Abstract
Background: Therapeutic drug monitoring of biological Tumor Necrosis Factor (TNF)-alpha inhibitors is of critical importance. In this study, the performance of practically advantageous chemiluminescent immunoassays of Theradiag, assessing Infliximab and Adalimumab serum concentrations and anti-drug antibodies (ADA) against these biologics, were compared to the Enzyme-Linked Immuno-Sorbent Assays (ELISAs) from Sanquin Diagnostics. Methods: Leftover serum samples (n = 80 for each parameter) from patients treated with Infliximab or Adalimumab were collected. Correlation and agreement analyses for serum concentration and ADAs, respectively, were performed. Both Theradiag ADA assays, an assay targeting both free and bound ADAs and an assay targeting solely free ADAs, were investigated and compared to the Sanquin Diagnostics ADA assay, targeting both free and bound ADAs. Results: Strong positive correlations were observed between the biologic concentration assessment of Infliximab (Spearman’s Rho = 0.91) and Adalimumab (Spearman’s Rho = 0.94). However, there appeared to be significant bias in the Theradiag assay when compared to Sanquin (Infliximab median (Confidence Interval (CI)) = 2.1 (1.7–2.6) µg/mL; Adalimumab median (CI) = 0.8 (0.5–0.9) µg/mL). Agreement analyses showed moderate to good agreement for the Theradiag and Sanquin Diagnostics ADA assays, when detecting both free and bound ADAs, for Infliximab (Cohen’s k = 0.717) and Adalimumab (Cohen’s k = 0.802). In contrast, the Theradiag ADA assay detecting solely free ADAs had zero to poor agreement for Infliximab (Cohen’s k = 0.458) and Adalimumab (Cohen’s k = 0.119), respectively. Conclusions: This study demonstrated strong correlations and good agreement between the Theradiag and Sanquin Diagnostics assays measuring Infliximab and Adalimumab serum concentrations and ADAs, both free and bound, against these biologics. Discordance analyses showed significantly decreased drug concentrations in the solely free assays, indicating that the combined detection of free and bound ADAs better aligns with drug levels.
1. Introduction
Anti-tumor necrosis factor-alpha (anti-TNF-alpha) agents have permanently modified the landscape of therapeutic options in multiple chronic auto-inflammatory diseases such as inflammatory bowel diseases (IBD), rheumatoid arthritis (RA), and psoriasis [1]. These monoclonal antibodies (mAbs) target TNF-alfa, an inflammatory cytokine abundantly generated in these diseases. The two most established anti-TNF-alpha biologics are Infliximab and Adalimumab. Infliximab is a chimeric mAb consisting of a murine Fab fragment combined with a human Fc fragment and is intravenously administered, while Adalimumab is a completely humanized IgG mAb, which is subcutaneously delivered.
Despite the fact that anti-TNF-alpha biologics have been proven to have significant therapeutic benefits in multiple chronic auto-inflammatory diseases, a significant proportion (up to 70%) of patients unfortunately lose therapeutic response over time due to the formation of anti-drug antibodies (ADAs) [2]. These ADAs can not only reduce drug efficacy by targeting the antigen-binding site of the biologic, therefore neutralizing the agent’s biological activity, but can also cause severe adverse events through the formation of pathogenic immune complexes [2,3,4,5]. Work has been carried out to investigate the effects of the epitopes of anti-drug antibodies against Infliximab [6]. However, it remains to be explored whether there are epitope differences in ADAs, reducing their efficacy, neutralizing therapeutic effects, and allowing the formation of pathogenic immune complexes. Nevertheless, therapeutic drug management, through assessing biologic concentration as well as ADA levels, is of pivotal importance when prescribing these medications [7].
Successful therapeutic drug management requires assays that are able to assess serum drug levels and ADAs accurately. To date, many different methods have been used to develop such assays, all with their own technical (dis)advantages and interference factors [8]. Enzyme-linked immunosorbent assays (ELISAs) are the most commonly used methods to assess biologic concentrations and ADAs, especially in The Netherlands. Despite their many advantages, ELISAs have certain limitations, such as their time-consuming procedure and batch-wise approach, causing low throughput and potentially therapeutic management delays [9]. Recently, Theradiag developed novel chemiluminescent i-Tracker immunoassays, potentially overcoming these limitations.
The goal of this study was to compare the Infliximab and Adalimumab concentration and ADA levels found using the chemiluminescent immunoassays (CLIA) of Theradiag or the ELISAs of Sanquin Diagnostics.
2. Methods
2.1. Patient Population and Sample Selection
Leftover samples from patients treated with Infliximab or Adalimumab were collected from 2021 to 2023 for concentration assessment and from 2016 to 2023 for ADA assessment. Inclusion required biologic concentration and/or ADA level assessment as part of their routine clinical care. To minimize selection bias and to assess the Theradiag assays across a broad biologic or ADA concentration range, systematic sampling by applying a standard sample interval was performed for the biologic concentration cohorts (Infliximab n = 80, Adalimumab n = 80), based on the clinically measured Infliximab or Adalimumab Sanquin concentration. For the ADA cohorts (anti-Infliximab n = 80, anti-Adalimumab n = 80), 10 negative samples were selected, followed by systematic sampling based on Sanquin ADA concentration for the remaining 70 samples. The 320 performed tests involved the samples of 293 individuals. The samples of 27 patients were included in more than 1 of the 4 cohorts.
Ethical approval was not required as the study was non-interventional, all data were completely anonymized and no patients were included who objected against further use of their biological samples. Additionally, the study was performed according to the Code of Conduct for Health Research and the Declaration of Helsinki.
2.2. Sample Collection
Serum samples were retrieved by collecting blood samples through venipuncture in BD Vacutainer SSTTM II Advance (REF: 367955) gel collection tubes. After the manufacturer recommended 30 min of clotting time, samples were centrifuged at 1885× g for 10 min. Subsequently, a serum aliquot (separated from the cells) was directly stored at −20 °C until analysis.
2.3. Biologic Concentration and Anti-Drug Antibody (ADA) Assays
Sanquin Diagnostics: Infliximab and Adalimumab concentration and ADA level assessment for routine clinical care were determined using the assays from Sanquin Diagnostics (Amsterdam, The Netherlands). It is important to note that the Sanquin Diagnostics ADA assays detected both free and bound ADAs against Infliximab or Adalimumab. The characteristics of these assays are described in Table 1.

Table 1.
Characteristics of the Infliximab and Adalimumab concentration and ADA assays of Sanquin Diagnostics.
Theradiag: The i-Tracker CLIAs of Theradiag (Croissy-Beaubourg, France), measured on the IDS-iSYS multi-discipline automated system analyzer, are the assays under investigation. In addition to the Infliximab and Adalimumab concentration assays, Theradiag has two types of ADA assays, namely commercially available assays detecting exclusively free ADAs and research-only assays detecting both free as well as bound ADAs against Infliximab or Adalimumab. The characteristics of these assays are described in Table 2.

Table 2.
Characteristics of the Infliximab and Adalimumab concentration and ADA assays of Theradiag.
2.4. Statistical Analysis
Results are presented as median [interquartile range (IQR)] or mean ± standard deviation, depending on Gaussian distribution.
The paired samples t-test was used to compare paired Gaussian distributed biologic concentrations between assays. A p-value of <0.05 was regarded as statistically significant.
Passing–Bablok regression, difference plots, and Spearman’s correlation analyses were performed to study the biologic concentration agreement between assays. A Spearman’s rho value of >0.7 was considered to be a highly positive correlation.
Cohen’s kappa was used as a statistical measure to quantify the level of agreement between the Sanquin ADA assays and the Theradiag ADA assays. ADA concentrations of ≥12 AU/mL and ≥10 ng/mL were considered to indicate a positive result for the presence of ADAs for Sanquin and Theradiag, respectively. A value between 0 and 0.20 indicated “no agreement”, 0.21–0.39 indicated “minimal agreement”, 0.40–0.59 indicated “weak agreement”, 0.60–0.79 indicated “moderate agreement”, 0.80–0.90 indicated “strong agreement”, and ≥0.90 indicated “almost perfect” [10].
All statistical analyses were performed using GraphPad Prism (version 5.04, La Jolla, CA, USA) and R (version 4.4.1) using package ggplot2 (version 3.5.1). The R code is included as Supplementary Information.
3. Results
3.1. Patient Population
The patients included in the distinct cohorts were from 42 ± 18 to 49 ± 18 years old and predominantly female. Most samples were collected from patients treated with a biologic by medical professionals in the gastrointestinal and liver diseases, rheumatology, ophthalmology, and pediatrics specialties (±94%). A minor proportion of the samples was collected from patients of the dermatology, pulmonology and plastic surgery departments (Table 3).

Table 3.
Baseline characteristics, including treating medical specialty, of the patients selected for biologic concentration and ADA agreement assessment.
3.2. Agreement between Infliximab and Adalimumab Concentration Assays
A broad spectrum of biologic concentrations ranging from 0.2 to 25 µg/mL, as based on the Sanquin assays, was studied, with a predominant focus on concentrations between 2 and 12 µg/mL (±75%). For Infliximab, the Sanquin median [IQR] concentration was 5.6 [3.1–8.5] µg/mL, while the Theradiag median [IQR] concentration was 8.1 [4.8–12.6] µg/mL (p < 0.001), revealing a median (CI) bias of 2.1 (1.7–2.6) µg/mL (Figure 1A). For Adalimumab, the Sanquin median [IQR] concentration was 6.8 [4.4–9.0] µg/mL, while the Theradiag median [IQR] concentration was 7.6 [5.3–10.1] µg/mL (p < 0.001), revealing a median (CI) bias of 0.8 (0.5–0.9) µg/mL (Figure 1C). Despite the statistically significant difference in biologic concentrations, there were strong positive correlations for both Infliximab (Spearman’s Rho = 0.91) and Adalimumab (Spearman’s Rho = 0.94) between the assays (Figure 1B,D).
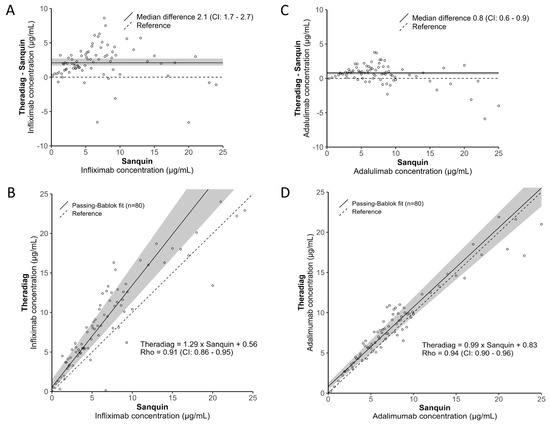
Figure 1.
Infliximab (A) bias and (B) linear correlation and Adalimumab (C) bias and (D) linear correlation analyses between the Sanquin Diagnostics and Theradiag assays.
3.3. Agreement between Infliximab and Adalimumab ADA Assays
The Theradiag ADA assays solely detecting free ADAs demonstrated weak to no agreement (Infliximab Cohen’s k = 0.458, Adalimumab Cohen’s k = 0.119), while there was moderate to strong agreement for the ADA assays detecting both free and bound ADAs (Infliximab Cohen’s k = 0.717, Adalimumab Cohen’s k = 0.802), when compared to the ADA assays of Sanquin (Table 4 and Table S1). Due to the limited sample availability, not all samples could be assessed for the research-only Theradiag ADA assays. The agreement difference was not explained by the difference in sample sets. The paired analyses demonstrated that the Infliximab Cohen’s k = 0.448 and Adalimumab Cohen’s k = 0.124.

Table 4.
Agreement analyses between the Sanquin Diagnostics ADA assays and Theradiag ADA (free and total) assays for Infliximab and Adalimumab.
3.4. ADA Assay Discordance Analyses
Discordance analyses between the Sanquin ADA assay and the Theradiag ADA assay detecting solely free ADAs revealed median [IQR] biologic concentrations below their therapeutic windows, as measured using the biologic concentration assays of both manufacturers (Table 5). All samples for both biologics were positive for ADAs according to the Sanquin ADA assay, while ADAs were absent, as determined by the Theradiag ADA assay. Additionally, there was considerably more discordance observed for Adalimumab (n = 45) than for Infliximab (n = 16).

Table 5.
Discordance analyses of the Theradiag (free) vs. Sanquin Diagnostics assay.
Comparing the discordance between the Theradiag ADA assay, detecting both free and bound ADAs, and the Sanquin assay demonstrated only four discordant samples for each biologic (Table 6). For Infliximab, the biologic concentrations as measured with the assays of both manufacturers were more in the range of their therapeutic windows. The Sanquin ADA assay concluded that there was an absence of ADAs, while the Theradiag ADA assay determined the presence of ADAs in these samples. For Adalimumab, two discordant samples had decreased biologic concentrations while the other two samples had adequate concentrations. All discordant samples were positive for ADAs according to the Sanquin assay, while they were negative according to the Theradiag assay.

Table 6.
Discordance analyses of the Theradiag (total) vs. Sanquin Diagnostics assay.
4. Discussion
Accurate therapeutic drug management, through assessing biologic concentration as well as ADA levels, is of pivotal importance when prescribing these medications. The present study investigated the biologic concentration and ADA assays of Theradiag and compared them to the assays of Sanquin Diagnostics in a patient population treated with Infliximab or Adalimumab. We report the following three major findings:
First, using a broad range of biologic concentrations, we demonstrated a strong correlation between the biologic concentration assays of Sanquin and Theradiag. This is in line with previous studies where the correlation between different methods has been shown to be relatively good [11]. However, there appears to be a significant bias in biologic concentration assessment, which was especially the case for Infliximab. This finding was also previously demonstrated by Berger et al. when comparing the CLIA (iTRACK10) to the ELISA (LISA-Tracker) tests of Theradiag, suggesting a methodological bias, amongst others [12]. Additionally, the bias is predominantly present for Infliximab, though concentration differences of more than 2 µg/mL between assays for multiple biologics have been previously observed [13]. However, as long as the same assay is used for follow-up, this restriction seems of limited clinical concern.
Second, the Theradiag ADA assays detecting solely free ADAs have weak to no agreement when compared to the ADA assay of Sanquin. From a technical perspective, this can, of course, be explained by the difference in analyte target. The absence of ADAs is measured by the Theradiag free ADA assays, but the presence of ADAs is measured by the Sanquin ADA assays, which demonstrated median biologic concentrations well below the therapeutic target. This suggests that the Sanquin ADA Theradiag free ADA assays do not detect relevant bound ADAs, causing decreased biologic concentration levels.
Third, the agreement between the Theradiag and Sanquin ADA assays detecting both free and bound ADAs is moderate to good. Antibodies are a highly variable mixture of molecules that are different in terms of epitope recognition, degree and type of glycosylation, isotype, and subclass distribution [14]. Additionally, each individual has their own antibody fingerprint, and the output of ADA assays depends on antibody characteristics, such as affinity and avidity, presented in non-uniform ways. All these factors prevent standardization and limit the comparability of results across assays [11,15].
4.1. Implications
The data presented in this retrospective assay comparison have important implications. Therapeutic drug management is heavily based on biological concentration and ADA assessment, in addition to clinical assessment. The observed correlation and agreement performance of the Theradiag biologic concentration and ADA (total) assays, as compared to the assays of Sanquin Diagnostics, grants the reliable use of Theradiag’s assays. Despite the Theradiag assays running on the automated platforms of Immunodiagnostic Systems (IDS), they cannot be combined with other tests and require system priming post-analyses. Nevertheless, implementing these assays would have practical advantages. The immunoassays have a faster turnaround time, are more suitable for high-throughput measurement, and require fewer technician resources. Also, this leads to enhanced therapeutic drug measurement, as sample collection, analysis and reporting, and an outpatient appointment can be scheduled on the same day. A point of attention is the observed bias in concentration assessment. Result interpretation should be carried out by taking the type of assay into account and will require careful communication with clinicians. Additionally, cost-effectiveness assessments will be required for clinical laboratories to investigate if the local patient population allows assay transition.
4.2. Limitations
This work is limited by the fact that selection criteria were used for the selection of patient samples which could lead to selection bias. However, the sample selection strategy that was applied minimized this bias. Second, the results in this study are only measured once. Nevertheless, the assays have been extensively validated by the manufacturers (intra and inter coefficients of variability (CVs) of approximately 5 and 10%, respectively), and quality controls were used, thereby diminishing this bias. Third, the current study’s results, for which only Theradiag ADA assays detecting both free and bound ADA were used, were not analyzed locally, but measured at Theradiag. However, the samples were anonymized and Theradiag did not have the results of the Theradiag free ADA and Sanquin ADA assays when analyzing these samples.
5. Conclusions
In conclusion, in this retrospective study, we demonstrated strong correlations and good agreement between the Theradiag and Sanquin Diagnostics assays measuring serum concentrations of Infliximab and Adalimumab and ADAs, both free and bound, against these biologics. The Theradiag ADA assay detecting solely free ADAs against Infliximab and Adalimumab had poor agreement with the assays of Sanquin Diagnostics when biologic concentrations were low, suggesting that these assays do not sufficiently detect clinically relevant ADAs. Despite good overall performance and correlations of the concentrations and ADA (total) assays, we recommend using the same assay format for long-term follow-up of patients treated with Infliximab and Adalimumab.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antib13030073/s1, Table S1: Detailed agreement analyses between the Sanquin Diagnostics ADA assays and Theradiag ADA (free and total) assays for Infliximab and Adalimumab.
Author Contributions
Conceptualization, J.G.M.C.D.; Methodology, J.G.M.C.D.; Validation, J.G.M.C.D.; Formal analysis, W.H.M.V. and S.S.A.; Investigation, S.S.A. and J.G.M.C.D.; Resources, J.G.M.C.D.; Data curation, W.H.M.V. and S.S.A.; Writing—original draft, W.H.M.V. and S.S.A.; Writing—review & editing, W.H.M.V., J.J.B.C.v.B. and J.G.M.C.D.; Visualization, W.H.M.V.; Supervision, J.G.M.C.D.; Project administration, S.S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical approval was not required, as the study was non-interventional. Additionally, the study was performed according to the Code of Conduct for Health Research and the Declaration of Helsinki.
Informed Consent Statement
The samples which were used for this investigation were collected in the context of individual patient healthcare. No additional venipuncture was performed and no additional blood tubes were collected. Thus, there was only the secondary use of human tissue. Additionally, as per hospital policy, healthcare providers ask for consent for the secondary use of patient samples. Patients who objected to secondary use were not included in this study. Moreover, all data were completely anonymized, in line with General Data Protection Regulation (GDPR) regulations.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials; further inquiries can be directed to the corresponding author/s.
Acknowledgments
The authors would like to thank M. Jobsen, C. Bijnens, and D. Jongen for sample handling and for performing the analyses.
Conflicts of Interest
There are no conflicts of interest to report regarding this manuscript.
References
- Colombel, J.F.; Sandborn, W.J.; Reinisch, W.; Mantzaris, G.J.; Kornbluth, A.; Rachmilewitz, D.; Lichtiger, S.; d’Haens, G.; Diamond, R.H.; Broussard, D.L.; et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N. Engl. J. Med. 2010, 362, 1383–1395. [Google Scholar] [CrossRef] [PubMed]
- Vaisman-Mentesh, A.; Gutierrez-Gonzalez, M.; DeKosky, B.J.; Wine, Y. The Molecular Mechanisms That Underlie the Immune Biology of Anti-drug Antibody Formation following Treatment with Monoclonal Antibodies. Front. Immunol. 2020, 11, 1951. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.; Nadler, S.G. Immunogenicity to Biotherapeutics—The Role of Anti-Drug Immune Complexes. Front. Immunol. 2016, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- van Schouwenburg, P.A.; Rispens, T.; Wolbink, G.J. Immunogenicity of anti-TNF biologic therapies for rheumatoid arthritis. Nat. Rev. Rheumatol. 2013, 9, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Hansel, T.T.; Kropshofer, H.; Singer, T.; Mitchell, J.A.; George, A.J. The safety and side effects of monoclonal antibodies. Nat. Rev. Drug Discov. 2010, 9, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Homann, A.; Rockendorf, N.; Kromminga, A.; Frey, A.; Jappe, U. B cell epitopes on infliximab identified by oligopeptide microarray with unprocessed patient sera. J. Transl. Med. 2015, 13, 339. [Google Scholar] [CrossRef] [PubMed]
- Shankar, G.; Devanarayan, V.; Amaravadi, L.; Barrett, Y.C.; Bowsher, R.; Finco-Kent, D.; Fiscella, M.; Gorovits, B.; Kirschner, S.; Moxness, M.; et al. Recommendations for the validation of immunoassays used for detection of host antibodies against biotechnology products. J. Pharm. Biomed. Anal. 2008, 48, 1267–1281. [Google Scholar] [CrossRef]
- Gorovits, B.; Baltrukonis, D.J.; Bhattacharya, I.; Birchler, M.A.; Finco, D.; Sikkema, D.; Vincent, M.S.; Lula, S.; Marshall, L.; Hickling, T.P. Immunoassay methods used in clinical studies for the detection of anti-drug antibodies to adalimumab and infliximab. Clin. Exp. Immunol. 2018, 192, 348–365. [Google Scholar] [CrossRef]
- Soenen, R.; Stove, C.; Capobianco, A.; De Schutter, H.; Dobbelaere, M.; Mahjor, T.; Follens, M.; Lambert, J.; Grine, L. Promising Tools to Facilitate the Implementation of TDM of Biologics in Clinical Practice. J. Clin. Med. 2022, 11, 3011. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Vande Casteele, N. Assays for measurement of TNF antagonists in practice. Frontline Gastroenterol. 2017, 8, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.E.; Gleizes, A.; Waeckel, L.; Roblin, X.; Krzysiek, R.; Hacein-Bey-Abina, S.; Soriano, A.; Paul, S. Validation Study of a New Random-Access Chemiluminescence Immunoassay Analyzer i-TRACK10((R)) to Monitor Infliximab and Adalimumab Serum trough Levels and Anti-Drug Antibodies. Int. J. Mol. Sci. 2022, 23, 9561. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Argüello, B.; del Agua, A.R.; Torres, N.; Monasterio, A.; Martínez, A.; Nagore, D. Comparison study of two commercially available methods for the determination of infliximab, adalimumab, etanercept and anti-drug antibody levels. Clin. Chem. Lab. Med. 2013, 51, e287–e289. [Google Scholar] [CrossRef] [PubMed]
- Damoiseaux, J. The perspective on standardisation and harmonisation: The viewpoint of the EASI president. Autoimmun. Highlights 2020, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- van Schouwenburg, P.A.; Kruithof, S.; Wolbink, G.; Wouters, D.; Rispens, T. Using monoclonal antibodies as an international standard for the measurement of anti-adalimumab antibodies. J. Pharm. Biomed. Anal. 2016, 120, 198–201. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).