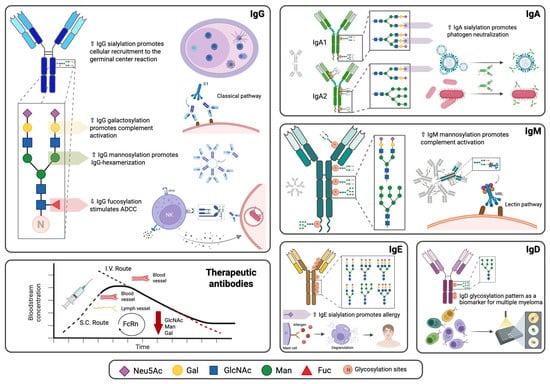
N-Glycosylation of Antibodies: Biological Effects During Infections and Therapeutic Applications
Abstract
1. Introduction
2. N-Glycosylation of the Fc Fragment of Antibodies
3. N-Glycosylation of the Fab Fragment of Antibodies
4. Biological Effects of N-Glycosylation of Antibodies
4.1. Effects of N-Glycosylation on Antibody Conformation
4.2. Effects of N-Glycosylation on Antibody Binding to Fc Receptors
4.3. Effects of N-Glycosylation on the Ability of Antibodies to Activate Complement
4.4. Effects of IgA N-Glycosylation on Its Ability to Neutralize the Adhesion of Bacteria and Viruses
5. Modification of Antibody N-Glycosylation During Infections
5.1. Modification of IgM N-Glycosylation in SARS-CoV-2 Infection
5.2. Modification of IgG N-Glycosylation in Tuberculosis
5.3. Modification of IgG N-Glycosylation in Viral Infections
6. Other Factors That Modify the N-Glycosylation of Antibodies
7. Vaccination-Induced Antibody N-Glycosylation Profiles
8. Importance of N-Glycosylation in Monoclonal Antibodies for Therapeutic Use
8.1. Effect of N-Glycosylation on the Pharmacokinetic Profile of Monoclonal Antibodies
8.2. Effect of N-Glycosylation on the Pharmacodynamic Profile of Monoclonal Antibodies
8.3. Effect of N-Glycosylation on the Safety Profile of Monoclonal Antibodies
9. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Damelang, T.; Brinkhaus, M.; van Osch, T.L.J.; Schuurman, J.; Labrijn, A.F.; Rispens, T.; Vidarsson, G. Impact of Structural Modifications of IgG Antibodies on Effector Functions. Front. Immunol. 2023, 14, 1304365. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Manabe, N.; Yamaguchi, Y. 3D Structures of Iga, Igm, and Components. Int. J. Mol. Sci. 2021, 22, 12776. [Google Scholar] [CrossRef]
- Kuijpers, T. Fc-Dependent Mechanisms of Action: Roles of FcγR and FcRn. Clin. Exp. Immunol. 2014, 178, 89–91. [Google Scholar] [CrossRef][Green Version]
- Bruhns, P.; Iannascoli, B.; England, P.; Mancardi, D.A.; Fernandez, N.; Jorieux, S.; Daë Ron, M. Specificity and Affinity of Human Fc Receptors and Their Polymorphic Variants for Human IgG Subclasses. Blood 2009, 113, 3716–3725. [Google Scholar] [CrossRef]
- McLean, M.R.; Lu, L.L.; Kent, S.J.; Chung, A.W. An Inflammatory Story: Antibodies in Tuberculosis Comorbidities. Front. Immunol. 2019, 10, 2846. [Google Scholar] [CrossRef] [PubMed]
- Napodano, C.; Marino, M.P.; Stefanile, A.; Pocino, K.; Scatena, R.; Gulli, F.; Rapaccini, G.L.; Delli Noci, S.; Capozio, G.; Rigante, D.; et al. Immunological Role of IgG Subclasses. Immunol. Investig. 2021, 50, 427–444. [Google Scholar] [CrossRef] [PubMed]
- Rispens, T.; Huijbers, M.G. The Unique Properties of IgG4 and Its Roles in Health and Disease. Nat. Rev. Immunol. 2023, 23, 763–778. [Google Scholar] [CrossRef]
- Ding, L.; Chen, X.; Cheng, H.; Zhang, T.; Li, Z. Advances in IgA Glycosylation and Its Correlation with Diseases. Front. Chem. 2022, 10, 974854. [Google Scholar] [CrossRef]
- Jones, K.; Savulescu, A.F.; Brombacher, F.; Hadebe, S. Immunoglobulin M in Health and Diseases: How Far Have We Come and What Next? Front. Immunol. 2020, 11, 595535. [Google Scholar] [CrossRef]
- Haslund-Gourley, B.; Woloszcuk, K.; Hou, J.; Connors, J.; Cusimano, G.; Bell, M.; Taramangalam, B.; Fourati, S.; Mege, N.; Bernui, M.; et al. IgM N-Glycosylation Correlates with COVID-19 Severity and Rate of Complement Deposition. Nat. Commun. 2023, 1, 404. [Google Scholar] [CrossRef]
- Arnold, J.N.; Radcliffe, C.M.; Wormald, M.R.; Royle, L.; Harvey, D.J.; Crispin, M.; Dwek, R.A.; Sim, R.B.; Rudd, P.M. The Glycosylation of Human Serum IgD and IgE and the Accessibility of Identified Oligomannose Structures for Interaction with Mannan-Binding Lectin1. J. Immunol. 2004, 173, 6831–6840. [Google Scholar] [CrossRef]
- Itoh, N.; Ohshima, Y. The Dual Aspects of IgD in the Development of Tolerance and the Pathogenesis of Allergic Diseases. Allergol. Int. 2023, 72, 227–233. [Google Scholar] [CrossRef]
- Shade, K.T.C.; Conroy, M.E.; Washburn, N.; Kitaoka, M.; Huynh, D.J.; Laprise, E.; Patil, S.U.; Shreffler, W.G.; Anthony, R.M. Sialylation of Immunoglobulin E Is a Determinant of Allergic Pathogenicity. Nature 2020, 582, 265–270. [Google Scholar] [CrossRef]
- Plattner, K.; Bachmann, M.F.; Vogel, M. On the Complexity of IgE: The Role of Structural Flexibility and Glycosylation for Binding Its Receptors. Front. Allergy 2023, 4, 1117611. [Google Scholar] [CrossRef]
- Nimmerjahn, F.; Vidarsson, G.; Cragg, M.S. Effect of Posttranslational Modifications and Subclass on IgG Activity: From Immunity to Immunotherapy. Nat. Immunol. 2023, 24, 1244–1255. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, P. O-GlcNAcylation and Immune Cell Signaling: A Review of Known and a Preview of Unknown. J. Biol. Chem. 2024, 300, 107349. [Google Scholar] [CrossRef] [PubMed]
- Schjoldager, K.T.; Narimatsu, Y.; Joshi, H.J.; Clausen, H. Global View of Human Protein Glycosylation Pathways and Functions. Nat. Rev. Mol. Cell Biol. 2020, 21, 729–749. [Google Scholar] [CrossRef] [PubMed]
- Freeze, H.H.; Boyce, M.; Zachara, N.E.; Hart, G.W.; Schnaar, R.L. Glycosylation Precursors. In Essentials of Glycobiology; Cold Spring Harbor Laboratory Press: Woodbury, NY, USA, 2022. [Google Scholar] [CrossRef]
- Jiménez del Val, I.; Constantinou, A.; Dell, A.; Haslam, S.; Polizzi, K.M.; Kontoravdi, C. A Quantitative and Mechanistic Model for Monoclonal Antibody Glycosylation as a Function of Nutrient Availability during Cell Culture. BMC Proc. 2013, 7, O10. [Google Scholar] [CrossRef]
- Wang, T.T. IgG Fc Glycosylation in Human Immunity. In Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2019; Volume 423, pp. 63–75. [Google Scholar]
- Hao, C.; Zou, Q.; Bai, X.; Shi, W. Effect of Glycosylation on Protein Folding: From Biological Roles to Chemical Protein Synthesis. iScience 2025, 28, 112605. [Google Scholar] [CrossRef]
- Trzos, S.; Link-Lenczowski, P.; Pocheć, E. The Role of N-Glycosylation in B-Cell Biology and IgG Activity. The Aspects of Autoimmunity and Anti-Inflammatory Therapy. Front. Immunol. 2023, 14, 1188838. [Google Scholar] [CrossRef]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in Health and Disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef]
- Stanley, P.; Moremen, K.W.; Lewis, N.E.; Taniguchi, N.; Aebi, M. N-Glycans. In Encyclopedia of Cell Biology: Volume 1–6, 2nd ed.; Cold Spring Harbor Laboratory Press: Woodbury, NY, USA, 2022; Volume 2, pp. 487–494. [Google Scholar]
- Hayes, J.M.; Cosgrave, E.F.J.; Struwe, W.B.; Wormald, M.; Davey, G.P.; Jefferis, R.; Rudd, P.M. Glycosylation and Fc Receptors. Curr. Top. Microbiol. Immunol. 2014, 382, 165–199. [Google Scholar]
- Esmail, S.; Manolson, M.F. Advances in Understanding N-Glycosylation Structure, Function, and Regulation in Health and Disease. Eur. J. Cell Biol. 2021, 100, 151186. [Google Scholar] [CrossRef]
- Irvine, E.B.; Alter, G. Understanding the Role of Antibody Glycosylation through the Lens of Severe Viral and Bacterial Diseases. Glycobiology 2021, 30, 241–253. [Google Scholar] [CrossRef]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG Subclasses and Allotypes: From Structure to Effector Functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef]
- Krištić, J.; Lauc, G. The Importance of IgG Glycosylation—What Did We Learn after Analyzing over 100,000 Individuals. Immunol. Rev. 2024, 328, 143–170. [Google Scholar] [CrossRef] [PubMed]
- Hui, G.K.; Wright, D.W.; Vennard, O.L.; Rayner, L.E.; Pang, M.; Yeo, S.C.; Gor, J.; Molyneux, K.; Barratt, J.; Perkins, S.J. The Solution Structures of Native and Patient Monomeric Human IgA1 Reveal Asymmetric Extended Structures: Implications for Function and IgAN Disease. Biochem. J. 2015, 471, 167–185. [Google Scholar] [CrossRef]
- Lombana, T.N.; Rajan, S.; Zorn, J.A.; Mandikian, D.; Chen, E.C.; Estevez, A.; Yip, V.; Bravo, D.D.; Phung, W.; Farahi, F.; et al. Production, Characterization, and In Vivo Half-Life Extension of Polymeric IgA Molecules in Mice. mAbs 2019, 11, 1122–1138. [Google Scholar] [CrossRef] [PubMed]
- Steffen, U.; Koeleman, C.A.; Sokolova, M.V.; Bang, H.; Kleyer, A.; Rech, J.; Unterweger, H.; Schicht, M.; Garreis, F.; Hahn, J.; et al. IgA Subclasses Have Different Effector Functions Associated with Distinct Glycosylation Profiles. Nat. Commun. 2020, 11, 120. [Google Scholar] [CrossRef] [PubMed]
- Shade, K.T.C.; Platzer, B.; Washburn, N.; Mani, V.; Bartsch, Y.C.; Conroy, M.; Pagan, J.D.; Bosques, C.; Mempel, T.R.; Fiebiger, E.; et al. A Single Glycan on IgE Is Indispensable for Initiation of Anaphylaxis. J. Exp. Med. 2015, 212, 457–467. [Google Scholar] [CrossRef]
- Mellis, S.J.; Baenziger, J.U. Structures of the Oligosaccharides Present at the Three Asparagine-Linked Glycosylation Sites of Human IgD. J. Biol. Chem. 1983, 258, 11546–11556. [Google Scholar] [CrossRef]
- Chen, J.; Fang, M.; Chen, X.; Yi, C.; Ji, J.; Cheng, C.; Wang, M.; Gu, X.; Sun, Q.; Gao, C. N-Glycosylation of Serum Proteins for the Assessment of Patients with IgD Multiple Myeloma. BMC Cancer 2017, 17, 881. [Google Scholar] [CrossRef]
- Van De Bovenkamp, F.S.; Derksen, N.I.L.; Ooijevaar-de Heer, P.; Van Schie, K.A.; Kruithof, S.; Berkowska, M.A.; Ellen van der Schoot, C.; IJspeert, H.; Van Der Burg, M.; Gils, A.; et al. Adaptive Antibody Diversification through N-Linked Glycosylation of the Immunoglobulin Variable Region. Proc. Natl. Acad. Sci. USA 2018, 115, 1901–1906. [Google Scholar] [CrossRef]
- Van de Bovenkamp, F.S.; Derksen, N.I.L.; van Breemen, M.J.; de Taeye, S.W.; Ooijevaar-de Heer, P.; Sanders, R.W.; Rispens, T. Variable Domain N-Linked Glycans Acquired during Antigen-Specific Immune Responses Can Contribute to Immunoglobulin G Antibody Stability. Front. Immunol. 2018, 9, 740. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Chen, X.; Zhao, C.; Liu, X.; Zhang, Z.; Li, T.; Sun, R.; Gu, H.; Gu, J. Sialylated Immunoglobulin G Can Neutralize Influenza Virus Infection through Receptor Mimicry. Oncotarget 2016, 7, 15606–15617. [Google Scholar] [CrossRef]
- Thaysen-Andersen, M.; Packer, N.H. Site-Specific Glycoproteomics Confirms That Protein Structure Dictates Formation of N-Glycan Type, Core Fucosylation and Branching. Glycobiology 2012, 22, 1440–1452. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Giddens, J.; Pincetic, A.; Lomino, J.V.; Ravetch, J.V.; Wang, L.X.; Bjorkman, P.J. Structural Characterization of Anti-Inflammatory Immunoglobulin G Fc Proteins. J. Mol. Biol. 2014, 426, 3166–3179. [Google Scholar] [CrossRef]
- Shade, K.T.; Conroy, M.E.; Anthony, R.M. IgE Glycosylation in Health and Disease. In Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2019; Volume 423, pp. 77–93. [Google Scholar]
- Zlatina, K.; Galuska, S.P. Immunoglobulin Glycosylation—An Unexploited Potential for Immunomodulatory Strategies in Farm Animals. Front. Immunol. 2021, 12, 753294. [Google Scholar] [CrossRef] [PubMed]
- Bournazos, S.; Gupta, A.; Ravetch, J.V. The Role of IgG Fc Receptors in Antibody-Dependent Enhancement. Nat. Rev. Immunol. 2020, 20, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Kao, D.; Danzer, H.; Collin, M.; Groß, A.; Eichler, J.; Stambuk, J.; Lauc, G.; Lux, A.; Nimmerjahn, F. A Monosaccharide Residue Is Sufficient to Maintain Mouse and Human IgG Subclass Activity and Directs IgG Effector Functions to Cellular Fc Receptors. Cell Rep. 2015, 13, 2376–2385. [Google Scholar] [CrossRef]
- Van Coillie, J.; Schulz, M.A.; Bentlage, A.E.H.; de Haan, N.; Ye, Z.; Geerdes, D.M.; van Esch, W.J.E.; Hafkenscheid, L.; Miller, R.L.; Narimatsu, Y.; et al. Role of N-Glycosylation in FcγRIIIa Interaction with IgG. Front. Immunol. 2022, 13, 987151. [Google Scholar] [CrossRef] [PubMed]
- Falconer, D.J.; Subedi, G.P.; Marcella, A.M.; Barb, A.W. Antibody Fucosylation Lowers the FcγRIIIa/CD16a Affinity by Limiting the Conformations Sampled by the N162-Glycan. ACS Chem. Biol. 2018, 13, 2179–2189. [Google Scholar] [CrossRef]
- Shinkawa, T.; Nakamura, K.; Yamane, N.; Shoji-Hosaka, E.; Kanda, Y.; Sakurada, M.; Uchida, K.; Anazawa, H.; Satoh, M.; Yamasaki, M.; et al. The Absence of Fucose but Not the Presence of Galactose or Bisecting N-Acetylglucosamine of Human IgG1 Complex-Type Oligosaccharides Shows the Critical Role of Enhancing Antibody-Dependent Cellular Cytotoxicity. J. Biol. Chem. 2003, 278, 3466–3473. [Google Scholar] [CrossRef]
- Dekkers, G.; Treffers, L.; Plomp, R.; Bentlage, A.E.H.; de Boer, M.; Koeleman, C.A.M.; Lissenberg-Thunnissen, S.N.; Visser, R.; Brouwer, M.; Mok, J.Y.; et al. Decoding the Human Immunoglobulin G-Glycan Repertoire Reveals a Spectrum of Fc-Receptor- and Complement-Mediated-Effector Activities. Front. Immunol. 2017, 8, 877. [Google Scholar] [CrossRef]
- Larsen, M.D.; Lopez-Perez, M.; Dickson, E.K.; Ampomah, P.; Tuikue Ndam, N.; Nouta, J.; Koeleman, C.A.M.; Ederveen, A.L.H.; Mordmüller, B.; Salanti, A.; et al. Afucosylated Plasmodium Falciparum-Specific IgG Is Induced by Infection but Not by Subunit Vaccination. Nat. Commun. 2021, 12, 5838. [Google Scholar] [CrossRef]
- Ackerman, M.E.; Crispin, M.; Yu, X.; Baruah, K.; Boesch, A.W.; Harvey, D.J.; Dugast, A.S.; Heizen, E.L.; Ercan, A.; Choi, I.; et al. Natural Variation in Fc Glycosylation of HIV-Specific Antibodies Impacts Antiviral Activity. J. Clin. Investig. 2013, 123, 2183–2192. [Google Scholar] [CrossRef]
- Bournazos, S.; Vo, H.T.M.; Duong, V.; Auerswald, H.; Ly, S.; Sakuntabhai, A.; Dussart, P.; Cantaert, T.; Ravetch, J.V. Antibody Fucosylation Predicts Disease Severity in Secondary Dengue Infection. Science 2021, 372, 1102–1105. [Google Scholar] [CrossRef]
- Larsen, M.D.; de Graaf, E.L.; Sonneveld, M.E.; Plomp, H.R.; Nouta, J.; Hoepel, W.; Chen, H.J.; Linty, F.; Visser, R.; Brinkhaus, M.; et al. Afucosylated IgG Characterizes Enveloped Viral Responses and Correlates with COVID-19 Severity. Science 2021, 371, eabc8378. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.L.; Lai, J.; Keck, R.; O’Connell, L.Y.; Hong, K.; Gloria Meng, Y.; Weikert, S.H.A.; Presta, L.G. Lack of Fucose on Human IgG1 N-Linked Oligosaccharide Improves Binding to Human FcγRIII and Antibody-Dependent Cellular Toxicity. J. Biol. Chem. 2002, 277, 26733–26740. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Nimmerjahn, F.; Ravetch, J. V Anti-Inflammatory Activity of Immunoglobulin G Resulting from Fc Sialylation. Science 2006, 313, 670–673. [Google Scholar] [CrossRef]
- Anthony, R.M.; Kobayashi, T.; Wermeling, F.; Ravetch, J.V. Intravenous Gammaglobulin Suppresses Inflammation through a Novel T H 2 Pathway. Nature 2011, 475, 110–114. [Google Scholar] [CrossRef]
- Vattepu, R.; Sneed, S.L.; Anthony, R.M. Sialylation as an Important Regulator of Antibody Function. Front. Immunol. 2022, 13, 818736. [Google Scholar] [CrossRef]
- Gao, C.; Chen, Q.; Hao, X.; Wang, Q. Immunomodulation of Antibody Glycosylation through the Placental Transfer. Int. J. Mol. Sci. 2023, 24, 16772. [Google Scholar] [CrossRef]
- Van Gool, M.M.J.; van Egmond, M. IgA and FcαRI: Versatile Players in Homeostasis, Infection, and Autoimmunity. Immunotargets Ther. 2020, 9, 351–372. [Google Scholar] [CrossRef]
- Gomes, M.M.; Wall, S.B.; Takahashi, K.; Novak, J.; Renfrow, M.B.; Herr, A.B. Analysis of IgA1 N-Glycosylation and Its Contribution to FcαRI Binding. Biochemistry 2008, 47, 11285–11299. [Google Scholar] [CrossRef]
- Göritzer, K.; Turupcu, A.; Maresch, D.; Novak, J.; Altmann, F.; Oostenbrink, C.; Obinger, C.; Strasser, R. Distinct Fcα Receptor N-Glycans Modulate the Binding Affinity to Immunoglobulin A (IgA) Antibodies. J. Biol. Chem. 2019, 294, 13995–14008. [Google Scholar] [CrossRef]
- Colucci, M.; Stöckmann, H.; Butera, A.; Masotti, A.; Baldassarre, A.; Giorda, E.; Petrini, S.; Rudd, P.M.; Sitia, R.; Emma, F.; et al. Sialylation of N-Linked Glycans Influences the Immunomodulatory Effects of IgM on T Cells. J. Immunol. 2015, 194, 151–157. [Google Scholar] [CrossRef]
- Wei, B.; Gao, X.; Cadang, L.; Izadi, S.; Liu, P.; Zhang, H.M.; Hecht, E.; Shim, J.; Magill, G.; Pabon, J.R.; et al. Fc Galactosylation Follows Consecutive Reaction Kinetics and Enhances Immunoglobulin G Hexamerization for Complement Activation. mAbs 2021, 13, 1893427. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, R.; Wormald, M.; Rudd, P.; Fischer, P.; Dwek, R.; Sim, R. Glycosylation Changes of IgG Associated with Rheumatooid Arthritis Can Activate Complement via the Mannose-Binding Protein. Nat. Med. 1995, 1, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Royle, L.; Roos, A.; Harvey, D.J.; Wormald, M.R.; Van Gijlswijk-Janssen, D.; Redwan, E.R.M.; Wilson, I.A.; Daha, M.R.; Dwek, R.A.; Rudd, P.M. Secretory IgA N- and O-Glycans Provide a Link between the Innate and Adaptive Immune Systems. J. Biol. Chem. 2003, 278, 20140–20153. [Google Scholar] [CrossRef] [PubMed]
- Goonatilleke, E.; Smilowitz, J.T.; Mariño, K.V.; German, B.J.; Lebrilla, C.B.; Barboza, M. Immunoglobulin A N-Glycosylation Presents Important Body Fluid-Specific Variations in Lactating Mothers. Mol. Cell. Proteom. 2019, 18, 2165–2177. [Google Scholar] [CrossRef]
- Schroten, H.; Stapper, C.; Plogmann, R.; Köhler, H.; Köhler, K.; Jö, J.; Hacker, J.; Hanisch, F.-G. Fab-Independent Antiadhesion Effects of Secretory Immunoglobulin A on S-Fimbriated Escherichia Coli Are Mediated by Sialyloligosaccharides. Infect. Immun. 1998, 66, 3971–3973. [Google Scholar] [CrossRef] [PubMed]
- Mathias, A.; Corthésy, B. N-Glycans on Secretory Component: Mediators of the Interaction between Secretory IgA and Gram-Positive Commensals Sustaining Intestinal Homeostasis. Gut Microbes 2011, 2, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.A.; Meyer, L.; Bianchi, M.; Turner, H.L.; Le, N.P.L.; Steck, M.; Wyrzucki, A.; Orlowski, V.; Ward, A.B.; Crispin, M.; et al. Glycosylation of Human IgA Directly Inhibits Influenza A and Other Sialic-Acid-Binding Viruses. Cell Rep. 2018, 23, 90–99. [Google Scholar] [CrossRef]
- Haycroft, E.R.; Damelang, T.; Lopez, E.; Rodgers, M.A.; Wines, B.D.; Hogarth, M.; Ameel, C.L.; Kent, S.J.; Scanga, C.A.; O’Connor, S.L.; et al. Antibody Glycosylation Correlates with Disease Progression in SIV-Mycobacterium Tuberculosis Coinfected Cynomolgus Macaques. Clin. Transl. Immunol. 2023, 12, e1474. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.L.; Chung, A.W.; Rosebrock, T.R.; Ghebremichael, M.; Yu, W.H.; Grace, P.S.; Schoen, M.K.; Tafesse, F.; Martin, C.; Leung, V.; et al. A Functional Role for Antibodies in Tuberculosis. Cell 2016, 167, 433–443.e14. [Google Scholar] [CrossRef]
- Grace, P.S.; Dolatshahi, S.; Lu, L.L.; Cain, A.; Palmieri, F.; Petrone, L.; Fortune, S.M.; Ottenhoff, T.H.M.; Lauffenburger, D.A.; Goletti, D.; et al. Antibody Subclass and Glycosylation Shift Following Effective TB Treatment. Front. Immunol. 2021, 12, 679973. [Google Scholar] [CrossRef]
- Schwedler, C.; Grzeski, M.; Kappert, K.; Rust, J.; Heymann, G.; Hoppe, B.; Blanchard, V. Coronavirus Disease 2019-Related Alterations of Total and Anti-Spike IgG Glycosylation in Relation to Age and Anti-Spike IgG Titer. Front. Microbiol. 2022, 13, 775186. [Google Scholar] [CrossRef]
- Pongracz, T.; Nouta, J.; Wang, W.; Van Meijgaarden, K.E.; Linty, F.; Vidarsson, G.; Joosten, S.A.; Ottenhoff, T.H.M.; Hokke, C.H.; De Vries, J.J.C.; et al. Immunoglobulin G1 Fc Glycosylation as an Early Hallmark of Severe COVID-19. eBioMedicine 2022, 78, 103957. [Google Scholar] [CrossRef]
- Hoepel, W.; Chen, H.-J.; Geyer, C.E.; Allahverdiyeva, S.; Manz, X.D.; de Taeye, S.W.; Aman, J.; Mes, L.; Steenhuis, M.; Griffith, G.R.; et al. High Titers and Low Fucosylation of Early Human Anti-SARS-CoV-2 IgG Promote Inflammation by Alveolar Macrophages. Sci. Transl. Med. 2021, 13, eabf8654. [Google Scholar] [CrossRef]
- Kljaković-Gašpić Batinjan, M.; Petrović, T.; Vučković, F.; Hadžibegović, I.; Radovani, B.; Jurin, I.; Đerek, L.; Huljev, E.; Markotić, A.; Lukšić, I.; et al. Differences in Immunoglobulin G Glycosylation Between Influenza and COVID-19 Patients. Engineering 2023, 26, 54–62. [Google Scholar] [CrossRef]
- Vadrevu, S.K.; Trbojevic-Akmacic, I.; Kossenkov, A.V.; Colomb, F.; Giron, L.B.; Anzurez, A.; Lynn, K.; Mounzer, K.; Landay, A.L.; Kaplan, R.C.; et al. Frontline Science: Plasma and Immunoglobulin G Galactosylation Associate with HIV Persistence during Antiretroviral Therapy. J. Leukoc. Biol. 2018, 104, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Giron, L.B.; Azzoni, L.; Yin, X.; Lynn, K.M.; Ross, B.N.; Fair, M.; Damra, M.; Sciorillo, A.C.; Liu, Q.; Jacobson, J.M.; et al. Hepatitis C Virus Modulates IgG Glycosylation in HIV Co-Infected Antiretroviral Therapy Suppressed Individuals. AIDS 2020, 34, 1461–1466. [Google Scholar] [CrossRef]
- Endy, T.P.; Nisalak, A.; Chunsuttitwat, S.; Vaughn, D.W.; Green, S.; Ennis, F.A.; Rothman, A.L.; Libraty, D.H. Relationship of Preexisting Dengue Virus (DV) Neutralizing Antibody Levels to Viremia and Severity of Disease in a Prospective Cohort Study of DV Infection in Thailand. J. Infect. Dis. 2004, 189, 990–1000. [Google Scholar] [CrossRef]
- Wang, T.T.; Sewatanon, J.; Memoli, M.J.; Wrammert, J.; Bournazos, S.; Bhaumik, S.K.; Pinsky, B.A.; Chokephaibulkit, K.; Onlamoon, N.; Pattanapanyasat, K.; et al. IgG Antibodies to Dengue Enhanced for FcgRIIIA Binding Determine Disease Severity Downloaded From. Science 2017, 355, 395–398. [Google Scholar] [CrossRef]
- Sastre, D.E.; Bournazos, S.; Du, J.; Boder, E.J.; Edgar, J.E.; Azzam, T.; Sultana, N.; Huliciak, M.; Flowers, M.; Yoza, L.; et al. Potent Efficacy of an IgG-Specific Endoglycosidase against IgG-Mediated Pathologies. Cell 2024, 187, 6994–7007.e12. [Google Scholar] [CrossRef]
- Mahan, A.E.; Jennewein, M.F.; Suscovich, T.; Dionne, K.; Tedesco, J.; Chung, A.W.; Streeck, H.; Pau, M.; Schuitemaker, H.; Francis, D.; et al. Antigen-Specific Antibody Glycosylation Is Regulated via Vaccination. PLoS Pathog. 2016, 12, e1005456. [Google Scholar] [CrossRef] [PubMed]
- Parekh, R.; Roitt, I.; Isenberg, D.; Dwek, R.; Rademacher, T. Age-Related Galactosylation of the N-Linked Oligosaccharides of Human Serum IgG. J. Exp. Med. 1988, 167, 1731–1736. [Google Scholar] [CrossRef] [PubMed]
- De Haan, N.; Reiding, K.R.; Driessen, G.; Van Der Burg, M.; Wuhrer, M. Changes in Healthy Human IgG Fc-Glycosylation after Birth and during Early Childhood. J. Proteome Res. 2016, 15, 1853–1861. [Google Scholar] [CrossRef]
- Sun, W.; Jian, X.; Zhang, J.; Meng, X.; Wang, H.; Zheng, D.; Wu, L.; Wang, Y. The Causality between Human Immunoglobulin G (IgG) N-Glycosylation and Aging: A Mendelian Randomization Study. Molecules 2024, 29, 1281. [Google Scholar] [CrossRef]
- Jennewein, M.F.; Kosikova, M.; Noelette, F.J.; Radvak, P.; Boudreau, C.M.; Campbell, J.D.; Chen, W.H.; Xie, H.; Alter, G.; Pasetti, M.F. Functional and Structural Modifications of Influenza Antibodies during Pregnancy. iScience 2022, 25, 104088. [Google Scholar] [CrossRef]
- Ruhaak, L.R.; Kim, K.; Stroble, C.; Taylor, S.L.; Hong, Q.; Miyamoto, S.; Lebrilla, C.B.; Leiserowitz, G. Protein-Specific Differential Glycosylation of Immunoglobulins in Serum of Ovarian Cancer Patients. J. Proteome Res. 2016, 15, 1002–1010. [Google Scholar] [CrossRef]
- Lomax-Browne, H.J.; Robertson, C.; Antonopoulos, A.; Leathem, A.J.C.; Haslam, S.M.; Dell, A.; Dwek, M.V. Serum IgA1 Shows Increased Levels of A2,6-Linked Sialic Acid in Breast Cancer. Interface Focus 2019, 9, 20180079. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cao, X.; Liu, C.; Li, W.; Zeng, W.; Li, B.; Chi, H.; Liu, M.; Qin, X.; Tang, L.; et al. N-Glycopeptide Signatures of IgA2 in Serum from Patients with Hepatitis b Virus-Related Liver Diseases. Mol. Cell. Proteom. 2019, 18, 2262–2272. [Google Scholar] [CrossRef]
- Wang, J.; Balog, C.I.A.; Stavenhagen, K.; Koeleman, C.A.M.; Scherer, H.U.; Selman, M.H.J.; Deelder, A.M.; Huizinga, T.W.J.; Toes, R.E.M.; Wuhrer, M. Fc-Glycosylation of IgG1 Is Modulated by B-Cell Stimuli. Mol. Cell. Proteom. 2011, 10, M110-004655. [Google Scholar] [CrossRef]
- Cao, Y.; Song, Z.; Guo, Z.; Zhao, X.; Gong, Y.; Zhao, K.; Qu, C.; Huang, Y.; Li, Y.; Gao, Y.; et al. Cytokines in the Immune Microenvironment Change the Glycosylation of IgG by Regulating Intracellular Glycosyltransferases. Front. Immunol. 2022, 12, 724379. [Google Scholar] [CrossRef]
- Lofano, G.; Gorman, M.J.; Yousif, A.S.; Yu, W.-H.; Fox, J.M.; Dugast, A.-S.; Ackerman, M.E.; Suscovich, T.J.; Weiner, J.; Barouch, D.; et al. Antigen-Specific Antibody Fc Glycosylation Enhances Humoral Immunity via the Recruitment of Complement. Sci. Immunol. 2018, 3, eaat7796. [Google Scholar] [CrossRef] [PubMed]
- Van Coillie, J.; Pongracz, T.; Rahmöller, J.; Chen, H.J.; Geyer, C.E.; van Vught, L.A.; Buhre, J.S.; Šuštić, T.; van Osch, T.L.J.; Steenhuis, M.; et al. The BNT162b2 MRNA SARS-CoV-2 Vaccine Induces Transient Afucosylated IgG1 in Naive but Not in Antigen-Experienced Vaccinees. eBioMedicine 2023, 87, 104408. [Google Scholar] [CrossRef] [PubMed]
- Selman, M.H.J.; De Jong, S.E.; Soonawala, D.; Kroon, F.P.; Adegnika, A.A.; Deelder, A.M.; Hokke, C.H.; Yazdanbakhsh, M.; Wuhrer, M. Changes in Antigen-Specific IgG1 Fc N-Glycosylation upon Influenza and Tetanus Vaccination. Mol. Cell. Proteom. 2012, 11, M111.014563. [Google Scholar] [CrossRef]
- Wang, T.T.; Maamary, J.; Tan, G.S.; Bournazos, S.; Davis, C.W.; Krammer, F.; Schlesinger, S.J.; Palese, P.; Ahmed, R.; Ravetch, J.V. Anti-HA Glycoforms Drive B Cell Affinity Selection and Determine Influenza Vaccine Efficacy. Cell 2015, 162, 160–169. [Google Scholar] [CrossRef]
- Buhre, J.S.; Pongracz, T.; Künsting, I.; Lixenfeld, A.S.; Wang, W.; Nouta, J.; Lehrian, S.; Schmelter, F.; Lunding, H.B.; Dühring, L.; et al. MRNA Vaccines against SARS-CoV-2 Induce Comparably Low Long-Term IgG Fc Galactosylation and Sialylation Levels but Increasing Long-Term IgG4 Responses Compared to an Adenovirus-Based Vaccine. Front. Immunol. 2023, 13, 1020844. [Google Scholar] [CrossRef]
- Van Coillie, J.; Pongracz, T.; Šuštić, T.; Wang, W.; Nouta, J.; Le Gars, M.; Keijzer, S.; Linty, F.; Cristianawati, O.; Keijser, J.B.D.; et al. Comparative Analysis of Spike-Specific IgG Fc Glycoprofiles Elicited by Adenoviral, MRNA, and Protein-Based SARS-CoV-2 Vaccines. iScience 2023, 26, 107619. [Google Scholar] [CrossRef]
- Farkash, I.; Feferman, T.; Cohen-Saban, N.; Avraham, Y.; Morgenstern, D.; Mayuni, G.; Barth, N.; Lustig, Y.; Miller, L.; Shouval, D.S.; et al. Anti-SARS-CoV-2 Antibodies Elicited by COVID-19 MRNA Vaccine Exhibit a Unique Glycosylation Pattern. Cell Rep. 2021, 37, 110114. [Google Scholar] [CrossRef]
- Bartsch, Y.C.; Eschweiler, S.; Leliavski, A.; Lunding, H.B.; Wagt, S.; Petry, J.; Lilienthal, G.M.; Rahmöller, J.; de Haan, N.; Hölscher, A.; et al. IgG Fc Sialylation Is Regulated during the Germinal Center Reaction Following Immunization with Different Adjuvants. J. Allergy Clin. Immunol. 2020, 146, 652–666.e11. [Google Scholar] [CrossRef] [PubMed]
- Vaccari, M.; Gordon, S.N.; Fourati, S.; Schifanella, L.; Liyanage, N.P.M.; Cameron, M.; Keele, B.F.; Shen, X.; Tomaras, G.D.; Billings, E.; et al. Adjuvant-Dependent Innate and Adaptive Immune Signatures of Risk of SIVmac251 Acquisition. Nat. Med. 2016, 22, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.K.H. The History of Monoclonal Antibody Development—Progress, Remaining Challenges and Future Innovations. Ann. Med. Surg. 2014, 3, 113–116. [Google Scholar] [CrossRef]
- Crescioli, S.; Kaplon, H.; Wang, L.; Visweswaraiah, J.; Kapoor, V.; Reichert, J.M. Antibodies to Watch in 2025. mAbs 2025, 17, 2443538. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, L.; Voglmeir, J. MAbs N-Glycosylation: Implications for Biotechnology and Analytics. Carbohydr. Res. 2022, 514, 108541. [Google Scholar] [CrossRef]
- Monoclonal Antibody Therapeutics Market Growth, Drivers, and Opportunities. Available online: https://www.marketsandmarkets.com/Market-Reports/monoclonal-antibody-mabs-therapeutics-market-115323820.html (accessed on 5 August 2025).
- Thakkar, S.; Chopra, A.; Nagendra, L.; Kalra, S.; Bhattacharya, S. Teplizumab in Type 1 Diabetes Mellitus: An Updated Review. touchREV. Endocrinol. 2023, 19, 22–30. [Google Scholar] [CrossRef]
- Xu, N.; Ma, C.; Ou, J.; Sun, W.W.; Zhou, L.; Hu, H.; Liu, X.M. Comparative Proteomic Analysis of Three Chinese Hamster Ovary (CHO) Host Cells. Biochem. Eng. J. 2017, 124, 122–129. [Google Scholar] [CrossRef]
- Kunert, R.; Reinhart, D. Advances in Recombinant Antibody Manufacturing. Appl. Microbiol. Biotechnol. 2016, 100, 3451–3461. [Google Scholar] [CrossRef]
- William, R.S.; Lila, M. Strohl Therapeutic Antibody Classes. In Therapeutic Antibody Engineering; Elsevier: Amsterdam, The Netherlands, 2012; pp. 197–595. [Google Scholar]
- Gangwar, N.; Dixit, N.; Rathore, A.S. N-Glycosylation Modulators for Targeted Manipulation of Glycosylation for Monoclonal Antibodies. Appl. Microbiol. Biotechnol. 2025, 109, 16. [Google Scholar] [CrossRef]
- Beck, A.; Liu, H. Macro-and Micro-Heterogeneity of Natural and Recombinant IgG Antibodies. Antibodies 2019, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Liu, L. Pharmacokinetics of Monoclonal Antibodies and Fc-Fusion Proteins. Protein Cell 2018, 9, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Lee, K.H. Characterization of Monoclonal Antibody Glycan Heterogeneity Using Hydrophilic Interaction Liquid Chromatography-Mass Spectrometry. Front. Bioeng. Biotechnol. 2022, 9, 805788. [Google Scholar] [CrossRef]
- Anthony, R.M.; Nimmerjahn, F.; Ashline, D.J.; Reinhold, V.N.; Paulson, J.C.; Ravetch, J.V. A Recombinant IgG Fc that Recapitulates the Anti-Inflammatory Activity of IVIG; Science: New York, NY, USA, 2008. [Google Scholar]
- Siebert, H.C.; Rosen, J.; Seyrek, K.; Kaltner, H.; André, S.; Bovin, N.V.; Nyholm, P.G.; Sinowatz, F.; Gabius, H.J. A2,3/A2,6-Sialylation of N-Glycans: Non-Synonymous Signals with Marked Developmental Regulation in Bovine Reproductive Tracts. Biochimie 2006, 88, 399–410. [Google Scholar] [CrossRef]
- Chung, C.Y.; Wang, Q.; Yang, S.; Yin, B.; Zhang, H.; Betenbaugh, M. Integrated Genome and Protein Editing Swaps α-2,6 Sialylation for α-2,3 Sialic Acid on Recombinant Antibodies from CHO. Biotechnol. J. 2017, 12, 1600502. [Google Scholar] [CrossRef]
- Lin, N.; Mascarenhas, J.; Sealover, N.R.; George, H.J.; Brooks, J.; Kayser, K.J.; Gau, B.; Yasa, I.; Azadi, P.; Archer-Hartmann, S. Chinese Hamster Ovary (CHO) Host Cell Engineering to Increase Sialylation of Recombinant Therapeutic Proteins by Modulating Sialyltransferase Expression. Biotechnol. Prog. 2015, 31, 334–346. [Google Scholar] [CrossRef]
- Tuntland, T.; Ethell, B.; Kosaka, T.; Blasco, F.; Zang, R.; Jain, M.; Gould, T.; Hoffmaster, K. Implementation of Pharmacokinetic and Pharmacodynamic Strategies in Early Research Phases of Drug Discovery and Development at Novartis Institute of Biomedical Research. Front. Pharmacol. 2014, 5, 174. [Google Scholar] [CrossRef] [PubMed]
- Lodge, J.; Kajtar, L.; Duxbury, R.; Hall, D.; Burley, G.A.; Cordy, J.; Yates, J.W.T.; Rattray, Z. Quantifying Antibody Binding: Techniques and Therapeutic Implications. mAbs 2025, 17, 2459795. [Google Scholar] [CrossRef]
- Reusch, J.; Andersen, J.T.; Rant, U.; Schlothauer, T. Insight into the Avidity–Affinity Relationship of the Bivalent, PH-Dependent Interaction between IgG and FcRn. mAbs 2024, 16, 2361585. [Google Scholar] [CrossRef] [PubMed]
- Betts, A.; Keunecke, A.; van Steeg, T.J.; van der Graaf, P.H.; Avery, L.B.; Jones, H.; Berkhout, J. Linear Pharmacokinetic Parameters for Monoclonal Antibodies Are Similar within a Species and across Different Pharmacological Targets: A Comparison between Human, Cynomolgus Monkey and HFcRn Tg32 Transgenic Mouse Using a Population-Modeling Approach. mAbs 2018, 10, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhou, Y.; Guo, L.; Feng, S. Biological Function of Sialic Acid and Sialylation in Human Health and Disease. Cell Death Discov. 2024, 10, 415. [Google Scholar] [CrossRef]
- Boune, S.; Hu, P.; Epstein, A.L.; Khawli, L.A. Principles of N-linked Glycosylation Variations of Igg-based Therapeutics: Pharmacokinetic and Functional Considerations. Antibodies 2020, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Keizer, R.J.; Huitema, A.D.R.; Schellens, J.H.M.; Beijnen, J.H. Clinical Pharmacokinetics of Therapeutic Monoclonal Antibodies. Clin. Pharmacokinet. 2010, 49, 493–507. [Google Scholar] [CrossRef]
- Datta-Mannan, A. Mechanisms Influencing the Pharmacokinetics and Disposition of Monoclonal Antibodies and Peptides. Drug Metab. Dispos. 2019, 47, 1100–1110. [Google Scholar] [CrossRef]
- Glassman, P.M.; Abuqayyas, L.; Balthasar, J.P. Assessments of Antibody Biodistribution. J. Clin. Pharmacol. 2015, 55, S29–S38. [Google Scholar] [CrossRef]
- Wolf, B.; Piksa, M.; Beley, I.; Patoux, A.; Besson, T.; Cordier, V.; Voedisch, B.; Schindler, P.; Stöllner, D.; Perrot, L.; et al. Therapeutic Antibody Glycosylation Impacts Antigen Recognition and Immunogenicity. Immunology 2022, 166, 380–407. [Google Scholar] [CrossRef]
- Matucci, A.; Nencini, F.; Vivarelli, E.; Bormioli, S.; Maggi, E.; Vultaggio, A. Immunogenicity-Unwanted Immune Responses to Biological Drugs–Can We Predict Them? Expert. Rev. Clin. Pharmacol. 2021, 14, 47–53. [Google Scholar] [CrossRef]
- Mastrangeli, R.; Audino, M.C.; Palinsky, W.; Broly, H.; Bierau, H. The Formidable Challenge of Controlling High Mannose-Type N-Glycans in Therapeutic MAbs. Trends Biotechnol. 2020, 38, 1154–1168. [Google Scholar] [CrossRef]

 ), Gal (
), Gal ( ), Man (
), Man ( ), GlcNAc (
), GlcNAc ( ). Created in https://BioRender.com (accessed on 19 August 2025).
). Created in https://BioRender.com (accessed on 19 August 2025).
 ), Gal (
), Gal ( ), Man (
), Man ( ), GlcNAc (
), GlcNAc ( ). Created in https://BioRender.com (accessed on 19 August 2025).
). Created in https://BioRender.com (accessed on 19 August 2025).
 ), Gal (
), Gal ( ), Man (
), Man ( ), Fuc (
), Fuc ( ), increase (↑), decrease (↓). Created in https://BioRender.com (accessed on 19 August 2025).
), increase (↑), decrease (↓). Created in https://BioRender.com (accessed on 19 August 2025).
 ), Gal (
), Gal ( ), Man (
), Man ( ), Fuc (
), Fuc ( ), increase (↑), decrease (↓). Created in https://BioRender.com (accessed on 19 August 2025).
), increase (↑), decrease (↓). Created in https://BioRender.com (accessed on 19 August 2025).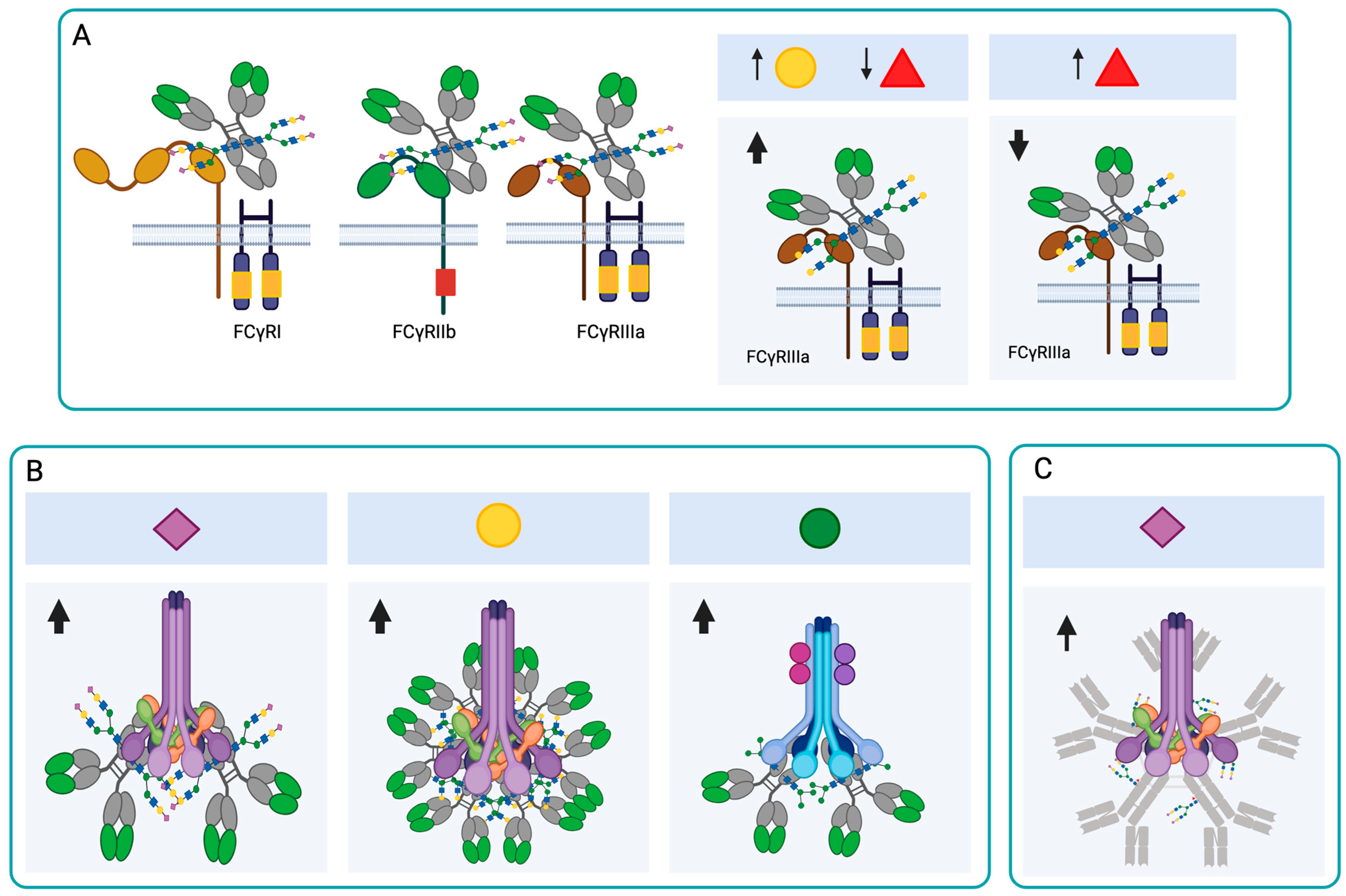
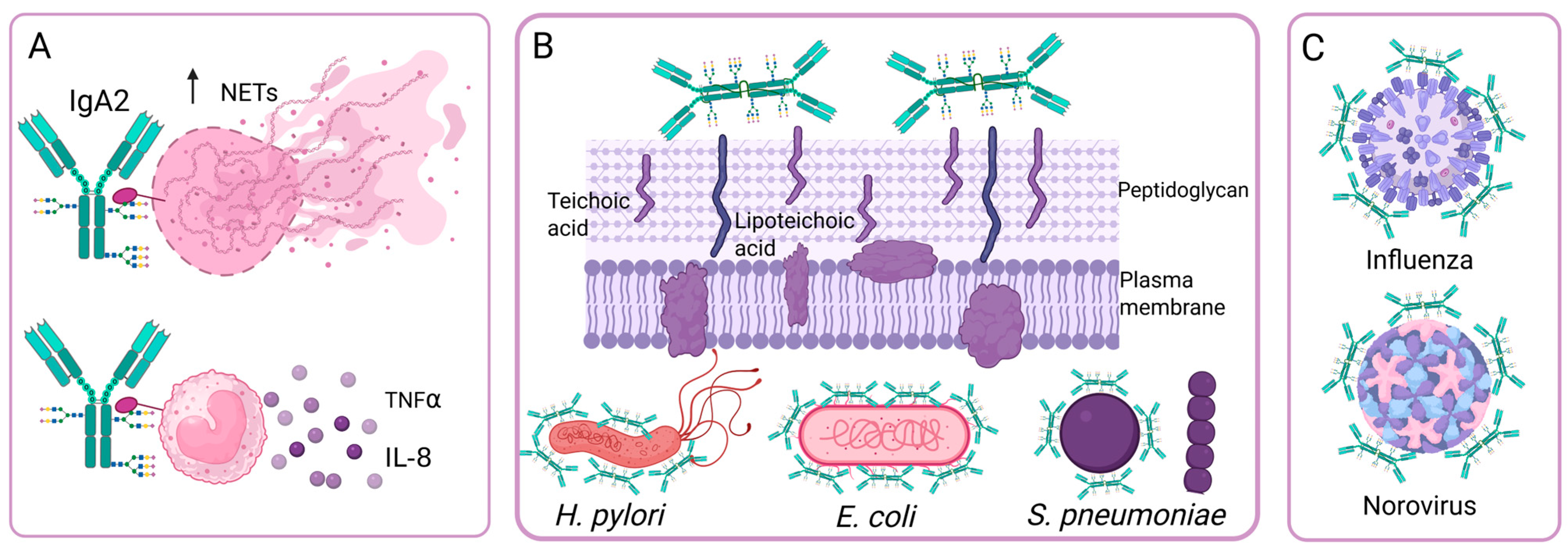
 ), Gal (
), Gal ( ), Man (
), Man ( ), GlcNAc (
), GlcNAc ( ), Fuc (
), Fuc ( ). The arrows indicate an increase or decrease in the indicated glycan, and the triangles indicate an increase or decrease in the antibody function. (C) Changes in the N-glycosylation profile of antibodies in response to vaccination. Created in https://BioRender.com (accessed on 19 August 2025).
). The arrows indicate an increase or decrease in the indicated glycan, and the triangles indicate an increase or decrease in the antibody function. (C) Changes in the N-glycosylation profile of antibodies in response to vaccination. Created in https://BioRender.com (accessed on 19 August 2025).
 ), Gal (
), Gal ( ), Man (
), Man ( ), GlcNAc (
), GlcNAc ( ), Fuc (
), Fuc ( ). The arrows indicate an increase or decrease in the indicated glycan, and the triangles indicate an increase or decrease in the antibody function. (C) Changes in the N-glycosylation profile of antibodies in response to vaccination. Created in https://BioRender.com (accessed on 19 August 2025).
). The arrows indicate an increase or decrease in the indicated glycan, and the triangles indicate an increase or decrease in the antibody function. (C) Changes in the N-glycosylation profile of antibodies in response to vaccination. Created in https://BioRender.com (accessed on 19 August 2025).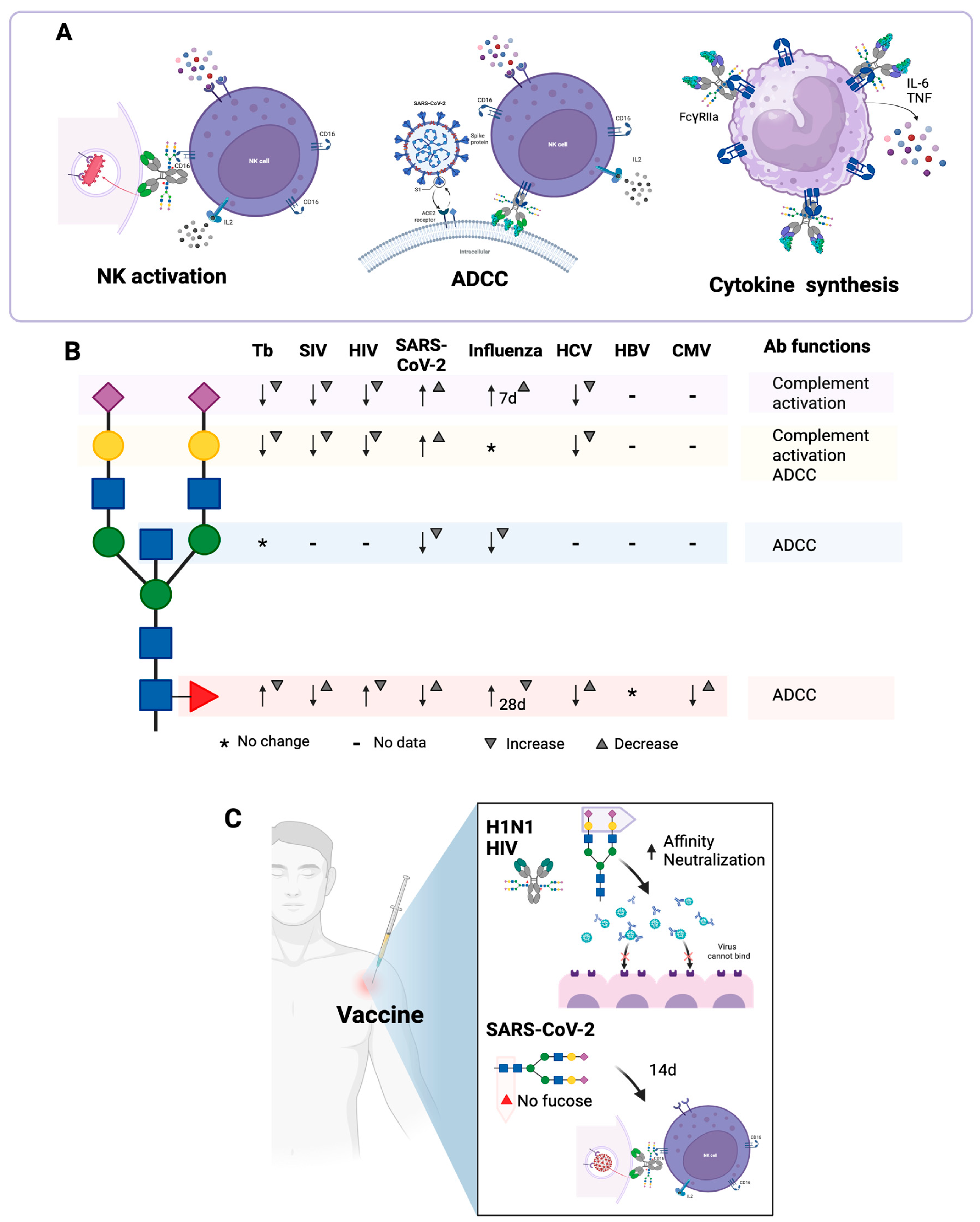
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castañeda-Casimiro, J.; Vallejo-Castillo, L.; Peregrino, E.S.; Hernández-Solis, A.; Vázquez-Flores, L.; Chacón-Salinas, R.; Wong-Baeza, I.; Serafín-López, J. N-Glycosylation of Antibodies: Biological Effects During Infections and Therapeutic Applications. Antibodies 2025, 14, 93. https://doi.org/10.3390/antib14040093
Castañeda-Casimiro J, Vallejo-Castillo L, Peregrino ES, Hernández-Solis A, Vázquez-Flores L, Chacón-Salinas R, Wong-Baeza I, Serafín-López J. N-Glycosylation of Antibodies: Biological Effects During Infections and Therapeutic Applications. Antibodies. 2025; 14(4):93. https://doi.org/10.3390/antib14040093
Chicago/Turabian StyleCastañeda-Casimiro, Jessica, Luis Vallejo-Castillo, Eliud S. Peregrino, Alejandro Hernández-Solis, Luis Vázquez-Flores, Rommel Chacón-Salinas, Isabel Wong-Baeza, and Jeanet Serafín-López. 2025. "N-Glycosylation of Antibodies: Biological Effects During Infections and Therapeutic Applications" Antibodies 14, no. 4: 93. https://doi.org/10.3390/antib14040093
APA StyleCastañeda-Casimiro, J., Vallejo-Castillo, L., Peregrino, E. S., Hernández-Solis, A., Vázquez-Flores, L., Chacón-Salinas, R., Wong-Baeza, I., & Serafín-López, J. (2025). N-Glycosylation of Antibodies: Biological Effects During Infections and Therapeutic Applications. Antibodies, 14(4), 93. https://doi.org/10.3390/antib14040093