Novel Anti-FOLR1 Antibody–Drug Conjugate MORAb-202 in Breast Cancer and Non-Small Cell Lung Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Reagents
2.2. Real-Time RT-PCR
2.3. Cell Proliferation Assay
2.4. Western Blot Analysis
2.5. RNA Interference
2.6. Transwell Co-Culture Assay
2.7. Flow Cytometric Analysis
2.8. Orthotopic Xenograft Mouse Studies
2.9. TP53 and PIK3CA Sequence Analysis
2.10. Statistical Analysis
3. Results
3.1. FOLR1 Expression Levels in Breast Cancer and NSCLC Cell Lines
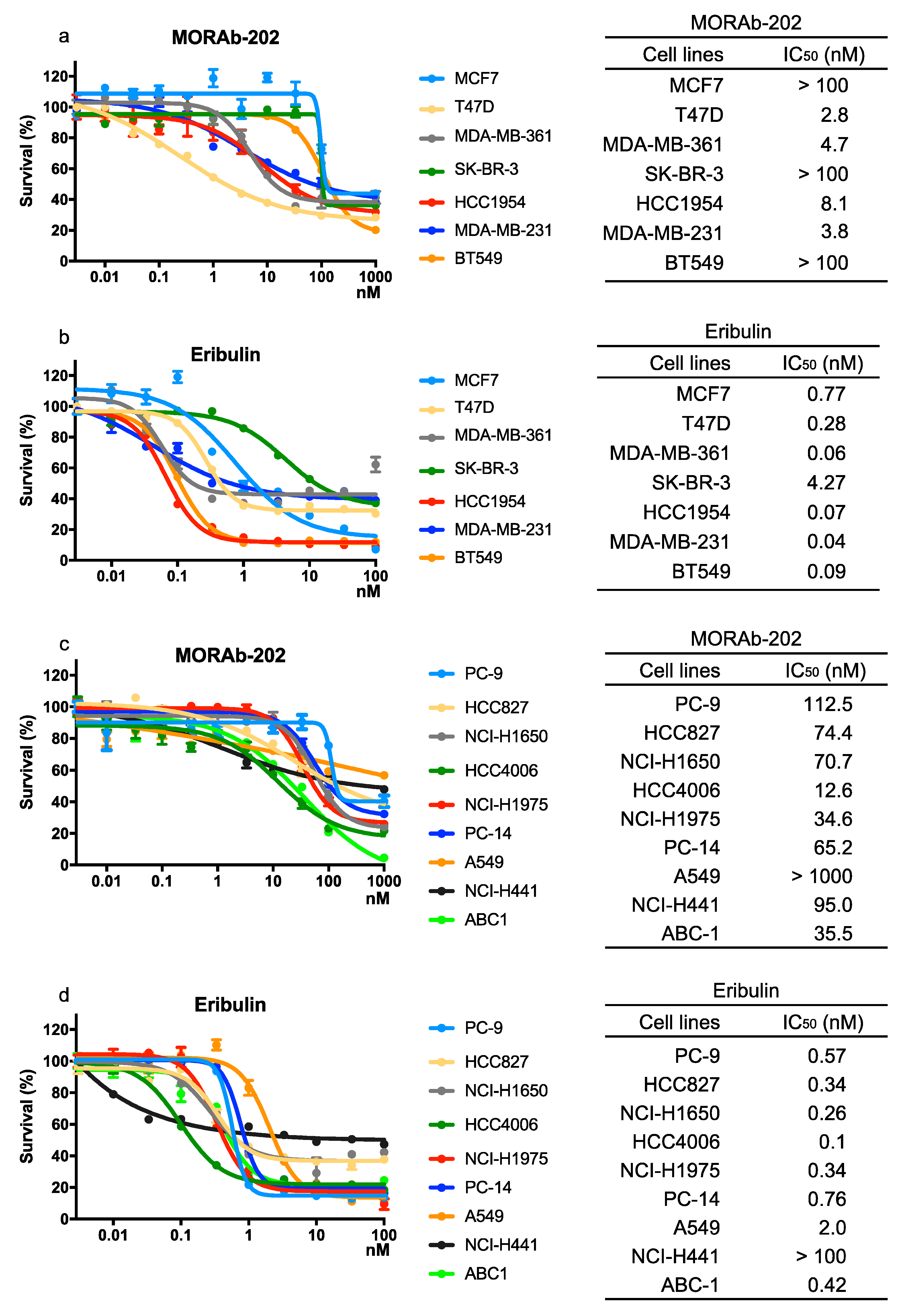
3.2. The Effect of MORAb-202 on Proliferation in Breast Cancer and NSCLC Cell Lines
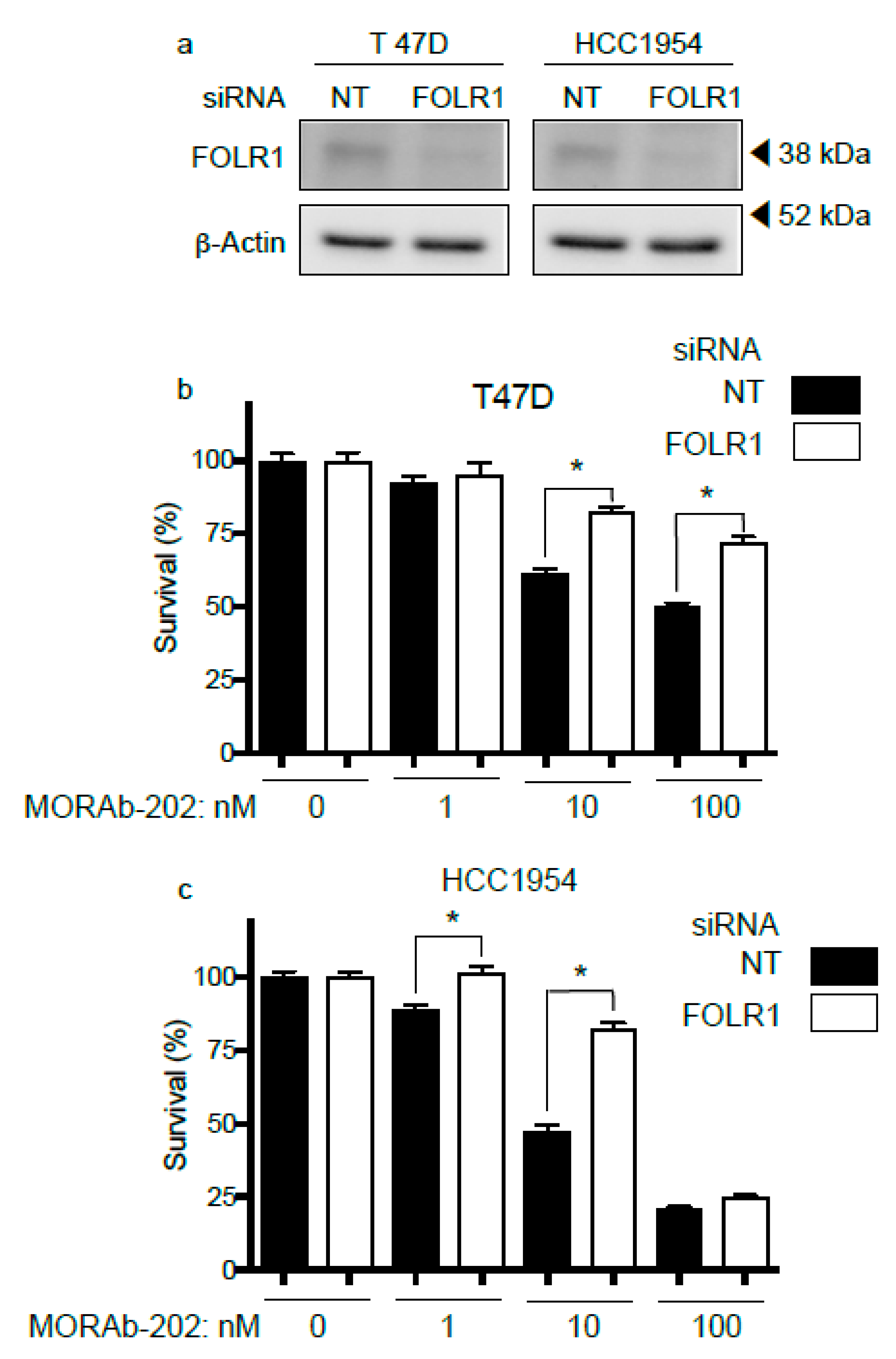
3.3. FOLR1 Attenuation Decreases the Effect of MORAb-202 on Cell Proliferation
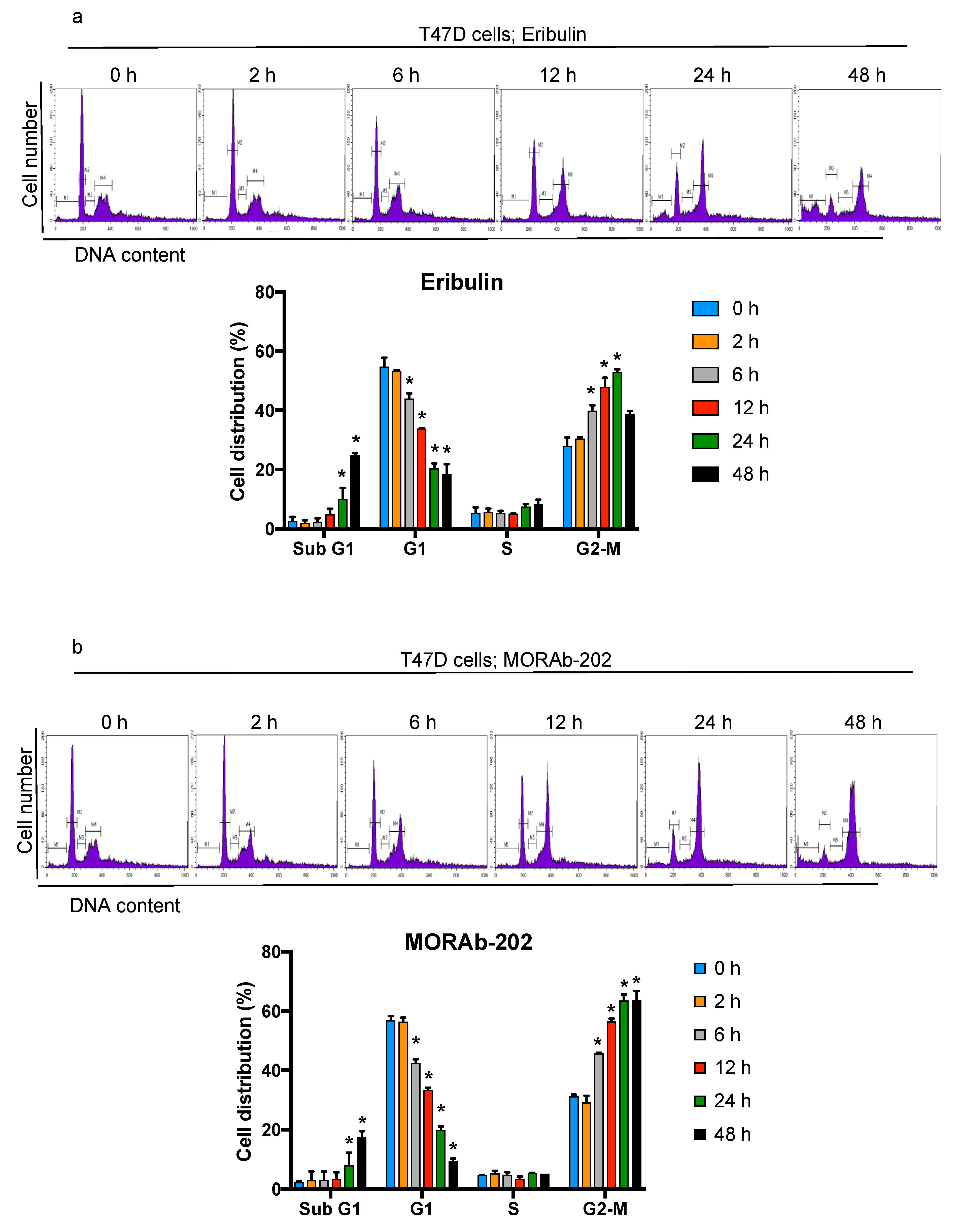
3.4. MORAb-202 Induced G2-M Phase Cell Cycle Arrest and Accumulation of Cells in the Sub-G1 Phase
3.5. MORAb-202 Inhibited Insensitive MCF7 Cell Proliferation in Co-Culture with HCC1954 Cells
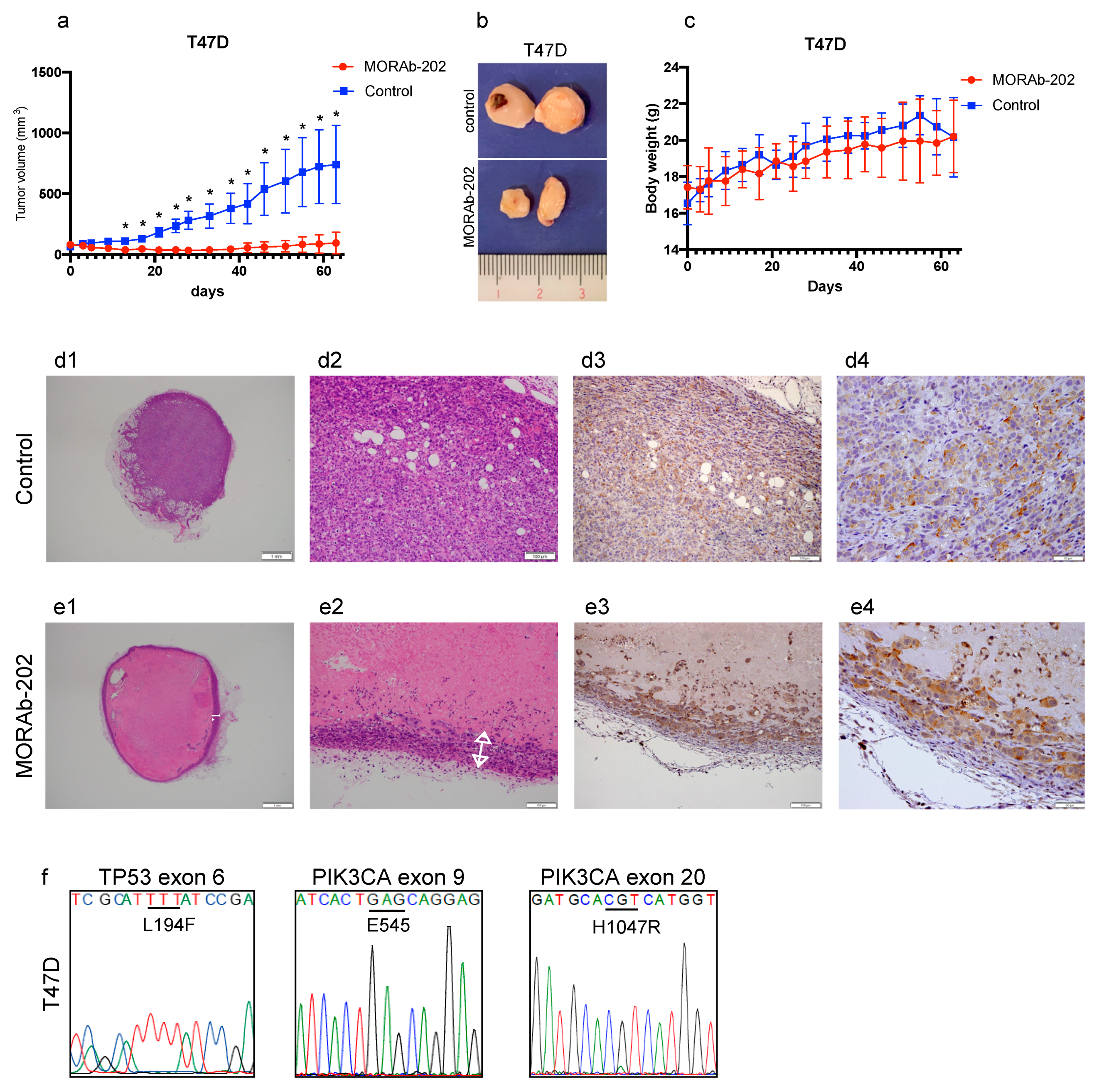
3.6. MORAb-202 Suppressed Orthotopic Xenograft Tumor Growth of T47D Cells, Which Are Sensitive to MORAb-202 In Vitro
3.7. MORAb-202 Suppressed Orthotopic Xenograft Tumor Growth of MCF7 Cells, Which Are Resistant to MORAb-202 In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carter, P.J.; Lazar, G.A. Next generation antibody drugs: Pursuit of the ‘high-hanging fruit’. Nat. Rev. Drug Discov. 2018, 17, 197–223. [Google Scholar] [CrossRef] [PubMed]
- Connors, J.M.; Jurczak, W.; Straus, D.J.; Ansell, S.M.; Kim, W.S.; Gallamini, A.; Younes, A.; Alekseev, S.; Illes, A.; Picardi, M.; et al. Brentuximab Vedotin with Chemotherapy for Stage III or IV Hodgkin’s Lymphoma. N. Engl. J. Med. 2018, 378, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N. Engl. J. Med. 2020, 382, 610–621. [Google Scholar] [CrossRef]
- Thomas, A.; Teicher, B.A.; Hassan, R. Antibody-drug conjugates for cancer therapy. Lancet Oncol. 2016, 17, e254–e262. [Google Scholar] [CrossRef]
- Cheng, X.; Li, J.; Tanaka, K.; Majumder, U.; Milinichik, A.Z.; Verdi, A.C.; Maddage, C.J.; Rybinski, K.A.; Fernando, S.; Fernando, D.; et al. MORAb-202, an Antibody-Drug Conjugate Utilizing Humanized Anti-human FRalpha Farletuzumab and the Microtubule-targeting Agent Eribulin, has Potent Antitumor Activity. Mol. Cancer Ther. 2018, 17, 2665–2675. [Google Scholar] [CrossRef]
- Kelemen, L.E. The role of folate receptor alpha in cancer development, progression and treatment: Cause, consequence or innocent bystander? Int. J. Cancer 2006, 119, 243–250. [Google Scholar] [CrossRef]
- Salazar, M.D.; Ratnam, M. The folate receptor: What does it promise in tissue-targeted therapeutics? Cancer Metastasis Rev. 2007, 26, 141–152. [Google Scholar] [CrossRef]
- Toffoli, G.; Cernigoi, C.; Russo, A.; Gallo, A.; Bagnoli, M.; Boiocchi, M. Overexpression of folate binding protein in ovarian cancers. Int. J. Cancer 1997, 74, 193–198. [Google Scholar] [CrossRef]
- Shi, H.; Guo, J.; Li, C.; Wang, Z. A current review of folate receptor alpha as a potential tumor target in non-small-cell lung cancer. Drug Des. Devel Ther. 2015, 9, 4989–4996. [Google Scholar] [CrossRef]
- Chen, Y.L.; Chang, M.C.; Huang, C.Y.; Chiang, Y.C.; Lin, H.W.; Chen, C.A.; Hsieh, C.Y.; Cheng, W.F. Serous ovarian carcinoma patients with high alpha-folate receptor had reducing survival and cytotoxic chemo-response. Mol. Oncol. 2012, 6, 360–369. [Google Scholar] [CrossRef]
- Konner, J.A.; Bell-McGuinn, K.M.; Sabbatini, P.; Hensley, M.L.; Tew, W.P.; Pandit-Taskar, N.; Els, N.V.; Phillips, M.D.; Schweizer, C.; Weil, S.C.; et al. Farletuzumab, a humanized monoclonal antibody against folate receptor alpha, in epithelial ovarian cancer: A phase I study. Clin. Cancer Res. 2010, 16, 5288–5295. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.K.; White, A.J.; Weil, S.C.; Phillips, M.; Coleman, R.L. Farletuzumab (a monoclonal antibody against folate receptor alpha) in relapsed platinum-sensitive ovarian cancer. Gynecol. Oncol. 2013, 129, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; Armstrong, D.; Scambia, G.; Teneriello, M.; Sehouli, J.; Schweizer, C.; Weil, S.C.; Bamias, A.; Fujiwara, K.; Ochiai, K.; et al. A Randomized, Double-Blind, Placebo-Controlled, Phase III Study to Assess Efficacy and Safety of Weekly Farletuzumab in Combination With Carboplatin and Taxane in Patients With Ovarian Cancer in First Platinum-Sensitive Relapse. J. Clin. Oncol. 2016, 34, 2271–2278. [Google Scholar] [CrossRef] [PubMed]
- Smith-Jones, P.M.; Pandit-Taskar, N.; Cao, W.; O’Donoghue, J.; Philips, M.D.; Carrasquillo, J.; Konner, J.A.; Old, L.J.; Larson, S.M. Preclinical radioimmunotargeting of folate receptor alpha using the monoclonal antibody conjugate DOTA-MORAb-003. Nucl. Med. Biol. 2008, 35, 343–351. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bai, R.L.; Paull, K.D.; Herald, C.L.; Malspeis, L.; Pettit, G.R.; Hamel, E. Halichondrin B and homohalichondrin B, marine natural products binding in the vinca domain of tubulin. Discovery of tubulin-based mechanism of action by analysis of differential cytotoxicity data. J. Biol. Chem. 1991, 266, 15882–15889. [Google Scholar] [CrossRef]
- Kuznetsov, G.; Towle, M.J.; Cheng, H.; Kawamura, T.; TenDyke, K.; Liu, D.; Kishi, Y.; Yu, M.J.; Littlefield, B.A. Induction of morphological and biochemical apoptosis following prolonged mitotic blockage by halichondrin B macrocyclic ketone analog E7389. Cancer Res. 2004, 64, 5760–5766. [Google Scholar] [CrossRef] [PubMed]
- Chanez, B.; Goncalves, A.; Badache, A.; Verdier-Pinard, P. Eribulin targets a ch-TOG-dependent directed migration of cancer cells. Oncotarget 2015, 6, 41667–41678. [Google Scholar] [CrossRef]
- Ueda, S.; Saeki, T.; Takeuchi, H.; Shigekawa, T.; Yamane, T.; Kuji, I.; Osaki, A. In vivo imaging of eribulin-induced reoxygenation in advanced breast cancer patients: A comparison to bevacizumab. Br. J. Cancer 2016, 114, 1212–1218. [Google Scholar] [CrossRef]
- Yoshida, T.; Ozawa, Y.; Kimura, T.; Sato, Y.; Kuznetsov, G.; Xu, S.; Uesugi, M.; Agoulnik, S.; Taylor, N.; Funahashi, Y.; et al. Eribulin mesilate suppresses experimental metastasis of breast cancer cells by reversing phenotype from epithelial-mesenchymal transition (EMT) to mesenchymal-epithelial transition (MET) states. Br. J. Cancer 2014, 110, 1497–1505. [Google Scholar] [CrossRef]
- Jain, S.; Vahdat, L.T. Eribulin mesylate. Clin. Cancer Res. 2011, 17, 6615–6622. [Google Scholar] [CrossRef]
- Benbow, S.J.; Wozniak, K.M.; Kulesh, B.; Savage, A.; Slusher, B.S.; Littlefield, B.A.; Jordan, M.A.; Wilson, L.; Feinstein, S.C. Microtubule-Targeting Agents Eribulin and Paclitaxel Differentially Affect Neuronal Cell Bodies in Chemotherapy-Induced Peripheral Neuropathy. Neurotox. Res. 2017, 32, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; O’Shaughnessy, J.; Loesch, D.; Blum, J.L.; Vahdat, L.T.; Petrakova, K.; Chollet, P.; Manikas, A.; Dieras, V.; Delozier, T.; et al. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): A phase 3 open-label randomised study. Lancet 2011, 377, 914–923. [Google Scholar] [CrossRef]
- Twelves, C.; Cortes, J.; Vahdat, L.; Olivo, M.; He, Y.; Kaufman, P.A.; Awada, A. Efficacy of eribulin in women with metastatic breast cancer: A pooled analysis of two phase 3 studies. Breast Cancer Res. Treat. 2014, 148, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Bryden, F.; Martin, C.; Letast, S.; Lles, E.; Vieitez-Villemin, I.; Rousseau, A.; Colas, C.; Brachet-Botineau, M.; Allard-Vannier, E.; Larbouret, C.; et al. Impact of cathepsin B-sensitive triggers and hydrophilic linkers on in vitro efficacy of novel site-specific antibody-drug conjugates. Org. Biomol. Chem. 2018, 16, 1882–1889. [Google Scholar] [CrossRef] [PubMed]
- Golfier, S.; Kopitz, C.; Kahnert, A.; Heisler, I.; Schatz, C.A.; Stelte-Ludwig, B.; Mayer-Bartschmid, A.; Unterschemmann, K.; Bruder, S.; Linden, L.; et al. Anetumab ravtansine: A novel mesothelin-targeting antibody-drug conjugate cures tumors with heterogeneous target expression favored by bystander effect. Mol. Cancer Ther. 2014, 13, 1537–1548. [Google Scholar] [CrossRef] [PubMed]
- Ratnam, M.; Marquardt, H.; Duhring, J.L.; Freisheim, J.H. Homologous membrane folate binding proteins in human placenta: Cloning and sequence of a cDNA. Biochemistry 1989, 28, 8249–8254. [Google Scholar] [CrossRef]
- Yan, W.; Ratnam, M. Preferred sites of glycosylphosphatidylinositol modification in folate receptors and constraints in the primary structure of the hydrophobic portion of the signal. Biochemistry 1995, 34, 14594–14600. [Google Scholar] [CrossRef]
- Shen, F.; Wang, H.; Zheng, X.; Ratnam, M. Expression levels of functional folate receptors alpha and beta are related to the number of N-glycosylated sites. Biochem. J. 1997, 327 Pt 3, 759–764. [Google Scholar] [CrossRef]
- Krypuy, M.; Ahmed, A.A.; Etemadmoghadam, D.; Hyland, S.J.; Australian Ovarian Cancer Study Group; DeFazio, A.; Fox, S.B.; Brenton, J.D.; Bowtell, D.D.; Dobrovic, A. High resolution melting for mutation scanning of TP53 exons 5–8. BMC Cancer 2007, 7, 168. [Google Scholar] [CrossRef]
- Wu, G.; Xing, M.; Mambo, E.; Huang, X.; Liu, J.; Guo, Z.; Chatterjee, A.; Goldenberg, D.; Gollin, S.M.; Sukumar, S.; et al. Somatic mutation and gain of copy number of PIK3CA in human breast cancer. Breast Cancer Res. 2005, 7, R609. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Huang, C.S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Tsurutani, J.; Takahashi, S.; Iwata, H.; Krop, I.E.; Redfern, C.; Sagara, Y.; Doi, T.; Park, H.; Murthy, R.K.; et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: A dose-expansion, phase 1 study. Lancet Oncol. 2019, 20, 816–826. [Google Scholar] [CrossRef]
- Necela, B.M.; Crozier, J.A.; Andorfer, C.A.; Lewis-Tuffin, L.; Kachergus, J.M.; Geiger, X.J.; Kalari, K.R.; Serie, D.J.; Sun, Z.; Moreno-Aspitia, A.; et al. Folate receptor-alpha (FOLR1) expression and function in triple negative tumors. PLoS ONE 2015, 10, e0122209. [Google Scholar] [CrossRef]
- Sadasivan, E.; Regec, A.; Rothenberg, S.P. The half-life of the transcript encoding the folate receptor alpha in KB cells is reduced by cytosolic proteins expressed in folate-replete and not in folate-depleted cells. Gene 2002, 291, 149–158. [Google Scholar] [CrossRef]
- Jansen, G.; Kathmann, I.; Rademaker, B.C.; Braakhuis, B.J.; Westerhof, G.R.; Rijksen, G.; Schornagel, J.H. Expression of a folate binding protein in L1210 cells grown in low folate medium. Cancer Res. 1989, 49, 1959–1963. [Google Scholar] [PubMed]
- Antony, A.; Tang, Y.S.; Khan, R.A.; Biju, M.P.; Xiao, X.; Li, Q.J.; Sun, X.L.; Jayaram, H.N.; Stabler, S.P. Translational upregulation of folate receptors is mediated by homocysteine via RNA-heterogeneous nuclear ribonucleoprotein E1 interactions. J. Clin. Investig. 2004, 113, 285–301. [Google Scholar] [CrossRef]
- Kelemen, L.E.; Sellers, T.A.; Keeney, G.L.; Lingle, W.L. Multivitamin and alcohol intake and folate receptor alpha expression in ovarian cancer. Cancer Epidemiol. Prev. Biomark. 2005, 14, 2168–2172. [Google Scholar] [CrossRef]
- Towle, M.J.; Salvato, K.A.; Budrow, J.; Wels, B.F.; Kuznetsov, G.; Aalfs, K.K.; Welsh, S.; Zheng, W.; Seletsky, B.M.; Palme, M.H.; et al. In vitro and in vivo anticancer activities of synthetic macrocyclic ketone analogues of halichondrin B. Cancer Res. 2001, 61, 1013–1021. [Google Scholar]
- Ogitani, Y.; Hagihara, K.; Oitate, M.; Naito, H.; Agatsuma, T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016, 107, 1039–1046. [Google Scholar] [CrossRef]
- Tong, C.W.S.; Wu, W.K.K.; Loong, H.H.F.; Cho, W.C.S.; To, K.K.W. Drug combination approach to overcome resistance to EGFR tyrosine kinase inhibitors in lung cancer. Cancer Lett. 2017, 405, 100–110. [Google Scholar] [CrossRef]
- Li, B.T.; Michelini, F.; Misale, S.; Cocco, E.; Baldino, L.; Cai, Y.; Shifman, S.; Tu, H.Y.; Myers, M.L.; Xu, C.; et al. HER2-Mediated Internalization of Cytotoxic Agents in ERBB2 Amplified or Mutant Lung Cancers. Cancer Discov. 2020, 10, 674–687. [Google Scholar] [CrossRef] [PubMed]
- Tsurutani, J.; Iwata, H.; Krop, I.; Janne, P.A.; Doi, T.; Takahashi, S.; Park, H.; Redfern, C.; Tamura, K.; Wise-Draper, T.M.; et al. Targeting HER2 with Trastuzumab Deruxtecan: A Dose-Expansion, Phase I Study in Multiple Advanced Solid Tumors. Cancer Discov. 2020, 10, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.A.P.; Oliveira, A.; Silva, R.C.; Lima, R.R.M.; Souto, F.O.; Baratti, M.O.; Carvalho, H.F.; Santos, B.S.; Filho, P.E.C.; Fontes, A. Evaluating internalization and recycling of folate receptors in breast cancer cells using quantum dots. J. Photochem. Photobiol. B 2020, 209, 111918. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsunaga, Y.; Yamaoka, T.; Ohba, M.; Miura, S.; Masuda, H.; Sangai, T.; Takimoto, M.; Nakamura, S.; Tsurutani, J. Novel Anti-FOLR1 Antibody–Drug Conjugate MORAb-202 in Breast Cancer and Non-Small Cell Lung Cancer Cells. Antibodies 2021, 10, 6. https://doi.org/10.3390/antib10010006
Matsunaga Y, Yamaoka T, Ohba M, Miura S, Masuda H, Sangai T, Takimoto M, Nakamura S, Tsurutani J. Novel Anti-FOLR1 Antibody–Drug Conjugate MORAb-202 in Breast Cancer and Non-Small Cell Lung Cancer Cells. Antibodies. 2021; 10(1):6. https://doi.org/10.3390/antib10010006
Chicago/Turabian StyleMatsunaga, Yuki, Toshimitsu Yamaoka, Motoi Ohba, Sakiko Miura, Hiroko Masuda, Takafumi Sangai, Masafumi Takimoto, Seigo Nakamura, and Junji Tsurutani. 2021. "Novel Anti-FOLR1 Antibody–Drug Conjugate MORAb-202 in Breast Cancer and Non-Small Cell Lung Cancer Cells" Antibodies 10, no. 1: 6. https://doi.org/10.3390/antib10010006
APA StyleMatsunaga, Y., Yamaoka, T., Ohba, M., Miura, S., Masuda, H., Sangai, T., Takimoto, M., Nakamura, S., & Tsurutani, J. (2021). Novel Anti-FOLR1 Antibody–Drug Conjugate MORAb-202 in Breast Cancer and Non-Small Cell Lung Cancer Cells. Antibodies, 10(1), 6. https://doi.org/10.3390/antib10010006






