Fine-Scale Organization and Dynamics of Matrix-Forming Species in Primary and Secondary Grasslands
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Field Sampling
2.3. Data Analysis
2.4. Testing for Significant Spatial Aggregation of Species Using Information Theory Models
- Hx can be calculated aswhere px is the probability of the presence of species x, and (1 − px) is the probability of its absence in the sample (for more details, see Supplementary Materials S3).Hx = − px ∗ log px − (1 − px) ∗ log (1 − px)
2.5. Quantifying Temporal Dependence in Time Series of Species Patterns Using Information Theory Models
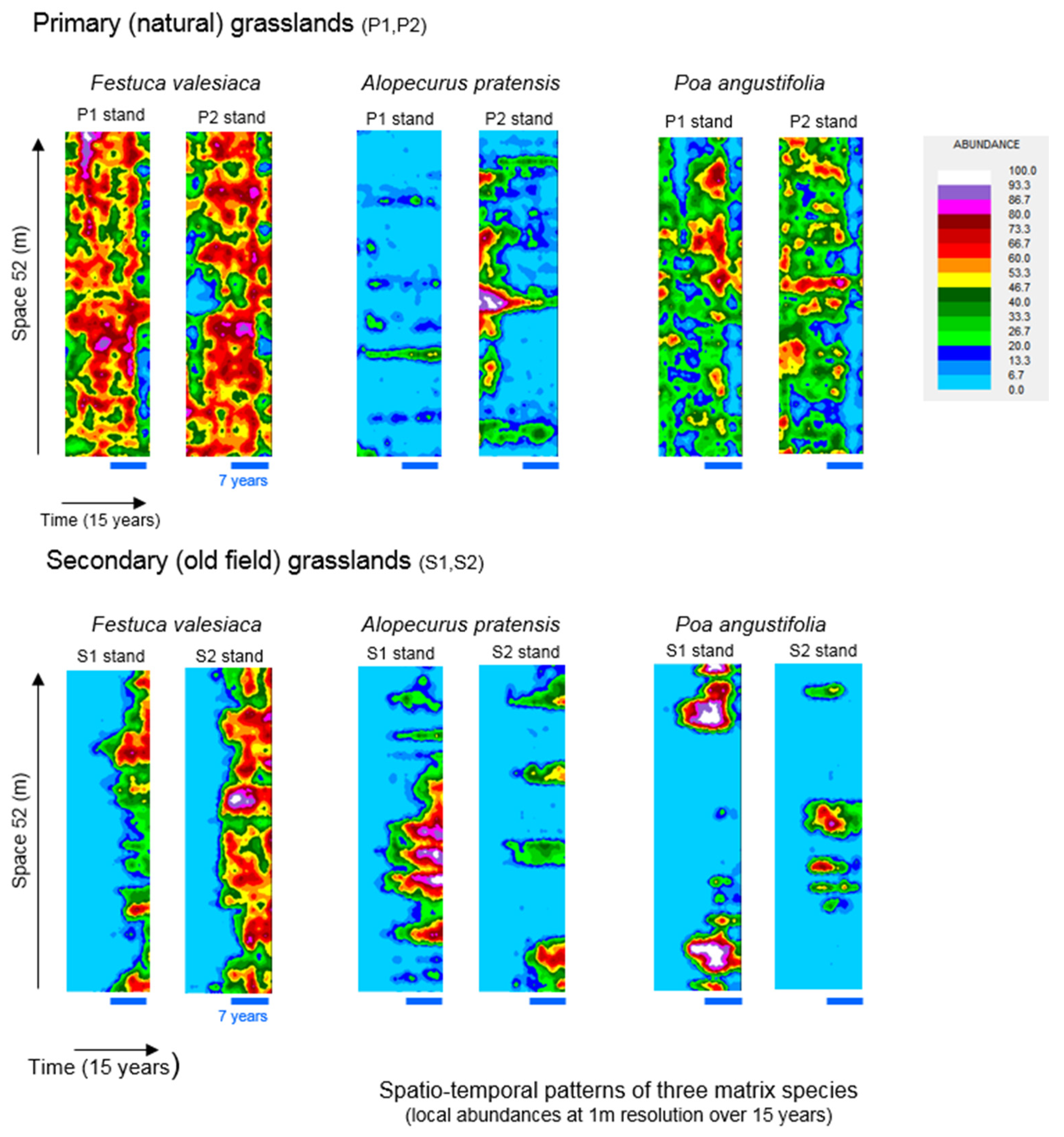
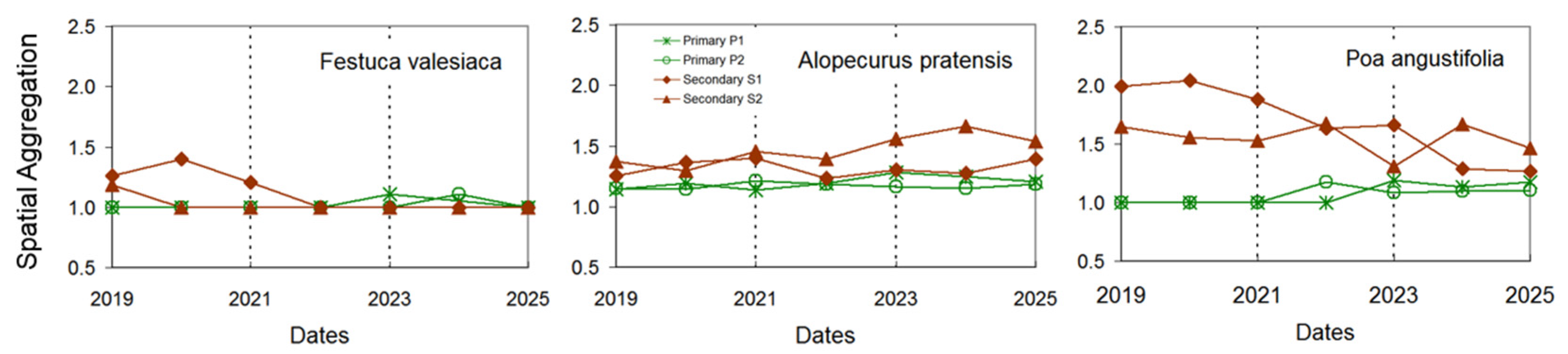
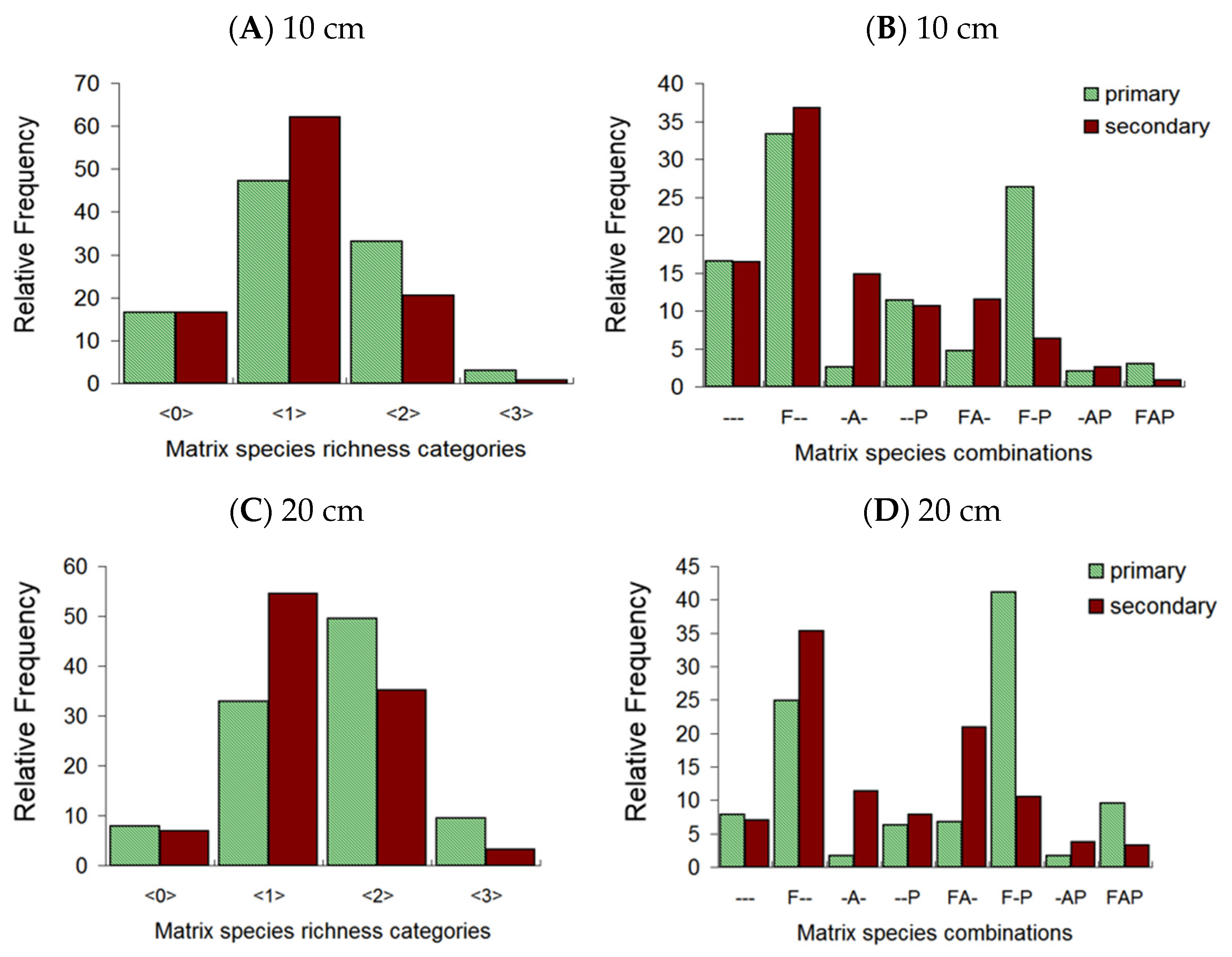
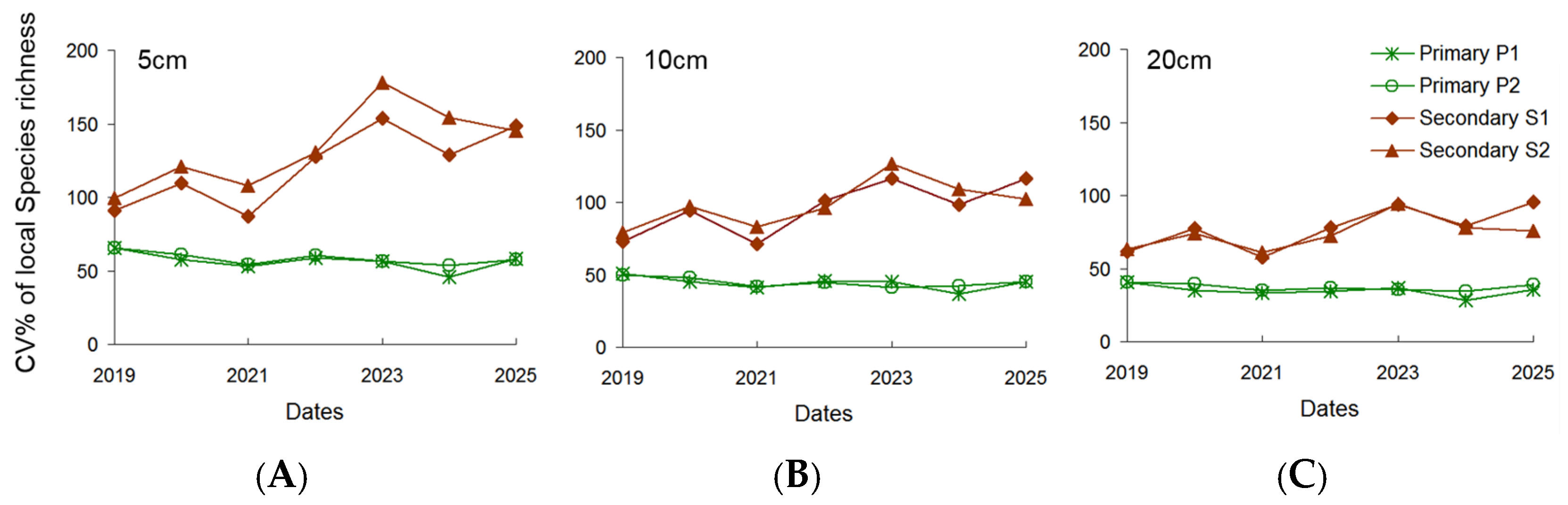
3. Results
4. Discussion
4.1. Spatial Patterns of Matrix Species
4.2. Temporal Dependence and Fine-Scale Dynamics
4.3. Co-Occurrence Patterns of Matrix Species and Responses of Subordinates
4.4. Limitations and Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Hx | Spatial entropy function |
| Iaggr | Aggregation index |
Appendix A


References
- Whittaker, R.H. Dominance and diversity in land plant communities. Science 1965, 147, 250–260. [Google Scholar] [CrossRef]
- Grime, J.P. Benefits of plant diversity to ecosystems: Immediate, filter and founder effects. J. Ecol. 1998, 86, 902–910. [Google Scholar] [CrossRef]
- Avolio, M.L.; Forrestel, E.J.; Chang, C.C.; La Pierre, K.J.; Burghardt, K.T.; Smith, M.D. Demystifying dominant species. New Phytol. 2019, 223, 1106–1126. [Google Scholar] [CrossRef]
- Smith, M.D.; Knapp, A.K. Dominant species maintain ecosystem function with non-random species loss. Ecol. Lett. 2003, 6, 509–517. [Google Scholar] [CrossRef]
- Wilsey, B.J. Productivity and Subordinate Species Response to Dominant Grass Species and Seed Source during Restoration. Restor. Ecol. 2010, 18, 628–637. [Google Scholar] [CrossRef]
- Kikvidze, Z.; Brooker, R.W.; Butterfield, B.J.; Callaway, R.M.; Cavieres, L.A.; Cook, B.J.; Lortie, C.J.; Michalet, R.; Pugnaire, F.I.; Xiao, S.; et al. The effects of foundation species on community assembly: A global study on alpine cushion plant communities. Ecology 2015, 96, 2064–2069. [Google Scholar] [CrossRef] [PubMed]
- Akatov, V.V.; Akatova, T.V.; Eskina, T.G.; Sazonets, N.M.; Chefranov, S.G. Dominants in plant communities: The nature of impact on biomass determines the thresholds of impact on local species richness. Biol. Bull. Rev. 2025, 15, 12–23. [Google Scholar] [CrossRef]
- Zhang, P.; Seabloom, E.W.; Foo, J.; MacDougall, A.S.; Harpole, W.S.; Adler, P.B.; Hautier, Y.; Eisenhauer, N.; Spohn, M.; Bakker, J.D.; et al. Dominant species predict plant richness and biomass in global grasslands. Nat. Ecol. Evol. 2025, 9, 924–936. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.K.; Ripplinger, J.; Collins, S.L. Species reordering, not changes in richness, drives long-term dynamics in grassland communities. Ecol. Lett. 2017, 20, 1556–1565. [Google Scholar] [CrossRef]
- Hou, G.; Shi, P.; Zhou, T.; Sun, J.; Zong, N.; Song, M.; Zhang, X. Dominant species play a leading role in shaping community stability in the northern Tibetan grasslands. J. Plant Ecol. 2023, 16, 110. [Google Scholar] [CrossRef]
- Adler, P.B.; HilleRisLambert, J.; Kyriakidis, P.C.; Guan, Q.; Levine, J.M. Climate variability has a stabilizing effect on the coexistence of prairie grasses. Proc. Natl. Acad. Sci. USA 2006, 103, 12793–12798. [Google Scholar] [CrossRef]
- Falinski, J.B. Vegetation Dynamics in Temperate Lowland Primeval Forests. Ecological Studies in Białowieza Forest; Springer: Dordrecht, The Netherlands, 1986; p. 537. [Google Scholar] [CrossRef]
- Bobiec, A. The mosaic diversity of field layer vegetation in the natural and exploited forests of Bialowieza. Plant Ecol. 1998, 136, 175–187. [Google Scholar] [CrossRef]
- Brearley, F.Q.; Prajadinata, S.; Kidd, P.S.; Proctor, J.; Suriantata. Structure and floristics of an old secondary rain forest in Central Kalimantan, Indonesia, and a comparison with adjacent primary forest. For. Ecol. Manag. 2004, 195, 385–397. [Google Scholar] [CrossRef]
- Korepin, A.A.; Kapitsa, E.A.; Shorohov, A.A. Half-Century Dynamics of Primary and Secondary Tree Stands in the Veps Forest Natural Reserve. Contemp. Probl. Ecol. 2024, 17, 996–1008. [Google Scholar] [CrossRef]
- Ehbrecht, M.; Seidel, D.; Annighöfer, P.; Kreft, H.; Köhler, M.; Zemp, D.C.; Puettmann, K.; Nilus, R.; Babweteera, F.; Willim, K.; et al. Global patterns and climatic controls of forest structural complexity. Nat. Commun. 2021, 12, 519. [Google Scholar] [CrossRef]
- Wang, T.; Dong, L.; Liu, Z. Stand structure is more important for forest productivity stability than tree, understory plant and soil biota species diversity. Front. For. Glob. Change 2024, 7, 1354508. [Google Scholar] [CrossRef]
- Illyés, E.; Chytrý, M.; Botta-Dukát, Z.; Jandt, U.; Škodová, I.; Janišová, M.; Willner, W.; Hájek, O. Semi-dry grasslands along a climatic gradient across Central Europe: Vegetation classification with validation. J. Veg. Sci. 2007, 18, 835–846. [Google Scholar] [CrossRef]
- Velev, N.; Apostolova, I. Successional changes of Nardus stricta communities in the Central Balkan Range (Bulgaria). Phytol. Balc. 2008, 14, 75–84. [Google Scholar]
- Dengler, J.; Janisová, M.; Török, P.; Wellstein, C. Biodiversity of Palaearctic grasslands: A synthesis. Agric. Ecosyst. Environ. 2015, 182, 1–14. [Google Scholar] [CrossRef]
- Biurrun, I.; Pielech, R.; Dembicz, I.; Gillet, F.; Kozub, Ł.; Marcenò, C.; Reitalu, T.; Van Meerbeek, K.; Guarino, R.; Chytrý, M.; et al. Benchmarking plant diversity of Palaearctic grasslands and other open habitats. J. Veg. Sci. 2021, 32, e13050. [Google Scholar] [CrossRef]
- Prach, K.; Török, P.; Bakker, J.D. Temperate grasslands. In Routledge Handbook of Ecological and Environmental Restoration; Taylor & Francis: Abingdon, UK, 2017; pp. 126–141. [Google Scholar]
- Greig-Smith, P. Pattern in vegetation. J. Ecol. 1979, 67, 755–779. [Google Scholar] [CrossRef]
- Kershaw, K.A. Pattern in vegetation and its causality. Ecology 1963, 44, 377–388. [Google Scholar] [CrossRef]
- Gibson, D.J. The relationship of sheep grazing and soil heterogeneity to plant spatial patterns in dune grassland. J. Ecol. 1988, 76, 233–252. [Google Scholar] [CrossRef]
- Seabloom, E.W.; Bjørnstad, O.N.; Bolker, B.M.; Reichman, O.J. Spatial signature of environmental heterogeneity, dispersal, and competition in successional grasslands. Ecol. Monogr. 2005, 75, 199–214. [Google Scholar] [CrossRef]
- Benot, M.L.; Bittebiere, A.K.; Ernoult, A.; Clément, B.; Mony, C. Fine-scale spatial patterns in grassland communities depend on species clonal dispersal ability and interactions with neighbours. J. Ecol. 2013, 101, 626–636. [Google Scholar] [CrossRef]
- De Benedictis, L.L.M.; Chelli, S.; Canullo, R.; Campetella, G. Measuring them all: Individual-based functional spatial patterns in mountain grasslands. J. Veg. Sci. 2025, 36, e70029. [Google Scholar] [CrossRef]
- Chesson, P.; Neuhauser, C. Intraspecific aggregation and species coexistence. Trends Ecol. Evol. 2002, 17, 210–211. [Google Scholar] [CrossRef]
- Bolker, B.M.; Pacala, S.W.; Neuhauser, C. Spatial dynamics in model plant communities: What do we really know? Am. Nat. 2003, 162, 135–148. [Google Scholar] [CrossRef]
- Wiegand, T.; Wang, X.; Anderson-Teixeira, K.J.; Bourg, N.A.; Cao, M.; Ci, X.; Davies, S.J.; Hao, Z.; Howe, R.W.; Kress, W.J.; et al. Consequences of spatial patterns for coexistence in species-rich plant communities. Nat. Ecol. Evol. 2021, 5, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Bartha, S. Composition, differentiation and dynamics in the forest steppe biome. In Slope Steppes, Loess Steppes and Forest Steppe Meadows in Hungary; Illyés, E., Bölöni, J., Eds.; MTA ÖBKI: Budapest, Hungary, 2007; pp. 194–210. [Google Scholar]
- Virágh, K.; Horváth, A.; Bartha, S.; Somodi, I. A multiscale methodological approach for monitoring the effectiveness of grassland management. Community Ecol. 2008, 9, 237–246. [Google Scholar] [CrossRef]
- Kéfi, S.; Guttal, V.; Brock, W.A.; Carpenter, S.R.; Ellison, A.M.; Livina, V.N.; Seekell, D.A.; Scheffer, M.; van Nes, E.H.; Dakos, V.; et al. Early warning signals of ecological transitions: Methods for spatial patterns. PLoS ONE 2014, 9, e92097. [Google Scholar] [CrossRef]
- Fehmi, J.S.; Bartolome, J.W. A grid-based method for sampling and analysing spatially ambiguous plants. J. Veg. Sci. 2001, 12, 467–472. [Google Scholar] [CrossRef]
- Bartha, S.; Campatella, G.; Canullo, R.; Bódis, J.; Mucina, L. On the importance of fine-scale spatial complexity in vegetation restoration. Int. J. Ecol. Environ. Sci. 2004, 30, 101–116. [Google Scholar]
- Chen, X.; Li, B.; Collins, S.L. Multiscale monitoring of a multispecies case study: Two grass species at Sevilleta. Plant Ecol. 2005, 179, 149–154. [Google Scholar] [CrossRef]
- Collins, S.L.; Xia, Y. Long-term dynamics and hotspots of change in a desert grassland plant community. Am. Nat. 2015, 185, E30–E43. [Google Scholar] [CrossRef] [PubMed]
- Dobson, A.P.; Bradshaw, A.D.; Baker, A.J.M. Hopes for the future: Restoration Ecology and Conservation Biology. Science 1997, 277, 515–522. [Google Scholar] [CrossRef]
- Hölzel, N.; Haub, C.; Ingelfinger, M.P.; Otte, A.; Pilipenko, V.N. The return of the steppe: Largescale restoration of degraded land in southern Russia during the post-Soviet era. J. Nat. Conserv. 2002, 10, 75–85. [Google Scholar] [CrossRef]
- Török, P.; Brudvig, L.A.; Kollmann, J.; Price, J.N.; Tóthmérész, B. The present and future of grassland restoration. Restor. Ecol. 2021, 29, e13378. [Google Scholar] [CrossRef]
- Sun, C.; Chai, Z.; Liu, G.; Xue, S. Changes in species diversity patterns and spatial heterogeneity during the secondary succession of grassland vegetation on the loess plateau, China. Front. Plant Sci. 2017, 8, 1465. [Google Scholar] [CrossRef]
- Zaplata, M.K.; Winter, S.; Fischer, A.; Kollmann, J.; Ulrich, W. Species-driven phases and increasing structure in early-successional plant communities. Am. Nat. 2013, 181, E17–E27. [Google Scholar] [CrossRef]
- Fukami, T. Historical contingency in community assembly: Integrating Niches, Species Pools, and Priority Effects. Annu. Rev. Ecol. Evol. Syst. 2015, 46, 1–23. [Google Scholar] [CrossRef]
- Myster, R.W.; Pickett, S.T.A. A comparison of rate of succession over 18 years in 10 contrasting old fields. Ecology 1994, 75, 387–392. [Google Scholar] [CrossRef]
- Anderson, K.J. Temporal patterns in rates of community change during succession. Am. Nat. 2007, 169, 780–793. [Google Scholar] [CrossRef]
- Wilson, J.B. Assembly rules in plant communities. In Ecological Assembly Rules: Perspectives, Advances, Retreats; Weiher, E., Keddy, P., Eds.; Cambridge University Press: Cambridge, UK, 1999; pp. 130–164. [Google Scholar] [CrossRef]
- Csathó, A.J.; Csathó, A.I. The floralist of the Külső-gulya meadow of Battonya-Tompapuszta (SE Hungary). Crisicum 2009, 5, 51–70. [Google Scholar]
- Podani, J. Analysis of mapped and simulated vegetation patterns by means of computerized sampling techniques. Acta Bot. Hung. 1984, 30, 403–425. [Google Scholar]
- Nilsson, I.N.; Nilsson, S.G. Experimental estimates of census efficiency and pseudoturnover on islands: Error trend and between-observer variation when recording vascular plants. J. Ecol. 1985, 73, 65–70. [Google Scholar] [CrossRef]
- Juhász-Nagy, P.; Podani, J. Information theory methods for the study of spatial processes and succession. Vegetatio 1983, 51, 129–140. [Google Scholar] [CrossRef]
- Juhász-Nagy, P. Spatial dependence of plant populations. Part 2. A family of new models. Acta Bot. Acad. Sci. Hung. 1984, 30, 363–402. [Google Scholar]
- Tsakalos, J.L.; Chelli, S.; Campetella, G.; Canullo, R.; Simonetti, E.; Bartha, S. Comspat: An R package to analyze within-community spatial organization using species combinations. Ecography 2022, 7, e06216. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Bartha, S.; Czárán, T.; Podani, J. Exploring plant community dynamics in abstract coenostate spaces. Abstr. Bot. 1998, 22, 49–66. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Greig-Smith, P. Quantitative Plant Ecology, 3rd ed.; Blackwell Sci. Pub.: London, UK, 1983; 359p. [Google Scholar]
- Dale, M.R.T.; Fortin, M.J. (Eds.) Spatial Analysis: A Guide for Ecologists; Cambridge Univ. Press: New York, NY, USA, 2005; 365p. [Google Scholar]
- Addicott, J.F.; Aho, J.M.; Antolin, M.F.; Padilla, D.K.; Richardson, J.S.; Soluk, D.A. Ecological neighborhoods: Scaling environmental patterns. Oikos 1987, 49, 340–346. [Google Scholar] [CrossRef]
- Czárán, T. Spatiotemporal Models of Population and Community Dynamics; Chapman & Hall: London, UK, 1998; 284p. [Google Scholar]
- Bevacqua, E.; Rakovec, O.; Schumacher, D.L.; Kumar, R.; Thober, S.; Samaniego, L.; Seneviratne, S.I.; Zscheischler, J. Direct and lagged climate change effects intensified the 2022 European drought. Nat. Geosci. 2024, 17, 1100–1107. [Google Scholar] [CrossRef]
- Szentes, S.; Sutyinszki, Z.; Szabó, G.; Zimmermann, Z.; Házi, J.; Wichmann, B.; Hufnágel, L.; Penksza, K.; Bartha, S. Grazed pannonian grassland beta-diversity changes due to C4 yellow bluestem. Cent. Eur. J. Biol. 2012, 7, 1055–1065. [Google Scholar] [CrossRef]
- Adler, P.B.; Lauenroth, W.K. Livestock exclusion increases the spatial heterogeneity of vegetation in Colorado shortgrass steppe. Appl. Veg. Sci. 2000, 3, 213–222. [Google Scholar] [CrossRef]
- Adler, P.B.; Raff, D.A.; Lauenroth, W.K. The effect of grazing on the spatial heterogeneity of vegetation. Oecologia 2001, 128, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Lepš, J.; Burianek, V. Interspecific associations in old field succession. In Spatial Processes in Plant Communities; Krahulec, F., Agnew, A.D.Q., Agnew, S., Willems, J.H., Eds.; SPB Publ: The Hague, The Netherlands, 1990; pp. 13–22. [Google Scholar]
- Herben, T.; During, H.J.; Krahulec, F. Spatiotemporal dynamics in mountain grasslands: Species autocorrelations in space and time. In Species Coexistence in Temperate Grasslands; Krahulec, F., Goldberg, D.E., Willems, J.H., Eds.; Opulus Press: Uppsala, Sweden, 1995; pp. 79–90. [Google Scholar]
- MacCain, K.N.S.; Baer, S.G.; Blair, J.M.; Wilson, G.W.T. Dominant grasses suppress local diversity in restored tallgrass prairie. Restor. Ecol. 2010, 18, 40–49. [Google Scholar] [CrossRef]
- Albertson, F.W.; Tomanek, G.W. Vegetation changes during a 30-year period in grassland communities near hays, Kansas. Ecology 1965, 46, 714–720. [Google Scholar] [CrossRef]
- Herben, T.; Mayerová, H.; Skálová, H.; Hadincová, V.; Pecháčková, S.; Krahulec, F. Long-term time series of legume cycles in a semi-natural montane grassland: Evidence for nitrogen-driven grass dynamics. Funct. Ecol. 2017, 31, 1430–1440. [Google Scholar] [CrossRef]
- Pickett, S.T.A. Succession: An evolutionary interpretation. Am. Nat. 1976, 110, 107–119. [Google Scholar] [CrossRef]
- Odum, E.P. The strategy of ecosystem development. Science 1969, 164, 262–270. [Google Scholar] [CrossRef] [PubMed]


| Primary Grasslands | Secondary Grasslands | |
|---|---|---|
| spatial dependence | nearly random patterns or fine-scale patchiness | very strong aggregations coarse scale patchiness |
| temporal dependence | weak appears at finer scales | strong stronger at coarser scales |
| fine-scale dynamics (rate of changes) | high rate of exchanges species specific rates gain and loss rates balanced | high rate of exchanges species specific rates gain and loss rates imbalanced |
| spatial diversity and co-occurrence of matrix species | spatially variable patterns of different 1-,2-,3-species combinations | monodominant patches are typical |
| pattern of subordinate species richness | temporally stable low spatial variability | temporally increasing high spatial variability |
| potential mechanisms effect of matrix species on subordinates | weak pattern selection homogeneous environment | spatially variable temporally stable strong pattern selection heterogeneous environment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartha, S.; Házi, J.; Purger, D.; Zimmermann, Z.; Szabó, G.; Guller, Z.E.; Csathó, A.I.; Csete, S. Fine-Scale Organization and Dynamics of Matrix-Forming Species in Primary and Secondary Grasslands. Land 2025, 14, 1736. https://doi.org/10.3390/land14091736
Bartha S, Házi J, Purger D, Zimmermann Z, Szabó G, Guller ZE, Csathó AI, Csete S. Fine-Scale Organization and Dynamics of Matrix-Forming Species in Primary and Secondary Grasslands. Land. 2025; 14(9):1736. https://doi.org/10.3390/land14091736
Chicago/Turabian StyleBartha, Sándor, Judit Házi, Dragica Purger, Zita Zimmermann, Gábor Szabó, Zsófia Eszter Guller, András István Csathó, and Sándor Csete. 2025. "Fine-Scale Organization and Dynamics of Matrix-Forming Species in Primary and Secondary Grasslands" Land 14, no. 9: 1736. https://doi.org/10.3390/land14091736
APA StyleBartha, S., Házi, J., Purger, D., Zimmermann, Z., Szabó, G., Guller, Z. E., Csathó, A. I., & Csete, S. (2025). Fine-Scale Organization and Dynamics of Matrix-Forming Species in Primary and Secondary Grasslands. Land, 14(9), 1736. https://doi.org/10.3390/land14091736









