Preventative Cancer Vaccine-Elicited Human Anti-MUC1 Antibodies Have Multiple Effector Functions
Abstract
1. Introduction
2. Materials and Methods
2.1. Antibodies
2.2. Cell Lines
2.3. Primary Cells
2.4. Lentiviral Vector Generation
2.5. Lentiviral Production
2.6. Lentiviral Transduction
2.7. Cell Sorting
2.8. Antibody Binding Assay
2.9. Antibody-Dependent Cellular Cytotoxicity Assay
2.10. Antibody-Dependent Cytokine Release Assay
2.11. Antibody-Dependent Cellular Phagocytosis Assay
2.12. Antibody-Dependent Trogocytosis/Trogoptosis Assay
2.13. Complement-Dependent Cytotoxicity Assay
2.14. Glycosylation Enzyme Inhibition
2.15. Glycopeptide ELISA
2.16. MCP-1 ELISA
2.17. Imaging Flow Cytometry
2.18. Data Analysis
3. Results
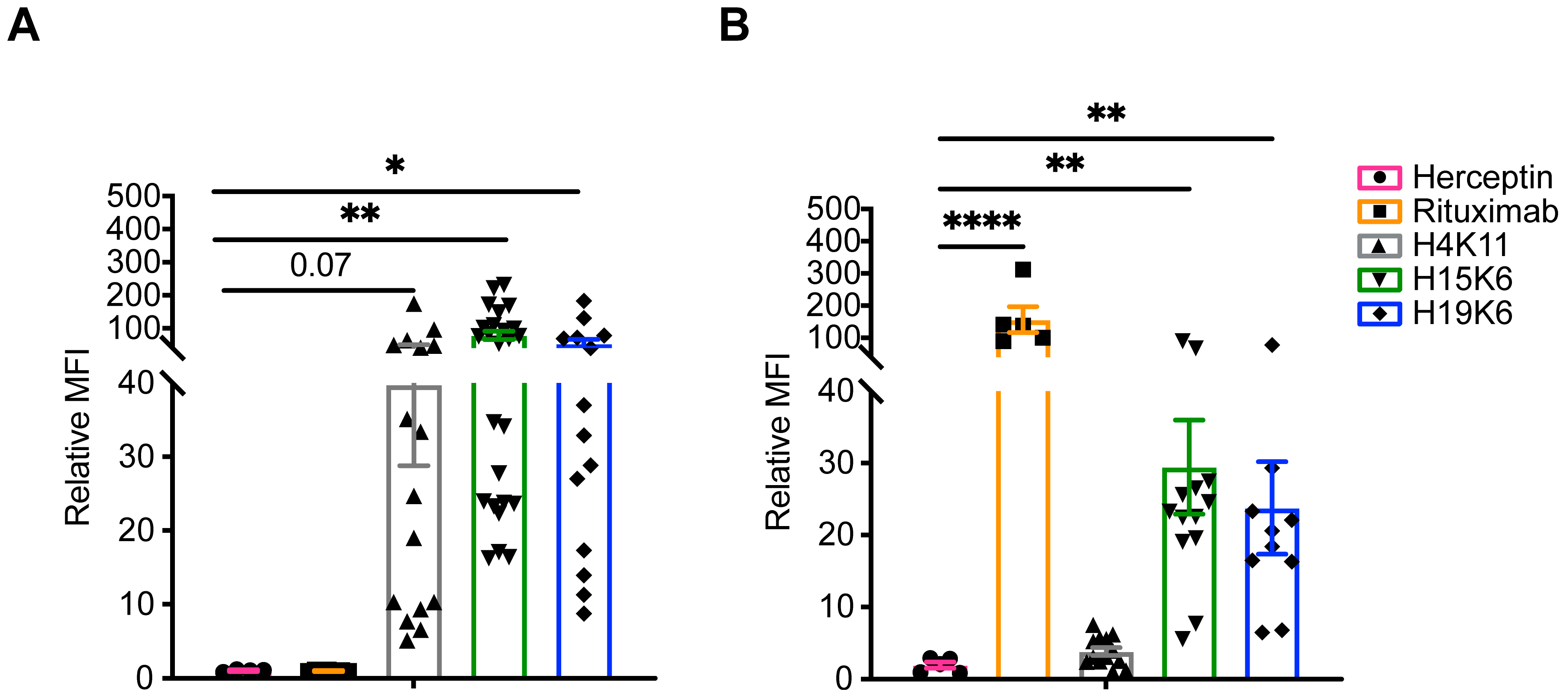
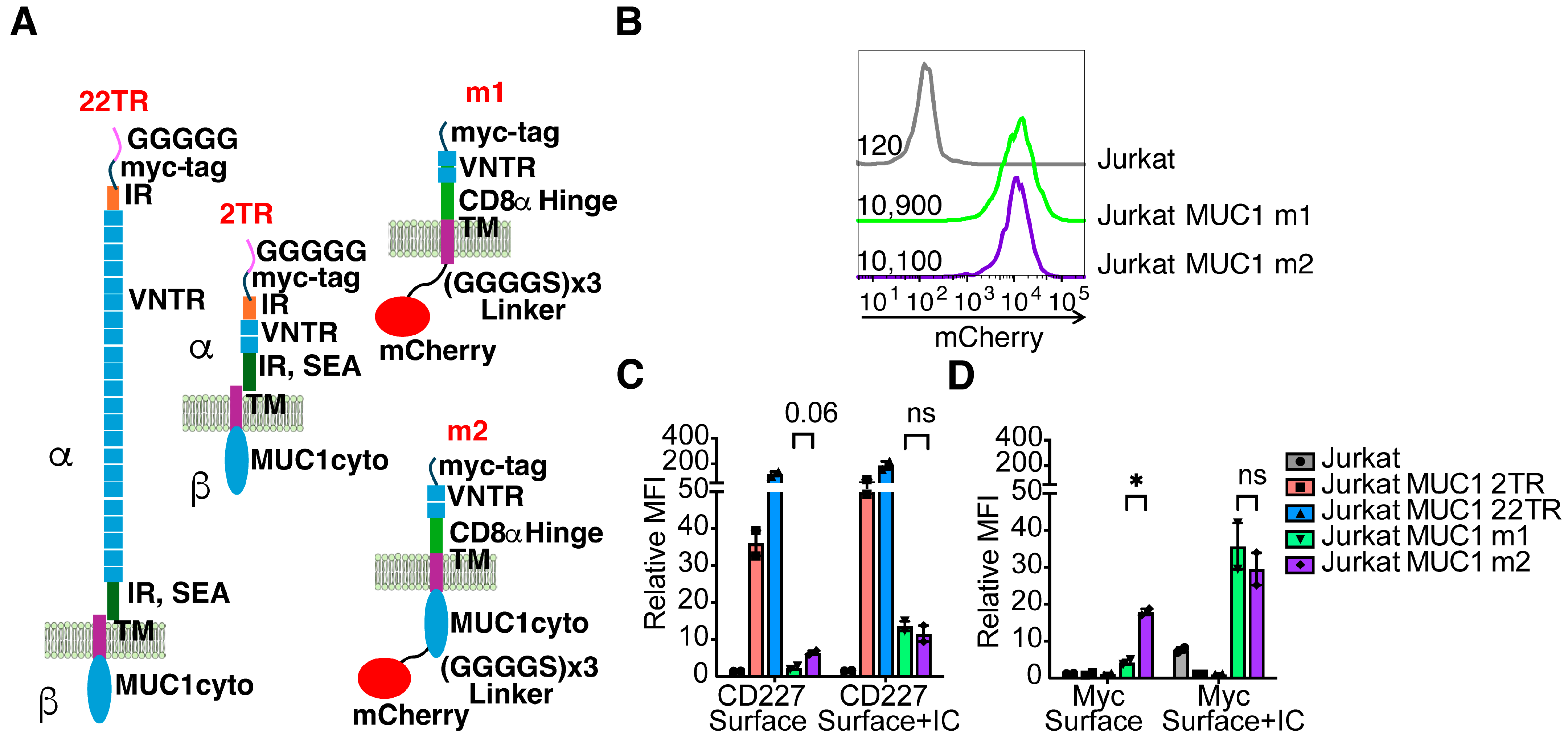
3.1. Characterization of the Target Cell Lines and Antibody Binding
3.2. Characterization of Anti-MUC1 Antibody-Mediated Effector Functions
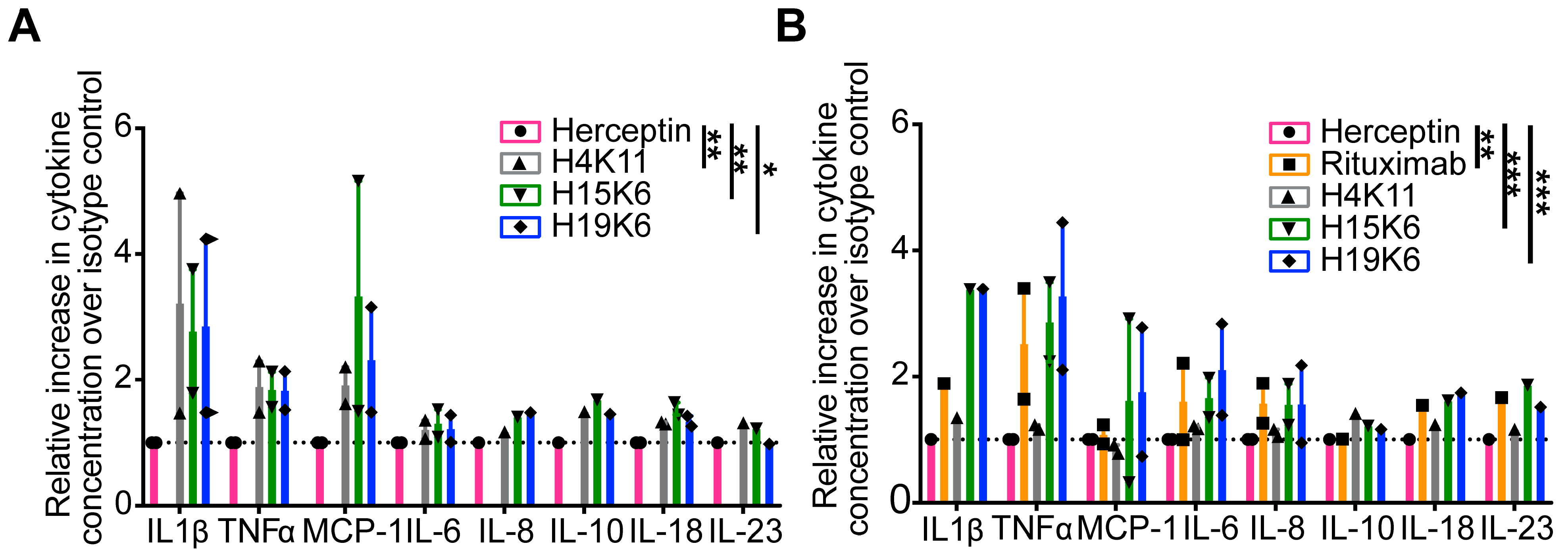
3.2.1. Cytokine Release
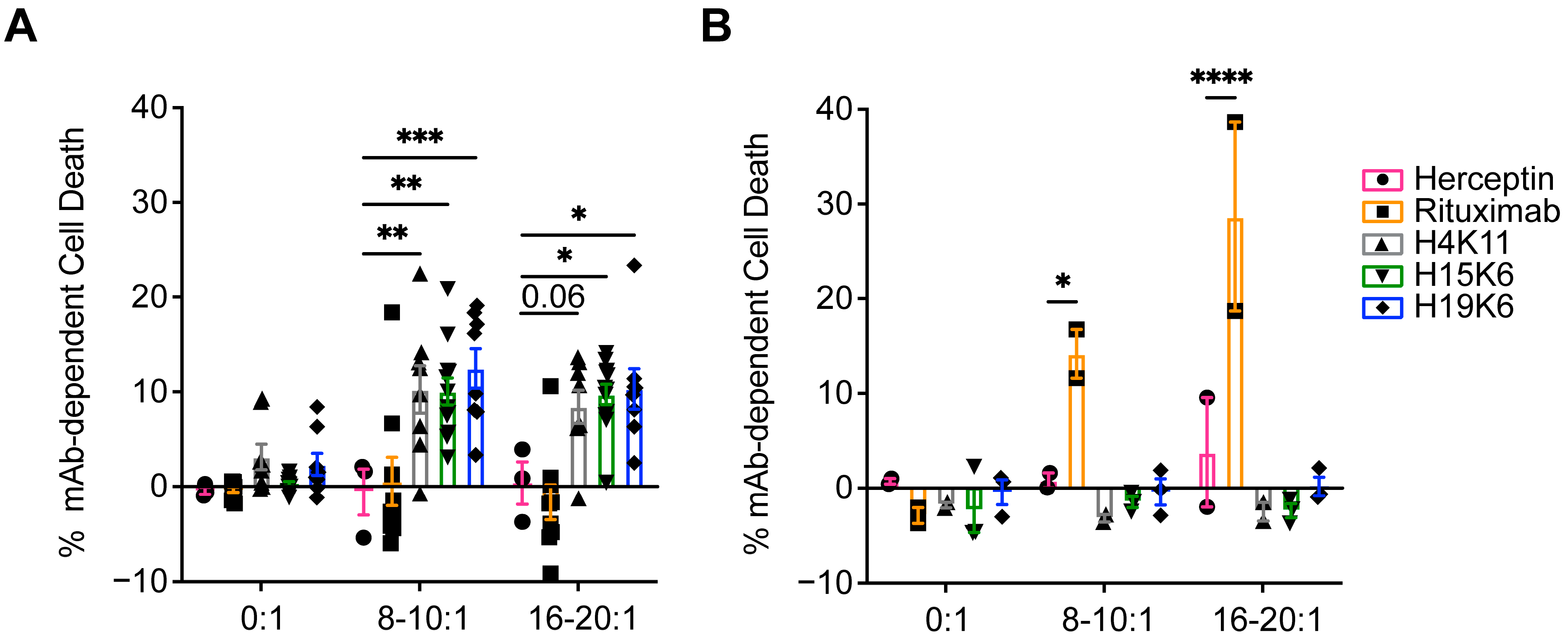
3.2.2. Antibody-Dependent Cytotoxicity (ADCC)
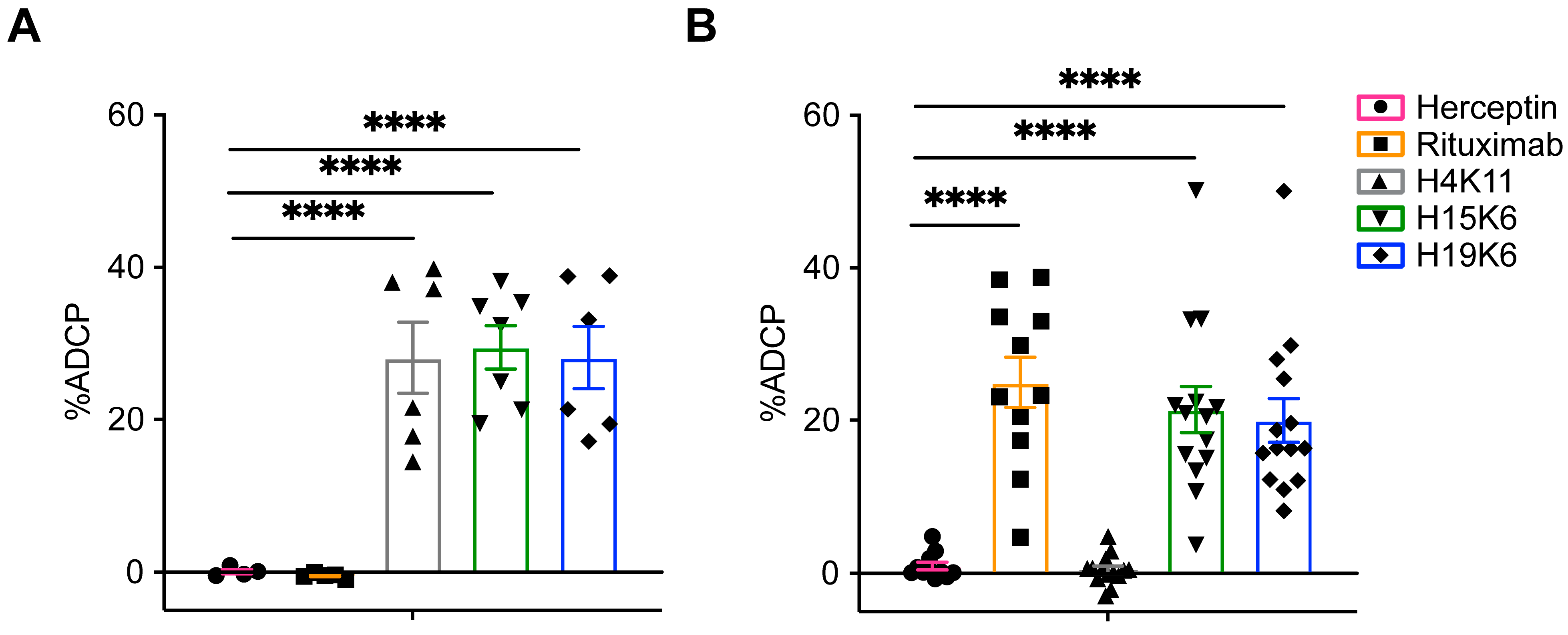
3.2.3. Antibody-Dependent Phagocytosis (ADCP)
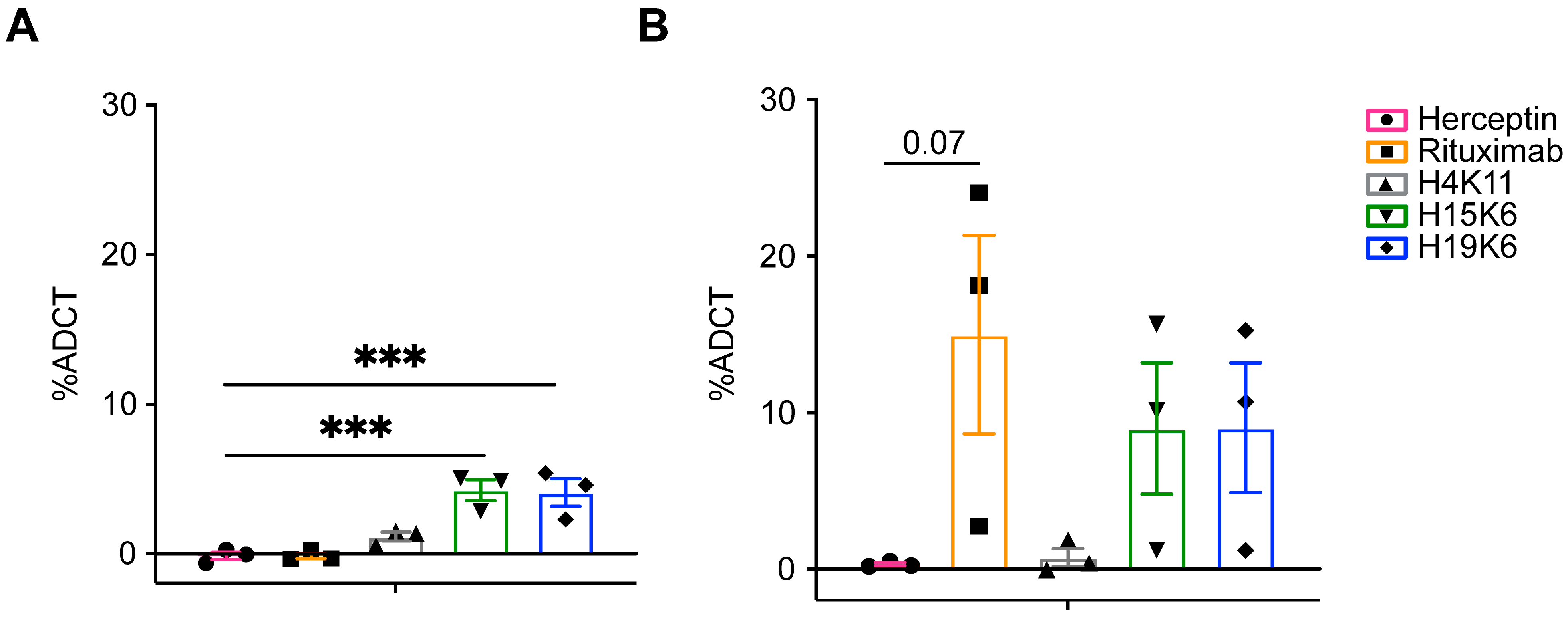
3.2.4. Antibody-Dependent Trogocytosis/Trogoptosis (ADCT)
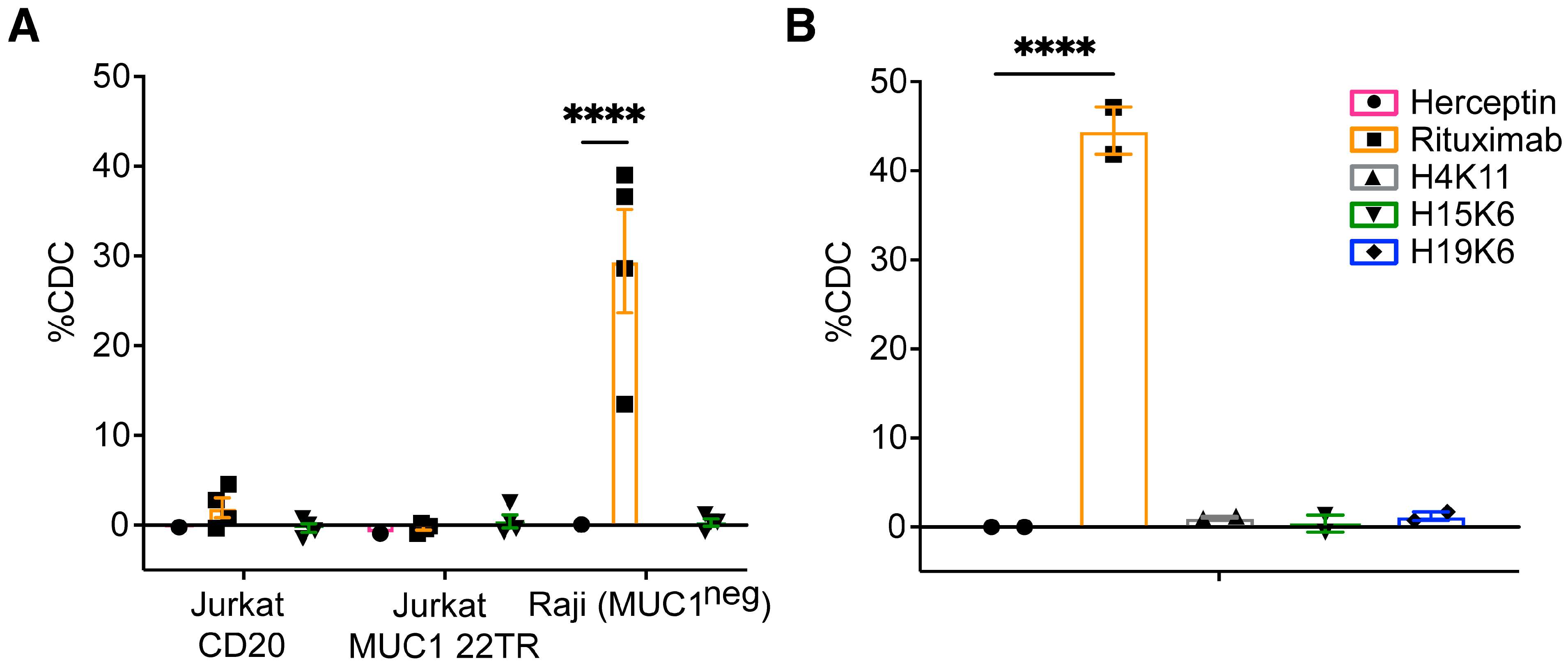
3.2.5. Antibody-Dependent Complement-Dependent Cytotoxicity (ADCDC)
3.3. Characterization of MUC1 Antigen Attributes That Impact mAb-Epitope Interactions and Effector Functions
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Nath, S.; Mukherjee, P. MUC1: A Multifaceted Oncoprotein with a Key Role in Cancer Progression. Trends Mol. Med. 2014, 20, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Gendler, S.J.; Lancaster, C.A.; Taylor-Papadimitriou, J.; Duhig, T.; Peat, N.; Burchell, J.; Pemberton, L.; Lalani, E.N.; Wilson, D. Molecular Cloning and Expression of Human Tumor-Associated Polymorphic Epithelial Mucin. J. Biol. Chem. 1990, 265, 15286–15293. [Google Scholar] [CrossRef] [PubMed]
- Beatty, P.L.; van der Geest, R.; Hashash, J.G.; Kimura, T.; Gutkin, D.; Brand, R.E.; Finn, O.J. Immunobiology and Immunosurveillance in Patients with Intraductal Papillary Mucinous Neoplasms (IPMNs), Premalignant Precursors of Pancreatic Adenocarcinomas. Cancer Immunol. Immunother. 2016, 65, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Saltos, A.; Khalil, F.; Smith, M.; Li, J.; Schell, M.; Antonia, S.J.; Gray, J.E. Clinical Associations of Mucin 1 in Human Lung Cancer and Precancerous Lesions. Oncotarget 2018, 9, 35666–35675. [Google Scholar] [CrossRef]
- Krishn, S.R.; Kaur, S.; Smith, L.M.; Johansson, S.L.; Jain, M.; Patel, A.; Gautam, S.K.; Hollingsworth, M.A.; Mandel, U.; Clausen, H.; et al. Mucins and Associated Glycan Signatures in Colon Adenoma-Carcinoma Sequence: Prospective Pathological Implication(s) for Early Diagnosis of Colon Cancer. Cancer Lett. 2016, 374, 304–314. [Google Scholar] [CrossRef]
- Ju, T.; Lanneau, G.S.; Gautam, T.; Wang, Y.; Xia, B.; Stowell, S.R.; Willard, M.T.; Wang, W.; Xia, J.Y.; Zuna, R.E.; et al. Human Tumor Antigens Tn and Sialyl Tn Arise from Mutations in Cosmc. Cancer Res. 2008, 68, 1636–1646. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Ni, W.; Tai, G. Expression of MUC1 in Different Tumours and Its Clinical Significance (Review). Mol. Clin. Oncol. 2022, 17, 161. [Google Scholar] [CrossRef]
- Stroopinsky, D.; Kufe, D.; Avigan, D. MUC1 in Hematological Malignancies. Leuk. Lymphoma 2016, 57, 2489–2498. [Google Scholar] [CrossRef]
- Beckwith, D.M.; Cudic, M. Tumor-Associated O-Glycans of MUC1: Carriers of the Glyco-Code and Targets for Cancer Vaccine Design. Semin. Immunol. 2020, 47, 101389. [Google Scholar] [CrossRef]
- Budiu, R.A.; Mantia-Smaldone, G.; Elishaev, E.; Chu, T.; Thaller, J.; McCabe, K.; Lenzner, D.; Edwards, R.P.; Vlad, A.M. Soluble MUC1 and Serum MUC1-Specific Antibodies Are Potential Prognostic Biomarkers for Platinum-Resistant Ovarian Cancer. Cancer Immunol. Immunother. 2011, 60, 975–984. [Google Scholar] [CrossRef]
- Hamanaka, Y.; Suehiro, Y.; Fukui, M.; Shikichi, K.; Imai, K.; Hinoda, Y. Circulating Anti-MUC1 IgG Antibodies as a Favorable Prognostic Factor for Pancreatic Cancer. Int. J. Cancer 2003, 103, 97–100. [Google Scholar] [CrossRef]
- Gao, T.; Cen, Q.; Lei, H. A Review on Development of MUC1-Based Cancer Vaccine. Biomed. Pharmacother. 2020, 132, 110888. [Google Scholar] [CrossRef]
- Kimura, T.; McKolanis, J.R.; Dzubinski, L.A.; Islam, K.; Potter, D.M.; Salazar, A.M.; Schoen, R.E.; Finn, O.J. MUC1 Vaccine for Individuals with Advanced Adenoma of the Colon: A Cancer Immunoprevention Feasibility Study. Cancer Prev. Res. 2013, 6, 18–26. [Google Scholar] [CrossRef]
- Lohmueller, J.J.; Sato, S.; Popova, L.; Chu, I.M.; Tucker, M.A.; Barberena, R.; Innocenti, G.M.; Cudic, M.; Ham, J.D.; Cheung, W.C.; et al. Antibodies Elicited by the First Non-Viral Prophylactic Cancer Vaccine Show Tumor-Specificity and Immunotherapeutic Potential. Sci. Rep. 2016, 6, 31740. [Google Scholar] [CrossRef]
- Shurer, C.R.; Kuo, J.C.-H.; Roberts, L.M.; Gandhi, J.G.; Colville, M.J.; Enoki, T.A.; Pan, H.; Su, J.; Noble, J.M.; Hollander, M.J.; et al. Physical Principles of Membrane Shape Regulation by the Glycocalyx. Cell 2019, 177, 1757–1770.e21. [Google Scholar] [CrossRef]
- Miller, M.L.; Finn, O.J. Flow Cytometry-Based Assessment of Direct-Targeting Anti-Cancer Antibody Immune Effector Functions. Methods Enzymol. 2020, 632, 431–456. [Google Scholar] [CrossRef]
- Matlung, H.L.; Babes, L.; Zhao, X.W.; van Houdt, M.; Treffers, L.W.; van Rees, D.J.; Franke, K.; Schornagel, K.; Verkuijlen, P.; Janssen, H.; et al. Neutrophils Kill Antibody-Opsonized Cancer Cells by Trogoptosis. Cell Rep. 2018, 23, 3946–3959.e6. [Google Scholar] [CrossRef]
- Ayyalasomayajula, R.; Boneva, I.; Ormaza, D.; Whyte, A., Jr.; Farook, K.; Gorlin, Z.; Yancey, E.; André, S.; Kaltner, H.; Cudic, M. Synthesis and Thermodynamic Evaluation of Sialyl-Tn MUC1 Glycopeptides Binding to Macrophage Galactose-Type Lectin. ChemBioChem 2024, 25, e202400391. [Google Scholar] [CrossRef]
- Beckwith, D.M.; FitzGerald, F.G.; Rodriguez Benavente, M.C.; Mercer, E.R.; Ludwig, A.-K.; Michalak, M.; Kaltner, H.; Kopitz, J.; Gabius, H.-J.; Cudic, M. Calorimetric Analysis of the Interplay between Synthetic Tn Antigen-Presenting MUC1 Glycopeptides and Human Macrophage Galactose-Type Lectin. Biochemistry 2021, 60, 547–558. [Google Scholar] [CrossRef]
- Giovannone, N.; Antonopoulos, A.; Liang, J.; Geddes Sweeney, J.; Kudelka, M.R.; King, S.L.; Lee, G.S.; Cummings, R.D.; Dell, A.; Barthel, S.R.; et al. Human B Cell Differentiation Is Characterized by Progressive Remodeling of O-Linked Glycans. Front. Immunol. 2018, 9, 2857. [Google Scholar] [CrossRef]
- Jerome, K.R.; Bu, D.; Finn, O.J. Expression of Tumor-Associated Epitopes on Epstein-Barr Virus-Immortalized B-Cells and Burkitt’s Lymphomas Transfected with Epithelial Mucin Complementary DNA. Cancer Res. 1992, 52, 5985–5990. [Google Scholar] [CrossRef]
- Wilkie, S.; van Schalkwyk, M.; Hobbs, S.; Davies, D.M.; van der Stegen, S.; Parente-Pereira, A.; Papa, S.; Box, C.; Eccles, S.; Maher, J. Dual Targeting of ErbB2 and MUC1 in Breast Cancer Using Chimeric Antigen Receptors Engineered to Provide Complementary Signaling. J. Clin. Immunol. 2012, 32, 1059–1070. [Google Scholar] [CrossRef]
- Levitin, F.; Stern, O.; Weiss, M.; Gil-Henn, C.; Ziv, R.; Prokocimer, Z.; Smorodinsky, N.I.; Rubinstein, D.B.; Wreschner, D.H. The MUC1 SEA Module Is a Self-Cleaving Domain. J. Biol. Chem. 2005, 280, 33374–33386. [Google Scholar] [CrossRef]
- Engelmann, K.; Kinlough, C.L.; Müller, S.; Razawi, H.; Baldus, S.E.; Hughey, R.P.; Hanisch, F.-G. Transmembrane and Secreted MUC1 Probes Show Trafficking-Dependent Changes in O-Glycan Core Profiles. Glycobiology 2005, 15, 1111–1124. [Google Scholar] [CrossRef]
- Altschuler, Y.; Kinlough, C.L.; Poland, P.A.; Bruns, J.B.; Apodaca, G.; Weisz, O.A.; Hughey, R.P. Clathrin-Mediated Endocytosis of MUC1 Is Modulated by Its Glycosylation State. Mol. Biol. Cell 2000, 11, 819–831. [Google Scholar] [CrossRef]
- Pan, H.; Colville, M.J.; Supekar, N.T.; Azadi, P.; Paszek, M.J. Sequence-Specific Mucins for Glycocalyx Engineering. ACS Synth. Biol. 2019, 8, 2315–2326. [Google Scholar] [CrossRef]
- Tsao, L.-C.; Force, J.; Hartman, Z.C. Mechanisms of Therapeutic Anti-Tumor Monoclonal Antibodies. Cancer Res. 2021, 81, 4641–4651. [Google Scholar] [CrossRef]
- Antibody Therapeutics Approved or in Regulatory Review in the EU or US. Available online: https://www.antibodysociety.org/resources/approved-antibodies/ (accessed on 26 July 2024).
- Sakahara, H.; Saga, T.; Onodera, H.; Yao, Z.; Nakamoto, Y.; Zhang, M.; Sato, N.; Nakada, H.; Yamashina, I.; Endo, K.; et al. Anti-Murine Antibody Response to Mouse Monoclonal Antibodies in Cancer Patients. Jpn. J. Cancer Res. 1997, 88, 895–899. [Google Scholar] [CrossRef]
- Lux, A.; Nimmerjahn, F. Of Mice and Men: The Need for Humanized Mouse Models to Study Human IgG Activity In Vivo. J. Clin. Immunol. 2013, 33, 4–8. [Google Scholar] [CrossRef]
- Loeff, F.C.; van Egmond, H.M.E.; Nijmeijer, B.A.; Falkenburg, J.H.F.; Halkes, C.J.; Jedema, I. Complement-Dependent Cytotoxicity Induced by Therapeutic Antibodies in B-Cell Acute Lymphoblastic Leukemia Is Dictated by Target Antigen Expression Levels and Augmented by Loss of Membrane-Bound Complement Inhibitors. Leuk. Lymphoma 2017, 58, 2185–2195. [Google Scholar] [CrossRef]
- Cleary, K.L.S.; Chan, H.T.C.; James, S.; Glennie, M.J.; Cragg, M.S. Antibody Distance from the Cell Membrane Regulates Antibody Effector Mechanisms. J. Immunol. 2017, 198, 3999–4011. [Google Scholar] [CrossRef]
- Tang, Y.; Lou, J.; Alpaugh, R.K.; Robinson, M.K.; Marks, J.D.; Weiner, L.M. Regulation of Antibody-Dependent Cellular Cytotoxicity by IgG Intrinsic and Apparent Affinity for Target Antigen. J. Immunol. 2007, 179, 2815–2823. [Google Scholar] [CrossRef]
- Yin, J.; Albers, A.J.; Smith, T.S.; Riddell, G.T.; Richards, J.O. Differential Regulation of Human Monocytes and NK Cells by Antibody-Opsonized Tumors. Cancer Immunol. Immunother. 2018, 67, 1239–1250. [Google Scholar] [CrossRef]
- Derer, S.; Bauer, P.; Lohse, S.; Scheel, A.H.; Berger, S.; Kellner, C.; Peipp, M.; Valerius, T. Impact of Epidermal Growth Factor Receptor (EGFR) Cell Surface Expression Levels on Effector Mechanisms of EGFR Antibodies. J. Immunol. 2012, 189, 5230–5239. [Google Scholar] [CrossRef]
- Malenge, M.M.; Patzke, S.; Ree, A.H.; Stokke, T.; Ceuppens, P.; Middleton, B.; Dahle, J.; Repetto-Llamazares, A.H.V. 177Lu-Lilotomab Satetraxetan Has the Potential to Counteract Resistance to Rituximab in Non-Hodgkin Lymphoma. J. Nucl. Med. 2020, 61, 1468–1475. [Google Scholar] [CrossRef]
- Czuczman, M.S.; Olejniczak, S.; Gowda, A.; Kotowski, A.; Binder, A.; Kaur, H.; Knight, J.; Starostik, P.; Deans, J.; Hernandez-Ilizaliturri, F.J. Acquirement of Rituximab Resistance in Lymphoma Cell Lines Is Associated with Both Global CD20 Gene and Protein Down-Regulation Regulated at the Pretranscriptional and Posttranscriptional Levels. Clin. Cancer Res. 2008, 14, 1561–1570. [Google Scholar] [CrossRef]
- Grazia Cifone, M.; Giacomelli, R.; Famularo, G.; Paolini, R.; Danese, C.; Napolitano, T.; Procopio, A.; Perego, A.M.; Santoni, A.; Tonietti, G. Natural Killer Activity and Antibody-Dependent Cellular Cytotoxicity in Progressive Systemic Sclerosis. Clin. Exp. Immunol. 1990, 80, 360–365. [Google Scholar] [CrossRef]
- Borgerding, A.; Hasenkamp, J.; Engelke, M.; Burkhart, N.; Trümper, L.; Wienands, J.; Glass, B. B-Lymphoma Cells Escape Rituximab-Triggered Elimination by NK Cells through Increased HLA Class I Expression. Exp. Hematol. 2010, 38, 213–221. [Google Scholar] [CrossRef]
- Hasenkamp, J.; Borgerding, A.; Wulf, G.; Uhrberg, M.; Jung, W.; Dingeldein, S.; Truemper, L.; Glass, B. Resistance against Natural Killer Cell Cytotoxicity: Analysis of Mechanisms. Scand. J. Immunol. 2006, 64, 444–449. [Google Scholar] [CrossRef]
- Xiao, H.; Woods, E.C.; Vukojicic, P.; Bertozzi, C.R. Precision Glycocalyx Editing as a Strategy for Cancer Immunotherapy. Proc. Natl. Acad. Sci. USA 2016, 113, 10304–10309. [Google Scholar] [CrossRef]
- van Rees, D.J.; Bouti, P.; Klein, B.; Verkuijlen, P.J.H.; van Houdt, M.; Schornagel, K.; Tool, A.T.J.; Venet, D.; Sotiriou, C.; El-Abed, S.; et al. Cancer Cells Resist Antibody-Mediated Destruction by Neutrophils through Activation of the Exocyst Complex. J. Immunother. Cancer 2022, 10, e004820. [Google Scholar] [CrossRef]
- van Rees, D.J.; Brinkhaus, M.; Klein, B.; Verkuijlen, P.; Tool, A.T.J.; Schornagel, K.; Treffers, L.W.; van Houdt, M.; Kater, A.P.; Vidarsson, G.; et al. Sodium Stibogluconate and CD47-SIRPα Blockade Overcome Resistance of Anti-CD20-Opsonized B Cells to Neutrophil Killing. Blood Adv. 2022, 6, 2156–2166. [Google Scholar] [CrossRef]
- Bakalar, M.H.; Joffe, A.M.; Schmid, E.M.; Son, S.; Podolski, M.; Fletcher, D.A. Size-Dependent Segregation Controls Macrophage Phagocytosis of Antibody-Opsonized Targets. Cell 2018, 174, 131–142.e13. [Google Scholar] [CrossRef]
- Cullen, S.P.; Martin, S.J. Mechanisms of Granule-Dependent Killing. Cell Death Differ. 2008, 15, 251–262. [Google Scholar] [CrossRef]
- Bramwell, M.E.; Wiseman, G.; Shotton, D.M. Electron-Microscopic Studies of the ca Antigen, Epitectin. J. Cell Sci. 1986, 86, 249–261. [Google Scholar] [CrossRef]
- Cragg, M.S.; Morgan, S.M.; Chan, H.T.C.; Morgan, B.P.; Filatov, A.V.; Johnson, P.W.M.; French, R.R.; Glennie, M.J. Complement-Mediated Lysis by Anti-CD20 mAb Correlates with Segregation into Lipid Rafts. Blood 2003, 101, 1045–1052. [Google Scholar] [CrossRef]
- Ringshausen, I.; Feuerstacke, Y.; Krainz, P.; den Hollander, J.; Hermann, K.; Buck, A.; Peschel, C.; Meyer Zum Bueschenfelde, C. Antifungal Therapy with Itraconazole Impairs the Anti-Lymphoma Effects of Rituximab by Inhibiting Recruitment of CD20 to Cell Surface Lipid Rafts. Cancer Res. 2010, 70, 4292–4296. [Google Scholar] [CrossRef][Green Version]
- van Meerten, T.; van Rijn, R.S.; Hol, S.; Hagenbeek, A.; Ebeling, S.B. Complement-Induced Cell Death by Rituximab Depends on CD20 Expression Level and Acts Complementary to Antibody-Dependent Cellular Cytotoxicity. Clin. Cancer Res. 2006, 12, 4027–4035. [Google Scholar] [CrossRef]
- Hanisch, F.-G.; Kinlough, C.L.; Staubach, S.; Hughey, R.P. MUC1 Membrane Trafficking: Protocols for Assessing Biosynthetic Delivery, Endocytosis, Recycling, and Release through Exosomes. Methods Mol. Biol. 2012, 842, 123–140. [Google Scholar] [CrossRef]
- Staubach, S.; Razawi, H.; Hanisch, F.-G. Proteomics of MUC1-Containing Lipid Rafts from Plasma Membranes and Exosomes of Human Breast Carcinoma Cells MCF-7. Proteomics 2009, 9, 2820–2835. [Google Scholar] [CrossRef]
- Lee, D.; Ahn, H.; Sim, H.-I.; Choi, E.; Choi, S.; Jo, Y.; Yun, B.; Song, H.K.; Oh, S.J.; Denda-Nagai, K.; et al. A CRISPR Activation Screen Identifies MUC-21 as Critical for Resistance to NK and T Cell-Mediated Cytotoxicity. J. Exp. Clin. Cancer Res. 2023, 42, 272. [Google Scholar] [CrossRef]
- Aldeghaither, D.S.; Zahavi, D.J.; Murray, J.C.; Fertig, E.J.; Graham, G.T.; Zhang, Y.-W.; O’Connell, A.; Ma, J.; Jablonski, S.A.; Weiner, L.M. A Mechanism of Resistance to Antibody-Targeted Immune Attack. Cancer Immunol. Res. 2019, 7, 230–243. [Google Scholar] [CrossRef]
- Kamber, R.A.; Nishiga, Y.; Morton, B.; Banuelos, A.M.; Barkal, A.A.; Vences-Catalán, F.; Gu, M.; Fernandez, D.; Seoane, J.A.; Yao, D.; et al. Inter-Cellular CRISPR Screens Reveal Regulators of Cancer Cell Phagocytosis. Nature 2021, 597, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Mercogliano, M.F.; De Martino, M.; Venturutti, L.; Rivas, M.A.; Proietti, C.J.; Inurrigarro, G.; Frahm, I.; Allemand, D.H.; Deza, E.G.; Ares, S.; et al. TNFα-Induced Mucin 4 Expression Elicits Trastuzumab Resistance in HER2-Positive Breast Cancer. Clin. Cancer Res. 2017, 23, 636–648. [Google Scholar] [CrossRef]
- Namba, M.; Hattori, N.; Hamada, H.; Yamaguchi, K.; Okamoto, Y.; Nakashima, T.; Masuda, T.; Sakamoto, S.; Horimasu, Y.; Miyamoto, S.; et al. Anti-KL-6/MUC1 Monoclonal Antibody Reverses Resistance to Trastuzumab-Mediated Antibody-Dependent Cell-Mediated Cytotoxicity by Capping MUC1. Cancer Lett. 2019, 442, 31–39. [Google Scholar] [CrossRef]
- Raina, D.; Uchida, Y.; Kharbanda, A.; Rajabi, H.; Panchamoorthy, G.; Jin, C.; Kharbanda, S.; Scaltriti, M.; Baselga, J.; Kufe, D. Targeting the MUC1-C Oncoprotein Downregulates HER2 Activation and Abrogates Trastuzumab Resistance in Breast Cancer Cells. Oncogene 2014, 33, 3422–3431. [Google Scholar] [CrossRef]
- Petricevic, B.; Laengle, J.; Singer, J.; Sachet, M.; Fazekas, J.; Steger, G.; Bartsch, R.; Jensen-Jarolim, E.; Bergmann, M. Trastuzumab Mediates Antibody-Dependent Cell-Mediated Cytotoxicity and Phagocytosis to the Same Extent in Both Adjuvant and Metastatic HER2/Neu Breast Cancer Patients. J. Transl. Med. 2013, 11, 307. [Google Scholar] [CrossRef]
- Dall’Ozzo, S.; Tartas, S.; Paintaud, G.; Cartron, G.; Colombat, P.; Bardos, P.; Watier, H.; Thibault, G. Rituximab-Dependent Cytotoxicity by Natural Killer Cells: Influence of FCGR3A Polymorphism on the Concentration-Effect Relationship. Cancer Res. 2004, 64, 4664–4669. [Google Scholar] [CrossRef]
- Kimura, H.; Sakai, K.; Arao, T.; Shimoyama, T.; Tamura, T.; Nishio, K. Antibody-Dependent Cellular Cytotoxicity of Cetuximab against Tumor Cells with Wild-Type or Mutant Epidermal Growth Factor Receptor. Cancer Sci. 2007, 98, 1275–1280. [Google Scholar] [CrossRef]
- Ruella, M.; Korell, F.; Porazzi, P.; Maus, M.V. Mechanisms of Resistance to Chimeric Antigen Receptor-T Cells in Haematological Malignancies. Nat. Rev. Drug Discov. 2023, 22, 976–995. [Google Scholar] [CrossRef]
- Chao, M.P.; Alizadeh, A.A.; Tang, C.; Myklebust, J.H.; Varghese, B.; Gill, S.; Jan, M.; Cha, A.C.; Chan, C.K.; Tan, B.T.; et al. Anti-CD47 Antibody Synergizes with Rituximab to Promote Phagocytosis and Eradicate Non-Hodgkin Lymphoma. Cell 2010, 142, 699–713. [Google Scholar] [CrossRef]
- Barkal, A.A.; Brewer, R.E.; Markovic, M.; Kowarsky, M.; Barkal, S.A.; Zaro, B.W.; Krishnan, V.; Hatakeyama, J.; Dorigo, O.; Barkal, L.J.; et al. CD24 Signalling through Macrophage Siglec-10 Is a Target for Cancer Immunotherapy. Nature 2019, 572, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Turaj, A.H.; Hussain, K.; Cox, K.L.; Rose-Zerilli, M.J.J.; Testa, J.; Dahal, L.N.; Chan, H.T.C.; James, S.; Field, V.L.; Carter, M.J.; et al. Antibody Tumor Targeting Is Enhanced by CD27 Agonists through Myeloid Recruitment. Cancer Cell 2017, 32, 777–791.e6. [Google Scholar] [CrossRef] [PubMed]
- Cornet, S.; Mathé, D.; Chettab, K.; Evesque, A.; Matera, E.-L.; Trédan, O.; Dumontet, C. Pegfilgrastim Enhances the Antitumor Effect of Therapeutic Monoclonal Antibodies. Mol. Cancer Ther. 2016, 15, 1238–1247. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.; Medarova, Z.; Potthast, A.; Dai, G. In Vivo Targeting of Underglycosylated MUC-1 Tumor Antigen Using a Multimodal Imaging Probe. Cancer Res. 2004, 64, 1821–1827. [Google Scholar] [CrossRef]
- Lau, S.K.; Weiss, L.M.; Chu, P.G. Differential Expression of MUC1, MUC2, and MUC5AC in Carcinomas of Various Sites: An Immunohistochemical Study. Am. J. Clin. Pathol. 2004, 122, 61–69. [Google Scholar] [CrossRef]
- Tone, M.; Diamond, L.E.; Walsh, L.A.; Tone, Y.; Thompson, S.A.; Shanahan, E.M.; Logan, J.S.; Waldmann, H. High Level Transcription of the Complement Regulatory Protein CD59 Requires an Enhancer Located in Intron 1. J. Biol. Chem. 1999, 274, 710–716. [Google Scholar] [CrossRef]
- Christmas, S.E.; De La Mata Espinosa, C.T.; Halliday, D.; Buxton, C.A.; Cummerson, J.A.; Johnson, P.M. Levels of Expression of Complement Regulatory Proteins CD46, CD55 and CD59 on Resting and Activated Human Peripheral Blood Leucocytes. Immunology 2006, 119, 522–528. [Google Scholar] [CrossRef]
- Bordron, A.; Bagacean, C.; Mohr, A.; Tempescul, A.; Bendaoud, B.; Deshayes, S.; Dalbies, F.; Buors, C.; Saad, H.; Berthou, C.; et al. Resistance to Complement Activation, Cell Membrane Hypersialylation and Relapses in Chronic Lymphocytic Leukemia Patients Treated with Rituximab and Chemotherapy. Oncotarget 2018, 9, 31590–31605. [Google Scholar] [CrossRef]
- Granoff, D.M. Relative Importance of Complement-Mediated Bactericidal and Opsonic Activity for Protection against Meningococcal Disease. Vaccine 2009, 27, B117–B125. [Google Scholar] [CrossRef]
- Teeling, J.L.; Mackus, W.J.M.; Wiegman, L.J.J.M.; van den Brakel, J.H.N.; Beers, S.A.; French, R.R.; van Meerten, T.; Ebeling, S.; Vink, T.; Slootstra, J.W.; et al. The Biological Activity of Human CD20 Monoclonal Antibodies Is Linked to Unique Epitopes on CD20. J. Immunol. 2006, 177, 362–371. [Google Scholar] [CrossRef]
- Ragupathi, G.; Liu, N.X.; Musselli, C.; Powell, S.; Lloyd, K.; Livingston, P.O. Antibodies against Tumor Cell Glycolipids and Proteins, but Not Mucins, Mediate Complement-Dependent Cytotoxicity. J. Immunol. 2005, 174, 5706–5712. [Google Scholar] [CrossRef]
- Moreno, L.; Pérez, C.; Zabaleta, A.; Manrique, I.; Alignani, D.; Ajona, D.; Blanco, L.; Lasa, M.; Maiso, P.; Rodriguez, I.; et al. The Mechanism of Action of the Anti-CD38 Monoclonal Antibody Isatuximab in Multiple Myeloma. Clin. Cancer Res. 2019, 25, 3176–3187. [Google Scholar] [CrossRef]
- Zhang, L.; Vlad, A.; Milcarek, C.; Finn, O.J. Human Mucin MUC1 RNA Undergoes Different Types of Alternative Splicing Resulting in Multiple Isoforms. Cancer Immunol. Immunother. 2012, 62, 423–435. [Google Scholar] [CrossRef]
- Gong, Y.; Klein Wolterink, R.G.J.; Gulaia, V.; Cloosen, S.; Ehlers, F.A.I.; Wieten, L.; Graus, Y.F.; Bos, G.M.J.; Germeraad, W.T.V. Defucosylation of Tumor-Specific Humanized Anti-MUC1 Monoclonal Antibody Enhances NK Cell-Mediated Anti-Tumor Cell Cytotoxicity. Cancers 2021, 13, 2579. [Google Scholar] [CrossRef]
- Liu, R.; Oldham, R.J.; Teal, E.; Beers, S.A.; Cragg, M.S. Fc-Engineering for Modulated Effector Functions—Improving Antibodies for Cancer Treatment. Antibodies 2020, 9, 64. [Google Scholar] [CrossRef]
- Cascio, S.; Zhang, L.; Finn, O.J. MUC1 Protein Expression in Tumor Cells Regulates Transcription of Proinflammatory Cytokines by Forming a Complex with Nuclear Factor-κB P65 and Binding to Cytokine Promoters: IMPORTANCE OF EXTRACELLULAR DOMAIN *. J. Biol. Chem. 2011, 286, 42248–42256. [Google Scholar] [CrossRef]
- Lohmueller, J.J.; Ham, J.D.; Kvorjak, M.; Finn, O.J. mSA2 Affinity-Enhanced Biotin-Binding CAR T Cells for Universal Tumor Targeting. Oncoimmunology 2017, 7, e1368604. [Google Scholar] [CrossRef] [PubMed]
- Morsut, L.; Roybal, K.T.; Xiong, X.; Gordley, R.M.; Coyle, S.M.; Thomson, M.; Lim, W.A. Engineering Customized Cell Sensing and Response Behaviors Using Synthetic Notch Receptors. Cell 2016, 164, 780–791. [Google Scholar] [CrossRef]
- Evan, G.I.; Lewis, G.K.; Ramsay, G.; Bishop, J.M. Isolation of Monoclonal Antibodies Specific for Human C-Myc Proto-Oncogene Product. Mol. Cell. Biol. 1985, 5, 3610–3616. [Google Scholar] [CrossRef]
- Pasqual, G.; Chudnovskiy, A.; Tas, J.M.J.; Agudelo, M.; Schweitzer, L.D.; Cui, A.; Hacohen, N.; Victora, G.D. Monitoring T Cell-Dendritic Cell Interactions in Vivo by Intercellular Enzymatic Labelling. Nature 2018, 553, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Shaner, N.C.; Campbell, R.E.; Steinbach, P.A.; Giepmans, B.N.G.; Palmer, A.E.; Tsien, R.Y. Improved Monomeric Red, Orange and Yellow Fluorescent Proteins Derived from Discosoma Sp. Red Fluorescent Protein. Nat. Biotechnol. 2004, 22, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Matz, M.V.; Fradkov, A.F.; Labas, Y.A.; Savitsky, A.P.; Zaraisky, A.G.; Markelov, M.L.; Lukyanov, S.A. Fluorescent Proteins from Nonbioluminescent Anthozoa Species. Nat. Biotechnol. 1999, 17, 969–973. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McKeague, M.L.; Lohmueller, J.; Dracz, M.T.; Saadallah, N.; Ricci, E.D.; Beckwith, D.M.; Ayyalasomayajula, R.; Cudic, M.; Finn, O.J. Preventative Cancer Vaccine-Elicited Human Anti-MUC1 Antibodies Have Multiple Effector Functions. Antibodies 2024, 13, 85. https://doi.org/10.3390/antib13040085
McKeague ML, Lohmueller J, Dracz MT, Saadallah N, Ricci ED, Beckwith DM, Ayyalasomayajula R, Cudic M, Finn OJ. Preventative Cancer Vaccine-Elicited Human Anti-MUC1 Antibodies Have Multiple Effector Functions. Antibodies. 2024; 13(4):85. https://doi.org/10.3390/antib13040085
Chicago/Turabian StyleMcKeague, Michelle L., Jason Lohmueller, Matthew T. Dracz, Najla Saadallah, Eric D. Ricci, Donella M. Beckwith, Ramya Ayyalasomayajula, Maré Cudic, and Olivera J. Finn. 2024. "Preventative Cancer Vaccine-Elicited Human Anti-MUC1 Antibodies Have Multiple Effector Functions" Antibodies 13, no. 4: 85. https://doi.org/10.3390/antib13040085
APA StyleMcKeague, M. L., Lohmueller, J., Dracz, M. T., Saadallah, N., Ricci, E. D., Beckwith, D. M., Ayyalasomayajula, R., Cudic, M., & Finn, O. J. (2024). Preventative Cancer Vaccine-Elicited Human Anti-MUC1 Antibodies Have Multiple Effector Functions. Antibodies, 13(4), 85. https://doi.org/10.3390/antib13040085






