Tree Roots Anchoring and Binding Soil: Reducing Landslide Risk in Indonesian Agroforestry
Abstract
:1. Introduction
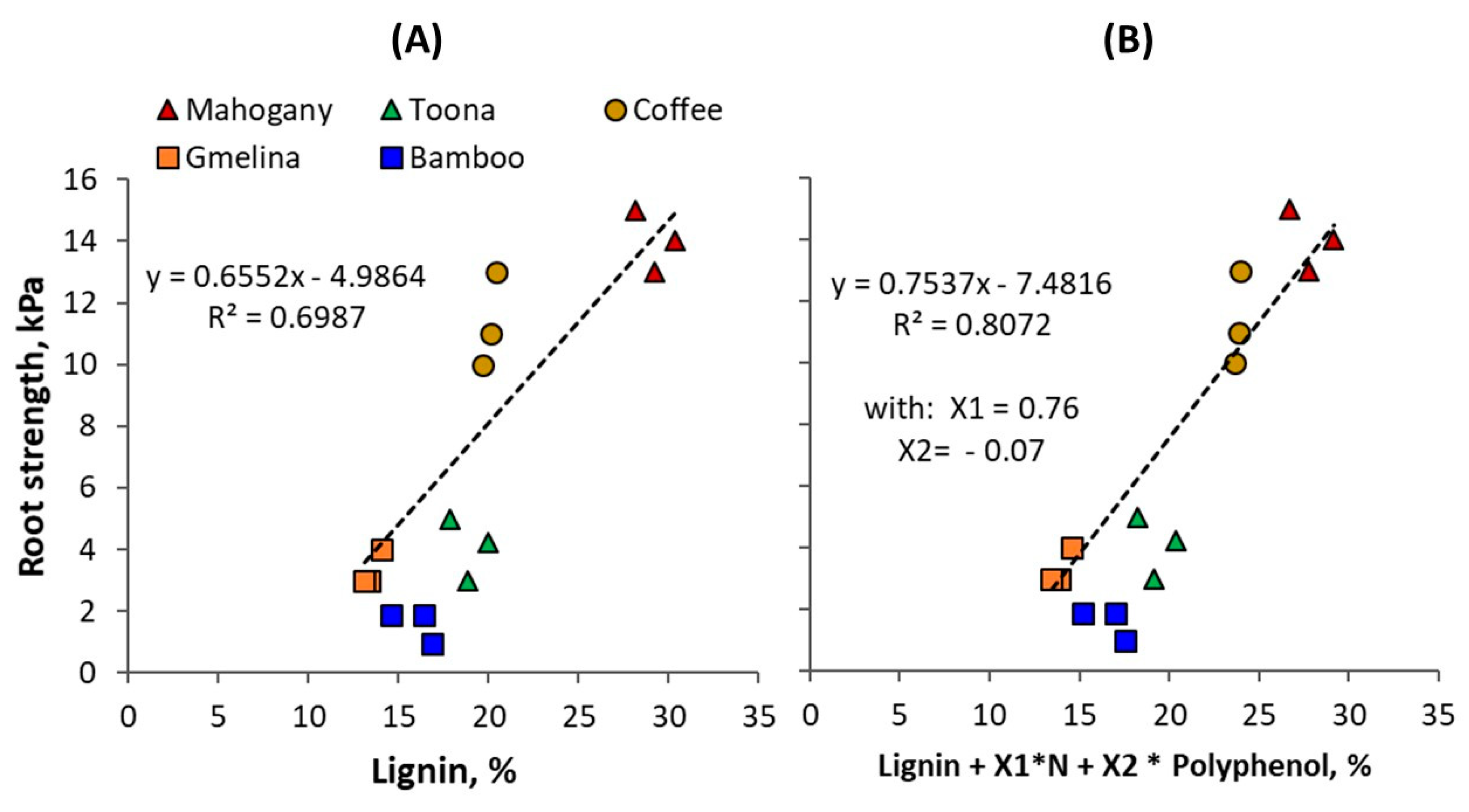
- The critical load where individual roots break is related to the root’s lignin content,
- The shear strength of a volume of soil increases proportionally to the number and strength of individual roots,
- With a similar overall ratio of woody root to stem cross sectional area, there are consistent differences between tree species in root development in the topsoil and at depth, that contribute to differences in soil binding and soil anchoring, respectively,
- Differences in the distribution of tree roots between species can be used to reduce landslide risks in the context of productive coffee agroforestry systems.
2. Materials and Methods
2.1. Location
2.2. Individual Root Strength in Relation to Root Properties
2.3. Role of Root Length Density in Soil Shear Strength
2.4. Inventory of Potential Tree Root System as an Anchor to Maintain Slope and Riverbank Stability
3. Results
3.1. Individual Root Strength in Relation to Root Properties
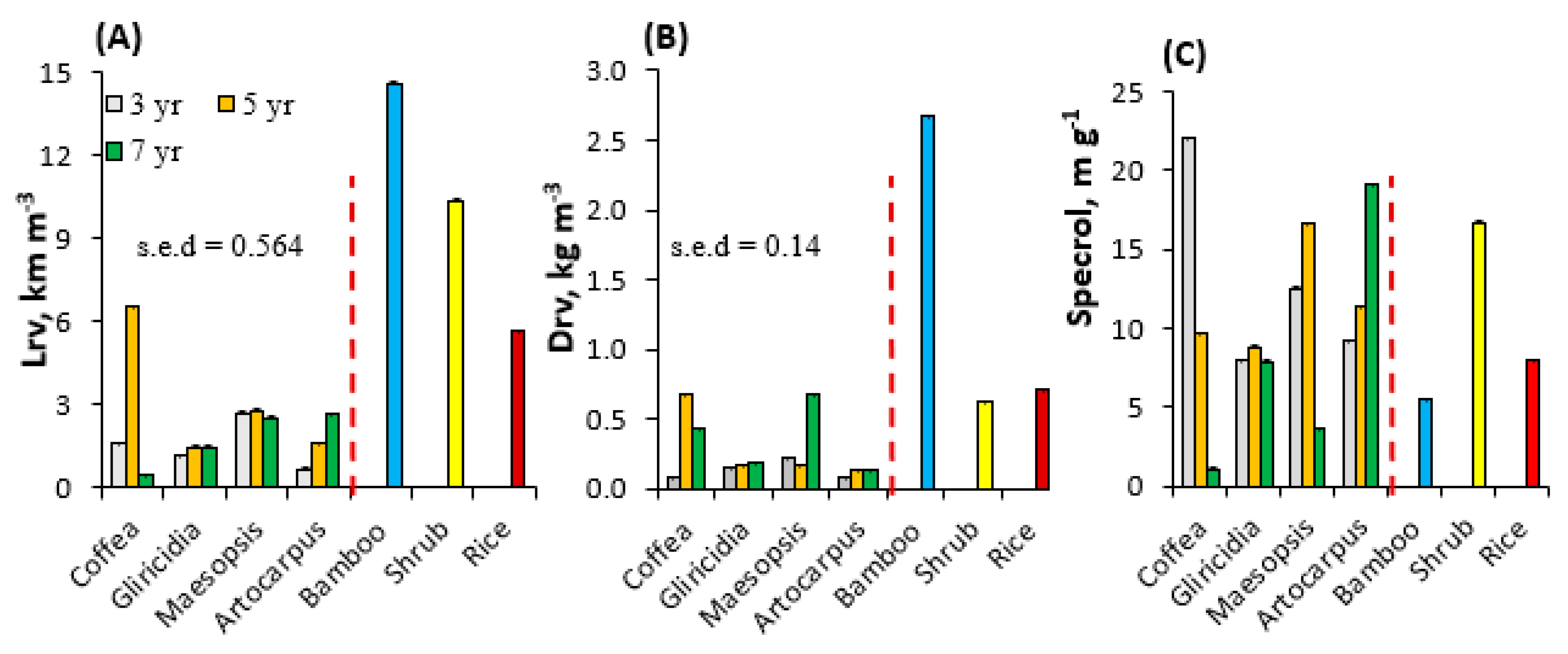
3.2. Tree Root Length Density (Lrv)
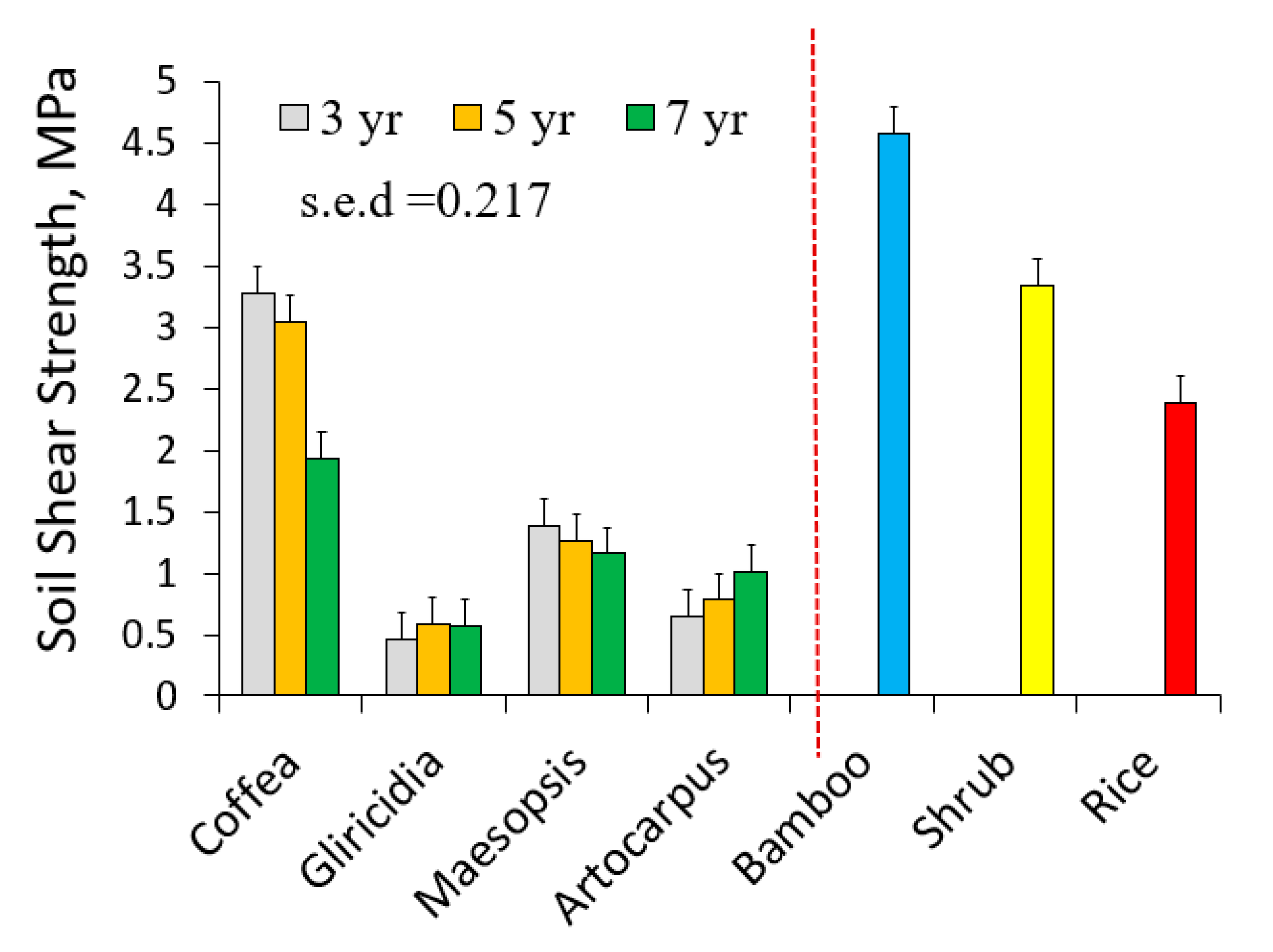
3.3. Soil Shear Strength
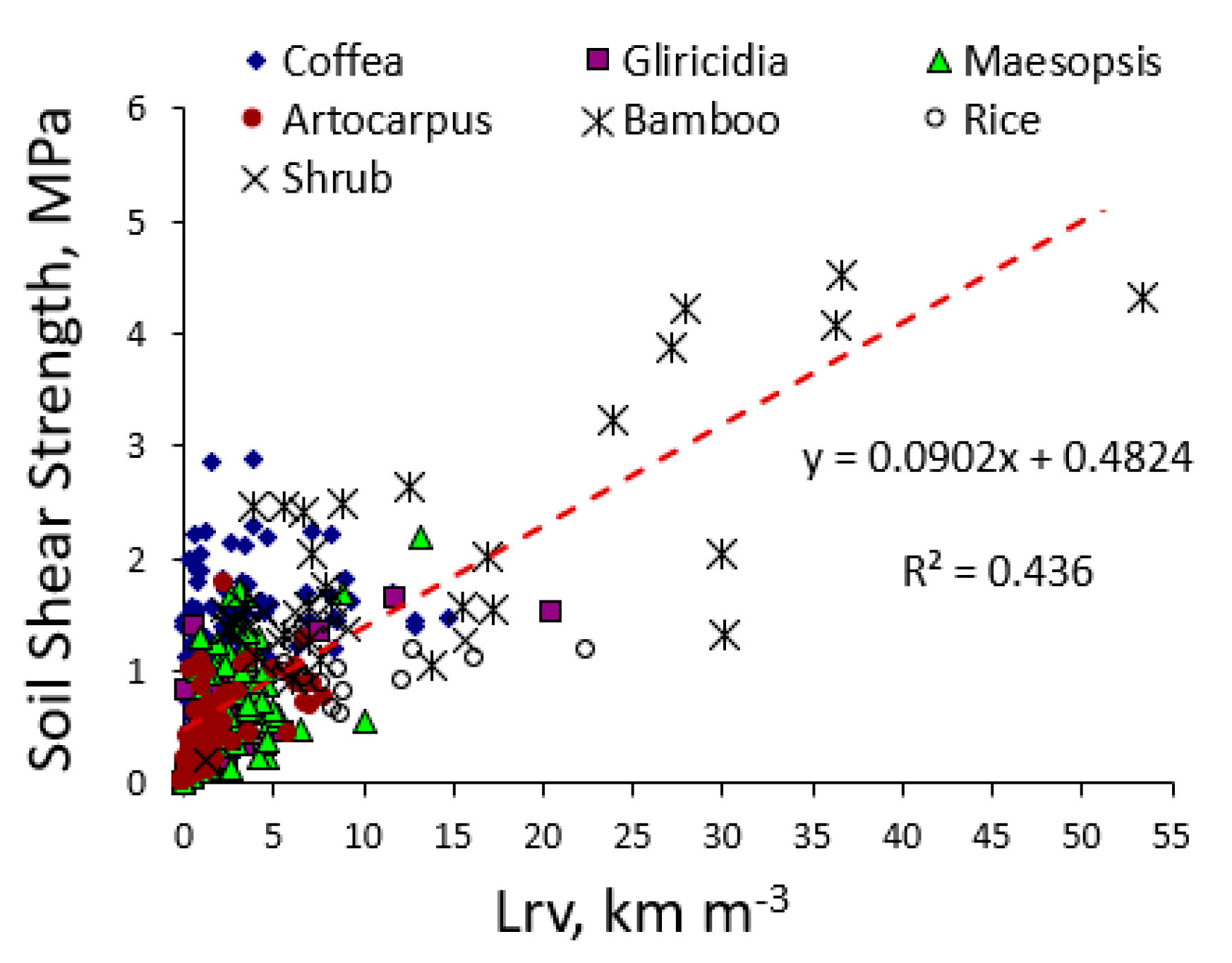
3.4. Soil Shear Strength in Relation to Root Length Density in the Topsoil
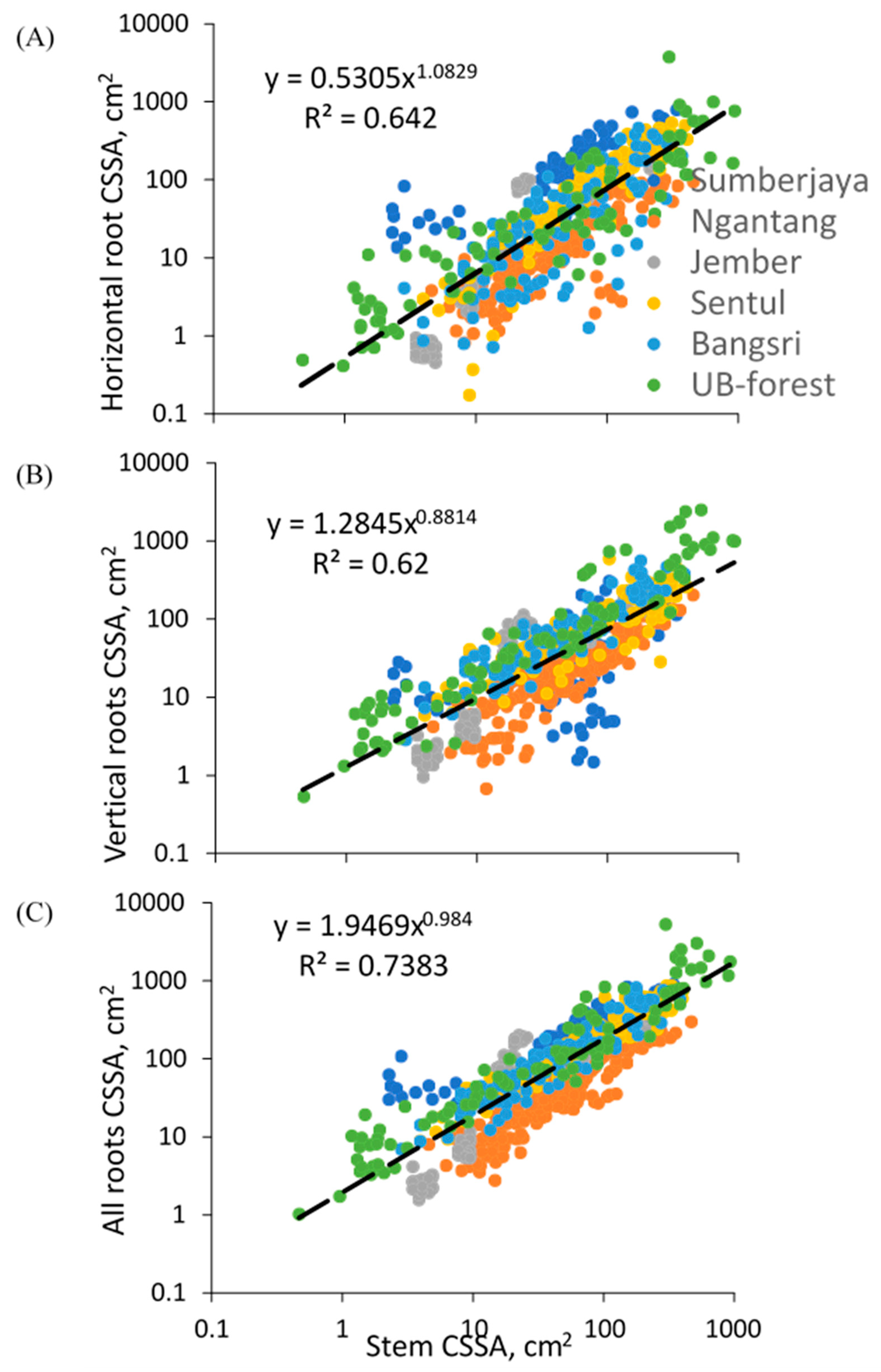
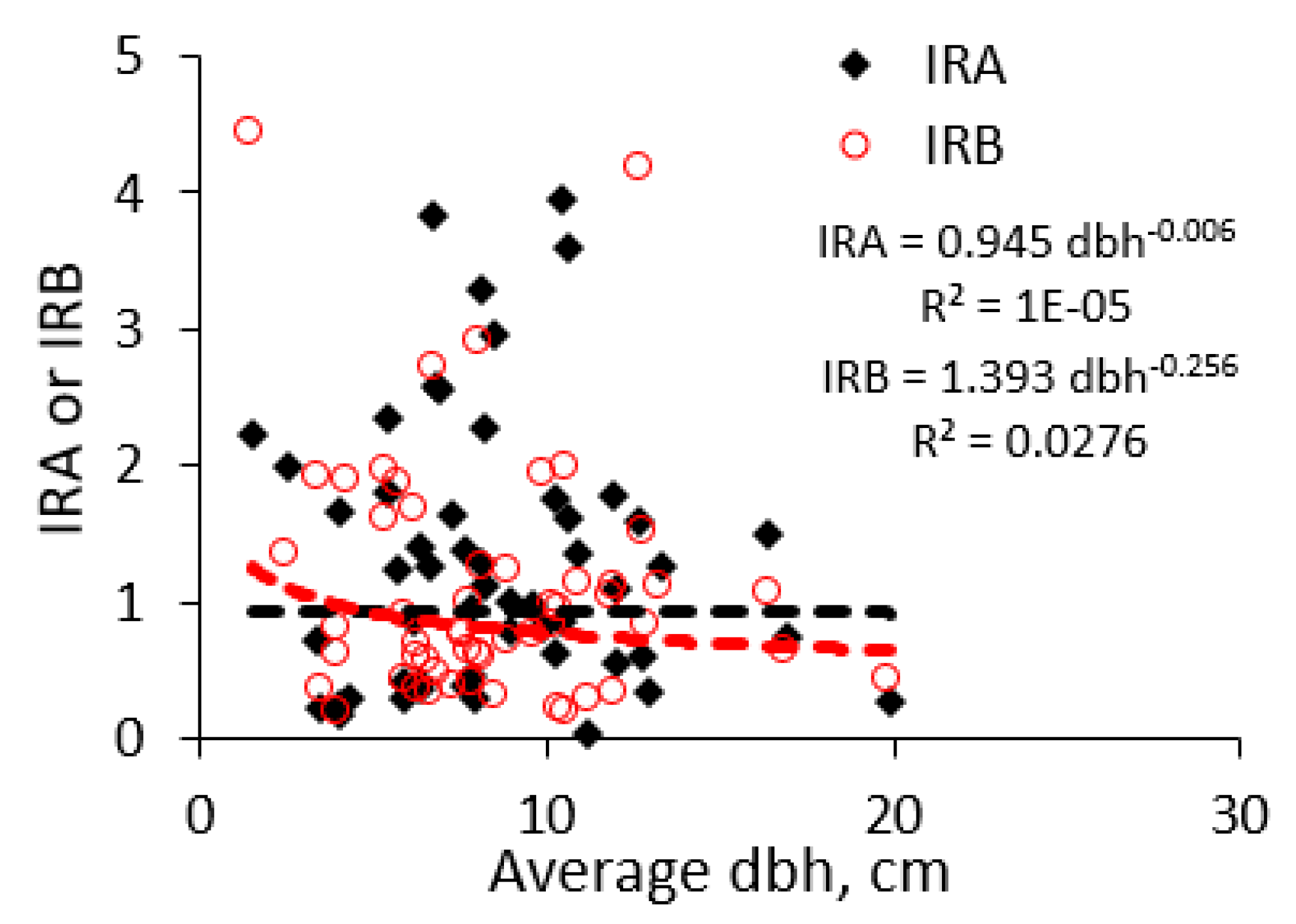
3.5. Allometry of Proximal Tree Root Systems
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Species | N | Dbh Avg | IRA Avg | IRB Avg | Quartile | Quartile |
|---|---|---|---|---|---|---|
| Mangifera indica | 6 | 11.2 | 0.04 | 0.29 | IRA Q1 | IRB Q1 |
| Citrus × sinensis | 6 | 4.0 | 0.18 | 0.19 | IRA Q1 | IRB Q1 |
| Malus domestica | 6 | 3.5 | 0.23 | 0.36 | IRA Q1 | IRB Q1 |
| Cassia fistula | 6 | 19.9 | 0.26 | 0.42 | IRA Q1 | IRB Q1 |
| Agathis dammara | 6 | 5.9 | 0.29 | 0.43 | IRA Q1 | IRB Q1 |
| Ficus benjamina | 6 | 7.9 | 0.29 | 0.41 | IRA Q1 | IRB Q1 |
| Annona muricata | 6 | 6.3 | 0.37 | 0.35 | IRA Q1 | IRB Q1 |
| Spathodea campanulata | 6 | 4.0 | 0.25 | 0.61 | IRA Q1 | IRB Q23 |
| Pinus merkusii | 11 | 12.9 | 0.34 | 0.83 | IRA Q1 | IRB Q23 |
| Melia azedarach | 11 | 7.6 | 0.40 | 0.75 | IRA Q1 | IRB Q23 |
| Jatropha curcas | 6 | 6.3 | 0.41 | 0.68 | IRA Q1 | IRB Q23 |
| Ceiba pentandra | 6 | 5.9 | 0.41 | 0.89 | IRA Q1 | IRB Q23 |
| Trema orientalis | 10 | 7.8 | 0.47 | 0.64 | IRA Q1 | IRB Q23 |
| Litsea garcia | 5 | 4.3 | 0.30 | 1.88 | IRA Q1 | IRB Q4 |
| Eucalyptus deglupta | 6 | 12.0 | 0.56 | 0.33 | IRA Q23 | IRB Q1 |
| Cinnamommum burmannii | 10 | 7.3 | 1.65 | 0.38 | IRA Q23 | IRB Q1 |
| Parkia speciosa | 19 | 12.8 | 0.59 | 1.50 | IRA Q23 | IRB Q23 |
| Sandoricum koetjape | 20 | 10.2 | 0.62 | 0.96 | IRA Q23 | IRB Q23 |
| Hevea brasiliensis | 10 | 16.9 | 0.75 | 0.64 | IRA Q23 | IRB Q23 |
| Aleurites moluccana | 15 | 8.9 | 0.80 | 0.71 | IRA Q23 | IRB Q23 |
| Durio zibethinus | 15 | 10.4 | 0.87 | 0.95 | IRA Q23 | IRB Q23 |
| Erythrina subumbrans | 20 | 7.8 | 0.95 | 0.98 | IRA Q23 | IRB Q23 |
| Ochroma pyramidale | 7 | 9.6 | 0.99 | 0.76 | IRA Q23 | IRB Q23 |
| Syzygium polyanthum | 5 | 8.9 | 1.00 | 1.218 | IRA Q23 | IRB Q23 |
| Swietenia mahagoni | 45 | 12.0 | 1.09 | 1.11 | IRA Q23 | IRB Q23 |
| Persea americana | 21 | 8.2 | 1.13 | 1.26 | IRA Q23 | IRB Q23 |
| Calliandra calothyrsus | 15 | 6.6 | 1.26 | 0.57 | IRA Q23 | IRB Q23 |
| Pangium edule | 23 | 13.3 | 1.27 | 1.11 | IRA Q23 | IRB Q23 |
| Maesopsis eminii | 31 | 10.9 | 1.36 | 1.14 | IRA Q23 | IRB Q23 |
| Nephelium lappaceum | 32 | 7.6 | 1.37 | 0.77 | IRA Q23 | IRB Q23 |
| Psidium guajava | 10 | 6.3 | 1.40 | 0.58 | IRA Q23 | IRB Q23 |
| Toona sureni | 25 | 16.4 | 1.50 | 1.07 | IRA Q23 | IRB Q23 |
| Coffea canephora var. robinson | 16 | 4.0 | 1.66 | 0.80 | IRA Q23 | IRB Q23 |
| Artocarpus communis | 4 | 10.2 | 1.76 | 0.83 | IRA Q23 | IRB Q23 |
| Tectona grandis | 9 | 11.9 | 1.79 | 1.03 | IRA Q23 | IRB Q23 |
| Homalantus populneus | 5 | 3.4 | 0.73 | 1.90 | IRA Q23 | IRB Q4 |
| Artocarpus heterophyllus | 35 | 9.9 | 0.82 | 1.93 | IRA Q23 | IRB Q4 |
| Hibiscus tiliaceus | 20 | 6.2 | 0.86 | 1.68 | IRA Q23 | IRB Q4 |
| Pterocarpus indicus | 5 | 5.7 | 1.25 | 1.86 | IRA Q23 | IRB Q4 |
| Paraserianthes falcataria | 15 | 8.1 | 1.28 | 2.91 | IRA Q23 | IRB Q4 |
| Leucaena leucocephala | 15 | 12.7 | 1.59 | 4.17 | IRA Q23 | IRB Q4 |
| Artocarpus elasticus | 4 | 10.6 | 1.62 | 1.99 | IRA Q23 | IRB Q4 |
| Quercus lineata | 4 | 6.9 | 2.57 | 0.48 | IRA Q4 | IRB Q1 |
| Macarangga triloba | 4 | 8.5 | 2.96 | 0.30 | IRA Q4 | IRB Q1 |
| Syzygium aqueum | 4 | 10.6 | 3.60 | 0.19 | IRA Q4 | IRB Q1 |
| Croton argyratus | 4 | 6.7 | 3.84 | 0.34 | IRA Q4 | IRB Q1 |
| Ficus padana | 4 | 10.4 | 3.96 | 0.22 | IRA Q4 | IRB Q1 |
| Coffea canephora var. robusta | 58 | 2.5 | 1.99 | 1.35 | IRA Q4 | IRB Q23 |
| Piper aduncum | 4 | 8.2 | 2.28 | 0.58 | IRA Q4 | IRB Q23 |
| Gmelina arborea | 4 | 8.1 | 3.28 | 0.62 | IRA Q4 | IRB Q23 |
| Michellia alba | 5 | 5.4 | 1.81 | 1.96 | IRA Q4 | IRB Q4 |
| Coffea arabica | 10 | 1.5 | 2.23 | 4.43 | IRA Q4 | IRB Q4 |
| Gliricidia sepium | 14 | 5.4 | 2.35 | 1.60 | IRA Q4 | IRB Q4 |
| Acacia auriculiformis | 16 | 6.8 | 2.58 | 2.72 | IRA Q4 | IRB Q4 |
References
- Sidle, R.C.; Dhakal, A.S. Recent advances in the spatial and temporal modeling of shallow landslides. In Proceedings of the 2003 MODSIM Conference, Townsville, Australia, 14–17 July 2003; pp. 602–607. [Google Scholar]
- Stokes, A.; Norris, J.E.; Van Beek, L.P.H.; Bogaard, T.; Cammeraat, E.; Mickovski, S.B.; Jenner, A.; Di Iorio, A.; Fourcaud, T. How vegetation reinforces soil on slopes. In Slope Stability and Erosion Control: Ecotechnological Solutions; Springer: Dordrecht, The Netherlands, 2008; pp. 65–118. [Google Scholar]
- Van Noordwijk, M.; Hairiah, K.; Lasco, R.; Tata, H.L. How can agroforestry be part of disaster risk management. In Sustainable Development through Trees on Farms: Agroforestry in Its Fifth Decade; Van Noordwijk, M., Ed.; World Agroforestry (ICRAF): Bogor, Indonesia, 2019; pp. 215–229. [Google Scholar]
- Pohl, M.; Alig, D.; Körner, C.; Rixen, C. Higher plant diversity enhances soil stability in disturbed alpine ecosystems. Plant Soil 2009, 324, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Alam, S.; Banjara, A.; Wang, J.; Patterson, W.B.; Baral, S. Novel Approach in Sampling and Tensile Strength Evaluation of Roots to Enhance Soil for Preventing Erosion. Open J. Soil Sci. 2018, 8, 330. [Google Scholar] [CrossRef] [Green Version]
- Giadrossich, F.; Schwarz, M.; Cohen, D.; Cislaghi, A.; Vergani, C.; Hubble, T.; Phillips, C.; Stokes, A. Methods to measure the mechanical behaviour of tree roots: A review. Ecol. Eng. 2017, 109, 256–271. [Google Scholar] [CrossRef]
- Iverson, R.M. Landslide triggering by rain infiltration. Water Resour. Res. 2000, 36, 1897–1910. [Google Scholar] [CrossRef] [Green Version]
- Micheli, E.R.; Kirchner, J.W. Effects of wet meadow riparian vegetation on streambank erosion. 2. Measurements of vegetated bank strength and consequences for failure mechanics. Earth Surf. Process Landf. 2002, 27, 687–697. [Google Scholar] [CrossRef]
- Roering, J.J.; Schmidt, K.M.; Stock, J.D.; Dietrich, W.E.; Montgomery, D.R. Shallow landsliding, root reinforcement, and the spatial distribution of trees in the Oregon Coast range. Can. Geotech. 2003, 40, 237–253. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.H.; Swanston, D.N. Risks of landslides in shallow soils and its relation to clearcutting in Southeastern Alaska. For. Sci. 1980, 26, 495–510. [Google Scholar] [CrossRef]
- O’Loughlin, C.L.; Watson, A.J. Root wood strength deterioration in radiate pine after clearfelling. N. Z. J. For. Sci. 1979, 9, 284–293. [Google Scholar]
- Wilkinson, P.L.; Anderson, M.G.; Lloyd, D.M. An integrated hydrological model for rain-induced landslide prediction. Earth Surf. Processes Landf. 2002, 27, 1285–1297. [Google Scholar] [CrossRef]
- Ziemer, R.R.; Swantson, D.N. Root Strength Changes after Logging in Southeast Alaska; USDA Forest Service Research Note PNW; Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station: Portland, OR, USA, 1997; Volume 306.
- Abe, K.; Ziemer, R.R. Effect of Tree Roots on Shallow-Seated Land Slides; USDA Forest Service Gen. Tech. Rep. PSW-GT130; International Union of Forestry Research: Vienna, Austria, 1991; pp. 11–20. [Google Scholar]
- Schiechtl, H.M. Bioengineering for Land Reclamation and Conservation; University of Alberta Press: Edmonton, AL, Canada, 1980. [Google Scholar]
- Shen, P.; Zhang, L.M.; Chen, H.X.; Gao, L. Role of vegetation restoration in mitigating hillslope erosion and debris flows. Eng. Geol. 2017, 216, 122–133. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, G.H. Quantifying the binding and bonding effects of plant roots on soil detachment by overland flow in 10 typical grasslands on the Loess Plateau. Soil Sci. Soc. Am. J. 2017, 81, 1567–1576. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Y.; Wang, Y.; Mao, Z.; Langendoen, E.J. How does root biodegradation after plant felling change root reinforcement to soil? Plant Soil 2020, 446, 211–227. [Google Scholar] [CrossRef]
- Watson, A.; Phillips, C.; Marden, M. Root strength, growth, and rates of decay: Root reinforcement changes of two tree species and their contribution to slope stability. Plant Soil 1999, 217, 39–47. [Google Scholar] [CrossRef]
- Hubble, T.C.T.; Docker, B.B.; Rutherfurd, I.D. The role of riparian trees in maintaining riverbank stability: A review of Australian experience and practice. Ecol. Eng. 2010, 36, 292–304. [Google Scholar] [CrossRef]
- Bischetti, G.B.; Chiaradia, E.A.; Simonato, T.; Speziali, B.; Vitali, B.; Vullo, P.; Zocco, A. Root Strength and Root Area Ratio of Forest Species in Lombardy (Northern Italy). Plant Soil 2005, 278, 11–22. [Google Scholar] [CrossRef]
- De Baets, S.; Poesen, J.; Reubens, B.; Wemans, K.; De Baerdemaeker, J.; Muys, B. Root tensile strength and root distribution of typical Mediterranean plant species and their contribution to soil shear strength. Plant Soil 2008, 305, 207–226. [Google Scholar] [CrossRef]
- Abernethy, B.; Rutherfurd, I.D. The distribution and strength of riparian tree roots in relation to riverbank reinforcement. Hydrol. Processes 2001, 15, 63–79. [Google Scholar] [CrossRef]
- Ekanayake, J.C.; Marden, M.; Watson, A.J.; Rowan, D. Tree roots and slope stability: A comparison between Pinus radiata and kanuka. N. Z. J. For. Sci. 1997, 27, 216–233. [Google Scholar]
- Genet, M.; Kokutse, N.; Stokes, A.; Fourcaud, T.; Cai, X.; Ji, J.; Mickovski, S. Root reinforcement in plantations of Cryptomeria japonica D. Don: Effect of tree age and stand structure on slope stability. For. Ecol. Manag. 2008, 256, 1517–1526. [Google Scholar] [CrossRef]
- Tosi, M. Root tensile strength relationships and their slope stability implications of three shrub species in the Northern Apennines (Italy). Geomorphology 2007, 87, 268–283. [Google Scholar] [CrossRef]
- Giadrossich, F.; Cohen, D.; Schwarz, M.; Ganga, A.; Marrosu, R.; Pirastru, M.; Capra, G.F. Large roots dominate the contribution of trees to slope stability. Earth Surf. Processes Landf. 2019, 44, 1602–1609. [Google Scholar] [CrossRef]
- Collison, A.J.C.; Anderson, M.G. Using a combined slope hydrology/stability model to identify suitable conditions for landslide prevention by vegetation in the humid tropics. Earth Surf. Processes Landf. 1996, 21, 737–747. [Google Scholar] [CrossRef]
- Nilaweera, N.S.; Nutalaya, P. Role of tree roots in slope stabilisation. Bull. Eng. Geol. Environ. 1999, 57, 337–342. [Google Scholar] [CrossRef]
- Madsen, C.; Potvin, C.; Hall, J.; Sinacore, K.; Turner, B.L.; Schnabel, F. Coarse root architecture: Neighbourhood and abiotic environmental effects on five tropical tree species growing in mixtures and monocultures. For. Ecol. Manag. 2020, 460, 117851. [Google Scholar] [CrossRef]
- Danjon, F.; Reubens, B. Assessing and analyzing 3D architecture of woody root systems, a review of methods and applications in tree and soil stability, resource acquisition and allocation. Plant Soil 2008, 303, 1–34. [Google Scholar] [CrossRef]
- Collison, A.; Pollen, N. The effects of riparian buffer strips on streambank stability: Root reinforcement, soil strength and growth rates. In Roots and Soil Management: Interaction between Roots and the Soil; Zobel, R.W., Wright, S.F., Eds.; American Society of Agronomy: Madison, WI, USA, 2005; Volume 48, pp. 15–56. [Google Scholar]
- Reubens, B.; Poesen, J.; Danjon, F.; Geudens, G.; Muys, B. The role of fine and coarse roots in shallow slope stability and soil erosion control with a focus on root system architecture: A review. Trees 2007, 21, 385–402. [Google Scholar] [CrossRef]
- Stokes, A.; Atger, C.; Bengough, A.G.; Fourcaud, T.; Sidle, R.C. Desirable plant root traits for protecting natural and engineered slopes against landslides. Plant Soil 2009, 324, 1–30. [Google Scholar] [CrossRef]
- Boldrin, D.; Leung, A.K.; Bengough, A.G. Root biomechanical properties during establishment of woody perennials. Ecol. Eng. 2017, 109, 196–206. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Harmo, M.E.; Griffiths, R.P. Decomposition and nitrogen release from decomposing woody roots in coniferous forests of the Pacific Northwest: A chronosequence approach. Can. J. For. Res. 1999, 31, 246–260. [Google Scholar] [CrossRef]
- Schroth, G. Decomposition and nutrient supply from biomass. In Trees, Crops and Soil Fertility: Concepts and Research Methods; Schroth, G., Sinclair, F.L., Eds.; CABI Publishing: Wallingford, UK, 2003; pp. 131–150. [Google Scholar]
- Hairiah, K.; Sulistyani, H.; Suprayogo, D.; Widianto Purnomosidhi, P.; Widodo, R.H.; Van Noordwijk, M. Litter layer residence time in forest and coffee agroforestry systems in Sumberjaya, West Lampung. For. Ecol. Manag. 2006, 224, 45–57. [Google Scholar] [CrossRef]
- Palm, C.A.; Sanchez, P.A. Nitrogen release from some tropical legumes as affected by lignin and polyphenol contents. Soil Biol. Biochem. 1991, 23, 83–88. [Google Scholar] [CrossRef]
- Genet, M.; Stokes, A.; Salin, F.; Mickovski, S.B.; Fourcaud, T.; Dumail, F.; van Beek, R. The Influence of Cellulose Content on Tensile Strength in Tree Roots. Plant Soil 2005, 278, 1–9. [Google Scholar] [CrossRef]
- Van Noordwijk, M.; Purnomosidhi, P. Root architecture in relation to tree-soil- crop interactions and shoot pruning in agroforestry. Agrofor. Syst. 1996, 30, 161–173. [Google Scholar] [CrossRef]
- Van Noordwijk, M.; Mulia, R. Functional branch analysis as tool for scaling above- and belowground trees for their additive and non-additive properties. Ecol. Model. 2002, 149, 41–51. [Google Scholar] [CrossRef]
- Schwarz, M.; Lehmann, P.; Or, D. Quantifying lateral root reinforcement in steep slopes—From a bundle of roots to tree stands. Earth Surf. Processes Landf. J. Br. Geomorphol. Res. Group 2010, 35, 354–367. [Google Scholar] [CrossRef]
- Nespoulous, J.; Merino-Martin, L.; Monnier, Y.; Bouchet, D.C.; Ramel, M.; Dombey, R.; Viennois, G.; Mao, Z.; Zhang, J.L.; Cao, K.F.; et al. Tropical forest structure and understorey determine subsurface flow through biopores formed by plant roots. Catena 2019, 181, 104061. [Google Scholar] [CrossRef]
- Van Noordwijk, M.; Agus, F.; Verbist, B.; Hairiah, K.; Tomich, T.P. Managing Watershed Services in Ecoagriculture Landscapes. In Farming with Nature: The Science and Practice of Ecoagriculture; Scherr, S.J., McNeely, J.A., Eds.; Island Press: Washington, DC, USA, 200; pp. 191–212. [Google Scholar]
- Burroughs, E.R.; Thomas, B.R. Declining Root Strength in Douglas-Fir after Felling as a Factor in Slope Stability; US Department Agriculture Forest Services Research Paper INT-190; Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station: Ogden, UT, USA, 1977.
- Ziemer, R.R. An apparatus to measure the crosscut shearing strength of roots. Can. J. For. Res. 1978, 8, 142–144. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Goering, H.K.; Van Soest, P.J. Forage Fiber Analysis; Agricultural Handbook no 379; USDA: Washington, DC, USA, 1970; p. 20.
- Anderson, J.M.; Ingram, J.S.I. Tropical Soil Biology and Fertility: A Handbook of Methods; CAB. International: Wallingford, UK, 1989; p. 171. [Google Scholar]
- Rose, C. An Introduction to the Environmental Physics of Soils, Water and Watershed; Cambridge University Press: Cambridge, UK, 2004; p. 65. [Google Scholar]
- Tennant, D. A test of a modified line intersect method of estimating root length. J. Ecol. 1975, 63, 995–1001. [Google Scholar] [CrossRef]
- Payne, R.W.; Lane, P.W.; Ainsley, A.E.; Bicknell, K.E.; Digby, P.G.N.; Harding, S.A.; Leech, P.K.; Simpson, H.R.; Todd, A.D.; Verrier, P.J.; et al. Genstat 5 Reference Manual; Clarendon Press: Oxford, UK, 1987. [Google Scholar]
- Van Noordwijk, M. Functional branch analysis to derive allometric equations. In Modelling Global Change Impacts on the Soil Environment: Report of Training Workshop 5–13 May 1998; IC-SEA Report No., 6; Murdiyarso, D., van Noordwijk, M., Suyamto, D.A., Eds.; BIOTROP-GCTE: Bogor, Indonesia; ICSEA: Lisbon, Portugal, 1999; pp. 77–80. [Google Scholar]
- Akinnifesi, F.K.; Rowe, E.C.; Livesley, S.J.; Kwesiga, F.R.; Van Lauwe, B.; Alegre, J.C. Tree root architecture. In Below-Ground Interactions in Tropical Agroecosystems. Concepts and Models with Multiple Plant Components; Van Noordwijk, M., Cadisch, G., Ong, C.K., Eds.; CABI Publishing: Wallingford, UK, 2004; pp. 61–82. [Google Scholar]
- Hairiah, K.; Widianto, W.; Utami, S.R.; Sunaryo, D.S.; Sitompul, S.M.; Lusiana, B.; van Noordwijk, M.; Cadish, G. Pengelolaan Tanah Masam Secara Biologi: Refleksi Pengelaman dari Lampung Utara; World Agroforestry Centre (ICRAF): Bogor, Indonesia, 2000; p. 184. [Google Scholar]
- Budidarsono, S.; Wijaya, K. Praktek Konservasi dalam Budidaya Kopi Robusta dan Keuntungan Petani. Agrivita 2004, 26, 107–117. [Google Scholar]
- Cammeraat, E.; van Beek, R.; Kooijman, A. Vegetation succession and its consequences for slope stability in SE Spain. Plant Soil 2005, 278, 135–147. [Google Scholar] [CrossRef]
- Stokes, A.; Mattheck, C. Variation of wood strength in tree roots. J. Exp. Bot. 1996, 47, 693–699. [Google Scholar] [CrossRef]
- Coster, C. Wortelstudien in de tropen. V. Gebergtehoutsoorten [Root studies in the Tropics. V. Tree species of the mountain region]. Tectona 1935, 28, 861–878. [Google Scholar]
- Van Noordwijk, M.; Lawson, G.; Hairiah, K.; Wilson, J. Root Distribution of Trees and Crops: Competition and/or Complementarity. Tree-Crop Interactions: Agroforestry in a Changing Climate; CABI: Wallingford, UK, 2015; pp. 221–257. [Google Scholar]
- Van Noordwijk, M.; Martikainen, P.; Bottner, P.; Cuevas, E.; Rouland, C.; Dhillion, S.S. Global change and root function. Glob. Change Biol. 1998, 4, 759–772. [Google Scholar] [CrossRef]
- Wibawa, G.; Hendratno, S.; van Noordwijk, M. Permanent smallholder rubber agroforestry systems in Sumatra, Indonesia. In Slash and Burn: The Search for Alternatives; Palm, C.A., Vosti, S.A., Sanchez, P.A., Ericksen, P.J., Juo, A.S.R., Eds.; Columbia University Press: New York, NY, USA, 2005; pp. 222–232. [Google Scholar]
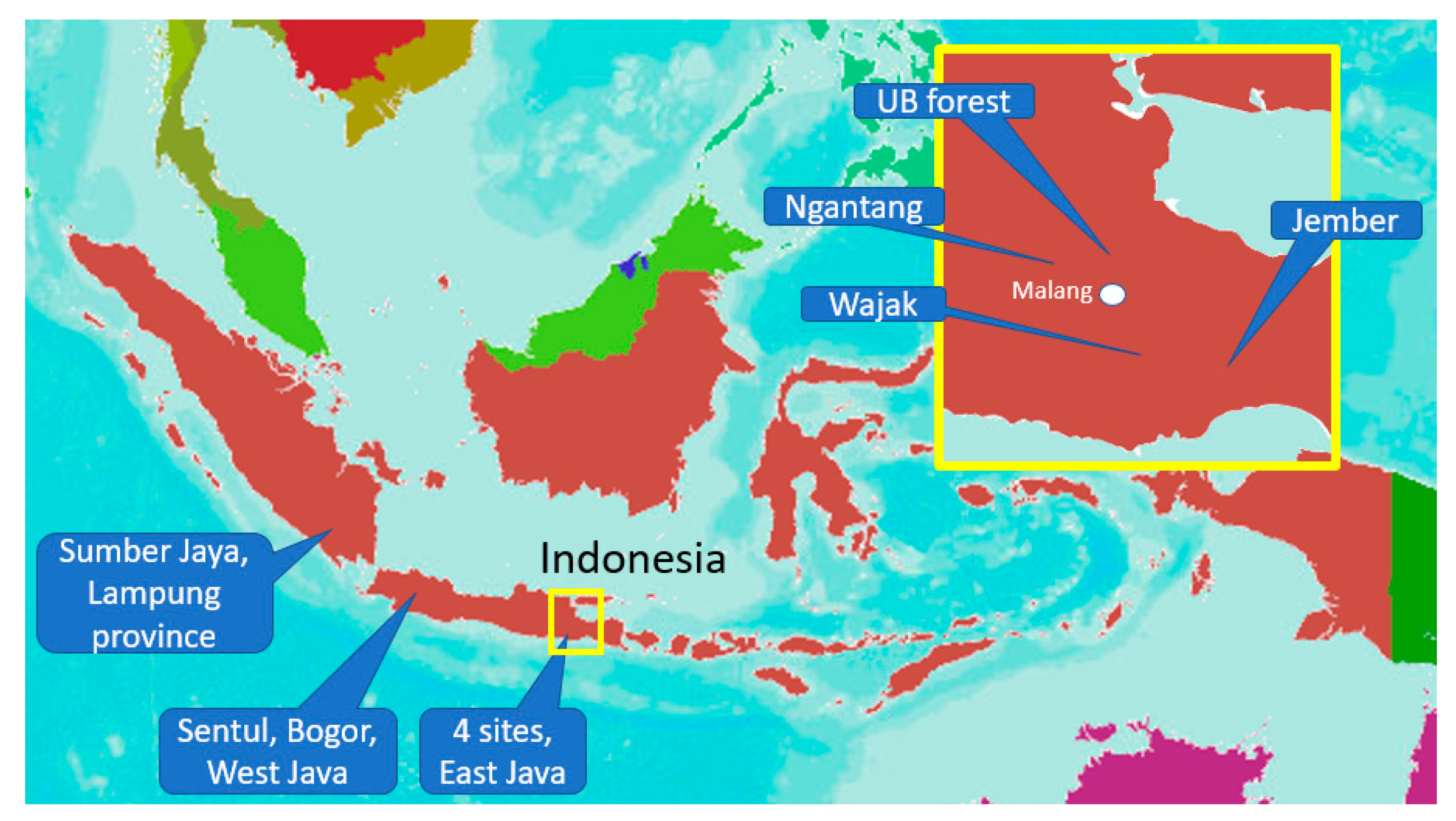
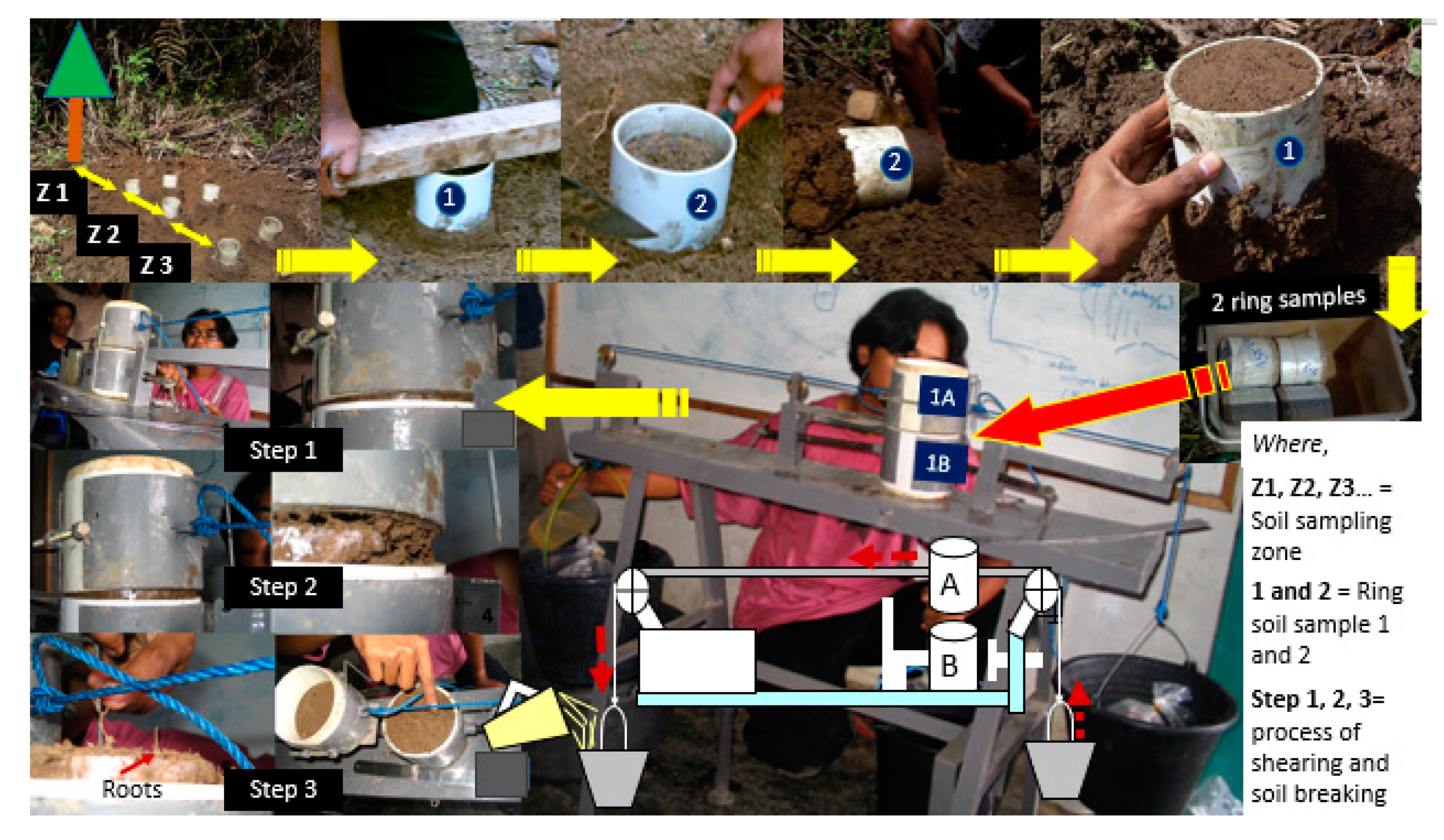
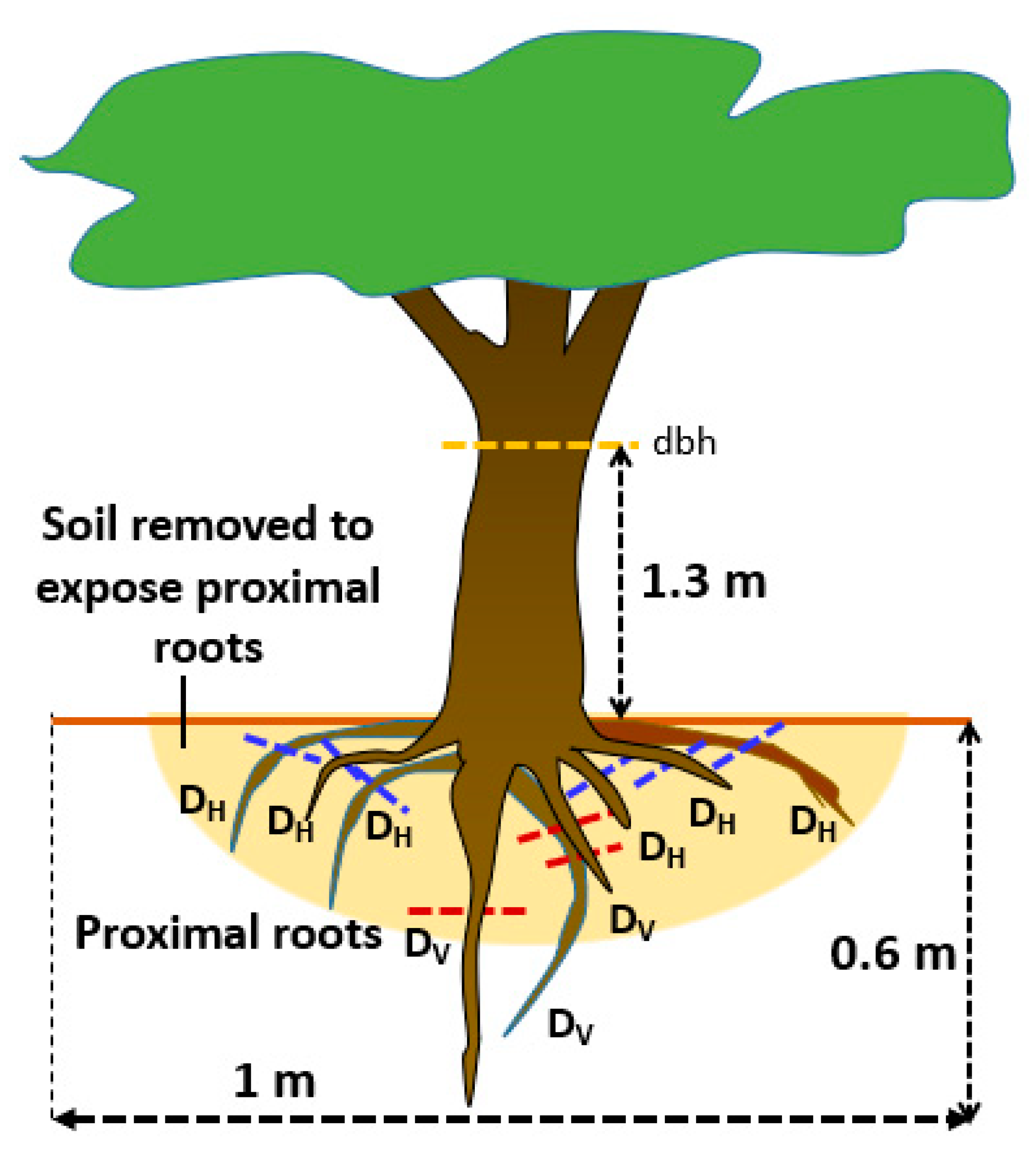

| Watershed and Time of Study | Geoposition | Altitude, m above Sea Level | Annual Rainfall, mm | Soil Type | Main Tree Crops |
|---|---|---|---|---|---|
| Way Besai, Sumber Jaya, West Lampung, (2005–2006) | 5°01′29.9″–5°02′34.2″ S, 104°25′46.5″–104°26′51.4″ E | 700–1700 | 2500–2614 | Inceptisol and Andisols | Coffea, Gliricidia, Persea, Durio, Artocarpus, Aleurites |
| Ciherang and Cibadak sub-watershed, Sentul Bogor, West Java (2007) | 6°35′37″–6°35′29″ S, 106°54′58″–106°55′47″ E | 1529 | 4000 | Inceptisol | Maesopsis, Pangium, Eugenia, Sandoricum |
| Kali Konto sub-watershed (Pujon and Ngantang village), Malang, East Java (2006–2007) | 7°46′03″–7°56′54″ S, 112°19′20″–112°29′55″ E. | 1150 | 1797–3151 | Inceptisol | Coffea, Durio, Persea, Gliricidia, Pinus, Maesopsis, Toona, Agathis |
| Kalisari sub-watershed (UB Forest), Malang, East Java (2019) | 7°49′30″–7°51′36″ S, 112°34′38″–112°36′53″ E | 900–1300 | 2005 | Inceptisol, Andisol | Pinus, Swietenia, Coffea, Gliricidia, Calliandra, Hibiscus, Parkia, Toona, Erythrina |
| Bangsri sub-watershed, Wajak, Malang, East Java (2018) | 8°7′29″–8°7′32″ S, 112°47′30″–112°48′30″ E | 660–720 | 2220–2314 | Inceptisol, Entisol | Persea, Durio, Paraserianthes, Swietenia, Pinus, Michelia |
| Upper Bedadung watershed, Jember, East Java (2006) | 7°59′66″–8°33′56″ S, 113°19′–114°02′30″ E, | 300–3000 | 2699 | Inceptisol and Andisols | Coffea, Leucaena, Swietenia, Hevea, Ochroma, |
| Litter Type | N (%) | L (%) | P (%) | L/N | P/N | (L + P)/N |
|---|---|---|---|---|---|---|
| Mahogany | 0.6 | 29.2 | 26.4 | 45.0 | 40.7 | 85.7 |
| Toona | 1.2 | 18.9 | 7.7 | 16.4 | 6.7 | 23.1 |
| Gmelina | 1.3 | 13.5 | 8.1 | 10.1 | 6.0 | 16.1 |
| Coffea | 5.4 | 20.1 | 5.4 | 3.7 | 1.0 | 4.7 |
| Bamboo | 1.0 | 16.0 | 1.6 | 16.5 | 1.6 | 18.2 |
| Stem CSSA, cm2 | Vertical (IRA) | Horizontal (IRB) | Root/Shoot CSSA | Vertical/Horizontal Ratio |
|---|---|---|---|---|
| 1 | 1.28 | 0.53 | 1.95 | 2.42 |
| 10 | 0.98 | 0.64 | 1.88 | 1.52 |
| 100 | 0.74 | 0.78 | 1.81 | 0.96 |
| 1000 | 0.57 | 0.94 | 1.74 | 0.60 |
| INDEX | IRB_Low (<0.5) | IRB_ Medium (0.5–1.5) | IRB_High (>1.5) |
|---|---|---|---|
| IRA_Low <0.5 | Mangifera indica, Citrus × sinensis, Malus domestica, Cassia fistula, Agathis dammara, Ficus benjamina, Annona muricata | Spathodea campanulata, Pinus merkusii, Melia azedarach, Jatropha curcas, Ceiba pentandra, Trema orientalis | Litsea garcia |
| IRA_Medium 0.5–1.8 | Eucalyptus deglupta, Cinnamommum burmannii | Parkia speciosa, Sandoricum koetjape, Hevea brasiliensis, Aleurites moluccana, Durio zibethinus, Erythrina subumbrans, Ochroma pyramidale, Syzygium polyanthum, Swietenia mahagoni, Persea americana, Calliandra calothyrsus, Pangium edule, Maesopsis eminii, Nephelium lappaceum, Psidium guajava, Toona sureni, Coffea canephora var. robinson, Artocarpus communis, Tectona grandis | Homalantus populneus, Artocarpus heterophyllus, Hibiscus tiliaceus, Pterocarpus indicus, Paraserianthes falcataria, Leucaena leucocephala, Artocarpus elasticus |
| IRA_High >1.8 | Quercus lineata, Macarangga triloba, Syzygium aqueum, Croton argyratus, Ficus padana | Coffea canephora var. robusta, Piper aduncum, Gmelina arborea | Michellia alba, Coffea arabica, Gliricidia sepium, Senna spectabilis |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hairiah, K.; Widianto, W.; Suprayogo, D.; Van Noordwijk, M. Tree Roots Anchoring and Binding Soil: Reducing Landslide Risk in Indonesian Agroforestry. Land 2020, 9, 256. https://doi.org/10.3390/land9080256
Hairiah K, Widianto W, Suprayogo D, Van Noordwijk M. Tree Roots Anchoring and Binding Soil: Reducing Landslide Risk in Indonesian Agroforestry. Land. 2020; 9(8):256. https://doi.org/10.3390/land9080256
Chicago/Turabian StyleHairiah, Kurniatun, Widianto Widianto, Didik Suprayogo, and Meine Van Noordwijk. 2020. "Tree Roots Anchoring and Binding Soil: Reducing Landslide Risk in Indonesian Agroforestry" Land 9, no. 8: 256. https://doi.org/10.3390/land9080256






