B Cell and Antibody Responses in Bovine Tuberculosis
Abstract
1. Introduction
2. General Aspects of B Cell and Antibody Responses in Human Tuberculosis
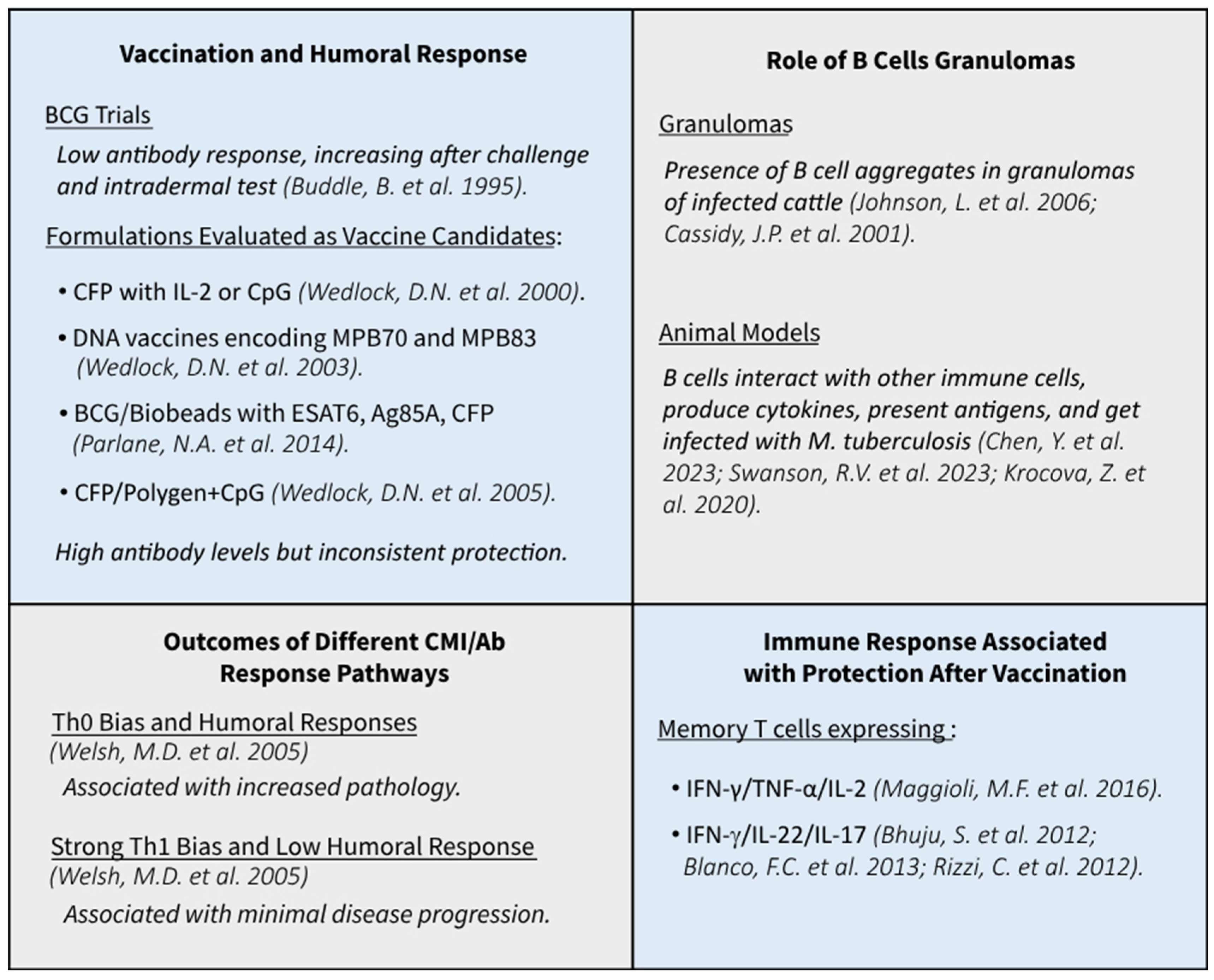
3. The Humoral Immune Response of Cattle upon Vaccination
4. The Humoral Immune Response of Cattle towards M. bovis in Experimental Infection
5. Role of B Cells in Tuberculosis
6. Diagnosis of Bovine Tuberculosis
7. Antibodies in the Diagnosis of Bovine Tuberculosis in Cattle and Other Animals
7.1. Whole M. bovis Antigen
7.2. Individual Antigens
7.3. Serological Tests Approved by the World Organisation for Animal Health
7.4. Serology for M. bovis-Infected Cattle That Escape Cellular Immune Tests
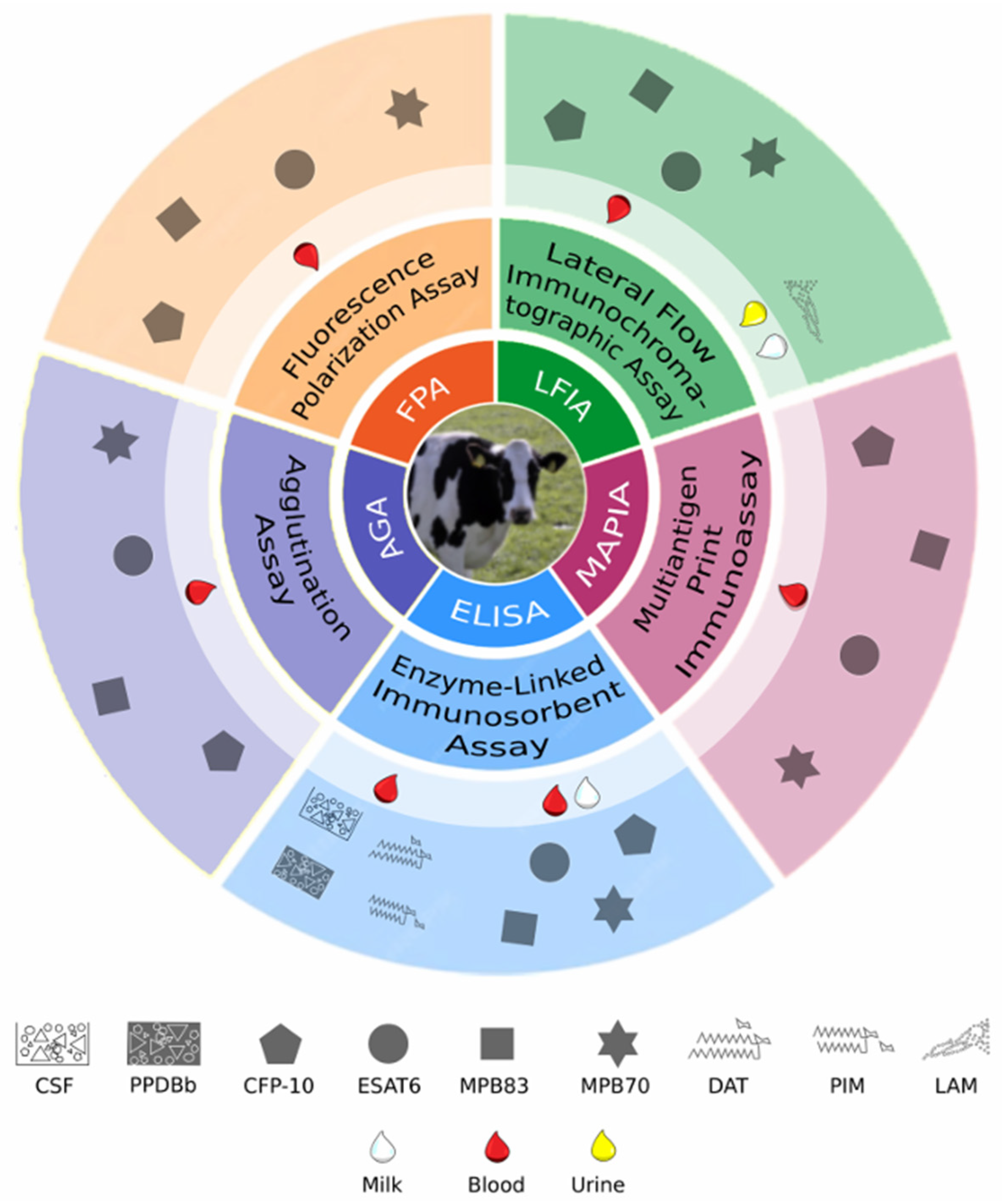
7.5. Serological Platforms
7.6. Antibody Detection in Milk and Urine Samples
7.7. Serological Diagnosis in Other Animals
7.8. Factors Influencing the Humoral Response to M. bovis in Cattle
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cadmus, S.; Akinseye, V.O.; van Soolingen, D. Mycobacterium bovis in humans and M. tuberculosis in animals in Nigeria: An overview from 1975–2014. Int. J. Tuberc. Lung Dis. 2019, 23, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, R.; Duffy, S.C.; Bin Rashid, H.; Gill, S.S.; Jabeen, C.; Arshad, N.; Umbreen, G.; Behr, M.A.; Kapur, V.; Chaudhry, M. Molecular detection and characterization of the Mycobacterium tuberculosis complex subspecies responsible for bovine tuberculosis in Punjab, Pakistan. Microbiol. Spectr. 2024, 12, e0269223. [Google Scholar] [CrossRef] [PubMed]
- Rahim, Z.; Thapa, J.; Fukushima, Y.; van der Zanden, A.G.M.; Gordon, S.V.; Suzuki, Y.; Nakajima, C. Tuberculosis Caused by Mycobacterium orygis in Dairy Cattle and Captured Monkeys in Bangladesh: A New Scenario of Tuberculosis in South Asia. Transbound. Emerg. Dis. 2017, 64, 1965–1969. [Google Scholar] [CrossRef] [PubMed]
- Blanco, F.; Garcia, J.S.Y.; Bigi, F. Recent advances in non-specific immune memory against bovine tuberculosis. Comp. Immunol. Microbiol. Infect. Dis. 2021, 75, 101615. [Google Scholar] [CrossRef]
- Guerra-Maupome, M.; Vang, D.X.; McGill, J.L. Aerosol vaccination with Bacille Calmette-Guerin induces a trained innate immune phenotype in calves. PLoS ONE 2019, 14, e0212751. [Google Scholar] [CrossRef]
- Suen, T.K.; Moorlag, S.J.C.F.M.; Li, W.; de Bree, L.C.J.; Koeken, V.A.C.M.; Mourits, V.P.; Dijkstra, H.; Lemmers, H.; Bhat, J.; Xu, C.-J.; et al. BCG vaccination induces innate immune memory in γδ T cells in humans. J. Leukoc. Biol. 2024, 115, 149–163. [Google Scholar] [CrossRef]
- Mihret, A. The role of dendritic cells in Mycobacterium tuberculosis infection. Virulence 2012, 3, 654–659. [Google Scholar] [CrossRef]
- Barnes, P.F.; Vankayalapati, R. Th1 and Th2 Cytokines in the Human Immune Response to Tuberculosis. In Tuberculosis and the Tubercle Bacillus; Wiley: Hoboken, NJ, USA, 2004; pp. 489–495. [Google Scholar]
- Balikã, Z.; Szereday, L.; Szekeres-Bartho, J. Th2 biased immune response in cases with active Mycobacterium tuberculosis infection and tuberculin anergy. FEMS Immunol. Med Microbiol. 1998, 22, 199–204. [Google Scholar] [CrossRef]
- Smith, K.; Kleynhans, L.; Warren, R.M.; Goosen, W.J.; Miller, M.A. Cell-Mediated Immunological Biomarkers and Their Diagnostic Application in Livestock and Wildlife Infected with Mycobacterium bovis. Front. Immunol. 2021, 12, 639605. [Google Scholar] [CrossRef] [PubMed]
- Welsh, M.D.; Cunningham, R.T.; Corbett, D.M.; Girvin, R.M.; McNair, J.; Skuce, R.A.; Bryson, D.G.; Pollock, J.M. Influence of pathological progression on the balance between cellular and humoral immune responses in bovine tuberculosis. Immunology 2005, 114, 101–111. [Google Scholar] [CrossRef]
- Maggioli, M.F.; Palmer, M.V.; Thacker, T.C.; Vordermeier, H.M.; McGill, J.L.; Whelan, A.O.; Larsen, M.H.; Jacobs, W.R., Jr.; Waters, W.R. Increased TNF-α/IFN-γ/IL-2 and Decreased TNF-α/IFN-γ Production by Central Memory T Cells Are Associated with Protective Responses against Bovine Tuberculosis Following BCG Vaccination. Front. Immunol. 2016, 7, 421. [Google Scholar] [CrossRef] [PubMed]
- Bhuju, S.; Aranday-Cortes, E.; Villarreal-Ramos, B.; Xing, Z.; Singh, M.; Vordermeier, H.M. Global Gene Transcriptome Analysis in Vaccinated Cattle Revealed a Dominant Role of IL-22 for Protection against Bovine Tuberculosis. PLoS Pathog. 2012, 8, e1003077. [Google Scholar] [CrossRef]
- Blanco, F.C.; Bianco, M.V.; Garbaccio, S.; Meikle, V.; Gravisaco, M.J.; Montenegro, V.; Alfonseca, E.; Singh, M.; Barandiaran, S.; Canal, A.; et al. Mycobacterium bovis Δmce2 double deletion mutant protects cattle against challenge with virulent M. bovis. Tuberculosis 2013, 93, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, C.; Bianco, M.V.; Blanco, F.C.; Soria, M.; Gravisaco, M.J.; Montenegro, V.; Vagnoni, L.; Buddle, B.; Garbaccio, S.; Delgado, F.; et al. Vaccination with a BCG Strain Overexpressing Ag85B Protects Cattle against Mycobacterium bovis Challenge. PLoS ONE 2012, 7, e51396. [Google Scholar] [CrossRef] [PubMed]
- Tanner, R.; Villarreal-Ramos, B.; Vordermeier, H.M.; McShane, H. The Humoral Immune Response to BCG Vaccination. Front. Immunol. 2019, 10, 1317. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.; Patel, S.; Comer, A.; Muneer, S.; Nawaz, U.; Quann, V.; Bansal, M.; Venketaraman, V. Role of B Cells in Mycobacterium Tuberculosis Infection. Vaccines 2023, 11, 955. [Google Scholar] [CrossRef] [PubMed]
- Lyashchenko, K.P.; Vordermeier, H.M.; Waters, W.R. Memory B cells and tuberculosis. Vet. Immunol. Immunopathol. 2020, 221, 110016. [Google Scholar] [CrossRef] [PubMed]
- Chandramuki, A.; Bothamley, G.H.; Brennan, P.J.; Ivanyi, J. Levels of antibody to defined antigens of Mycobacterium tuberculosis in tuberculous meningitis. J. Clin. Microbiol. 1989, 27, 821–825. [Google Scholar] [CrossRef]
- Achkar, J.M.; Chan, J.; Casadevall, A. B cells and antibodies in the defense against Mycobacterium tuberculosis infection. Immunol. Rev. 2015, 264, 167–181. [Google Scholar] [CrossRef]
- Eleftheriadis, T.; Tsiaga, P.; Antoniadi, G.; Liakopoulos, V.; Kortsaris, A.; Giannatos, E.; Barbutis, K.; Stefanidis, I.; Vargemezis, V. The Value of Serum Antilipoarabinomannan Antibody Detection in the Diagnosis of Latent Tuberculosis in Hemodialysis Patients. Am. J. Kidney Dis. 2005, 46, 706–712. [Google Scholar] [CrossRef]
- Chan, E.D.; Reves, R.; Belisle, J.T.; Brennan, P.J.; Hahn, W.E. Diagnosis of Tuberculosis by a Visually Detectable Immunoassay for Lipoarabinomannan. Am. J. Respir. Crit. Care Med. 2000, 161, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Moreno, C.; Narayan, V.; Akbar, M.S.; Ahmed, S.; Costello, A.M.L.; Abou-Zeid, C.; Rook, G.; Stanford, J.; Moreno, C. Does antibody to mycobacterial antigens, including lipoarabinomannan, limit dissemination in childhood tuberculosis? Trans. R. Soc. Trop. Med. Hyg. 1992, 86, 686–692. [Google Scholar]
- Hur, Y.-G.; Kim, A.; Kang, Y.A.; Kim, A.S.; Kim, D.Y.; Kim, Y.; Kim, Y.; Lee, H.; Cho, S.-N. Evaluation of Antigen-Specific Immunoglobulin G Responses in Pulmonary Tuberculosis Patients and Contacts. J. Clin. Microbiol. 2015, 53, 904–909. [Google Scholar] [CrossRef][Green Version]
- Perley, C.C.; Frahm, M.; Click, E.M.; Dobos, K.M.; Ferrari, G.; Stout, J.E.; Frothingham, R. The Human Antibody Response to the Surface of Mycobacterium tuberculosis. PLoS ONE 2014, 9, e98938. [Google Scholar] [CrossRef] [PubMed]
- Kunnath-Velayudhan, S.; Davidow, A.L.; Wang, H.-Y.; Molina, D.M.; Huynh, V.T.; Salamon, H.; Pine, R.; Michel, G.; Perkins, M.D.; Xiaowu, L.; et al. Proteome-Scale Antibody Responses and Outcome of Mycobacterium tuberculosis Infection in Nonhuman Primates and in Tuberculosis Patients. J. Infect. Dis. 2012, 206, 697–705. [Google Scholar] [CrossRef]
- Lu, L.L.; Chung, A.W.; Rosebrock, T.R.; Ghebremichael, M.; Yu, W.H.; Grace, P.S.; Schoen, M.K.; Tafesse, F.; Martin, C.; Leung, V.; et al. A Functional Role for Antibodies in Tuberculosis. Cell 2016, 167, 433–443.e14. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, S.; Yamabe, M.; Yamaguchi, Y.; Kobayashi, Y.; Konno, T.; Tada, K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 1980, 26, 171–176. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.-X.; Wang, B.; Fu, L.; Liu, G.; Lu, Y.; Cao, M.; Huang, H.; Javid, B. Latently and uninfected healthcare workers exposed to TB make protective antibodies against Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2017, 114, 5023–5028. [Google Scholar] [CrossRef]
- de Vallière, S.; Abate, G.; Blazevic, A.; Heuertz, R.M.; Hoft, D.F. Enhancement of Innate and Cell-Mediated Immunity by Antimycobacterial Antibodies. Infect. Immun. 2005, 73, 6711–6720. [Google Scholar] [CrossRef]
- Jacobs, A.J.; Mongkolsapaya, J.; Screaton, G.R.; McShane, H.; Wilkinson, R.J. Antibodies and tuberculosis. Tuberculosis 2016, 101, 102–113. [Google Scholar] [CrossRef]
- Rijnink, W.F.; Ottenhoff, T.H.; Joosten, S.A. B-Cells and Antibodies as Contributors to Effector Immune Responses in Tuberculosis. Front. Immunol. 2021, 12, 640168. [Google Scholar] [CrossRef]
- Chen, Y.; Bharrhan, S.; Xu, J.; Sharma, T.; Wang, Y.; Salgame, P.; Zhang, J.; Nargan, K.; Steyn, A.J.C.; Maglione, P.J.; et al. B cells promote granulomatous inflammation during chronic Mycobacterium tuberculosis infection in mice. PLoS Pathog. 2023, 19, e1011187. [Google Scholar] [CrossRef] [PubMed]
- Linge, I.; Tsareva, A.; Kondratieva, E.; Dyatlov, A.; Hidalgo, J.; Zvartsev, R.; Apt, A. Pleiotropic Effect of IL-6 Produced by B-Lymphocytes During Early Phases of Adaptive Immune Responses against TB Infection. Front. Immunol. 2022, 13, 750068. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Juarrero, M.; Turner, O.C.; Turner, J.; Marietta, P.; Brooks, J.V.; Orme, I.M. Temporal and Spatial Arrangement of Lymphocytes with in Lung Granulomas Induced by Aerosol Infection with Mycobacterium tuberculosis. Infect. Immun. 2001, 69, 1722–1728. [Google Scholar] [CrossRef] [PubMed]
- Krause, R.; Ogongo, P.; Tezera, L.; Ahmed, M.; Mbano, I.; Chambers, M.; Ngoepe, A.; Magnoumba, M.; Muema, D.; Karim, F.; et al. B cell heterogeneity in human tuberculosis highlights compartment-specific phenotype and functional roles. Commun. Biol. 2024, 7, 584. [Google Scholar] [CrossRef]
- Ulrichs, T.; A Kosmiadi, G.; Trusov, V.; Jörg, S.; Pradl, L.; Titukhina, M.; Mishenko, V.; Gushina, N.; Kaufmann, S.H. Human tuberculous granulomas induce peripheral lymphoid follicle-like structures to orchestrate local host defence in the lung. J. Pathol. 2004, 204, 217–228. [Google Scholar] [CrossRef]
- Joosten, S.A.; van Meijgaarden, K.E.; del Nonno, F.; Baiocchini, A.; Petrone, L.; Vanini, V.; Smits, H.H.; Palmieri, F.; Goletti, D.; Ottenhoff, T.H.M. Patients with Tuberculosis Have a Dysfunctional Circulating B-Cell Compartment, Which Normalizes following Successful Treatment. PLoS Pathog. 2016, 12, e1005687. [Google Scholar] [CrossRef]
- Buddle, B.; de Lisle, G.; Pfeffer, A.; Aldwell, F. Immunological responses and protection against Mycobacterium bovis in calves vaccinated with a low dose of BCG. Vaccine 1995, 13, 1123–1130. [Google Scholar] [CrossRef]
- Wedlock, D.N.; Vesosky, B.; Skinner, M.A.; de Lisle, G.W.; Orme, I.M.; Buddle, B.M. Vaccination of Cattle with Mycobacterium bovis Culture Filtrate Proteins and Interleukin-2 for Protection against Bovine Tuberculosis. Infect. Immun. 2000, 68, 5809–5815. [Google Scholar] [CrossRef]
- Wedlock, D.N.; Skinner, M.A.; Parlane, N.A.; Vordermeier, H.M.; Hewinson, R.G.; De Lisle, G.W.; Buddle, B. Vaccination with DNA vaccines encoding MPB70 or MPB83 or a MPB70 DNA prime-protein boost does not protect cattle against bovine tuberculosis. Tuberculosis 2003, 83, 339–349. [Google Scholar] [CrossRef]
- Wedlock, D.N.; Skinner, M.A.; De Lisle, G.W.; Vordermeier, H.M.; Hewinson, R.G.; Hecker, R.; Hecker, R.; Hurk, S.v.D.L.-V.D.; Babiuk, L.; Buddle, B. Vaccination of cattle with Mycobacterium bovis culture filtrate proteins and CpG oligodeoxynucleotides induces protection against bovine tuberculosis. Vet. Immunol. Immunopathol. 2005, 106, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Vordermeier, H.M.; Lowrie, D.B.; Hewinson, R.G. Improved immunogenicity of DNA vaccination with mycobacterial HSP65 against bovine tuberculosis by protein boosting. Vet. Microbiol. 2003, 93, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Lyashchenko, K.; Whelan, A.O.; Greenwald, R.; Pollock, J.M.; Andersen, P.; Hewinson, R.G.; Vordermeier, H.M. Association of Tuberculin-Boosted Antibody Responses with Pathology and Cell-Mediated Immunity in Cattle Vaccinated with Mycobacterium bovis BCG and Infected with M. bovis. Infect. Immun. 2004, 72, 2462–2467. [Google Scholar] [CrossRef] [PubMed]
- Lesellier, S.; Corner, L.; Costello, E.; Lyashchenko, K.; Greenwald, R.; Esfandiari, J.; Singh, M.; Hewinson, R.G.; Chambers, M.; Gormley, E. Immunological responses and protective immunity in BCG vaccinated badgers following endobronchial infection with Mycobacterium bovis. Vaccine 2009, 27, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Whelan, A.O.; Wright, D.C.; Chambers, M.A.; Singh, M.; Hewinson, R.G.; Vordermeier, H.M. Evidence for enhanced central memory priming by live Mycobacterium bovis BCG vaccine in comparison with killed BCG formulations. Vaccine 2008, 26, 166–173. [Google Scholar] [CrossRef]
- Parlane, N.A.; Shu, D.; Subharat, S.; Wedlock, D.N.; Rehm, B.H.A.; de Lisle, G.W.; Buddle, B.M. Revaccination of Cattle with Bacille Calmette-Guérin Two Years after First Vaccination when Immunity Has Waned, Boosted Protection against Challenge with Mycobacterium bovis. PLoS ONE 2014, 9, e106519. [Google Scholar] [CrossRef]
- Johnson, L.; Gough, J.; Spencer, Y.; Hewinson, G.; Vordermeier, M.; Wangoo, A. Immunohistochemical markers augment evaluation of vaccine efficacy and disease severity in bacillus Calmette–Guerin (BCG) vaccinated cattle challenged with Mycobacterium bovis. Vet. Immunol. Immunopathol. 2006, 111, 219–229. [Google Scholar] [CrossRef]
- Cassidy, J.P.; Bryson, D.G.; Gutiérrez Cancela, M.M.; Forster, F.; Pollock, J.M.; Neill, S.D. Lymphocyte Subtypes in Experimentally Induced Early-stage Bovine Tuberculous Lesions. J. Comp. Pathol. 2001, 124, 46–51. [Google Scholar] [CrossRef]
- Swanson, R.V.; Gupta, A.; Foreman, T.W.; Lu, L.; Choreno-Parra, J.A.; Mbandi, S.K.; Rosa, B.A.; Akter, S.; Das, S.; Ahmed, M.; et al. Antigen-specific B cells direct T follicular-like helper cells into lymphoid follicles to mediate Mycobacterium tuberculosis control. Nat. Immunol. 2023, 24, 855–868. [Google Scholar] [CrossRef]
- Krocova, Z.; Plzakova, L.; Pavkova, I.; Kubelkova, K.; Macela, A.; Ozanic, M.; Marecic, V.; Mihelcic, M.; Santic, M. The role of B cells in an early immune response to Mycobacterium bovis. Microb. Pathog. 2020, 140, 103937. [Google Scholar] [CrossRef]
- Palmer, M.V.; Whipple, D.L.; Olsen, S.C.; Jacobson, R.H. Cell mediated and humoral immune responses of white-tailed deer experimentally infected with Mycobacterium bovis. Res. Vet. Sci. 2000, 68, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Hunter, L.; Hingley-Wilson, S.; Stewart, G.R.; Sharpe, S.A.; Salguero, F.J. Dynamics of Macrophage, T and B Cell Infiltration with in Pulmonary Granulomas Induced by Mycobacterium tuberculosis in Two Non-Human Primate Models of Aerosol Infection. Front. Immunol. 2022, 12, 776913. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.L. The Pathogenesis of Tuberculosis–The Koch Phenomenon Reinstated. Pathogens 2020, 9, 813. [Google Scholar] [CrossRef] [PubMed]
- Stanford, J. Koch’s phenomenon: Can it be corrected? Tubercle 1991, 72, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Bezos, J.; Casal, C.; Romero, B.; Schroeder, B.; Hardegger, R.; Raeber, A.J.; López, L.; Rueda, P.; Domínguez, L. Current ante-mortem techniques for diagnosis of bovine tuberculosis. Res. Vet. Sci. 2014, 97, S44–S52. [Google Scholar] [CrossRef]
- Pollock, J.M.; McNair, J.; Welsh, M.D.; Girvin, R.M.; Kennedy, H.E.; Mackie, D.P.; Neill, S.D. Immune responses in bovine tuberculosis. Tuberculosis 2001, 81, 103–107. [Google Scholar] [CrossRef]
- Ritacco, V.; Lopez, B.; De Kantor, I.; Barrera, L.; Errico, F.; Nader, A. Reciprocal cellular and humoral immune responses in bovine tuberculosis. Res. Vet. Sci. 1991, 50, 365–367. [Google Scholar] [CrossRef]
- Fifis, T.; Corner, L.A.; Rothel, J.S.; Wood, P.R. Cellular and Humoral Immune Responses of Cattle to Purified Mycobacterium bovis Antigens. Scand. J. Immunol. 1994, 39, 267–274. [Google Scholar] [CrossRef]
- Harboe, M.; Wiker, H.G.; Duncan, J.R.; Garcia, M.M.; Dukes, T.W.; Brooks, B.W.; Turcotte, C.; Nagai, S. Protein G-based enzyme-linked immunosorbent assay for anti-MPB70 antibodies in bovine tuberculosis. J. Clin. Microbiol. 1990, 28, 913–921. [Google Scholar] [CrossRef]
- Holder, T.; Srinivasan, S.; McGoldrick, A.; Williams, G.A.; Palmer, S.; Clarke, J.; O’brien, A.; Conlan, A.J.K.; Juleff, N.; Vordermeier, H.M.; et al. Temporal dynamics of the early immune response following Mycobacterium bovis infection of cattle. Sci. Rep. 2024, 14, 2600. [Google Scholar] [CrossRef]
- Ritacco, V.; de Kantor, I.N.; Barrera, L.; Nader, A.; Bernardelli, A.; Torrea, G.; Errico, F.; Fliess, E. Assessment of the Sensitivity and Specificity of Enzyme-Linked Immunosorbent Assay (ELISA) for the Detection of Mycobacterial Antibodies in Bovine Tuberculosis. J. Vet. Med. Ser. B 1987, 34, 119–125. [Google Scholar] [CrossRef]
- Ritacco, V.; López, B.; Barrera, L.; Nader, A.; Fliess, E.; de Kantor, I.N. Further Evaluation of an Indirect Enzyme-Linked Immunosorbent Assay for the Diagnosis of Bovine Tuberculosis. J. Vet. Med. Ser. B 1990, 37, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Auer, L.A. Assessment of an enzyme linked immunosorbent assay for the detection of cattle infected with Mycobacterium bovis. Aust. Vet. J. 1987, 64, 172–176. [Google Scholar] [CrossRef]
- Plackett, P.; Ripper, J.; Corner, L.; Small, K.; Witte, K.D.; Melville, L.; Hides, S.; Wood, P. An ELISA for the detection of anergic tuberculous cattle. Aust. Vet. J. 1989, 66, 15–19. [Google Scholar] [CrossRef]
- Wiker, H.G.; Harboe, M.; Nagai, S.; Patarroyo, M.E.; Ramirez, C.; Cruz, N. MPB59, a Widely Cross-Reacting Protein of Mycobacterium bovis BCG. Int. Arch. Allergy Immunol. 1986, 81, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Fifis, T.; Costopoulos, C.; Corner, L.; Wood, P. Serological reactivity to Mycobacterium bovis protein antigens in cattle. Vet. Microbiol. 1992, 30, 343–354. [Google Scholar] [CrossRef]
- Waters, W.R.; Palmer, M.V.; Stafne, M.R.; Bass, K.E.; Maggioli, M.F.; Thacker, T.C.; Linscott, R.; Lawrence, J.C.; Nelson, J.T.; Esfandiari, J.; et al. Effects of Serial Skin Testing with Purified Protein Derivative on the Level and Quality of Antibodies to Complex and Defined Antigens in Mycobacterium bovis-Infected Cattle. Clin. Vaccine Immunol. 2015, 22, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Harboe, M.; Wiker, H.G.; Lachmann, P.J. Carrier Effect of Concanavalin A-Reactive and -Non-Reactive Material in Tuberculin PPD. Scand. J. Immunol. 1990, 32, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Fifis, T.; Plackett, P.; Corner, L.A.; Wood, P.R. Purification of a Major Mycobacterium bovis Antigen for the Diagnosis of Bovine Tuberculosis. Scand. J. Immunol. 1989, 29, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.S.; Shin, A.-R.; Son, Y.-J.; Kim, J.-M.; Jang, Y.; Kim, S.; Lee, K.-I.; Choi, C.H.; Park, J.-K.; Kim, H.-J. An evaluation of the use of immunoglobulin A antibody response against mycobacterial antigens for the diagnosis of Mycobacterium bovis infection in cattle. J. Vet. Diagn. Investig. 2015, 27, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Sridhara, A.A.; Johnathan-Lee, A.; Elahi, R.; Sikar-Gang, A.; Lambotte, P.; Esfandiari, J.; de Juan, L.; Gortazar, C.; Marpe, B.N.; Thacker, T.C.; et al. Potential for improved detection of bovine tuberculosis by targeting combined blood biomarkers in multi-test algorithms. Vet. Immunol. Immunopathol. 2022, 248, 110419. [Google Scholar] [CrossRef] [PubMed]
- Lyashchenko, K.P.; Grandison, A.; Keskinen, K.; Sikar-Gang, A.; Lambotte, P.; Esfandiari, J.; Ireton, G.C.; Vallur, A.; Reed, S.G.; Jones, G.; et al. Identification of Novel Antigens Recognized by Serum Antibodies in Bovine Tuberculosis. Clin. Vaccine Immunol. 2017, 24, e00259-17. [Google Scholar] [CrossRef] [PubMed]
- Green, L.R.; Jones, C.C.; Sherwood, A.L.; Garkavi, I.V.; Cangelosi, G.A.; Thacker, T.C.; Palmer, M.V.; Waters, W.R.; Rathe, C.V. Single-Antigen Serological Testing for Bovine Tuberculosis. Clin. Vaccine Immunol. 2009, 16, 1309–1313. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moens, C.; Filée, P.; Boes, A.; Alie, C.; Dufrasne, F.; André, E.; Marché, S.; Fretin, D. Identification of New Mycobacterium bovis antigens and development of a multiplexed serological bead-immunoassay for the diagnosis of bovine tuberculosis in cattle. PLoS ONE 2023, 18, e0292590. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, S.D.; Grodi, H.A.; Kaneene, J.B. An Evaluation of MAPIA in Michigan as an Ante-Mortem Supplemental Test for Use in Suspect Tuberculosis Cattle. Vet. Med. Int. 2012, 2012, 674368. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Surujballi, O.P.; Romanowska, A.; Sugden, E.A.; Turcotte, C.; Jolley, M.E. A fluorescence polarization assay for the detection of antibodies to Mycobacterium bovis in cattle sera. Vet. Microbiol. 2002, 87, 149–157. [Google Scholar] [CrossRef]
- Mcnair, J.; Corbett, D.M.; Girvin, R.M.; Mackie, D.P.; Pollock, J.M. Characterization of the Early Antibody Response in Bovine Tuberculosis: MPB83 is an Early Target with Diagnostic Potential. Scand. J. Immunol. 2001, 53, 365–371. [Google Scholar] [CrossRef]
- Marassi, C.; Medeiros, L.; McNair, J.; Lilenbaum, W. Use of recombinant proteins MPB70 or MPB83 as capture antigens in ELISAs to confirm bovine tuberculosis infections in Brazil. Acta Trop. 2011, 118, 101–104. [Google Scholar] [CrossRef]
- Amadori, M.; Lyashchenko, K.P.; Gennaro, M.L.; Pollock, J.M.; Zerbini, I. Use of recombinant proteins in antibody tests for bovine tuberculosis. Vet. Microbiol. 2002, 85, 379–389. [Google Scholar] [CrossRef]
- Lightbody, K.A.; Skuce, R.A.; Neill, S.D.; Pollock, J.M. Mycobacterial antigen-specific antibody responses in bovine tuberculosis: An ELISA with potential to confirm disease status. Vet. Rec. 1998, 142, 295–300. [Google Scholar]
- Casal, C.; Infantes, J.; Risalde, M.; Díez-Guerrier, A.; Domínguez, M.; Moreno, I.; Romero, B.; de Juan, L.; Sáez, J.; Juste, R.; et al. Antibody detection tests improve the sensitivity of tuberculosis diagnosis in cattle. Res. Vet. Sci. 2017, 112, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Guo, S.; Wang, C.; Shao, M.; Zhang, X.; Guo, Y.; Gong, Q. A novel fusion protein-based indirect enzyme-linked immunosorbent assay for the detection of bovine tuberculosis. Tuberculosis 2007, 87, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Souza, I.I.F.; Melo, E.S.P.; Ramos, C.A.N.; Farias, T.A.; Osório, A.L.A.R.; Jorge, K.S.G.; Vidal, C.E.; Silva, A.S.; Silva, M.R.; O Pellegrin, A.; et al. Screening of recombinant proteins as antigens in indirect ELISA for diagnosis of bovine tuberculosis. SpringerPlus 2012, 1, 77. [Google Scholar] [CrossRef] [PubMed]
- Waters, W.R.; Buddle, B.M.; Vordermeier, H.M.; Gormley, E.; Palmer, M.V.; Thacker, T.C.; Bannantine, J.P.; Stabel, J.R.; Linscott, R.; Martel, E.; et al. Development and Evaluation of an Enzyme-Linked Immunosorbent Assay for Use in the Detection of Bovine Tuberculosis in Cattle. Clin. Vaccine Immunol. 2011, 18, 1882–1888. [Google Scholar] [CrossRef] [PubMed]
- Moens, C.; Saegerman, C.; Fretin, D.; Marché, S. Field evaluation of two commercial serological assays for detecting bovine tuberculosis. Res. Vet. Sci. 2023, 159, 125–132. [Google Scholar] [CrossRef]
- Carneiro, P.A.M.; de Moura Sousa, E.; Viana, R.B.; Monteiro, B.M.; do Socorro Lima Kzam, A.; de Souza, D.C.; Coelho, A.S.; Filho, J.D.R.; Jordao, R.S.; Tavares, M.R.M.; et al. Study on supplemental test to improve the detection of bovine tuberculosis in individual animals and herds. BMC Vet. Res. 2021, 17, 137. [Google Scholar] [CrossRef]
- McCallan, L.; Brooks, C.; Barry, C.; Couzens, C.; Young, F.J.; McNair, J.; Byrne, A.W. Serological test performance for bovine tuberculosis in cattle from herds with evidence of on-going infection in Northern Ireland. PLoS ONE 2021, 16, e0245655. [Google Scholar] [CrossRef]
- Veerasami, M.; Reddy, D.S.K.; Sugumar, P.; Naidu, S.S.; Bahekar, V.; Kumar, E.K.M.; Mukherjee, F.; Rana, S.K.; Chandran, D.; Das, D.; et al. Multi-antigen print immunoassay for seroepidemiological surveillance of bovine tuberculosis on Indian cattle farms. Vet. Ital. 2012, 48, 253–267. [Google Scholar]
- Jolley, M.E.; Nasir, M.S.; Surujballi, O.P.; Romanowska, A.; Renteria, T.B.; De la Mora, A.; Lim, A.; Bolin, S.R.; Michel, A.L.; Kostovic, M.; et al. Fluorescence polarization assay for the detection of antibodies to Mycobacterium bovis in bovine sera. Vet. Microbiol. 2007, 120, 113–121. [Google Scholar] [CrossRef]
- Sridhara, A.A.; Johnathan-Lee, A.; Elahi, R.; Lambotte, P.; Esfandiari, J.; Boschiroli, M.L.; Kerr, T.J.; Miller, M.A.; Holder, T.; Jones, G.; et al. Differential detection of IgM and IgG antibodies to chimeric antigens in bovine tuberculosis. Vet. Immunol. Immunopathol. 2022, 253, 110499. [Google Scholar] [CrossRef]
- Zewude, A.; Mohammed, T.; Terfassa, L.; Hunt, W.G.; Pan, X.; Balada-Llasat, J.M.; Gebreyes, W.; Torrelles, J.B.; Wang, S.-H.; Ameni, G. Evaluation of Mycobacterium tuberculosis lipoarabinomannan antigen assay and rapid serology blood test for the diagnosis of bovine tuberculosis in Ethiopia. BMC Vet. Res. 2019, 15, 359. [Google Scholar] [CrossRef] [PubMed]
- Fontana, S.; Pacciarini, M.; Boifava, M.; Pellesi, R.; Casto, B.; Gastaldelli, M.; Koehler, H.; Pozzato, N.; Casalinuovo, F.; Boniotti, M.B. Development and evaluation of two multi-antigen serological assays for the diagnosis of bovine tuberculosis in cattle. J. Microbiol. Methods 2018, 153, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Alonso, N.; Griffa, N.; Moyano, R.D.; Mon, M.L.; Olivieri, M.A.C.; Barandiaran, S.; Vivot, M.M.; Fiorini, G.; Canal, A.M.; Santangelo, M.P.; et al. Development of a lateral flow immunochromatography test for the rapid detection of bovine tuberculosis. J. Immunol. Methods 2020, 491, 112941. [Google Scholar] [CrossRef] [PubMed]
- Waters, W.R.; Vordermeier, H.M.; Rhodes, S.; Khatri, B.; Palmer, M.V.; Maggioli, M.F.; Thacker, T.C.; Nelson, J.T.; Thomsen, B.V.; Robbe-Austerman, S.; et al. Potential for rapid antibody detection to identify tuberculous cattle with non-reactive tuberculin skin test results. BMC Vet. Res. 2017, 13, 164. [Google Scholar] [CrossRef] [PubMed]
- Whelan, C.; Whelan, A.O.; Shuralev, E.; Kwok, H.F.; Hewinson, G.; Clarke, J.; Vordermeier, H.M. Performance of the Enferplex TB Assay with Cattle in Great Britain and Assessment of Its Suitability as a Test to Distinguish Infected and Vaccinated Animals. Clin. Vaccine Immunol. 2010, 17, 813–817. [Google Scholar] [CrossRef]
- Whelan, C.; Shuralev, E.; Kwok, H.F.; Kenny, K.; Duignan, A.; Good, M.; Davis, W.C.; Clarke, J. Use of a multiplex enzyme-linked immunosorbent assay to detect a subpopulation of Mycobacterium bovis–infected animals deemed negative or inconclusive by the single intradermal comparative tuberculin skin test. J. Vet. Diagn. Investig. 2011, 23, 499–503. [Google Scholar]
- Watt, N.; Harkiss, G.; Hayton, A.; Clarke, J.; O’Brien, A. Identify missed TB cattle with Enferplex. Vet. Rec. 2019, 185, 733. [Google Scholar] [CrossRef]
- Casal, C.; Díez-Guerrier, A.; Álvarez, J.; Rodriguez-Campos, S.; Mateos, A.; Linscott, R.; Martel, E.; Lawrence, J.C.; Whelan, C.; Clarke, J.; et al. Strategic use of serology for the diagnosis of bovine tuberculosis after intradermal skin testing. Vet. Microbiol. 2014, 170, 342–351. [Google Scholar]
- Koo, H.C.; Park, Y.H.; Ahn, J.; Waters, W.R.; Palmer, M.V.; Hamilton, M.J.; Barrington, G.; Mosaad, A.A.; Park, K.T.; Jung, W.K.; et al. Use of rMPB70 Protein and ESAT-6 Peptide as Antigens for Comparison of the Enzyme-Linked Immunosorbent, Immunochromatographic, and Latex Bead Agglutination Assays for Serodiagnosis of Bovine Tuberculosis. J. Clin. Microbiol. 2005, 43, 4498–4506. [Google Scholar] [CrossRef]
- Lyashchenko, K.P.; Sikar-Gang, A.; Sridhara, A.A.; Johnathan-Lee, A.; Elahi, R.; Lambotte, P.; Esfandiari, J.; Duthie, M.; Reed, S.G.; Jones, G.; et al. Novel polyprotein antigens designed for improved serodiagnosis of bovine tuberculosis. Vet. Immunol. Immunopathol. 2021, 240, 110320. [Google Scholar] [CrossRef]
- Backus, K.M.; Dolan, M.A.; Barry, C.S.; Joe, M.; McPhie, P.; Boshoff, H.I.M.; Lowary, T.L.; Davis, B.G.; E Barry, C. The Three Mycobacterium tuberculosis Antigen 85 Isoforms Have Unique Substrates and Activities Determined by Non-active Site Regions. J. Biol. Chem. 2014, 289, 25041–25053. [Google Scholar] [CrossRef] [PubMed]
- Babaki, M.K.Z.; Soleimanpour, S.; Rezaee, S.A. Antigen 85 complex as a powerful Mycobacterium tuberculosis immunogene: Biology, immune-pathogenicity, applications in diagnosis, and vaccine design. Microb. Pathog. 2017, 112, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Wiker, H.G.; Harboe, M. The antigen 85 complex: A major secretion product of Mycobacterium tuberculosis. Microbiol. Rev. 1992, 56, 648–661. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Hu, T.; Xia, A.; Li, X.; Liu, Z.; Min, J.; He, J.; Meng, C.; Yin, Y.; Chen, X.; et al. Generation of Monoclonal Antibodies against Ag85A Antigen of Mycobacterium tuberculosis and Application in a Competitive ELISA for Serodiagnosis of Bovine Tuberculosis. Front. Vet. Sci. 2017, 4, 107. [Google Scholar] [CrossRef] [PubMed]
- Lilenbaum, W.; Pessolani, M.C.V.; Fonseca, L.S. The Use of Ag85 Complex as Antigen in ELISA for the Diagnosis of Bovine Tuberculosis in Dairy Cows in Brazil. J. Vet. Med. Ser. B 2001, 48, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, C.; Govaerts, M.; Meikle, V.; Vallecillo, A.J.; Gutierrez-Pabello, J.A.; Suarez-Güemes, F.; McNair, J.; Cataldi, A.; Espitia, C.; Andersen, P.; et al. Optimizing Antigen Cocktails for Detection of Mycobacterium bovis in Herds with Different Prevalences of Bovine Tuberculosis: ESAT6-CFP10 Mixture Shows Optimal Sensitivity and Specificity. J. Clin. Microbiol. 2006, 44, 4326–4335. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Feng, C.G.; Britton, W.J. CD4+ and CD8+T Cells Mediate Adoptive Immunity to Aerosol Infection of Mycobacterium bovis Bacillus Calmette-Guérin. J. Infect. Dis. 2000, 181, 1846–1849. [Google Scholar] [CrossRef]
- Pollock, J.M.; Andersen, P. Predominant recognition of the ESAT-6 protein in the first phase of interferon with Mycobacterium bovis in cattle. Infect. Immun. 1997, 65, 2587–2592. [Google Scholar] [CrossRef]
- Kunnath-Velayudhan, S.; Salamon, H.; Wang, H.-Y.; Davidow, A.L.; Molina, D.M.; Huynh, V.T.; Cirillo, D.M.; Michel, G.; Talbot, E.A.; Perkins, M.D.; et al. Dynamic antibody responses to the Mycobacterium tuberculosis proteome. Proc. Natl. Acad. Sci. USA 2010, 107, 14703–14708. [Google Scholar] [CrossRef]
- Elnaggar, M.M.; Abdellrazeq, G.S.; Sester, M.; Khaliel, S.A.; Singh, M.; Torky, H.A.; Davis, W.C. Development of an improved ESAT-6 and CFP-10 peptide-based cytokine flow cytometric assay for bovine tuberculosis. Comp. Immunol. Microbiol. Infect. Dis. 2015, 42, 1–7. [Google Scholar] [CrossRef]
- Vordermeier, H.M.; Whelan, A.; Cockle, P.J.; Farrant, L.; Palmer, N.; Hewinson, R.G. Use of Synthetic Peptides Derived from the Antigens ESAT-6 and CFP-10 for Differential Diagnosis of Bovine Tuberculosis in Cattle. Clin. Diagn. Lab. Immunol. 2001, 8, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Waters, W.R.; Palmer, M.V.; Thacker, T.C.; Payeur, J.B.; Harris, N.B.; Minion, F.C.; Greenwald, R.; Esfandiari, J.; Andersen, P.; McNair, J.; et al. Immune Responses to Defined Antigens of Mycobacterium bovis in Cattle Experimentally Infected with Mycobacterium kansasii. Clin. Vaccine Immunol. 2006, 13, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Wiker, H.G. MPB70 and MPB83—Major Antigens of Mycobacterium bovis. Scand. J. Immunol. 2009, 69, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Sidders, B.; Pirson, C.; Hogarth, P.J.; Hewinson, R.G.; Stoker, N.G.; Vordermeier, H.M.; Ewer, K. Screening of Highly Expressed Mycobacterial Genes Identifies Rv3615c as a Useful Differential Diagnostic Antigen for the Mycobacterium tuberculosis Complex. Infect. Immun. 2008, 76, 3932–3939. [Google Scholar] [CrossRef]
- Wiker, H.G.; Lyashchenko, K.P.; Aksoy, A.M.; Lightbody, K.A.; Pollock, J.M.; Komissarenko, S.V.; Bobrovnik, S.O.; Kolesnikova, I.N.; Mykhalsky, L.O.; Gennaro, M.L.; et al. Immunochemical Characterization of the MPB70/80 and MPB83 Proteins of Mycobacterium bovis. Infect. Immun. 1998, 66, 1445–1452. [Google Scholar] [CrossRef]
- Cataldi, A.; Romano, M.; Bigi, F. A western blot characterization of Mycobacterium bovis antigens recognized by cattle sera. Res. Microbiol. 1994, 145, 689–698. [Google Scholar] [CrossRef]
- Bigi, F.; Espitia, C.; Alito, A.; Zumarraga, M.; Romano, M.I.; Cravero, S.; Cataldi, A. A novel 27 kDa lipoprotein antigen from Mycobacterium bovis. Microbiology 1997, 143, 3599–3605. [Google Scholar] [CrossRef]
- Hovav, A.-H.; Mullerad, J.; Davidovitch, L.; Fishman, Y.; Bigi, F.; Cataldi, A.; Bercovier, H. The Mycobacterium tuberculosis Recombinant 27-Kilodalton Lipoprotein Induces a Strong Th1-Type Immune Response Deleterious to Protection. Infect. Immun. 2003, 71, 3146–3154. [Google Scholar] [CrossRef]
- Jackson, M.; McNeil, M.R.; Brennan, P.J. Progress in Targeting Cell Envelope Biogenesis in Mycobacterium Tuberculosis. Futur. Microbiol. 2013, 8, 855–875. [Google Scholar] [CrossRef]
- Turner, J.; Torrelles, J.B. Mannose-capped lipoarabinomannan in Mycobacterium tuberculosis pathogenesis. Pathog. Dis. 2018, 76, fty026. [Google Scholar] [CrossRef]
- Marais, B.J. Improved Urine Lipoarabinomannan (LAM) Tests: The Answer for Child Tuberculosis Diagnosis? Clin. Infect. Dis. 2020, 72, e289–e290. [Google Scholar] [CrossRef] [PubMed]
- Koets, A.P.; Esker, M.H.v.D.; Riepema, K.; Bakker, D. The Role of Phosphatidylinositol Mannosides in the Serological Diagnosis of Mycobacterial Infections. Vet. Sci. 2019, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Ostyn, A.; Lanéelle, M.; Thorel, M. Glycolipid antigen for use in diagnostic assays for bovine tuberculosis. Res. Microbiol. 1997, 148, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Borsuk, S.; Newcombe, J.; Mendum, T.A.; Dellagostin, O.A.; McFadden, J. Identification of proteins from tuberculin purified protein derivative (PPD) by LC-MS/MS. Tuberculosis 2009, 89, 423–430. [Google Scholar] [CrossRef]
- Moens, C.; Saegerman, C.; Fretin, D.; Marché, S. Performance of two commercial serological assays for bovine tuberculosis using plasma samples. Vet. Immunol. Immunopathol. 2023, 263, 110644. [Google Scholar] [CrossRef]
- Whelan, C.; Shuralev, E.; O’Keeffe, G.; Hyland, P.; Kwok, H.F.; Snoddy, P.; O’Brien, A.; Connolly, M.; Quinn, P.; Groll, M.; et al. Multiplex Immunoassay for Serological Diagnosis of Mycobacterium bovis Infection in Cattle. Clin. Vaccine Immunol. 2008, 15, 1834–1838. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Tellechea, J.; Marín, J.F.G. Evaluation of cellular and serological diagnostic tests for the detection of Mycobacterium bovis-infected goats. Vet. Microbiol. 1998, 62, 281–290. [Google Scholar] [CrossRef]
- Hanna, J.; Neill, S.; O’Brien, J. ELISA tests for antibodies in experimental bovine tuberculosis. Vet. Microbiol. 1992, 31, 243–249. [Google Scholar] [CrossRef]
- Palmer, M.V.; Waters, W.R.; Thacker, T.C.; Greenwald, R.; Esfandiari, J.; Lyashchenko, K.P. Effects of Different Tuberculin Skin-Testing Regimens on Gamma Interferon and Antibody Responses in Cattle Experimentally Infected with Mycobacterium bovis. Clin. Vaccine Immunol. 2006, 13, 387–394. [Google Scholar] [CrossRef]
- Waters, W.R.; Palmer, M.V.; Thacker, T.C.; Bannantine, J.P.; Vordermeier, H.M.; Hewinson, R.G.; Greenwald, R.; Esfandiari, J.; McNair, J.; Pollock, J.M.; et al. Early Antibody Responses to Experimental Mycobacterium bovis Infection of Cattle. Clin. Vaccine Immunol. 2006, 13, 648–654. [Google Scholar] [CrossRef]
- Koni, A.; Juma, A.; Morini, M.; Nardelli, S.; Connor, R.; Koleci, X. Assessment of an ELISA method to support surveillance of bovine tuberculosis in Albania. Ir. Vet. J. 2015, 69, 11. [Google Scholar] [CrossRef] [PubMed]
- Griffa, N.; Moyano, R.D.; Canal, A.M.; Travería, G.E.; Santangelo, M.P.; Alonso, N.; Romano, M. Development and diagnostic validation of an ELISA based on an antigenic mixture for the detection of bovine tuberculosis. Vet. J. 2020, 256, 105426. [Google Scholar] [CrossRef] [PubMed]
- Garbaccio, S.G.; Garro, C.J.; Delgado, F.O.; Tejada, G.A.; Eirin, M.E.; Huertas, P.S.; Leon, E.; Zumárraga, M. Enzyme-linked immunosorbent assay as complement of intradermal skin test for the detection of Mycobacterium bovis infection in cattle. Tuberculosis 2019, 117, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, R.; Lyashchenko, O.; Esfandiari, J.; Miller, M.; Mikota, S.; Olsen, J.H.; Ball, R.; Dumonceaux, G.; Schmitt, D.; Moller, T.; et al. Highly Accurate Antibody Assays for Early and Rapid Detection of Tuberculosis in African and Asian Elephants. Clin. Vaccine Immunol. 2009, 16, 605–612. [Google Scholar] [CrossRef][Green Version]
- Lyashchenko, K.P.; Sikar-Gang, A.; Sridhara, A.A.; Johnathan-Lee, A.; Elahi, R.; Greenwald, R.; Lambotte, P.; Esfandiari, J.; Roos, E.O.; Kerr, T.J.; et al. Use of blood matrices and alternative biological fluids for antibody detection in animal tuberculosis. Vet. Immunol. Immunopathol. 2021, 239, 110303. [Google Scholar] [CrossRef]
- El-Sayyad, G.S.; Hasan, O.F.; Saad, M.A.M.; El-Batal, A.I. Improving the diagnosis of bovine tuberculosis using gold nanoparticles conjugated with purified protein derivative: Special regard to staphylococcal protein A and streptococcal protein G. Environ. Sci. Pollut. Res. 2021, 28, 29200–29220. [Google Scholar] [CrossRef] [PubMed]
- Javed, R.; Narang, D.; Kaur, P.; Chandra, M.; Filia, G.; Singh, S. A fluorescence polarization assay using recombinant protein ESAT-6 for the detection of antibodies against pathogenic Mycobacterium bovis in bovine. Iran. J. Vet. Res. 2022, 23, 204–209. [Google Scholar]
- Byrne, W.J.; Ball, H.J.; Brice, N.; McCormack, R.; Baker, S.E.; Ayling, R.D.; Nicholas, R.A.J. Application of an indirect ELISA to milk samples to identify cows with Mycoplasma bovis mastitis. Vet. Rec. 2000, 146, 368–369. [Google Scholar] [CrossRef]
- Sergeant, E.; Nielsen, S.; Toft, N. Evaluation of test-strategies for estimating probability of low prevalence of paratuberculosis in Danish dairy herds. Prev. Vet. Med. 2008, 85, 92–106. [Google Scholar] [CrossRef]
- Thoen, C.O.; Haas, C.A.; Angus, R.; Townsend, A.S. Evaluation of a potassium chloride extract of Brucella abortus in an ELISA for detecting Brucella antibodies in bulk tank milk samples from cows. Vet. Microbiol. 1995, 45, 185–189. [Google Scholar] [CrossRef]
- Lombard, J.E.; Byrem, T.M.; Wagner, B.A.; McCluskey, B.J. Comparison of Milk and Serum Enzyme–Linked Immunosorbent Assays for Diagnosis of Mycobacterium Avium Subspecies Paratuberculosis Infection in Dairy Cattle. J. Vet. Diagn. Investig. 2006, 18, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, R.W.; Whitlock, R.H.; Buckley, C.L.; Spencer, P.A. Evaluation of a Commercial Enzyme-Linked Immunosorbent Assay for the Diagnosis of Paratuberculosis in Dairy Cattle. J. Vet. Diagn. Investig. 1995, 7, 488–493. [Google Scholar] [CrossRef]
- Jeon, B.-Y.; Kim, S.-C.; Je, S.; Kwak, J.; Cho, J.-E.; Woo, J.-T.; Seo, S.; Shim, H.-S.; Park, B.-O.; Lee, S.-S.; et al. Evaluation of enzyme-linked immunosorbent assay using milk samples as a potential screening test of bovine tuberculosis of dairy cows in Korea. Res. Vet. Sci. 2010, 88, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Buddle, B.M.; Wilson, T.; Luo, D.; Voges, H.; Linscott, R.; Martel, E.; Lawrence, J.C.; Neill, M.A. Evaluation of a Commercial Enzyme-Linked Immunosorbent Assay for the Diagnosis of Bovine Tuberculosis from Milk Samples from Dairy Cows. Clin. Vaccine Immunol. 2013, 20, 1812–1816. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhao, Y.; Zhang, Z.; Yan, L.; Li, J.; Chen, Y.; Hu, C.; Robertson, I.D.; Guo, A.; Aleri, J. Evaluation of an ELISA for the diagnosis of bovine tuberculosis using milk samples from dairy cows in China. Prev. Vet. Med. 2022, 208, 105752. [Google Scholar] [CrossRef] [PubMed]
- O’brien, A.; Hayton, A.; Cutler, K.; Adler, A.; Shaw, D.J.; Clarke, J.; Watt, N.; Harkiss, G.D. Diagnostic accuracy of the Enferplex Bovine TB antibody test using individual milk samples from cattle. PLoS ONE 2024, 19, e0301609. [Google Scholar] [CrossRef]
- Lawn, S.D. Point-of-care detection of lipoarabinomannan (LAM) in urine for diagnosis of HIV-associated tuberculosis: A state of the art review. BMC Infect. Dis. 2012, 12, 103. [Google Scholar] [CrossRef]
- Kelley, H.V.; Waibel, S.M.; Sidiki, S.; Tomatis-Souverbielle, C.; Scordo, J.M.; Hunt, W.G.; Barr, N.; Smith, R.; Silwani, S.N.; Averill, J.J.; et al. Accuracy of Two Point-of-Care Tests for Rapid Diagnosis of Bovine Tuberculosis at Animal Level using Non-Invasive Specimens. Sci. Rep. 2020, 10, 5441. [Google Scholar] [CrossRef]
- Chambers, M.A. Review of the Diagnosis and Study of Tuberculosis in Non-Bovine Wildlife Species Using Immunological Methods. Transbound. Emerg. Dis. 2009, 56, 215–227. [Google Scholar] [CrossRef]
- Thomas, J.; Balseiro, A.; Gortázar, C.; Risalde, M.A. Diagnosis of tuberculosis in wildlife: A systematic review. Vet. Res. 2021, 52, 31. [Google Scholar] [CrossRef]
- Gowtage-Sequeira, S.; Paterson, A.; Lyashchenko, K.P.; Lesellier, S.; Chambers, M.A. Evaluation of the CervidTB STAT-PAK for the Detection of Mycobacterium bovis Infection in Wild Deer in Great Britain. Clin. Vaccine Immunol. 2009, 16, 1449–1452. [Google Scholar] [CrossRef]
- Lyashchenko, K.; Greenwald, R.; Esfandiari, J.; Chambers, M.; Vicente, J.; Gortazar, C.; Santos, N.; Correia-Neves, M.; Buddle, B.; Jackson, R.; et al. Animal-side serologic assay for rapid detection of Mycobacterium bovis infection in multiple species of free-ranging wildlife. Vet. Microbiol. 2008, 132, 283–292. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, D.J.; Schmitt, S.M.; Lyashchenko, K.P.; Waters, W.R.; Berry, D.E.; Palmer, M.V.; McNair, J.; Greenwald, R.; Esfandiari, J.; Cosgrove, M.K. Evaluation of blood assays for detection of Mycobacterium bovis in white-tailed deer (Odocoileus virginianus) in Michigan. J. Wildl. Dis. 2009, 45, 153–164. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Buddle, B.M.; Wilson, T.; Denis, M.; Greenwald, R.; Esfandiari, J.; Lyashchenko, K.P.; Liggett, S.; Mackintosh, C.G. Sensitivity, Specificity, and Confounding Factors of Novel Serological Tests Used for the Rapid Diagnosis of Bovine Tuberculosis in Farmed Red Deer (Cervus elaphus). Clin. Vaccine Immunol. 2010, 17, 626–630. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Buddle, B.M.; Mackintosh, C.G. Improving the diagnosis of bovine tuberculosis in farmed deer. Vet. Rec. 2017, 180, 66–67. [Google Scholar] [CrossRef]
- Aurtenetxe, O.; Barral, M.; Vicente, J.; de la Fuente, J.; Gortázar, C.; A Juste, R. Development and validation of an enzyme-linked immunosorbent assay for antibodies against Mycobacterium bovisin european wild boar. BMC Vet. Res. 2008, 4, 43. [Google Scholar] [CrossRef]
- Cardoso-Toset, F.; Luque, I.; Carrasco, L.; Jurado-Martos, F.; Risalde, M.A.; Venteo, A.; Infantes-Lorenzo, J.A.; Bezos, J.; Rueda, P.; Tapia, I.; et al. Evaluation of five serologic assays for bovine tuberculosis surveillance in domestic free-range pigs from southern Spain. Prev. Vet. Med. 2017, 137, 101–104. [Google Scholar] [CrossRef]
- Ippolito, D.; Boniotti, M.B.; Fiasconaro, M.; Fontana, S.; Boifava, M.; Ciarello, F.P.; Amato, B.; Pacciarini, M.; Presti, V.D.M.L. Development and evaluation of a multi-antigen serological assay for the intra-vitam diagnosis of Tuberculosis caused by Mycobacterium bovis in pigs. J. Immunol. Methods 2022, 503, 113234. [Google Scholar] [CrossRef]
- Miller, M.A.; Gortazar, C.; Roos, E.O.; Risalde, M.A.; Johnathan-Lee, A.; Sridhara, A.A.; Lyashchenko, K.P. Serological reactivity to MPB83 and CFP10/ESAT-6 antigens in three suid hosts of Mycobacterium bovis infection. Vet. Microbiol. 2019, 235, 285–288. [Google Scholar] [CrossRef]
- Infantes-Lorenzo, J.A.; Whitehead, C.E.; Moreno, I.; Bezos, J.; Roy, A.; Domínguez, L.; Domínguez, M.; Salguero, F.J. Development and Evaluation of a Serological Assay for the Diagnosis of Tuberculosis in Alpacas and Llamas. Front. Vet. Sci. 2018, 5, 189. [Google Scholar] [CrossRef]
- van der Heijden, E.M.; Cooper, D.V.; Rutten, V.P.; Michel, A.L. Mycobacterium bovis prevalence affects the performance of a commercial serological assay for bovine tuberculosis in African buffaloes. Comp. Immunol. Microbiol. Infect. Dis. 2020, 70, 101369. [Google Scholar] [CrossRef] [PubMed]
- Lyashchenko, K.P.; Pollock, J.M.; Colangeli, R.; Gennaro, M.L. Diversity of Antigen Recognition by Serum Antibodies in Experimental Bovine Tuberculosis. Infect. Immun. 1998, 66, 5344–5349. [Google Scholar] [CrossRef]
- Chai, J.; Wang, Q.; Qin, B.; Wang, S.; Wang, Y.; Shahid, M.; Liu, K.; Zhang, Y.; Qu, W. Association of NOS2A gene polymorphisms with susceptibility to bovine tuberculosis in Chinese Holstein cattle. PLoS ONE 2021, 16, e0253339. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Gandham, S.; Rana, A.; Maity, H.K.; Sarkar, U.; Dey, B. Divergent proinflammatory immune responses associated with the differential susceptibility of cattle breeds to tuberculosis. Front. Immunol. 2023, 14, 1199092. [Google Scholar] [CrossRef] [PubMed]
- Raphaka, K.; Matika, O.; Sánchez-Molano, E.; Mrode, R.; Coffey, M.P.; Riggio, V.; Glass, E.J.; Woolliams, J.A.; Bishop, S.C.; Banos, G. Genomic regions underlying susceptibility to bovine tuberculosis in Holstein-Friesian cattle. BMC Genet. 2017, 18, 27. [Google Scholar] [CrossRef] [PubMed]
- Pelayo, M.C.G.; Garcia, J.N.; Golby, P.; Pirson, C.; Ewer, K.; Vordermeier, M.; Hewinson, R.G.; Gordon, S.V. Gene expression profiling and antigen mining of the tuberculin production strain Mycobacterium bovis AN5. Vet. Microbiol. 2009, 133, 272–277. [Google Scholar] [CrossRef]
- Flores-Gonzalez, J.; Ramón-Luing, L.A.; Romero-Tendilla, J.; Urbán-Solano, A.; Cruz-Lagunas, A.; Chavez-Galan, L. Latent Tuberculosis Patients Have an Increased Frequency of IFN-γ-Producing CD5+ B Cells, Which Respond Efficiently to Mycobacterial Proteins. Pathogens 2023, 12, 818. [Google Scholar] [CrossRef]
- Silva, E. Evaluation of an enzyme-linked immunosorbent assay in the diagnosis of bovine tuberculosis. Vet. Microbiol. 2001, 78, 111–117. [Google Scholar] [CrossRef]
- Wadhwa, A.; Johonson, R.E.; Eda, K.; Waters, W.R.; Palmer, M.V.; Bannantine, J.P.; Eda, S. Evaluation of ethanol vortex ELISA for detection of bovine tuberculosis in cattle and deer. BMC Vet. Res. 2014, 10, 147. [Google Scholar] [CrossRef]
- Cho, Y.S.; Lee, S.E.; Woo, J.T.; Oh, J.; Choi, H.W.; Kwon, J.H.; Kim, J.T.; Ha, G.; Jung, S. Comparing recombinant mpb70/sahh and native 20-kda protein for detecting bovine tuberculosis using elisa. J. Vet. Med. Sci. 2020, 82, 1631–1638. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klepp, L.I.; Blanco, F.C.; Bigi, M.M.; Vázquez, C.L.; García, E.A.; Sabio y García, J.; Bigi, F. B Cell and Antibody Responses in Bovine Tuberculosis. Antibodies 2024, 13, 84. https://doi.org/10.3390/antib13040084
Klepp LI, Blanco FC, Bigi MM, Vázquez CL, García EA, Sabio y García J, Bigi F. B Cell and Antibody Responses in Bovine Tuberculosis. Antibodies. 2024; 13(4):84. https://doi.org/10.3390/antib13040084
Chicago/Turabian StyleKlepp, Laura Inés, Federico Carlos Blanco, María Mercedes Bigi, Cristina Lourdes Vázquez, Elizabeth Andrea García, Julia Sabio y García, and Fabiana Bigi. 2024. "B Cell and Antibody Responses in Bovine Tuberculosis" Antibodies 13, no. 4: 84. https://doi.org/10.3390/antib13040084
APA StyleKlepp, L. I., Blanco, F. C., Bigi, M. M., Vázquez, C. L., García, E. A., Sabio y García, J., & Bigi, F. (2024). B Cell and Antibody Responses in Bovine Tuberculosis. Antibodies, 13(4), 84. https://doi.org/10.3390/antib13040084