Diagnosis of Systemic Rheumatic Disease Using the Connective Tissue Disease Screen
Abstract
1. Introduction
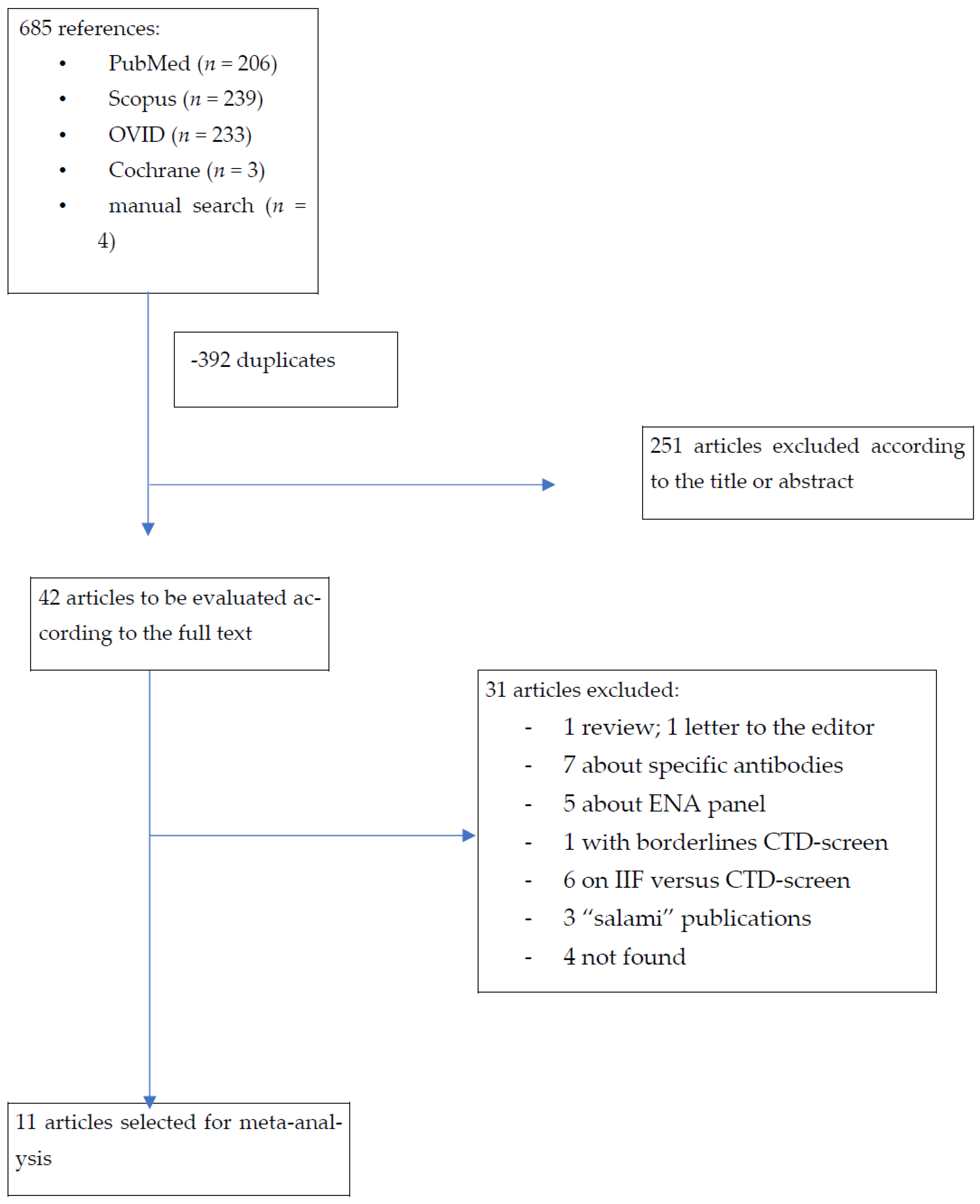
2. Materials and Methods

Statistical Analysis
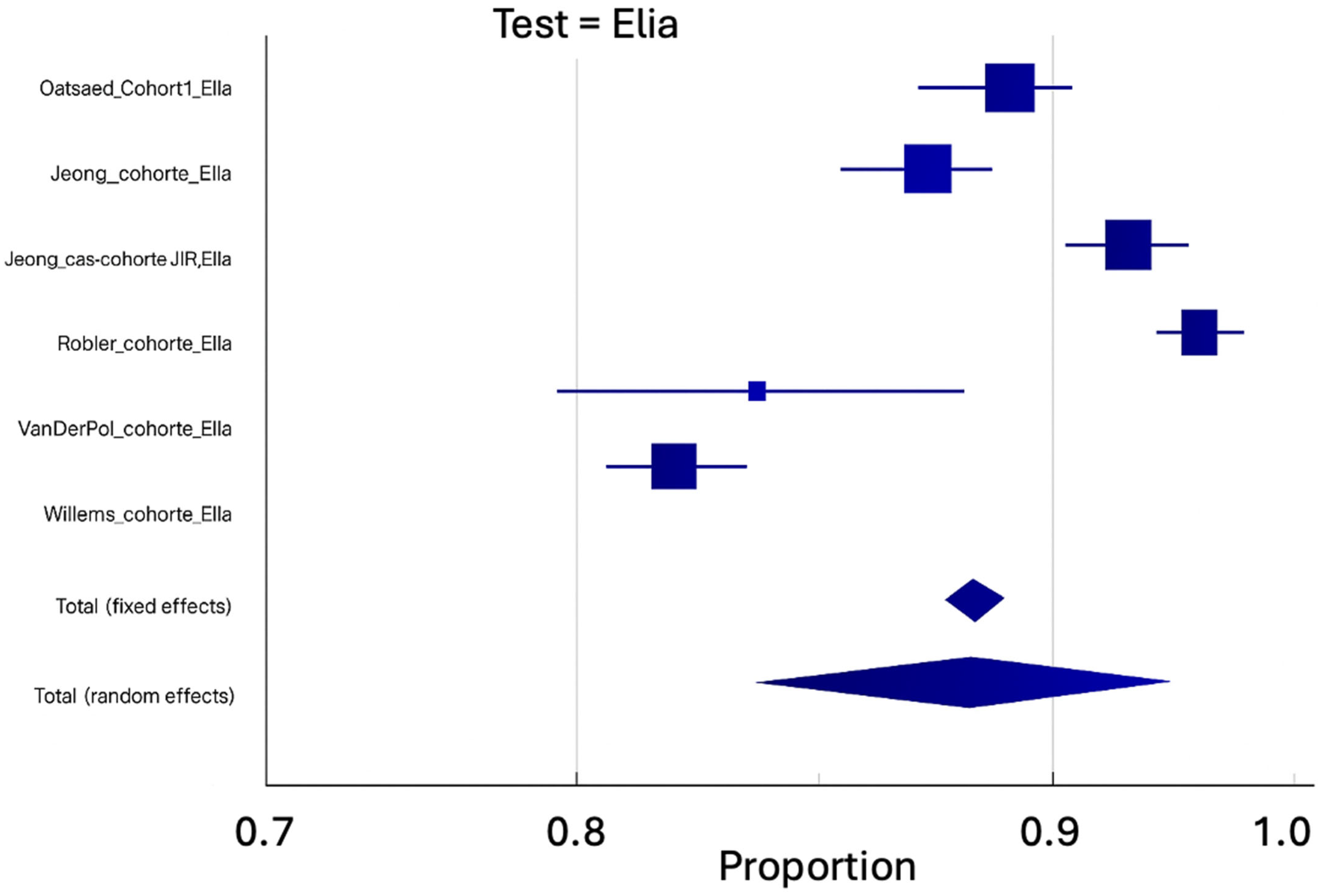
3. Results
4. Discussion
- Peripheral/homogeneous patterns are strongly associated with anti-dsDNA antibodies and systemic lupus erythematosus.
- Centromere patterns are highly suggestive of limited systemic sclerosis.
- Nucleolar patterns are characteristic of systemic sclerosis, particularly diffuse cutaneous forms.
- Speckled patterns encompass various specificities including anti-Sm, anti-RNP, anti-SSA/Ro, and anti-SSB/La antibodies.
- Clinical Assessment: Comprehensive evaluation including symptoms, physical examination, and organ system involvement.
- Pretest Probability Determination: Low probability—avoid testing; intermediate–high probability—proceed with a multi-parameter approach.
- Multi-Parameter Testing: CTD screen + CBC with calculated ratios + inflammatory markers.
- Result Integration: Combined interpretation of serological and hematologic findings.
- Confirmation Strategy: Positive CTD screen confirmed with IIF and expert pattern interpretation.
- Clinical Correlation: Rheumatological integration of all findings with clinical presentation.
- Monitoring Framework: Disease-specific, organ-based follow-up rather than repeat CTD screening.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mulhearn, B.; Tansley, S.L.; McHugh, N.J. Autoantibodies in connective tissue disease. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101462. [Google Scholar] [CrossRef] [PubMed]
- Didier, K.; Bolko, L.; Giusti, D.; Toquet, S.; Robbins, A.; Antonicelli, F.; Servettaz, A. Autoantibodies Associated With Connective Tissue Diseases: What Meaning for Clinicians? Front. Immunol. 2018, 9, 541. [Google Scholar] [CrossRef]
- Pepmueller, P.H. Undifferentiated Connective Tissue Disease, Mixed Connective Tissue Disease, and Overlap Syndromes in Rheumatology. Mo. Med. 2016, 113, 136–140. [Google Scholar] [PubMed]
- Bossuyt, X.; De Langhe, E.; Borghi, M.O.; Meroni, P.L. Understanding and interpreting antinuclear antibody tests in systemic rheumatic diseases. Nat. Rev. Rheumatol. 2020, 16, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Yang, H.; Hwang, H. Evaluation of an automated connective tissue disease screening assay in Korean patients with systemic rheumatic diseases. PLoS ONE 2017, 12, e0173597. [Google Scholar] [CrossRef]
- Volkmann, E.R.; Taylor, M.; Ben-Artzi, A. Using the antinuclear antibody test to diagnose rheumatic diseases: When does a positive test warrant further investigation? South. Med. J. 2012, 105, 100–104. [Google Scholar] [CrossRef]
- Abeles, A.M.; Abeles, M. The clinical utility of a positive antinuclear antibody test result. Am. J. Med. 2013, 126, 342–348. [Google Scholar] [CrossRef]
- Willems, P.; De Langhe, E.; Claessens, J.; Westhovens, R.; Van Hoeyveld, E.; Poesen, K.; Vanderschueren, S.; Blockmans, D.; Bossuyt, X. Screening for connective tissue disease-associated antibodies by automated immunoassay. Clin. Chem. Lab. Med. 2018, 56, 909–918. [Google Scholar] [CrossRef]
- Robier, C.; Amouzadeh-Ghadikolai, O.; Stettin, M.; Reicht, G. Comparison of the Clinical Utility in the Detection of Anti-Nuclear Antibodies Between the Elia CTD Screen and Indirect Immunofluorescence on Hep-2 Cells: A Review of the Literature. Isr. Med. Assoc. J. IMAJ 2018, 20, 700–702. [Google Scholar]
- Jeong, S.; Hwang, H.; Roh, J.; Shim, J.E.; Kim, J.; Kim, G.-T.; Tag, H.-S.; Kim, H.-S. Evaluation of an Automated Screening Assay, Compared to Indirect Immunofluorescence, an Extractable Nuclear Antigen Assay, and a Line Immunoassay in a Large Cohort of Asian Patients with Antinuclear Antibody-Associated Rheumatoid Diseases: A Multicenter Retrospective Study. J. Immunol. Res. 2018, 2018, 9094217. [Google Scholar]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M. QUADAS-2 Group QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Bizzaro, N.; Brusca, I.; Previtali, G.; Alessio, M.G.; Daves, M.; Platzgummer, S.; Cinquanta, L.; Paura, G.; Infantino, M.; Manfredi, M.; et al. The association of solid-phase assays to immunofluorescence increases the diagnostic accuracy for ANA screening in patients with autoimmune rheumatic diseases. Autoimmun. Rev. 2018, 17, 541–547. [Google Scholar] [CrossRef]
- Robier, C.; Amouzadeh-Ghadikolai, O.; Stettin, M.; Reicht, G. Comparison of the clinical utility of the Elia CTD Screen to indirect immunofluorescence on Hep-2 cells. Clin. Chem. Lab. Med. 2016, 54, 1365–1370. [Google Scholar] [CrossRef]
- Op De Beeck, K.; Vermeersch, P.; Verschueren, P.; Westhovens, R.; Mariën, G.; Blockmans, D.; Bossuyt, X. Detection of antinuclear antibodies by indirect immunofluorescence and by solid phase assay. Autoimmun. Rev. 2011, 10, 801–808. [Google Scholar] [CrossRef]
- van der Pol, P.; Bakker-Jonges, L.E.; Kuijpers, J.H.S.A.M.; Schreurs, M.W.J. Analytical and clinical comparison of two fully automated immunoassay systems for the detection of autoantibodies to extractable nuclear antigens. Clin. Chim. Acta Int. J. Clin. Chem. 2018, 476, 154–159. [Google Scholar] [CrossRef]
- Claessens, J.; Belmondo, T.; De Langhe, E.; Westhovens, R.; Poesen, K.; Hüe, S.; Blockmans, D.; Mahler, M.; Fritzler, M.J.; Bossuyt, X. Solid phase assays versus automated indirect immunofluorescence for detection of antinuclear antibodies. Autoimmun. Rev. 2018, 17, 533–540. [Google Scholar] [CrossRef]
- Bentow, C.; Lakos, G.; Rosenblum, R.; Bryant, C.; Seaman, A.; Mahler, M. Clinical performance evaluation of a novel, automated chemiluminescent immunoassay, QUANTA Flash CTD Screen Plus. Immunol. Res. 2015, 61, 110–116. [Google Scholar] [CrossRef]
- Alsaed, O.S.; Alamlih, L.I.; Al-Radideh, O.; Chandra, P.; Alemadi, S.; Al-Allaf, A.-W. Clinical utility of ANA-ELISA vs. ANA-immunofluorescence in connective tissue diseases. Sci. Rep. 2021, 11, 8229. [Google Scholar] [CrossRef]
- Lopez-Hoyos, M.; Rodriguez-Valverde, V.; Martinez-Taboada, V. Performance of Antinuclear Antibody Connective Tissue Disease Screen. Ann. N. Acad. Sci. 2007, 1109, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Pisetsky, D.S. Antinuclear antibody testing—Misunderstood or misbegotten? Nat. Rev. Rheumatol. 2017, 13, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Panimolle, F.; Tiberti, C.; Spaziani, M.; Riitano, G.; Lucania, G.; Anzuini, A.; Lenzi, A.; Gianfrilli, D.; Sorice, M.; Radicioni, A.F. Non-organ-specific autoimmunity in adult 47, XXY Klinefelter patients and higher-grade X-chromosome aneuploidies. Clin. Exp. Immunol. 2021, 205, 316–325. [Google Scholar] [CrossRef] [PubMed]



| All Connective Tissue Diseases | Systemic Lupus Erythematosus | Primary Sjogren’s Syndrome | Systemic Scleroderma | Dermato-Polymyositis | Undifferentiated Connective Tissue Diseases | Controls Without Connective Tissue Diseases | Blood Donor | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | CTDpos | total | CTDpos | total | CTDpos | total | CTDpos | total | CTD pos | total | CTD pos | total | CTD neg | total | CTD neg | total |
| Bizarro Cohort study Elia-QuantaFlash | 282 | 368 | 91 | 123 | 111 | 128 | 47 | 66 | 25 | 39 | 8 | 12 | ||||
| Willems Cohort study Elia | 170 | 216 | 62 | 83 | 43 | 45 | 54 | 63 | 12 | 17 | 8 | 8 | 1804 | 2197 | ||
| Robier Cohort study Elia | 65 | 85 | 21 | 28 | 17 | 17 | 10 | 11 | 1 | 4 | 4 | 1523 | 1623 | |||
| Van der Pool Cohort study Elia | 66 | 72 | 43 | 44 | 13 | 16 | 2 | 4 | 4 | 4 | 4 | 4 | 210 | 250 | ||
| Van der Pool Cohort study QuantaFlash | 71 | 72 | 44 | 44 | 15 | 16 | 4 | 4 | 4 | 4 | 4 | 4 | 190 | 250 | ||
| Van der Pool Case–control study Elia | 104 | 120 | 36 | 40 | 33 | 34 | 17 | 23 | 18 | 23 | ||||||
| Van der Pool Case–control study QuantaFlash | 101 | 120 | 38 | 40 | 32 | 34 | 17 | 23 | 14 | 23 | ||||||
| Lopez-Hoyos Case–control study Varelisa | 193 | 254 | 152 | 202 | 30 | 41 | 11 | 11 | 198 | 218 | 95 | 105 | ||||
| Bentow Case–control study QuantaFlash | 139 | 178 | 79 | 98 | 24 | 30 | 21 | 30 | 15 | 20 | 192 | 204 | 140 | 146 | ||
| Jeong Cohort study Elia | 46 | 62 | 31 | 35 | 2 | 2 | 13 | 23 | 898 | 1031 | ||||||
| Jeong Cohort study Elia | 91 | 112 | 57 | 67 | 16 | 19 | 13 | 21 | 5 | 5 | 924 | 1003 | ||||
| Claessens Case–control study Elia | 386 | 480 | 95 | 119 | 59 | 65 | 181 | 220 | 25 | 50 | 26 | 26 | 748 | 767 | 276 | 279 |
| Claessens Case–control study QuantaFlash | 412 | 480 | 102 | 119 | 59 | 65 | 192 | 220 | 33 | 50 | 26 | 26 | 675 | 767 | 262 | 279 |
| Op De Beeck Case–control study Elia | 171 | 236 | 59 | 80 | 32 | 36 | 50 | 69 | 11 | 28 | 13 | 13 | 409 | 422 | 145 | 149 |
| Olsaed Cohort study Elia | 150 | 201 | ? | 142 | ? | 24 | ? | 15 | ? | 10 | 1112 | 1257 | ||||
| Author and Test | Year | Country And Study | Quality Assessment of the Studies (QUADAS2) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Risk of Bias | Applicability Concerns | ||||||||
| Patients’ Selection | Index Test | Standard Reference | Flow and Timing | Patients’ Selection | Index Test | Standard Reference | |||
| Bizzaro Elia-Quanta | 2018 | Italy Cohort study |  |  |  |  |  |  |  |
| Willems Elia | 2018 | Belgium Cohort study |  |  |  |  |  |  |  |
| Robier Elia | 2016 | Austria Cohort study |  |  |  |  |  |  |  |
| Van der Pool Elia | 2018 | Netherlands Cohort study |  |  |  |  |  |  |  |
| Van der Pool Quanta | 2018 | Netherlands Cohort study |  |  |  |  |  |  |  |
| Van der Pool Elia | 2018 | Netherlands Case–control study |  |  |  |  |  |  |  |
| Van der Pool Quanta | 2018 | Netherlands Case–control study |  |  |  |  |  |  |  |
| Lopez-Hoyos Varelisa | 2007 | Spain Case–control study |  |  |  |  |  |  |  |
| Bentow Quanta | 2015 | International Case–control study |  |  |  |  |  |  |  |
| Jeong Elia | 2018 | Korea Cohort study | ? |  |  |  | ? |  |  |
| Jeong Elia | 2017 | Korea Cohort study | ? |  |  |  | ? |  |  |
| Claessens Elia | 2018 | International Case–control study |  |  |  |  |  |  |  |
| Claessens Quanta | 2018 | International Case–control study |  |  |  |  |  |  |  |
| Op De Beeck Elia | 2011 | Belgium Case–control study |  |  |  |  |  |  |  |
| Alsaed Elia | 2018 | Qatar Cohort |  |  |  |  |  |  |  |
 High
High  low ? unclear.
low ? unclear.| ELIA© CTD-SCREEN | ||||||||
| Sensitivity for… | Number of Studies | Number of Cases | Pooled Sensitivity | IC95% | Q | I2 | Egger | Begg |
| Connective tissue disease diagnosis | 9 | 1584 | 79.36 | 75.61–82.88 | 23.54 | 66.02% | NS | NS |
| Lupus erythematosus diagnosis | 8 | 496 | 82.98 | 76.49–88.60 | 22.52 | 68.92% | NS | NS |
| Primary Sjogren’s syndrome diagnosis | 7 | 232 | 91.43 | 86.69–95.21 | 8.2356 | 27.15% | NS | NS |
| Systemic scleroderma diagnosis | 7 | 411 | 77.44 | 70.40–87.78 | 11.21 | 46.46% | NS | NS |
| Autoimmune myositis diagnosis | 6 | 123 | 60.95 | 43.13–77.37 | 16.624 | 69.92 | NS | NS |
| Sharp’s syndrome diagnosis | 7 | 83 | 93.05 | 77.92–99.80 | 24.13 | 75.14 | NS | NS |
| Specificity for … | ||||||||
| Clinic controls (no connective tissue disease) | 7 | 8550 | 91.05 | 86.59–94.69 | 298.65 | 97.66 | NS | NS |
| Healthy controls (blood donor) | 2 | 428 | 98.18 | 96.09–99.49 | 1.7005 | 41.19 | p <0.001 | NS |
| Total pooled controls | 8 | 8122 | 90.79 | 86.69–94.20 | 233.28 | 97 | NS | NS |
| ELIA© Pooled Cohorts | Number of Studies | Number of Cases | Pooled % | IC95% | Q | I2 | Egger | Begg |
| Prevalence of connective tissue disease | 6 | 8109 | 10.22 | 6.90–14.10 | 141.63 | 96.47 | NS | NS |
| Sensitivity | 6 | 748 | 79.25 | 74.26–83.84 | 12.64 | 60.43 | NS | NS |
| Specificity | 6 | 7361 | 88.3 | 83.81–92.14 | 151.71 | 96.7 | NS | NS |
| QUANTAFLASH© CTD-SCREEN | ||||||||
| Sensitivity for… | Number of Studies | Number of Cases | Pooled Sensitivity | IC95% | Q | I2 | Egger | Begg |
| Connective tissue disease diagnosis | 4 | 850 | 87.23 | 79.10–93.58 | 25.28 | 88.13 | NS | NS |
| Lupus erythematosus diagnosis | 4 | 301 | 91.13 | 80.51–97.83 | 20.94 | 85.68 | NS | NS |
| Primary Sjogren’s syndrome diagnosis | 4 | 145 | 89.1 | 83.31–93.79 | 3.2471 | 7.61 | NS | NS |
| Systemic scleroderma diagnosis | 4 | 277 | 80.52 | 68.07–90.48 | 7.892 | 61.93% | NS | NS |
| Autoimmune myositis diagnosis | 3 | 77 | 68.205 | 52.30–82.18 | 3.36 | 40.62% | NS | NS |
| Sharp’s syndrome diagnosis | 3 | 50 | 91.65 | 67.02–99.94 | 9.21 | 78.28 | NS | NS |
| Specificity for … | ||||||||
| Clinic controls (no connective tissue disease) | 3 | 796 | 83.56 | 75.18–90.50 | 13.3791 | 85.05 | NS | NS |
| Healthy controls (blood donor) | 2 | 425 | 94.474 | 92.11–96.44 | 0.5871 | 0 | p <0.001 | NS |
| Total pooled controls | 3 | 1221 | 87.14 | 77.18–94.56 | 35.15 | 94.31 | NS | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapuczinski, A.; Parisis, D.; Kassab, N.; Smet, J.; Soyfoo, M. Diagnosis of Systemic Rheumatic Disease Using the Connective Tissue Disease Screen. Antibodies 2025, 14, 56. https://doi.org/10.3390/antib14030056
Kapuczinski A, Parisis D, Kassab N, Smet J, Soyfoo M. Diagnosis of Systemic Rheumatic Disease Using the Connective Tissue Disease Screen. Antibodies. 2025; 14(3):56. https://doi.org/10.3390/antib14030056
Chicago/Turabian StyleKapuczinski, Abeline, Dorian Parisis, Nour Kassab, Julie Smet, and Muhammad Soyfoo. 2025. "Diagnosis of Systemic Rheumatic Disease Using the Connective Tissue Disease Screen" Antibodies 14, no. 3: 56. https://doi.org/10.3390/antib14030056
APA StyleKapuczinski, A., Parisis, D., Kassab, N., Smet, J., & Soyfoo, M. (2025). Diagnosis of Systemic Rheumatic Disease Using the Connective Tissue Disease Screen. Antibodies, 14(3), 56. https://doi.org/10.3390/antib14030056





