Merged 2 × 300 Reads | Correct Merged Sequences * | By Searching only CDR3 | Searching the full VHO |
| 1 | 33,933,864 | 33,327,254 (98.2%) | 21,370,246 (64.1%) | 9 | 2 |
| 2 | 40,256,678 | 39,223,082 (97.4%) | 13,862,286 (35.3%) | 19 | 1 |
| Tumor Associated Antigen | VHO | VHO-Fc | Binding to Protein (BLI and/or ELISA) | Epitope/Activitybins | ||
|---|---|---|---|---|---|---|
| Candidates | Expressed | Human | Monkey | Mouse/Rat | (at Least) | |
| TAA1 | 64 | 65% | 90% | 90% | 7 ^ | |
| TAA2 | 64 | 84% | 85% | 83% | 92% | 10 |
| TAA3 | 48 | 67% | 99% | 66% | 50% | 15 |
| TAA4 | 80 | 96% | 85% | 83% | 10 | |
| TAA5 | 110 | 96% | 99% | 99% | 99% | 12 |
| TAA6 | 110 | 99% | 97% | 98% | 65% | 11 |
| TAA7 | 128 | 90% | 76% | 87% | 13 | |
| TAA8 | 64 | 97% | 94% | 97% | 22% | 4 ^ |
| TAA9 | 64 | 92% | 97% | 89% | 9 ^ | |
| TAA10 | 64 | 81% | 75% | 35% | 89% | 10 ^ |
| TAA11 | 64 | 72% | 85% | 78% | 8 ^ | |
| TAA12 | 54 | 87% | 96% | 10 ^ | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tornetta, M.A.; Whitaker, B.P.; Cantwell, O.M.; Haytko, P.N.; Pisors, E.D.; Zhou, F.; Chiu, M.L. Guidelines in the Preparation of Fully Synthetic, Human Single-Domain Antibody Phage Display Libraries. Antibodies 2025, 14, 71. https://doi.org/10.3390/antib14030071
Tornetta MA, Whitaker BP, Cantwell OM, Haytko PN, Pisors ED, Zhou F, Chiu ML. Guidelines in the Preparation of Fully Synthetic, Human Single-Domain Antibody Phage Display Libraries. Antibodies. 2025; 14(3):71. https://doi.org/10.3390/antib14030071
Chicago/Turabian StyleTornetta, Mark A., Brian P. Whitaker, Olivia M. Cantwell, Peter N. Haytko, Eileen D. Pisors, Fulai Zhou, and Mark L. Chiu. 2025. "Guidelines in the Preparation of Fully Synthetic, Human Single-Domain Antibody Phage Display Libraries" Antibodies 14, no. 3: 71. https://doi.org/10.3390/antib14030071
APA StyleTornetta, M. A., Whitaker, B. P., Cantwell, O. M., Haytko, P. N., Pisors, E. D., Zhou, F., & Chiu, M. L. (2025). Guidelines in the Preparation of Fully Synthetic, Human Single-Domain Antibody Phage Display Libraries. Antibodies, 14(3), 71. https://doi.org/10.3390/antib14030071







