AeroHydro Culture: An Integrated Approach to Improve Crop Yield and Ecological Restoration Through Root–Microbe Symbiosis in Tropical Peatlands
Abstract
1. Introduction
2. Materials and Methods
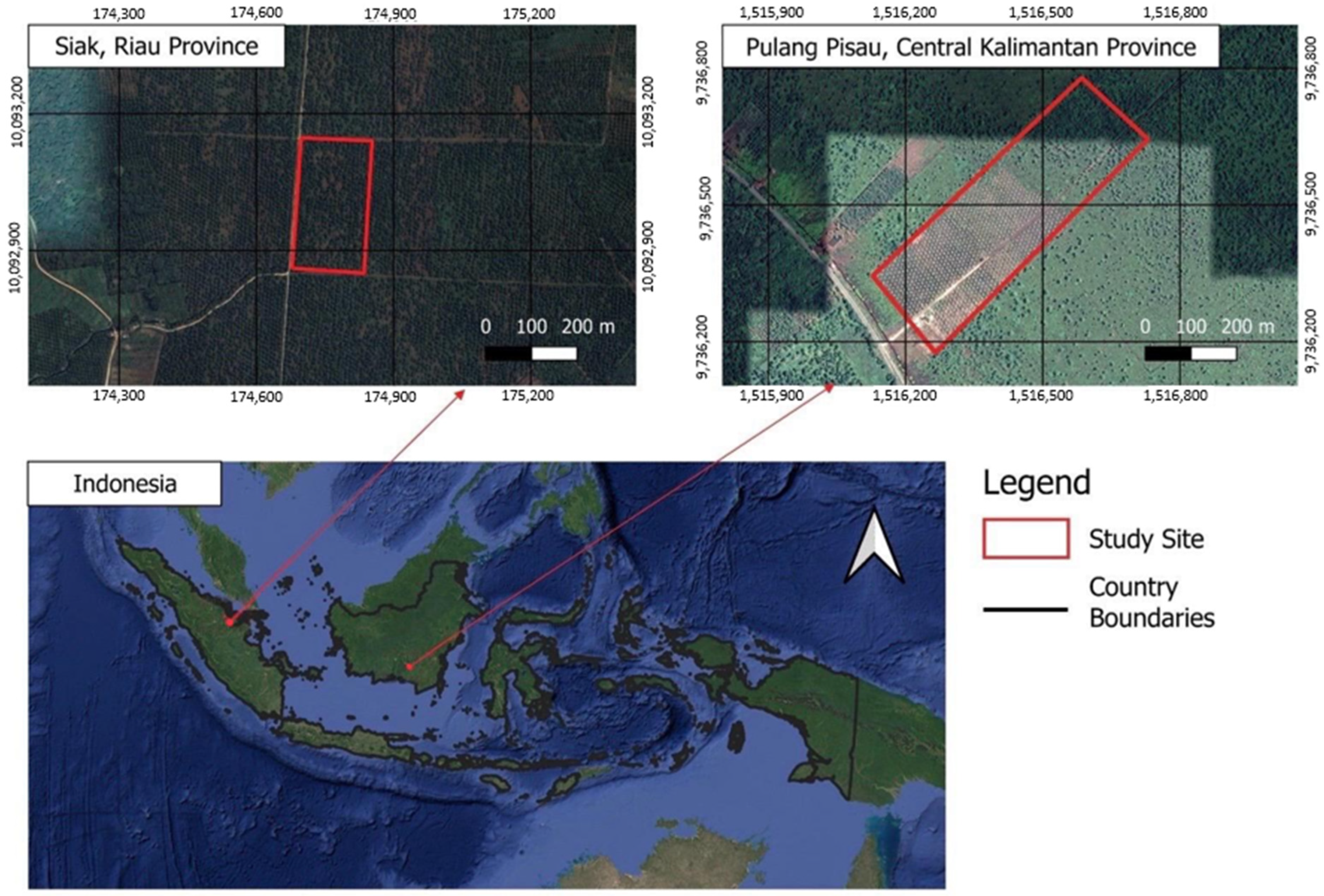
2.1. Study Sites
2.2. AHC Media Preparation and Application
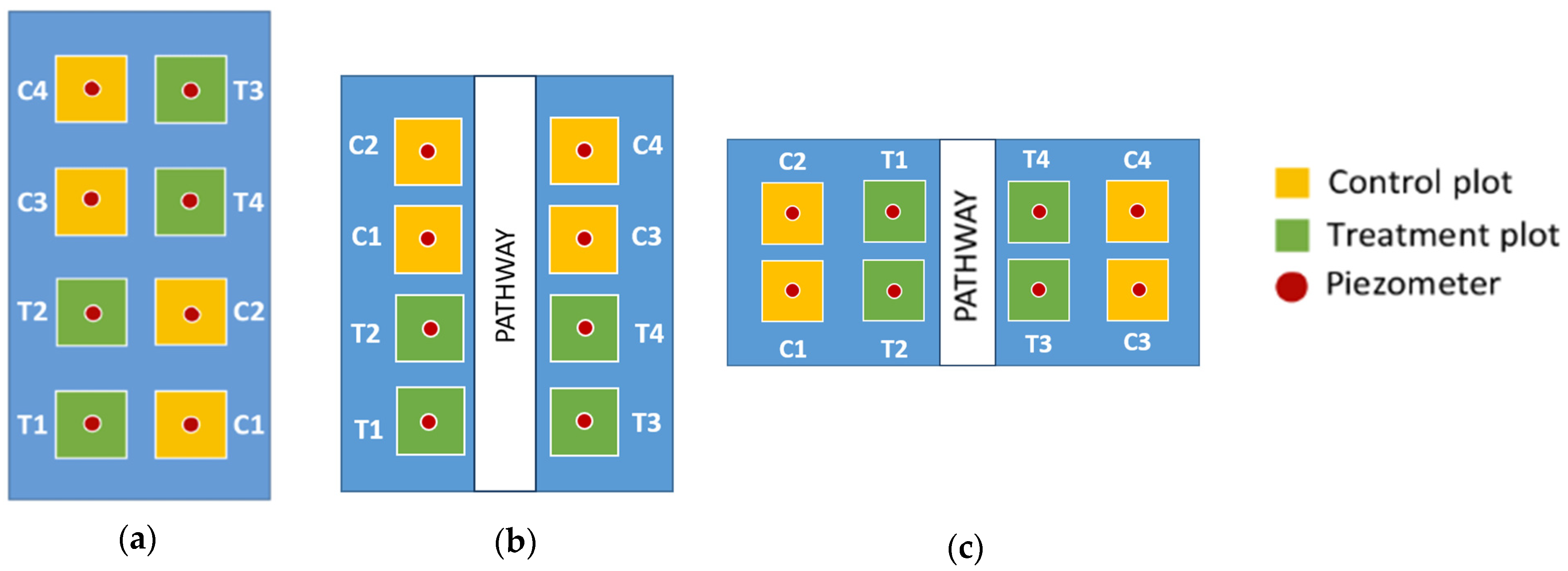
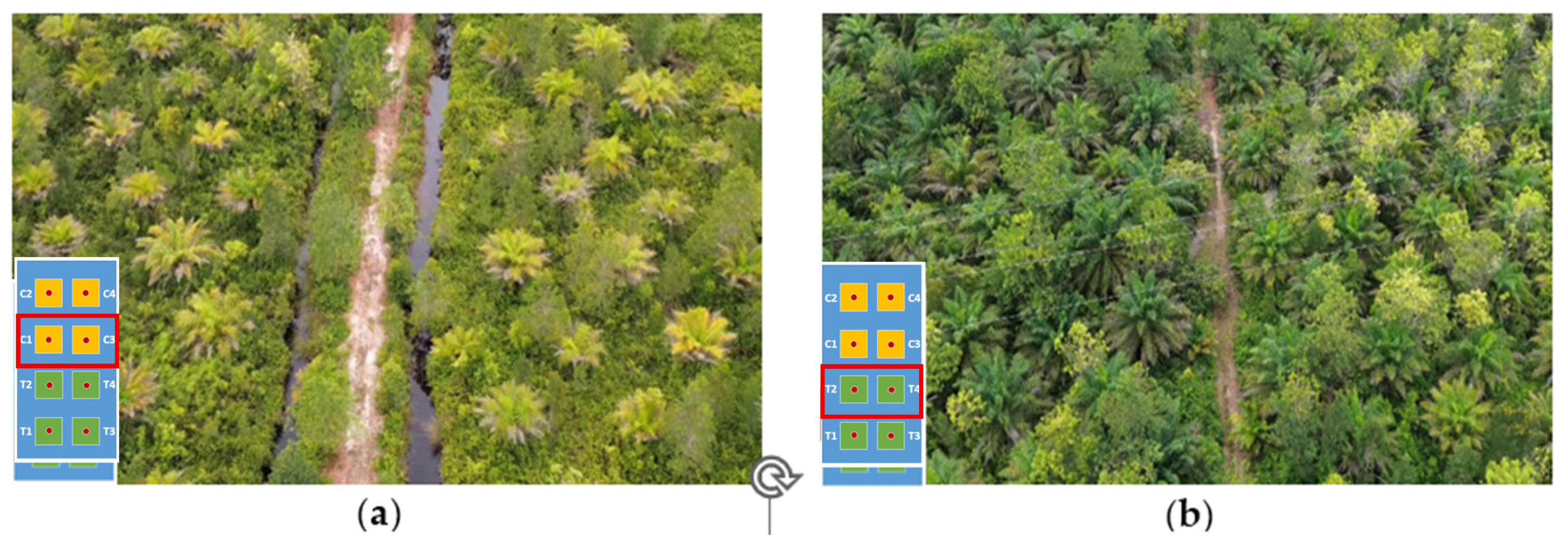
2.3. Experimental Design
2.4. Assessment Timeline
2.5. Plant Trait Assessment
2.6. Arbuscular Mycorrhizae Colonization
2.7. Soil Microbial Activity Assessment
2.8. Yield Measurement
2.9. Statistical Analysis
3. Results
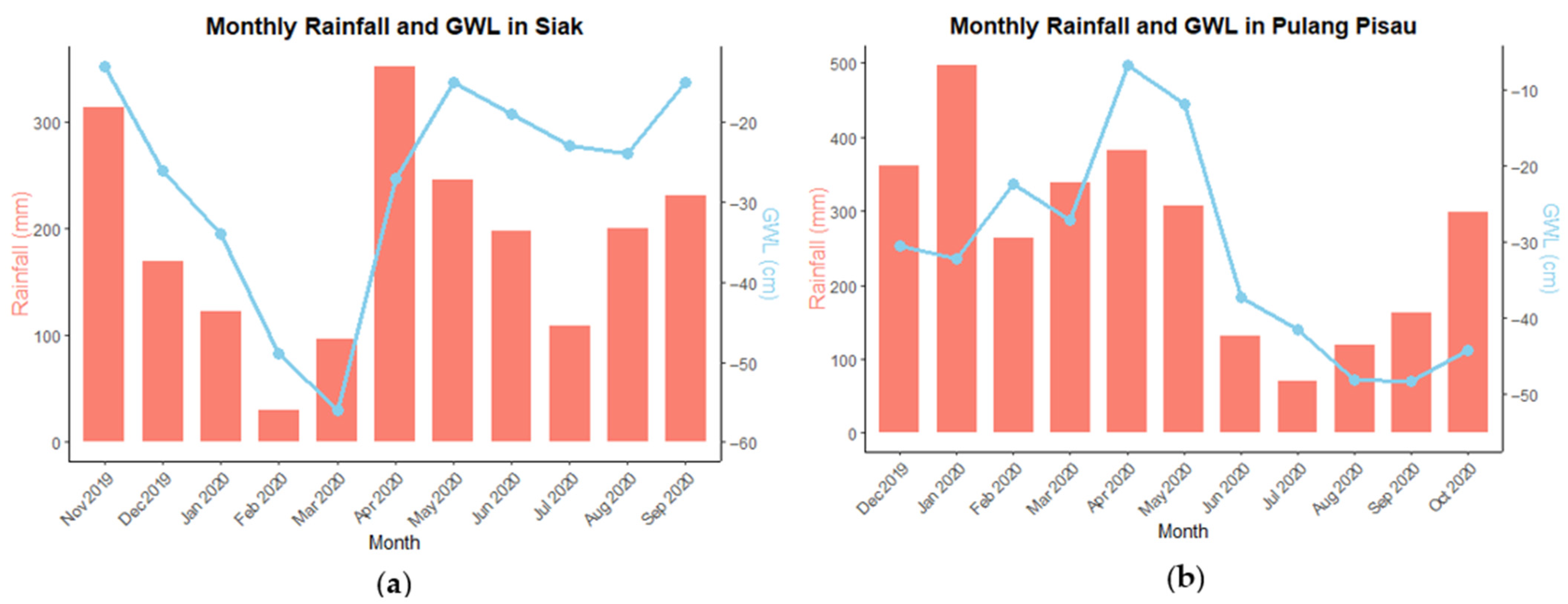
3.1. Groundwater Level
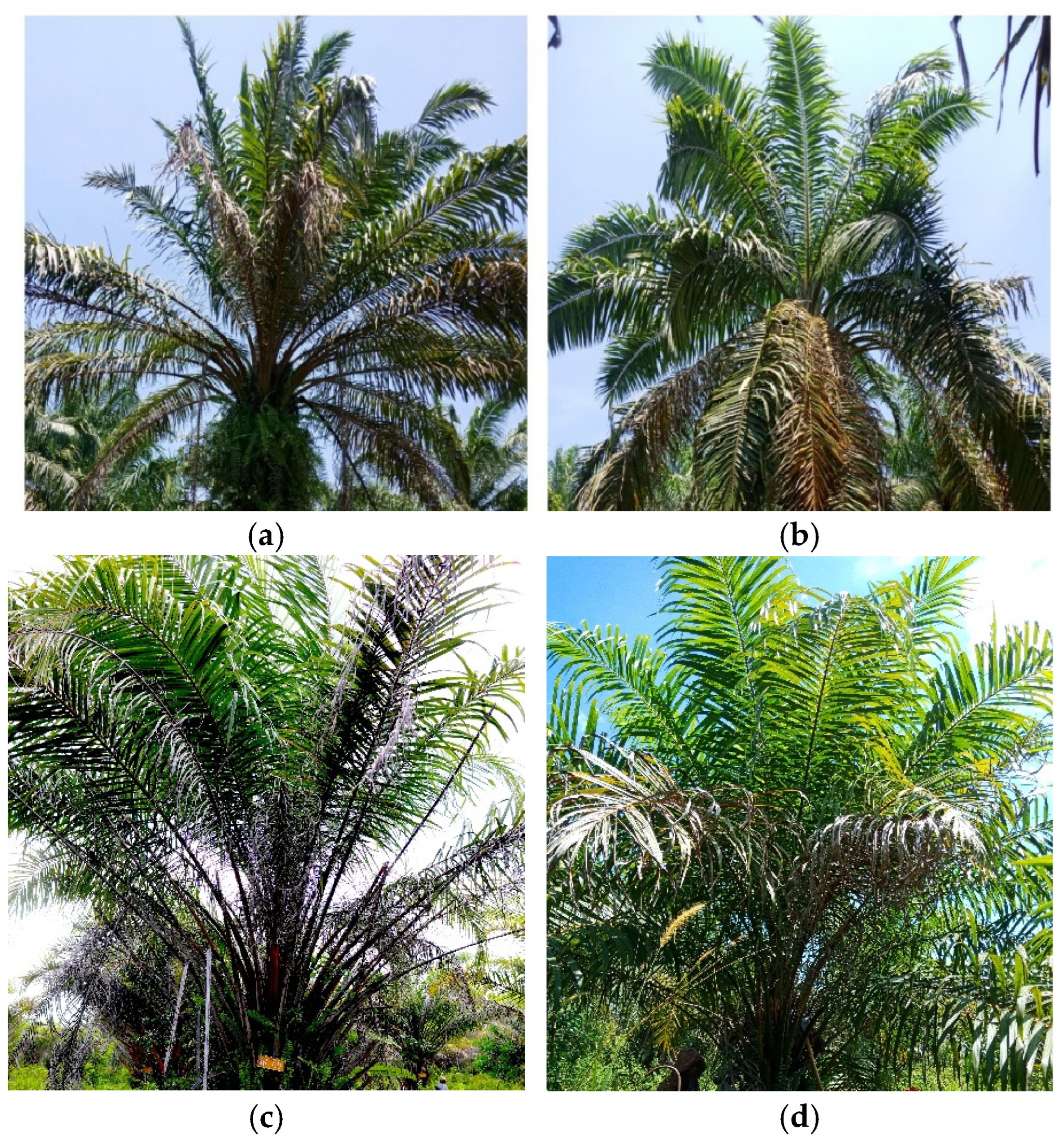
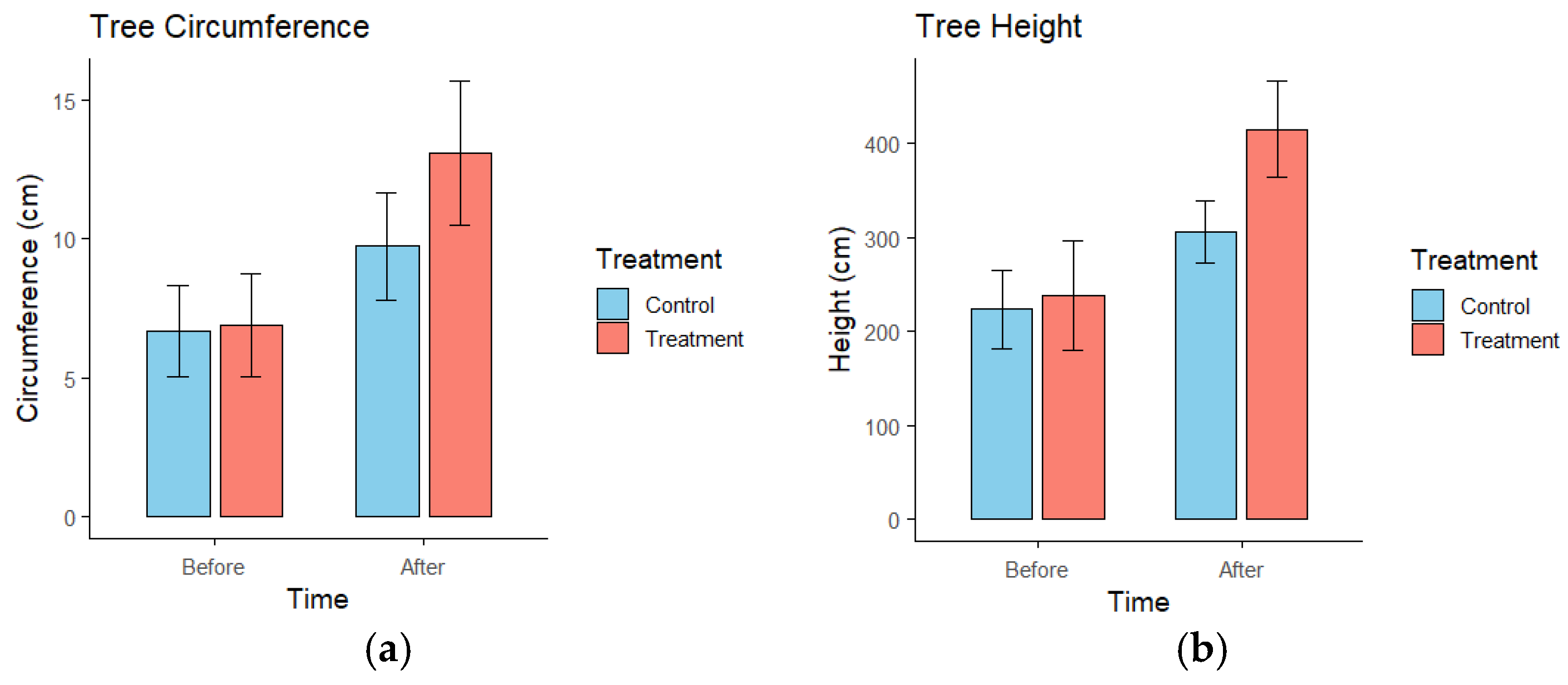
3.2. Plant Morphology
3.3. Leaf Nutrient Content
3.4. Chlorophyll Content
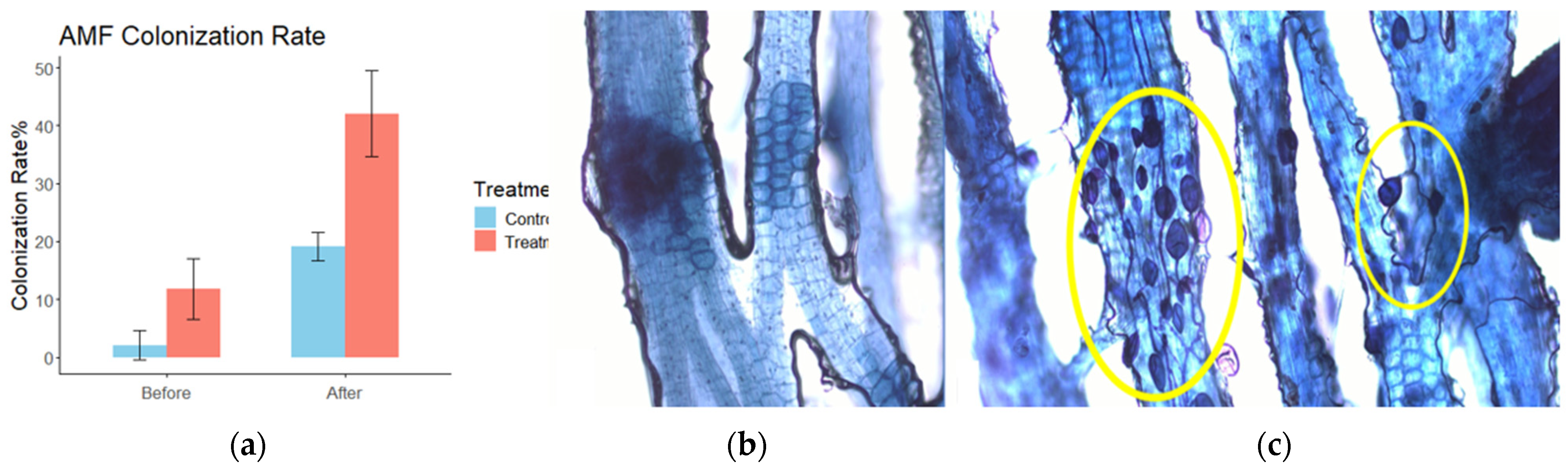
3.5. AMF Colonization
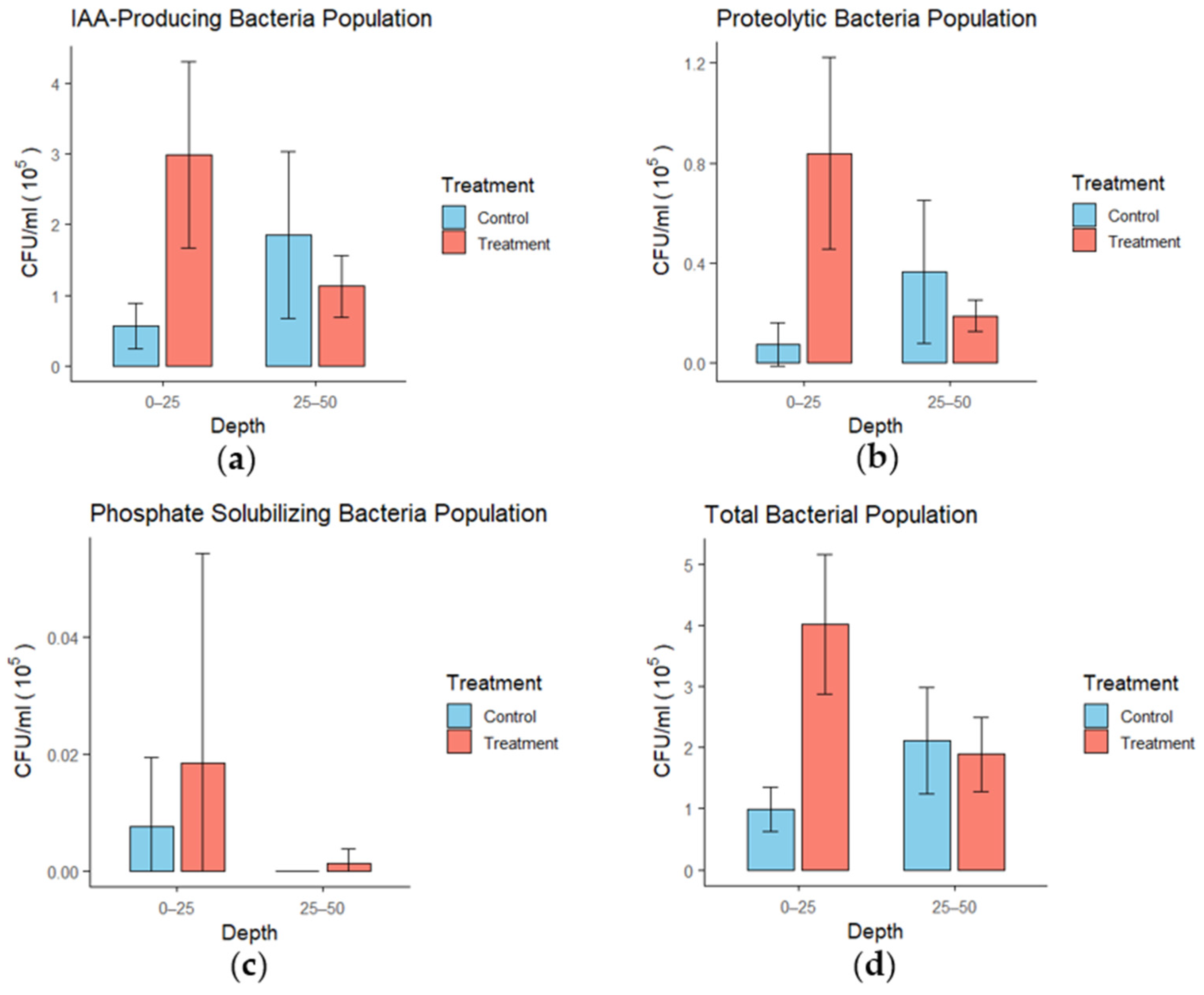
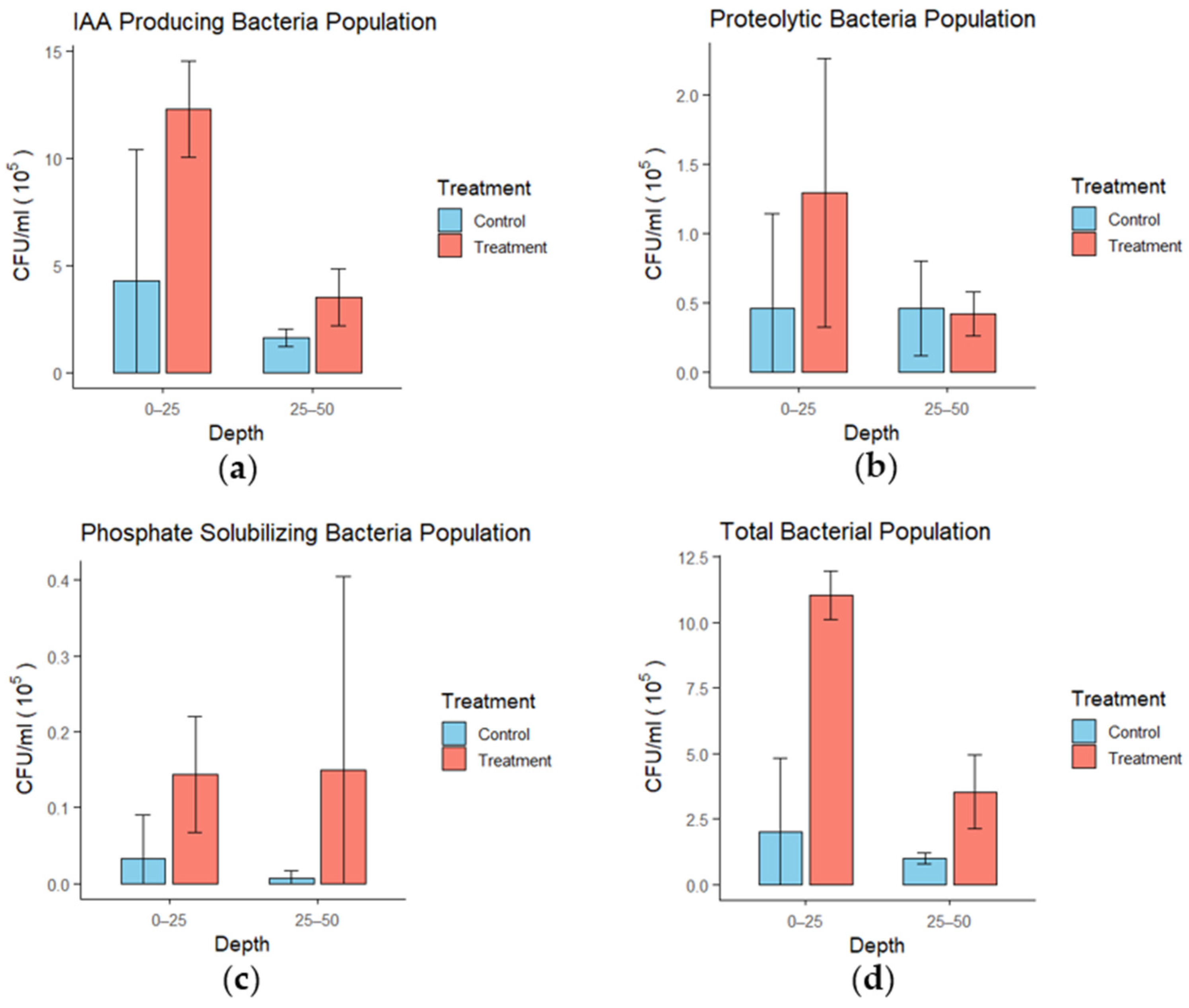
3.6. Soil Microbial Activity
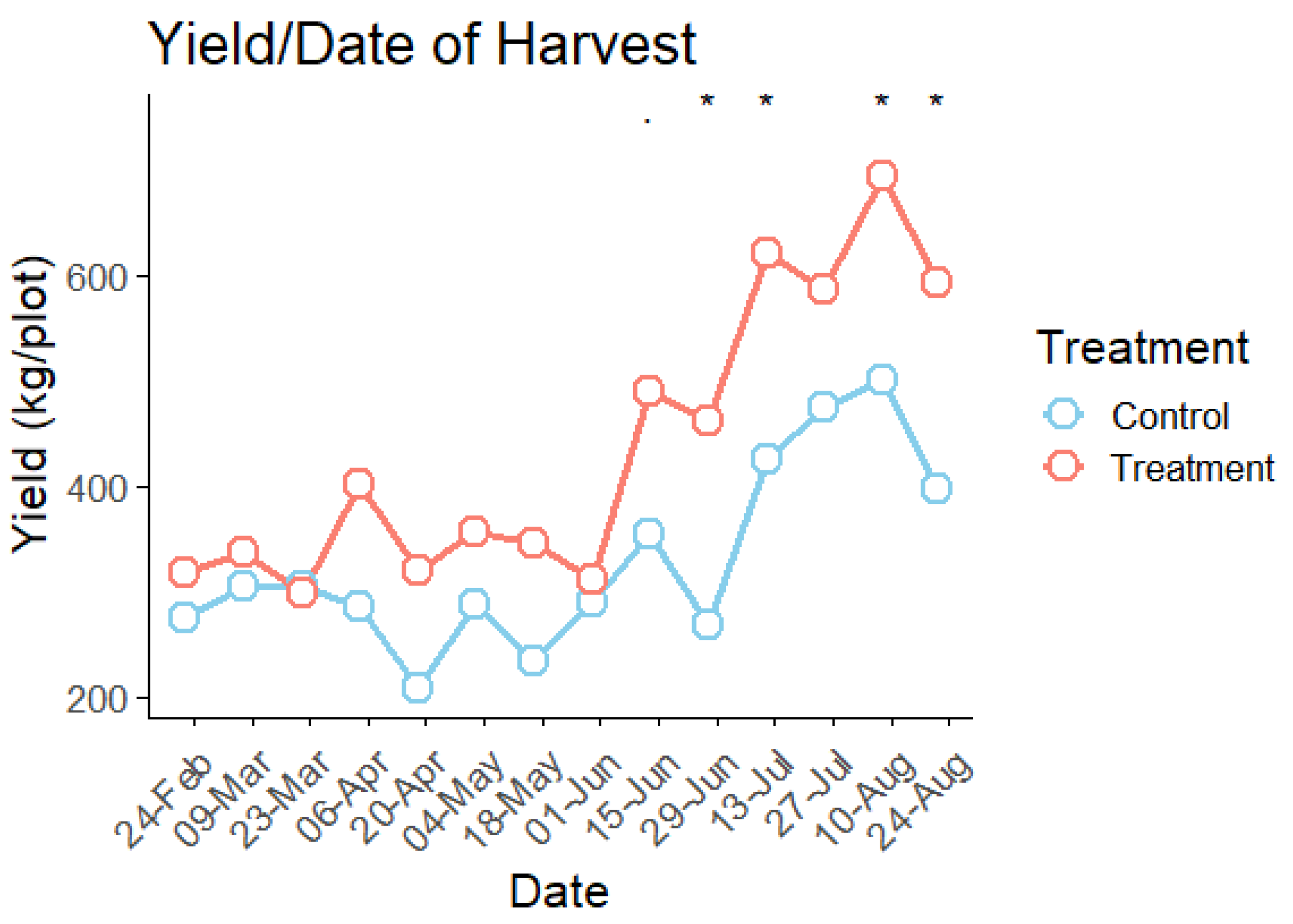
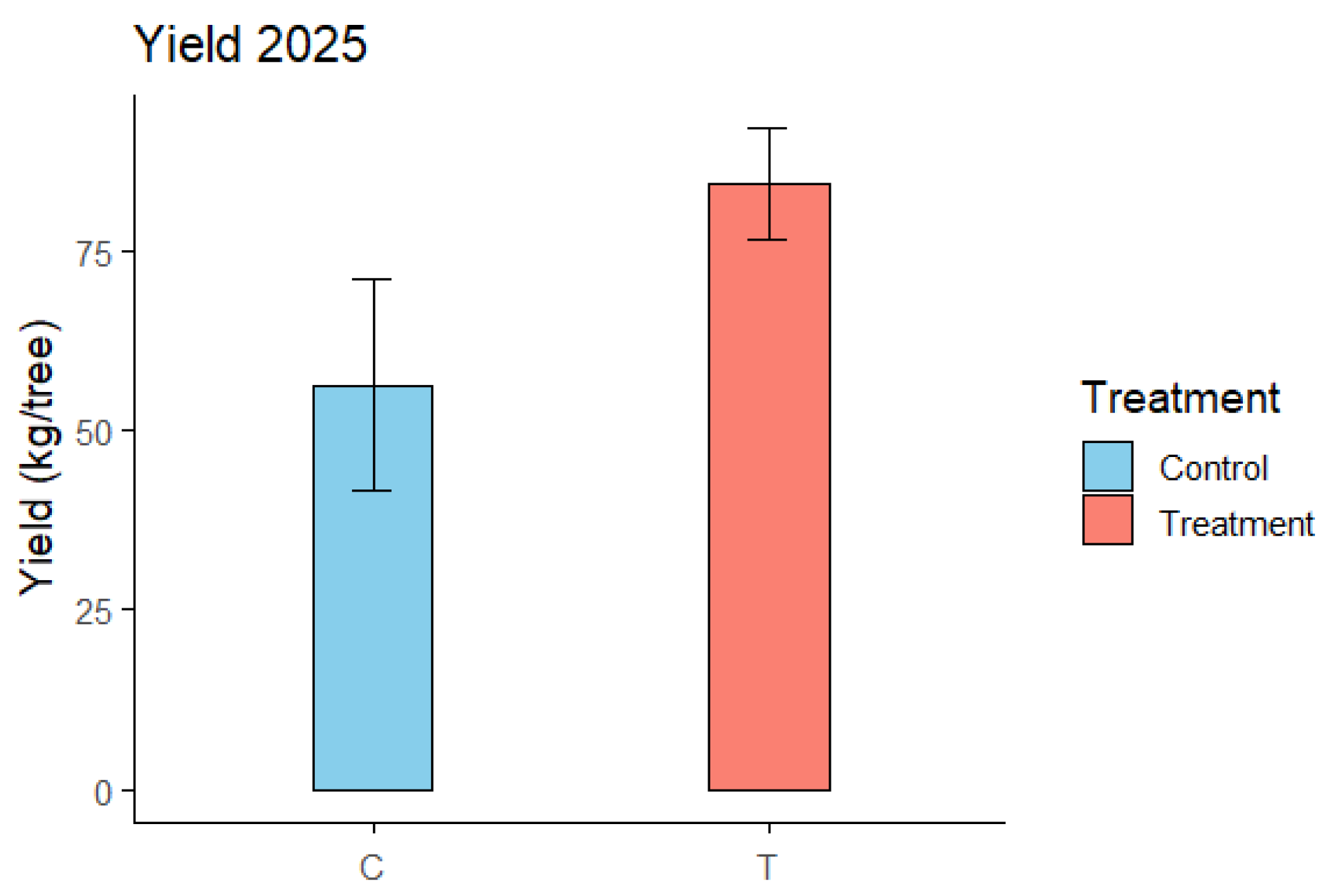
3.7. Yield Analysis
4. Discussion
4.1. AHC Application Impact
4.2. Hydrological Anomaly and AHC System Resilience
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMF | Arbuscular mycorrhizae |
| PGPR | Plant Growth-Promoting Rhizobacteria |
| AHC | AeroHydro Culture |
| TSA | Tryptic soy agar |
| IAA | Indole-3-acetic acid |
| LMM | Linear mixed model |
| ANOVA | Analysis of variance |
| GWL | Groundwater level |
| NbS | Nature based solution |
References
- Page, S.E.; Rieley, J.O.; Banks, C.J. Global and Regional Importance of the Tropical Peatland Carbon Pool. Glob. Chang. Biol. 2011, 17, 798–818. [Google Scholar] [CrossRef]
- Peatlands in Indonesia—ASEAN Haze Portal. Available online: https://hazeportal.asean.org/peatlands-in-sea/peatlands-in-indonesia/ (accessed on 8 April 2025).
- Anda, M.; Ritung, S.; Suryani, E.; Sukarman; Hikmat, M.; Yatno, E.; Mulyani, A.; Subandiono, R.E.; Suratman; Husnain. Revisiting Tropical Peatlands in Indonesia: Semi-Detailed Mapping, Extent and Depth Distribution Assessment. Geoderma 2021, 402, 115235. [Google Scholar] [CrossRef]
- Jaenicke, J.; Rieley, J.O.; Mott, C.; Kimman, P.; Siegert, F. Determination of the Amount of Carbon Stored in Indonesian Peatlands. Geoderma 2008, 147, 151–158. [Google Scholar] [CrossRef]
- Meijaard, E.; Brooks, T.M.; Carlson, K.M.; Slade, E.M.; Garcia-Ulloa, J.; Gaveau, D.L.A.; Lee, J.S.H.; Santika, T.; Juffe-Bignoli, D.; Struebig, M.J.; et al. The Environmental Impacts of Palm Oil in Context. Nat. Plants 2020, 6, 1418–1426. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Page, S.E.; Cobb, A.R.; Lee, J.S.H.; Jovani-Sancho, A.J.; Sjögersten, S.; Jaya, A.; Aswandi; Wardle, D.A. Degradation of Southeast Asian Tropical Peatlands and Integrated Strategies for Their Better Management and Restoration. J. Appl. Ecol. 2021, 58, 1370–1387. [Google Scholar] [CrossRef]
- Favre, F.; Tessier, D.; Abdelmoula, M.; Génin, J.M.; Gates, W.P.; Boivin, P. Iron Reduction and Changes in Cation Exchange Capacity in Intermittently Waterlogged Soil. Eur. J. Soil Sci. 2002, 53, 175–183. [Google Scholar] [CrossRef]
- Dasgupta, S.; Siegel, D.I.; Zhu, C.; Chanton, J.P.; Glaser, P.H. Geochemical Mixing in Peatland Waters: The Role of Organic Acids. Wetlands 2015, 35, 567–575. [Google Scholar] [CrossRef]
- Gandois, L.; Teisserenc, R.; Cobb, A.R.; Chieng, H.I.; Lim, L.B.L.; Kamariah, A.S.; Hoyt, A.; Harvey, C.F. Origin, Composition, and Transformation of Dissolved Organic Matter in Tropical Peatlands. Geochim. Cosmochim. Acta 2014, 137, 35–47. [Google Scholar] [CrossRef]
- Freeman, B.W.J.; Evans, C.D.; Musarika, S.; Morrison, R.; Newman, T.R.; Page, S.E.; Wiggs, G.F.S.; Bell, N.G.A.; Styles, D.; Wen, Y.; et al. Responsible Agriculture Must Adapt to the Wetland Character of Mid-latitude Peatlands. Glob. Chang. Biol. 2022, 28, 3795–3811. [Google Scholar] [CrossRef]
- Cooper, H.V.; Evers, S.; Aplin, P.; Crout, N.; Dahalan, M.P.B.; Sjogersten, S. Greenhouse Gas Emissions Resulting from Conversion of Peat Swamp Forest to Oil Palm Plantation. Nat. Commun. 2020, 11, 407. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Boodoo, K.S.; Knorr, K.; Glatzel, S. Assessing the Impact of Land Use on Peat Degradation in Bogs in the Enns Valley, Austria. Soil Use Manag. 2025, 41, e70013. [Google Scholar] [CrossRef]
- Wetlands International. Indonesia’s Largest Pulp-for-Paper Producer Risks Defaulting on Sustainability Commitments Due to Bad Peatland Management. Wetlands International, 20 April 2016. Available online: https://www.wetlands.org/indonesias-largest-pulp-for-paper-producer-risks-defaulting-on-sustainability-commitments-due-to-bad-peatland-management/ (accessed on 6 June 2025).
- Ma, L.; Zhu, G.; Chen, B.; Zhang, K.; Niu, S.; Wang, J.; Ciais, P.; Zuo, H. A Globally Robust Relationship between Water Table Decline, Subsidence Rate, and Carbon Release from Peatlands. Commun. Earth Environ. 2022, 3, 254. [Google Scholar] [CrossRef]
- Hadi, A.; Inubushi, K.; Purnomo, E.; Razie, F.; Yamakawa, K.; Tsuruta, H. Effect of Land-Use Changes on Nitrous Oxide (N2O) Emission from Tropical Peatlands. Chemosphere-Glob. Change Sci. 2000, 2, 347–358. [Google Scholar] [CrossRef]
- Peatland Restoration Agency of the Republic of Indonesia (BRG). Three Years of Peatland Restoration in Indonesia; Peatland Restoration Agency: Jakarta, Indonesia, 2019.
- Tan, Z.D.; Lupascu, M.; Wijedasa, L.S. Paludiculture as a Sustainable Land Use Alternative for Tropical Peatlands: A Review. Sci. Total Environ. 2021, 753, 142111. [Google Scholar] [CrossRef] [PubMed]
- Yuwati, T.W.; Rachmanadi, D.; Pratiwi; Turjaman, M.; Indrajaya, Y.; Nugroho, H.Y.S.H.; Qirom, M.A.; Narendra, B.H.; Winarno, B.; Lestari, S.; et al. Restoration of Degraded Tropical Peatland in Indonesia: A Review. Land 2021, 10, 1170. [Google Scholar] [CrossRef]
- Kitson, E.; Bell, N.G.A. The Response of Microbial Communities to Peatland Drainage and Rewetting. A Review. Front. Microbiol. 2020, 11, 582812. [Google Scholar] [CrossRef]
- Wetadewi, R.I.; Osaki, M.; Turjaman, M.; Antonius, S.; Goenadi, D.H.; Nursyamsi, D.; Maswar; Surayah, L.; Kato, T. Principles of AeroHydro Culture. In Tropical Peatland Eco-Management; Osaki, M., Tsuji, N., Foead, N., Rieley, J., Eds.; Springer: Singapore, 2021; pp. 249–283. ISBN 978-981-334-654-3. [Google Scholar]
- Turjaman, M.; Osaki, M. The Role of Mycorrhizal Fungi for Supporting AeroHydro Culture in Tropical Peatland. In Tropical Peatland Eco-Management; Osaki, M., Tsuji, N., Foead, N., Rieley, J., Eds.; Springer: Singapore, 2021; pp. 285–299. ISBN 978-981-334-654-3. [Google Scholar]
- Antonius, S.; Agustiyani, D.; Dewi, T.K.; Laili, N.; Osaki, M. Plant Growth-Promoting Rhizobacteria (PGPR) and Compost Materials for AeroHydro Culture. In Tropical Peatland Eco-Management; Osaki, M., Tsuji, N., Foead, N., Rieley, J., Eds.; Springer: Singapore, 2021; pp. 301–325. ISBN 978-981-334-654-3. [Google Scholar]
- Turjaman, M.; Siregar, C.A.; Wahyuni, T.; Silsigia, S.; Hidayat, A.; Aryanto; Rahayu, L.M.; Putri, N.A.; Kato, T.; Tsuji, N.; et al. An Innovative Restoration Technology for Tropical Peatlands: AeroHydro Culture (AHC). In Tropical Peatland Eco-Evaluation; Osaki, M., Tsuji, N., Kato, T., Sulaiman, A., Eds.; Springer Nature: Singapore, 2023; pp. 139–161. ISBN 978-981-99-6790-2. [Google Scholar]
- Lihan, S.; Benet, F.; Awang Husaini, A.A.S.; Apun, K.; Roslan, H.A.; Hassan, H. Isolation and Identification of Plant Growth Promoting Rhizobacteria from Sago Palm (Metroxylon Sagu, Rottb.). Trop. Life Sci. Res. 2021, 32, 39–51. [Google Scholar] [CrossRef]
- Asano, K.; Kagong, W.V.A.; Mohammad, S.M.B.; Sakazaki, K.; Talip, M.S.A.; Sahmat, S.S.; Chan, M.K.Y.; Isoi, T.; Kano-Nakata, M.; Ehara, H. Arbuscular Mycorrhizal Communities in the Roots of Sago Palm in Mineral and Shallow Peat Soils. Agriculture 2021, 11, 1161. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, H.; Zhang, W.; Liu, K.; Liu, M.; Shao, X. Cooperation between Arbuscular Mycorrhizal Fungi and Plant Growth-Promoting Bacteria and Their Effects on Plant Growth and Soil Quality. PeerJ 2022, 10, e13080. [Google Scholar] [CrossRef] [PubMed]
- Siregar, M.A.; Santri, J.A.; Aksani, D.; Maas, A.; Nurudin, M. Analysis of FTIR Spectroscopic Data to Observe Hydrophilic and Hydrophobic Levels of Peat in Hemic and Sapric Maturity. IOP Conf. Ser. Earth Environ. Sci. 2022, 1025, 012026. [Google Scholar] [CrossRef]
- Veloo, R.; Ranst, E.V.; Selliah, P. Peat Characteristics and Its Impact on Oil Palm Yield. NJAS Wagening. J. Life Sci. 2015, 72–73, 33–40. [Google Scholar] [CrossRef]
- Azizan, S.N.F.; Murakami, S.; McTaggart, I.; Yusof, N.; Sha’arani, S.; Hara, H.; Noborio, K. Changes in Specific Microbial Groups Characterize the Impact of Land Conversion to Oil Palm Plantations on Peat. Front. For. Glob. Chang. 2024, 7, 1305491. [Google Scholar] [CrossRef]
- Ratnaningsih, H.R.; Noviana, Z.; Dewi, T.K.; Loekito, S.; Wiyono, S.; Gafur, A.; Antonius, S. IAA and ACC Deaminase Producing-Bacteria Isolated from the Rhizosphere of Pineapple Plants Grown under Different Abiotic and Biotic Stresses. Heliyon 2023, 9, e16306. [Google Scholar] [CrossRef]
- Badan Pusat Statistik Provinsi Riau. Curah Hujan—Tabel Statistik. Available online: https://riau.bps.go.id/id/statistics-table/2/MTQ1IzI=/curah-hujan.html (accessed on 3 April 2025).
- Badan Pusat Statistik Provinsi Kalimantan Tengah. Jumlah Curah Hujan Menurut Stasiun Pengamatan—Tabel Statistik. Available online: https://kalteng.bps.go.id/id/statistics-table/2/MTAwOSMy/jumlah-curah-hujan-menurut-stasiun-pengamatan--mm-bulan-.html (accessed on 3 April 2025).
- Thompson-Morrison, H.; Ariantiningsih, F.; Arief, S.M.; Gaw, S.; Robinson, B. Chemical Elements in Elaeis Guineensis Materials and Derived Oil. Sci. Rep. 2024, 14, 1836. [Google Scholar] [CrossRef]
- Chaddy, A.; Melling, L.; Ishikura, K.; Hatano, R. Soil N2O Emissions under Different N Rates in an Oil Palm Plantation on Tropical Peatland. Agriculture 2019, 9, 213. [Google Scholar] [CrossRef]
- Dubos, B.; Baron, V.; Bonneau, X.; Dassou, O.; Flori, A.; Impens, R.; Ollivier, J.; Pardon, L. Precision Agriculture in Oil Palm Plantations: Diagnostic Tools for Sustainable N and K Nutrient Supply. OCL 2019, 26, 5. [Google Scholar] [CrossRef]
- Giehl, R.F.H.; Von Wirén, N. Hydropatterning—How Roots Test the Waters. Science 2018, 362, 1358–1359. [Google Scholar] [CrossRef] [PubMed]
- Daniel, K.; Hartman, S. How Plant Roots Respond to Waterlogging. J. Exp. Bot. 2024, 75, 511–525. [Google Scholar] [CrossRef]
- Geng, S.; Lin, Z.; Xie, S.; Xiao, J.; Wang, H.; Zhao, X.; Zhou, Y.; Duan, L. Ethylene Enhanced Waterlogging Tolerance by Changing Root Architecture and Inducing Aerenchyma Formation in Maize Seedlings. J. Plant Physiol. 2023, 287, 154042. [Google Scholar] [CrossRef]
- Herman, R.P.; Provencio, K.R.; Torrez, R.J.; Seager, G.M. Effect of Water and Nitrogen Additions on Free-Living Nitrogen Fixer Populations in Desert Grass Root Zones. Appl. Environ. Microbiol. 1993, 59, 3021–3026. [Google Scholar] [CrossRef]
- Phosri, C.; Rodriguez, A.; Sanders, I.R.; Jeffries, P. The Role of Mycorrhizas in More Sustainable Oil Palm Cultivation. Agric. Ecosyst. Environ. 2010, 135, 187–193. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D. Introduction. In Mycorrhizal Symbiosis, 3rd ed.; Smith, S.E., Read, D., Eds.; Academic Press: London, UK, 2008; pp. 1–9. ISBN 978-0-12-370526-6. [Google Scholar][Green Version]
- Hasan, A.; Tabassum, B.; Hashim, M.; Khan, N. Role of Plant Growth Promoting Rhizobacteria (PGPR) as a Plant Growth Enhancer for Sustainable Agriculture: A Review. Bacteria 2024, 3, 59–75. [Google Scholar] [CrossRef]
- He, S.; Li, L.; Lv, M.; Wang, R.; Wang, L.; Yu, S.; Gao, Z.; Li, X. PGPR: Key to Enhancing Crop Productivity and Achieving Sustainable Agriculture. Curr. Microbiol. 2024, 81, 377. [Google Scholar] [CrossRef]
- Yonebayashi, K. Studies on Sustainable Land Use and Soil Ecosystems in Tropical Peat Land. Tropics 2006, 15, 313–320. [Google Scholar] [CrossRef]
- Wall, A. Effect of Removal of Logging Residue on Nutrient Leaching and Nutrient Pools in the Soil after Clearcutting in a Norway Spruce Stand. For. Ecol. Manag. 2008, 256, 1372–1383. [Google Scholar] [CrossRef]
- Iskandar, I.; Lestari, D.O.; Saputra, A.D.; Setiawan, R.Y.; Wirasatriya, A.; Susanto, R.D.; Mardiansyah, W.; Irfan, M.; Rozirwan; Setiawan, J.D.; et al. Extreme Positive Indian Ocean Dipole in 2019 and Its Impact on Indonesia. Sustainability 2022, 14, 15155. [Google Scholar] [CrossRef]



| Dosage/Sack | ||
|---|---|---|
| Ingredients | Unit | Amount |
| NPK + Mg + B (briquettes) | Kg | 0.500 |
| Biochar | Kg | 0.125 |
| Zeolite | Kg | 0.188 |
| K+ (granules) | Kg | 0.160 |
| Compost | Kg | 20.00 |
| Bio-organic fertilizer | L | 0.500 |
| Arbuscular mycorrhizal fungi + zeolite carrier | Kg | 0.250 |
| Parameter | Result | Unit | Method | ||
|---|---|---|---|---|---|
| Siak | Pulang Pisau | ||||
| C—Organic | 24.5 | 30.6 | % | Ash/Gravimetry | |
| C/N | 15.0 | 9.00 | - | - | |
| Water content | 37.6 | 71.6 | % | Gravimetry/Oven | |
| Macro nutrient | N | 1.67 | 3.51 | % | Kjeldahl/Distillation |
| P2O5 | 5.12 | 0.78 | % | HNO3/Spectrophotometry | |
| K2O | 1.82 | 0.60 | % | HNO3/F-AAS | |
| Micro nutrient | Fe total | 8.15 | 1.03 | ppm | HNO3/F-AAS |
| Fe available | 310 | 575 | ppm | HNO3/F-AAS | |
| Zn total | 355 | 132 | ppm | HNO3/F-AAS | |
| pH H2O | 7.40 | 8.30 | - | Potentiometry/pH Meter | |
| Heavy Metal | As | Not detected | Not detected | ppm | HNO3/F-AAS |
| Hs | Not detected | Not detedted | ppm | HNO3/F-AAS | |
| Pb | 36.0 | 9.00 | ppm | HNO3/F-AAS | |
| Cd | 1.10 | 0.30 | ppm | HNO3/F-AAS | |
| Cr | 99.0 | 3.00 | ppm | HNO3/F-AAS | |
| Ni | 32.0 | 11.0 | ppm | HNO3/F-AAS | |
| numDf | denDF | Sum sq | Mean Sq | f Value | p Value | |
|---|---|---|---|---|---|---|
| Circumference | ||||||
| Treatment | 1 | 57 | 63.72 | 63.72 | 14.986 | 0.0003 *** |
| Time | 1 | 57 | 427.81 | 427.81 | 100.609 | 3.364 × 10−14 *** |
| Treatment: Time | 1 | 57 | 48.67 | 48.67 | 11.446 | 0.0013 ** |
| Height | ||||||
| Treatment | 1 | 73 | 76,589 | 76,589 | 16.073 | 0.0001 *** |
| Time | 1 | 73 | 337,831 | 337,831 | 70.897 | 2.287 × 10−12 *** |
| Treatment: Time | 1 | 73 | 44,053 | 44,053 | 9.245 | 0.0033 ** |
| Siak | Pulang Pisau | |||
|---|---|---|---|---|
| Nutrient | Control | Treatment | Control | Treatment |
| N (%) | 2.71 | 2.54 | 2.14 | 1.80 |
| P (%) | 0.23 | 0.24 | 0.17 | 0.16 |
| K (%) | 0.77 | 0.80 | 0.66 | 0.59 |
| Ca (%) | 0.73 | 0.71 | 0.49 | 0.55 |
| Mg (%) | 0.59 | 0.61 | 0.73 | 0.82 |
| Cu (ppm) | 2.68 | 2.43 | 84.5 | 87.1 |
| Zn (ppm) | 13.1 | 10.5 | 42.7 | 73.6 |
| B (ppm) | 9.35 | 9.83 | 12.2 | 12.1 |
| Mn (ppm) | 392 | 382 | 441.1 | 492 |
| numDf | denDF | Sum sq | Mean Sq | F Value | p Value | |
|---|---|---|---|---|---|---|
| Treatment | 1 | 76 | 79.142 | 79.142 | 3.7463 | 0.0566 |
| Time | 1 | 76 | 81.669 | 81.669 | 3.8659 | 0.0529 |
| Treatment: Time | 1 | 76 | 212.650 | 212.650 | 10.0661 | 0.0022 ** |
| numDf | denDF | Sum sq | Mean Sq | F Value | (Pr > F) | |
|---|---|---|---|---|---|---|
| Treatment | 1 | 57 | 0.0189062 | 0.0189062 | 7.2060 | 0.0095 ** |
| Time | 1 | 57 | 0.0090250 | 0.0090250 | 3.4398 | 0.0688 |
| Treatment: Time | 1 | 57 | 0.0039062 | 0.0039062 | 1.4888 | 0.2274 |
| numDf | denDF | Sum sq | Mean Sq | f Value | p Value | |
|---|---|---|---|---|---|---|
| Treatment | 1 | 33 | 3342.3 | 3342.3 | 26.0441 | 1.360 × 10−5 *** |
| Time | 1 | 33 | 7163.9 | 7163.9 | 55.8220 | 1.374 × 10−8 *** |
| Treatment: Time | 1 | 33 | 744.7 | 744.7 | 5.8025 | 0.0218 * |
| numDf | denDF | Sum sq | Mean Sq | f Value | p Value | |
|---|---|---|---|---|---|---|
| Treatment | 1 | 33 | 2322.8 | 2322.8 | 25.019 | 1.834 × 10−5 *** |
| Time | 1 | 33 | 2973.1 | 2973.1 | 32.023 | 2.637 × 10−6 *** |
| Treatment: Time | 1 | 33 | 1640.7 | 1640.7 | 17.672 | 0.0002 *** |
| numDf | denDF | Sum sq | Mean Sq | f Value | p Value | |
|---|---|---|---|---|---|---|
| IAA-Producing Bacteria | ||||||
| Treatment | 1 | 57 | 14.450 | 14.450 | 5.3095 | 0.0249 * |
| Depth | 1 | 57 | 1.653 | 1.653 | 0.6074 | 0.4389 |
| Treatment: Depth | 1 | 57 | 49.613 | 49.613 | 18.2295 | 7.493 × 10−5 *** |
| Proteolytic Bacteria | ||||||
| Treatment | 1 | 57 | 1.7258 | 1.7258 | 4.5317 | 0.0376 * |
| Depth | 1 | 57 | 0.6570 | 0.6570 | 1.7253 | 0.1943 |
| Treatment: Depth | 1 | 57 | 4.3945 | 4.3945 | 11.5395 | 0.0012 ** |
| Phosphate Solubilizing Bacteria | ||||||
| Treatment | 1 | 73 | 0.0008 | 0.0008 | 0.7911 | 0.3767 |
| Depth | 1 | 73 | 0.0031 | 0.0031 | 3.2295 | 0.0765 |
| Treatment: Depth | 1 | 73 | 0.0005 | 0.0005 | 0.5012 | 0.4812 |
| Total Bacterial Population | ||||||
| Treatment | 1 | 73 | 39.200 | 39.200 | 11.2937 | 0.0012 ** |
| Depth | 1 | 73 | 5.000 | 5.000 | 1.4405 | 0.2339 |
| Treatment: Depth | 1 | 73 | 52.812 | 52.812 | 15.2155 | 0.0002 *** |
| numDf | denDF | Sum sq | Mean Sq | f Value | p Value | |
|---|---|---|---|---|---|---|
| IAA-Producing Bacteria | ||||||
| Treatment | 1 | 44 | 292.55 | 292.55 | 10.2282 | 0.0026 ** |
| Depth | 1 | 44 | 391.02 | 391.02 | 13.6711 | 0.0006 *** |
| Treatment: Depth | 1 | 44 | 111.02 | 111.02 | 3.8816 | 0.0551 |
| Proteolytic Bacteria | ||||||
| Treatment | 1 | 44 | 1.7248 | 1.7248 | 2.3903 | 0.1293 |
| Depth | 1 | 44 | 2.1199 | 2.1199 | 2.9378 | 0.0936 |
| Treatment: Depth | 1 | 44 | 2.1199 | 2.1199 | 2.9378 | 0.0936 |
| Phosphate Solubilizing Bacteria | ||||||
| Treatment | 1 | 35 | 0.1725 | 0.1725 | 2.5160 | 0.1218 |
| Depth | 1 | 35 | 0.0003 | 0.0003 | 0.0038 | 0.9515 |
| Treatment: Depth | 1 | 35 | 0.0059 | 0.0059 | 0.0857 | 0.7715 |
| Total Bacterial Population | ||||||
| Treatment | 1 | 44 | 384.22 | 384.22 | 32.718 | 8.694 × 10−7 *** |
| Depth | 1 | 44 | 207.29 | 207.29 | 17.652 | 0.0001 *** |
| Treatment: Depth | 1 | 44 | 118.91 | 118.91 | 10.126 | 0.0027 ** |
| numDf | denDF | Sum sq | Mean Sq | F Value | (Pr > F) | |
|---|---|---|---|---|---|---|
| Treatment | 1 | 51 | 2324 | 2324 | 0.3110 | 0.5795 |
| Days | 1 | 51 | 419917 | 419917 | 56.1987 | 8.96 × 10−10 *** |
| Treatment:Days | 1 | 51 | 38640 | 38640 | 5.1713 | 0.0272 * |
| numDf | denDF | Sum sq | Mean Sq | F Value | (Pr > F) | |
|---|---|---|---|---|---|---|
| Treatment | 1 | 11 | 4697 | 4697 | 22.532 | 0.0006 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verchius, E.; Miyazawa, K.; Wetadewi, R.I.; Turjaman, M.; Antonius, S.; Segah, H.; Dewi, T.K.; Sutisna, E.; Wahyuni, T.; Goenadi, D.H.; et al. AeroHydro Culture: An Integrated Approach to Improve Crop Yield and Ecological Restoration Through Root–Microbe Symbiosis in Tropical Peatlands. Land 2025, 14, 1823. https://doi.org/10.3390/land14091823
Verchius E, Miyazawa K, Wetadewi RI, Turjaman M, Antonius S, Segah H, Dewi TK, Sutisna E, Wahyuni T, Goenadi DH, et al. AeroHydro Culture: An Integrated Approach to Improve Crop Yield and Ecological Restoration Through Root–Microbe Symbiosis in Tropical Peatlands. Land. 2025; 14(9):1823. https://doi.org/10.3390/land14091823
Chicago/Turabian StyleVerchius, Eric, Kae Miyazawa, Rahmawati Ihsani Wetadewi, Maman Turjaman, Sarjiya Antonius, Hendrik Segah, Tirta Kumala Dewi, Entis Sutisna, Tien Wahyuni, Didiek Hadjar Goenadi, and et al. 2025. "AeroHydro Culture: An Integrated Approach to Improve Crop Yield and Ecological Restoration Through Root–Microbe Symbiosis in Tropical Peatlands" Land 14, no. 9: 1823. https://doi.org/10.3390/land14091823
APA StyleVerchius, E., Miyazawa, K., Wetadewi, R. I., Turjaman, M., Antonius, S., Segah, H., Dewi, T. K., Sutisna, E., Wahyuni, T., Goenadi, D. H., Putri, N. A., Silsigia, S., Kato, T., Dohong, A., Takahashi, H., Nursyamsi, D., Kubo, H., Tsuji, N., & Osaki, M. (2025). AeroHydro Culture: An Integrated Approach to Improve Crop Yield and Ecological Restoration Through Root–Microbe Symbiosis in Tropical Peatlands. Land, 14(9), 1823. https://doi.org/10.3390/land14091823









