Interspecies Scaling of Antibody–Drug Conjugates (ADC) for the Prediction of Human Clearance
Abstract
:1. Introduction
- To apply the ROE only to those drugs that had at least three animal species.
- Considering a real life situation where three animal species may not be available, two-animal species allometric scaling was performed to evaluate if it was possible to predict human clearance of ADCs with two-species allometric scaling using only simple allometry.
- The authors Li et al. used a single species scaling for the prediction of human clearance of ADCs. The species used by the authors was monkey. Although it is widely believed that the predicted human clearance can be fairly accurate using the monkey clearance values alone, there is no analysis with other species such as rat and mice for ADCs. Therefore, in this study, the clearance data from mice or rat were used to evaluate if the predicted human clearance values of ADCs are comparable with the monkey.
- Li et al. [9] used three allometric exponents (0.75, 0.85, and 1.0) for a single-species scaling for monkeys. The exponent 0.75 is a theoretical allometric exponent, exponent 0.85 was taken from Deng et al. [6,7] for the prediction of human clearance from monkey data, and the authors explored exponent 1.0. A study by Oitate et al. [8] indicated that the exponent 0.79 was the most suitable exponent for the prediction of human clearance of antibodies from monkey. Therefore, in this study, exponent 0.79 was also used for a single-species scaling by rounding it to 0.80.
2. Methods
2.1. Simple Allometry
2.2. Product of MLP and Clearance (MLP × Clearance)
2.3. Product of Brain Weight and Clearance (Br WT × Clearance)
2.4. Two-Species Scaling
2.5. One-Species Scaling
2.6. Statistical Analysis
3. Results
3.1. Three-Species Allometric Scaling
3.2. Application of MLP:
3.3. Two-Species Allometric Scaling
3.4. One-Species Allometric Scaling
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lambert, J.M.; Charles, Q.; Morris, C.Q. Antibody–Drug Conjugates (ADCs) for Personalized Treatment of Solid Tumors: A Review. Adv. Ther. 2017, 34, 1015–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuo, P. Capturing the Magic Bullet: Pharmacokinetic Principles and Modeling of Antibody-Drug Conjugates. AAPS J. 2020, 22, 105. [Google Scholar] [PubMed]
- Mahmood, I. Application of allometric principles for the prediction of pharmacokinetics in human and veterinary drug development. Adv. Drug Deliv. Rev. 2007, 59, 1177–1192. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, I. Interspecies scaling of protein drugs: Prediction of clearance from animals to humans. J. Pharm. Sci. 2004, 93, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, I. Pharmacokinetic allometric scaling of antibodies: Application to the first-in-human dose estimation. J. Pharm. Sci. 2009, 98, 3850–3861. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Iyer, S.; Theil, F.P.; Mortensen, D.L.; Fielder, P.J.; Prabhu, S. Projecting human pharmacokinetics of therapeutic antibodies from non-clinical data: What have we learned? MAbs 2011, 3, 61–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, J.; Zhou, H.; Jiao, Q.; Davis, H.M. Interspecies scaling of therapeutic monoclonal antibodies: Initial look. J. Clin. Pharmacol. 2009, 49, 1382–1402. [Google Scholar] [CrossRef] [PubMed]
- Oitate, M.; Masubuchi, N.; Ito, T.; Yabe, Y.; Karibe, T.; Aoki, T.; Murayama, N.; Kurihara, A.; Okudaira, N.; Izumi, T. Prediction of human pharmacokinetics of therapeutic monoclonal antibodies from simple allometry of monkey data. Drug Metab Pharmacokinet. 2011, 26, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, C.; Deng, R.; Leipold, D.; Li, D.; Latifi, B.; Gao, Y.; Zhang, C.; Li, Z.; Miles, D.; et al. Prediction of Human Pharmacokinetics of Antibody-Drug Conjugates from Nonclinical Data. Clin. Transl. Sci. 2019, 12, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, I.; Balian, J.D. Interspecies scaling: Predicting clearance of drugs in humans. Three different approaches. Xenobiotica 1996, 26, 887–895. [Google Scholar] [PubMed]
- Goteti, K.; Garner, C.E.; Mahmood, I. Prediction of human drug clearance from two species: A comparison of several allometric methods. J. Pharm. Sci. 2010, 99, 1601–1613. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Rubinfeld, B.; Zhang, C.; Firestein, R.; Harstad, E.; Roth, L.; Tsai, S.P.; Schutten, M.; Xu, K.; Hristopoulos, M.; et al. Preclinical Development of an Anti-NaPi2b (SLC34A2) Antibody-Drug Conjugate as a Therapeutic for Non-Small Cell Lung and Ovarian Cancers. Clin. Cancer Res. 2015, 21, 5139–5150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Lee, D.; Dere, R.C.; Zheng, B.; Yu, S.F.; Fuh, F.K.; Kozak, K.R.; Chung, S.; Yadav, D.B.; Nazzal, D.; et al. Evaluation and use of an anti-cynomolgus monkey CD79b surrogate antibody-drug conjugate to enable clinical development of polatuzumab vedotin. Br. J. Pharmacol. 2019, 176, 3805–3818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, D.K.; Haddish-Berhane, N.; Betts, A. Bench to bedside translation of antibody drug conjugates using a multiscale mechanistic PK/PD model: A case study with brentuximab-vedotin. J. Pharmacokinet. Pharmacodyn. 2012, 39, 643–659. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Zhou, C.; Li, D.; Cai, H.; Sukumaran, S.; Carrasco-Triguero, M.; Saad, O.; Nazzal, D.; Lowe, C.; Ramanujan, S.; et al. Preclinical and translational pharmacokinetics of a novel THIOMAB antibody-antibiotic conjugate against Staphylococcus aureus. MAbs 2019, 11, 1162–1174. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Lehar, S.; Guierrez, J.; Rosenberger, C.M.; Ljumanovic, N.; Dinoso, J.; Koppada, N.; Hong, K.; Baruch, A.; Carrasco-Triguero, M.; et al. Pharmacokinetics and pharmacodynamics of DSTA4637A: A novel THIOMAB antibody antibiotic conjugate against Staphylococcus aureus in mice. MAbs 2016, 8, 1612–1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peck, M.; Rothenberg, M.E.; Deng, R.; Lewin-Koh, N.; She, G.; Kamath, A.V.; Carrasco-Triguero, M.; Saad, O.; Castro, A.; Teufel, L.; et al. A phase 1, randomized, single ascending-dose study to investigate the safety, tolerability, and pharmacokinetics of DSTA4637S, an anti-Staphylococcus aureus THIOMABTM antibody-antibiotic conjugate, in healthy volunteers. Antimicrob. Agents Chemother. 2019, 63, e02588-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leal, M.; Wentland, J.; Han, X.; Zhang, Y.; Rago, B.; Duriga, N.; Spriggs, F.; Kadar, E.; Song, W.; McNally, J.; et al. Preclinical Development of an anti-5T4 Antibody−Drug Conjugate: Pharmacokinetics in Mice, Rats, and NHP and Tumor/Tissue Distribution in Mice. Bioconjugate Chem. 2015, 26, 2223–2232. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, G.I.; Vaishampayan, U.N.; LoRusso, P. First-in-human trial of an anti-5T4 antibody-monomethylauristatin conjugate, PF-06263507, in patients with advanced solid tumors. Investig. New Drugs. 2017, 35, 315–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Drugs | CL (mL/day) | Drugs | CL (mL/day) |
|---|---|---|---|
| DNIB0600A | Polatuzumab vedotin | ||
| Mouse | 0.18 | Mouse | 0.11 |
| Rat * | 3.98 | Rat * | 4 |
| Monkey | 46.55 | Monkey | 24.2 |
| Human | 854 | Human | 1015 |
| DMOT4039A | Pinatuzumab vedotin | ||
| Mouse | 0.19 | Mouse | 0.12 |
| Monkey | 96.6 | Monkey | 32.9 |
| Human | 1400 | Human | 966 |
| DSTP3086S | Brentuximab vedotin | ||
| Mouse | 0.20 | Mouse * | 0.50 |
| Rat | 2.37 | Rat | 2.25 |
| Monkey | 46.9 | Monkey | 51.1 |
| Human | 574 | Human | 742 |
| T-DM1 (total antibody) | T-DM1 (conjugate) | ||
| Mouse | 0.16 | Mouse | 0.38 |
| Rat | 1.62 | Rat | 4.6 |
| Monkey | 16.1 | Monkey | 41.9 |
| Human | 343 | Human | 600 |
| Thiomab (New) total antibody | Thiomab (New) conjugate | ||
| Mouse | 0.10 | Mouse | 0.40 |
| Rat | 2.15 | Rat | 6.03 |
| Monkey | 20.37 | Monkey | 54.25 |
| Human | 200 | Human | 759 |
| Anti-5T4 (New) (total antibody) | Anti-5T4 (New) (conjugate) | ||
| Mouse | 0.39 | Mouse | 0.68 |
| Rat | 3.3 | Rat | 4.8 |
| Monkey | 27.1 | Monkey | 52.6 |
| Human | 360 | Human | 700 |
| ADC1 | |||
| Mouse | 0.13 | ||
| Monkey | 36.7 | ||
| Human | 756 | ||
| Drugs | Coefficient | Exponent | Observed | Predicted | Ratio * |
|---|---|---|---|---|---|
| DSTP3086S (total antibody) | |||||
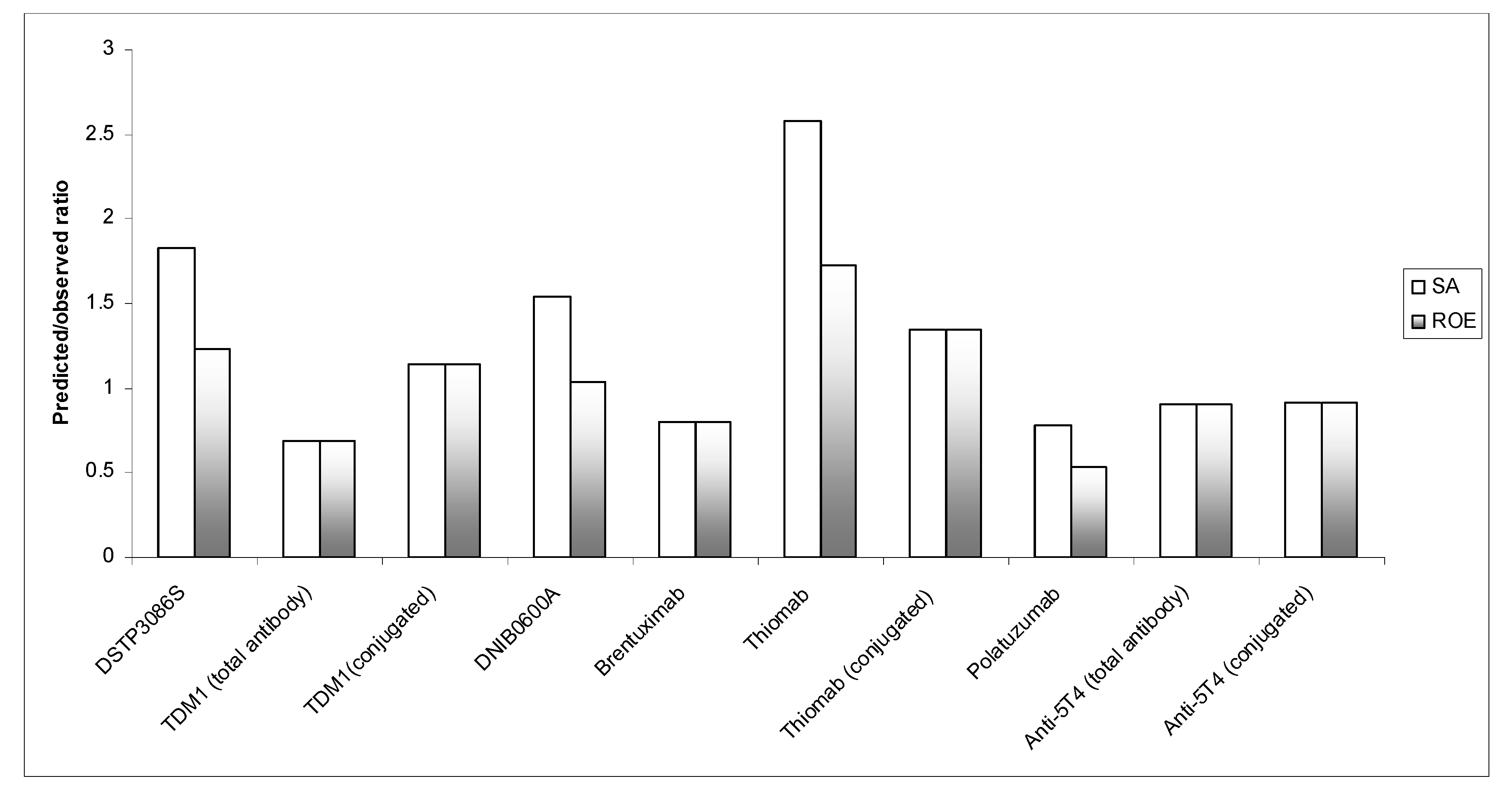
| Simple | 11.7 | 1.06 | 574 | 1052 | 1.83 |
| Brain weight | 155.7 | 2.06 | 574 | 709 | 1.23 |
| T-DM1 (total antibody) | |||||
| Simple | 5.37 | 0.89 | 343 | 236 | 0.69 |
| T-DM1 (conjugate) | |||||
| Simple | 14.3 | 0.91 | 600 | 683 | 1.14 |
| DNIB0600A (rat data were added to Li et al.’s original data) (total antibody) | |||||
| Simple | 13.7 | 1.07 | 854 | 1313 | 1.54 |
| Brain weight | 182.5 | 2.08 | 854 | 886 | 1.04 |
| Brentuximab vedotin (mouse data were added to Li et al.’s original data) (total antibody) | |||||
| Simple | 13 | 0.90 | 742 | 595 | 0.80 |
| Thiomab (total antibody) | |||||
| Simple | 6.5 | 1.03 | 200 | 517 | 2.58 |
| Brain weight | 86.9 | 2.03 | 200 | 345 | 1.73 |
| Thiomab (conjugate) | |||||
| Simple | 18.3 | 0.95 | 759 | 1027 | 1.35 |
| Polatuzumab vedotin (total antibody) (rat data were added to Li et al.’s original data) (total antibody) | |||||
| Simple | 8.91 | 1.06 | 1015 | 795 | 0.78 |
| Brain weight | 119 | 2.06 | 1015 | 537 | 0.53 |
| Anti-5T4 (total antibody) ** | |||||
| Simple | 9.9 | 0.82 | 360 | 323 | 0.90 |
| Anti-5T4 (conjugate) ** | |||||
| Simple | 17.3 | 0.85 | 700 | 640 | 0.91 |
| Drugs | Exponent | Observed | Predicted | Predicted |
|---|---|---|---|---|
| 3 species, SA | SA | MLP | ||
| Brentuximab vedotin | ||||
| Methods | 0.90 | 742 | 595 | 274 |
| Ratio | 0.80 | 0.37 | ||
| T-DM1 (total antibody) | ||||
| Methods | 0.89 | 343 | 236 | 111 |
| Ratio | 0.69 | 0.32 | ||
| T-DM1 (conjugate) | ||||
| Methods | 0.91 | 600 | 683 | 319 |
| Ratio | 1.14 | 0.53 | ||
| Thiomab (conjugate) | ||||
| Methods | 0.95 | 759 | 1027 | 478 |
| Ratio | 1.35 | 0.63 | ||
| Anti-5T4 (total antibody) | ||||
| Methods | 0.82 | 360 | 323 | 151 |
| Ratio | 0.90 | 0.42 | ||
| Anti-5T4 (conjugate) | ||||
| Methods | 0.85 | 700 | 640 | 288 |
| Ratio | 0.91 | 0.41 | ||
| Drugs | Coefficient | Exponent | Predicted | Observed | Ratio * |
|---|---|---|---|---|---|
| DNIB0600A | |||||
| Mouse, monkey | 12.1 | 1.08 | 1190 | 854 | 1.39 |
| Mouse, rat | 21.8 | 1.23 | 3981 | 854 | 4.66 |
| Rat, monkey | 14.5 | 0.93 | 753 | 854 | 0.88 |
| DMOT4039A | |||||
| Mouse, monkey | 21.3 | 1.21 | 3640 | 1400 | 2.60 |
| Polatuzumab vedotin | |||||
| Mouse, monkey | 6.6 | 1.04 | 543 | 1015 | 0.53 |
| Mouse, rat | 28.8 | 1.42 | 12,007 | 1015 | 11.83 |
| Rat, monkey | 10.3 | 0.68 | 185 | 1015 | 0.18 |
| Pinatuzumab vedotin | |||||
| Mouse, monkey | 8.46 | 1.08 | 832 | 966 | 0.86 |
| ADC1 | |||||
| Mouse, monkey | 9.38 | 1.09 | 962 | 756 | 1.27 |
| Brentuximab vedotin | |||||
| Mouse, monkey | 16.6 | 0.89 | 728 | 742 | 0.98 |
| Mouse, rat | 5.1 | 0.59 | 63 | 742 | 0.08 |
| Rat, monkey | 11.6 | 1.18 | 1745 | 742 | 2.35 |
| DSTP3086S | |||||
| Mouse, monkey | 12.4 | 1.09 | 1277 | 574 | 2.22 |
| Mouse, rat | 9.3 | 0.98 | 598 | 574 | 1.04 |
| Rat, monkey | 11.4 | 1.13 | 1386 | 574 | 2.41 |
| T-DM1 | |||||
| Mouse, monkey | 5.3 | 0.89 | 231 | 343 | 0.67 |
| Mouse, rat | 5.4 | 0.87 | 219 | 343 | 0.64 |
| Rat, monkey | 5.8 | 0.92 | 291 | 343 | 0.85 |
| Thiomab | |||||
| Mouse, monkey | 5.6 | 1.03 | 445 | 200 | 2.23 |
| Mouse, rat | 11.6 | 1.21 | 1982 | 200 | 9.91 |
| Rat, monkey | 7 | 0.85 | 259 | 200 | 1.30 |
| Anti-5T4 | |||||
| Mouse, monkey | 9.7 | 0.82 | 316 | 360 | 0.88 |
| Mouse, rat | 10.7 | 0.85 | 388 | 360 | 1.08 |
| Rat, monkey | 10 | 0.80 | 299 | 360 | 0.83 |
| Species | Predicted | Prediction Ratio | ||||||
|---|---|---|---|---|---|---|---|---|
| E 0.75 | E 0.80 | E 0.85 | E 1.0 | E 0.75 | E 0.80 | E 0.85 | E 1.0 | |
| DNIB0600A (observed human CL = 854 mL/day) | ||||||||
| Mouse | 82 | 123 | 185 | 630 | 0.10 | 0.14 | 0.22 | 0.74 |
| Rat | 272 | 361 | 479 | 1114 | 0.32 | 0.42 | 0.56 | 1.30 |
| Monkey | 440 | 511 | 594 | 931 | 0.52 | 0.60 | 0.70 | 1.09 |
| DMOT4039A (observed human CL = 1400 mL/day) | ||||||||
| Mouse | 86 | 130 | 196 | 665 | 0.06 | 0.09 | 0.14 | 0.48 |
| Monkey | 914 | 1061 | 1233 | 1932 | 0.65 | 0.76 | 0.88 | 1.38 |
| DSTP3086S (observed human CL = 574 mL/day) | ||||||||
| Mouse | 90 | 135 | 204 | 693 | 0.16 | 0.24 | 0.35 | 1.21 |
| Rat | 163 | 215 | 286 | 665 | 0.28 | 0.38 | 0.50 | 1.16 |
| Monkey | 444 | 515 | 598 | 938 | 0.77 | 0.90 | 1.04 | 1.63 |
| TDM1 (observed human CL = 343 mL/day) | ||||||||
| Mouse | 73 | 109 | 165 | 560 | 0.21 | 0.32 | 0.48 | 1.63 |
| Rat | 111 | 147 | 195 | 455 | 0.32 | 0.43 | 0.57 | 1.33 |
| Monkey | 152 | 177 | 205 | 322 | 0.44 | 0.52 | 0.60 | 0.94 |
| Polatuzumab vedotin (observed human CL = 1015 mL/day) | ||||||||
| Mouse | 46 | 70 | 105 | 356 | 0.05 | 0.07 | 0.10 | 0.35 |
| Rat | 185 | 245 | 325 | 756 | 0.18 | 0.24 | 0.32 | 0.74 |
| Monkey | 199 | 231 | 268 | 420 | 0.20 | 0.23 | 0.26 | 0.41 |
| Pinatuzumab vedotin (observed human CL = 966 mL/day) | ||||||||
| Mouse | 56 | 83 | 126 | 427 | 0.06 | 0.09 | 0.13 | 0.44 |
| Monkey | 311 | 361 | 420 | 658 | 0.32 | 0.37 | 0.43 | 0.68 |
| ADC1 (observed human CL = 756 mL/day) | ||||||||
| Mouse | 60 | 90 | 136 | 462 | 0.08 | 0.12 | 0.18 | 0.61 |
| Monkey | 348 | 404 | 469 | 735 | 0.46 | 0.53 | 0.62 | 0.97 |
| Brentuximab vedotin (observed human CL = 742 mL/day) | ||||||||
| Mouse | 55 | 82 | 123 | 420 | 0.07 | 0.11 | 0.17 | 0.57 |
| Rat | 154 | 204 | 271 | 630 | 0.21 | 0.28 | 0.36 | 0.85 |
| Monkey | 483 | 561 | 652 | 1022 | 0.65 | 0.76 | 0.88 | 1.38 |
| Thiomab (observed human CL = 200 mL/day) | ||||||||
| Mouse | 44 | 66 | 99 | 338 | 0.22 | 0.33 | 0.50 | 1.69 |
| Rat | 147 | 195 | 259 | 602 | 0.74 | 0.98 | 1.29 | 3.01 |
| Monkey | 193 | 224 | 260 | 407 | 0.96 | 1.12 | 1.30 | 2.04 |
| Anti-5T4 (observed human CL = 360 mL/day) | ||||||||
| Mouse | 177 | 266 | 400 | 1361 | 0.49 | 0.74 | 1.11 | 3.78 |
| Rat | 226 | 299 | 397 | 924 | 0.63 | 0.83 | 1.10 | 2.57 |
| Monkey | 256 | 297 | 345 | 541 | 0.71 | 0.83 | 0.96 | 1.50 |
| ADCs | Observed CL | Predicted CL (mL/day) | Predicted Ratio | ||
|---|---|---|---|---|---|
| (mL/Day) | Exponent 1.0 | Average * | Exponent 1.0 | Average * | |
| DNIB0600A | 854 | 630 | 726 | 0.74 | 0.85 |
| DMOT4039A | 1400 | 665 | 766 | 0.48 | 0.55 |
| DSTP3086S | 574 | 693 | 799 | 1.21 | 1.43 |
| TDM1 | 343 | 560 | 645 | 1.63 | 1.94 |
| Polatuzumab | 1015 | 356 | 411 | 0.35 | 0.42 |
| Pinatuzumab | 966 | 427 | 492 | 0.44 | 0.52 |
| ADC1 | 756 | 462 | 532 | 0.61 | 0.72 |
| Brentuximab | 742 | 420 | 484 | 0.57 | 0.67 |
| Thiomab | 200 | 338 | 390 | 1.69 | 1.99 |
| Anti-5T4 | 360 | 1361 | 1618 | 3.78 | 4.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmood, I. Interspecies Scaling of Antibody–Drug Conjugates (ADC) for the Prediction of Human Clearance. Antibodies 2021, 10, 1. https://doi.org/10.3390/antib10010001
Mahmood I. Interspecies Scaling of Antibody–Drug Conjugates (ADC) for the Prediction of Human Clearance. Antibodies. 2021; 10(1):1. https://doi.org/10.3390/antib10010001
Chicago/Turabian StyleMahmood, Iftekhar. 2021. "Interspecies Scaling of Antibody–Drug Conjugates (ADC) for the Prediction of Human Clearance" Antibodies 10, no. 1: 1. https://doi.org/10.3390/antib10010001
APA StyleMahmood, I. (2021). Interspecies Scaling of Antibody–Drug Conjugates (ADC) for the Prediction of Human Clearance. Antibodies, 10(1), 1. https://doi.org/10.3390/antib10010001




