Abstract
Immunotherapy has emerged as an alternative strategy to treat malignancies in addition to conventional radio- and chemotherapy. There has been a plethora of evidence that the immune system is able to control tumor outgrowth and a number of strategies have been put forward to utilize this ability for immunotherapy. However, some of these strategies have not been very efficient and their success has been limited by tumor evasion mechanisms. A promising approach to engage effector cells of the immune system overcoming some of the escape mechanisms has been introduced more than two decades ago. This approach is based on bispecific antibodies. Here we summarize the evolution of bispecific antibodies, their improvement, remaining obstacles and some controversial reports.
1. Introduction
The idea of immunotherapy is based on the premise that the immune system can recognize and eradicate malignant cells. The concept of tumor immunosurveillance was introduced last century [1,2,3] and for over 50 years has been an object of controversy [4,5]. However in the past two decades, due to the advances in mouse genetics, data collected in many laboratories [6,7,8,9,10,11] have shown that in mice deficient in key immunologic molecules, the development of both chemically induced and spontaneous tumors is enhanced, and thus demonstrated the ability of the immune system to control outgrowth of malignancies [12,13].
The concept of immunosurveillance has also been supported by a number of clinical observations in humans, such as cases of spontaneous tumor regression [14,15], the increased risk of tumor development in immunosuppressed patients [16,17], as well as improved prognosis related to the presence of tumor reactive T and B cells [18,19,20].
The discovery in 2001 that the immune system controls not only tumor growth but also shapes its immunogenicity [4,11] prompted a major revision of the cancer immunosurveillance hypothesis [21].
Today immunosurveillance is considered the stage of a long-lasting complex interaction between the immune system and the tumor, termed cancer immunoediting [12,22,23], in which molecules and cells of both innate and adaptive immunity work together to detect and eradicate the malignancy before the tumor becomes eventually clinically apparent [13].
There are a number of mechanisms involved in the alerting of the immune system to the presence of a growing tumor early during cancer development, namely damage-associated molecular pattern molecules (DAMPs) [24,25], released either directly from the dying tumor cells or damaged tissues ingrown by invasive tumors. DAMPs can be detected by different receptor types inducing a type I interferon answer [26,27]. Another mechanism involves stress induced ligands (MIC A/B, ULBPs, etc.) expressed on the surface of the malignant cells, which can bind to activating receptors on NK cells. NK cells play an important role in tumor eradication and release of proinflammatory cytokines, which in turn contribute to induction of adaptive anti-tumor immune responses [21,28]. All these mechanisms can lead to activation of dendritic cells and the induction of an adaptive immune response. In order for the adaptive immune system to react against a tumor, the latter must express antigens that are either specific or at least over expressed in the tumor and are termed tumor-associated antigens (TAAs). The presentation of TAA derived peptides can promote the generation of TAA-specific tumor-reactive effector CD4+ and CD8+ T cells (21).
Activated TAA-specific T cells play a major role in the control of tumor growth either by differentiating into cytotoxic CD8+ T lymphocytes (CTL), which can recognize and directly kill tumor cells presenting peptides of the corresponding TAA via MHC (Major histocompatibility complex) class I molecules, or by becoming cytokine (i.e., IFN-γ, IL-2) secreting CD4+ helper T cells which can stimulate the activity of CTLs, macrophages, induce an antibody response etc. [29,30], or themselves can contribute to the eradication of the malignancy [31,32].
The important role which T and NK cells play in immunosurveillance prompted the realization of the potential of these cells in immunotherapy. In recent decades various therapeutic approaches have been developed to utilize T and NK cells’ ability to control tumor growth, such as vaccination and adoptive transfer of autologous ex vivo expanded or genetically modified T and NK cells [33,34,35,36,37,38,39,40,41,42]. Unfortunately, the therapeutic effects were limited. The low response rates might be explained by the various mechanisms utilized by the aberrant cells to evade immune recognition or to inhibit the immune response, including downregulation of MHC molecules or downmodulation of proteins involved in the antigen processing and presentation machinery [43], diminished expression or shedding of ligands for activating NK cell receptors, or the presence of immunosuppressive molecules, such as TGF-β, IL-10, FasL, PD-L1/B7-H1, or IDO [44,45,46] in the tumor microenvironment.
A promising way to bypass certain evasion mechanisms and to utilize efficiently the potential of the effector mechanisms of the immune system in immunotherapy could be to target and destroy tumor cells with monoclonal antibodies (mabs) or antibody based constructs against TAAs expressed on the surface of the malignant cells.
2. Monoclonal Antibody Based Therapy
Mabs are considered the ‘magic bullets’ in cancer immunotherapy due to their high specificity and ability to target the aberrant cell in a very selective manner.
There are a number of mechanisms used by abs to trigger tumor cell death. They can block ligand-receptor interactions involved in growth and survival pathways. In addition mabs can invoke innate immune effector mechanisms via their Fc portion either by engaging the soluble factors of the complement to trigger complement-mediated cytotoxicity (CMC) or by ligating activating Fc receptors on the surface of NK cells, macrophages and dendritic cells [47], resulting in antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP) [48,49,50].
The development of the hybridoma technique in 1975 [51], allowing the relatively easy production of murine mabs specific for a wide variety of targets, enabled the exploration of their therapeutic potential. But two decades of advances in immunology and molecular biology were needed to overcome the major limitations of murine mabs, such as inefficient effector functions, high immunogenicity, and short half-lives, by using genetic engineering to generate chimeric [52], humanized [53] or fully human abs [54,55], and to reach their true potential [56,57]. In order to further improve the antibody treatment efficiency other approaches, such as conjugating mabs with toxins, cytotoxic drugs or radioisotopes [58,59] have also been utilized.
Until today a series of mabs were developed for cancer therapy [60] targeting various tumor targets such as CD20 [61,62], CD33 [63], human epidermal growth factor receptor 2 (Her2/neu) [64,65], CD52 [66], vascular endothelial growth factor (VEGF) [67] and epidermal growth factor receptor (EGFR) [68,69]. Unfortunately, even though they have shown significant clinical results, especially in hematological malignancies, none of them were able to treat cancer as single agent [70].
A lot of major limitations are associated with the application of mabs for cancer therapy, which were highlighted by several clinical and animal studies. One of these limitations is the size of the mabs. Although their molecular weight of 150 kDa improves their pharmacokinetic properties, in the case of solid tumors it decreases the penetration and the retention of the therapeutic antibody in the malignant tissues [71] thus reducing the efficiency of the treatment.
Other limitations of mabs are based on their mode of action. Abs used for interfering with the survival and growth of the cancer cells might block redundant pathways, thus having poor effect on the death of the aberrant cells [70]. The efficiency of abs relying on triggering the innate immune mechanisms via their Fc region can be hindered by suboptimal interaction of Fc part of the mab with the Fc receptors of the immune effector cells, due to alternative glycosylation of the Fc fragment [72] or by the competition with the circulating IgGs [73]. Fc receptor polymorphism can also negatively affect the clinical outcome of the antibody therapy [74,75], as can the ligation of inhibitory Fc receptor [76] expressed on B-cells, neutrophils, macrophages and dendritic cells, which negatively regulates effector functions [57]. Moreover, mabs cannot recruit cytotoxic T cells, due to their lack of Fc receptors, thus omitting one of the most potent effector mechanisms of the immune system. Using toxins and radioisotopes conjugated to mabs indeed overcomes some of these limitations and enhances the efficiency of the therapy, but they also carry a significant drawback associated with high toxicity to the healthy tissues and hence to the patients [59,77,78].
Already in the 1980s it was hypothesized [79] that bispecific antibody molecules that can recruit selectively an effector mechanism to a defined cancer target can overcome the major shortcomings of mabs, while taking advantage of their specificity [70,80].
Such a bispecific antibody (bsAb) can bind simultaneously a tumor antigen on the target cell and an activating receptor on the effector cell, triggering efficient effector cell activation and resulting in the eradication of the malignant cell. The activating receptor of choice on the surface of T cells is the CD3 complex, due to its expression on all T cells and its ability to provide strong triggering mechanisms. Nevertheless, bsAbs targeting other effector cells, such as NK cells, macrophages, and neutrophils have also been developed, using the respective Fc receptors (FcγRIII, FcγRI, FcαR) as trigger molecules [81,82]. The targets on the aberrant cells are generally selected among TAAs of hematological malignancies, such as CD20, CD19, CD33 and CD30, or of different solid tumors, including carcinoembryonic antigen (CEA), prostate stem cell antigen (PSCA), prostate specific membrane antigen (PSMA), epithelial cell adhesion molecule (EpCAM), EGFR and Her2/neu [83,84,85,86,87,88,89,90,91,92].
Even though the idea of bsAbs and the used target antigens for both effector and tumor cells remained relatively constant over the years, their format has undergone significant evolution (Figure 1) driven by the advances in technology and influenced by the requirements for efficient clinical outcome.
3. Evolution of Bispecific Antibodies
The earliest bsAbs were generated either by chemical cross-linking of whole antibodies or parts of them (e.g., Fab fragments), or by fusion of two hybridomas resulting in hybrid hybridoma (quadroma), secreting bispecific IgG molecules [93]. The limited efficiency of most of the first generation of bsAbs [94] was attributed to two major drawbacks of these molecules: The first was connected with the production approach. It was difficult to generate large, homogeneous batches of a well-defined and clinically useful product, due to the random combination of two mabs in chemical cross-linking, or the random association of two different heavy and two different light chains within one cell, in the case of quadroma technique, which resulted in a mixture of functional and non-functional molecules. The second limitation was connected with the reduced efficacy of the murine fragments, resulting from the induction of human anti-mouse antibody (HAMA) responses against the murine bsAbs. Moreover some of the molecules triggered Fc-mediated side effects, such as cytokine release syndrome, thrombocytopenia and leukopenia. Therefore, the maximal applicable dose of bsAbs was limited and the possibility of multiple administrations was excluded [80].
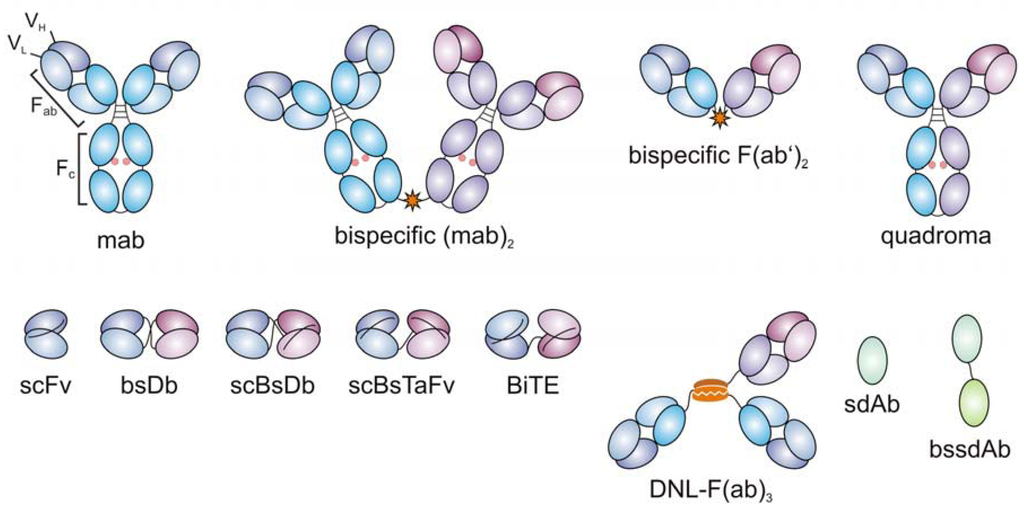
Figure 1.
Evolution of bispecific antibodies. First bispecific antibodies were developed by chemical cross-linking of monoclonal antibodies (mabs) or of Fab fragments, or by quadroma technology (upper row). Recombinant antibody engineering allowed for the generation of small recombinant bispecific antibodies comprising the variable heavy (VH) and light (VL) domains of the parental mabs (lower row). scFv: single-chain fragment variable; bsDb: bispecific diabody; scBsDb: single-chain bispecific diabody; scBsTaFv: single-chain bispecific tandem variable domain; DNL-(Fab)3: dock-and-lock trivalent Fab; sdAb: single-domain antibody; bssdAb: bispecific single-domain antibody (only formats discussed in this manuscript are included, additional formats reviewed in [81]).
These observations prompted the need to set a number of requirements for clinically useful bsAbs [95]. BsAbs should possess high affinity and selectivity for the TAA. They should be non-immunogenic, and should have a defined structure. In addition, bsAbs should bind monovalently to the effector cells to avoid inappropriate activation in the absence of the target cells. Moreover they should not contain an Fc-region in order to prevent Fc-mediated side effects, and their size should allow efficient penetration into tumor tissues, without affecting the pharmacokinetic properties in a way limiting the therapeutic effects.
In the nineties, advances in antibody engineering provided novel approaches of design and development of recombinant antibody constructs which can overcome the drawbacks of the bsAbs produced by chemical cross-linking or the quadroma technique, as well as fulfill the above mentioned requirements. Since then, a wide variety of different recombinant bsAb formats were developed [96]. One of them is the bispecific diabody (bsDb) format. BsDbs are produced from two different single chain fragment variable (scFv) fragments, comprising the heavy variable domain of one and the light variable domain of the other paternal mab. In these scFvs the polypeptide linker connecting the variable domains is reduced to about five amino acid residues [97], thus forcing the crossover pairing of the two scFv polypeptide chains. Even though such bsDbs can be produced with high yield in bacteria, significant drawbacks of this approach are their reduced stability and the presence of inactive homodimers along with the functional heterodimers [98]. In part these problems were overcome by introducing artificial cysteine residues that can be oxidized leading to stable disulfide bridges between the two scFvs in a diabody. A more promising format was developed by fusing the two antibody domains resulting in single-chain bsAbs. In general, such single-chain bsAbs consist of two variable heavy and two variable light chains which can be rearranged in many different ways with respect to the order of the variable domains and, in addition to the size and sequence of the linker elements in between the antibody domains. In the single-chain bispecific diabody (scBsDb) format, one of the binding moieties, in the form of a scFv, is inserted into the linker between the variable heavy- and light-chain portions of the other scFv [90,99,100]. Alternatively, the two different scFvs can be arranged in a row, by fusing one to the C-terminus of the other, with the help of a polypeptide linker [101], forming a single-chain bispecific tandem fragment variable (scBsTaFv). In this case, the two scFvs present in the scBsTaFv form separate folding entities. Different linkers varying in the length and complexity can be used to connect the two scFv fragments, as long as they do not interfere with the proper folding and the functionality of the resulting molecule [102,103,104]. Another approach for the generation of bispecific and trivalent molecules was recently developed. The so called dock-and-lock (DNL) method is based on homo- and heterodimerization of the dimerization and docking domain (DDD) of human cAMP-dependent protein kinase A and the anchoring domain (AD) from A-kinase anchor protein (AKAP). When a Fab fragment recognizing the first antigen is fused to the AD and the Fab fragment specific for the second antigen is attached to DDD (forming homodimers in the cell), the DDD dimer spontaneously associates with the AD. Upon association the covalent complex which is stable for more than a week at 37 °C in human serum is created due to the formation of two disulfide bonds [105].
In the last few years an additional format of recombinant antibodies–single-domain antibodies (sdAbs)–was established, by eliminating one of the partner domains from a variable fragment (Fv) [106]. These can be generated by selecting individual recombinant variable domains either cloned from spleen of immunized mice [107], or identified by screening human phage-display libraries. Furthermore, a source of sdAbs can be the naturally occurring heavy chain Abs (hcAbs), which can be found in the serum of camelids, lacking the first constant domain of the heavy chain and the complete light chain [108]. The variable heavy domain of these hcAbs (VHH) can be subjected to humanization and thus used for the development of so called nanobodies (Nb) [109]. In addition, single domains can be selected by exploring various protein scaffolds, which have been established as antibody mimetics [110,111]. Due to their simple structure sdAbs and antibody mimetics are very easy to manipulate, engineer and produce. It is even possible to fuse two single domains to generate bispecific molecules [112]. However, there are also certain drawbacks associated with these molecules. Their small size (molecular weight often below 20 kDa) can hinder their therapeutic efficiency, since they are rapidly cleared from the circulation.
4. Recombinant Bispecific Antibodies for Targeted Tumor Therapy
Various formats of recombinant bispecific antibodies have been introduced and there are a number of studies, which demonstrated their efficiency in targeting malignancies in preclinical and clinical settings [87]. There have been several reports showing potent anti-tumor response of bsDbs and scBsDbs, such as CD19xCD3 and CD19xCD16 bsDbs, displaying synergistic effect in the eradication of aberrant cells in non-Hodgkin’s lymphoma [113], or EGFRxCD3 Db, which efficiently eliminated tumors in xenografted mice retargeting lymphokine activated killer cells [114], or PSMAxCD3 bsDb, used for the treatment of xenografted mice bearing prostate cancer cells [115], as well as scBsDb CD3xPSCA also targeting prostate cancer [90] and many others [81]. So far, no bispecific ab in a bsDb or scBsDb format has been put forward into clinical trials [70], even though they have shown great potential as therapeutic compounds.
The other major format of recombinant single-chain bispecific constructs has also been extensively studied, namely the single-chain tandem antibodies. There have been several reports describing different tandem abs, for example CD3xCD33 scBsTaFv targeting efficiently blasts derived from AML patients [92], or the tandems PSMAxCD3 and CD3-PSCA, which potently redirect T cells to prostate cancer cells [90,91]. Another interesting example for a bispecific tandem antibody is rM28 which recognizes the co-stimulatory receptor CD28 as an effector molecule and melanoma-associated proteoglycan NG2 as a tumor-associated target. This molecule spontaneously forms stable dimers and induces target cell restricted T cell activation independent of the TCR/CD3 complex, triggering effective cancer cell lysis by so called “targeted super-agonistic stimulation” [116]. Recently, this effective mode of action was also utilized in a scBsTaFv targeting lymphoma cells by exchanging the anti-NG2 moiety with an anti-CD20 scFv, showing the reproducibility of this approach [117]. Phase I/II clinical studies investigating the safety and efficiency of rM28 have been initiated in 2005, however some concerns were raised, due to the systemic T-cell activation and severe cytokine release syndrome induced when six healthy volunteers were injected with monospecific “super-agonistic” CD28 antibody [118]. Nevertheless, it was shown that the “supra-agonistic” CD28 stimulation by rM28 is strictly target-cell restricted over a wide concentration range [119].
Tandem scFvs consisting of an anti-CD3 and an anti-TAA domain are also termed bispecific T cell engagers (BiTEs). Usually, BiTEs are generated by fusing an anti-CD3 scFv to an anti-TAA scFv via a short five amino acid long linker elements. With the exception of the recently described CD3xCD33 and CD3-PSCA [89,90,92] scBsTaFvs BiTEs are commonly constructed starting from anti-CD3 mabs with strong T cell activation capabilities such as the anti-CD3 mab OKT3. The first description of such a tandem antibody targeting EpCAM as a TAA was published in 1995. Redirection of unstimulated human PBMC toward TAA positive tumor cells resulted in high cytotoxicity even at very low concentrations of the bsAb [120].When later the anti-EpCAM scFv was exchanged by an anti-CD19 scFv a novel BiTE with also outstanding properties was generated [121]. Since then, Baeuerle and coworkers have demonstrated the fascinating properties of bispecific abs in this format [122,123]. Currently, two BiTEs are undergoing clinical studies—CD3xCD19 (blinatumomab or MT103) and EpCAMxCD3 (MT110). Blinatumomab is the most advanced BiTE in clinical trials and has been studied as a treatment for lymphoma and leukemia. The Phase I studies demonstrated that even low doses (5 µg/m2) led to elimination of the aberrant cells in the blood of relapsed NHL patients and all patients treated with 60 µg/m2 of the MT103 experienced tumor regression [124]. Because of its small size blinatumomab has a short serum half-life and in order to achieve the required concentration continuous infusion was required. To demonstrate a significant effect in patients cumulative doses of several milligrams were needed, whereas conventional antibody treatment requires grams of the compound per treatment cycle.
Currently, MT103 is also being tested in Phase II trials in patients with B-precursor lymphoblastic leukemia (B-ALL) with minimal residual disease (MRD). In 80% of the 20 patients treated T cells activated by blinatumomab were able to locate and eradicate the rare disseminated tumor cells in the bone marrow, rendering the patients MRD negative. 78% of the patients were relapse free after a follow up of 405 days. MT103 was also able to engage T cells to eradicate chemotherapy-resistant tumor cells, which can otherwise cause clinical relapse. In the Phase II trial adverse events, such as lymphopenia, were also observed, but they were completely reversible [125].
There are several new BiTEs in the process of development, utilizing either humanized or fully human scFvs, which are cross-reactive with orthologous antigens in non-human primates, allowing the direct determination of the safety and the pharmacokinetics of the respective BiTE (e.g., CD33, melanoma associated chondroitin sulfate proteoglycan), or by reformatting approved therapeutic antibodies as BiTE molecules (e.g., panitumumab, cetuximab, etc.) [70].
Taken together, until now, a series of recombinant single-chain bispecific abs have been created either in the diabody or various tandem formats. Both formats seem to have advantages and disadvantages. E.g., scBsDbs appear to be more protease-resistant although experimental evidence has so far not been provided. On the other hand the antibody domains in scBsDbs might be less flexible than in scBsTaFvs. Controversial data had been published in the literature with respect to the efficacy of scBsDbs versus scBsTaFvs. In a first side by side comparison it was shown that scBsTaFvs are far more superior to scBsDbs [126]. However, the antibody components in this manuscript were not completely identical and, thus, a clear-cut conclusion remained open. In a more recent study, we presented scBsDbs and scBsTaFvs both targeting PSCA which were prepared from the same antibody domains [90]. The direct comparison of these bispecific abs in both formats did not show obvious differences. However, the bispecific ab in the tandem format could be further improved by modifying the linker elements and the order of the heavy and light chains and also easily be humanized [89]. The same was true for a CD3xCD33 scBsTaFv [89]. In contrast, until now we failed to improve their respective bispecific counterparts in the scBsDb format [127]. The more rigid structure of a scBsDb may be responsible for these problems and may limit the chance of improving bispecific abs in this format. However, our experience does not necessarily mean that this must be true for all bispecific abs in the scBsDb format.
Another unexpected effect which we observed during optimization of bispecific abs is shown in Figure 2. When we altered the order of the heavy and light chain of the first scFv in an antibody in the tandem format this had not only an effect on the binding affinity of the first domain to its target antigen: Unexpectedly, it also effected the binding affinity of the second antibody domain although this domain was not modified at all (Figure 2A,B, and black graph versus red graph). The same was true when the linker size in one of the scFv domains was altered which also effected the binding capability of the second unmodified scFv domain (Figure 2A,C, and black graph versus blue graph). Also rearranging the two scFv domains in a different order had dramatic effects on the binding capability (Figure 2C,D, and blue graph versus purple graph). It is of interest to mention that the scBsDb (Figure 2E, green graph) showed a balanced binding towards both epitopes. In spite of this, the killing capability was impaired [127]. In summary, these data indicate the difficulties of improving the functionality of a bispecifc ab molecule. At least currently there are no common rules helping to predict the best structure of a novel bispecific ab, thus every novel ab requires an individual optimization. Unfortunately, this optimization process is time consuming and thus expensive. One has also to keep these in mind when replacing one of the domains, e.g., the anti-human CD3 domain with an anti-mouse CD3 domain, e.g., if a safety study in an immunocompetent mouse or monkey model is considered. Such a construct may be helpful for collecting mechanistic data. However, it might have completely unpredictable properties with respect to the capability to mediate the killing of tumor cells via redirected human T cells and even more important to side effects such as the risk of cytokine storms in humans.
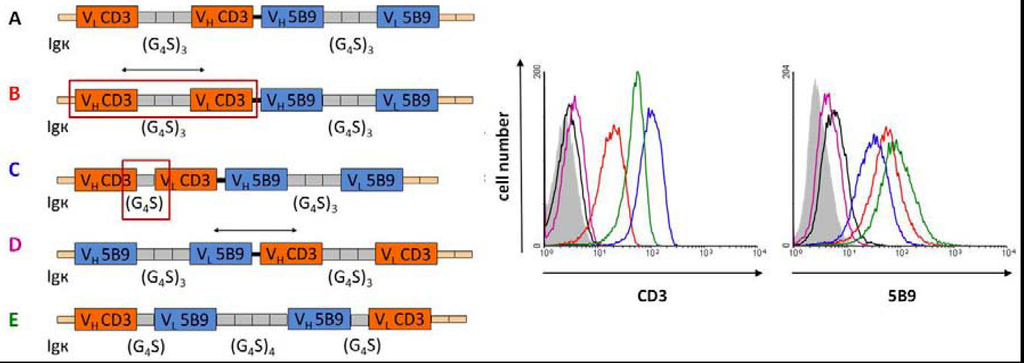
Figure 2.
Effect of the heavy (VH) and light (VL) chain domains as well as the linker lengths on the binding capabilities of single-chain bispecific abs; G4S: block of four glycine and one serine residues as a peptide linker; Igκ: leader sequence.
5. Mechanism of Action of the Bispecific Antibodies
Several publications in the last few years have offered an insight in the mechanism utilized by bsAbs to mediate recognition and eradication of the malignant cells by T cells (Figure 3). It has been shown for BiTEs and other bispecific molecules that they function as adaptor molecules between the T and the tumor cells that bring them closer together and trigger activation of the signaling cascade of the T cell receptor (TCR) complex facilitated by the binding of the bispecific abs to the CD3 component of the receptor (Figure 3A). Since the activation is based on CD3 and not on the highly variable TCR, bispecific abs can redirect all antigen experienced CD4+ and CD8+ T cells [91,127,128,129,130] in the patient against the aberrant cells independent of their specificity. The activation of the T cells results only from the polyvalent ligation of CD3 [70,90] that induces the formation of a transient cytolytic synapse between the cytotoxic T cells and the target cells (Figure 3B and Figure 4) [104,131]. As a consequence granzyme and perforine containing granules fuse with the T cell membrane, and release their contents towards the target cell. The perforine forms pores in the cancer cell membrane, facilitating the entry of the granzymes, that in turn triggers apoptosis of the tumor cell by activating the caspase pathway (Figure 3B) [123,130,131,132]. Besides mediating cancer cell death bispecific abs contribute to the potent activation of the killer T cell. Activation markers like CD69 and CD25 are markedly upregulated. In addition, T cells transiently release proinflammatory cytokines (i.e., IFN-γ, TNF-α, IL-2, etc.) and start to proliferate, which can increase their number in the target tissue [70,130,133] (see also Figure 5). As already mentioned, the formed synapses are transient and after eradicating the target cells activated T cells can move on to the next target cell and continue killing in a “serial killing” manner. This was suggested by the efficiency of the target cell elimination even at low effector to target cell ratios and was visualized by video-assisted microscopy [134]. Since the formation of the bispecific ab mediated cytolytic synapse is independent of the expression of MHC class I molecules [131], their utilization as therapeutic compounds is not influenced by the antigen presentation machinery and therefore can overcome some of the major immune evasion mechanisms, which normally interfere with immunotherapeutic efficacy in case of other cancer immunotherapy approaches based on specific T cell responses [123].
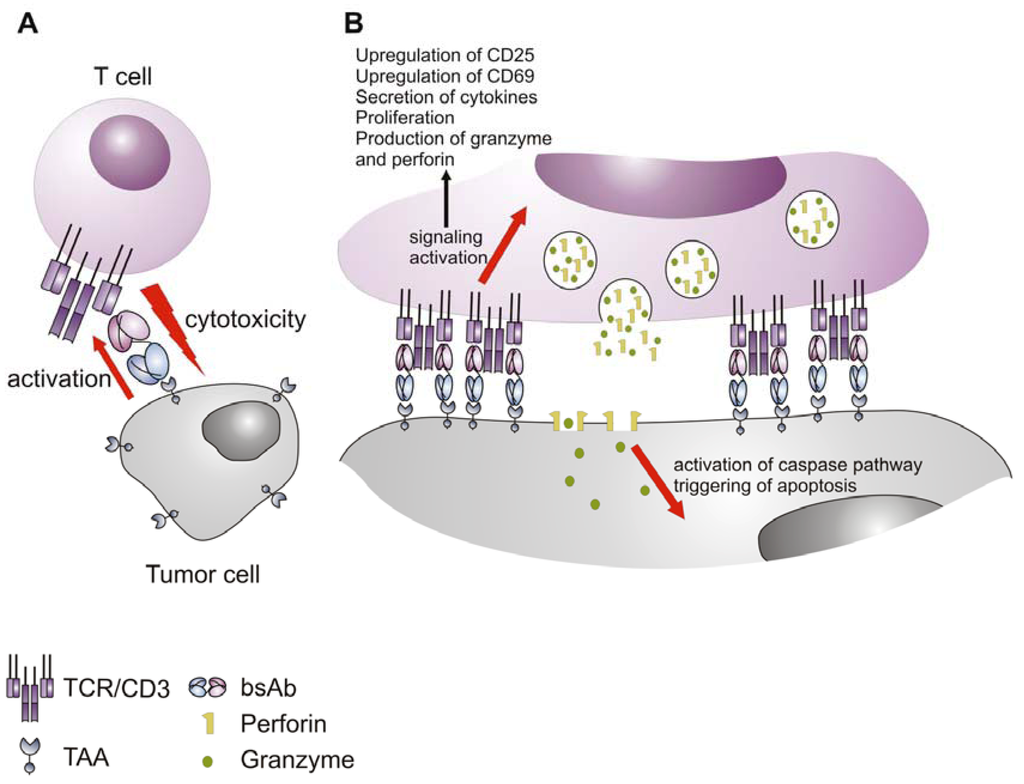
Figure 3.
Mode of action of bispecific antibodies. (A) The bispecific antibody functions as an adaptor molecule between the T cell and the tumor cell, cross-linking the two cells and triggering CD3-mediated T cell activation, leading to lysis of the tumor cell; (B) The killing of the tumor cell is a result of the formation of cytolytic synapse, whereafter activated T cells release granules containing toxic payload of perforine and granzyme, which trigger apoptosis in the tumor cell. TCR: T cell receptor complex; TAA: tumor-associated antigen; bsAb: bispecific antibody.
Another highlight of bispecific abs, which is mainly contributed to the BiTEs but is also observed for other bispecific molecules, is that they can activate T cells without the need of a co-stimulatory signal. There are currently two theories that try to give an explanation for this phenomenon. One is associated with the possibility of co-signaling, which can occur upon cross-linking of the target and effector cell via the bispecific ab, that is mediated by the interaction between CD28 and B7, known to be expressed on some malignant cells. However, some bispecific abs targeting a variety of tumor cells that do not express B7 molecules show similar efficacy [70]. The other theory is based on the observation that the activity of bsAbs is mediated by effector memory T cells, which do not require CD28 co-stimulation during secondary responses, whereas naïve T cells do not contribute to the killing of the target cells [70,121], thus explaining the lack of necessity for further co-stimulation. A possible explanation for these findings comes from the observation, that CD28 triggering results in a rather quantitative amplification of TCR initiated signaling pathways instead of stimulating additional unique signaling pathways, thus, if the signaling threshold for activation in different T cell populations varies (e.g., memory versus naïve), potent triggering of the TCR-CD3 pathway via bsAbs might be sufficient to initiate activation without any additional costimulatory signals in certain T cell populations. This arguments are in line with observations, that under certain circumstances even memory T cells need costimulatory assistances for full blown activation [135].
It was also recently shown that bispecific abs targeting CD3 as an effector molecule can activate not only effector CD8+ and CD4+ T cells, but redirect regulatory T cells (Tregs) as well [133,136]. As shown in Figure 4, Tregs are not only cross-linked by bispecific abs with target cells. The cross-linkage also results in an immune synapse-like interaction as in case of classical effector T cells. Activation of Tregs could have a detrimental effect on the efficiency of the tumor cell targeting in tumors where Tregs have accumulated. However, in clinical trials so far bispecific abs did not show a reduced efficacy in cancer treatment. One possible explanation may be that CD8+ T cells are capable to start killing immediately after cross-linkage with a tumor cell via a bsAb while there is a gap of about five hours for conventional CD4+ T cells until they have achieved their killing capability. This gap is most likely due to the fact that only CD8+ T cells have preformed perforine and granzyme molecules. A delayed onset of response may also be true for CD4+ regulatory T cells. According to a recent abstract, Tregs may even be converted into killer cells by the cross-linkage with a bispecific ab in BiTE format [134]. It should, however, be mentioned that isolated Tregs are usually contaminated with effector T cells. Moreover, freshly isolated Tregs have different properties compared to expanded Tregs. One obvious example is shown in Figure 5: While freshly isolated Tregs secrete IL-10, expanded Tregs fail to do so. Consequently, future studies using more carefully characterized Treg preparations are required to show whether or not co-ligated Tregs can indeed efficiently work as killer cells. At least when Tregs were co-injected with effector T cells in an animal model the tumor growth was clearly accelerated by Tregs in the presence of a bispecific co-ligating ab and not improved [133]. Moreover, Tregs transduced with a chimeric antigen receptor were also capable to restore tumor growth [137]. Thus, according to these studies it appears rather unlikely that Tregs have a major contribution in killing of tumor cells after cross-linkage via bispecific abs. If so, it may become necessary to develop strategies to circumvent the potential activation of Tregs when malignant diseases are targeted with CD3-engaging bispecific abs. However, in view of the importance of Tregs in establishing and maintaining peripheral tolerance, an antigen specific retargeting of Tregs using bispecific abs may provide a promising therapeutic opportunity for the treatment of autoimmunity and graft rejection [133].
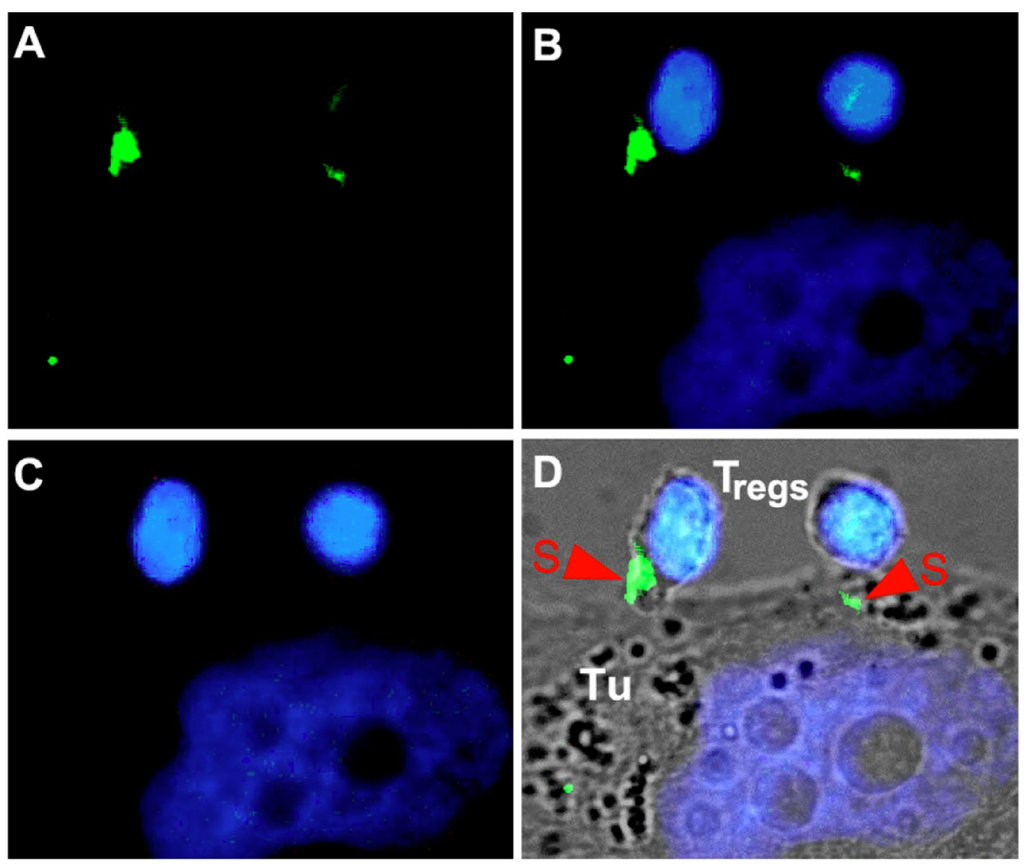
Figure 4.
Formation of immune synapse (S)-like structures by cross-linked Tregs with tumor (Tu) cells. (A) GFP labeled bispecific antibody. (B) overlay of DAPI staining (C) with GFP signal (A). (D) overlay of (B) with the corresponding phase contrast image.
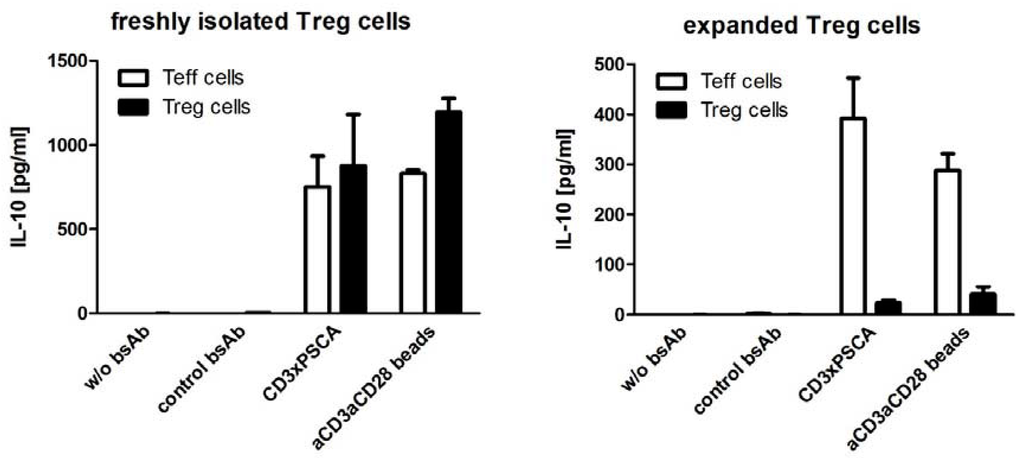
Figure 5.
Comparison of IL-10 release from freshly isolated and expanded Tregs after cross-linkage with tumor cells via bispecific abs.
6. Immunoligands
As already mentioned, besides T cells, NK cells are another class of immune effector cells which have great potential in immunotherapy. In addition to the Fc receptors on their surface, which can trigger ADCC, NK cells possess a number of activating receptors that can be involved in the detection of malignant cells [138,139]. One of these receptors, the activating receptor NKG2D (natural-killer group 2, member D) plays an important role in the NK immune response to tumors. The interest towards NKG2D is raised by the fact that its ligands (MIC A/B, and ULBPs) are frequently expressed by aberrant cells and in malignant tissues, but are rarely detected on the surface of their healthy counterparts. Therefore, NKG2D can mediate efficient anti-tumor response without damaging the normal tissues. However, some malignant cells use downregulation or shedding of these molecules as an evasion mechanism [140,141]. Based on the observation that tumor cells expressing high levels of NKG2D ligands were more susceptible to NK cell mediated killing, whereas tumor cells expressing low or intermediate levels of NKG2D ligands were less immunogenic [142], a promising strategy to activate efficiently anti-tumor immune responses and to overcome some of the escape mechanisms would be to increase the density of NKG2D ligands on tumor cells. One possible strategy would be to “decorate” the surface of the malignant cells with these ligands. This can be achieved by creating a recombinant protein, which comprises a single-chain antibody or an antibody fragment (Fab) against a TAA fused to such a ligand molecule. In this way tumor cells expressing a certain TAA can be specifically targeted and thereby be sensitized to NK cell mediated eradication. There have been several reports for aforesaid bispecific immunoligands, such as Fab fragment recognizing CD20 (targeting NHL) fused to MICA [143], or anti-CD33 scFv (targeting AML) or anti-CD138 (targeting multiple myeloma) fused to ULBP-2 [92,144], showing that indeed, antibody-mediated coating of tumor cells with NKG2D ligands triggers efficient NKG2D-dependent NK cell lysis of the target cells, therefore implying that cell activation via NKG2D has a potential in mediating an immune response to a broad number of tumors [145].
Furthermore, NKG2D is expressed on the surface of CD8+ T cells as well, and provides an important co-stimulatory signal to these cells [146]. Therefore, the combination of such an immunoligand with a T cell engaging bispecific ab in view of redirecting both T and NK cells against target cells expressing a TAA may have synergistic effects, resulting in an improved cytotoxicity and increase of secretion of proinflammatory cytokines, which could be important for breaking tolerance of the effector cells established in the malignant milieu [92]. In addition, co-signaling via NKG2D in T cells leads to upregulation of the additional co-stimulatory molecule 4-1BB [147], which has the ability to reverse inhibition of CD8+ T-cell responses mediated by TGF-β1—a factor responsible for tumor immune escape [148,149]. Therefore, the combination of bispecific abs with immunoligands might have the potential not only to improve the cytotoxicity of NK and T cells, but also to help the modulation of the immune response in a way to overcome some of the evasion mechanisms utilized by tumors to escape recognition and killing.
Another class of antibody derivatives also holds great potential to act as immunomodulators, the so called immunocytokines. Many proinflammatory cytokines such as IL-2, IL-12, IL-15 and GM-CSF have demonstrated potent anti-tumor activity and have the ability to enhance the immunogenicity of certain tumor types [150,151]. Unfortunately, preclinical and clinical studies have shown limited success due to a number of drawbacks, such as rapid blood clearance of cytokines and their lack of tumor specificity, and the need of high dose administration to ensure sufficient concentration of the cytokine in the tumor microenvironment to trigger an efficient immune response. Furthermore, the systemic administration of high doses of these cytokines have been associated with severe toxic effects such as tachycardia, hypotension, respiratory failure, vascular permeability, anemia, fevers and chills [152,153,154], and in some cases fatal consequences [155]. Therefore, the antibody-based targeted delivery of cytokines to the tumor environment is a promising strategy to enhance the therapeutic efficiency and improve the safety of these potent anti-cancer agents [156,157].
There have been several examples of fusing mabs or scFvs specific for different TAAs i.e. CD20, CD30, glycosphingolipid GD2, Her2, EpCAM, extra-domain B of fibronectin (EDB), etc., to a number of cytokines (IL-2, IL-12, IL-15, GM-CSF), which have yielded impressive results in preclinical studies, and several of these constructs are currently under investigation in the clinic (reviewed extensively in [158]).
7. Improving the Efficacy and Pharmacokinetics
One of the major limitations for the efficacy of murine mabs and their derivatives appears to be their immunogenicity and the induction of HAMA responses although this was recently challenged: While the treatment with catumaxomab resulted in the development of HAMAs patients who developed HAMAs sooner derived greater benefit from the treatment [159]. On the other hand there is certainly no doubt, that the occurrence of HAMAs increases the risk of anaphylactic reactions as already known from the earliest passive vaccination attempts based on antisera developed in animals. It was reported that the replacement of the constant (C) regions of the heavy and the light chains by human C regions can already help to overcome this problem or at least to reduce the immunogenicity of murine abs. However, in some cases this exchange is not sufficient, since the variable regions can also be immunogenic [160,161,162]. Therefore, this limitation might be valid for the recombinant single-chain abs as well and strategies to overcome it have to be considered. One way to reduce the immunogenicity of a bispecific ab would be the humanization of the variable domains comprising it. This can be done by grafting the complementarity determining regions (CDRs) of the murine variable domain into the best fitting human framework regions [163,164]. Other options are the selection of the variable domains from a human phage display library [165,166] or to isolate them from mabs raised in transgenic mice in which the murine immunoglobulin genes have been disrupted and replaced with human immunoglobulin gene clusters [55,167].
Another potential limitation of the recombinant bispecific abs is their short half-life, resulting from their small size (~50–60 kDa). Unlike mabs, which have a half-life of several days, bispecific abs are retained in the circulation only for a few hours. As seen during the clinical trials with BiTE blinatumomab, it has a half-life of two hours and in order to ensure sufficient concentration for efficient response an application form of continuous intravenous infusion over four to eight weeks per cycle was necessary [122]. Therefore, there is a need to increase the half-life of these molecules and thus to facilitate application and improve efficacy. Several strategies to this end have been proposed [168]. It has been shown that the fusion of single-chain bispecific abs to human serum albumin (HSA) or to an albumin-binding domain derived from streptococcal protein G, resulted in a significantly increased half-life [169,170]. This strategy is based on the observation that albumin has a similar half-life as IgGs and takes advantage of recycling process mediated via neonatal Fc receptor (FcRn) in the endosomal compartment of endothelial cells after endocytosis. During this process a pH-dependent binding of IgGs and albumin to FcRn diverts the bound proteins from lysosomal degradation and results in their recycling back into the blood plasma [81].
In addition to the prolonged time in the circulation, HSA-ab fusion proteins have demonstrated increased accumulation in the malignant tissue of tumor bearing mice [171]. Recently, human domain abs and Nbs binding to HSA have been identified, and their fusion to other therapeutic proteins resulted in improved retention in the circulation [172,173], therefore giving rise to a new strategy for extension of the half-life. An alternative strategy, which takes advantage of the same mechanism, is fusion of the therapeutic protein to Fc region, which in addition leads to homodimerization. This approach might be of interest in the cases where increased avidity is required to improve the functionality of the therapeutic compound [174], whereas HSA fusion is more suitable in the cases where a monovalent binding is required to avoid target cell independent activation of the effector cells.
Another option to prolong the half-life of single-chain bispecific abs would be to increase their hydrodynamic radius, which can be done by chemical conjugation to polyethylene glycol (PEG) chain [81]. Results to this effect have been shown for a number of recombinant antibody constructs [175]. However, the addition of PEG to recombinant abs can interfere with their antigen binding activity [176]. Furthermore, conjugation of PEG to single-chain bispecifc ab can significantly reduce the ability of the construct to trigger target cell dependent T cell cytotoxicity, even though its binding capability to the target and effector cell was not affected [171,175].
8. Experimental Section
Expression and isolation of bispecific antibodies was performed as described in [80]. Epifluorescence analysis using directly labeled bispecific antibodies was performed as described in [104]. Killing assays and cytokine measurements were performed as described, e.g., in [133].
9. Conclusions
BsAbs have undergone a significant evolution since the 1980s, when the idea was formulated for the first time. Advances in immunology, molecular biology and antibody engineering, as well as deeper understanding of the molecular mechanisms governing effector cell activation were necessary to overcome the obstacles and limitations faced by the first bsAbs. In the past few years, bsAbs have shown great potential in immunotherapy. Several molecules have demonstrated promising anti-tumor potential including in first clinical trials [123] and even one bsAb (catumaxomab) has been approved for cancer therapy [177]. Whether these bispecific abs will establish themselves as single agents or as adjuvants for conventional tumor treatment (e.g., chemotherapy) remains to be seen.
Most of the developed recombinant bsAbs targeting leukemias have shown very efficient anti-cancer effect, and even though bispecific compounds targeting TAA expressed on solid tumors have also been very efficient in preclinical studies, further investigations are needed to determine if they will have the same clinical success. Furthermore, there are still a few challenges with respect to pharmacokinetics and efficiency which need to be overcome. However, with all the work and improvements already achieved in this field one can expect that soon there will be more bsAbs entering into clinical practice.
Acknowledgments
The Robert Pfleger Stiftung and the DFG research center and cluster of excellence for Regenerative Therapies Dresden provided financial support for this publication.
References
- Ehrlich, P. Ueber den jetzigen stand der Karzinomforschung. Ned. Tijdschr. Geneeskd. 1909, 273–290. [Google Scholar]
- Burnet, M. Cancer: A biological approach. I. The processes of control. Br. Med. J. 1957, 1, 779–786. [Google Scholar] [CrossRef]
- Thomas, L. Reactions to homologous tissue antigens in relation to hypersensitivity [Discussion]. In Cellular and Humoral Aspects of the Hypersensitive States; Lawrence, H.S., Ed.; Hoeber-Harper: New York, NY, USA, 1959; pp. 529–533. [Google Scholar]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef]
- Parish, C.R. Cancer immunotherapy: The past, the present and the future. Immunol. Cell Biol. 2003, 81, 106–113. [Google Scholar] [CrossRef]
- Dighe, A.S.; Richards, E.; Old, L.J.; Schreiber, R.D. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN gamma receptors. Immunity 1994, 1, 447–456. [Google Scholar] [CrossRef]
- van den Broek, M.E.; Kagi, D.; Ossendorp, F.; Toes, R.; Vamvakas, S.; Lutz, W.K.; Melief, C.J.; Zinkernagel, R.M.; Hengartner, H. Decreased tumor surveillance in perforin-deficient mice. J. Exp. Med. 1996, 184, 1781–1790. [Google Scholar] [CrossRef]
- Kaplan, D.H.; Shankaran, V.; Dighe, A.S.; Stockert, E.; Aguet, M.; Old, L.J.; Schreiber, R.D. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc. Natl. Acad. Sci. USA 1998, 95, 7556–7561. [Google Scholar]
- Smyth, M.J.; Thia, K.Y.; Street, S.E.; Cretney, E.; Trapani, J.A.; Taniguchi, M.; Kawano, T.; Pelikan, S.B.; Crowe, N.Y.; Godfrey, D.I. Differential tumor surveillance by natural killer (NK) and NKT cells. J. Exp. Med. 2000, 191, 661–668. [Google Scholar] [CrossRef]
- Smyth, M.J.; Thia, K.Y.; Street, S.E.; MacGregor, D.; Godfrey, D.I.; Trapani, J.A. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J. Exp. Med. 2000, 192, 755–760. [Google Scholar] [CrossRef]
- Shankaran, V.; Ikeda, H.; Bruce, A.T.; White, J.M.; Swanson, P.E.; Old, L.J.; Schreiber, R.D. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 2001, 410, 1107–1111. [Google Scholar] [CrossRef]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004, 21, 137–148. [Google Scholar] [CrossRef]
- Vesely, M.D.; Kershaw, M.H.; Schreiber, R.D.; Smyth, M.J. Natural innate and adaptive immunity to cancer. Annu. Rev. Immunol. 2011, 29, 235–271. [Google Scholar] [CrossRef]
- Halliday, G.M.; Patel, A.; Hunt, M.J.; Tefany, F.J.; Barnetson, R.S. Spontaneous regression of human melanoma/nonmelanoma skin cancer: Association with infiltrating CD4+ T cells. World J. Surg. 1995, 19, 352–358. [Google Scholar] [CrossRef]
- Iihara, K.; Yamaguchi, K.; Nishimura, Y.; Iwasaki, T.; Suzuki, K.; Hirabayashi, Y. Spontaneous regression of malignant lymphoma of the breast. Pathol. Int. 2004, 54, 537–542. [Google Scholar] [CrossRef]
- Penn, I. Tumors of the immunocompromised patient. Annu. Rev. Med. 1988, 39, 63–73. [Google Scholar] [CrossRef]
- Buell, J.F.; Gross, T.G.; Woodle, E.S. Malignancy after transplantation. Transplantation 2005, 80, S254–S264. [Google Scholar] [CrossRef]
- Clemente, C.G.; Mihm, M.C., Jr.; Bufalino, R.; Zurrida, S.; Collini, P.; Cascinelli, N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer 1996, 77, 1303–1310. [Google Scholar] [CrossRef]
- Scanlan, M.J.; Simpson, A.J.; Old, L.J. The cancer/testis genes: Review, standardization, and commentary. Cancer Immun. 2004, 4, 1. [Google Scholar]
- Haanen, J.B.; Baars, A.; Gomez, R.; Weder, P.; Smits, M.; de Gruijl, T.D.; von Blomberg, B.M.; Bloemena, E.; Scheper, R.J.; van Ham, S.M.; et al. Melanoma-specific tumor-infiltrating lymphocytes but not circulating melanoma-specific T cells may predict survival in resected advanced-stage melanoma patients. Cancer Immunol. Immunother. 2006, 55, 451–458. [Google Scholar] [CrossRef]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity's roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar]
- Smyth, M.J.; Dunn, G.P.; Schreiber, R.D. Cancer immunosurveillance and immunoediting: The roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv. Immunol. 2006, 90, 1–50. [Google Scholar] [CrossRef]
- Swann, J.B.; Smyth, M.J. Immune surveillance of tumors. J. Clin. Invest. 2007, 117, 1137–1146. [Google Scholar] [CrossRef]
- Rubartelli, A.; Lotze, M.T. Inside, outside, upside down: Damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 2007, 28, 429–436. [Google Scholar] [CrossRef]
- Sims, G.P.; Rowe, D.C.; Rietdijk, S.T.; Herbst, R.; Coyle, A.J. HMGB1 and RAGE in inflammation and cancer. Annu. Rev. Immunol. 2010, 28, 367–388. [Google Scholar] [CrossRef]
- Dunn, G.P.; Bruce, A.T.; Sheehan, K.C.; Shankaran, V.; Uppaluri, R.; Bui, J.D.; Diamond, M.S.; Koebel, C.M.; Arthur, C.; White, J.M.; et al. A critical function for type I interferons in cancer immunoediting. Nat. Immunol. 2005, 6, 722–729. [Google Scholar] [CrossRef]
- Smith, P.L.; Lombardi, G.; Foster, G.R. Type I interferons and the innate immune response—More than just antiviral cytokines. Mol. Immunol. 2005, 42, 869–877. [Google Scholar] [CrossRef]
- Guerra, N.; Tan, Y.X.; Joncker, N.T.; Choy, A.; Gallardo, F.; Xiong, N.; Knoblaugh, S.; Cado, D.; Greenberg, N.M.; Raulet, D.H. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity 2008, 28, 571–580. [Google Scholar] [CrossRef]
- Smyth, M.J.; Godfrey, D.I.; Trapani, J.A. A fresh look at tumor immunosurveillance and immunotherapy. Nat. Immunol. 2001, 2, 293–299. [Google Scholar] [CrossRef]
- Chan, C.W.; Housseau, F. The 'kiss of death' by dendritic cells to cancer cells. Cell Death Differ. 2008, 15, 58–69. [Google Scholar] [CrossRef]
- Appay, V. The physiological role of cytotoxic CD4(+) T-cells: The holy grail? Clin. Exp. Immunol. 2004, 138, 10–13. [Google Scholar] [CrossRef]
- Quezada, S.A.; Simpson, T.R.; Peggs, K.S.; Merghoub, T.; Vider, J.; Fan, X.; Blasberg, R.; Yagita, H.; Muranski, P.; Antony, P.A.; et al. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J. Exp. Med. 2010, 207, 637–650. [Google Scholar]
- Law, T.M.; Motzer, R.J.; Mazumdar, M.; Sell, K.W.; Walther, P.J.; O'Connell, M.; Khan, A.; Vlamis, V.; Vogelzang, N.J.; Bajorin, D.F. Phase III randomized trial of interleukin-2 with or without lymphokine-activated killer cells in the treatment of patients with advanced renal cell carcinoma. Cancer 1995, 76, 824–832. [Google Scholar] [CrossRef]
- Imai, C.; Iwamoto, S.; Campana, D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood 2005, 106, 376–383. [Google Scholar] [CrossRef]
- Morgan, R.A.; Dudley, M.E.; Wunderlich, J.R.; Hughes, M.S.; Yang, J.C.; Sherry, R.M.; Royal, R.E.; Topalian, S.L.; Kammula, U.S.; Restifo, N.P.; et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 2006, 314, 126–129. [Google Scholar]
- Ljunggren, H.G.; Malmberg, K.J. Prospects for the use of NK cells in immunotherapy of human cancer. Nat. Rev. Immunol. 2007, 7, 329–339. [Google Scholar] [CrossRef]
- Cartellieri, M.; Bachmann, M.; Feldmann, A.; Bippes, C.; Stamova, S.; Wehner, R.; Temme, A.; Schmitz, M. Chimeric antigen receptor-engineered T cells for immunotherapy of cancer. J. Biomed. Biotechnol. 2010, 2010. [Google Scholar] [CrossRef]
- Cartellieri, M.; Michalk, I.; von Bonin, M.; Kruger, T.; Stamova, S.; Koristka, S.; Arndt, C.; Feldmann, A.; Schmitz, M.; Wermke, M.; et al. Chimeric Antigen Receptor-Engineered T Cells for Immunotherapy of Acute Myeloid Leukemia. Blood 2011, 118, 1124–1125. [Google Scholar]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489. [Google Scholar]
- Park, T.S.; Rosenberg, S.A.; Morgan, R.A. Treating cancer with genetically engineered T cells. Trends Biotechnol. 2011, 29, 550–557. [Google Scholar] [CrossRef]
- Schwartzentruber, D.J.; Lawson, D.H.; Richards, J.M.; Conry, R.M.; Miller, D.M.; Treisman, J.; Gailani, F.; Riley, L.; Conlon, K.; Pockaj, B.; et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N. Engl. J. Med. 2011, 364, 2119–2127. [Google Scholar]
- Stroncek, D.F.; Berger, C.; Cheever, M.A.; Childs, R.W.; Dudley, M.E.; Flynn, P.; Gattinoni, L.; Heath, J.R.; Kalos, M.; Marincola, F.M.; et al. New directions in cellular therapy of cancer: A summary of the summit on cellular therapy for cancer. J. Transl. Med. 2012, 10, 48. [Google Scholar] [CrossRef]
- Rivoltini, L.; Canese, P.; Huber, V.; Iero, M.; Pilla, L.; Valenti, R.; Fais, S.; Lozupone, F.; Casati, C.; Castelli, C.; et al. Escape strategies and reasons for failure in the interaction between tumour cells and the immune system: How can we tilt the balance towards immune-mediated cancer control? Expert Opin. Biol. Ther. 2005, 5, 463–476. [Google Scholar] [CrossRef]
- Ferrone, S.; Whiteside, T.L. Tumor microenvironment and immune escape. Surg. Oncol. Clin. N. Am. 2007, 16, 755–774, viii. [Google Scholar] [CrossRef]
- Rabinovich, G.A.; Gabrilovich, D.; Sotomayor, E.M. Immunosuppressive strategies that are mediated by tumor cells. Annu. Rev. Immunol. 2007, 25, 267–296. [Google Scholar] [CrossRef]
- Groth, A.; Kloss, S.; von Strandmann, E.P.; Koehl, U.; Koch, J. Mechanisms of tumor and viral immune escape from natural killer cell-mediated surveillance. J. Innate Immun. 2011, 3, 344–354. [Google Scholar] [CrossRef]
- Weiner, L.M.; Murray, J.C.; Shuptrine, C.W. Antibody-based immunotherapy of cancer. Cell 2012, 148, 1081–1084. [Google Scholar] [CrossRef]
- Raju, T.S. Terminal sugars of Fc glycans influence antibody effector functions of IgGs. Curr. Opin. Immunol. 2008, 20, 471–478. [Google Scholar] [CrossRef]
- Chan, A.C.; Carter, P.J. Therapeutic antibodies for autoimmunity and inflammation. Nat. Rev. Immunol. 2010, 10, 301–316. [Google Scholar] [CrossRef]
- Jiang, X.R.; Song, A.; Bergelson, S.; Arroll, T.; Parekh, B.; May, K.; Chung, S.; Strouse, R.; Mire-Sluis, A.; Schenerman, M. Advances in the assessment and control of the effector functions of therapeutic antibodies. Nat. Rev. Drug Discov. 2011, 10, 101–111. [Google Scholar]
- Kohler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef]
- Neuberger, M.S.; Williams, G.T.; Mitchell, E.B.; Jouhal, S.S.; Flanagan, J.G.; Rabbitts, T.H. A hapten-specific chimaeric IgE antibody with human physiological effector function. Nature 1985, 314, 268–270. [Google Scholar]
- Jones, P.T.; Dear, P.H.; Foote, J.; Neuberger, M.S.; Winter, G. Replacing the complementarity-determining regions in a human antibody with those from a mouse. Nature 1986, 321, 522–525. [Google Scholar] [CrossRef]
- Hoogenboom, H.R.; Chames, P. Natural and designer binding sites made by phage display technology. Immunol. Today 2000, 21, 371–378. [Google Scholar] [CrossRef]
- Lonberg, N. Human monoclonal antibodies from transgenic mice. Handb. Exp. Pharmacol. 2008, 69–97. [Google Scholar] [CrossRef]
- Reichert, J.M.; Rosensweig, C.J.; Faden, L.B.; Dewitz, M.C. Monoclonal antibody successes in the clinic. Nat. Biotechnol. 2005, 23, 1073–1078. [Google Scholar] [CrossRef]
- Chames, P.; Van Regenmortel, M.; Weiss, E.; Baty, D. Therapeutic antibodies: Successes, limitations and hopes for the future. Br. J. Pharmacol. 2009, 157, 220–233. [Google Scholar] [CrossRef]
- Cheson, B.D. Radioimmunotherapy of non-Hodgkin's lymphomas. Curr. Drug Targets 2006, 7, 1293–1300. [Google Scholar] [CrossRef]
- Carter, P.J.; Senter, P.D. Antibody-drug conjugates for cancer therapy. Cancer J. 2008, 14, 154–169. [Google Scholar] [CrossRef]
- Lum, L.G.; Thakur, A. Targeting T cells with bispecific antibodies for cancer therapy. BioDrugs 2011, 25, 365–379. [Google Scholar] [CrossRef]
- McLaughlin, P.; Grillo-Lopez, A.J.; Link, B.K.; Levy, R.; Czuczman, M.S.; Williams, M.E.; Heyman, M.R.; Bence-Bruckler, I.; White, C.A.; Cabanillas, F.; et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: Half of patients respond to a four-dose treatment program. J. Clin. Oncol. 1998, 16, 2825–2833. [Google Scholar]
- Witzig, T.E.; White, C.A.; Gordon, L.I.; Wiseman, G.A.; Emmanouilides, C.; Murray, J.L.; Lister, J.; Multani, P.S. Safety of yttrium-90 ibritumomab tiuxetan radioimmunotherapy for relapsed low-grade, follicular, or transformed non-hodgkin's lymphoma. J. Clin. Oncol. 2003, 21, 1263–1270. [Google Scholar]
- Bross, P.F.; Beitz, J.; Chen, G.; Chen, X.H.; Duffy, E.; Kieffer, L.; Roy, S.; Sridhara, R.; Rahman, A.; Williams, G.; et al. Approval summary: Gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin. Cancer Res. 2001, 7, 1490–1496. [Google Scholar]
- Cobleigh, M.A.; Vogel, C.L.; Tripathy, D.; Robert, N.J.; Scholl, S.; Fehrenbacher, L.; Wolter, J.M.; Paton, V.; Shak, S.; Lieberman, G.; et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J. Clin. Oncol. 1999, 17, 2639–2648. [Google Scholar]
- Romond, E.H.; Perez, E.A.; Bryant, J.; Suman, V.J.; Geyer, C.E., Jr.; Davidson, N.E.; Tan-Chiu, E.; Martino, S.; Paik, S.; Kaufman, P.A.; et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N. Engl. J. Med. 2005, 353, 1673–1684. [Google Scholar] [CrossRef]
- Lundin, J.; Kimby, E.; Bjorkholm, M.; Broliden, P.A.; Celsing, F.; Hjalmar, V.; Mollgard, L.; Rebello, P.; Hale, G.; Waldmann, H.; et al. Phase II trial of subcutaneous anti-CD52 monoclonal antibody alemtuzumab (Campath-1H) as first-line treatment for patients with B-cell chronic lymphocytic leukemia (B-CLL). Blood 2002, 100, 768–773. [Google Scholar] [CrossRef]
- Rhee, J.; Hoff, P.M. Angiogenesis inhibitors in the treatment of cancer. Expert Opin. Pharmacother. 2005, 6, 1701–1711. [Google Scholar] [CrossRef]
- Snyder, L.C.; Astsaturov, I.; Weiner, L.M. Overview of monoclonal antibodies and small molecules targeting the epidermal growth factor receptor pathway in colorectal cancer. Clin. Colorectal. Cancer 2005, 5 (Suppl. 2), S71–S80. [Google Scholar] [CrossRef]
- Patel, D.K. Clinical use of anti-epidermal growth factor receptor monoclonal antibodies in metastatic colorectal cancer. Pharmacotherapy 2008, 28, 31S–41S. [Google Scholar] [CrossRef]
- Chames, P.; Baty, D. Bispecific antibodies for cancer therapy: The light at the end of the tunnel? MAbs 2009, 1, 539–547. [Google Scholar] [CrossRef]
- Beckman, R.A.; Weiner, L.M.; Davis, H.M. Antibody constructs in cancer therapy: Protein engineering strategies to improve exposure in solid tumors. Cancer 2007, 109, 170–179. [Google Scholar] [CrossRef]
- Shinkawa, T.; Nakamura, K.; Yamane, N.; Shoji-Hosaka, E.; Kanda, Y.; Sakurada, M.; Uchida, K.; Anazawa, H.; Satoh, M.; Yamasaki, M.; et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J. Biol. Chem. 2003, 278, 3466–3473. [Google Scholar]
- Preithner, S.; Elm, S.; Lippold, S.; Locher, M.; Wolf, A.; da Silva, A.J.; Baeuerle, P.A.; Prang, N.S. High concentrations of therapeutic IgG1 antibodies are needed to compensate for inhibition of antibody-dependent cellular cytotoxicity by excess endogenous immunoglobulin G. Mol. Immunol. 2006, 43, 1183–1193. [Google Scholar] [CrossRef]
- Cartron, G.; Dacheux, L.; Salles, G.; Solal-Celigny, P.; Bardos, P.; Colombat, P.; Watier, H. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood 2002, 99, 754–758. [Google Scholar] [CrossRef]
- Weng, W.K.; Levy, R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J. Clin. Oncol. 2003, 21, 3940–3947. [Google Scholar] [CrossRef]
- Nimmerjahn, F.; Ravetch, J.V. Antibodies, Fc receptors and cancer. Curr. Opin. Immunol. 2007, 19, 239–245. [Google Scholar] [CrossRef]
- Xie, H.; Blattler, W.A. In vivo behaviour of antibody-drug conjugates for the targeted treatment of cancer. Expert Opin. Biol. Ther. 2006, 6, 281–291. [Google Scholar] [CrossRef]
- Steiner, M.; Neri, D. Antibody-radionuclide conjugates for cancer therapy: Historical considerations and new trends. Clin. Cancer Res. 2011, 17, 6406–6416. [Google Scholar] [CrossRef]
- Staerz, U.D.; Kanagawa, O.; Bevan, M.J. Hybrid antibodies can target sites for attack by T cells. Nature 1985, 314, 628–631. [Google Scholar] [CrossRef]
- Kontermann, R.E. Recombinant bispecific antibodies for cancer therapy. Acta Pharmacol. Sin. 2005, 26, 1–9. [Google Scholar] [CrossRef]
- Muller, D.; Kontermann, R.E. Bispecific antibodies for cancer immunotherapy: Current perspectives. BioDrugs 2010, 24, 89–98. [Google Scholar] [CrossRef]
- Singer, H.; Kellner, C.; Lanig, H.; Aigner, M.; Stockmeyer, B.; Oduncu, F.; Schwemmlein, M.; Stein, C.; Mentz, K.; Mackensen, A.; et al. Effective elimination of acute myeloid leukemic cells by recombinant bispecific antibody derivatives directed against CD33 and CD16. J. Immunother. 2010, 33, 599–608. [Google Scholar] [CrossRef]
- Silla, L.M.; Chen, J.; Zhong, R.K.; Whiteside, T.L.; Ball, E.D. Potentiation of lysis of leukaemia cells by a bispecific antibody to CD33 and CD16 (Fc gamma RIII) expressed by human natural killer (NK) cells. Br. J. Haematol. 1995, 89, 712–718. [Google Scholar]
- Cochlovius, B.; Kipriyanov, S.M.; Stassar, M.J.; Christ, O.; Schuhmacher, J.; Strauss, G.; Moldenhauer, G.; Little, M. Treatment of human B cell lymphoma xenografts with a CD3 x CD19 diabody and T cells. J. Immunol. 2000, 165, 888–895. [Google Scholar]
- Blanco, B.; Holliger, P.; Vile, R.G.; Alvarez-Vallina, L. Induction of human T lymphocyte cytotoxicity and inhibition of tumor growth by tumor-specific diabody-based molecules secreted from gene-modified bystander cells. J. Immunol. 2003, 171, 1070–1077. [Google Scholar]
- Schlereth, B.; Fichtner, I.; Lorenczewski, G.; Kleindienst, P.; Brischwein, K.; da Silva, A.; Kufer, P.; Lutterbuese, R.; Junghahn, I.; Kasimir-Bauer, S.; et al. Eradication of tumors from a human colon cancer cell line and from ovarian cancer metastases in immunodeficient mice by a single-chain Ep-CAM-/CD3-bispecific antibody construct. Cancer Res. 2005, 65, 2882–2889. [Google Scholar] [CrossRef]
- Muller, D.; Kontermann, R.E. Recombinant bispecific antibodies for cellular cancer immunotherapy. Curr. Opin. Mol. Ther. 2007, 9, 319–326. [Google Scholar]
- Kiessling, A.; Fussel, S.; Wehner, R.; Bachmann, M.; Wirth, M.P.; Rieber, E.P.; Schmitz, M. Advances in specific immunotherapy for prostate cancer. Eur. Urol. 2008, 53, 694–708. [Google Scholar]
- Arndt, C.; Feldmann, A.; Koristka, S.; Michalk, I.; Cartellieri, M.; Stamova, S.; von Bonin, M.; Bornhauser, M.; Ehninger, G.; Bachmann, M. Redirection of Immune Effector Cells by Bispecific Antibody Systems for the Treatment of Acute Myeloid Leukemia. Blood 2011, 118, 663–664. [Google Scholar]
- Feldmann, A.; Stamova, S.; Bippes, C.C.; Bartsch, H.; Wehner, R.; Schmitz, M.; Temme, A.; Cartellieri, M.; Bachmann, M. Retargeting of T cells to prostate stem cell antigen expressing tumor cells: Comparison of different antibody formats. Prostate 2011, 71, 998–1011. [Google Scholar] [CrossRef]
- Fortmuller, K.; Alt, K.; Gierschner, D.; Wolf, P.; Baum, V.; Freudenberg, N.; Wetterauer, U.; Elsasser-Beile, U.; Buhler, P. Effective targeting of prostate cancer by lymphocytes redirected by a PSMA x CD3 bispecific single-chain diabody. Prostate 2011, 71, 588–596. [Google Scholar] [CrossRef]
- Stamova, S.; Cartellieri, M.; Feldmann, A.; Bippes, C.C.; Bartsch, H.; Wehner, R.; Schmitz, M.; von Bonin, M.; Bornhauser, M.; Ehninger, G.; et al. Simultaneous engagement of the activatory receptors NKG2D and CD3 for retargeting of effector cells to CD33-positive malignant cells. Leukemia 2011, 25, 1053–1056. [Google Scholar]
- Milstein, C.; Cuello, A.C. Hybrid hybridomas and their use in immunohistochemistry. Nature 1983, 305, 537–540. [Google Scholar] [CrossRef]
- Kufer, P.; Lutterbuse, R.; Baeuerle, P.A. A revival of bispecific antibodies. Trends Biotechnol. 2004, 22, 238–244. [Google Scholar] [CrossRef]
- Segal, D.M.; Weiner, G.J.; Weiner, L.M. Bispecific antibodies in cancer therapy. Curr. Opin. Immunol. 1999, 11, 558–562. [Google Scholar]
- Kriangkum, J.; Xu, B.; Nagata, L.P.; Fulton, R.E.; Suresh, M.R. Bispecific and bifunctional single chain recombinant antibodies. Biomol. Eng. 2001, 18, 31–40. [Google Scholar] [CrossRef]
- Holliger, P.; Prospero, T.; Winter, G. "Diabodies": Small bivalent and bispecific antibody fragments. Proc. Natl. Acad. Sci. USA 1993, 90, 6444–6448. [Google Scholar]
- Lawrence, L.J.; Kortt, A.A.; Iliades, P.; Tulloch, P.A.; Hudson, P.J. Orientation of antigen binding sites in dimeric and trimeric single chain Fv antibody fragments. FEBS Lett. 1998, 425, 479–484. [Google Scholar] [CrossRef]
- Volkel, T.; Korn, T.; Bach, M.; Muller, R.; Kontermann, R.E. Optimized linker sequences for the expression of monomeric and dimeric bispecific single-chain diabodies. Protein Eng. 2001, 14, 815–823. [Google Scholar] [CrossRef]
- Bippes, C.C.; Feldmann, A.; Stamova, S.; Cartellieri, M.; Schwarzer, A.; Wehner, R.; Schmitz, M.; Rieber, E.P.; Zhao, S.; Schakel, K.; et al. A novel modular antigen delivery system for immuno targeting of human 6-sulfo LacNAc-positive blood dendritic cells (SlanDCs). PLoS One 2011, 6, e16315. [Google Scholar]
- Kontermann, R.E.; Korn, T.; Jerome, V. Recombinant adenoviruses for in vivo expression of antibody fragments. Methods Mol. Biol. 2003, 207, 421–433. [Google Scholar]
- Ren-Heidenreich, L.; Davol, P.A.; Kouttab, N.M.; Elfenbein, G.J.; Lum, L.G. Redirected T-cell cytotoxicity to epithelial cell adhesion molecule-overexpressing adenocarcinomas by a novel recombinant antibody, E3Bi, in vitro and in an animal model. Cancer 2004, 100, 1095–1103. [Google Scholar] [CrossRef]
- Stamova, S.; Cartellieri, M.; Feldmann, A.; Arndt, C.; Koristka, S.; Bartsch, H.; Bippes, C.C.; Wehner, R.; Schmitz, M.; von Bonin, M.; et al. Unexpected recombinations in single chain bispecific anti-CD3-anti-CD33 antibodies can be avoided by a novel linker module. Mol. Immunol. 2011, 49, 474–482. [Google Scholar] [CrossRef]
- Stamova, S.; Feldmann, A.; Cartellieri, M.; Arndt, C.; Koristka, S.; Apel, F.; Wehner, R.; Schmitz, M.; Bornhauser, M.; von Bonin, M.; et al. Generation of single-chain bispecific green fluorescent protein fusion antibodies for imaging of antibody-induced T cell synapses. Anal. Biochem. 2012, 423, 261–268. [Google Scholar]
- Rossi, E.A.; Goldenberg, D.M.; Cardillo, T.M.; McBride, W.J.; Sharkey, R.M.; Chang, C.H. Stably tethered multifunctional structures of defined composition made by the dock and lock method for use in cancer targeting. Proc. Natl. Acad. Sci. USA 2006, 103, 6841–6846. [Google Scholar]
- Saerens, D.; Ghassabeh, G.H.; Muyldermans, S. Single-domain antibodies as building blocks for novel therapeutics. Curr. Opin. Pharmacol. 2008, 8, 600–608. [Google Scholar] [CrossRef]
- Ward, E.S.; Gussow, D.; Griffiths, A.D.; Jones, P.T.; Winter, G. Binding activities of a repertoire of single immunoglobulin variable domains secreted from Escherichia coli. Nature 1989, 341, 544–546. [Google Scholar]
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hamers, C.; Songa, E.B.; Bendahman, N.; Hamers, R. Naturally occurring antibodies devoid of light chains. Nature 1993, 363, 446–448. [Google Scholar]
- Harmsen, M.M.; De Haard, H.J. Properties, production, and applications of camelid single-domain antibody fragments. Appl. Microbiol. Biotechnol. 2007, 77, 13–22. [Google Scholar] [CrossRef]
- Gill, D.S.; Damle, N.K. Biopharmaceutical drug discovery using novel protein scaffolds. Curr. Opin. Biotechnol. 2006, 17, 653–658. [Google Scholar] [CrossRef]
- Gebauer, M.; Skerra, A. Engineered protein scaffolds as next-generation antibody therapeutics. Curr. Opin. Chem. Biol. 2009, 13, 245–255. [Google Scholar] [CrossRef]
- Friedman, M.; Lindstrom, S.; Ekerljung, L.; Andersson-Svahn, H.; Carlsson, J.; Brismar, H.; Gedda, L.; Frejd, F.Y.; Stahl, S. Engineering and characterization of a bispecific HER2 × EGFR-binding affibody molecule. Biotechnol. Appl. Biochem. 2009, 54, 121–131. [Google Scholar] [CrossRef]
- Kipriyanov, S.M.; Cochlovius, B.; Schafer, H.J.; Moldenhauer, G.; Bahre, A.; Le Gall, F.; Knackmuss, S.; Little, M. Synergistic antitumor effect of bispecific CD19 × CD3 and CD19 x CD16 diabodies in a preclinical model of non-Hodgkin's lymphoma. J. Immunol. 2002, 169, 137–144. [Google Scholar]
- Asano, R.; Sone, Y.; Makabe, K.; Tsumoto, K.; Hayashi, H.; Katayose, Y.; Unno, M.; Kudo, T.; Kumagai, I. Humanization of the bispecific epidermal growth factor receptor × CD3 diabody and its efficacy as a potential clinical reagent. Clin. Cancer Res. 2006, 12, 4036–4042. [Google Scholar] [CrossRef]
- Buhler, P.; Wolf, P.; Gierschner, D.; Schaber, I.; Katzenwadel, A.; Schultze-Seemann, W.; Wetterauer, U.; Tacke, M.; Swamy, M.; Schamel, W.W.; et al. A bispecific diabody directed against prostate-specific membrane antigen and CD3 induces T-cell mediated lysis of prostate cancer cells. Cancer Immunol. Immunother. 2008, 57, 43–52. [Google Scholar] [CrossRef]
- Grosse-Hovest, L.; Hartlapp, I.; Marwan, W.; Brem, G.; Rammensee, H.G.; Jung, G. A recombinant bispecific single-chain antibody induces targeted, supra-agonistic CD28-stimulation and tumor cell killing. Eur. J. Immunol. 2003, 33, 1334–1340. [Google Scholar] [CrossRef]
- Otz, T.; Grosse-Hovest, L.; Hofmann, M.; Rammensee, H.G.; Jung, G. A bispecific single-chain antibody that mediates target cell-restricted, supra-agonistic CD28 stimulation and killing of lymphoma cells. Leukemia 2009, 23, 71–77. [Google Scholar] [CrossRef]
- Suntharalingam, G.; Perry, M.R.; Ward, S.; Brett, S.J.; Castello-Cortes, A.; Brunner, M.D.; Panoskaltsis, N. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 2006, 355, 1018–1028. [Google Scholar] [CrossRef]
- Grosse-Hovest, L.; Wick, W.; Minoia, R.; Weller, M.; Rammensee, H.G.; Brem, G.; Jung, G. Supraagonistic, bispecific single-chain antibody purified from the serum of cloned, transgenic cows induces T-cell-mediated killing of glioblastoma cells in vitro and in vivo. Int. J. Cancer 2005, 117, 1060–1064. [Google Scholar] [CrossRef]
- Mack, M.; Riethmuller, G.; Kufer, P. A small bispecific antibody construct expressed as a functional single-chain molecule with high tumor cell cytotoxicity. Proc. Natl. Acad. Sci. USA 1995, 92, 7021–7025. [Google Scholar] [CrossRef]
- Loffler, A.; Kufer, P.; Lutterbuse, R.; Zettl, F.; Daniel, P.T.; Schwenkenbecher, J.M.; Riethmuller, G.; Dorken, B.; Bargou, R.C. A recombinant bispecific single-chain antibody, CD19 × CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood 2000, 95, 2098–2103. [Google Scholar]
- Baeuerle, P.A.; Reinhardt, C. Bispecific T-cell engaging antibodies for cancer therapy. Cancer Res. 2009, 69, 4941–4944. [Google Scholar] [CrossRef]
- Nagorsen, D.; Baeuerle, P.A. Immunomodulatory therapy of cancer with T cell-engaging BiTE antibody blinatumomab. Exp. Cell Res. 2011, 317, 1255–1260. [Google Scholar] [CrossRef]
- Bargou, R.; Leo, E.; Zugmaier, G.; Klinger, M.; Goebeler, M.; Knop, S.; Noppeney, R.; Viardot, A.; Hess, G.; Schuler, M.; et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science 2008, 321, 974–977. [Google Scholar]
- Topp, M.S.; Kufer, P.; Gokbuget, N.; Goebeler, M.; Klinger, M.; Neumann, S.; Horst, H.A.; Raff, T.; Viardot, A.; Schmid, M.; et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J. Clin. Oncol. 2011, 29, 2493–2498. [Google Scholar]
- Molhoj, M.; Crommer, S.; Brischwein, K.; Rau, D.; Sriskandarajah, M.; Hoffmann, P.; Kufer, P.; Hofmeister, R.; Baeuerle, P.A. CD19-/CD3-bispecific antibody of the BiTE class is far superior to tandem diabody with respect to redirected tumor cell lysis. Mol. Immunol. 2007, 44, 1935–1943. [Google Scholar]
- Feldmann, A.; Arndt, C.; Töpfer, K.; Stamova, S.; Krone, C.M.; Koristka, S.; Michalk, I.; Lindemann, D.; Schmitz, M.; Temme, A.; et al. Novel humanized and highly efficient bispecific antibodies mediate killing of prostate stem cell antigen-expressing tumor cells by CD8+ and CD4+ T Cells. J. Immunol. 2012, in press.. [Google Scholar]
- Haagen, I.A.; de Lau, W.B.; Bast, B.J.; Geerars, A.J.; Clark, M.R.; de Gast, B.C. Unprimed CD4+ and CD8+ T cells can be rapidly activated by a CD3 × CD19 bispecific antibody to proliferate and become cytotoxic. Cancer Immunol. Immunother. 1994, 39, 391–396. [Google Scholar] [CrossRef]
- Dreier, T.; Lorenczewski, G.; Brandl, C.; Hoffmann, P.; Syring, U.; Hanakam, F.; Kufer, P.; Riethmuller, G.; Bargou, R.; Baeuerle, P.A. Extremely potent, rapid and costimulation-independent cytotoxic T-cell response against lymphoma cells catalyzed by a single-chain bispecific antibody. Int. J. Cancer 2002, 100, 690–697. [Google Scholar] [CrossRef]
- Buhler, P.; Molnar, E.; Dopfer, E.P.; Wolf, P.; Gierschner, D.; Wetterauer, U.; Schamel, W.W.; Elsasser-Beile, U. Target-dependent T-cell activation by coligation with a PSMA × CD3 diabody induces lysis of prostate cancer cells. J. Immunother. 2009, 32, 565–573. [Google Scholar] [CrossRef]
- Offner, S.; Hofmeister, R.; Romaniuk, A.; Kufer, P.; Baeuerle, P.A. Induction of regular cytolytic T cell synapses by bispecific single-chain antibody constructs on MHC class I-negative tumor cells. Mol. Immunol. 2006, 43, 763–771. [Google Scholar] [CrossRef]
- Wolf, E.; Hofmeister, R.; Kufer, P.; Schlereth, B.; Baeuerle, P.A. BiTEs: Bispecific antibody constructs with unique anti-tumor activity. Drug Discov. Today 2005, 10, 1237–1244. [Google Scholar]
- Koristka, S.; Cartellieri, M.; Theil, A.; Feldmann, A.; Arndt, C.; Stamova, S.; Michalk, I.; Topfer, K.; Temme, A.; Kretschmer, K.; et al. Retargeting of human regulatory T cells by single-chain bispecific antibodies. J. Immunol. 2012, 188, 1551–1558. [Google Scholar]
- Hoffmann, P.; Hofmeister, R.; Brischwein, K.; Brandl, C.; Crommer, S.; Bargou, R.; Itin, C.; Prang, N.; Baeuerle, P.A. Serial killing of tumor cells by cytotoxic T cells redirected with a CD19-/CD3-bispecific single-chain antibody construct. Int. J. Cancer 2005, 115, 98–104. [Google Scholar] [CrossRef]
- Boesteanu, A.C.; Katsikis, P.D. Memory T cells need CD28 costimulation to remember. Semin. Immunol. 2009, 21, 69–77. [Google Scholar] [CrossRef]
- Koristka, S.; Cartellieri, M.; Theil, A.; Arndt, C.; Feldmann, A.; Michalk, I.; Schmitz, M.; Kretschmer, K.; Bornhauser, M.; Ehninger, G.; et al. Antigen-Specific Redirection of Human Regulatory T Cells by Bispecific Antibodies. Blood 2011, 118, 1725–1726. [Google Scholar]
- Hombach, A.A.; Kofler, D.; Rappl, G.; Abken, H. Redirecting human CD4+CD25+ regulatory T cells from the peripheral blood with pre-defined target specificity. Gene Ther. 2009, 16, 1088–1096. [Google Scholar] [CrossRef]
- Lanier, L.L. Natural killer cell receptor signaling. Curr. Opin. Immunol. 2003, 15, 308–314. [Google Scholar]
- Moretta, L.; Moretta, A. Unravelling natural killer cell function: Triggering and inhibitory human NK receptors. EMBO J. 2004, 23, 255–259. [Google Scholar] [CrossRef]
- Salih, H.R.; Rammensee, H.G.; Steinle, A. Cutting edge: Down-regulation of MICA on human tumors by proteolytic shedding. J. Immunol. 2002, 169, 4098–4102. [Google Scholar]
- Fuertes, M.B.; Girart, M.V.; Molinero, L.L.; Domaica, C.I.; Rossi, L.E.; Barrio, M.M.; Mordoh, J.; Rabinovich, G.A.; Zwirner, N.W. Intracellular retention of the NKG2D ligand MHC class I chain-related gene A in human melanomas confers immune privilege and prevents NK cell-mediated cytotoxicity. J. Immunol. 2008, 180, 4606–4614. [Google Scholar]
- Diefenbach, A.; Jensen, E.R.; Jamieson, A.M.; Raulet, D.H. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature 2001, 413, 165–171. [Google Scholar]
- Germain, C.; Larbouret, C.; Cesson, V.; Donda, A.; Held, W.; Mach, J.P.; Pelegrin, A.; Robert, B. MHC class I-related chain A conjugated to antitumor antibodies can sensitize tumor cells to specific lysis by natural killer cells. Clin. Cancer Res. 2005, 11, 7516–7522. [Google Scholar]
- von Strandmann, E.P.; Hansen, H.P.; Reiners, K.S.; Schnell, R.; Borchmann, P.; Merkert, S.; Simhadri, V.R.; Draube, A.; Reiser, M.; Purr, I.; et al. A novel bispecific protein (ULBP2-BB4) targeting the NKG2D receptor on natural killer (NK) cells and CD138 activates NK cells and has potent antitumor activity against human multiple myeloma in vitro and in vivo. Blood 2006, 107, 1955–1962. [Google Scholar]
- Nausch, N.; Cerwenka, A. NKG2D ligands in tumor immunity. Oncogene 2008, 27, 5944–5958. [Google Scholar]
- Groh, V.; Rhinehart, R.; Randolph-Habecker, J.; Topp, M.S.; Riddell, S.R.; Spies, T. Costimulation of CD8alphabeta T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat. Immunol. 2001, 2, 255–260. [Google Scholar] [CrossRef]
- Kim, Y.J.; Han, M.K.; Broxmeyer, H.E. 4-1BB regulates NKG2D costimulation in human cord blood CD8+ T cells. Blood 2008, 111, 1378–1386. [Google Scholar]
- Doubrovina, E.S.; Doubrovin, M.M.; Vider, E.; Sisson, R.B.; O'Reilly, R.J.; Dupont, B.; Vyas, Y.M. Evasion from NK cell immunity by MHC class I chain-related molecules expressing colon adenocarcinoma. J. Immunol. 2003, 171, 6891–6899. [Google Scholar]
- Kim, Y.J.; Stringfield, T.M.; Chen, Y.; Broxmeyer, H.E. Modulation of cord blood CD8+ T-cell effector differentiation by TGF-beta1 and 4-1BB costimulation. Blood 2005, 105, 274–281. [Google Scholar] [CrossRef]
- Parmiani, G.; Rivoltini, L.; Andreola, G.; Carrabba, M. Cytokines in cancer therapy. Immunol. Lett. 2000, 74, 41–44. [Google Scholar] [CrossRef]
- Masztalerz, A.; Van Rooijen, N.; Den Otter, W.; Everse, L.A. Mechanisms of macrophage cytotoxicity in IL-2 and IL-12 mediated tumour regression. Cancer Immunol. Immunother. 2003, 52, 235–242. [Google Scholar]
- Emminger, W.; Emminger-Schmidmeier, W.; Peters, C.; Susani, M.; Hawliczek, R.; Hocker, P.; Gadner, H. Capillary leak syndrome during low dose granulocyte-macrophage colony-stimulating factor (rh GM-CSF) treatment of a patient in a continuous febrile state. Blut 1990, 61, 219–221. [Google Scholar] [CrossRef]
- Stern, A.C.; Jones, T.C. The side-effect profile of GM-CSF. Infection 1992, 20 (Suppl. 2), S124–S127. [Google Scholar] [CrossRef]
- Schwartz, R.N.; Stover, L.; Dutcher, J. Managing toxicities of high-dose interleukin-2. Oncology (Williston Park) 2002, 16, 11–20. [Google Scholar]
- Leonard, J.P.; Sherman, M.L.; Fisher, G.L.; Buchanan, L.J.; Larsen, G.; Atkins, M.B.; Sosman, J.A.; Dutcher, J.P.; Vogelzang, N.J.; Ryan, J.L. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood 1997, 90, 2541–2548. [Google Scholar]
- Neri, D.; Bicknell, R. Tumour vascular targeting. Nat. Rev. Cancer 2005, 5, 436–446. [Google Scholar] [CrossRef]
- Schrama, D.; Reisfeld, R.A.; Becker, J.C. Antibody targeted drugs as cancer therapeutics. Nat. Rev. Drug Discov. 2006, 5, 147–159. [Google Scholar] [CrossRef]
- Ortiz-Sanchez, E.; Helguera, G.; Daniels, T.R.; Penichet, M.L. Antibody-cytokine fusion proteins: Applications in cancer therapy. Expert Opin. Biol. Ther. 2008, 8, 609–632. [Google Scholar] [CrossRef]
- Ott, M.G.; Marme, F.; Moldenhauer, G.; Lindhofer, H.; Hennig, M.; Spannagl, R.; Essing, M.M.; Linke, R.; Seimetz, D. Humoral response to catumaxomab correlates with clinical outcome: Results of the pivotal phase II/III study in patients with malignant ascites. Int. J. Cancer 2012, 130, 2195–2203. [Google Scholar]
- Meredith, R.F.; Khazaeli, M.B.; Plott, W.E.; Saleh, M.N.; Liu, T.; Allen, L.F.; Russell, C.D.; Orr, R.A.; Colcher, D.; Schlom, J.; et al. Phase I trial of iodine-131-chimeric B72.3 (human IgG4) in metastatic colorectal cancer. J. Nucl. Med. 1992, 33, 23–29. [Google Scholar]
- Steffens, M.G.; Boerman, O.C.; Oosterwijk-Wakka, J.C.; Oosterhof, G.O.; Witjes, J.A.; Koenders, E.B.; Oyen, W.J.; Buijs, W.C.; Debruyne, F.M.; Corstens, F.H.; et al. Targeting of renal cell carcinoma with iodine-131-labeled chimeric monoclonal antibody G250. J. Clin. Oncol. 1997, 15, 1529–1537. [Google Scholar]
- Pavlinkova, G.; Colcher, D.; Booth, B.J.; Goel, A.; Wittel, U.A.; Batra, S.K. Effects of humanization and gene shuffling on immunogenicity and antigen binding of anti-TAG-72 single-chain Fvs. Int. J. Cancer 2001, 94, 717–726. [Google Scholar] [CrossRef]
- Verhoeyen, M.; Milstein, C.; Winter, G. Reshaping human antibodies: Grafting an antilysozyme activity. Science 1988, 239, 1534–1536. [Google Scholar]
- Jolliffe, L.K. Humanized antibodies: Enhancing therapeutic utility through antibody engineering. Int. Rev. Immunol. 1993, 10, 241–250. [Google Scholar] [CrossRef]
- Marks, J.D.; Hoogenboom, H.R.; Bonnert, T.P.; McCafferty, J.; Griffiths, A.D.; Winter, G. By-passing immunization. Human antibodies from V-gene libraries displayed on phage. J. Mol. Biol. 1991, 222, 581–597. [Google Scholar] [CrossRef]
- Vaughan, T.J.; Williams, A.J.; Pritchard, K.; Osbourn, J.K.; Pope, A.R.; Earnshaw, J.C.; McCafferty, J.; Hodits, R.A.; Wilton, J.; Johnson, K.S. Human antibodies with sub-nanomolar affinities isolated from a large non-immunized phage display library. Nat. Biotechnol. 1996, 14, 309–314. [Google Scholar] [CrossRef]
- Weiner, L.M. Fully human therapeutic monoclonal antibodies. J. Immunother. 2006, 29, 1–9. [Google Scholar] [CrossRef]
- Kontermann, R.E. Strategies to extend plasma half-lives of recombinant antibodies. BioDrugs 2009, 23, 93–109. [Google Scholar] [CrossRef]
- Muller, D.; Karle, A.; Meissburger, B.; Hofig, I.; Stork, R.; Kontermann, R.E. Improved pharmacokinetics of recombinant bispecific antibody molecules by fusion to human serum albumin. J. Biol. Chem. 2007, 282, 12650–12660. [Google Scholar]
- Stork, R.; Muller, D.; Kontermann, R.E. A novel tri-functional antibody fusion protein with improved pharmacokinetic properties generated by fusing a bispecific single-chain diabody with an albumin-binding domain from streptococcal protein G. Protein Eng. Des. Sel. 2007, 20, 569–576. [Google Scholar] [CrossRef]
- Stork, R.; Campigna, E.; Robert, B.; Muller, D.; Kontermann, R.E. Biodistribution of a bispecific single-chain diabody and its half-life extended derivatives. J. Biol. Chem. 2009, 284, 25612–25619. [Google Scholar]
- Holt, L.J.; Basran, A.; Jones, K.; Chorlton, J.; Jespers, L.S.; Brewis, N.D.; Tomlinson, I.M. Anti-serum albumin domain antibodies for extending the half-lives of short lived drugs. Protein Eng. Des. Sel. 2008, 21, 283–288. [Google Scholar] [CrossRef]
- Tijink, B.M.; Laeremans, T.; Budde, M.; Stigter-van Walsum, M.; Dreier, T.; de Haard, H.J.; Leemans, C.R.; van Dongen, G.A. Improved tumor targeting of anti-epidermal growth factor receptor Nanobodies through albumin binding: Taking advantage of modular Nanobody technology. Mol. Cancer Ther. 2008, 7, 2288–2297. [Google Scholar] [CrossRef]
- Marvin, J.S.; Zhu, Z. Recombinant approaches to IgG-like bispecific antibodies. Acta Pharmacol. Sin. 2005, 26, 649–658. [Google Scholar] [CrossRef]
- Stork, R.; Zettlitz, K.A.; Muller, D.; Rether, M.; Hanisch, F.G.; Kontermann, R.E. N-Glycosylation as novel strategy to improve pharmacokinetic properties of bispecific single-chain diabodies. J. Biol. Chem. 2008, 283, 7804–7812. [Google Scholar]
- Chapman, A.P. PEGylated antibodies and antibody fragments for improved therapy: A review. Adv. Drug Deliv. Rev. 2002, 54, 531–545. [Google Scholar] [CrossRef]
- Seimetz, D.; Lindhofer, H.; Bokemeyer, C. Development and approval of the trifunctional antibody catumaxomab (anti-EpCAM × anti-CD3) as a targeted cancer immunotherapy. Cancer Treat. Rev. 2010, 36, 458–467. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).