Benefits Are Limited with High Nitrogen Fertiliser Rates in Kikuyu-Ryegrass Pasture Systems
Abstract
:1. Introduction
2. Results and Discussion
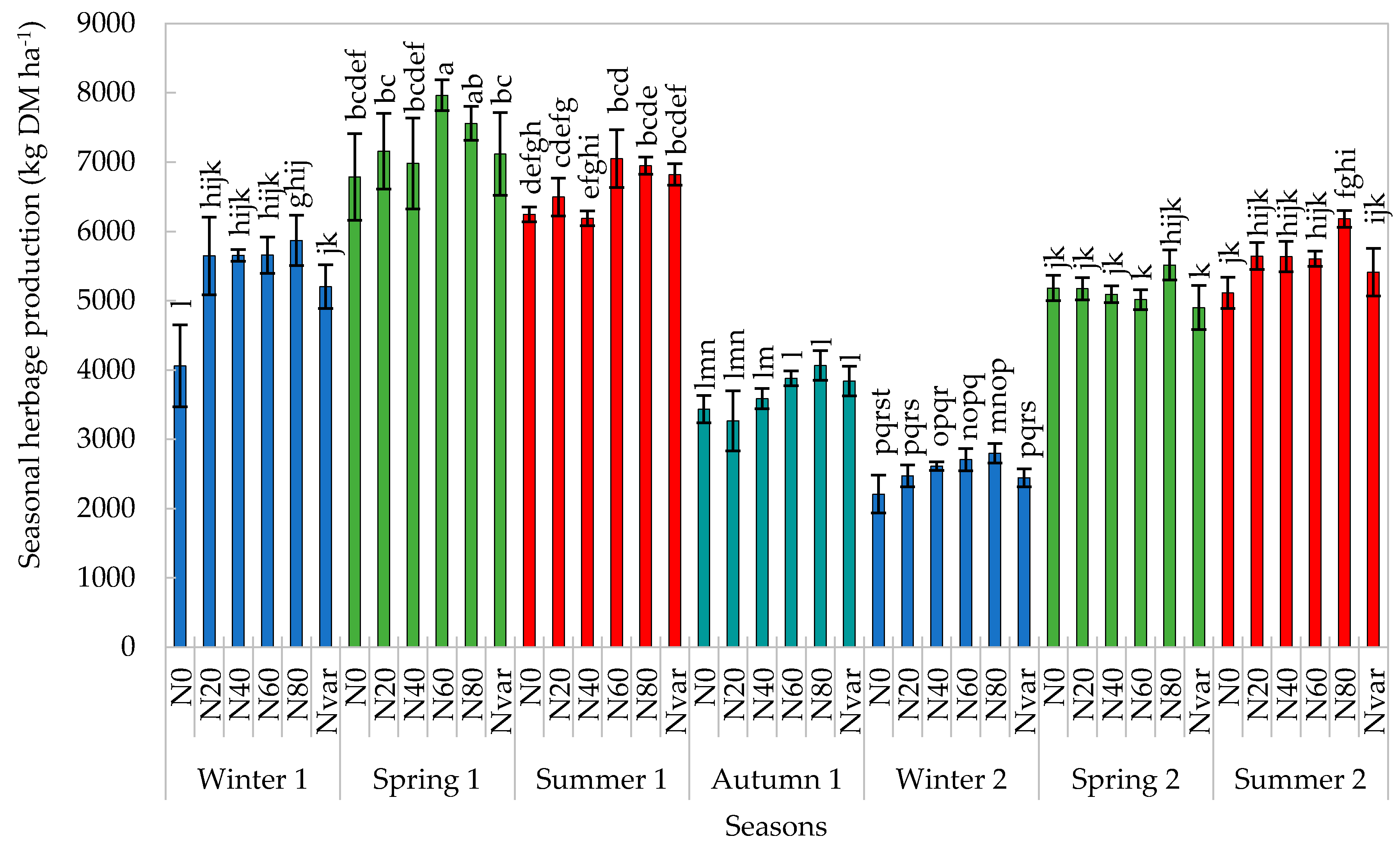
2.1. Herbage Production
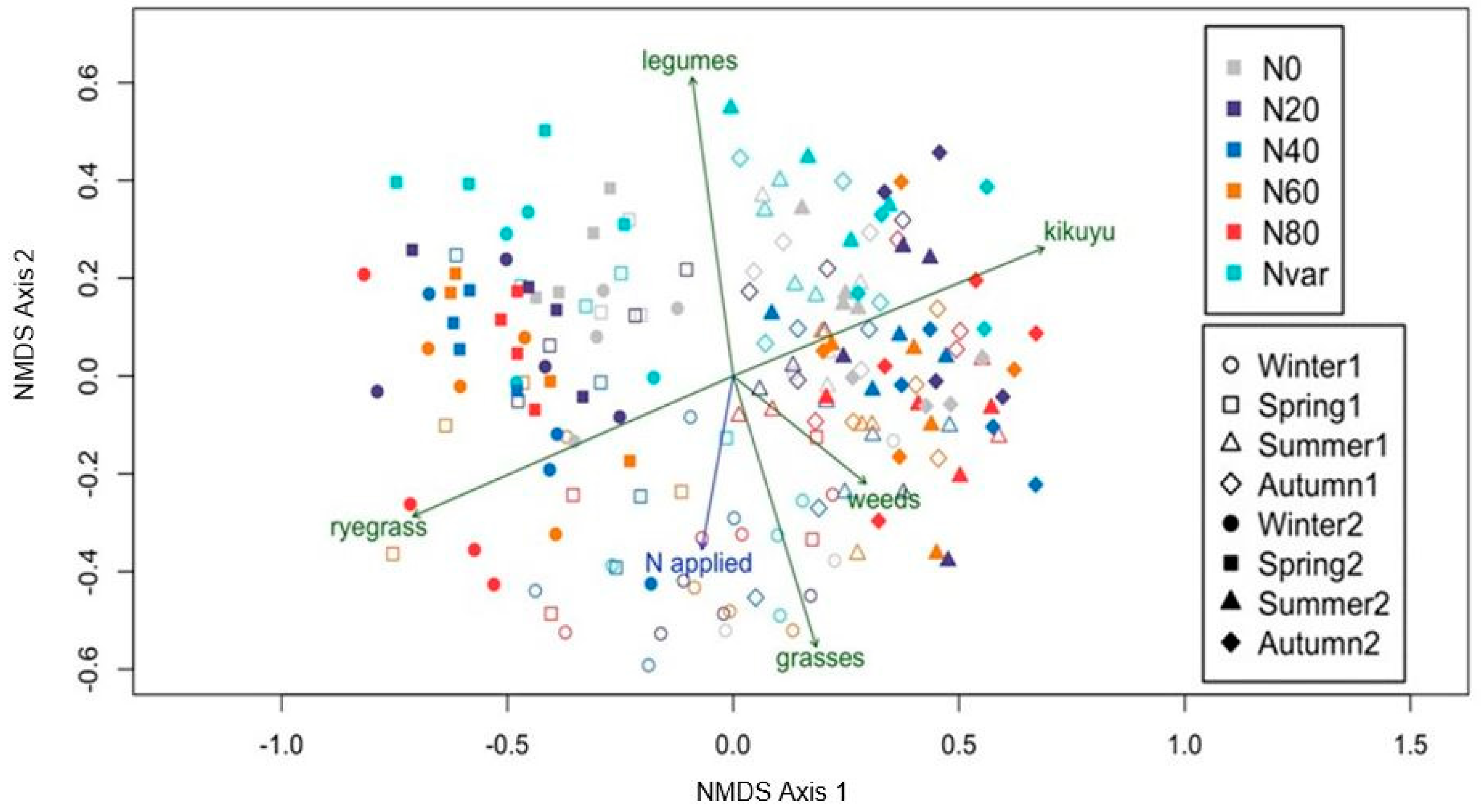
2.2. Botanical Composition
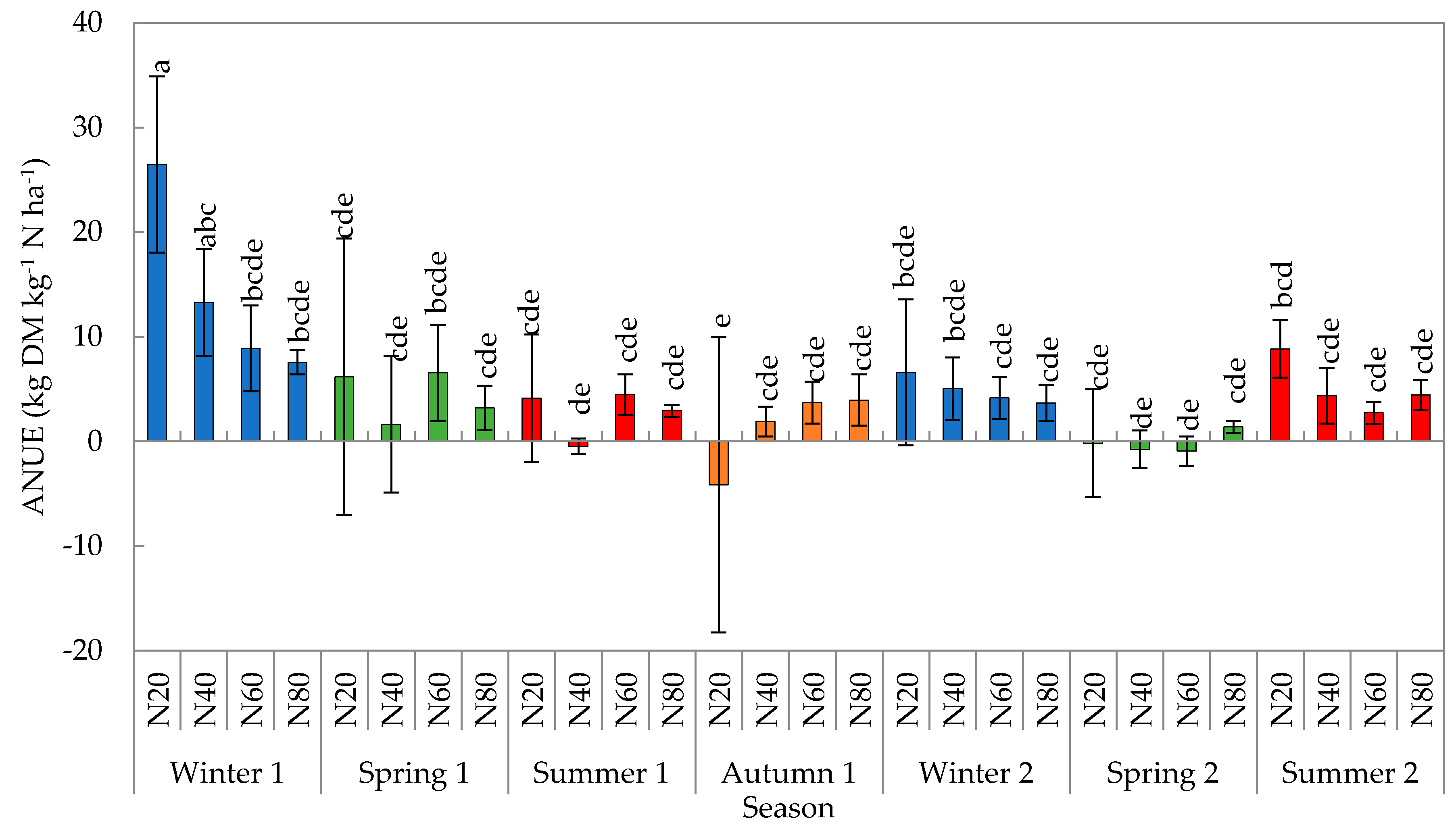
2.3. Agronomic Nitrogen Use Efficiency
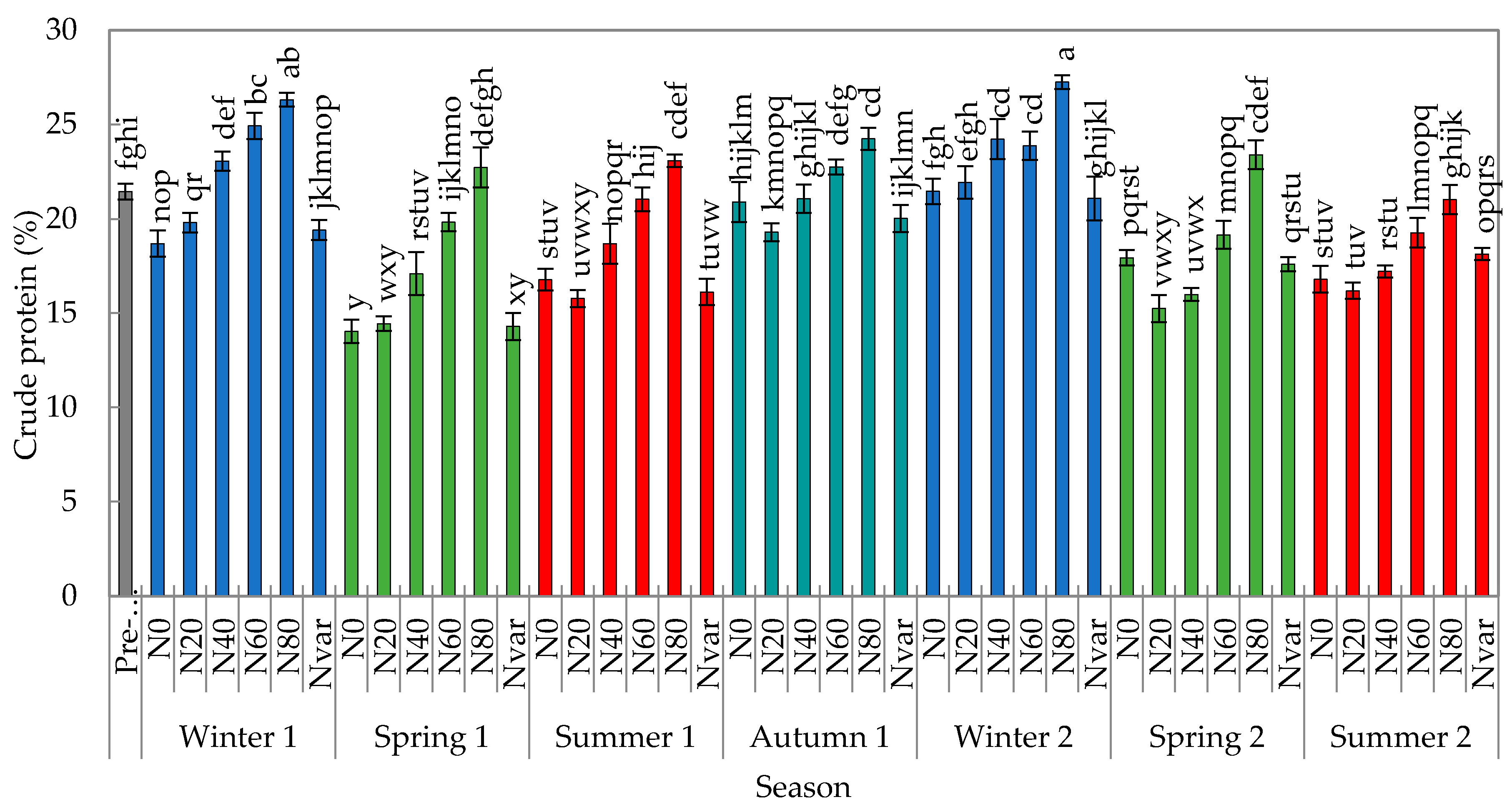
2.4. Crude Protein Content of Herbage
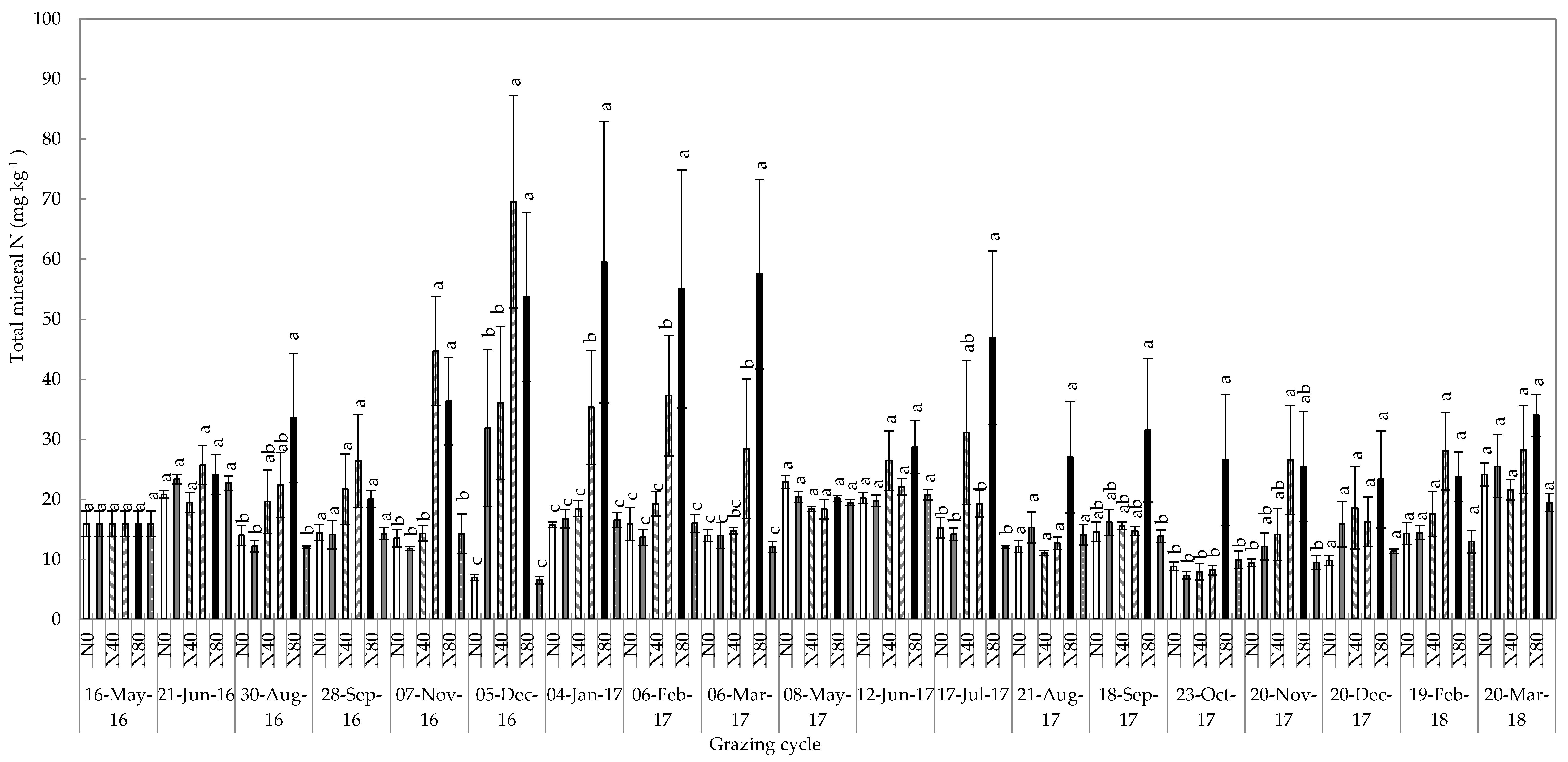
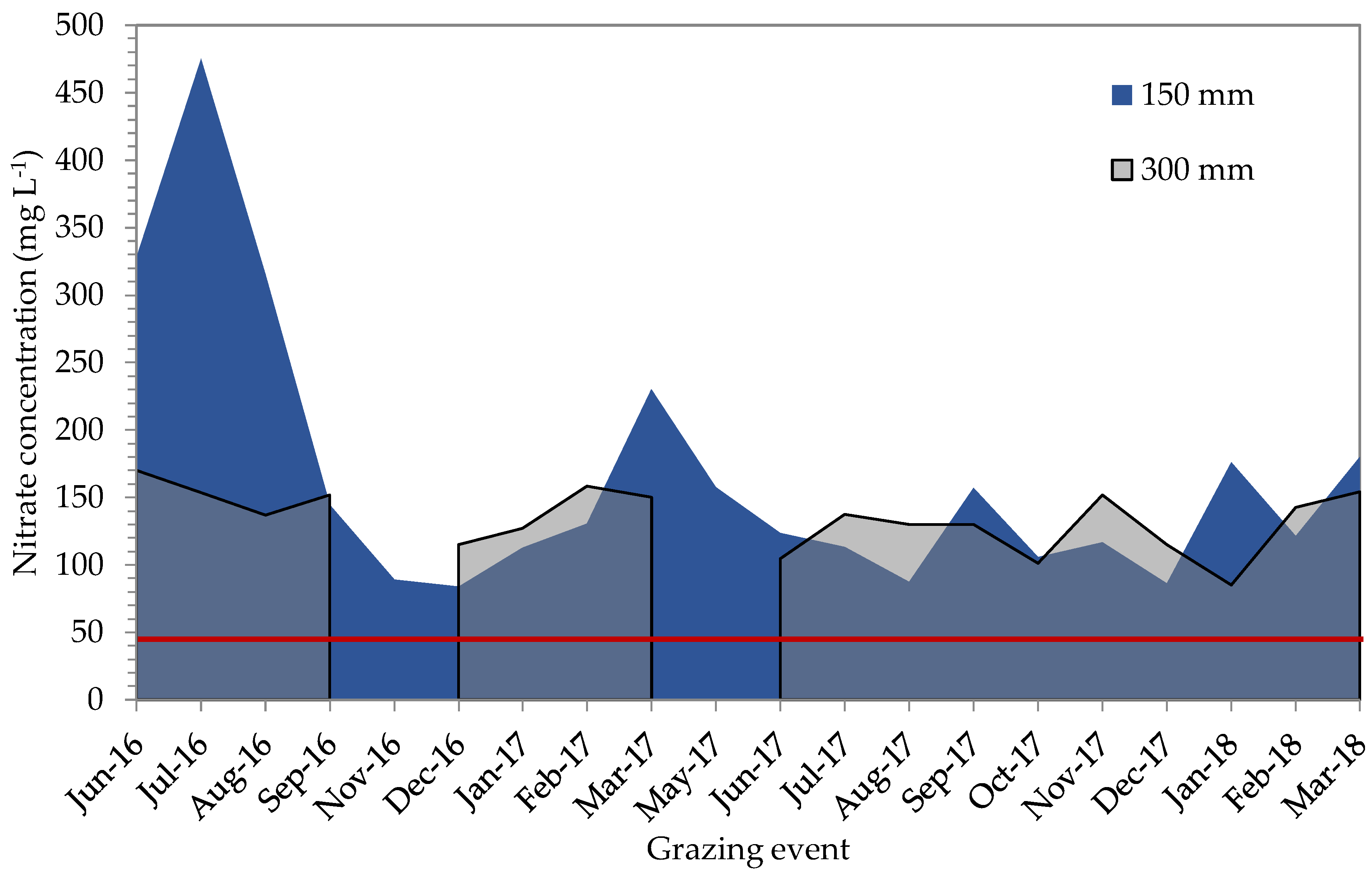
2.5. Total Mineral Nitrogen Content of the Soil
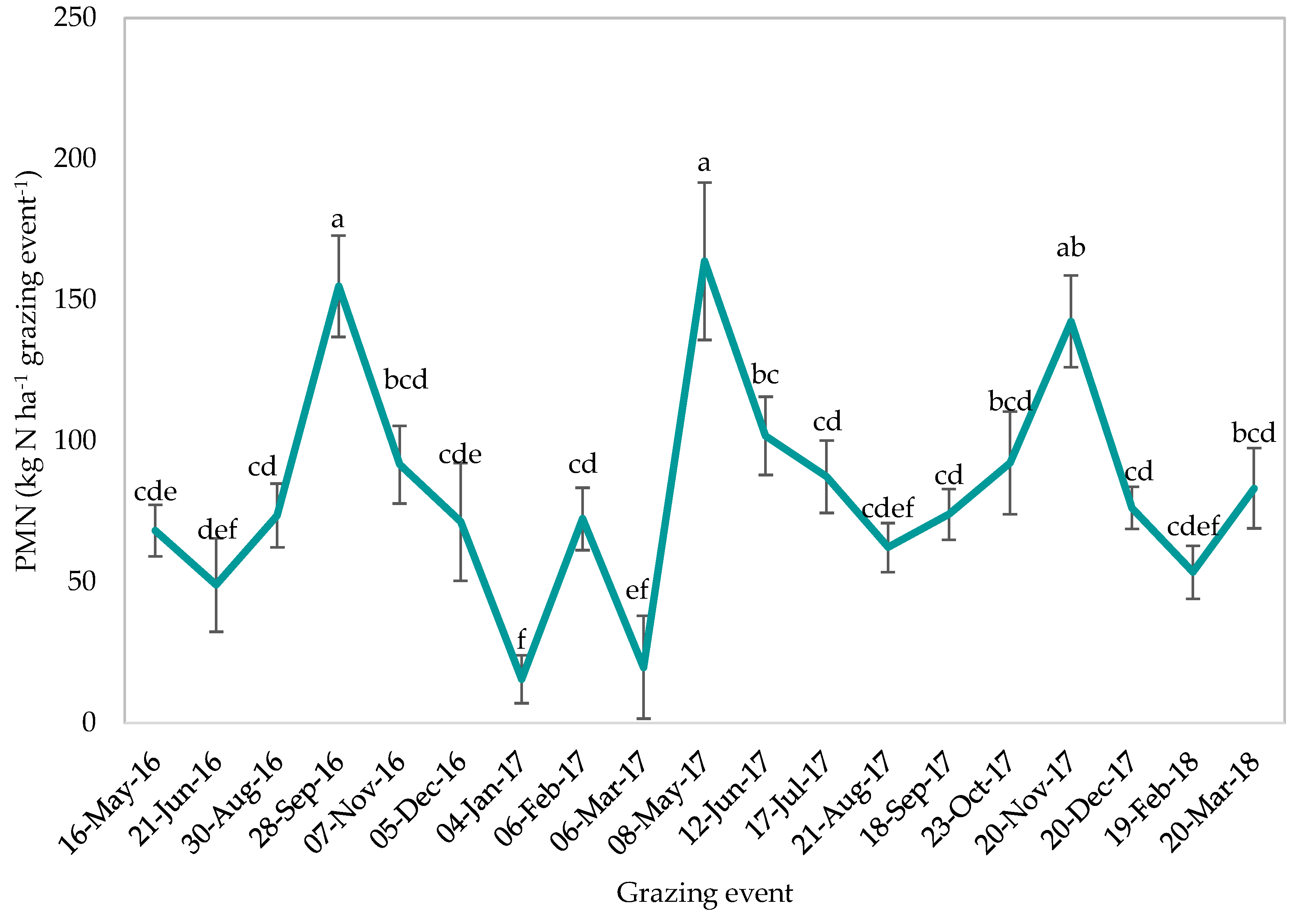
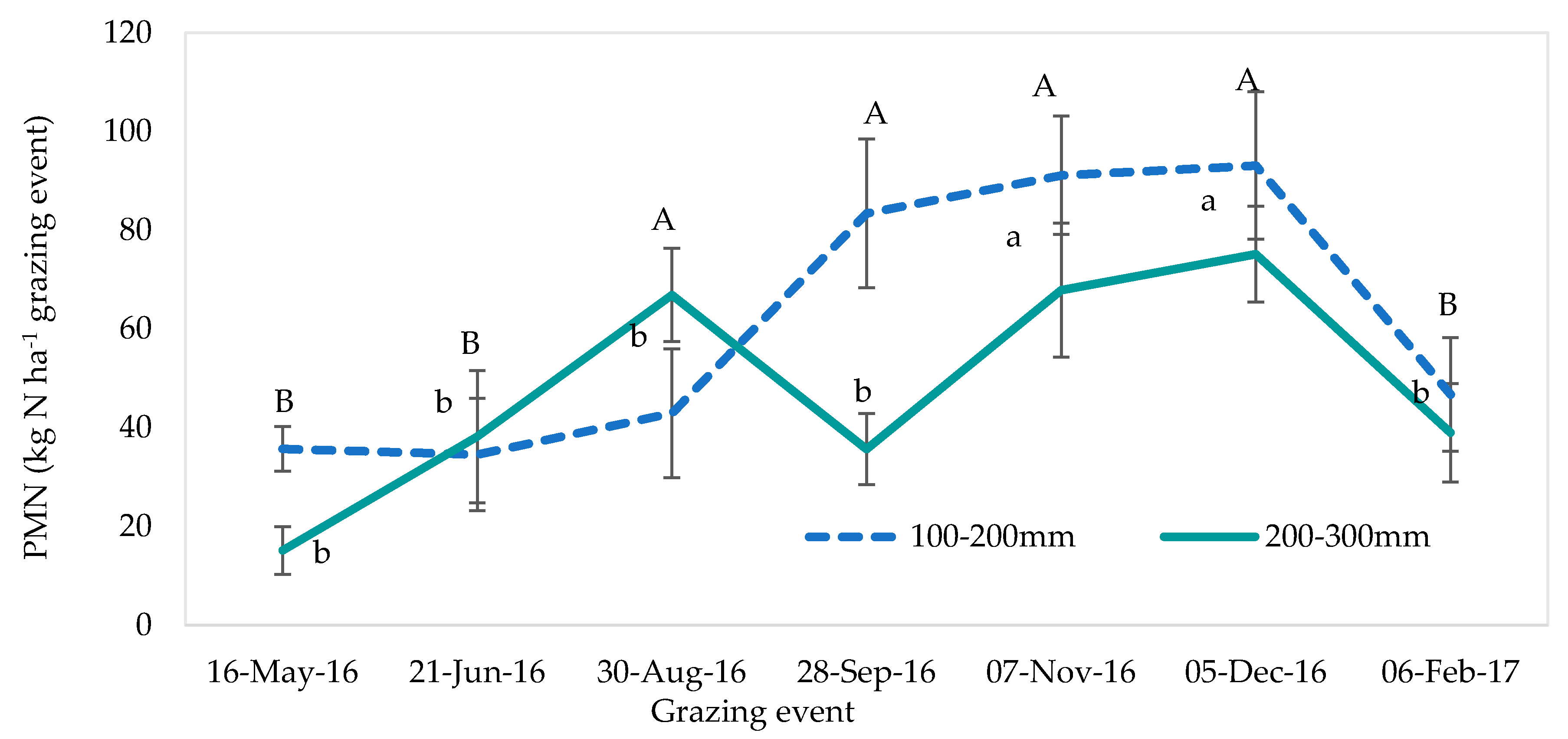
2.6. Potentially Mineralisable Nitrogen in the Soil
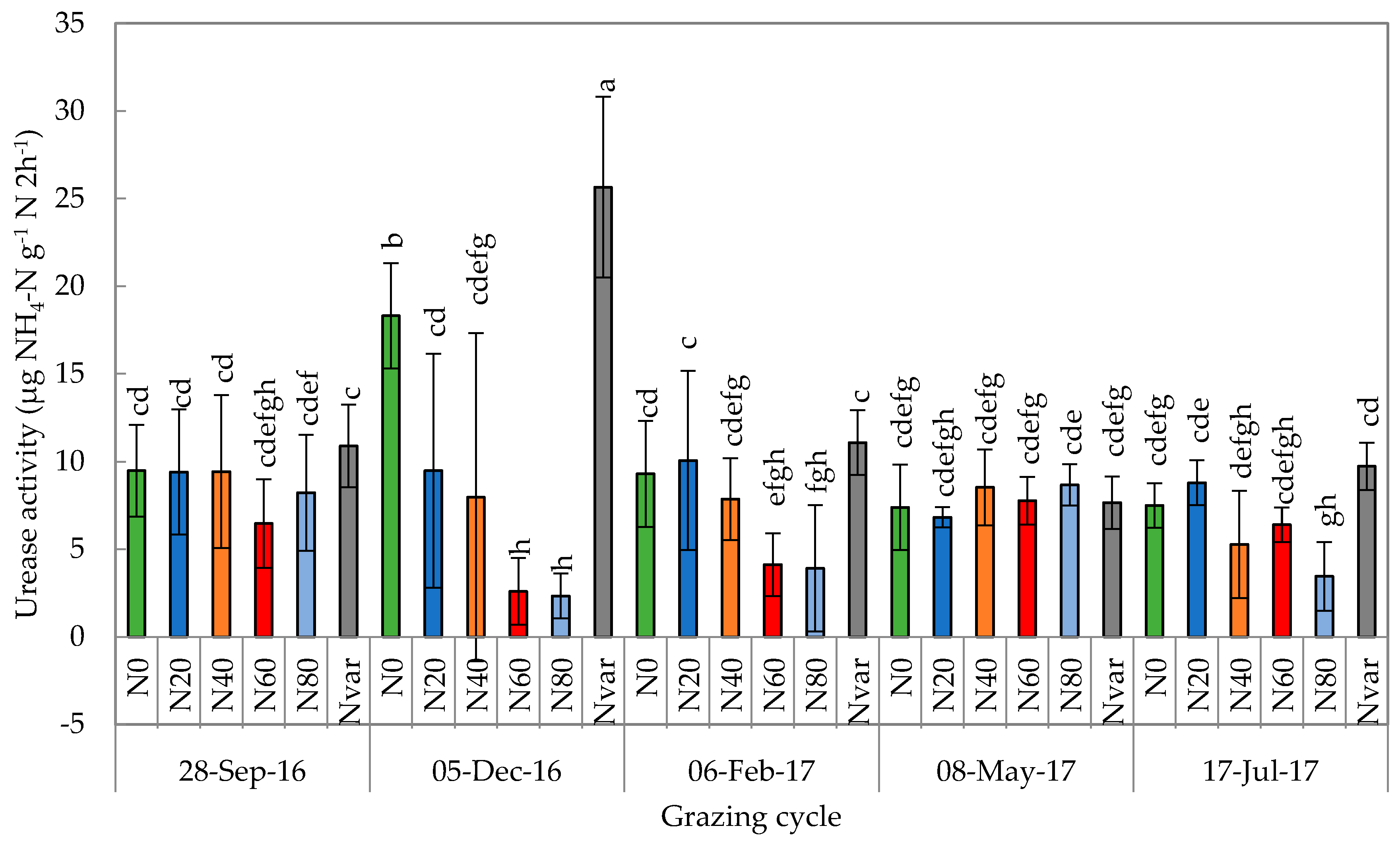
2.7. Urease Activity
2.8. Synthesis
3. Materials and Methods
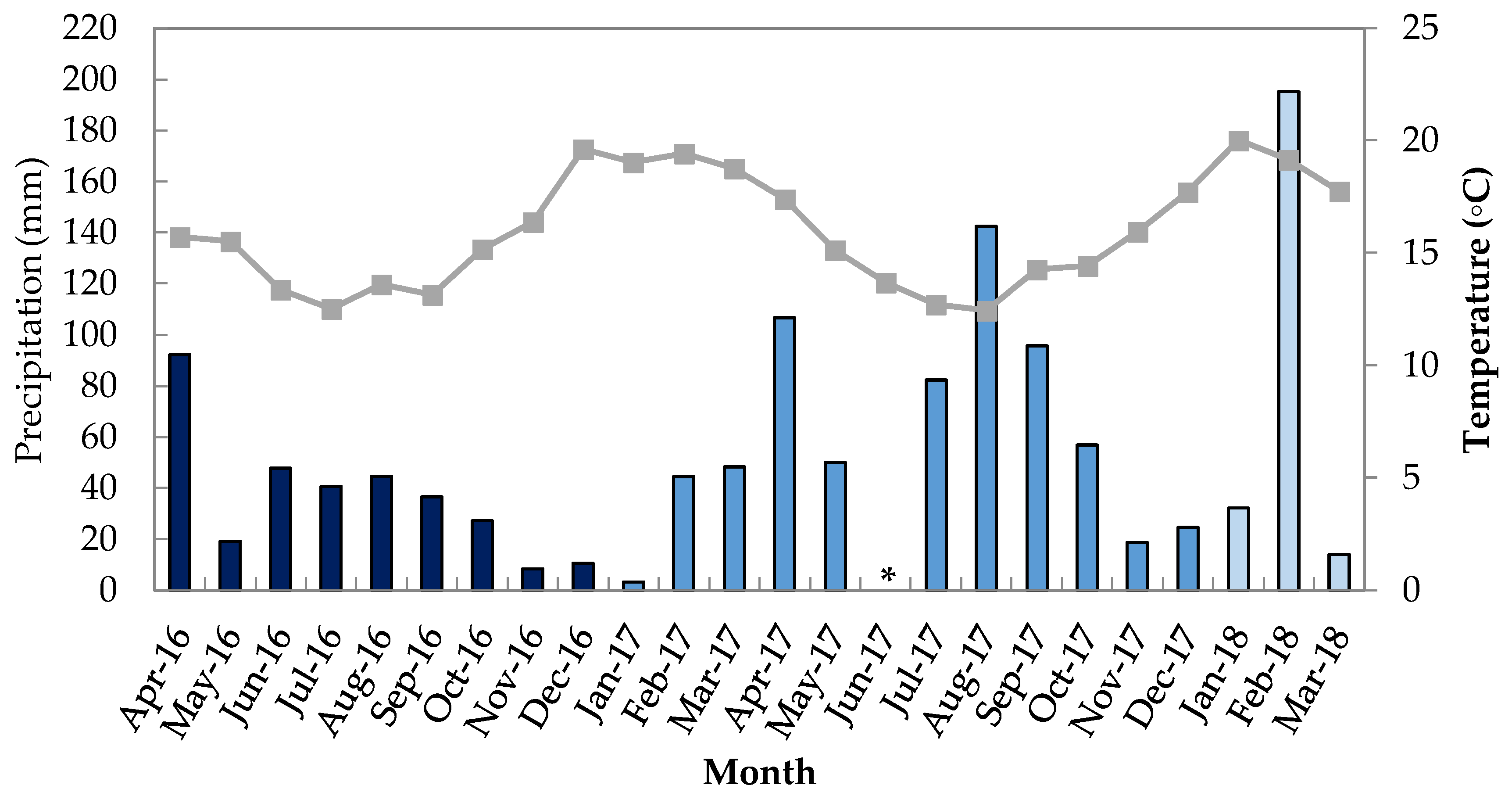
3.1. Site Description
3.2. Experimental Layout and Treatments
3.3. Pasture Management
3.4. Sampling and Analysis
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Garcia, S.C.; Islam, M.R.; Clark, C.E.F.; Martin, P.M. Kikuyu-based pasture for dairy production: A review. Crop Pasture Sci. 2014, 65, 787–797. [Google Scholar] [CrossRef]
- Marais, J.P. Factors affecting the nutritive value of kikuyu grass. Trop. Grassl. 2001, 35, 65–84. [Google Scholar]
- van der Colf, J.; Botha, P.R.; Meeske, R.; Truter, W.F. Seasonal dry matter production, botanical composition and forage quality of kikuyu over-sown with annual or perennial ryegrass. Afr. J. Range Forage Sci. 2015, 32, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Botha, P.R.; Zulu, L.B.; van der Colf, J.; Swanepoel, P.A. Production potential of Italian and Westerwolds ryegrass established at different planting dates. Afr. J. Range Forage Sci. 2015, 32, 153–159. [Google Scholar] [CrossRef]
- Botha, P.; Meeske, R.; Snyman, H. Kikuyu over-sown with ryegrass and clover: Dry matter production, botanical composition and nutritional value. Afr. J. Range Forage Sci. 2008, 25, 93–101. [Google Scholar] [CrossRef]
- Brock, J.L.; Kane, G.J. Variability in establishing white clover in pastures on farms. Proc. N. Z. Grassl. Assoc. 2003, 65, 223–228. [Google Scholar]
- Schlueter, D.; Tracy, B. Sowing Method Effects on Clover Establishment into Permanent Pasture. Agron. J. 2011, 104, 1217–1222. [Google Scholar] [CrossRef] [Green Version]
- Caradus, J.R.; Woodfield, D. Overview and vision for white clover white clover: New Zealand’s competitive edge. Spec. Publ.-Agron. Soc. N. Z. 1995, 11, 1–6. [Google Scholar]
- Beyers, C.D.L. Die bemesting van aangeplante weidings. Weidings Pastures. Winterreën Spes. Uitgawe Elsenbg. Dep. Agric. 1994, 5, 86–93. [Google Scholar]
- Romera, A.J.; Levy, G.; Beukes, P.C.; Clark, D.A.; Glassey, C.B. A urine patch framework to simulate nitrogen leaching on New Zealand dairy farms. Nutr. Cycl. Agroecosyst. 2012, 92, 329–346. [Google Scholar] [CrossRef]
- Christie, K.M.; Smith, A.P.; Rawnsley, R.P.; Harrison, M.T.; Eckard, R.J. Simulated seasonal responses of grazed dairy pastures to nitrogen fertilizer in SE Australia: Pasture production. Agric. Syst. 2018, 166, 36–47. [Google Scholar] [CrossRef]
- Swanepoel, P.A.; du Preez, C.C.; Botha, P.R.; Snyman, H.A. A critical view on the soil fertility status of minimum-till kikuyu–ryegrass pastures in South Africa. Afr. J. Range Forage Sci. 2015, 32, 113–124. [Google Scholar] [CrossRef]
- Sistani, K.R.; Adeli, A.; Tewolde, H. Apparent use efficiency of nitrogen and phosphorus from litter applied to bermudagrass. Commun. Soil Sci. Plant Anal. 2010, 41, 1873–1884. [Google Scholar] [CrossRef]
- Fertasa, (Fertilizer Association of Southern Africa). Fertilizer Handbook, 8th ed.; Fertilizer Association of Southern Africa: Pretoria, South Africa, 2016. [Google Scholar]
- UN. Transforming our World: The 2030 Agenda For Sustainable Development; United Nations: New York, NY, USA, 2015. [Google Scholar]
- Pannell, D.J. Economic perspectives on nitrogen in farming systems: Managing trade-offs between production, risk and the environment. Soil Res. 2017, 55, 473–478. [Google Scholar] [CrossRef]
- Monjardino, M.; McBeath, T.; Ouzman, J.; Llewellyn, R.; Jones, B. Farmer risk-aversion limits closure of yield and profit gaps: A study of nitrogen management in the southern Australian wheatbelt. Agric. Syst. 2015, 137, 108–118. [Google Scholar] [CrossRef]
- Sutton, M.A.; Oenema, O.; Erisman, J.W.; Leip, A.; van Grinsven, H.; Winiwarter, W. Too much of a good thing. Nature 2011, 472, 159–161. [Google Scholar] [CrossRef] [Green Version]
- Foote, K.J.; Joy, M.K.; Death, R.G. New Zealand Dairy Farming: Milking Our Environment for All Its Worth. Environ. Manag. 2015, 56, 709–720. [Google Scholar] [CrossRef]
- Adenuga, A.; Davis, J.; Hutchinson, G.; Donnellan, T.; Patton, M. Valuing Agricultural Externalities: Nitrogen Surplus in the Dairy Sector on the Island of Ireland. In Proceedings of the 30th International Conference of Agricultural Economists (IAAE), Vancouver, BC, Canada, 28 July–2 August 2018. [Google Scholar]
- Pretty, J.N.; Brett, C.; Gee, D.; Hine, R.E.; Mason, C.F.; Morison, J.I.L.; Raven, H.; Rayment, M.D.; Van Der Bijl, G. An assessment of the total external costs of UK agriculture. Agric. Syst. 2000, 65, 113–136. [Google Scholar] [CrossRef]
- Swanepoel, P.A.; Botha, P.R.; Snyman, H.A.; Preez, C.C. Impact of cultivation method on productivity and botanical composition of a kikuyu—Ryegrass pasture. Afr. J. Range Forage Sci. 2014, 31, 215–220. [Google Scholar] [CrossRef]
- van der Colf, J. The evaluation of annual ryegrass varieties in the southern Cape: 2014 to 2015. In Proceedings of the Inligtingsdag│Information Day, Outeniqua Research, George, South Africa, 19 October 2016; pp. 15–20. [Google Scholar]
- Botha, P.; Meeske, R.; Snyman, H. Kikuyu over-sown with ryegrass and clover: Grazing capacity, milk production and milk composition. Afr. J. Range Forage Sci. 2008, 25, 103–110. [Google Scholar] [CrossRef]
- Sun, X.; Luo, N.; Longhurst, B.; Luo, J. Fertiliser Nitrogen and Factors Affecting Pasture Responses. Open Agric. J. 2008, 2, 35–42. [Google Scholar] [CrossRef]
- Enriquez-Hidalgo, D.; Gilliland, T.J.; Hennessy, D. Herbage and nitrogen yields, fixation and transfer by white clover to companion grasses in grazed swards under different rates of nitrogen fertilization. Grass Forage Sci. 2016, 71, 559–574. [Google Scholar] [CrossRef]
- Clark, D.A.; Harris, S.L. White clover or nitrogen fertiliser for dairying? Spec. Publ. Agron. Soc. N. Z. 1996, 6, 107–114. [Google Scholar]
- Bolland, M.D.A.; Guthridge, I.F. Responses of intensively grazed dairy pastures to applications of fertiliser nitrogen in south-western Australia. Aust. J. Exp. Agric. 2007, 47, 927–941. [Google Scholar] [CrossRef]
- Smith, A.P.; Christie, K.M.; Rawnsley, R.P.; Eckard, R.J. Fertiliser strategies for improving nitrogen use efficiency in grazed dairy pastures. Agric. Syst. 2018, 165, 274–282. [Google Scholar] [CrossRef]
- Nevens, F.; Rehuel, D. Effects of cutting, grazing grass swards on herbage yield, N uptake and residual soil N at different levels of N fertilization. Grass Forage Sci. 2003, 58, 431–449. [Google Scholar] [CrossRef] [Green Version]
- Malcolm, B.J.; Moir, J.L.; Cameron, K.C.; Di, H.J.; Edwards, G.R. Influence of plant growth and root architecture of Italian ryegrass (Lolium multiflorum) and tall fescue (Festuca arundinacea) on N recovery during winter. Grass Forage Sci. 2015, 70, 600–610. [Google Scholar] [CrossRef]
- Goold, G.J. Effect of nitrogen and cutting interval on production of grass species swards in Northland, New Zealand. N. Z. J. Exp. Agric. 1979, 7, 353–359. [Google Scholar] [CrossRef]
- NRC, (National Research Council). Nutrient Requirements of Dairy Cattle, 7th ed.; National Academy Press: Washington, DC, USA, 2001.
- Veterinary Medicine: A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs and Goats, 10th ed.; Radostits, O.M.; Gay, C.C.; Hinchcliff, K.W.; Constable, P.D. (Eds.) Saunders Elsevier: London, UK, 2006; ISBN 9788578110796. [Google Scholar]
- Olmos Colmenero, J.J.; Broderick, G.A. Effect of dietary crude protein concentration on milk production and nitrogen utilization in lactating dairy cows. J. Dairy Sci. 2006, 89, 1704–1712. [Google Scholar] [CrossRef] [Green Version]
- Reid, M.; O’Donovan, M.; Elliott, C.T.; Bailey, J.S.; Watson, C.J.; Lalor, S.T.J.; Corrigan, B.; Fenelon, M.A.; Lewis, E. The effect of dietary crude protein and phosphorus on grass-fed dairy cow production, nutrient status, and milk heat stability. J. Dairy Sci. 2015, 98, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Huddell, A.M.; Galford, G.L.; Tully, K.L.; Crowley, C.; Palm, C.A.; Neill, C.; Hickman, J.E.; Menge, D.N.L. Meta-analysis on the potential for increasing nitrogen losses from intensifying tropical agriculture. Glob. Chang. Biol. 2020, 26, 1668–1680. [Google Scholar] [CrossRef] [PubMed]
- Maire, J.; Krol, D.; Pasquier, D.; Cowan, N.; Skiba, U.; Rees, R.M.; Reay, D.; Lanigan, G.J.; Richards, K.G. Nitrogen fertiliser interactions with urine deposit affect nitrous oxide emissions from grazed grasslands. Agric. Ecosyst. Environ. 2020, 290, 106784. [Google Scholar] [CrossRef]
- Ledgard, S.F.; Penno, J.W.; Sprosen, M.S. Nitrogen inputs and losses from clover/grass pastures grazed by dairy cows, as affected by nitrogen fertilizer application. J. Agric. Sci. 1999, 132, 215–225. [Google Scholar] [CrossRef]
- Di, H.J.; Cameron, K.C. Nitrate leaching in temperate agroecosystems: Sources, factors and mitigating strategies. Nutr. Cycl. Agroecosyst. 2002, 64, 237–256. [Google Scholar] [CrossRef]
- Directive, N. Council Directive 91/676/EEC of 12 December 1991 concerning the protection of waters against pollution caused by nitrates from agricultural sources. Off. J. 1991, 375, 1–8. [Google Scholar]
- DWAF (Department of Water Affairs and Forestry, South Africa). South African Water Quality Guidelines: Volume 1 Domestic Use; Department of Water Affairs and Forestry Private: Pretoria, South Africa, 1996; Volume 1, ISBN 0798853387.
- Fulkerson, W.J.; Lowe, K.F.; Hume, D.E. Perennial Forage and Pasture Crops—Establishment and Maintenance. Science 2011, 2, 586–593. [Google Scholar]
- Swanepoel, P.A.; Habig, J.; du Preez, C.C.; Snyman, H.A.; Botha, P.R. Tillage effects, soil quality and production potential of kikuyu–ryegrass pastures in South Africa. Grass Forage Sci. 2017, 72, 308–321. [Google Scholar] [CrossRef]
- Saha, S.; Prakash, V.; Kundu, S.; Kumar, N.; Mina, B.L. Soil enzymatic activity as affected by long term application of farm yard manure and mineral fertilizer under a rainfed soybean-wheat system in N-W Himalaya. Eur. J. Soil Biol. 2008, 44, 309–315. [Google Scholar] [CrossRef]
- Pascual, J.A.; García, C.; Hernandez, T. Lasting microbiological and biochemical effects of the addition of municipal solid waste to an arid soil. Biol. Fertil. Soils 1999, 30, 1–6. [Google Scholar] [CrossRef]
- Zaman, M.; Cameron, K.C.; Di, H.J.; Inubushi, K. Navigating wall-sized displays with the gaze: A proposal for cultural heritage. Nutr. Cycl. Agroecosyst. 2002, 63, 275–290. [Google Scholar] [CrossRef]
- Liu, E.; Yan, C.; Mei, X.; He, W.; Bing, S.H.; Ding, L.; Liu, Q.; Liu, S.; Fan, T. Long-term effect of chemical fertilizer, straw, and manure on soil chemical and biological properties in northwest China. Geoderma 2010, 158, 173–180. [Google Scholar] [CrossRef]
- Becker, F.; MacLaren, C.; Brink, C.J.; Jacobs, K.; Roux, M.R.; Swanepoel, P.A. High nitrogen rates do not increase canola yield and may affect soil bacterial functioning. Agron. J. 2020, 112, 523–536. [Google Scholar] [CrossRef]
- Shalloo, L.; Donovan, M.O.; Leso, L.; Werner, J.; Ruelle, E.; Geoghegan, A.; Delaby, L.; Leary, N.O. Review: Grass-based dairy systems, data and precision technologies. Animal 2018, 12, S262–S271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, L.W.; Hayes, R.C.; Pembleton, K.G.; Waters, C.M. Opportunities and challenges in Australian grasslands: Pathways to achieve future sustainability and productivity imperatives. Crop Pasture Sci. 2014, 65, 489–507. [Google Scholar] [CrossRef]
- Gargiulo, J.I.; Eastwood, C.R.; Garcia, S.C.; Lyons, N.A. Dairy farmers with larger herd sizes adopt more precision dairy technologies. J. Dairy Sci. 2018, 101, 5466–5473. [Google Scholar] [CrossRef]
- IUSS Working Group. World Reference Base for Soil Resources 2014, Update 2015 International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; FAO: Rome, Italy, 2015. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 9th ed.; Department of Agriculture: Washington, DC, USA, 2003. [Google Scholar]
- Soil Classification Working Group. Soil Classification: A Taxonomic System for South Africa: Memoirs on the Agricultural Natural Resources of South Africa No. 15; Department of Agricultural Development: Pretoria, South Africa, 1991. [Google Scholar]
- Stevens, J.; Stirzaker, R. Wetting Front Detector Transfer of Technology. WRC Report No. KV 246/10; Water Research Comission: Pretoria, South Africa, 2010. [Google Scholar]
- Fessehazion, M.K.; Abraha, A.B.; Everson, C.S.; Truter, W.F.; Annandale, J.G.; Moodley, M. Water Use and Nitrogen Application for Irrigation Management of Annual Ryegrass and Kikuyu Pasture Production; Water Research Comission: Pretoria, South Africa, 2012. [Google Scholar]
- Stirzaker, R.J. When to turn the water off: Scheduling micro-irrigation with a wetting front detector. Irrig. Sci. 2003, 22, 177–185. [Google Scholar] [CrossRef]
- Fessehazion, M.K.; Stirzaker, R.J.; Annandale, J.G.; Everson, C.S. Improving nitrogen and irrigation water use efficiency through adaptive management: A case study using annual ryegrass. Agric. Ecosyst. Environ. 2011, 141, 350–358. [Google Scholar] [CrossRef] [Green Version]
- Botha, P. Factors influencing the persistence and production potential of kikuyu (Pennisetum clandestinum) over-sown with different ryegrass and clover species in the southern Cape. Agriprobe 2009, 6, 4–9. [Google Scholar]
- van der Colf, J.; Botha, P.R.; Meeske, R.; Truter, W.F. Grazing capacity, milk production and milk composition of kikuyu over-sown with annual or perennial ryegrass. Afr. J. Range Forage Sci. 2015, 32, 143–151. [Google Scholar] [CrossRef] [Green Version]
- van der Colf, J. The Production Potential of Kikuyu (Pennisetum Clandestinum) Pastures Over-Sown with Ryegrass (Lolium spp.); University of Pretoria: Pretoria, South Africa, 2011. [Google Scholar]
- AOAC. Official Method of Analysis 988.05, 17th ed.; Association of Official Analytical Chemists, Inc.: Rockville, MD, USA, 2000. [Google Scholar]
- Cataldo, D.; Haroon, H.; Schrader, L.; Young, V. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Keeney, D.R.; Nelson, D.W. Nitrogen-Inorganic Forms. In Methods of Soil Analysis: Part 2, Chemical and Microbiological Properties—Agronomy Monograph no. 9; ASA-SSSA, 677 S.; Segoe Rd.: Madison, WI, USA, 1982. [Google Scholar]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Kotzé, E.; Sandhage-Hofmann, A.; Amelung, W.; Oomen, R.J.; du Preez, C.C. Soil microbial communities in different rangeland management systems of a sandy savanna and clayey grassland ecosystem, South Africa. Nutr. Cycl. Agroecosyst. 2017, 107, 227–245. [Google Scholar]
- Glass, G.V.; Peckham, P.D.; Sanders, J.R. Consequences of failure to meet assumptions underlying the fixed effects analyses of variance and covariance. Rev. Educ. Res. 1972, 42, 237–288. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 7th ed.; Iowa State University Press: Ames, IA, USA, 1980. [Google Scholar]
- Statistica (Data Analysis Software System), Version 13; TIBCO Software: Palo Alto, CA, USA, 2017.
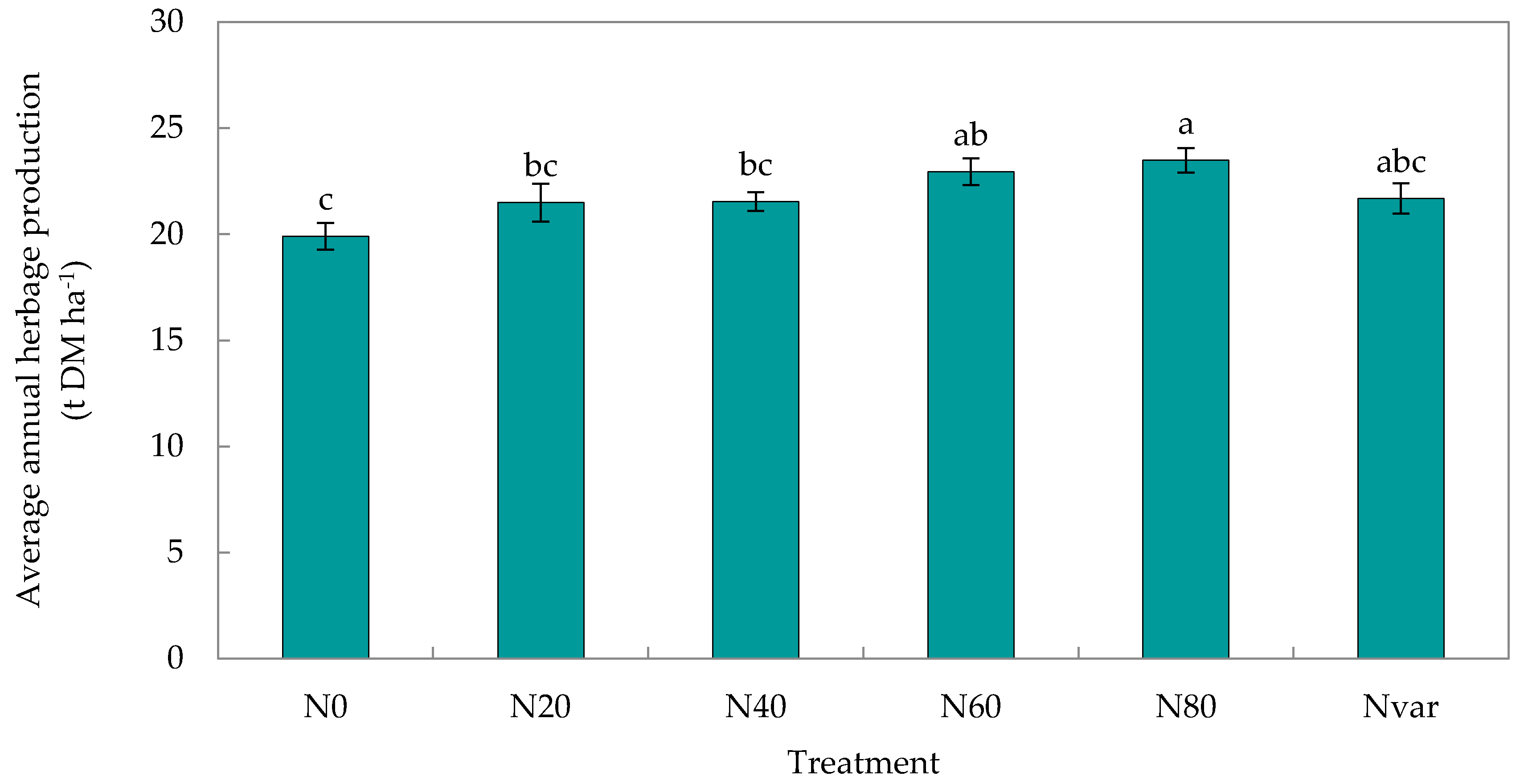
| N Application after Every Grazing (kg N ha−1 grazing −1) | Total Annual N Application (kg N ha−1 year−1) | ||
|---|---|---|---|
| Treatment | Year 1 | Year 2 | |
| N0 | 0 | 0 | 0 |
| N20 | 20 | 200 | 220 |
| N40 | 40 | 400 | 440 |
| N60 | 60 | 600 | 660 |
| N80 | 80 | 800 | 880 |
| Nvar | Dependent on soil water nitrate concentration | 10–35 | 0–50 |
| Number of grazing events | 11 | 12 | |
| Days in production year | 356 | 379 | |
| Variable | F-Statistic | p-Value | |
|---|---|---|---|
| Herbage Production | |||
| Treatment | 3.47 | 0.023 | |
| Season | 314.63 | <0.001 | |
| Treatment * season | 1.02 | 0.455 | |
| Agronomic N use efficiency | |||
| Treatment | 0.47 | 0.712 | |
| Season | 4.79 | <0.001 | |
| Treatment * season | 0.84 | 0.663 | |
| Crude protein | |||
| Treatment | 25.65 | <0.001 | |
| Season | 31.20 | <0.001 | |
| Treatment * season | 4.47 | <0.001 | |
| Total mineral soil N | |||
| Treatment | 12.51 | <0.001 | |
| Time of grazing event | 4.02 | <0.001 | |
| Treatment * Time of grazing event | 2.04 | <0.001 | |
| Potentially mineralisable N (0–100 mm) | |||
| Treatment | 0.17 | 0.971 | |
| Time of grazing event | 4.45 | <0.001 | |
| Treatment * Time of grazing event | 0.88 | 0.764 | |
| Potentially mineralisable N (100–200 mm) | |||
| Treatment | 0.38 | 0.855 | |
| Time of grazing event | 4.39 | <0.001 | |
| Treatment * Time of grazing event | 0.63 | 0.928 | |
| Potentially mineralisable N (200–300 mm) | |||
| Treatment | 1.24 | 0.333 | |
| Time of grazing event | 4.99 | <0.001 | |
| Treatment * Time of grazing event | 0.68 | 0.886 | |
| Urease activity | |||
| Treatment | 9.67 | <0.001 | |
| Time of grazing event | 4.43 | 0.008 | |
| Treatment * Time of grazing event | 5.59 | <0.001 | |
| 6 | 12 | 18 | 24 | ||||
| B1 Nvar | B2 N0 | B3 N20 | B4 N40 | ||||
| 5 | 11 | 17 | 23 | ||||
| B1 N20 | B2 N60 | B3 N0 | B4 Nvar | ||||
| 4 | 10 | 16 | 22 | ||||
| B1 N80 | B2 N20 | B3 Nvar | B4 N0 | ||||
| 3 | 9 | 15 | 21 | ||||
| B1 N0 | B2 N80 | B3 N40 | B4 N20 | ||||
| 2 | 8 | 14 | 20 | ||||
| B1 N60 | B2 N40 | B3 N60 | B4 N80 | ||||
| 1 | 7 | 13 | 19 | ||||
| B1 N40 | B2 Nvar | B3 N80 | B4 N60 |
| Initial Nitrate Concentration Range of 150 † mm WFD ‡ (mg L−1) | Adapted Nitrate Concentration Range of 150 mm WFD (mg L−1) | Nitrogen Application Rate (kg N ha−1 grazing−1) |
|---|---|---|
| <25 | <50 | 50 |
| 25–50 | 50–75 | 25 |
| >50 | >75 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viljoen, C.; van der Colf, J.; Swanepoel, P.A. Benefits Are Limited with High Nitrogen Fertiliser Rates in Kikuyu-Ryegrass Pasture Systems. Land 2020, 9, 173. https://doi.org/10.3390/land9060173
Viljoen C, van der Colf J, Swanepoel PA. Benefits Are Limited with High Nitrogen Fertiliser Rates in Kikuyu-Ryegrass Pasture Systems. Land. 2020; 9(6):173. https://doi.org/10.3390/land9060173
Chicago/Turabian StyleViljoen, Charné, Janke van der Colf, and Pieter Andreas Swanepoel. 2020. "Benefits Are Limited with High Nitrogen Fertiliser Rates in Kikuyu-Ryegrass Pasture Systems" Land 9, no. 6: 173. https://doi.org/10.3390/land9060173






