Sustainability Analysis of Prosopis juliflora (Sw.) DC Based Restoration of Degraded Land in North India
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field Study and Soil Sampling
2.3. Soil Analysis
2.4. Biodiversity Indices under P. juliflora-Invaded and Non-Invaded Areas
2.5. Analysis of P. juliflora Invasion on Local Livelihood
2.6. Invasion Mapping and Data Analysis
3. Results
3.1. The Current Status of P. juliflora Invasion: Global and Indian Scenario
3.2. Effect of P. juliflora Invasion on Soil Quality
3.3. Effect of P. juliflora Invasion on Local Plant Biodiversity
3.4. Effect of P. juliflora Invasion on Local Livelihood
4. Discussion
4.1. Effect of P. juliflora Invasion on Soil Quality
4.2. Effect of P. juliflora Invasion on Local Plant Biodiversity
4.3. Effect of P. juliflora Invasion on Local Livelihood
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rakshit, A.; Sarkar, B.; Abhilash, P.C. Soil Amendments for Sustainability: Challenges and Perspectives, 1st ed.; CRC Press: Boca Raton, FL, USA, 2018; p. 428. [Google Scholar]
- Edrisi, S.A.; Abhilash, P.C. Book review: Socio-economic impacts of bioenergy production. Front. Bioeng. Biotechnol. 2015, 3, 174. [Google Scholar] [CrossRef]
- Plieninger, T.; Gaertner, M. Harnessing degraded lands for biodiversity conservation. J. Nat. Conserv. 2011, 19, 18–23. [Google Scholar] [CrossRef]
- Nkonya, E.; Mirzabaev, A.; Von Braun, J. Economics of Land Degradation and Improvement: A Global Assessment for Sustainable Development; Springer: Cham, Switzerland, 2016; p. 686. [Google Scholar]
- Tripathi, V.; Edrisi, S.A.; O’Donovan, A.; Gupta, V.K.; Abhilash, P.C. Bioremediation for Fueling the Biobased Economy. Trend Biotechnol. 2016, 34, 775–777. [Google Scholar] [CrossRef] [PubMed]
- Dubey, R.K.; Tripathi, V.; Edrisi, S.A.; Bakshi, M.; Dubey, P.K.; Singh, A.; Verma, J.P.; Singh, A.; Sarma, B.K.; Raskhit, A.; et al. Role of Plant Growth Promoting Microorganisms in Sustainable Agriculture and Environmental Remediation. In Advances in PGPR Research; Sarma, B.K., Singh, H.B., Keswani, C., Eds.; Centre for Agriculture and Biosciences International (CABI): Wallingford, UK, 2017; p. 466. ISBN 9781786390325. [Google Scholar]
- Edrisi, S.A.; Tripathi, V.; Abhilash, P.C. Performance analysis and soil quality indexing for Dalbergia sissoo Roxb. grown in marginal and degraded land of eastern Uttar Pradesh, India. Land 2019, 8, 63. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, Y.; Wu, Z.; Lv, T. A Bibliometric Analysis on Land Degradation: Current Status, Development, and Future Directions. Land 2020, 9, 28. [Google Scholar] [CrossRef]
- ICAR. Degraded and Wastelands of India; Indian Council of Agricultural Research: New Delhi, India, 2010; p. 167. Available online: https://icar.org.in/files/Degraded-and-Wastelands.pdf (accessed on 10 January 2020).
- ICRISAT. Agri-Buzz Blog: Bioreclamation of Degraded Lands: Restoring Roots, Rights and Resilience in Niger. Available online: https://www.icrisat.org/bioreclamation-of-degraded-lands-restoring-roots-rights-and-resilience-in-niger/ (accessed on 16 October 2017).
- Besseau, P.; Graham, S.; Christophersen, T. (Eds.) Restoring Forests and Landscapes: The Key to a Sustainable Future; Global Partnership on Forest and Landscape Restoration (GPFLR): Vienna, Austria, 2018; p. 25. [Google Scholar]
- Keesstra, S.; Mol, G.; de Leeuw, J.; Okx, J.; Molenaar, C.; de Cleen, M.; Visser, S. Soil-Related Sustainable Development Goals: Four Concepts to Make Land Degradation Neutrality and Restoration Work. Land 2018, 7, 133. [Google Scholar] [CrossRef]
- Tianjiao, F.; Wei, W.; Liding, C.; Keesstra, S.D.; Yang, Y. Effects of land preparation and plantings of vegetation on soil moisture in a hilly loess catchment in China. Land Degrad. Dev. 2018, 29, 1427–1441. [Google Scholar] [CrossRef]
- Novara, A.; Pulido, M.; Rodrigo-Comino, J.; Di Prima, S.; Smith, P.; Gristina, L.; Gimenez-Morera, A.; Terol, E.; Salesa, D.; Keesstra, S. Long-term organic farming on a citrus plantation results in soil organic carbon recovery. Cuad. Investig. Geogr. 2019, 45, 271–286. [Google Scholar] [CrossRef]
- Edrisi, S.A.; Abhilash, P.C. Sustainable bioenergy production from woody biomass: Prospects and promises. J. Clean. Prod. 2015, 102, 558–559. [Google Scholar] [CrossRef]
- Edrisi, S.A.; Abhilash, P.C. Exploring marginal and degraded lands for biomass and bioenergy production: An Indian scenario. Renew. Sustain. Energy Rev. 2016, 54, 1537–1551. [Google Scholar] [CrossRef]
- Lefcheck, J.S.; Byrnes, J.E.; Isbell, F.; Gamfeldt, L.; Griffin, J.N.; Eisenhauer, N.; Hensel, M.J.; Hector, A.; Cardinale, B.J.; Duffy, J.E. Biodiversity enhances ecosystem multifunctionality across trophic levels and habitats. Nat. Commun. 2015, 6, 6936. [Google Scholar] [CrossRef] [PubMed]
- Paini, D.R.; Sheppard, A.W.; Cook, D.C.; De Barro, P.J.; Worner, S.P.; Thomas, M.B. Global threat to agriculture from invasive species. Proc. Nat. Acad. Sci. USA 2016, 113, 7575–7579. [Google Scholar] [CrossRef] [PubMed]
- Early, R.; Bradley, B.A.; Dukes, J.S.; Lawler, J.J.; Olden, J.D.; Blumenthal, D.M.; Gonzalez, P.; Grosholz, E.D.; Ibañez, I.; Miller, L.P.; et al. Global threats from invasive alien species in the twenty-first century and national response capacities. Nat. Commum. 2016, 7, 12485. [Google Scholar] [CrossRef] [PubMed]
- Hanley, N.; Roberts, M. The economic benefits of invasive species management. People Nat. 2019, 1, 124–137. [Google Scholar] [CrossRef]
- MNRE. National Policy on Biofuels, Ministry of New and Renewable Energy; Government of India: New Delhi, India, 2009.
- Baka, J. What wastelands? A critique of biofuel policy discourse in South India. Geoforum 2014, 54, 315–323. [Google Scholar] [CrossRef]
- El-Keblawy, A.; Al-Rawai, A. Impacts of the invasive exotic Prosopis juliflora (Sw.) D.C. on the native flora and soils of the UAE. Plant Ecol. 2007, 190, 23–35. [Google Scholar] [CrossRef]
- El-Fadl, M.A. Management of Prosopis Juliflora for Use in Agroforestry Systems in the Sudan. Bachelor’s Thesis, University of Helsinki, Helsinki, Finland, 1997; p. 107. [Google Scholar]
- Kaur, R.; Gonzales, W.L.; Llambi, L.D.; Soriano, P.J.; Callaway, R.M.; Rout, M.E.; Gallaher, T.J.; Inderjit. Community Impacts of Prosopis juliflora Invasion: Biogeographic and Congeneric Comparisons. PLoS ONE 2012, 7, e44966. [Google Scholar] [CrossRef]
- Al-Humaid, A.I.; Warrag, M.O.A. Allelopathic effects of mesquite (Prosopis juliflora) foliage on seed germination and seedling growth of bermudagrass (Cynodon dactylon). J. Arid Environ. 1998, 38, 237–243. [Google Scholar] [CrossRef]
- Maundu, P.; Kibet, S.; Morimoto, Y.; Imbumi, M.; Adeka, R. Impact of Prosopis juliflora on Kenya’s semi-arid and arid ecosystems and local livelihoods. Biodivers 2009, 10, 33–50. [Google Scholar] [CrossRef]
- Gallaher, T.; Merlin, M. Biology and impacts of Pacific Island invasive species Prosopis pallida and Prosopis juliflora (Algar roba, Mesquite, Kiawe) (Fabaceae). Pac. Sci. 2010, 64, 489–526. [Google Scholar] [CrossRef]
- Dzikiti, S.; Schachtschneider, K.; Naiken, V.; Gush, M.; Moses, G.; Le Maitre, D.C. Water relations and the effects of clearing invasive Prosopis trees on groundwater in an arid environment in the Northern Cape, South Africa. J. Arid Environ. 2013, 90, 103–113. [Google Scholar] [CrossRef]
- Pasiecznik, N.; Felker, P.; Harris, P.; Harsh, L.; Cruz, G.; Tewari, J.; Cadoret, K.; Maldonado, L. The Prosopis Juliflora-Prosopis Pallida Complex: A Monograph; HDRA: Coventry, UK, 2001; p. 172. [Google Scholar]
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species: A Selection from the Global Invasive Species Database; Invasive Species Specialist Group; IUCN: Auckland, New Zealand, 2000; Volume 12, p. 12. [Google Scholar]
- Shackleton, R.T.; Le Maitre, D.C.; Pasiecznik, N.M.; Richardson, D.M. Prosopis: A global assessment of the biogeography, benefits, impacts and management of one of the world’s worst woody invasive plant taxa. AoB Plants 2014, 6, plu027. [Google Scholar] [CrossRef]
- Shiferaw, H.; Bewket, W.; Alamirew, T.; Zeleke, G.; Teketay, D.; Bekele, K.; Schaffner, U.; Eckert, S. Implications of land use/land cover dynamics and Prosopis invasion on ecosystem service values in Afar Region, Ethiopia. Sci. Total Environ. 2019, 675, 354–366. [Google Scholar] [CrossRef]
- Shackleton, R.T.; Le Maitre, D.C.; Van Wilgen, B.W.; Richardson, D.M. The impact of invasive alien Prosopis species (mesquite) on native plants in different environments in South Africa. S. Afr. Bot. 2015, 97, 25–31. [Google Scholar] [CrossRef]
- Keller, P.; Lodge, M.; Lewis, M.; Shogren, J. Bioeconomics of Invasive Species: Integrating Ecology, Economics, Policy, and Management; University of Oxford: Oxford, UK, 2010. [Google Scholar]
- Prasad, M.N.V.; Tewari, J.C. Prosopis juliflora (SW) DC: Potential for Bioremediation and Bioeconomy. In Bioremediation and Bioeconomy; Prasad, M.N.V., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 49–76. [Google Scholar]
- El-Fadl, M.A.; Luukkanen, O. Field studies on ecological strategies of Prosopis juliflora in a dry land ecosystem: 1. A leaf gas exchange approach. J. Arid Environ. 2006, 66, 1–15. [Google Scholar] [CrossRef]
- Zimmermann, H.G. Biological control of Prosopis, Prosopis spp. (Fabaceae), in South Africa. Agric. Ecosyst. Environ. 1991, 37, 175–186. [Google Scholar] [CrossRef]
- Choge, S.K.; Ngujiri, F.D.; Kuria, M.N.; Busaka, E.A.; Muthondeki, J.K. The Status and Impact of Prosopis spp.; KEFRI: Kenya, Nairobi, 2002. [Google Scholar]
- Laxen, J. Is Prosopis a curse or a blessing? An ecological and economic analysis of an invasive alien tree species in Sudan. In Tropical Forestry Reports; Lukkanan, O., Ed.; VITRI, University of Helsinki: Helsinki, Finland, 2007; pp. 1–199. [Google Scholar]
- CAZRI. Prosopis juliflora: A Valuable Species for Arid and Semi-arid Tropics. In Discussion Papers for National Workshop on Prqsopis juliflora: Past, Present and Future; ICAR-CAZRI (Indian Council of Agricultural Research, Central Arid Zone Research Institute): Jodhpur, India, 2011; pp. 7–44. [Google Scholar]
- Pysek, P.; Richardson, D.M.; Rejmanek, M.; Webster, G.L.; Williamson, M.; Kirschner, J. Alien plants in checklists and floras: Towards better communication between taxonomists and ecologists. Taxon 2004, 53, 131–143. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, K.A.; Ahmad, V.U.; Oazi, S. Antibacterial activity of juliflorine isolated from Prosopis juliflora. Planta Med. 1986, 52, 285–288. [Google Scholar] [CrossRef]
- Bhojvaid, P.P.; Timmer, V.R. Soil dynamics in an age sequence of Prosopis juliflora planted for sodic soil restoration in India. For. Ecol. Manag. 1998, 106, 181–193. [Google Scholar] [CrossRef]
- Prabha, D.S.; Dahms, H.U.; Malliga, P. Pharmacological potentials of phenolic compounds from Prosopis spp.-a. J. Coast. Life Med. 2014, 2, 918–924. [Google Scholar] [CrossRef]
- McLean, E.O. Soil pH and lime requirement. In Methods of Soil Analysis. Part 2, 2nd ed.; Page, A.L., Ed.; ASA and SSSA: Madison, WI, USA, 1982; pp. 199–224. [Google Scholar]
- Blake, G.R.; Harte, K.H. Bulk density. In Methods of Soil Analysis. Part 1. Physical and Mineralogical Methods, 2nd ed.; Klute, A., Ed.; American Society of Agronomy and Soil Science Society of America: Madison, WI, USA, 1986; pp. 363–375. [Google Scholar]
- Toth, S.J.; Prince, A.L. Estimation of cation-exchange capacity and exchangeable Ca, K, and Na contents of soils by flame photometer techniques. Soil Sci. 1949, 67, 439–446. [Google Scholar] [CrossRef]
- Walkley, A. A critical examination of a rapid method for determining organic carbon in soils: Effect of variations in digestion conditions and of organic soil constituents. Soil Sci. 1947, 63, 251–264. [Google Scholar] [CrossRef]
- Kalra, Y.P.; Maynard, D.G. Methods Manual for Forest Soil and Plant Analysis; Information Report NOR-X-319; Northern Forestry Centre: Edmonton, AB, Canada, 1991; p. 116. [Google Scholar]
- Subbiah, B.V.; Asija, G.L. A rapid procedure for the determination of available nitrogen in soils. Curr. Sci. 1956, 25, 259–260. [Google Scholar]
- Olsen, S.R.; Sommers, L.E. Phosphorus. Methods of Soil Analysis, Part 2—Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Ed.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982. [Google Scholar]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Casida, L.E., Jr.; Klein, D.A.; Santoro, T. Soil dehydrogenase activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Murthy, H.M.R.; Manomani, H.K. Aerobic degradation of technical hexachlorocyclohexane by a defined microbial consortium. J. Hazard. Mater. 2007, 149, 18–25. [Google Scholar] [CrossRef]
- Adhikari, M.; Nagata, S.; Adhikari, M. Rural household and forest: An evaluation of household’s dependency on community forest in Nepal. J. For. Res. 2004, 9, 33–44. [Google Scholar] [CrossRef]
- Maikhuri, R.K.; Semwal, R.L.; Singh, A.; Nautiyal, M.C. Wild fruits as a contribution to sustainable rural development: A case study from the Garhwal Himalaya. Int. J. Sust. Dev. World Ecol. 1994, 1, 56–68. [Google Scholar] [CrossRef]
- Sundriyal, R.C.; Sharma, E. Anthropogenic pressure on tree structure and biomass in the temperate forest of Mamlay watershed in Sikkim. For. Ecol. Manag. 1996, 81, 113–134. [Google Scholar] [CrossRef]
- IBM SPSS. IBM SPSS Statistics 19: A Brief Guide; SPSS Inc.: Chicago, IL, USA, 2010. [Google Scholar]
- SPSS. SPSS Version 16.0.2; SPSS Inc.: Chicago, IL, USA, 2008; Available online: https://dl.acm.org/doi/abs/10.5555/542499 (accessed on 26 January 2020).
- Waksman, S.A. Microbiological analysis of soil as an index of soil quality. III. Influence of fertilization upon numbers of microorganisms in soil. Soil Sci. 1992, 14, 321–346. [Google Scholar] [CrossRef]
- Mahanta, S.K. Economic Balanced Rations for Dairy Animals; ICAR-Indian Grassland and Fodder Research Institute: Jhansi, India, 2017; p. 35. [Google Scholar]
- Câmara, A.C.L.; Afonso, J.A.B.; Riet-Correa, F. Rational uses of mesquite (Prosopis juliflora) and the importance of spontaneous poisoning by the pods in ruminants from Pernambuco. In Poisoning by Plants, Mycotoxins and Related Toxins; Riet-Correa, F., Pfister, J., Schild, A.L., Eds.; CAB International: London, UK 2011; pp. 295–301. [Google Scholar]
- Wardle, D.A.; Bardgett, R.D.; Callaway, R.M.; Van der Putten, W.H. Terrestrial ecosystem responses to species gains and losses. Science 2011, 332, 1273–1277. [Google Scholar] [CrossRef] [PubMed]
- Trenchard, L.J.; Harris, P.J.C.; Smith, S.J.; Pasiecznik, N.M. A review of ploidy in the genus Prosopis (Leguminosae). Bot. J. Linn. Soc. 2008, 156, 425–438. [Google Scholar] [CrossRef]
- Seastedt, T.R.; Callaway, R.M.; Pollock, J.; Kaur, J. Allelopathy and plant invasions: Traditional, congeneric, and biogeographical approaches. Biol. Invasions 2008, 10, 875–890. [Google Scholar] [CrossRef]
- Nakano, H.; Fujii, Y.; Suzuki, T.; Yamada, K.; Kosemura, S.; Yamamura, S.; Hasegawa, K. A growth-inhibitory substance exuded from freeze-dried mesquite (Prosopis juliflora (Sw.) DC.) leaves. Plant Growth Regul. 2001, 33, 165–168. [Google Scholar] [CrossRef]
- Nakano, H. Plant growth inhibitors from mesquite (Prosopis juliflora). In Desert Plants; Ramawat, K.G., Ramawat, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 341–352. [Google Scholar]
- Ahmad, V.U.; Mohammad, Z.G. Studies on the structure of juliflorine. J. Chem. Soc. Pak. 1979, 1, 137–138. [Google Scholar]
- Ott-Longoni, R.; Viswanathan, N.; Hesse, M. Die Konstitution des Alkaloides Juliprosopin aus Prosopis juliflora A. DC. Helv. Chim. Acta 1980, 63, 2119–2129. [Google Scholar] [CrossRef]
- Ahmad, V.U.; Sultana, A.; Qazi, S. Alkaloids from the leaves of Prosopis juliflora. J. Nat. Prod. 1989, 52, 497–501. [Google Scholar] [CrossRef]
- Datwyler, P.; Ott-Longoni, R.; Schopp, E.; Hesse, M. Uber Juliprosin, ein weiteres Alkaloid aus Prosopis juliflora A. DC. Helv. Chim. Acta 1981, 64, 1959–1963. [Google Scholar] [CrossRef]
- Tapia, A.; Feresin, G.E.; Bustos, D.; Astudillo, L.; Theoduloz, C.; Schmeda-Hirschmann, G. Biologically active alkaloids and a free radical scavenger from Prosopis species. J. Ethnopharmacol. 2000, 71, 241–246. [Google Scholar] [CrossRef]
- Aqeel, A.; Khursheed, A.K.; Viqaruddin, A.; Sabiha, Q. Antimicrobial activity of julifloricine isolated from Prosopis juliflora. Arzneim. Forsch. Drug Res. 1989, 39, 652–655. [Google Scholar]
- Ayanu, Y.; Jentsch, A.; Müller-Mahn, D.; Rettberg, S.; Romankiewicz, C.; Koellner, T. Ecosystem engineer unleashed: Prosopis juliflora threatening ecosystem services? Reg. Environ. Chang. 2014, 15, 155–167. [Google Scholar] [CrossRef]
- Admasu, D. Invasive Plants and Food Security: The case of Prosopis juliflora in the Afar region of Ethiopia. FARM-Africa, IUCN Report. 2008. Available online: http://cmsdata.iucn.org/downloads/invasive_plants_ and_food_security___final.pdf (accessed on 3 March 2019).
- Amdihun, A.; Tadesse, G.; Peden, D.; Getahun, Y. Invasive Plant Prosopis Juliflora Expansion on Farm and Grazing Land in Ethiopia: A Threat to Pastoral Grazing Land; International Livestock Research Institute: Addis Ababa, Ethiopia, 2010; Available online: http://www.docstoc.com/docs/23533265/Prosopis-Juliflora-distribution-in-Awash-River-Basin-Ethiopia (accessed on 22 March 2011).
- Berhanu, A.; Tesfaye, G. The Prosopis dilemma, impacts on dryland biodiversity and some controlling methods. J. Drylands 2006, 1, 158–164. [Google Scholar]
- Yohannes, T.; Awas, T.; Demisew, S. Survey and documentation of the potential and actual invasive alien plant species and other biological threats to biodiversity in Awash National Park, Ethiopia. Manag. Biol. Invasions 2011, 2, 3–14. [Google Scholar] [CrossRef][Green Version]
- Wamburu, R.W.; Kareru, P.G.; Mbaria, J.M.; Nyaga, G.; Rechab, S.O. Spectrometric detection of organic compounds and toxicity of ethanolic leaves extracts of Prosopis juliflora. Chem. Mater. Res. 2015, 7, 21–25. [Google Scholar]
- Silva, V.D.A.; Pitanga, B.P.S.; Nascimento, R.P.; Souza, C.S.; Coelho, P.L.; Menezes, F.N.; Silva, A.M.M.; Costa, M.F.D.; El-Bacha, R.S.; Velozo, E.S.; et al. Juliprosopine and juliprosine from Prosopis juliflora leaves induce mitochondrial damage and cytoplasmic vacuolation on co-cultured glial cells and neurons. Chem. Res. Toxicol. 2013, 26, 1810–1820. [Google Scholar] [CrossRef]
- Wamburu, R.W.; Kareru, P.G.; Mbaria, J.M.; Njonge, F.K.; Nyaga, G.; Rechab, S.O. Acute and sub-Acute toxicological evaluation of ethanolic leaves extract of Prosopis juliflora (Fabaceae). J. Nat. Sci. Res. 2013, 3, 8–15. [Google Scholar]
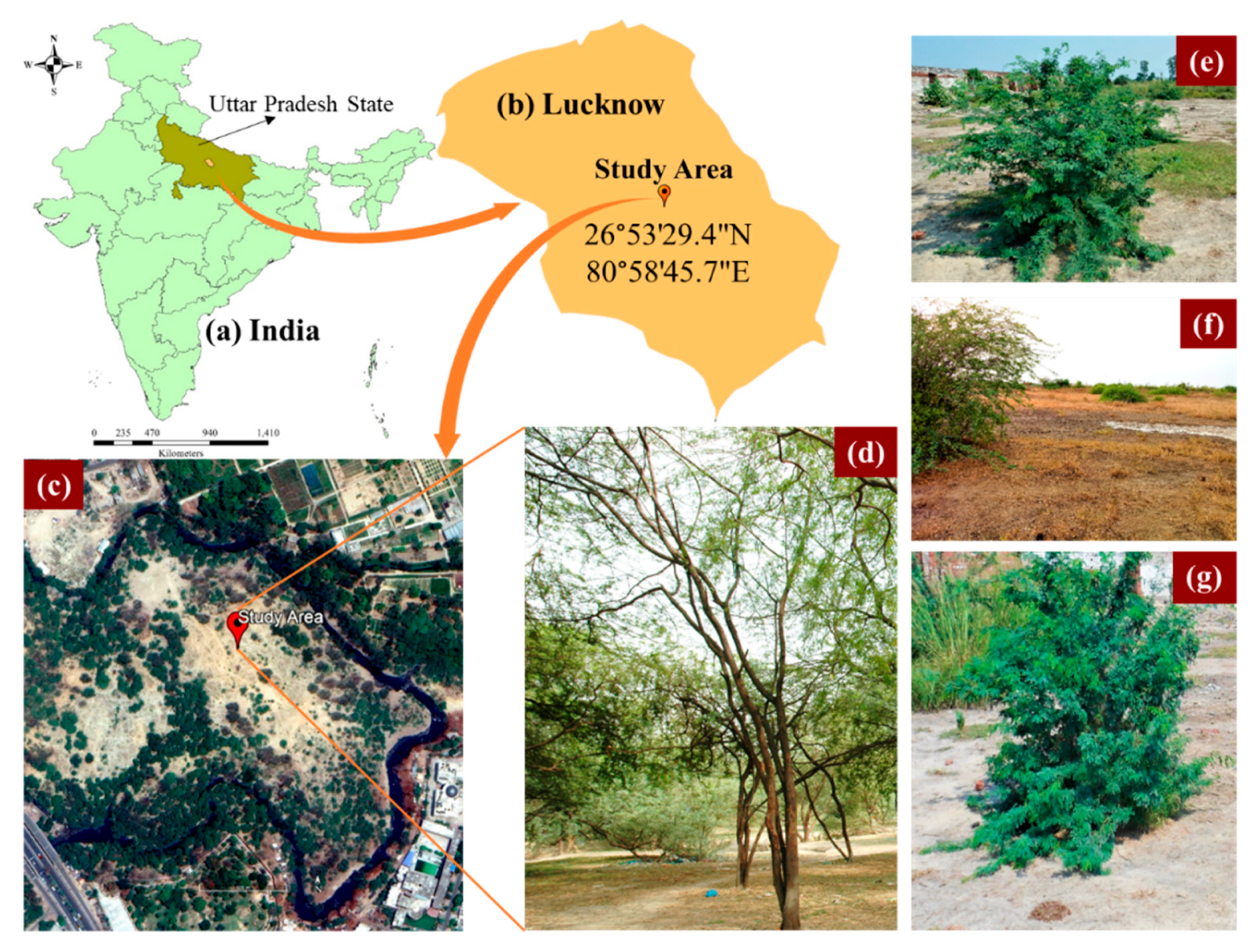
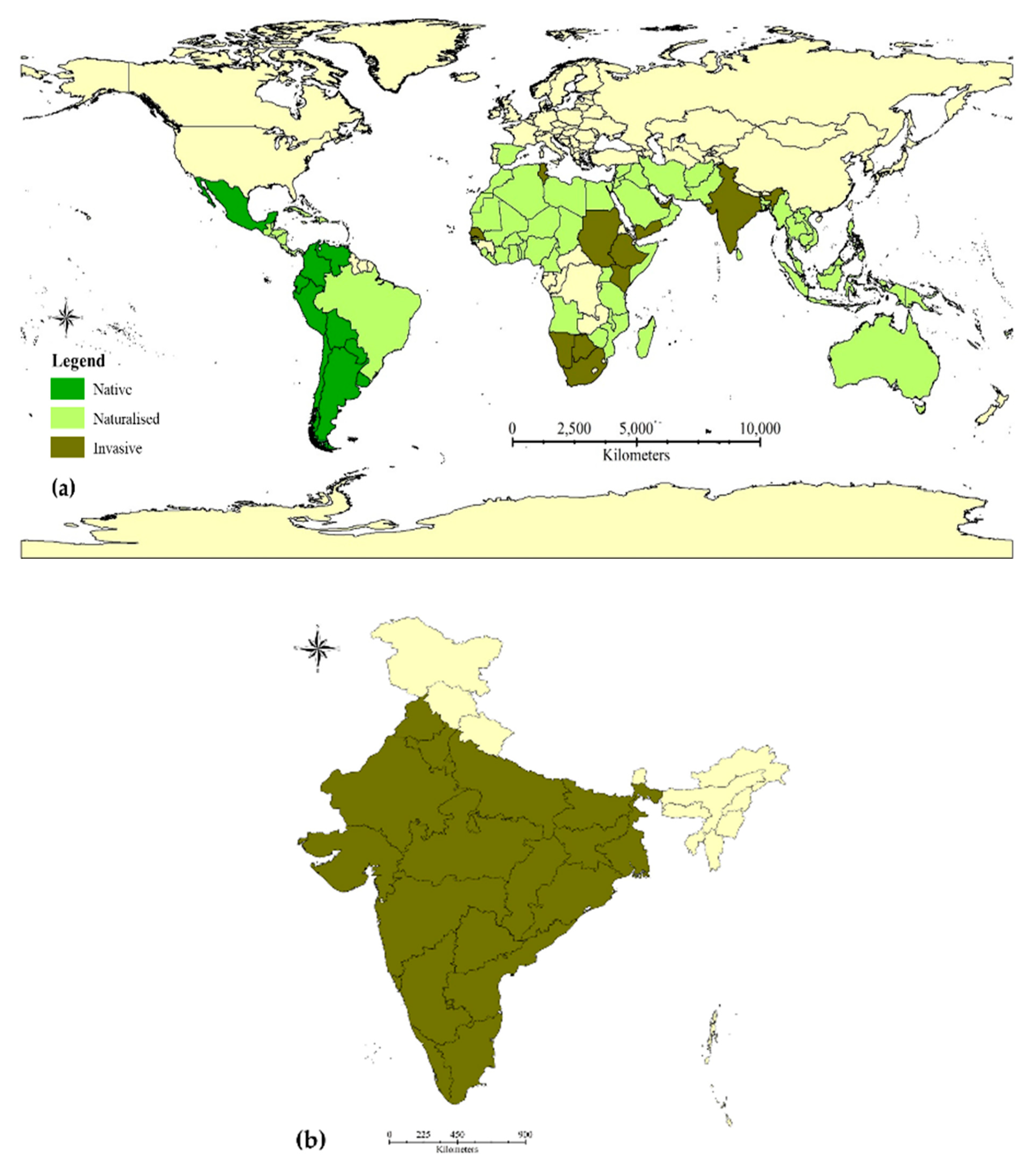
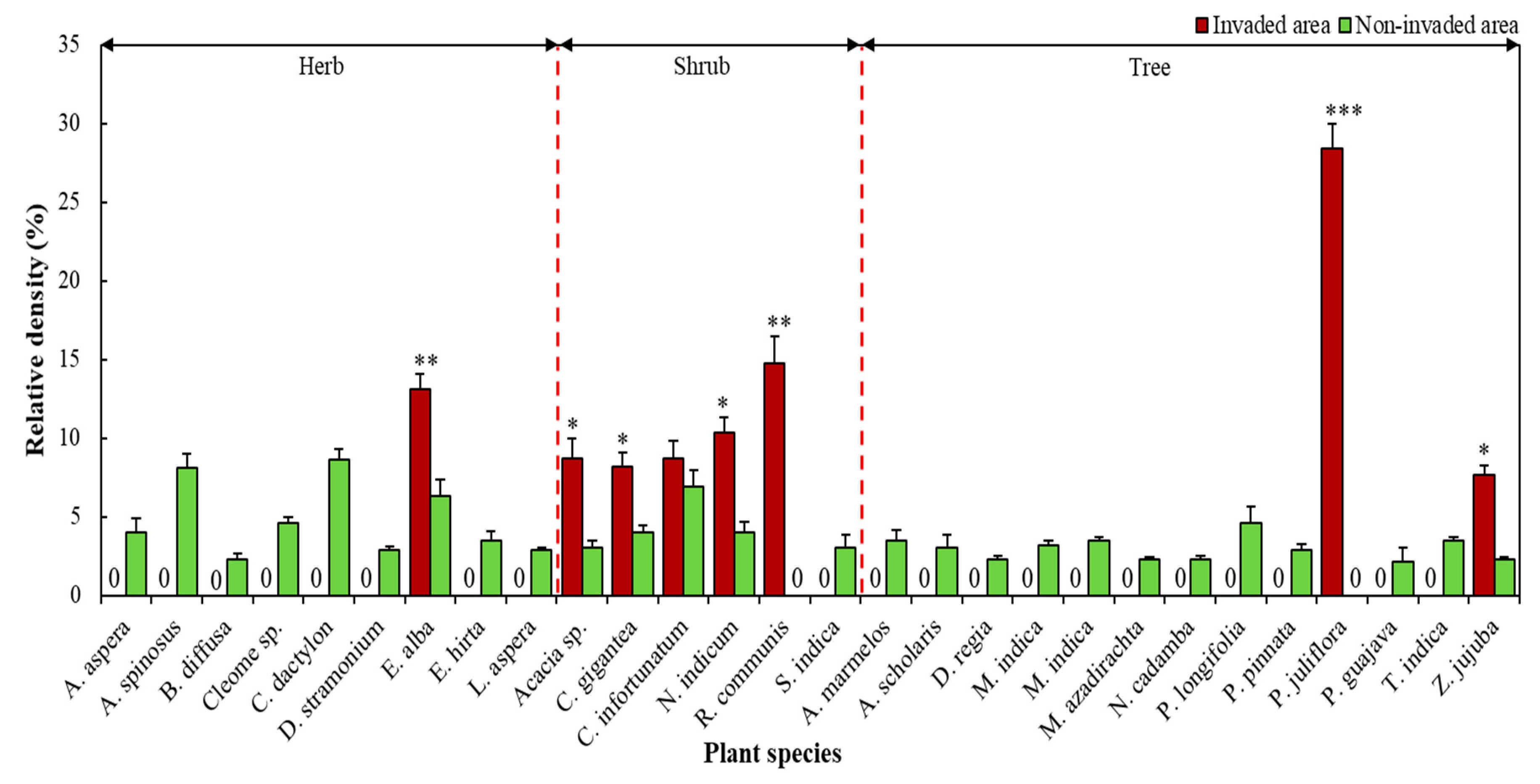


| S. No. | Land-Use of the Block * | (Area in ha) |
| 1. | Total block area | 12,564.94 |
| 2. | Cultivable area | 5688.15 |
| 3. | Forest area | 3.70 |
| 4. | Area under non-agricultural uses | 1746.70 |
| 5. | Barren and un-cultivable land | 462.40 |
| 6. | Permanent pastures and other grazing lands | 74.20 |
| 7. | Land under miscellaneous tree crops, etc. | 139.10 |
| 8. | Culturable wasteland | 597.30 |
| 9. | Fallow lands other than current fallows | 2398.9 |
| 10. | Current fallows | 1455.40 |
| Demography of the Block * | (numbers) | |
| 11. | Number of households | 24,845 |
| 12. | Total population | 137,251 |
| Demography of study area (P. juliflora-invaded and non-invaded) # | (numbers) | |
| 13. | Households in P. juliflora-invaded area | 21 |
| 14. | Households in non-invaded area | 36 |
| 15. | Population in P. juliflora-invaded area | 129 |
| 16 | Population in non-invaded area | 218 |
| 17. | Livestock in P. juliflora-invaded area | 11 |
| 18. | Livestock in non-invaded area | 30 |
| Item | Content/Purpose of the Questions |
|---|---|
| (A) Age and sex | Respondent’s age and sex |
| (B) Literacy level | The literacy level of the respondent and their understanding regarding Prosopis juliflora and other local/introduced species |
| (C) Resource utilisation type | The type of resources extracted/collected by the local residents from both invaded and non-invaded areas Fuelwood Fodder Medicinal Fencing Leaf litters Fruits/Edibles Other Utility |
| (D) Process of resource extraction/collection | The way by which the local residents extracted/collected/utilized the resources from both invaded and non-invaded areas Felling Chopping Lopping Plucking Grazing |
| (E) Frequency of the extraction | The frequency of the extraction/collection of the resources from both invaded and non-invaded areas Daily Weekly Fortnightly Monthly Once in a while Seasonally Annually |
| (F) Duration of the extraction | The duration of the extraction/collection of the resources from both invaded and non-invaded areas Summer Winter Rainy Annual |
| Parameters | Invaded area | Non-invaded (Open Area) |
|---|---|---|
| pH | 7.06 ± 0.15 | 7.05 ± 0.13 |
| EC (ds m−1) | 0.16 ± 0.11 | 0.27 ± 0.04* |
| BD (g cm−3) | 1.28 ± 0.06 | 1.30 ± 0.04 |
| Water holding capacity (%) | 57.97 ± 2.83* | 41.22 ± 0.50 |
| Cation Exchange Capacity (cmol kg−1) | 17.50 ± 0.60 | 16.77 ± 0.41 |
| Total organic carbon (g kg−1) | 8.93 ± 0.81* | 5.01 ± 0.42 |
| Soil organic matter (g kg−1) | 15.39 ± 1.40* | 8.64 ± 0.72 |
| Total nitrogen (g kg−1) | 0.85 ± 0.21** | 0.21 ± 0.07 |
| Available nitrogen (g kg−1) | 0.31 ± 0.05** | 0.09 ± 0.04 |
| Total phosphorus (g kg−1) | 0.17 ± 0.03** | 0.04 ± 0.01 |
| C:N ratio | 10.94 ± 2.37 | 26.79 ± 8.05** |
| Microbial biomass carbon (μg g−1) | 127.20 ± 12.08 | 119.10 ± 9.08 |
| Microbial biomass nitrogen (μg g−1) | 12.50 ± 1.07* | 9.46 ± 1.00 |
| Colony forming unit (106 g−1) | 19.28 ± 0.80 | 30.41 ± 4.55* |
| Soil dehydrogenase activity (μg g−1 h−1) | 16.31 ± 2.74* | 8.75 ± 0.69 |
| Site | Resource Utilisation | Plant Species | Extraction Process * | Extraction Period |
|---|---|---|---|---|
| P. juliflora-invaded area | Fuelwood (bark/logs)/ Fodder/ Fencing. Pods/fruits | Acacia sp. | F,C,L | Summer and winter |
| Prosopis juliflora | F,L | |||
| Ziziphus jujuba | F,C | |||
| Prosopis juliflora | F,C | |||
| Prosopis juliflora | F,C | As requirement | ||
| Prosopis juliflora | P,L | Summer | ||
| Ziziphus jujuba | P,L | |||
| Non-invaded area | Fuelwood (barks/logs)/ Fodder/ Medicinal uses/ Fencing/ Fruits/edibles | Acacia sp. | F,C,L | Summer and winter |
| Madhuca indica | F,C,L | |||
| Mangifera indica | F,C,L | |||
| Melia azadirachta | F,C,L | |||
| Pongamia pinnata | F,C,L | |||
| Polyalthia longifolia | C,L | |||
| Achyranthes aspera | G | |||
| Alstonia scholaris | F,G | |||
| Boerhavia diffusa | F,G | |||
| Cleome sp. | F,G | |||
| Clerodendrum infortunatum | C,G,Co | |||
| Cynodon dactylon | F,G | |||
| Eclipta alba | F,G | |||
| Euphorbia hirta | F,G | |||
| Leucas aspera | P,U | Summer and winter | ||
| Melia azadirachta | F,C,L | |||
| Delonix regia | F,C | As requirement | ||
| Polyalthia longifolia | F,C | |||
| Neolamarckia cadamba | P,L | Entire season | ||
| Aegle marmelos | P,L | |||
| Amaranthus spinosus | P,L |
| Sl No | Particulars | Study Area | |
|---|---|---|---|
| P. juliflora-Invaded Area | Non-Invaded Area | ||
| 1 | Fodder collected $ (kg household-1 d-1) | 1.69 ± 0.61 | 4.36 ± 1.74** |
| 2 | Fodder demand # (kg household-1 y-1) | 860.67 ± 135.24 | 1368.20 ± 214.99** |
| 3 | Fodder supply (kg household-1 y-1) | 440.70 ± 159.76 | 1526.00 ± 143.13** |
| 4 | Total deficit † (kg household-1 y-1) | 419.97 ± 85.06** | −157.80 ± 67.41 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edrisi, S.A.; El-Keblawy, A.; Abhilash, P.C. Sustainability Analysis of Prosopis juliflora (Sw.) DC Based Restoration of Degraded Land in North India. Land 2020, 9, 59. https://doi.org/10.3390/land9020059
Edrisi SA, El-Keblawy A, Abhilash PC. Sustainability Analysis of Prosopis juliflora (Sw.) DC Based Restoration of Degraded Land in North India. Land. 2020; 9(2):59. https://doi.org/10.3390/land9020059
Chicago/Turabian StyleEdrisi, Sheikh Adil, Ali El-Keblawy, and Purushothaman Chirakkuzhyil Abhilash. 2020. "Sustainability Analysis of Prosopis juliflora (Sw.) DC Based Restoration of Degraded Land in North India" Land 9, no. 2: 59. https://doi.org/10.3390/land9020059
APA StyleEdrisi, S. A., El-Keblawy, A., & Abhilash, P. C. (2020). Sustainability Analysis of Prosopis juliflora (Sw.) DC Based Restoration of Degraded Land in North India. Land, 9(2), 59. https://doi.org/10.3390/land9020059








