A Cassette Vector System for the Rapid Cloning and Production of Bispecific Tetravalent Antibodies
Abstract
:1. Introduction
2. Results and Discussion
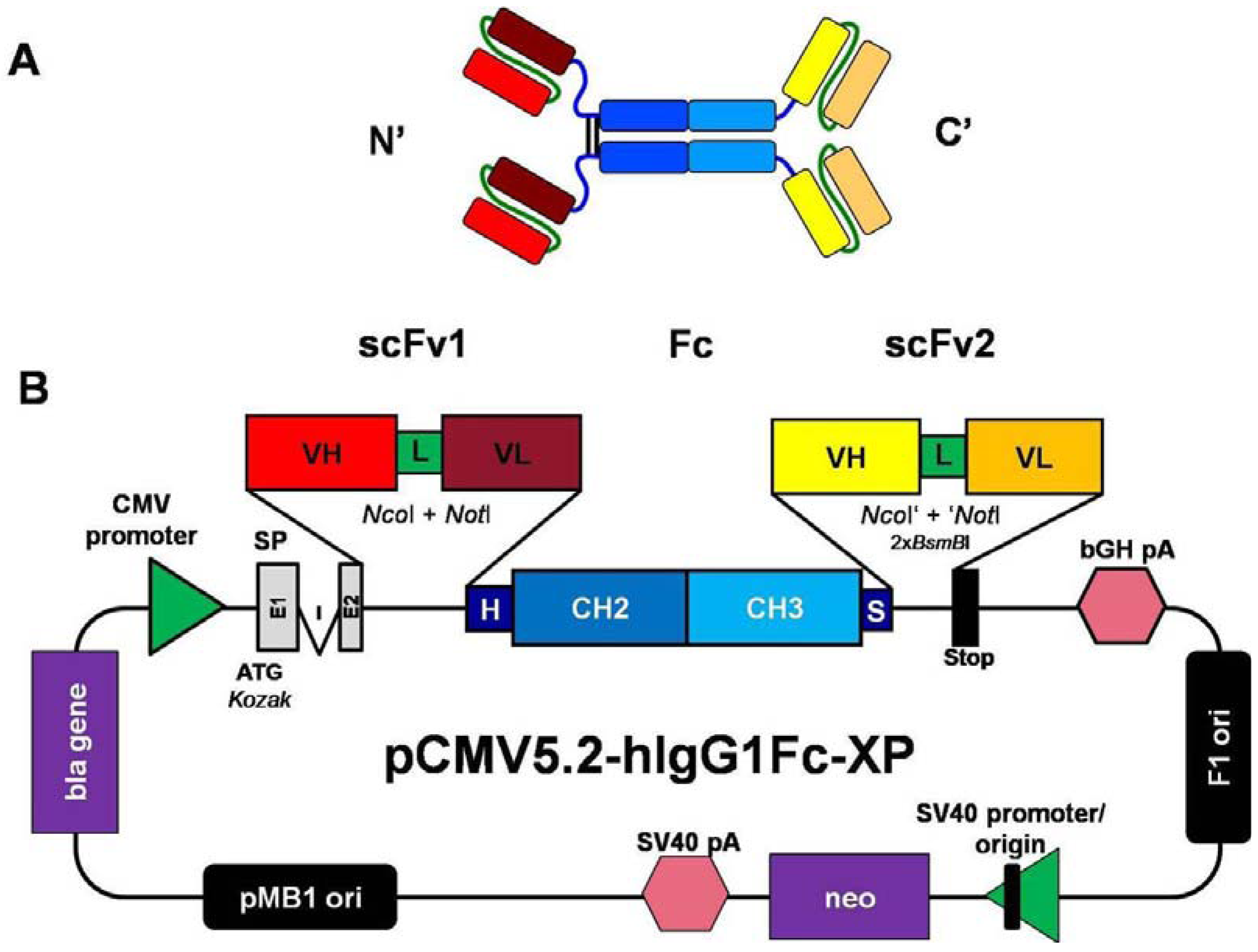
2.1. Construction of Mammalian Expression Vectors for the Production of scFv-Fc-scFv Antibodies
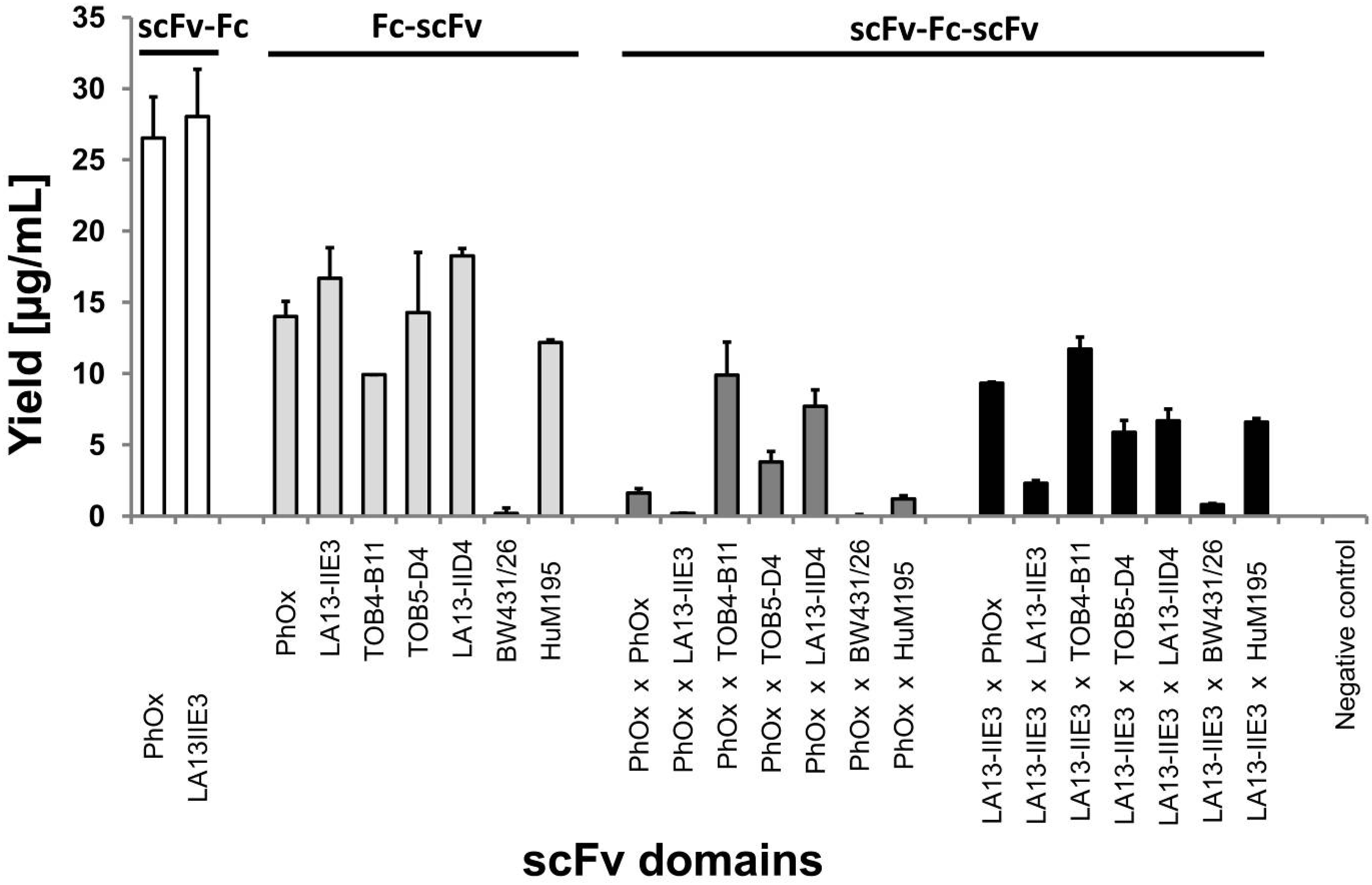
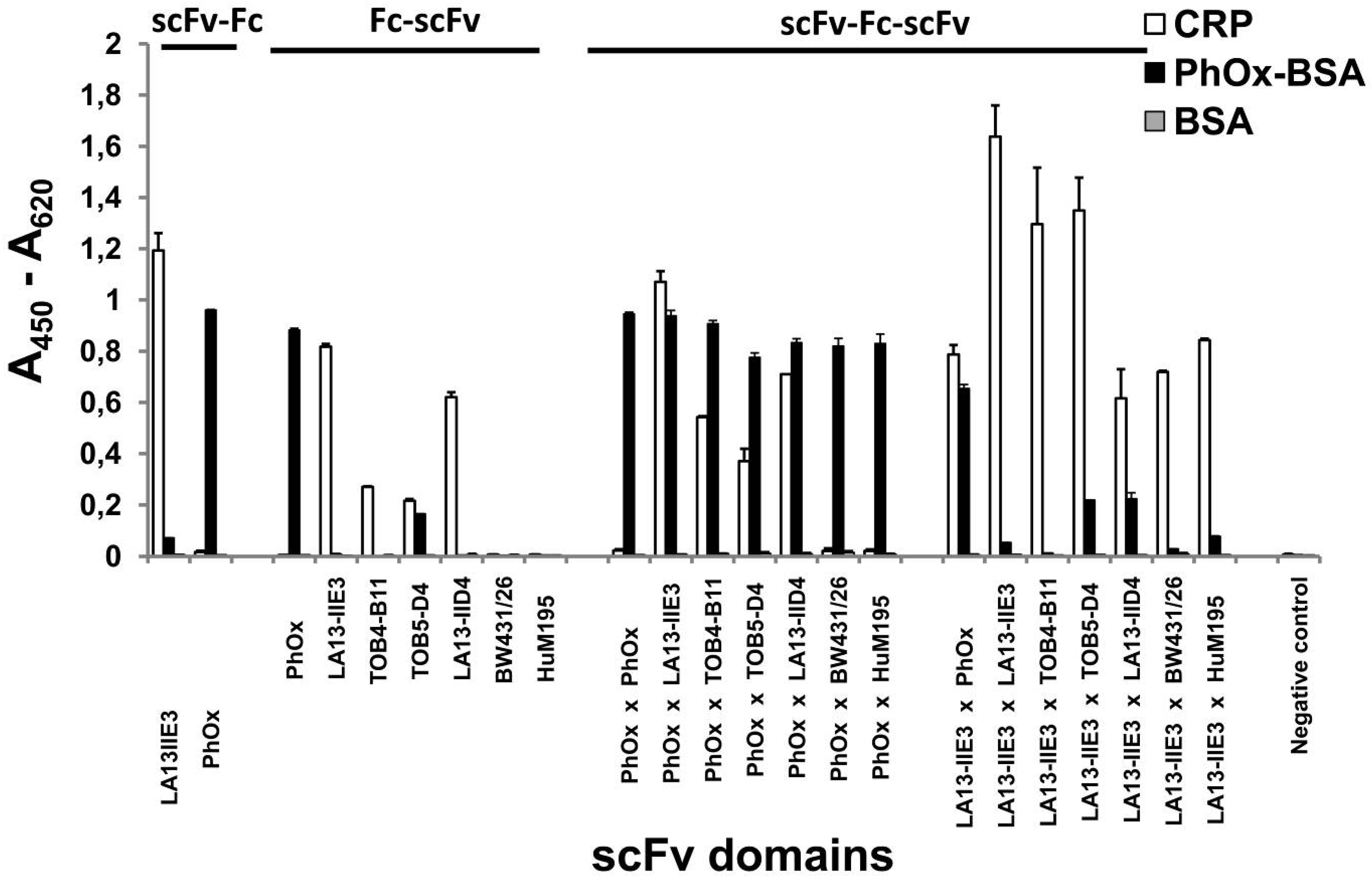
2.2. Production of scFv-Fc-scFvs Depends on the Employed scFv Domains
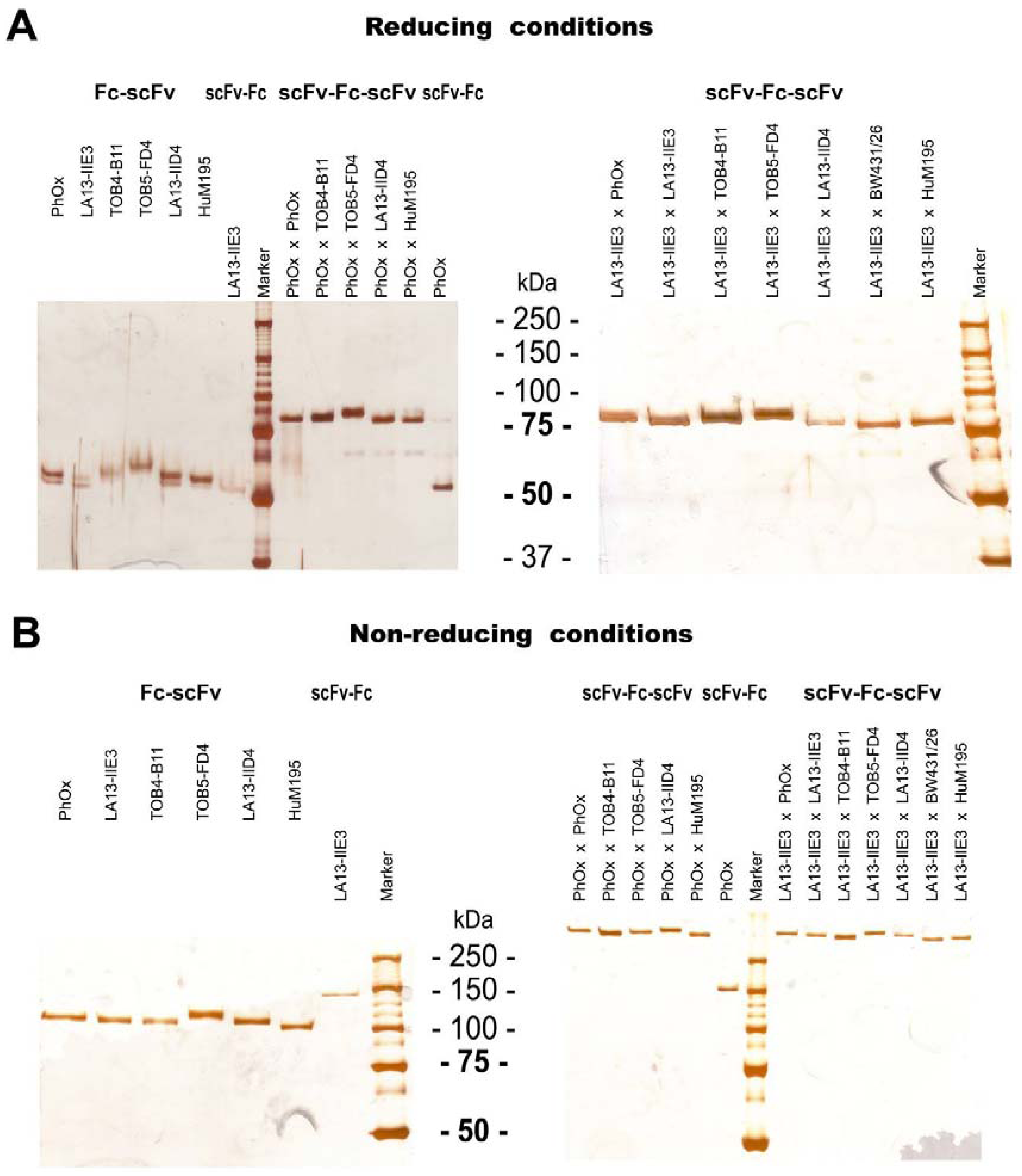
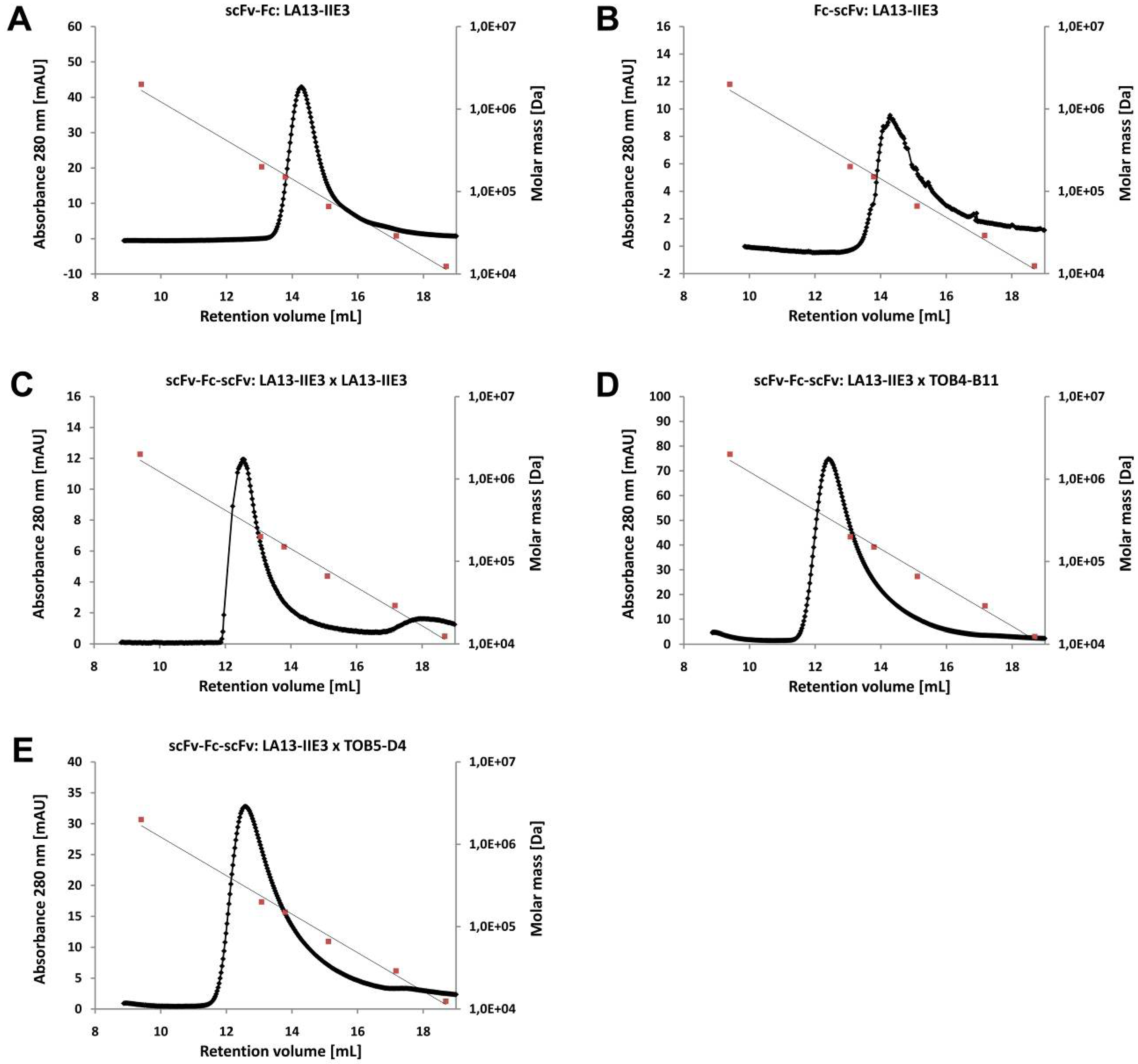
2.3. ScFv, Fc-scFv and scFv-Fc-scFv Antibodies Exclusively form Homodimers
| Construct | scFv2 | ||
|---|---|---|---|
| Homodimer [kDa] | Molar mass [kDa] | ||
| scFv-Fc | 103.8 | 112.4 | |
| Fc-scFv | LA13-IIE3 | 107.7 | 115.1 |
| scFv-Fc-scFv | LA13-IIE3 | 156.4 | 175.4 |
| scFv-Fc-scFv | TOB4-B11 | 159.2 | 179.5 |
| scFv-Fc-scFv | TOB5-D4 | 162.7 | 175.4 |
2.4. ScFv-Fc-scFv Antibodies Provide Dual Antigen Specificity
2.6. Avidity Effects of Tetravalent Antigen Binding by scFv-Fc-scFv Antibodies

| Antibody construct | scFv- | Fc | -scFv | EC50 [M] * | Increase of CRP binding (fold) ** |
|---|---|---|---|---|---|
| scFv *** | LA13-IIE3 | - | - | 2 × 10−8 | 1 |
| scFv-Fc | LA13-IIE3 | + | - | 4 × 10−10 | 50 |
| Fc-scFv | - | + | LA13-IIE3 | 1 × 10−9 | 20 |
| scFv-Fc-scFv | LA13-IIE3 | + | LA13-IIE3 | 4 × 10−11 | 500 |
| scFv-Fc-scFv | LA13-IIE3 | + | TOB4-B11 | 5 × 10−11 | 400 |
| scFv-Fc-scFv | LA13-IIE3 | + | TOB5-D4 | 4 × 10−10 | 50 |
2.7. Tetravalent scFv-Fc-scFv Antibodies Have Slower off-Rates Compared to Bivalent and Monovalent Antibody Formats
| Antibody construct | kon | koff | Rmax | Chi2 | KD * | ||||
|---|---|---|---|---|---|---|---|---|---|
| scFv- | Fc | -scFv | [1/Ms] | [1/s] | [RU] | [M] | Ratio to scFv | Ratio to scFv-Fc | |
| LA13-IIE3 ** | - | - | 1.6 × 105 | 1.6 × 10−3 | 160 | 16,6 | 1.0 × 10−8 | 1.0 | - |
| LA13-IIE3 | + | - | 2.1 × 106 | 5.1 × 10−3 | 154 | 65,1 | 2.5 × 10−9 | 4.2 | 1.0 |
| LA13-IIE3 | + | LA13-IIE3 | 4.3 × 106 | 3.1 × 10−4 | 216 | 65,4 | 7.2 × 10−11 | 141.9 | 34.1 |
| LA13-IIE3 | + | TOB4-B11 | 1.2 × 106 | 6.3 × 10−4 | 131 | 116,0 | 5.2 × 10−10 | 19.8 | 4.8 |
| LA13-IIE3 | + | TOB5-D4 | 1.6 × 106 | 7.8 × 10−4 | 157 | 131 | 5.0 × 10−10 | 20.8 | 5.0 |
| Antibody construct | kon1 | koff1 | Rmax | Chi2 | ||
|---|---|---|---|---|---|---|
| scFv- | Fc | -scFv | [1/Ms] | [1/s] | [RU] | |
| LA13-IIE3 | + | - | 9.5 × 105 | 6.5 × 10−3 | 178 | 6.93 |
| LA13-IIE3 | + | LA13-IIE3 | 1.2 × 106 | 2.7 × 10−3 | 378 | 12.3 |
| LA13-IIE3 | + | TOB4-B11 | 6.3 × 105 | 1.2 × 10−3 | 161 | 22.6 |
| LA13-IIE3 | + | TOB5-D4 | 8.0 × 105 | 1.4 × 10−3 | 191 | 36.7 |
3. Experimental Section
3.1. Single Chain (sc)Fv Antibody Gene Fragments
3.2. Selection of scFvs against Human CRP by Phage Display
3.3. ScFv Production and Purification
3.4. Construction of pCMV2.2- and pCMV5.2-hIgG1Fc-XP Vectors and the scFv-Fc, Fc-scFv and scFv-Fc-scFv Antibody Constructs
3.5. Antigens
3.6. Cell Culture
3.7. Transient Transfection and Production in HEK293T Cells
3.8. Quantification of Antibodies by IgG/Fc Capture ELISA
3.9. Protein Purification with Protein A Magnetic Beads and by Protein A Filtration
3.10. SDS-PAGE and Silver Staining
3.11. Analytical Size Exclusion Chromatograpy (SEC)
3.12. Antigen ELISA
3.13. Affinity and Kinetics Measurements
4. Conclusions
Acknowledgments
References and Notes
- Hust, M.; Meyer, T.; Voedisch, B.; Rülker, T.; Thie, H.; El-Ghezal, A.; Kirsch, M.I.; Schütte, M.; Helmsing, S.; Meier, D.; et al. A human scFv antibody generation pipeline for proteome research. J. Biotechnol. 2011, 152, 159–170. [Google Scholar]
- Thie, H.; Voedisch, B.; Dübel, S.; Hust, M.; Schirrmann, T. Affinity maturation by phage display. Methods Mol. Biol 2009, 525, 309–322, xv. [Google Scholar] [CrossRef]
- Thie, H.; Toleikis, L.; Li, J.; von Wasielewski, R.; Bastert, G.; Schirrmann, T.; Esteves, I.T.; Behrens, C.K.; Fournes, B.; Fournier, N.; et al. Rise and fall of an anti-MUC1 specific antibody. PLoS One 2011, 6, e15921. [Google Scholar]
- Kipriyanov, S.M.; Little, M.; Kropshofer, H.; Breitling, F.; Gotter, S.; Dübel, S. Affinity enhancement of a recombinant antibody: Formation of complexes with multiple valency by a single-chain Fv fragment-core streptavidin fusion. Protein Eng. 1996, 9, 203–211. [Google Scholar]
- Thie, H.; Binius, S.; Schirrmann, T.; Hust, M.; Dübel, S. Multimerization domains for antibody phage display and antibody production. N. Biotechnol. 2009, 26, 314–321. [Google Scholar] [CrossRef]
- Schirrmann, T.; Büssow, K. Transient production of scFv-Fc fusion proteins in mammalian cells. In Antibody Engineering; Kontermann, R., Dübel, S., Eds.; Springer Berlin Heidelberg: Berlin, Germany, 2010; pp. 387–398. [Google Scholar]
- Schirrmann, T.; Menzel, C.; Hust, M.; Prilop, J.; Jostock, T.; Dübel, S. Oligomeric forms of single chain immunoglobulin (scIgG). MAbs 2010, 2, 73–76. [Google Scholar] [CrossRef]
- McCall, A.M.; Adams, G.P.; Amoroso, A.R.; Nielsen, U.B.; Zhang, L.; Horak, E.; Simmons, H.; Schier, R.; Marks, J.D.; Weiner, L.M. Isolation and characterization of an anti-CD16 single-chain Fv fragment and construction of an anti-HER2/neu/anti-CD16 bispecific scFv that triggers CD16-dependent tumor cytolysis. Mol. Immunol. 1999, 36, 433–445. [Google Scholar] [CrossRef]
- Hammond, S.A.; Lutterbuese, R.; Roff, S.; Lutterbuese, P.; Schlereth, B.; Bruckheimer, E.; Kinch, M.S.; Coats, S.; Baeuerle, P.A.; Kufer, P.; et al. Selective targeting and potent control of tumor growth using an EphA2/CD3-bispecific single-chain antibody construct. Cancer Res. 2007, 67, 3927–3935. [Google Scholar]
- Mølhøj, M.; Crommer, S.; Brischwein, K.; Rau, D.; Sriskandarajah, M.; Hoffmann, P.; Kufer, P.; Hofmeister, R.; Baeuerle, P.A. CD19-/CD3-bispecific antibody of the BiTE class is far superior to tandem diabody with respect to redirected tumor cell lysis. Mol. Immunol. 2007, 44, 1935–1943. [Google Scholar]
- Schanzer, J.; Jekle, A.; Nezu, J.; Lochner, A.; Croasdale, R.; Dioszegi, M.; Zhang, J.; Hoffmann, E.; Dormeyer, W.; Stracke, J.; et al. Development of tetravalent, bispecific CCR5 antibodies with antiviral activity against CCR5 monoclonal antibody-resistant HIV-1 strains. Antimicrob. Agents Chemother. 2011, 55, 2369–2378. [Google Scholar] [CrossRef]
- Mabry, R.; Gilbertson, D.G.; Frank, A.; Vu, T.; Ardourel, D.; Ostrander, C.; Stevens, B.; Julien, S.; Franke, S.; Meengs, B.; et al. A dual-targeting PDGFRbeta/VEGF-A molecule assembled from stable antibody fragments demonstrates anti-angiogenic activity in vitro and in vivo. MAbs 2010, 2, 20–34. [Google Scholar] [CrossRef]
- Cao, Y.; Lam, L. Bispecific antibody conjugates in therapeutics. Adv. Drug Deliv. Rev. 2003, 55, 171–197. [Google Scholar] [CrossRef]
- Müller, D.; Kontermann, R.E. Bispecific antibodies for cancer immunotherapy: Current perspectives. BioDrugs 2010, 24, 89–98. [Google Scholar] [CrossRef]
- Linke, R.; Klein, A.; Seimetz, D. Catumaxomab. MAbs 2010, 2, 129–136. [Google Scholar] [CrossRef]
- Bargou, R.; Leo, E.; Zugmaier, G.; Klinger, M.; Goebeler, M.; Knop, S.; Noppeney, R.; Viardot, A.; Hess, G.; Schuler, M.; et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science 2008, 321, 974–977. [Google Scholar]
- Schaefer, W.; Regula, J.T.; Bähner, M.; Schanzer, J.; Croasdale, R.; Dürr, H.; Gassner, C.; Georges, G.; Kettenberger, H.; Imhof-Jung, S.; et al. Immunoglobulin domain crossover as a generic approach for the production of bispecific IgG antibodies. Proc. Natl. Acad. Sci. USA 2011, 108, 11187–11192. [Google Scholar]
- Muda, M.; Gross, A.W.; Dawson, J.P.; He, C.; Kurosawa, E.; Schweickhardt, R.; Dugas, M.; Soloviev, M.; Bernhardt, A.; Fischer, D.; et al. Therapeutic assessment of SEED: A new engineered antibody platform designed to generate mono- and bispecific antibodies. Protein Eng. Des. Sel. 2011, 24, 447–454. [Google Scholar] [CrossRef]
- Asano, R.; Watanabe, Y.; Kawaguchi, H.; Fukazawa, H.; Nakanishi, T.; Umetsu, M.; Hayashi, H.; Katayose, Y.; Unno, M.; Kudo, T.; et al. Highly effective recombinant format of a humanized IgG-like bispecific antibody for cancer immunotherapy with retargeting of lymphocytes to tumor cells. J. Biol. Chem. 2007, 282, 27659–27665. [Google Scholar]
- Wu, C.; Ying, H.; Grinnell, C.; Bryant, S.; Miller, R.; Clabbers, A.; Bose, S.; McCarthy, D.; Zhu, R.-R.; Santora, L.; et al. Simultaneous targeting of multiple disease mediators by a dual-variable-domain immunoglobulin. Nat. Biotechnol. 2007, 25, 1290–1297. [Google Scholar] [CrossRef]
- Michaelson, J.S.; Demarest, S.J.; Miller, B.; Amatucci, A.; Snyder, W.B.; Wu, X.; Huang, F.; Phan, S.; Gao, S.; Doern, A.; et al. Anti-tumor activity of stability-engineered IgG-like bispecific antibodies targeting TRAIL-R2 and LTbetaR. MAbs 2009, 1, 128–141. [Google Scholar] [CrossRef]
- Dong, J.; Sereno, A.; Aivazian, D.; Langley, E.; Miller, B.R.; Snyder, W.B.; Chan, E.; Cantele, M.; Morena, R.; Joseph, I.B.J.K.; et al. A stable IgG-like bispecific antibody targeting the epidermal growth factor receptor and the type I insulin-like growth factor receptor demonstrates superior anti-tumor activity. MAbs 2011, 3, 273–288. [Google Scholar] [CrossRef]
- Alt, M.; Müller, R.; Kontermann, R.E. Novel tetravalent and bispecific IgG-like antibody molecules combining single-chain diabodies with the immunoglobulin gamma1 Fc or CH3 region. FEBS Lett. 1999, 454, 90–94. [Google Scholar] [CrossRef]
- Connelly, R.J.; Hayden, M.S.; Scholler, J.K.; Tsu, T.T.; Dupont, B.; Ledbetter, J.A.; Kanner, S.B. Mitogenic properties of a bispecific single-chain Fv-Ig fusion generated from CD2-specific mAb to distinct epitopes. Int. Immunol. 1998, 10, 1863–1872. [Google Scholar] [CrossRef]
- Mabry, R.; Lewis, K.E.; Moore, M.; McKernan, P.A.; Bukowski, T.R.; Bontadelli, K.; Brender, T.; Okada, S.; Lum, K.; West, J.; et al. Engineering of stable bispecific antibodies targeting IL-17A and IL-23. Protein Eng. Des. Sel. 2010, 23, 115–127. [Google Scholar] [CrossRef]
- Jendreyko, N.; Popkov, M.; Beerli, R.R.; Chung, J.; McGavern, D.B.; Rader, C.; Barbas, C.F., 3rd. Intradiabodies, bispecific, tetravalent antibodies for the simultaneous functional knockout of two cell surface receptors. J. Biol. Chem. 2003, 278, 47812–47819. [Google Scholar]
- Schirrmann, T.; Pecher, G. Human natural killer cell line modified with a chimeric immunoglobulin T-cell receptor gene leads to tumor growth inhibition in vivo. Cancer Gene Ther. 2002, 9, 390–398. [Google Scholar] [CrossRef]
- Schirrmann, T.; Pecher, G. Specific targeting of CD33(+) leukemia cells by a natural killer cell line modified with a chimeric receptor. Leuk. Res. 2005, 29, 301–306. [Google Scholar] [CrossRef]
- Jostock, T.; Vanhove, M.; Brepoels, E.; Van Gool, R.; Daukandt, M.; Wehnert, A.; Van Hegelsom, R.; Dransfield, D.; Sexton, D.; Devlin, M.; et al. Rapid generation of functional human IgG antibodies derived from Fab-on-phage display libraries. J. Immunol. Methods 2004, 289, 65–80. [Google Scholar] [CrossRef]
- Li, J.; Menzel, C.; Meier, D.; Zhang, C.; Dübel, S.; Jostock, T. A comparative study of different vector designs for the mammalian expression of recombinant IgG antibodies. J. Immunol. Methods 2007, 318, 113–124. [Google Scholar] [CrossRef]
- Goldsmith, M.E.; Konigsberg, W.H. Adsorption protein of the bacteriophage fd: Isolation, molecular properties, and location in the virus. Biochemistry 1977, 16, 2686–2694. [Google Scholar] [CrossRef]
- Al-Halabi, L.; Balck, A.; Michalzik, M.; Fröde, D.; Büttgenbach, S.; Hust, M.; Schirrmann, T.; Dübel, S. Recombinant antibody fragments allow repeated measurements of C-reactive protein with a quartz crystal microbalance immunosensor. J. Biotechnol. 2012. submitted. [Google Scholar]
- Marks, J.D.; Griffiths, A.D.; Malmqvist, M.; Clackson, T.P.; Bye, J.M.; Winter, G. By-passing immunization: building high affinity human antibodies by chain shuffling. Biotechnology (N.Y.) 1992, 10, 779–783. [Google Scholar] [CrossRef]
- Hust, M.; Toleikis, L.; Dübel, S. Antibody phage display. Handbook of therapeutic antibodies. In Handbook of Therapeutic Antibodies; Wiley-VCH: Weinheim, Germany, 2007; Volume 1, pp. 45–68. [Google Scholar]
- Hust, M.; Dübel, S.; Schirrmann, T. Selection of recombinant antibodies from antibody gene libraries. Methods Mol. Biol 2007, 408, 243–255. [Google Scholar] [CrossRef]
- Hust, M.; Steinwand, M.; Al-Halabi, L.; Helmsing, S.; Schirrmann, T.; Dübel, S. Improved microtitre plate production of single chain Fv fragments in Escherichia coli. N. Biotechnol. 2009, 25, 424–428. [Google Scholar] [CrossRef]
- Schmiedl, A.; Breitling, F.; Winter, C.H.; Queitsch, I.; Dübel, S. Effects of unpaired cysteines on yield, solubility and activity of different recombinant antibody constructs expressed in E. coli. J. Immunol. Methods 2000, 242, 101–114. [Google Scholar]
- Jordan, E.; Hust, M.; Roth, A.; Biedendieck, R.; Schirrmann, T.; Jahn, D.; Dübel, S. Production of recombinant antibody fragments in Bacillus megaterium. Microb. Cell Fact. 2007, 6, 2. [Google Scholar] [CrossRef]
- Rülker, T.; Meier, D.; Schirrmann, T. Quantification of human IgG and related Fc fusion proteins by a human IgG/Fc capture ELISA. In Antibody Engineering; Kontermann, R., Dübel, S., Eds.; Springer Berlin Heidelberg: Berlin, Germany, 2010; pp. 743–748. [Google Scholar]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Blum, H.; Beier, H.; Gross, H.J. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 1987, 8, 93–99. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pohl, S.C.; Schwarz, S.; Frenzel, A.; Schirrmann, T. A Cassette Vector System for the Rapid Cloning and Production of Bispecific Tetravalent Antibodies. Antibodies 2012, 1, 19-38. https://doi.org/10.3390/antib1010019
Pohl SC, Schwarz S, Frenzel A, Schirrmann T. A Cassette Vector System for the Rapid Cloning and Production of Bispecific Tetravalent Antibodies. Antibodies. 2012; 1(1):19-38. https://doi.org/10.3390/antib1010019
Chicago/Turabian StylePohl, Stefanie Claudia, Steffi Schwarz, André Frenzel, and Thomas Schirrmann. 2012. "A Cassette Vector System for the Rapid Cloning and Production of Bispecific Tetravalent Antibodies" Antibodies 1, no. 1: 19-38. https://doi.org/10.3390/antib1010019
APA StylePohl, S. C., Schwarz, S., Frenzel, A., & Schirrmann, T. (2012). A Cassette Vector System for the Rapid Cloning and Production of Bispecific Tetravalent Antibodies. Antibodies, 1(1), 19-38. https://doi.org/10.3390/antib1010019




