Teprotumumab for Thyroid Eye Disease: Mechanism, Clinical Efficacy, and Current Challenges
Abstract
1. Introduction
2. Methods
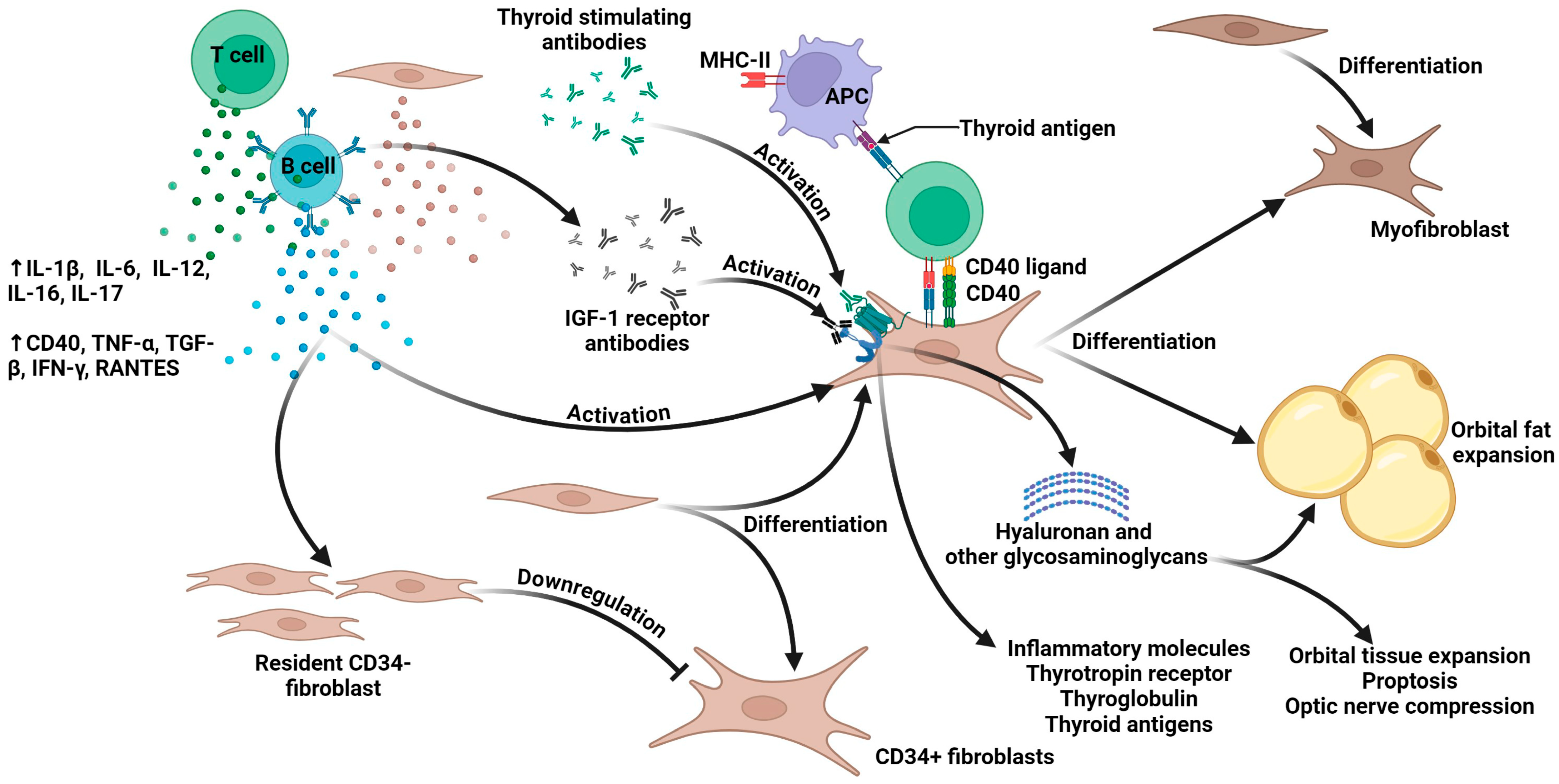
3. The Pathophysiology of Thyroid Eye Disease
4. The Traditional Clinical Management of TED
5. The Pharmacology of Teprotumumab
6. Clinical Translation of Teprotumumab: From Trials to Practice
6.1. Evidence from Pivotal Clinical Trials and FDA Approval
6.2. Real-World Evidence and Expanding Applications
7. Current Challenges in Teprotumumab Treatment
7.1. Safety Concerns and Adverse Events Profile
7.2. Economic Burden and Cost-Effectiveness Considerations
7.3. Treatment Durability and Insufficient Long-Term Data
8. Other Emerging IGF-1R Inhibitors for TED
9. Limitations
10. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rashad, R.; Pinto, R.; Li, E.; Sohrab, M.; Distefano, A.G. Thyroid Eye Disease. Life 2022, 12, 2084. [Google Scholar] [CrossRef]
- Ugradar, S.; Kang, J.; Kossler, A.L.; Zimmerman, E.; Braun, J.; Harrison, A.R.; Bose, S.; Cockerham, K.; Douglas, R.S. Teprotumumab for the treatment of chronic thyroid eye disease. Eye 2022, 36, 1553–1559. [Google Scholar] [CrossRef]
- Mallika, P.; Tan, A.; Aziz, S.; Alwi, S.S.; Chong, M.; Vanitha, R.; Intan, G. Thyroid associated ophthalmopathy—A review. Malays. Fam. Physician 2009, 4, 8–14. [Google Scholar]
- Nie, T.; Lamb, Y.N. Teprotumumab: A Review in Thyroid Eye Disease. Drugs 2022, 82, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Scarabosio, A.; Surico, P.L.; Singh, R.B.; Tereshenko, V.; Musa, M.; D’Esposito, F.; Russo, A.; Longo, A.; Gagliano, C.; Agosti, E.; et al. Thyroid Eye Disease: Advancements in Orbital and Ocular Pathology Management. J. Pers. Med. 2024, 14, 776. [Google Scholar] [CrossRef]
- Markham, A. Teprotumumab: First Approval. Drugs 2020, 80, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Douglas, R.S.; Kahaly, G.J.; Patel, A.; Sile, S.; Thompson, E.H.Z.; Perdok, R.; Fleming, J.C.; Fowler, B.T.; Marcocci, C.; Marinò, M.; et al. Teprotumumab for the Treatment of Active Thyroid Eye Disease. N. Engl. J. Med. 2020, 382, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Smith Terry, J.; Kahaly George, J.; Ezra Daniel, G.; Fleming James, C.; Dailey Roger, A.; Tang Rosa, A.; Harris Gerald, J.; Antonelli, A.; Salvi, M.; Goldberg Robert, A.; et al. Teprotumumab for Thyroid-Associated Ophthalmopathy. N. Engl. J. Med. 2017, 376, 1748–1761. [Google Scholar] [CrossRef]
- Householder, N.A.; Ray, C. Teprotumumab’s Impact on Proptosis in Long-duration Thyroid Eye Disease: A Systematic Review and Meta-analysis. touchREVIEWS Endocrinol. 2024, 20, 100–109. [Google Scholar] [CrossRef]
- Abumohssin, A.G.; Alshareef, R.A.; Aljohani, S.; Alqutub, A.; Alqutub, A. Comparative efficacy and safety of rituximab, tocilizumab, and teprotumumab in Graves’ orbitopathy: A systematic review and meta-analysis. Eye 2025. [Google Scholar] [CrossRef]
- Huang, W.; Ou, X.; Lin, S.; Lin, W.; Chen, G.; Huang, H.; Wen, J. Efficacy and Safety of Teprotumumab in Thyroid Eye Disease: A Systematic Review and Meta-Analysis. Endocr. Pract. 2025, 31, 640–649. [Google Scholar] [CrossRef]
- Kamoi, K.; Uchimaru, K.; Tojo, A.; Watanabe, T.; Ohno-Matsui, K. HTLV-1 uveitis and Graves’ disease presenting with sudden onset of blurred vision. Lancet 2022, 399, 60. [Google Scholar] [CrossRef]
- Kamoi, K.; Watanabe, T.; Uchimaru, K.; Okayama, A.; Kato, S.; Kawamata, T.; Kurozumi-Karube, H.; Horiguchi, N.; Zong, Y.; Yamano, Y.; et al. Updates on HTLV-1 Uveitis. Viruses 2022, 14, 794. [Google Scholar] [CrossRef] [PubMed]
- Kamoi, K. Current challenges facing the clinical treatment for HTLV-1 ocular manifestations. Expert Rev. Ophthalmol. 2023, 18, 405–419. [Google Scholar] [CrossRef]
- Yang, M.; Kamoi, K.; Zong, Y.; Zhang, J.; Zou, Y.; Ohno-Matsui, K. Ripasudil as a Potential Therapeutic Agent in Treating Secondary Glaucoma in HTLV-1-Uveitis: An In Vitro Analysis. Int. J. Mol. Sci. 2024, 25, 3229. [Google Scholar] [CrossRef]
- Kamoi, K.; Okayama, A.; Izumo, S.; Hamaguchi, I.; Uchimaru, K.; Tojo, A.; Watanabe, T.; Ohno-Matsui, K. Tackling HTLV-1 infection in ophthalmology: A nationwide survey of ophthalmic care in an endemic country, Japan. Br. J. Ophthalmol. 2020, 104, 1647–1651. [Google Scholar] [CrossRef]
- Shan, S.J.C.; Douglas, R.S. The Pathophysiology of Thyroid Eye Disease. J. Neuro-Ophthalmol. 2014, 34, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Maurya, V.K.; Kumar, S.; Ankita; Kaur, A.; Saxena, S.K. Clinical management and therapeutic strategies for the thyroid-associated ophthalmopathy: Current and future perspectives. Curr. Eye Res. 2020, 45, 1325–1341. [Google Scholar] [CrossRef]
- Janssen, J.A.M.J.L.; Smith, T.J. Lessons Learned from Targeting IGF-I Receptor in Thyroid-Associated Ophthalmopathy. Cells 2021, 10, 383. [Google Scholar] [CrossRef]
- García-Mato, Á.; Cervantes, B.; Murillo-Cuesta, S.; Rodríguez-de la Rosa, L.; Varela-Nieto, I. Insulin-like Growth Factor 1 Signaling in Mammalian Hearing. Genes 2021, 12, 1553. [Google Scholar] [CrossRef]
- Yakar, S.; Adamo, M.L. Insulin-like growth factor 1 physiology: Lessons from mouse models. Endocrinol. Metab. Clin. N. Am. 2012, 41, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Adams, T.E.; Epa, V.C.; Garrett, T.P.J.; Ward, C.W. Structure and function of the type 1 insulin-like growth factor receptor. Cell. Mol. Life Sci. CMLS 2000, 57, 1050–1093. [Google Scholar] [CrossRef]
- Bailes, J.; Soloviev, M. Insulin-Like Growth Factor-1 (IGF-1) and Its Monitoring in Medical Diagnostic and in Sports. Biomolecules 2021, 11, 217. [Google Scholar] [CrossRef] [PubMed]
- Khong, J.J.; McNab, A.A.; Ebeling, P.R.; Craig, J.E.; Selva, D. Pathogenesis of thyroid eye disease: Review and update on molecular mechanisms. Br. J. Ophthalmol. 2016, 100, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.S.; Kikkawa, D.O. Thyroid eye disease: From pathogenesis to targeted therapies. Taiwan J. Ophthalmol. 2022, 12, 3–11. [Google Scholar] [CrossRef]
- Shu, X.; Shao, Y.; Chen, Y.; Zeng, C.; Huang, X.; Wei, R. Immune checkpoints: New insights into the pathogenesis of thyroid eye disease. Front. Immunol. 2024, 15, 1392956. [Google Scholar] [CrossRef]
- Fang, S.; Lu, Y.; Huang, Y.; Zhou, H.; Fan, X. Mechanisms That Underly T Cell Immunity in Graves’ Orbitopathy. Front. Endocrinol. 2021, 12, 648732. [Google Scholar] [CrossRef]
- Wiersinga, W.M. Etiology and Pathogenesis of Graves’ Orbitopathy. In Surgery in and Around the Orbit: CrossRoads; Gooris, P.J.J., Mourits, M.P., Bergsma, J.E., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 279–285. [Google Scholar]
- Salvi, M.; Vannucchi, G.; Beck-Peccoz, P. Potential utility of rituximab for Graves’ orbitopathy. J. Clin. Endocrinol. Metab. 2013, 98, 4291–4299. [Google Scholar] [CrossRef]
- Murdock, J.; Nguyen, J.; Hurtgen, B.J.; Andorfer, C.; Walsh, J.; Lin, A.; Tubbs, C.; Erickson, K.; Cockerham, K. The role of IL-6 in thyroid eye disease: An update on emerging treatments. Front. Ophthalmol. 2025, 5, 1544436. [Google Scholar] [CrossRef]
- Smith, T.J. Understanding Pathogenesis Intersects With Effective Treatment for Thyroid Eye Disease. J. Clin. Endocrinol. Metab. 2022, 107 (Suppl. 1), S13–S26. [Google Scholar] [CrossRef]
- Bartalena, L.; Kahaly, G.J.; Baldeschi, L.; Dayan, C.M.; Eckstein, A.; Marcocci, C.; Marinò, M.; Vaidya, B.; Wiersinga, W.M.; EUGOGO. The 2021 European Group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur. J. Endocrinol. 2021, 185, G43–G67. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, L. Advances of IGF-1R inhibitors in Graves’ ophthalmopathy. Int. Ophthalmol. 2024, 44, 435. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Mester, T.; Raychaudhuri, N.; Kauh, C.Y.; Gupta, S.; Smith, T.J.; Douglas, R.S. Teprotumumab, an IGF-1R Blocking Monoclonal Antibody Inhibits TSH and IGF-1 Action in Fibrocytes. J. Clin. Endocrinol. Metab. 2014, 99, E1635–E1640. [Google Scholar] [CrossRef] [PubMed]
- Fernando, R.; Caldera, O.; Smith, T.J. Therapeutic IGF-I receptor inhibition alters fibrocyte immune phenotype in thyroid-associated ophthalmopathy. Proc. Natl. Acad. Sci. USA 2021, 118, e2114244118. [Google Scholar] [CrossRef]
- Krieger, C.C.; Sui, X.; Kahaly, G.J.; Neumann, S.; Gershengorn, M.C. Inhibition of TSH/IGF-1 Receptor Crosstalk by Teprotumumab as a Treatment Modality of Thyroid Eye Disease. J. Clin. Endocrinol. Metab. 2022, 107, e1653–e1660. [Google Scholar] [CrossRef]
- Burch, H.B.; Perros, P.; Bednarczuk, T.; Cooper, D.S.; Dolman, P.J.; Leung, A.M.; Mombaerts, I.; Salvi, M.; Stan, M.N. Management of thyroid eye disease: A Consensus Statement by the American Thyroid Association and the European Thyroid Association. Eur. Thyroid J. 2022, 11, e220189. [Google Scholar] [CrossRef]
- Xin, Y.; Xu, F.; Gao, Y.; Bhatt, N.; Chamberlain, J.; Sile, S.; Hammel, S.; Holt, R.J.; Ramanathan, S. Pharmacokinetics and Exposure-Response Relationship of Teprotumumab, an Insulin-Like Growth Factor-1 Receptor-Blocking Antibody, in Thyroid Eye Disease. Clin. Pharmacokinet. 2021, 60, 1029–1040. [Google Scholar] [CrossRef]
- Douglas, R.S.; Kossler, A.L.; Abrams, J.; Briceño, C.A.; Gay, D.; Harrison, A.; Lee, M.; Nguyen, J.; Joseph, S.S.; Schlachter, D.; et al. Expert Consensus on the Use of Teprotumumab for the Management of Thyroid Eye Disease Using a Modified-Delphi Approach. J. Neuroophthalmol. 2022, 42, 334–339. [Google Scholar] [CrossRef]
- Kahaly, G.J.; Douglas, R.S.; Holt, R.J.; Sile, S.; Smith, T.J. Teprotumumab for patients with active thyroid eye disease: A pooled data analysis, subgroup analyses, and off-treatment follow-up results from two randomised, double-masked, placebo-controlled, multicentre trials. Lancet Diabetes Endocrinol. 2021, 9, 360–372. [Google Scholar] [CrossRef]
- Douglas, R.S.; Kahaly, G.J.; Ugradar, S.; Elflein, H.; Ponto, K.A.; Fowler, B.T.; Dailey, R.; Harris, G.J.; Schiffman, J.; Tang, R.; et al. Teprotumumab Efficacy, Safety, and Durability in Longer-Duration Thyroid Eye Disease and Re-treatment: OPTIC-X Study. Ophthalmology 2022, 129, 438–449. [Google Scholar] [CrossRef]
- Diniz, S.B.; Cohen, L.M.; Roelofs, K.A.; Rootman, D.B. Early Experience With the Clinical Use of Teprotumumab in a Heterogenous Thyroid Eye Disease Population. Ophthalmic Plast. Reconstr. Surg. 2021, 37, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Sears, C.M.; Wang, Y.; Bailey, L.A.; Turbin, R.; Subramanian, P.S.; Douglas, R.; Cockerham, K.; Kossler, A.L. Early efficacy of teprotumumab for the treatment of dysthyroid optic neuropathy: A multicenter study. Am. J. Ophthalmol. Case Rep. 2021, 23, 101111. [Google Scholar] [CrossRef] [PubMed]
- Ugradar, S.; Braun, J.; Wang, Y.; Zimmerman, E.; Douglas, R.S. Facial and Eyelid Changes in Thyroid Eye Disease Are Reversed by Teprotumumab. Plast. Reconstr. Surg. Glob. Open 2021, 9, e3809. [Google Scholar] [CrossRef]
- Wang, Y.; Mester, T.; Ugradar, S.; Douglas, R. Teprotumumab for the Treatment of Thyroid Eye Disease: Clinical Experience from Expanded Access Program (EAP). Investig. Ophthalmol. Vis. Sci. 2021, 62, 3339. [Google Scholar]
- Douglas, R.S.; Wang, Y.; Bruch, J.; Anzeljc, A.; Sile, S.; Vescio, T.; Phelps, P.; Ramesh, S.; Wulc, A.; Meador, A.; et al. Teprotumumab use in a real-world setting: Expanded access program findings. Investig. Ophthalmol. Vis. Sci. 2021, 62, 3345. [Google Scholar]
- Ugradar, S.; Douglas, R. RF35 | PSAT268 Reversal of Graves’ Disease Associated Facial Volume Expansion and Eyelid Changes Following Teprotumumab Therapy. J. Endocr. Soc. 2022, 6 (Suppl. 1), A861–A862. [Google Scholar] [CrossRef]
- Adetunji, M.O.; Nguyen, B.J.; McGeehan, B.; Tamhankar, M.A.; Briceño, C.A. Effect of teprotumumab on intraocular pressure in thyroid-associated ophthalmopathy. Taiwan J. Ophthalmol. 2022, 12, 325–329. [Google Scholar] [CrossRef]
- Kotwal, A. Real-World Use of Teprotumumab and Tocilizumab in Moderate-to-Severe Steroid-Resistant Thyroid Eye Disease. Clin. Thyroidol. 2023, 35, 364–367. [Google Scholar] [CrossRef]
- Ho, T.C.; Maamari, R.N.; Kossler, A.L.; Sears, C.M.; Freitag, S.K.; Reshef, E.R.; Shinder, R.; Rootman, D.B.; Diniz, S.B.; Kahana, A.; et al. Outcomes of Patients With Thyroid Eye Disease Partially Treated With Teprotumumab. Ophthalmic Plast. Reconstr. Surg. 2023, 39, 150–155. [Google Scholar] [CrossRef]
- Douglas, R.S.; Couch, S.; Wester, S.T.; Fowler, B.T.; Liu, C.Y.; Subramanian, P.S.; Tang, R.; Nguyen, Q.T.; Maamari, R.N.; Ugradar, S.; et al. Efficacy and Safety of Teprotumumab in Patients With Thyroid Eye Disease of Long Duration and Low Disease Activity. J. Clin. Endocrinol. Metab. 2023, 109, 25–35. [Google Scholar] [CrossRef]
- Shah, S.A.; Amarikwa, L.; Sears, C.M.; Clauss, K.D.; Rajjoub, R.D.; Kang, J.Y.; Tamhankar, M.A.; Briceño, C.A.; Harrison, A.R.; Dosiou, C.; et al. Teprotumumab-Related Adverse Events in Thyroid Eye Disease: A Multicenter Study. Ophthalmology 2024, 131, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Men, C.J.; Amarikwa, L.; Pham, B.; Sears, C.; Clauss, K.; Lee, B.W.; Lee, W.W.; Pasol, J.; Ugradar, S.; Shinder, R.; et al. Teprotumumab for the Treatment of Recalcitrant Thyroid Eye Disease. Ophthalmic Plast. Reconstr. Surg. 2024, 40, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Hiromatsu, Y.; Ishikawa, E.; Kozaki, A.; Takahashi, Y.; Tanabe, M.; Hayashi, K.; Imagawa, Y.; Kaneda, K.; Mimura, M.; Dai, X.; et al. A randomised, double-masked, placebo-controlled trial evaluating the efficacy and safety of teprotumumab for active thyroid eye disease in Japanese patients. Lancet Reg. Health West. Pac. 2025, 55, 101464. [Google Scholar] [CrossRef]
- Ugradar, S.; Parunakian, E.; Malkhasyan, E.; Raika, P.; Tolentino, J.; Kossler, A.L.; Cockerham, K.; Amarikwa, L.; Weinberg, D.A.; Douglas, R.S. Teprotumumab for thyroid eye disease in patients with hypothyroid/euthyroid state: A multicenter case series. Graefe’s Arch. Clin. Exp. Ophthalmol. 2025, 263, 225–230. [Google Scholar] [CrossRef]
- Ugradar, S.; Parunakian, E.; Malkhasyan, E.; Chiou, C.A.; Walsh, H.L.; Tolentino, J.; Wester, S.T.; Freitag, S.K.; Douglas, R.S. The Rate of Re-treatment in Patients Treated with Teprotumumab: A Multicenter Study of 119 Patients with 1 Year of Follow-up. Ophthalmology 2025, 132, 92–97. [Google Scholar] [CrossRef]
- Lustig-Barzelay, Y.; Yagoda, D.; Zunz, E.; Hamed-Azzam, S.; Avisar, I.; Kehat-Ophir, S.; Gur, Z.; Cukierman-Yaffe, T.; Agmon-Levin, N.; Landau-Prat, D.; et al. Time to improvement following teprotumumab treatment of thyroid eye disease: Real world experience. Graefe’s Arch. Clin. Exp. Ophthalmol. 2025. [Google Scholar] [CrossRef]
- Zong, Y.; Miyagaki, M.; Yang, M.; Zhang, J.; Zou, Y.; Ohno-Matsui, K.; Kamoi, K. Ophthalmic Use of Targeted Biologics in the Management of Intraocular Diseases: Current and Emerging Therapies. Antibodies 2024, 13, 86. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Kamoi, K.; Miyagaki, M.; Zhang, J.; Yang, M.; Zou, Y.; Ohno-Matsui, K. Applications of Biological Therapy for Latent Infections: Benefits and Risks. Int. J. Mol. Sci. 2024, 25, 9184. [Google Scholar] [CrossRef]
- Kulbay, M.; Tanya, S.M.; Tuli, N.; Dahoud, J.; Dahoud, A.; Alsaleh, F.; Arthurs, B.; El-Hadad, C. A Comprehensive Review of Thyroid Eye Disease Pathogenesis: From Immune Dysregulations to Novel Diagnostic and Therapeutic Approaches. Int. J. Mol. Sci. 2024, 25, 11628. [Google Scholar] [CrossRef]
- Ugradar, S.; Shi, L.; Wang, Y.; Mester, T.; Yang, H.; Douglas, R.S. Teprotumumab for non-inflammatory thyroid eye disease (TED): Evidence for increased IGF-1R expression. Eye 2021, 35, 2607–2612. [Google Scholar] [CrossRef]
- Blandford, A.D.; Zhang, D.; Chundury, R.V.; Perry, J.D. Dysthyroid optic neuropathy: Update on pathogenesis, diagnosis, and management. Expert Rev. Ophthalmol. 2017, 12, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Douglas, R.S.; Dailey, R.; Subramanian, P.S.; Barbesino, G.; Ugradar, S.; Batten, R.; Qadeer, R.A.; Cameron, C. Proptosis and Diplopia Response With Teprotumumab and Placebo vs the Recommended Treatment Regimen With Intravenous Methylprednisolone in Moderate to Severe Thyroid Eye Disease: A Meta-analysis and Matching-Adjusted Indirect Comparison. JAMA Ophthalmol. 2022, 140, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Kahaly, G.J.; Xi, A.; Barretto, N.; Patel, H.; Qashqai, A.; Shokoohi, M.; Spin, P.; Holt, R.J. Teprotumumab Improves Quality of Life in Thyroid Eye Disease: Meta-analysis and Matching-adjusted Indirect Comparison. J. Endocr. Soc. 2025, 9, bvaf063. [Google Scholar] [CrossRef]
- Stan, M.N.; Krieger, C.C. The Adverse Effects Profile of Teprotumumab. J. Clin. Endocrinol. Metab. 2023, 108, e654–e662. [Google Scholar] [CrossRef] [PubMed]
- TEPEZZA-Prescribing-Information.pdf. Available online: https://fda.report/DailyMed/3e6c54a1-cefd-4a5b-a855-ab9f268b6cce (accessed on 24 May 2025).
- Amarikwa, L.; Mohamed, A.; Kim, S.H.; Kossler, A.L.; Dosiou, C. Teprotumumab-Related Hyperglycemia. J. Clin. Endocrinol. Metab. 2023, 108, 858–864. [Google Scholar] [CrossRef]
- Hoang, T.D.; Nguyen, N.T.; Chou, E.; Shakir, M.K. Rapidly progressive cognitive decline associated with teprotumumab in thyroid eye disease. BMJ Case Rep. 2021, 14, e242153. [Google Scholar] [CrossRef]
- Yee, M.D.; McCarthy, J.; Quinn, B.; Surani, A. Teprotumumab-Induced Encephalopathy: A Rare Side Effect of a Novel Therapeutic. WMJ 2023, 122, 134–137. [Google Scholar]
- Martel, A.; Rocher, F.; Gerard, A. Teprotumumab for the Treatment of Thyroid Eye Disease: Why Should We Keep Our Eyes “Wide Open”?—A Clinical and Pharmacovigilance Point of View. J. Pers. Med. 2024, 14, 1027. [Google Scholar] [CrossRef]
- Shah, S.A.; Lu, T.; Yu, M.; Hiniker, S.; Dosiou, C.; Kossler, A.L. Comparison of treatment cost and quality-of-life impact of thyroid eye disease therapies. Investig. Ophthalmol. Vis. Sci. 2022, 63, 4002-A0344. [Google Scholar]
- Rosenblatt, T.R.; Chiou, C.A.; Yoon, M.K.; Wolkow, N.; Lee, N.G.; Freitag, S.K. Proptosis Regression After Teprotumumab Treatment for Thyroid Eye Disease. Ophthalmic Plast. Reconstr. Surg. 2024, 40, 187–191. [Google Scholar] [CrossRef]
- Amgen Study of Teprotumumab in Participants with Thyroid Eye Disease (TED) (NCT05002998). Available online: https://clinicaltrials.gov/study/NCT05002998 (accessed on 24 May 2025).
- Viridian Announces Positive Data from Ongoing Phase 1/2 Trial Evaluating VRDN-001 in Patients Diagnosed with Chronic TED. Available online: https://www.ophthalmologytimes.com/view/viridian-announces-positive-data-from-ongoing-phase-1-2-trial-evaluating-vrdn-001-in-patients-diagnosed-with-chronic-ted (accessed on 22 June 2025).
- Jiayun Men, C.; Kossler, A.L. Advances in the Medical Treatment of Thyroid Eye Disease: Current and Emerging Therapies. touchREVIEWS Ophthalmol. 2024, 18, 33–40. [Google Scholar] [CrossRef]
- Salvi, M.; Foster, K.; Dickinson, B.; Matthew, A.; Vijayaraghavan, J.; Michalsky, C.; Bedian, V. VRDN-003, a full antagonist antibody to IGF-1R for thyroid eye disease (TED): Phase 1 results show potential for subcutaneous administration. Presented at the 46th Annual Meeting of the European Thyroid Association (ETA) 2024, Athens, Greece, 7–10 September 2024; Volume 101, p. OP-13-01, Endocrine Abstracts. [Google Scholar] [CrossRef]
- Luffy, M.; Ganz, A.-L.; Wagner, S.; Wolf, J.; Ropertz, J.; Zeidan, R.; Kent, J.D.; Douglas, R.S.; Kahaly, G.J. Linsitinib inhibits proliferation and induces apoptosis of both IGF-1R and TSH-R expressing cells. Front. Immunol. 2024, 15, 1488220. [Google Scholar] [CrossRef] [PubMed]
- Ugradar, S.; Kostick, D.; Spadaro, J.; Grover, A.; Imm, S.; Chesler, S.; Mpofu, S.; Khong, J. Preliminary Safety and Efficacy of Subcutaneous Lonigutamab (anti-IGF-1R) From a Phase 1/2 Proof of Concept Study in Patients With Thyroid Eye Disease. J. Endocr. Soc. 2024, 8, A1067–A1068. [Google Scholar] [CrossRef]
| Treatment Phase | Disease Stage | Therapeutic Approach | Intervention | Clinical Indications | Key Considerations |
|---|---|---|---|---|---|
| Initial Management | All Stages | Foundational Measures |
| All newly diagnosed TED cases |
|
| Ocular Support |
| Universal TED presentation | Heightened vigilance for keratopathy | ||
| Behavioral Modification |
| All TED patients | Tobacco use exacerbates disease progression | ||
| Specialist Consultation | Referral to TED multidisciplinary center | Moderate to severe or progressive disease | Facilitates timely, tailored management | ||
| Mild TED | Active Phase | Pharmacotherapy |
| Mild active TED, particularly in endemic selenium-deficient areas |
|
| Surveillance |
| Stable, minimally symptomatic presentations | Escalate therapy if progression occurs | ||
| Inactive Phase | Reconstructive Intervention |
| Persistent retraction or cosmetic impairment | Requires ≥ 6 months of thyroid stability | |
| Moderate-to-Severe TED | Active Phase | Intravenous Corticosteroids |
| Active inflammatory moderate to severe TED |
|
| Biologic Agents |
|
| Limited longitudinal data on efficacy | ||
| Radiotherapy |
| Progressive ocular motility impairment or steroid contraindications |
| ||
| Inactive Phase | Surgical Correction |
| Quiescent disease (≥6 months) with residual functional/aesthetic deficits |
| |
| Sight-Threatening TED | Active Phase | Urgent Medical Therapy |
| Dysthyroid optic neuropathy or acute visual decline | Surgical decompression if inadequate response within 2 weeks |
| Emergency Surgery |
| Vision deterioration despite medical therapy | Requires subspecialty orbital surgical expertise | ||
| Corneal Salvage |
| Impending corneal perforation or ulceration | Surgical delay risks irreversible visual loss |
| Study Design | Sample Size | Patient Characteristics | Treatment Dosage | Primary Outcomes | Secondary Outcomes | Notes | Study |
|---|---|---|---|---|---|---|---|
| Cross-sectional cohort | 21 | Heterogeneous TED including three DON cases | 8 doses | 71.4% achieved ≥2 mm proptosis reduction | CAS −2.2, motility +16.9° | DON cases improved | Diniz et al., 2021 [42] |
| Multicenter case series | 10 | DON patients in whom conventional therapy failed | 8 infusions | VA improvement = 0.87 logMAR | Proptosis −4.7 mm, CAS −5.25 | Rapid DON improvement | Sears et al., 2021 [43] |
| Prospective longitudinal | 23 | Patients with TED accompanied by facial/eyelid changes | 8 infusions | Reduction in facial soft tissue volume | Improvement in eyelid position | Orbital soft tissue expansion was also reduced | Ugradar et al., 2021 [44] |
| Retrospective EAP study | 13 | Active moderate–severe TED | 8 infusions (77%) | Proptosis −4.6 mm | CAS −4.0, light sensitivity −9.1 | Expanded access program | Wang et al., 2021 [45] |
| EAP study | 22 | Active moderate–severe TED | 8 infusions (86%) | QOL improvement | Not specified | All patients reported AEs; mainly muscle spasms (n = 11), fatigue (n = 10) | Douglas et al., 2021 [46] |
| Prospective longitudinal | 43 | Active TED with facial changes | 8 infusions | Facial volume reduction (mean decrease = 8.4 mL) | Proptosis improvement | Pan-facial assessment | Ugradar & Douglas, 2022 [47] |
| Retrospective review | 31 | Chronic TED (>2 years) | Mean: 7 infusions | Proptosis −3.5 mm | 67% diplopia improvement | Teprotumumab treatment had therapeutic efficacy in patients with chronic TED | Ugradar et al., 2022 [2] |
| Retrospective review | 17 | TED with IOP concerns | Mean: 12 weeks | Mean IOP was decreased at last record of follow-up by 4.9 mm Hg | Not specified | Teprotumumab treatment reduced IOP | Adetunji et al., 2022 [48] |
| Retrospective study | 37 | Steroid-naive and steroid-resistant TED | Up to 8 infusions | 81% proptosis response in steroid-resistant group | 45% diplopia resolution, 86% disease inactivation | Comparison with tocilizumab | Kotwal et al., 2023 [49] |
| Observational cross-sectional | 74 | Active (n = 62) and minimal-activity (n = 12) TED; treatment interrupted | Average of 4.2 infusions | Proptosis: −2.9 mm (active), −2.8 mm (minimal) | CAS −3.4 | COVID-19 interrupted this study | Ho et al., 2023 [50] |
| RCT | 62 | Chronic/low-activity TED (2–10 years) | 8 infusions | Proptosis: −2.41 mm vs. −0.92 mm(placebo) | Not specified | First chronic placebo-controlled trial with TED | Douglas et al., 2023 [51] |
| Multicenter retrospective | 131 | All stages/activity levels of TED | ≥4 infusions | 76% achieved proptosis −3.0 mm | 3.2-point average CAS reduction, GDS improved by at least 1 point for 50% | Comprehensive AE study | Shah et al., 2024 [52] |
| Multicenter retrospective | 66 | Recalcitrant TED for which conventional therapy failed | ≥4 infusions | 85.9% proptosis response | CAS 93.8%, diplopia 69.1% | Poor response post-decompression | Men et al., 2024 [53] |
| RCT | 54 | Active moderate–severe TED in Japanese cohort | 8 infusions | 89% vs. 11% (placebo) proptosis responses | 78% vs. 4% (placebo) overall responses | First Japanese RCT | Hironmatsu et al., 2025 [54] |
| Multicenter case series | 26 | Hypothyroid/euthyroid TED | 8 infusions | Proptosis −2.7 mm | CAS and diplopia improvements | Non-hyperthyroid TED | Ugradar et al., 2025 [55] |
| Multicenter retrospective | 119 | Complete treatment with 1-year follow-up | 8 infusions | 24% re-treatment rate | Not specified | Age was the only significant driver of re-treatment | Ugradar et al., 2025 [56]. |
| Retrospective cohort study | 32 | Failed intravenous glucocorticoid treatment; four with prior decompression surgery | 8 infusions | Proptosis: R −2.4 mm, L −2.0 mm | Improvement in diplopia | One case of teprotumumab-induced encephalopathy was reported and successfully treated using plasma exchange; decompression surgery history did not affect efficacy | Lustig-Barzelay et al., 2025 [57] |
| Author (Year) | Patients Treated with Teprotumumab | Total AE Rate | Common AEs (>10%) | Serious AEs | Special Notes |
|---|---|---|---|---|---|
| Ho et al., 2023 [50] | 74 | Initial: 66% Final: 29% | - Muscle spasms (27%) - Alopecia (18%) - Hyperglycemia (14%) - Hearing changes (11%) - Fatigue (9%) - GI discomfort (8%) | Three new diabetes cases; one severe hyperglycemia case (>700 mg/dL) | - AEs decreased over time - No new DON cases during interruption - One patient discontinued treatment due to hyperglycemia—three patients required oral diabetes medication |
| Diniz et al., 2021 [42] | 21 | 85.7% | - Fatigue (43%) - Muscle spasms (33%) - Dysgeusia (26%) - Nausea (19%) - Weight loss (14%) - Hearing issues (14%) - Hyperglycemia (14%) | Three cases requiring diabetes medication | - Most AEs were of a grade 1–2 severity - One discontinuation due to multiple AEs - Two/three hyperglycemia cases in non-diabetic patients - Age may be risk factor for hearing issues |
| Lustig-Barzelay et al., 2025 [57] | 32 | Not specified | - Myalgia (n = 12%) - Hyperglycemia (n = 9%) - Diarrhea (n = 9%) - Hearing issues (n = 12%) | One case of encephalopathy | - Encephalopathy successfully treated with plasmapheresis - Real-world Israeli cohort experience |
| Douglas et al., 2021 [46] | 22 | 100% | - Muscle spasms (50%) - Fatigue (45%) - Hypoacusis (23%)–headache (23%) - Nausea (23%) - Extremity pain (18%)–alopecia (18%) - Hypertension (18%) | One case of appendicitis (deemed unrelated) | - Multiple other AEs reported in smaller numbers (n = 3): dry skin, diarrhea, tinnitus, myalgia, increased lacrimation, hypogeusia |
| Kotwal et al., 2023 [49] | 37 | 76% | - Hearing changes (46%) - Hyperglycemia (23%) | Not specified | - Compared with the tocilizumab group which reported no AEs |
| Shah et al., 2024 [52] | 131 | 82% | - Musculoskeletal (58.0%) - GI (38%) - Skin (38%) - Ear/hearing (31%) - Nervous system (21%) - Metabolic (15%)–reproductive (12%) | 8.4% (11/131) severe AEs | - Mean AE onset: 7.9 weeks - Mean duration: 17.6 weeks - 46% had persistent AEs at last follow- up - 12.2% discontinued therapy (hearing loss n = 4, IBD n = 2, hyperglycemia n = 1, muscle spasms n = 1, multiple AEs n = 8) |
| Hironmatsu et al., 2025 [54] | 27 | Not specified | - Hyperglycemia (22%) - Hearing impairment (15%) | Not specified | - The Japanese population - Placebo-controlled comparison available |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zong, Y.; Qiu, S.; Yang, M.; Zhang, J.; Zou, Y.; Jing, Y.; Ohno-Matsui, K.; Kamoi, K. Teprotumumab for Thyroid Eye Disease: Mechanism, Clinical Efficacy, and Current Challenges. Antibodies 2025, 14, 55. https://doi.org/10.3390/antib14030055
Zong Y, Qiu S, Yang M, Zhang J, Zou Y, Jing Y, Ohno-Matsui K, Kamoi K. Teprotumumab for Thyroid Eye Disease: Mechanism, Clinical Efficacy, and Current Challenges. Antibodies. 2025; 14(3):55. https://doi.org/10.3390/antib14030055
Chicago/Turabian StyleZong, Yuan, Shuang Qiu, Mingming Yang, Jing Zhang, Yaru Zou, Yuxin Jing, Kyoko Ohno-Matsui, and Koju Kamoi. 2025. "Teprotumumab for Thyroid Eye Disease: Mechanism, Clinical Efficacy, and Current Challenges" Antibodies 14, no. 3: 55. https://doi.org/10.3390/antib14030055
APA StyleZong, Y., Qiu, S., Yang, M., Zhang, J., Zou, Y., Jing, Y., Ohno-Matsui, K., & Kamoi, K. (2025). Teprotumumab for Thyroid Eye Disease: Mechanism, Clinical Efficacy, and Current Challenges. Antibodies, 14(3), 55. https://doi.org/10.3390/antib14030055





