Abstract
Amphibians are particularly vulnerable to hydric stress due to their permeable skin, biphasic life cycle, and strong dependence on aquatic and moist terrestrial environments. In the Northwestern Mediterranean Basin—one of Europe’s most climate-sensitive regions—the intensification of droughts associated with climate change poses a critical threat to amphibian populations. Increased aridification, either due to higher temperatures or to more frequent, prolonged, and severe drought episodes, can affect both aquatic and terrestrial life stages, directly altering breeding opportunities, larval development, post-metamorphic survival, and dispersal capacity. This review aims to gather and synthesize current knowledge on the ecological, physiological, and demographic impacts of drought on amphibians of the Northwestern Mediterranean across habitat types, including ephemeral ponds, permanent water bodies, lotic systems, and terrestrial landscapes, including a final section on possible mitigation actions. Drought-induced shifts in hydroperiod can drastically reduce reproductive success and accelerate larval development with fitness consequences while, on land, desiccation risk and habitat degradation could limit access to refugia and fragment populations by reducing structural connectivity. These environmental constraints are compounded by the interactions between drought and emerging infectious diseases. We discuss the current knowledge on how chytrid fungi (Batrachochytrium dendrobatidis and B. salamandrivorans) and ranaviruses may respond to temperature and moisture regimes, and how drought may affect their transmission dynamics, host susceptibility, and pathogen persistence. In these cases, microbiome disruption, pollutant concentration, and increased contact rates between species may amplify disease outbreaks under dry conditions, but a better understanding of the multifactorial effects of drought on amphibian biology and disease ecology is needed for predicting species vulnerability, identifying high-risk populations, and guiding future conservation and management strategies in Mediterranean environments.
1. Introduction
Climate change due to increases in human greenhouse gas emissions since the beginning of the industrial revolution up to the present day is now considered one of the major threats to biodiversity and one which underlies several reported extinction events [1,2,3]. Climate is rapidly changing, and the trend towards increased global temperatures has been forecast to continue for the coming decades [4], together with the risk of droughts. Model projections for the 2090s indicate that the proportion of the global land surface under extreme drought is predicted to increase by a factor of 10 to 30. Although species have demonstrated resilience to important climate shifts throughout evolutionary history, the current rate of global change is challenging the ability of organisms to adapt rapidly. This poses serious risks to wildlife communities, potentially driving biodiversity losses [5]. Importantly, human-induced climate change is not only altering the mean temperature but also the patterns of precipitation and evapotranspiration, including extreme weather events such as heatwaves, floods, wildfires and droughts [6,7,8,9]. In addition, global trends towards aridification can further compromise biodiversity through changes in land use and overexploitation of hydric resources, leading to unpredictable changes in water variability and availability [10,11].
Droughts, characterized by anomalously low precipitation, abnormal soil moisture deficits, and excessive evapotranspiration [8,12,13], are a major environmental stressor with diverse potential effects on wildlife communities. They can impact wildlife directly by inducing physiological stress that affects survival and fecundity. Additionally, they exert indirect effects by altering habitat structure [14], resource availability [15], and interspecific interactions. These can include shifts in microbial soil composition [16], forest die-offs [17], the proliferation of invasive species [18], and the destabilization of species interactions due to changes in competition and trophic dynamics [19,20]. Drought effects depend on duration and intensity [15,16,17,18,19,20,21]. In this sense, the percentage of land under extreme drought, defined as the 1% most severe drought conditions that occurred between 1950 and 2000, is expected to rise from 1% today to 30% by the end of the 21st century according to climate models [22]. Estimates for the southwestern United States [23] suggest that several ecoregions will see large increases in drought exposure, with 29 out of 84 ecoregions being subject to increases of over 200%, and 19 ecoregions being subject to increases of over 400%. Concerning the effects of extreme weather and climate events [15], increases in their frequency, severity and/or duration can trigger resource bottlenecks that act as powerful demographic constraints on terrestrial fauna, often exacerbating other anthropic pressures such as land use change and driving populations to local extinction or marked declines. Bottlenecks caused by drought were found for most taxa, but interestingly they accounted for all three recorded impacts on amphibians.
Climate change is impacting water availability in the regions surrounding the Mediterranean Sea. The Western Mediterranean has been identified [8,24,25,26,27] as one of the regions with a moisture deficit from its North Atlantic oceanic moisture source. Most studies show negative consequences of climate change in soil moisture, aquifer recharge, irrigation demand, hydrological extremes (droughts, low flows), and water and soil quality (nutrient concentration, soil salinity, soil erosion). Projections generally agree on an increased duration, frequency and severity of droughts in the Mediterranean Basin [13,28]. However, recent studies [29] show that precipitation trends over the past one and a half century are negligible, and that the precipitation in the area is dominated by high temporal variability. Despite this, increasing aridity in the region is likely to be unavoidable and to be driven primarily by rising temperatures and enhanced evapotranspiration. In this sense, Mediterranean climate change has been observed at a magnitude exceeding global means [30]. The regional temperature increase is higher than the global mean temperature increase, with a peak at 1.9 °C above the 1950 temperature in 2022. Thus, it is important to highlight that, regardless of the presence, or not, of significant trends in precipitation in the Mediterranean Basin, the region is undergoing a progressive process of climatic aridification. This trend is primarily driven by an increase in atmospheric evaporative demand associated with rising temperatures and potentially compounded by changes in precipitation patterns. As a result, the region is experiencing increasingly severe ecological and agricultural droughts [8,12,13].
The Western Mediterranean Basin is not only home to droughts but also to a great use of water resources. For instance, Western Mediterranean countries are among the most productive agricultural suppliers, thanks mainly to the combination of high temperatures and the irrigation of water-consuming crops. Withdrawals for irrigation are high because of the evaporative demand for crops grown in the Mediterranean climate [31]. The demand for irrigation water in the eastern and southern countries is often over 50% and reaches almost 90% in some countries [32]. In these countries, agriculture is the primary water-consuming sector, with high-demand crops. In addition, the great increase in tourism in recent decades [33] poses an added pressure on the consumption of water resources for personal and recreational activities, mainly in summer [33], which is not only the driest and hottest season, but also the season for which its climate is becoming even more arid [34]. Heatwaves and droughts interact with various water uses, sometimes creating a positive feedback loop. For example, during droughts, agricultural water consumption often increases, further exacerbating water scarcity [34]. Additionally, the increase in forest cover in non-cultivated areas can also decrease the amount of water that reaches streams, ponds, and can become groundwater. In Catalonia (Northeastern Spain), the average annual temperature has already increased by 1.9 °C and the average annual rainfall has decreased by about 104 mm compared to the middle of the 20th century [35], with an increased duration and intensity of drought periods, while the increase in forest cover has greatly increased. In fact, the FOREStime report [36] estimates a 29% reduction in blue water between 1990 and 2014 in the Northeastern Iberian Peninsula, including Catalonia. All this leads to a scenario of great water scarcity, similar to that of other areas with a similar climate (e.g., California, see [17,36], where less water will be available for different uses, while the increased vegetation cover will demand more water to compensate for a warmer and drier atmosphere.
Amphibians are among the most widely threatened animal groups, and a recently updated assessment [37] reports that their conservation status keeps worsening globally. Most species lay eggs in the water, which hatch into aquatic larvae that metamorphose into land-living juveniles. Drought can heavily impact amphibian species that rely on water availability for larval development, with direct and indirect effects on larval survival and post-metamorphic fitness [38,39]. Soil moisture is important also for most amphibian post-metamorphic age-classes, which rely on underground microrefugia [40], or for many amphibian species which obtain water directly from the substrate [41]. Thus, droughts are related to increased mortality rates in amphibian populations [42], altered community compositions [10,43,44], and even decreases in biodiversity and functional diversity [45]. Thus, while amphibian species may have to endure smaller increases in drought episodes compared to the other vertebrate groups [23], this may nevertheless imply much more detrimental effects in amphibians than on other faunal groups.
Drought is expected to have the most severe consequences on amphibians [46]. According to [47], drought events have increased in several areas with high amphibian diversity, including much of the Amazon basin, the Atlantic Forest of Brazil, Madagascar, the southwestern United States, northern Mexico, and continental Europe [47]. Despite this, surprisingly in amphibians, the risks associated with more intense or recurrent dry periods remain relatively understudied compared to other groups [48].
Concerning the Northwestern Mediterranean Basin, while local species are adapted to survive and endure these temporarily arid conditions, it remains uncertain whether they can adapt to an increasing frequency and intensity of dry periods under changing water availability conditions [49]. In Europe, the frequency and magnitude of droughts and heatwaves have increased over the last 50 years, especially in the southern and western regions [50,51]. These events, in addition to the levels of water demand by human activities, will drive water scarcity in the Northwestern Mediterranean Basin, determining an important part of the negative effects on the amphibian communities, which will synergistically act together with other factors, such as habitat loss and fragmentation, pollutants, emerging diseases, and invasive species.
In this review, we assess the potential impact of the reduction of water availability and the possible increase in severe droughts in the amphibian community of the Northwestern Mediterranean Basin, highlighting the risks for this crucial element of fresh-water biodiversity in the long-term. We focus on how the loss of superficial water and moisture can affect amphibian assemblages in lentic, lotic and terrestrial habitats, and on how it can interact with the spread of emergent diseases, like chytridiomycosis and ranavirosis, which could be a very important conservation issue in the future. This review evaluates the current state of knowledge on the impacts of drought on amphibian populations in a region projected to experience increased aridification. While anthropogenic climate change imposes multiple stressors, drought—despite its potentially profound ecological consequences—has received relatively limited and fragmented attention compared to temperature increases. Notably, although many amphibian species in the Mediterranean and broader European context can tolerate a wide range of temperatures, drought is likely to impose more stringent constraints on their distribution, survival, and reproductive success. Our objective is to compile and synthesize existing evidence on how amphibian populations and communities are likely to be affected by drought, and to explore potential responses. We draw on studies from the global literature deemed relevant to the northwestern Mediterranean, selected for their taxonomic or climatic similarity, while also considering insights from other systems. This review represents the first comprehensive assessment of the potential effects of drought as a central driver shaping amphibian communities in this region.
2. General Susceptibility of the Amphibian Community to Drought
The amphibian community inhabiting the Northwestern Mediterranean basin lives under the domain of the Mediterranean climate with hot and dry summers (Figure 1), mild wet winters and high spatio-temporal variability of precipitation, which is mainly concentrated in autumn and secondarily in spring. Usually, the amphibian community in this area is subject to two well-characterised hydrological phases: a wet period, mainly from autumn to spring, and a dry period concentrated mainly in summer. However, there is great inter-annual variability in the duration and intensity of these periods. The adaptability of the local amphibian community to changes in the wet–dry regime probably depends on many intrinsic and extrinsic factors, determining different susceptibilities (Table 1 and Supplementary Material Figure S1 for graphical representation). These different susceptibilities most probably imply a phylogenetic signal, similar to that found in thermal tolerances [52]. Table 1 summarizes an objective assessment, as far as possible, of the vulnerability of amphibian genera in Northwestern Europe to drought, given projected increases in drought frequency and intensity. Seven biological or ecological life-history traits were selected as key determinants of drought susceptibility. Each trait was scored from 0 to 1 or 2, based on expert judgement and available biological data, to standardize risk estimates and minimize subjectivity in evaluating their influence on survival during prolonged or frequent droughts.

Table 1.
Semi-quantitative assessment of susceptibility to drought for the different genera present in the Northwestern Mediterranean. Values range from 0 (Low Susceptibility) to 10 (High susceptibility). Susceptibility assessments are based on species’ biological traits related to drought sensitivity, each variable scoring from 0 to 1 or 2. Variables and scoring criteria were: larval period (0 = a few weeks; 1 = up to 3–4 months; 2 = ≥5 months), breeding season (0 = autumn, winter, or year-round; 1 = spring; 2 = summer), reproductive strategy timing (0 = prolonged; 1 = explosive), reproductive strategy flexibility (0 = flexible; 1 = fixed), pond permanency preference (0 = ephemeral; 1 = temporary; 2 = permanent), longevity (0 = short, <10 years including immature stage; 1 = long), and clutch size (0 = large; 1 = small).
Since aridity periods are predicted to increase in frequency and intensity [13,28], this will put a strain on those species that are not plastic or adapted enough to face the diminished water availability for the Mediterranean Region. Refs. [24,26,47,53] identify Western Europe as one of the regions in which amphibian species are most exposed to droughts (Figure 1).
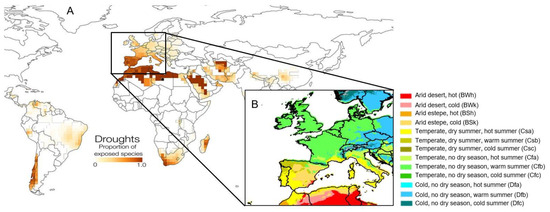
Figure 1.
(A): Proportion of amphibian species exposed to drought per 2° global grid cell according to [47], partial and directly extracted from Figure 5 in [47]. (B): Köppen–Geiger climate classification in the studied area. Extracted from [54] and CC BY 4.0, https://commons.wikimedia.org/w/index.php?curid=146711567 (accessed on 5 August 2025).
By the end of the 21st century, the combined effects of rising temperatures, reduced precipitation, and increasing pressure on water resources are expected to drive the expansion of the Mediterranean region’s drier ecosystems into areas further north and at higher altitudes [55]. It has been observed that many species of amphibians in the Iberian Peninsula have already moved up in altitude towards cooler and more moist environments [56]. Changes in predicted water availability to the end of 21st century for the Western Mediterranean (Figure 2, data extracted from [26]) predict a general decrease in water availability and an increase in water demand.
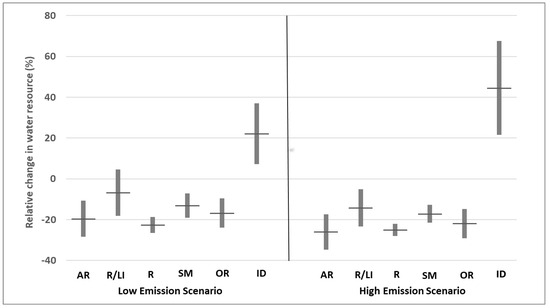
Figure 2.
Predicted change in water resource category (in %) to 2050–2100, from low and high scenarios of greenhouse emissions. AR: Aquifer recharge, R/LI: Reservoir/Lake Inflow, R: Runoff, SM: Soil Moisture, OR: Other Resources and ID: Irrigation Demand. Boxes indicate a confidence interval of 95%, black horizontal lines indicate means. The figure has been made by extracting data from Supplementary Materials (Tables S1 and S2) in [26] and tailored to include only data referring to the Northwestern Mediterranean Basin.
Local anuran larvae are typically primary consumers or opportunistic omnivores, whereas urodele larvae tend to be primarily predatory [39]. More importantly, the length of the larval phase frequently determines species-specific habitat requirements [39,57,58]. Pond drying is one of the main risks for amphibians in these communities. Species have specific requirements regarding the hydroperiod, which defines a trade-off between predation, competition, and desiccation risk [38]. For instance, the preferred hydroperiod can be short to avoid potential predators like fish or large dragonfly larvae but must be long enough to allow larval development to reach completion, if not met, at the cost of high levels of larval mortality and reproductive failure. Thus, prolonged periods of drought will probably affect the rates of colonization and extinction, driving changes in occupancy and metapopulation dynamics in some species [46,59,60], e.g., in the observed fecundity and survival at different ontogenetic stages in Bombina variegata [61], where population growth is strongly influenced by drought frequency. Decreases in fecundity can also be accompanied by changes in larval survival, larval size, growth and time of metamorphosis [62,63]. At the landscape level, droughts can alter amphibian ranges and breeding phenology with consequences for reproductive success [64].
On the other hand, most pond-breeding adult amphibians spend little time in breeding ponds, and most of their lifetime is spent in terrestrial habitats that may, or not, be directly adjacent to the breeding site. Each species and age-class may show different strategies to survive during severe droughts in terrestrial habitats. The intrinsic dispersal capacity of each species is important for migrating to areas with water, even over large distances. Theoretically, high dispersal rates, high fecundity, and a short lifespan are associated with metapopulations experiencing unpredictable environments and presumably those more adapted and resilient to droughts, while a very low dispersal rate, low fecundity and a long lifespan are associated with populations occupying predictable environments [61]. Accordingly, small toadlets and adults of Epidalea calamita migrate distances of several hundred meters within a short time [65,66], which may significantly help prevent local extinction in sink populations. Hyla molleri and Pelobates cultripes are capable of covering accumulated displacements up to 3.5 and 1.8 km respectively [67]. Similarly, Pelophylax perezi has high dispersal capacities [68] that allow the species to maintain genetic structure between far waterbodies. Contrarily however, other amphibian species have low dispersal capacities but may be very resilient thanks to longer lifespans. It has been suggested that high survival of adult D. fuscus [69], coupled with their temporary emigration, may compensate for the negative effects of drought on larvae and facilitate resilience of this species to drought conditions. In fact, long lifespans were identified as the main factor allowing long-term persistence of a population or marbled salamander under the stress of occasional catastrophic reproductive failures due to low precipitation [70]. Similarly, urodeles in the Northwestern Mediterranean can live more than 10 years [71,72,73,74], which could be a crucial feature in allowing a population to overcome long droughts.
In most amphibian species only a small fraction of the population performs long-distance dispersal events, while short distance displacements are much more frequent [66,68,75,76]. Usually, this small proportion of the population is enough to maintain genetic and metapopulational structures. However, droughts also produce habitat loss and fragmentation [39], hindering the connectivity along breeding sites, which is an important factor which could determine the survival of metapopulations. Landscape configuration conditions colonization and extinction probabilities and is very important for the persistence of “sink” populations [77,78].
Finally, changes in temperature or precipitation can potentially influence the timing of amphibian reproduction [57,79]. An earlier onset of spring breeding behaviour correlated with a warming climate has been observed among various pond-breeding frogs and toads in Europe [80,81,82,83,84]. The authors of [85], through a meta-analysis of global amphibian phenological data, concluded that this group is strongly influenced by climate change. Although they did not identify consistent patterns in demographic parameters such as abundance, survival, breeding success, or morphology, they found that drought and elevated temperatures can significantly alter amphibian phenology—advancing, delaying, or even preventing the onset of breeding. Such shifts in breeding timing can disrupt ecosystem processes and force larval development to occur under suboptimal conditions, leading to increased intraspecific competition and reduced resource availability [84].
3. Potential Effects of Extreme Droughts
3.1. Lakes and Ponds
The hydrology of lakes and ponds, whether from natural or human origin, is generally characterized by dependence on the hydrological and rainfall regime in one small area. That is, if we exclude large artificial reservoirs and the mountain lakes located in the main West European mountain ranges, most of the lentic environments on the Western Mediterranean area are quite sensitive to long periods of drought due to natural climate variations, anthropogenic climate change, or water overexploitation.
Most amphibians present in the Western Mediterranean can reproduce in lentic environments. It is usually assumed that these communities are structured along a series of abiotic and biotic gradients that determine the abundance of each species [86,87,88]. Among all factors, hydroperiod is arguably considered the most relevant. Studies on amphibian communities in temperate regions agree that pond breeding amphibian communities are organized along the hydroperiod gradient, ranging from permanent to temporary and ephemeral ponds (see e.g., [86,89,90]). While long hydroperiods support more complex faunal communities and promote the selection of predator-avoidance traits in amphibian larvae [59], ephemeral environments select for traits related to rapid development and efficient foraging instead, as the risk of reproductive failure primarily depends on hydroperiod length [91]. In an intermediate situation, temporary ponds show varying levels of desiccation and predation risk, with potentially important competition levels. In Mediterranean regions, temporary ponds are the main breeding habitats for several amphibian species [91,92]. In these environments, the alternation of dry and wet periods determines the resident community, as both terrestrial and aquatic species need to have strategies to resist dry periods and droughts, such as the ability to migrate, advance metamorphosis, increase growth rate or resistance behaviours. This combination of factors makes Mediterranean temporary ponds unique in their high biodiversity, becoming specially protected systems listed as priority habitats by the Habitats Directive code 3170 [93].
The position of a pond each year along this gradient largely depends on short- and long-term rainfall patterns, creating an unpredictability to which many species have adapted. This includes the demonstration of several types of phenotypic plasticity in larvae, and an important ability to optimize selection of oviposition sites and flexible strategies in the timing of the onset of breeding in adults [57,91,94,95,96,97,98]. For instance, most temperate amphibians can show antipredator morphologies if there are predators present [99] or accelerate the timing of metamorphosis to escape drying waters. However, all these shifts have trade-offs, and in the case of premature metamorphosis developmental acceleration comes at the cost of reduced body size, altered body proportions, and possible impairments of the immune function in the recently metamorphosed individual [38,59,89], which may even have downstream effects on susceptibility to disease [64,100].
Worryingly, prolonged droughts will directly and repeatedly affect the hydroperiod gradient that underpins this diversity and structures most amphibian communities, and plasticity not only entails costs but also has limits, meaning that several species may be pushed beyond their adaptive capacity if droughts become more frequent and intense [101]. While the Western Mediterranean basin is characterized by pronounced interannual variability in both the amount and timing of rainfall, it is already known that extreme events can strongly shape community composition. During a prolonged period of abnormally low rainfall in Doñana Natural Park (2011–2022), an exacerbated unpredictability in the onset of inundation of the temporary ponds led to interannual differences in amphibian community composition [39]. In the long term, an increased frequency or length of droughts either tend to favour the most dominant species [58,102,103] or lead to a direct loss of available habitats and to a reduction in abundance and diversity of amphibians [39,43,60,104,105].
On the positive side, droughts can lead to the disappearance of invasive fish species from many sites, often without subsequent recolonization, which is associated with reduced amphibian occupancy in areas where these exotics are present. This suggests that droughts may offer a window of opportunity for invasive species control, which may be one of the main problems for amphibian population dynamics [10,106]. For instance, [107] modelled the effects of various threats on the larval development of Epidalea calamita—a species that breeds mainly in ephemeral and temporary ponds—and found that predation pressure from invasive species had a stronger negative impact on tadpole guilds than changes in temperature or precipitation. This highlights that the presence of invasive predators poses a greater threat to the species than climate change itself. Supporting this, recent monitoring in Catalonian natural parks showed this species increased both in abundance and occupancy during the 2021–2024 drought period [108]. Conversely, if droughts do not reduce the presence of invasive fish and the remaining aquatic habitats continue to host these species, the combined stressors may lead to sharp declines in several amphibian populations. The same study [108], along with others, found that species dependent on longer hydroperiods were negatively affected in the short term by the reduced availability of suitable habitats, in some cases resulting in local extinctions [10,70,109].
Beyond the direct effects of altered hydroperiods (Figure 3), many other ecological factors associated with drying habitats can indirectly affect amphibian behaviour, physiology, and fitness [110]. Amphibians that breed in ephemeral or temporary ponds are especially exposed to fluctuations in temperature and precipitation, which can alter larval density, food availability, and host–pathogen interactions [46,64]. When these changes lead to metamorphosis at smaller body sizes [38,60,90], this will most probably entail costs in terms of survival in the first terrestrial life stages [111]. Shifts in reproductive phenology can also influence the outcome of competitive and predatory interactions, thereby potentially altering community dynamics in assemblages of co-existing species, beyond direct effects on individual mortality and survival [112,113]. If multiple species that previously segregated temporally are increasingly forced to breed synchronously, this may intensify both interspecific competition and predation pressure—particularly if predators are also constrained to breed or forage within the shortened periods of inundation—potentially leading to unpredictable shifts in community dynamics.
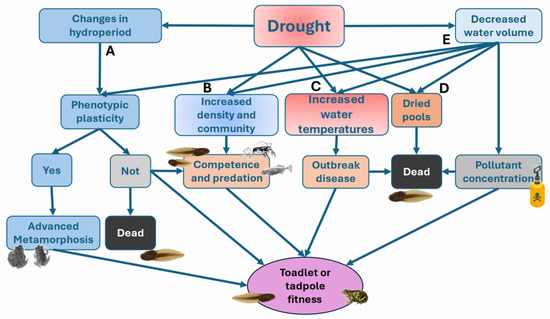
Figure 3.
Schematic diagram of the main pathways via which drought may change tadpole and toadlet fitness. A: Drought reduces hydroperiod forcing tadpoles to modify growth or larval development by means of phenotypic plasticity. B: Drought increases predation and intra- and interspecific competition. C: Increase of water temperatures can favor the outbreak of emergent diseases. D: Drought directly causes amphibian mortality when ponds or stream pools dry out, leading to the loss of entire cohorts. E: The decrease of water volume increases the pollutant concentration. E also has effects on B, C and D. The disease pathways are shown in detail in Figure 4.
In coastal freshwater ecosystems, the effects of drought can be particularly severe due to salinization. Proximity to the sea can lead to increased salt concentrations in aquatic habitats during drought periods [114,115,116]. Although salinization also occurs inland, mainly as a result of aquifer overexploitation and prolonged droughts [117,118] in coastal wetlands, it is especially exacerbated by decreasing precipitation, rising temperatures, and seawater intrusion. Drought periods disrupt the balance and dynamics between fresh and saltwater in coastal areas, a process that can drive significant changes in amphibian and aquatic communities [119]. Most amphibians in Western Europe have complex life cycles that include aquatic stages—eggs, larvae, and in some species, even adults—that are frequently exposed to increasingly saline conditions. Some species can reproduce in slightly brackish or low-salinity coastal habitats [115], but amphibians are generally highly susceptible to elevated salinity due to their permeable skin and limited osmoregulatory capacity [120].
Salinity can negatively impact reproductive performance, reducing sperm viability, fecundity, and embryo survival, with early life stages being particularly vulnerable [116,121,122]. Even moderate salinity can prolong development and alter hormone activity in larvae, depressing thyroid function, affecting osmoregulatory hormones, and altering antioxidant responses [120,123,124,125]. These physiological disruptions can lead to slower growth, reduced activity and foraging efficiency, and ultimately extended larval periods [126,127,128,129,130,131,132]. Larvae exposed to salinity may also exhibit reduced swimming performance and speed, increasing their susceptibility to predation [132,133,134,135]. As a result, repeated increases in salinity can compromise the normal survival of cohorts because saline and drought-stressed conditions will coincide with shorter hydroperiods and increased predator density. In Western Europe, certain populations of Pleurodeles waltl, Triturus marmoratus, T. cristatus, Lissotriton helveticus, Discoglossus pictus, D. galganoi, Pelobates cultripes, P. fuscus, Hyla meridionalis, Epidalea calamita, Bufo spinosus, Bufotes balearicus, B. viridis and Pelophylax perezi have demonstrated a degree of physiological adaptation to osmotically stressful environments, allowing some populations to persist in mildly or occasionally saline habitats [114,116,126,136,137,138,139], but no amphibian species is known to permanently inhabit highly saline environments.
3.2. Rivers, Streams, and Brooks
Under Mediterranean climate conditions, most watercourses exhibit a seasonal flow regime, with a wet phase occurring during late autumn, winter, and spring, and a dry phase that typically takes place in summer [140,141]. While permanent rivers in the region experience substantial fluctuations in discharge, they rarely dry out completely—the dry phase in these systems usually corresponds to reduced flow or water volume. In contrast, temporary rivers may break into a series of disconnected pools or even dry up entirely for part of the year. At the most extreme end of the gradient, ephemeral streams flow only briefly, typically following rainfall events [141,142]. Most of the research has historically focused on permanent rivers, despite the fact that temporary rivers are common watercourses throughout the world [142,143,144]. They are especially abundant in the Mediterranean basin, where they are currently more widely studied [145].
It is commonly accepted that temporary rivers support lower overall biodiversity than permanent ones, as the latter tend to exhibit more complex physical structures and contain a wider variety of microhabitats [146]. Temporary rivers are characterized by marked interannual variation in the onset and duration of their dry phases, which can jeopardize the survival of species not adapted to enduring prolonged droughts or flash floods. Due to these constraints, temporary rivers can host unique biodiversity and often act as refugia for specialized species [147]. Their intermittent nature may also make them more resistant to biological invasions, such as those by exotic fish [148,149], but see [150,151]. Similarly to temporary ponds, these systems can be frequently considered ‘hotspots’ of regional biodiversity and can play a key role in maintaining the ecological integrity of river networks [147]. Nevertheless, many are under significant pressure due to water overexploitation [152,153]. Climate change models predict a global increase in the extent of intermittent rivers, with formerly perennial rivers in some regions already transitioning to temporary flow regimes [142,154]. However, in this context temporary rivers themselves are becoming increasingly vulnerable. The combined effects of reduced precipitation and growing human water demands are intensifying the frequency and duration of no-flow periods in many areas [155,156]. Intensifying drought conditions are expected to reduce hydrological connectivity, limiting species dispersal and disrupting metapopulation dynamics [157]. Even species typically regarded as resilient may be at risk if extended dry phases—or shifts in their seasonal timing—become decoupled from species’ life-history strategies [158,159].
Although the Western Mediterranean does not host a high number of stream-specialist amphibians, those that do occur typically have high conservation value and occupy unique ecological niches. Newts of the genera Calotriton and Euproctus are typically protected under national or regional conservation frameworks, yet they face increasing threats—that similarly affect other stream-dwelling anurans, such as Rana pyrenaica—from rising water temperatures, more frequent extreme rainfall events, the introduction of non-native fish species into the few stream sections that still retain water, and water overexploitation [160,161,162]. In contrast to species inhabiting lentic habitats, those adapted to lotic environments tend to have more limited dispersal abilities [163,164,165]. This is likely because riverine systems are geologically more stable, and thus, evolutionary pressures have not favoured long-distance dispersal [165,166,167]. As running waters are generally more persistent than small lentic water bodies, populations within them are less reliant on dispersal for survival, often resulting in higher levels of genetic isolation, even at small distances [163,168]. Conversely, species from lentic environments tend to exhibit greater dispersal capabilities due to the unpredictable hydroperiods associated with rainfall dependency and, on average, occupy larger geographical ranges than species restricted to more stable lotic systems [166]. As a result, the increasing disruption of water continuity within and among river systems—primarily driven by more frequent droughts—may have severe consequences for the diversity and persistence of lotic-adapted species. A notable example is the Montseny Brook Newt (Calotriton arnoldi), listed as Critically Endangered, which is currently facing an extremely vulnerable situation in parts of its already restricted range due to markedly reduced stream flows [162]. This decline in water availability is likely to be the result of interacting pressures, including climate change, land-use transformations, and excessive water abstraction [162]. Moreover, the situation is exacerbated by the species’ extremely limited dispersal capacity and the near-total absence of connectivity among its fragmented populations [168], creating a highly precarious scenario for long-term survival in some brooks.
On the other hand, for more generalist amphibian species that inhabit both lentic and lotic environments, the main stressors and ecological trade-offs across biotic and abiotic gradients in river systems may broadly mirror those observed in ponds. In lentic habitats, permanent waters typically harbour a higher density of amphibian predators and competitors, while temporary systems are associated with greater desiccation risk [169]. However, unlike the more predictable gradient found in ponds, temporary rivers can also support fish species that are adapted to survive in isolated pools during dry periods [147,170]. Moreover, due to their limited baseflow and rapid hydrologic response to rainfall, temporary streams often experience more pronounced flood peaks than perennial systems [171,172,173]. In a recent study on the distribution of generalist amphibians in a Mediterranean stream system, [169] identified water availability as the primary factor driving both abundance and diversity. Although water availability was positively correlated with fish presence, the study suggests that, in temporary rivers, amphibians that tend to avoid fish in pond environments may successfully evade predation by exploiting microhabitats shaped by natural river dynamics—features that are largely absent in ponds. While this points to a potentially favourable scenario for amphibians able to inhabit temporary river systems, the study also found that water availability clearly determined the presence of species typically associated with permanent aquatic habitats, pointing that droughts may affect these species in a manner similar to how they impact fish populations, in some cases even leading to local extinctions [109].
In addition to reductions in water quantity, climate change also poses significant threats to water quality. During dry spells, the capacity of temporary streams and rivers to dilute pollutants is substantially reduced, impairing their ability to buffer the effects of continuous wastewater discharges. While effluent volumes typically remain stable throughout the year, the dilution potential of receiving water bodies is highly variable leading to sharp increases in pollutant concentrations during drought events [174]. This issue is not adequately addressed in current water management frameworks [175]. Among pollutants, glyphosate has received particular attention in amphibian research, with sublethal effects documented across all developmental stages [176]. However, the extent to which glyphosate-based herbicides and other pesticides contribute to amphibian declines remains unclear, largely due to a lack of data on actual exposure levels in natural populations [176]. Although the dynamics of pesticide presence and concentration in Mediterranean freshwater systems are not yet fully understood [177], there is growing concern that prolonged droughts may exacerbate the accumulation and persistence of these substances, thereby intensifying their ecological impact [178].
Finally, it is important to note that, even though amphibians also use temporary rivers and in most cases are more able to colonize them than fish, they are yet not considered in the environmental quality indices of rivers and streams (Ecological Quality Ratios-EQRs), based on ecological status classifications of the Water Framework Directive (WFD), Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 (https://eur-lex.europa.eu/eli/dir/2000/60/oj), (accessed on 5 August 2025).
3.3. Terrestrial Habitat
Most adult amphibians in the Western Mediterranean spend the majority of the year in terrestrial habitats, returning to aquatic environments only during the breeding season. In terrestrial settings, they are particularly vulnerable to water scarcity due to their permeable skin and reliance on moist microhabitats for hydration and cutaneous respiration. During droughts or extended dry periods, dehydration stress increases, compromising physiological processes such as gas exchange, locomotion, and aerobic activity [41]. Reduced soil moisture and the desiccation of terrestrial refuges can lower survival rates of post-metamorphic and adult individuals. For instance, in Bombina variegata, a severe drought led to a 12% and 10% reduction in juvenile and adult survival, respectively [61]. Similarly, in Rana sylvatica, adult survival was positively correlated with monthly rainfall [62]. In Ambystoma tigrinum [179], male survival during a drought year in breeding ponds was up to 54% lower compared to survival in adjacent forest habitats. Recent modelling studies further underscore the impact of climate change on amphibian activity levels. The authors of [46] showed that, under future scenarios combining increased temperatures and drought conditions, the potential activity of different frog ecotypes was markedly reduced. Notably, reduced rainfall had a greater negative effect on activity than temperature increases alone. Stream-dwelling and semiaquatic ecotypes were particularly assessed to be susceptible to desiccation stress, likely due to their higher dependency on continuous water availability compared to arboreal or fossorial species. Droughts are also likely to reduce dispersal opportunities by limiting surface activity, thereby decreasing the probability of successful emigration and colonization of new habitats [109].
Beyond the immediate limitation posed by reduced atmospheric moisture, extreme droughts can have long-term impacts on the terrestrial habitats inhabited by adult amphibians. Severe and recurrent drought events lead to widespread tree mortality and alter forest structure [44,180,181], which can significantly affect survival and activity during the most vulnerable life stages of some species [182,183,184]. Certain terrestrial microhabitats—such as dead logs, woody debris, and fallen trees that retain soil moisture—can function as hydrological refuges, reducing mortality among both juveniles and adults [184]. Thus, on a more positive note, some drought episodes may increase the volume of deadwood, a key component of forest maturity, e.g., [185,186]. This deadwood contributes to soil moisture retention and enhances the forest’s capacity to withstand future droughts, while supporting the diversity of understory organisms [187]. However, the broader trend in a context of increasing drought and fire frequency is the replacement of moist forest ecosystems by scrublands, or the shift from drought-sensitive to drought-adapted tree species. These changes typically result in more open habitats, where amphibians are more exposed to desiccation stress [188,189,190]. Among the habitats most critical in this changing landscape are riparian environments, which represent ecotones between terrestrial and aquatic systems and maintain unique moist conditions even within a fully Mediterranean context [191]. These areas provide essential terrestrial refuges for both adults and juveniles, as well as foraging and dispersal routes for many amphibian and non-amphibian species. Riparian habitats are therefore crucial for amphibian persistence during drought periods [192,193] and help buffer the impacts of increasing droughts by enhancing connectivity and ecosystem resilience [194]. In fact, such environments now serve as the last refuges for several populations of Atlantic or montane amphibian species persisting under Mediterranean climatic conditions, such as several locations of Rana iberica or low-elevation populations of Calotriton asper, and deserve special attention from a conservation perspective.
Droughts also increase the risk of forest fires. Drier and hotter conditions, combined with the expected accumulation of forest deadwood, are likely to raise the frequency and severity of wildfires [4,195,196,197]. In the Western Mediterranean Basin, before 1970, wildfires were primarily driven by fuel accumulation resulting from farmland abandonment and rural depopulation [196]. However, since 1970, wildfires have become more strongly linked to climatic variables associated with drought, particularly high temperatures and low precipitation, with significant fire–climate relationships in the same region in recent decades [198]. The effects of wildfires on amphibian populations have been studied worldwide, but mainly in biomes classified as temperate forests [184,198,199]. Interestingly, in a review in 2021 [199], no evidence of a predictable response to anuran communities to fire in any biome is reported, finding that 20% of studies reported positive effects, 26% observed negative effects and the majority of 47% of the studies did not detect a significant effect of fire on anuran assemblages. Thus, while fire can impact amphibian communities through both direct and indirect mechanisms, the final outcomes may depend on fire- and species-specific singularities. Thus, the impact of wildfires on amphibians should be assessed separately for short- and long-term on individuals, species and habitats [200,201]. While direct mortality occurs during the fire, the main long-term effects result from rapid changes in habitat structure [202].
Fires cause direct mortality of amphibians in their terrestrial phase, indirect mortality or displacement due to sudden habitat alteration, and larval mortality due to chemical changes in breeding ponds, or by direct loss of breeding sites through siltation caused by slope erosion [201]. After these initial effects, forest fires reduce soil water retention capacity and interception (green water), and increase soil erosion and evaporation [203], which collectively create a post-fire environment similar to aridification or drought conditions.
In the Western Mediterranean, this usually promotes expansion of scrubland at the expense of forest cover [188]. If fires are occasional, this can lead to an interesting spatial mosaic of burned and unburned habitats hosting different faunal communities [204,205]. However, if fires are repeated or larger in extension, a shift in dominant species may occur, a fact that has already been hinted in amphibian communities of the area, in which Mediterranean amphibian species are promoted by fires in areas limiting with the Atlantic bioregion [202]. This is supported by the close link between vegetation structure and faunal assemblages in burned landscapes [206,207]. For instance, the decline in the number of pond-breeding species in a Mediterranean Area which hosted some vegetation-associated species was inversely related to the percentage of vegetation burned [201]. Conversely, an increase in species richness and larval densities was observed in unburned areas, likely due to adult migration from nearby burned zones. In the longer term, if the reduction in vegetation cover is permanent, this can lead to decreased soil moisture, which is detrimental to amphibians that rely on moist environments [48,207,208,209]. Therefore, probably the main effect of fire in the Western Mediterranean amphibian communities will be when acting synergistically with droughts [188,189], accelerating the substitution of habitats and facilitating or accelerating community shifts. In this context, Atlantic or sub-mediterranean species marginally inhabiting the Mediterranean biome might be the most impacted by the increase of forest fires. Species like Lissotriton helveticus or Rana iberica [202], which are of Atlantic affinity but maintain populations under Mediterranean climate, or vegetation- or forest-associated species like Hyla meridionalis [210] or, for instance, Salamandra salamandra [201], will probably be the most severely affected.
4. Emerging Diseases and Drought
Currently, fifteen emerging diseases have been described in European amphibians [211]. Among these, two are of fungal origin—Batrachochytrium dendrobatidis (Bd, [212]) and Batrachochytrium salamandrivorans (Bsal, [213])—which cause the disease chytridiomycosis, and one is of viral origin (Ranavirus, [214]). Together, these three pathogens account for the majority of mortality events associated with emerging diseases in European amphibians. In the Western Mediterranean, several chytridiomycosis outbreaks have been historically recorded, related to either Bd (e.g., [215,216]), Bsal [217] or Ranaviruses [218]. The intensity, prevalence, and lethality of these pathogens depend on both intrinsic species-related factors—such as individual susceptibility, microbiota composition, and immune status—and environmental factors, including temperature, precipitation, altitude, and drought, among others (Figure 4). These factors can act independently, but they are often interrelated, acting simultaneously and sometimes synergistically or in a mutually dependent manner. This is not only for the direct effects of diseases but also for possible synergies with other stressors. For instance, an increase in death rates in adult specimens could result in a truncated age structure, which may erode the capacity of populations to overcome consecutive recruitment failures during long droughts [219]. To date, few studies have investigated how environmental stressors such as drought may interact with infectious diseases and drought condition influences on host microhabitat use, disease dynamics and inhibitory function of cutaneous bacterial communities [110,220].
4.1. Batrachochytrium Dendrobatidis (Bd)
The growth and survival of Batrachochytrium dendrobatidis (Bd) are strongly temperature-dependent. Its optimal growth range lies between 17 °C and 25 °C, while temperatures below 10 °C and above 28 °C significantly reduce or halt growth. Zoospores die within 4 h at 37 °C [220,221,222,223,224,225]. Bd is also highly sensitive to desiccation, exhibiting 100% mortality after just 3 h of complete drying. In addition, the pathogen poorly tolerates salinity; exposure to a 5% NaCl solution is lethal [220,226]. Beyond these physical constraints, other environmental factors may influence Bd virulence, prevalence, and host susceptibility [219]. These characteristics, together with intraspecific variability (e.g., [227]) and interspecific or individual differences in host response (e.g., [228]), make it particularly challenging to predict the pathogen’s effects on individual fitness and population dynamics.
Theoretically, ephemeral and temporary ponds should remain free of Bd during extended dry periods, whereas permanent ponds can act as pathogen reservoirs [229]. From this perspective, severe droughts could limit Bd dispersal and persistence. However, zoospores may survive in microscale moist environments—or even in fog or cloud layers under very humid conditions [230,231]—and, most importantly, the fungus can persist on the skin of amphibians during their terrestrial phase, or in/on other aquatic or semi-aquatic animal structures [230,232,233]. This allows Bd to recolonize ponds once water becomes available again, often from nearby aquatic habitats [232]. For instance, recently the painted frog (Discoglossus pictus)—an introduced species in northeastern Spain and southeastern France—has been identified as a potential Bd reservoir for ephemeral and temporary aquatic habitats, potentially facilitating the persistence of Bd in such environments in which this species usually breeds. Very high infection loads have been reported in the species [234] and, according to [235], the usually syntopic Natterjack toad Epidalea calamita exhibits higher zoospore loads (as estimated by qPCR) when in sympatry with D. pictus.
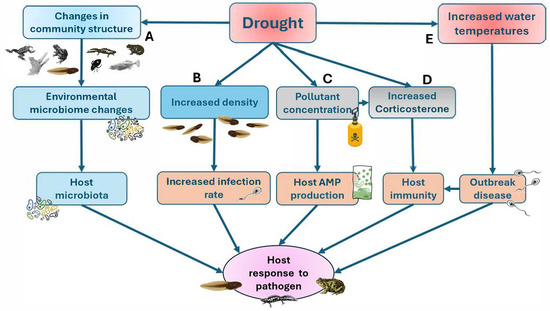
Figure 4.
Schematic diagram of the pathways via which droughts may change host response to disease. A: Drought produces changes in pond communities and alters skin microbiome compromising host response to the pathogen. B: Higher density of tadpoles increases infection rates. C: Drought increases pollutant concentration affecting host AMP (antimicrobial peptide) production and host immunity, D: Drought reduces host immunity by means of increased corticosterone. E: The increase in water temperature produced by drought facilitates the outbreak of emerging diseases. Modified from [110].
Interestingly, the dynamics of Bd may differ in lotic systems (streams and rivers) and in more aquatic species, where the pathogen appears to spread more effectively during summer, when anurans concentrate in and around the remaining water sources [236]. Under these conditions, the probability of zoospore transmission increases due to higher animal density, and mass mortality events are more likely, as infection intensity is usually elevated [236,237]. Conversely, during wetter periods, increased water flow in streams may dilute zoospore concentrations, thus reducing transmission efficiency [238]. Nevertheless, the effect of dilution may be counteracted by humid conditions, which could enhance transmission through alternative infection routes [236].
In addition to the pathogen’s capacity to persist and recolonize, the host’s ability to mount an immune response adds further complexity to future disease dynamics. For example, infection load in individuals of Bufo spinosus was positively correlated with the time individuals spent in aquatic environments [239]. Moreover, drought conditions may reduce the abundance of Bd-inhibiting bacteria in the amphibian skin microbiome, rendering toadlets more vulnerable to infection, [240]. Finally, similar to the trade-offs seen with premature metamorphosis—such as smaller body size or reduced immune function—early emergence may increase susceptibility to pathogens [64]. These findings highlight the complex, multifactorial responses of native amphibians to severe drought events in relation to Bd (see e.g., [241]), underlining the need to evaluate multiple, interacting stressors when assessing the drivers of amphibian reproductive and disease patterns.
4.2. Batrachochytrium Salamandrivorans Bsal
In Europe, Bsal has been detected in captive Salamandra salamandra populations in Belgium, Germany, Spain, the Netherlands, and the United Kingdom, and in wild populations in some regions of Belgium, Germany, and the Netherlands. In the Western Mediterranean basin, Bsal has so far only been detected in wild populations of Triturus marmoratus and Salamandra salamandra at a single locality in Spain, likely introduced via exotic species [217]. The Western Mediterranean hosts a high diversity of urodeles, many of which show high susceptibility to Batrachochytrium salamandrivorans (Bsal), at least under laboratory conditions (see Supplementary Materials Table S1).
The outlook for Bsal under rising temperature conditions is less favourable than for Bd, as Bsal’s optimal growth occurs between 10 °C and 15 °C, and its zoospores die at temperatures of 25 °C or higher [242,243]. Under global warming scenarios, it is assumed that, under both realistic (+2 °C) and extreme (+4 °C) warming conditions, Bsal will exhibit minimal growth rates during the summer [244]—the driest period in the Mediterranean. Thus, in principle extremely hot and dry periods will be the most detrimental to Bsal survival. However, Bsal has a dual transmission, with environmentally resistant non-motile encysted spores in addition to the motile zoospores, which are similar to those of B. dendrobatidis’ [242,245]. It is still unclear if the resistant encysted spores may display resistance to dry periods, but some data suggest that the pathogen may be a relatively poor disperser [246]. Bsal infection is transmitted through direct contact between individuals or via water. The authors of [243] found that the probability of transmission upon contact between infected and uninfected newts was very high (>90%), even at early stages of infection. Although reducing host density and increasing habitat complexity may help reduce transmission, such conditions are rarely met during drought periods, when individuals tend to aggregate in limited moist refuges. For example, [247] modelled Bsal growth for Salamandra salamandra, predicting that optimal pathogen proliferation should occur in summer and autumn—precisely when Mediterranean amphibian activity is typically minimal until the first autumn rains. Under this scenario, species- or population-specific responses to Bsal infection during droughts remain uncertain, and the timing and intensity of possible dispersion events and outbreaks under a Mediterranean climate will likely depend on the composition and connectivity of the local amphibian community, plus the duration and onset of rainfall or drought conditions. Therefore, it is important to remark that, while the biology and transmission pathways of Bsal are beginning to be understood, our capacity to predict how it will respond to environmental change—especially under Mediterranean drought scenarios—is still unknown.
4.3. Ranaviruses
Ranaviruses are also considered as emerging pathogens, meaning that their impact on amphibian populations is recent and mostly linked to increases in host range, geographic spread, or incidence (e.g., [248]). There is strong evidence of host switching between vertebrate classes, including amphibians, reptiles, and fish [248,249,250], with reports of simultaneous infections in sympatric fish and frog populations [251,252]. In the Iberian Peninsula, the Common Midwife Toad Virus (CMTV), together with Frog Virus 3 (FV3), has caused mass mortality events since the late 1980s. Climate warming may be triggering CMTV outbreaks [253], supporting the hypothesis of its endemic status in the region.
Climate change is widely recognised as a driver of disease emergence, particularly in regions where host–pathogen associations were well established [254,255]. Rising temperatures, increased frequency of droughts, and shifts in seasonal rainfall patterns can significantly alter ranavirus transmission dynamics. Higher temperatures have been associated with increased virus replication, incidence, and mortality [255], and outbreaks have become more frequent during historic warming events such as those in the 1990s. Modelling studies predict that future warming and more frequent droughts will expand the temporal and spatial extent of ranavirus outbreaks, potentially severely affecting larval recruitment [253,254,255]. Interestingly, although outbreaks are generally more frequent at high temperatures [253], some studies report greater lethality of infections at lower temperatures [256,257], indicating that temperature effects may vary depending on host species, life stage, and strain.
Environmental stressors associated with climate change—such as drought, contamination, and altered microbial environments—may further increase susceptibility to ranavirus. Amphibian larvae exposed to pesticides in water are more likely to contract and develop severe infections than those in uncontaminated habitats [258]. Reduced water levels concentrate contaminants, potentially leading to immunosuppression and heightened infection risk [259,260,261,262,263]. In parallel, drought can alter skin microbiome composition, which plays a key role in immune defense. Studies have shown that the stability of the amphibian skin microbiome depends on the environmental microbial reservoir, which can be disrupted by climatic and anthropogenic pressures [264,265]. This disruption may increase inter- and intraspecific variation in susceptibility to ranavirus—or other pathogen—infections across different populations.
Similarly to chytrid fungus, drought may also indirectly promote or hinder host contact by concentrating amphibians in fewer aquatic habitats—thereby facilitating interspecific interactions and increasing the likelihood of host switching—or diminishing amphibian stays in the water and reducing infections. In Mediterranean regions. desiccation risk in temporary and ephemeral ponds can induce premature metamorphosis in most species, but this could increase disease vulnerability in tadpoles at Gosner s44–46 stages, which are shown to be particularly vulnerable to ranavirus infection due to intrinsic immunosuppression during metamorphosis [266,267].
Finally, climate warming is also expected to facilitate ranavirus spread to higher altitudes. Rana temporaria, a species living near the southern limit of its range in Mediterranean mountain areas, has been shown to be highly susceptible to ranavirus, and could experience an increase in disease linked mortality in future warming scenarios [268]. Despite well-documented outbreaks and severe population declines in Iberia in recent years [217], the long-term epidemiology of ranavirus is a complex interaction among phenology, population dynamics, habitat heterogeneity, and climate change [218,219,262], which deserves to be further studied. Thus, similarly to the previous cases, studies addressing how increased drought interacts with disease dynamics—especially in amphibian communities of Mediterranean ecosystems—are still largely absent, which poses a significant knowledge gap.
5. Mitigation
Although halting a global phenomenon such as climate change lies largely beyond the reach of most biologists, mitigation strategies can be feasible and effective when efforts are well targeted—for instance, toward protected species or particularly vulnerable amphibian communities. To date, few published studies have documented real-world experiences of mitigating the impacts of extreme drought on amphibians (see [269,270] and references therein), despite widespread recognition of the urgent need to address this issue [271]. Most existing proposals and case studies focus on the creation, restoration, or enhancement of habitats [269] to support the persistence of target species or communities during drought periods, generally reporting positive responses to increased hydroperiods from amphibian populations [270]. Although specific projects aimed at drought mitigation for amphibian conservation in the Mediterranean region are largely absent from the scientific literature, several related useful experiences merit consideration.
At broader spatial scales, efforts to mitigate the impacts of drought on amphibians align closely with overarching goals for sustainable water use. A key priority should be the active management of aquifer recharge (e.g., [39,272]), together with the implementation, improvement, and monitoring of environmental flow regimes in water courses (e.g., [273,274]). Indeed, maintaining adequate environmental flows has been identified as one of the main global priorities in reversing the ongoing decline in freshwater biodiversity [275]. Ensuring the sustainable use of water resources would not only help prevent the degradation and loss of key flagship wetlands for amphibian conservation—driven by the combined pressures of drought and overextraction [39]—but would also benefit ecosystems throughout the middle and lower sections of entire river catchments.
At intermediate spatial scales, where efforts focus on specific areas or populations, mitigation strategies should address habitat loss resulting from prolonged drought, water overexploitation, or the combination of these factors with other synergistic pressures. This is typically achieved through the restoration, improvement, or creation of additional habitats that enhance the survival prospects of remaining local populations [276,277], or even by enabling translocations to newly-created suitable sites [278]. In the northwestern Mediterranean region, although not specifically aimed at drought mitigation, successful examples of man-made ponds have been reported, with rapid colonization by most amphibian species present in the area [279]. More importantly, future restoration projects should explicitly account for climate change and the increasing frequency of extreme events, as such events can compromise conservation outcomes if not anticipated [277]. In fact, a recent study by [269] found that, while amphibian conservation was the most commonly reported central aim among European pond restoration practitioners, climate change was perceived as the greatest current threat to the success of these efforts—ironically, a factor that could have been integrated into planning.
At fine spatial scales, microhabitat-focused interventions can also enhance amphibian community resilience to extreme droughts. This can be achieved by increasing the availability of humid refugia [184] such as logs, maintaining vegetated patches that remain green during dry periods, even in the absence of standing water [276], or by distributing coarse woody debris across the landscape to ensure a network of microhabitats [280].
As a final remark, while mid- and small-scale mitigation efforts may help avoid irreversible losses for amphibian populations in the Northwestern Mediterranean, it is important to emphasize that, most probably, only large-scale interventions addressing sustainable water management and climate change can truly reverse the ongoing decline in freshwater biodiversity in the region—including that of amphibians.
6. Conclusions
This review highlights that the effects of increasing drought frequency and intensity on amphibian populations in the Northwestern Mediterranean are heterogeneous across habitats, species, and life stages. While dry periods are predicted to increase in both frequency and intensity in the region [13,28], and most authors [24,26,47,53] identify this as one of the areas where amphibian species are most exposed to droughts, our understanding of the potential consequences of these changes remains incomplete. General physiological and ecological sensitivities to water scarcity are well documented, but detailed empirical evidence on species-and habitat-specific responses for taxa native to the Mediterranean Basin is still limited. In many cases, inferences must be drawn from research conducted in regions with comparable taxa or climatic conditions.
In lentic systems, hydroperiod shortening is confirmed as the principal driver of community change, with species requiring extended larval development or the presence of water during summer being most at risk. Plastic responses such as accelerated metamorphosis may buffer short-term impacts for most species but incur fitness costs that could compromise long-term population persistence. Although droughts can temporarily alleviate pressure from invasive fish, this advantage depends on subsequent recolonization dynamics of both amphibian and fish communities. Systematic, long-term monitoring of pond communities under extreme climatic events remains scarce.
In lotic environments, species adapted to permanent or cold-water streams (e.g., Calotriton sp., Euproctus sp.) show particularly low dispersal capacity and high vulnerability to flow interruption [161,162,164]. Habitat fragmentation and hydrological disconnection during prolonged droughts threaten these endemic taxa with local extinctions. Studies on temporary river systems suggest some resilience through microhabitat use, yet the influence of flow intermittence on amphibian populations—and on predator dynamics—within these systems remains far from fully understood [169,170,171].
Terrestrial habitats also present significant challenges, as reduced soil moisture and refuge availability heighten desiccation risk, restrict movement and, in some cases, directly increase mortality and limit recruitment. Riparian habitats stand out as essential drought refugia, yet their ecological condition is deteriorating. The interaction between drought and fire could accelerate shifts in community composition towards more xeric-adapted species, with forest-associated amphibians disproportionately affected [184,202,210].
Emerging diseases add a further layer of complexity that is still poorly explored. Regarding Bd, drought may reduce the persistence of free-living stages but also foster aggregation, increasing transmission risk, especially when facilitated by potential super-shedder species such as Discoglossus pictus [234]. For Bsal, information under Mediterranean climatic conditions is virtually absent, and its ability to survive dry periods through resistant spores remains unresolved [247]. Ranaviruses may expand in range and incidence under warming–drought scenarios, but the effects of hydroperiod and larval developmental stage on outbreak severity warrant further investigation. Overall, the combined influence of drought and disease remains to be further understood [258].
Finally, although mitigation strategies such as habitat restoration, artificial pond creation, and microhabitat enhancement show potential at a local scale, their documented implementation in the Mediterranean context is infrequent. Expanding efforts to the landscape scale, ensuring the sustainable use of water, incorporating climate projections into restoration planning, and safeguarding critical hydrological refugia, will be essential to ensure the long-term conservation of amphibians in this region.
According to [79,110], a multidisciplinary approach is essential to understanding the direct and indirect effects of climate change, and specifically of droughts, on amphibians.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/land14081668/s1, Figure S1: Susceptibility to drought for the different genera; Table S1: Susceptibility of emerging diseases.
Author Contributions
Conceptualization, A.M. and E.P.-B.; validation, A.M. and E.P.-B.; investigation, A.M. and E.P.-B.; resources, A.M. and E.P.-B.; writing—original draft preparation, A.M. and E.P.-B.; writing—review and editing, A.M. and E.P.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Bd | Batrachochytrium dendrobatidis |
| Bsal | Batrachochytrium salamandrivorans |
References
- Thomas, C.; Cameron, A.; Green, R.; Boulangeat, I.; Lafourcade, B.; Araujo, M.B. Extinction risk from climate change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Parmesan, C. Ecological and Evolutionary Responses to Recent Climate Change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Thuiller, W.; Lavergne, S.; Roquet, C.; Boulangeat, I.; Lafourcade, B.; Araujo, M.B. Consequences of climate change on the tree of life in Europe. Nature 2011, 470, 531–534. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2023: Synthesis Report. In Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023; 184p. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Scholes, R.J.; Agard, J.; Archer, E.; Arneth, A.; Bai, X.; Barnes, D.; Burrows, M.; Chan, L.; Cheung, W.L.; et al. IPBES-IPCC Co-Sponsored Workshop Report on Biodiversity and Climate Change; IPBES: Bonn, Germany; IPCC: Geneva, Switzerland, 2021. [Google Scholar] [CrossRef]
- Dai, A. Increasing drought under global warming in observations and models. Nat. Clim. Chang. 2013, 3, 52–58. [Google Scholar] [CrossRef]
- Chen, D.; Norris, J.; Thackeray, C.; Hall, A. Increasing precipitation whiplash in climate change hotspots. Environ. Res. Lett. 2022, 17, 124011. [Google Scholar] [CrossRef]
- Jézéquel, A.; Faranda, D.; Drobinski, P.; Lionello, P. Extreme Event Attribution in the Mediterranean. Int. J. Climatol. 2025, 45, e8799. [Google Scholar] [CrossRef]
- Swain, D.L.; Prein, A.F.; Abatzoglou, J.T.; Albano, C.M.; Brunner, M.; Diffenbaugh, N.S.; Singh, D.; Skinner, C.B.; Touma, D. Hydroclimate volatility on a warming Earth. Nat. Rev. Earth Environ. 2025, 6, 35–50. [Google Scholar] [CrossRef]
- Moss, W.E.; McDevitt-Galles, T.; Muths, E.; Bobzien, S.; Purificato, J.; Johnson, P. Resilience of native amphibian communities following catastrophic drought: Evidence from a decade of regional-scale monitoring. Biol. Conserv. 2021, 263, 109352. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, T.; Wu, P. Anthropogenic amplification of precipitation variability over the past century. Science 2024, 385, 427–432. [Google Scholar] [CrossRef]
- Lionello, P.; Scarascia, L. The Relation of Climate Extremes With Global Warming in the Mediterranean Region and Its North Versus South Contrast. Reg. Environ. Chang. 2020, 20, 31. [Google Scholar] [CrossRef]
- Tramblay, Y.; Koutroulis, A.; Samaniego, L.; Vicente-Serrano, S.M.; Volaire, F.; Boone, A.; Le Page, M.; Llasat, M.C.; Arbelgel, C.; Burak, S.; et al. Challenges for drought assessment in the Mediterranean region under future climate scenarios. Earth-Sci. Rev. 2020, 210, 103348. [Google Scholar] [CrossRef]
- Wen, L.; Saintilan, N.; Reid, J.R.; Colloff, M.J. Changes in distribution of waterbirds following prolonged drought reflect habitat availability in coastal and inland regions. Ecol. Evol. 2016, 6, 6672–6689. [Google Scholar] [CrossRef]
- Maron, M.; McAlpine, C.A.; Watson, J.E.; Maxwell, S.; Barnard, P. Climate-induced resource bottlenecks exacerbate species vulnerability: A review. Divers. Distrib. 2015, 21, 731–743. [Google Scholar] [CrossRef]
- Canarini, A.; Schmidt, H.; Fuchslueger, L.; Martin, V.; Herbold, C.V.; Zezula, D.; Gundler, P.; Hasibeder, R.; Jecmenica, M.; Bahn, M.; et al. Ecological memory of recurrent drought modifies soil processes via changes in soil microbial community. Nat. Commun. 2021, 12, 5308. [Google Scholar] [CrossRef] [PubMed]
- Steel, Z.L.; Jones, G.M.; Collins, B.M.; Green, R.; Koltunov, A.; Purcell, K.L.; Sawyer, S.C.; Slaton, M.R.; Stephens, S.L.; Stine, P.; et al. Mega-disturbances cause rapid decline of mature conifer forest habitat in California. Ecol. Appl. 2023, 33, e2763. [Google Scholar] [CrossRef] [PubMed]
- Everard, K.; Seabloom, E.W.; Harpole, W.S.; De Mazancourt, C. Plant water use affects competition for nitrogen: Why drought favors invasive species in California. Am. Nat. 2010, 175, 85–97. [Google Scholar] [CrossRef]
- Cavin, L.; Mountford, E.P.; Peterken, G.F.; Jump, A.S. Extreme drought alters competitive dominance within and between tree species in a mixed forest stand. Funct. Ecol. 2013, 27, 1424–1435. [Google Scholar] [CrossRef]
- Ledger, M.E.; Brown, L.E.; Edwards, F.K.; Milner, A.M.; Woodward, G. Drought alters the structure and functioning of complex food webs. Nat. Clim. Chang. 2013, 3, 223–227. [Google Scholar] [CrossRef]
- Prugh, L.R.; Deguines, N.; Grinath, J.B.; Suding, K.N.; Bean, W.T.; Stafford, R.; Brashare, J.S. Ecological winners and losers of extreme drought in California. Nat. Clim Chang. 2018, 8, 819–824. [Google Scholar] [CrossRef]
- Burke, E.J.; Brown, S.J.; Christidis, N. Modeling the recent evolution of global drought and projections for the twenty-first century with the Hadley Centre climate model. J. Hydrometeorol. 2006, 7, 1113–1125. [Google Scholar] [CrossRef]
- Van den Bosch, M.; Costanza, J.K.; Peek, R.A.; Mola, J.M.; Steel, Z.L. Climate change scenarios forecast increased drought exposure for terrestrial vertebrates in the contiguous United States. Commun. Earth Environ. 2024, 5, 708. [Google Scholar] [CrossRef]
- Iglesias, A.; Garrote, L.; Flores, F.; Moneo, M. Challenges to manage the risk of water scarcity and climate change in the Mediterranean. Water Resour. Manag. 2007, 21, 775–788. [Google Scholar] [CrossRef]
- García-Ruiz, J.M.; López-Moreno, J.I.; Vicente-Serrano, S.M.; Lasanta-Martínez, T.; Beguería, S. Mediterranean water resources in a global change scenario. Earth Sci. Rev. 2011, 105, 121–139. [Google Scholar] [CrossRef]
- Eekhout, J.P.C.; Nunes, J.P.; Tramblay, Y.; de Vente, J. Severe Impacts on Water Resources Projected for the Mediterranean Basin. WIREs Water 2025, 12, e70012. [Google Scholar] [CrossRef]
- Gimeno-Sotelo, L.; Sorí, R.; Nieto, R.; Vicente-Serrano, S.M.; Gimeno, L. Unravelling the origin of the atmospheric moisture deficit that leads to droughts. Nat. Water 2024, 2, 242–253. [Google Scholar] [CrossRef]
- Barrera-Escoda, A.; Gonçalves, M.; Guerreiro, D.; Cunillera, J.; Baldasano, J.M. Projections of temperature and precipitation extremes in the North Western Mediterranean Basin by dynamical downscaling of climate scenarios at high resolution (1971–2050). Clim. Chang. 2014, 122, 567–582. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Tramblay, Y.; Reig, F.; González-Hidalgo, J.C.; Beguería, S.; Brunetti, M.; Kalin, K.C.; Patalen, L.; Kržič, A.; Lionello, P.; et al. High temporal variability not trend dominates Mediterranean precipitation. Nature 2025, 639, 658–666. [Google Scholar] [CrossRef]
- Drobinski, P.; Azzopardi, B.; Ben Janet Allal, H.; Bouchet, V.; Civel, E.; Creti, A.; Duic, N.; Fylaktos, N.; Mutale, J.; Pariente-David, S.; et al. Energy Transition in the Mediterranean. In Climate and Environmental Change in the Mediterranean Basin—Current Situation and Risks for the Future. First Mediterranean Assessment Report; Cramer, W., Guiot, J., Marini, K., Eds.; Union for the Mediterranean, Plan Bleu, UNEP/MAP: Marseille, France, 2020; pp. 265–322. [Google Scholar] [CrossRef]
- Thivet, G.; Fernández, S. Water Demand Management: The Mediterranean Experience; Technical Focus Paper. Global Partnership Group; Plan Bleu: Marseille, France, 2020; 74p, Available online: https://planbleu.org/en/publications/water-demand-management-the-mediterranean-experience/ (accessed on 15 June 2025).
- Blinda, M.; Margat, J. Ressources et Demandes en eau en Région Méditerranéenne. Situations et Perspectives. In Proceedings of Congrès Mondial de L’eau; Plan Bleu: Marserille, France, 2008; 14p, Available online: https://iwra.org/proceedings/congress/resource/abs326_article.pdf (accessed on 5 August 2025).
- Toth, E.; Neri, M. Tourism Water Demand Modelling in Mediterranean Cities Under Current and Future Climate; EGU General Assembly: Vienna, Austria, 2024; p. EGU24-18575. [Google Scholar] [CrossRef]
- Howard, B. California Drought Spurs Groundwater Drilling Boom in Central Valley. National Geographic. 2014. Available online: https://www.nationalgeographic.com/culture/article/140815-central-valley-california-drilling-boom-groundwater-drought-wells (accessed on 16 August 2014).
- BAIC. Butlletí Anual d’Indicadors Climàtics (BAIC 2023). Servei Meteorològic de Catalunya 2023, Report. 127p. Available online: https://www.meteo.cat/wpweb/climatologia/butlletins-i-episodis-meteorologics/butlleti-anual-dindicadors-climatics/ (accessed on 15 June 2025).
- Blanqué, M.; de Cáceres, M.; García-Valdés, M.; Martínez-Vilalta, J.; Roces-Díaz, J.V.; Vayreda, J. FOREStime. Canvis dels Serveis Ecosistèmics dels Boscos de Catalunya al Llarg dels Darrers 25 Anys (Període 1990–2014). Oficina Catalana del Canvi Climàtic-CREAF-CTFC. Report. 35p. Available online: https://canviclimatic.gencat.cat/web/.content/02_OFICINA/publicacions/publicacions_de_canvi_climatic/Estudis_i_docs_adaptacio/FORESTIME.PDF (accessed on 4 June 2025).
- Luedtke, J.A.; Chanson, J.; Neam, K.; Hobin, L.; Maciel, A.O.; Catenazzi, A.; Borzee, A.; Hamidy, A.; Aowphol, A.; Jean, A.; et al. Ongoing declines for the world’s amphibians in the face of emerging threats. Nature 2023, 622, 308–314. [Google Scholar] [CrossRef]
- Richter-Boix, A.; Llorente, G.A.; Montori, A. Effects of phenotypic plasticity on post-metamorphic traits during pre-metamorphic stages in the anuran Pelodytes punctatus. Evol. Ecol. Res. 2006, 8, 309–320. Available online: https://www.evolutionary-ecology.com/issues/v08n02/iiar1861.pdf (accessed on 20 April 2025).
- Díaz-Paniagua, C.; Florencio, M.; de Felipe, M.; Ramírez-Soto, M.; Román, I.; Arribas, R. Groundwater decline has negatively affected the well-preserved amphibian community of Doñana National Park (SW Spain). Amphib.-Reptil. 2024, 45, 205–217. [Google Scholar] [CrossRef]
- Giacometti, D.; Tattersall, G.J. Putting the energetic-savings hypothesis underground: Fossoriality does not affect metabolic rates in amphibians. Evol. Ecol. 2023, 37, 761–777. [Google Scholar] [CrossRef]
- Hillman, S.S.; Withers, P.C.; Drews, R.C.; Hillyard, S.D. Ecological and Environmental Physiology of Amphibians; Oxford University Press: Oxford, UK, 2008. [Google Scholar] [CrossRef]
- Hillman, J.C.; Hillman, A.K.K. Mortality of wildlife in Nairobi National Park, during the drought of 1973–1974. Afr. J. Ecol. 1977, 15, 1–18. [Google Scholar] [CrossRef]
- McMenamin, S.K.; Hadly, E.A.; Wright, C.K. Climatic change and wetland desiccation cause amphibian decline in Yellowstone National Park. Proc. Natl. Acad. Sci. USA 2008, 105, 16988–16993. [Google Scholar] [CrossRef] [PubMed]
- Batllori, E.; Lloret, F.; Aakala, T.; Zeemann, B. Forest and woodland replacement patterns following drought-related mortality. Proc. Natl. Acad. Sci. USA 2020, 117, 29720–29729. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Gutiérrez, J.; Malhi, Y.; Lewis, S.L.; Fauset, S.; Adu-Bredu, S.; Affum-Baffoe, K.; Baker, T.R.; Gvozdevaite, A.; Hubau, W.; Moore, S.; et al. Long-term droughts may drive drier tropical forests towards increased functional, taxonomic and phylogenetic homogeneity. Nat. Commun. 2020, 11, 3346. [Google Scholar] [CrossRef]
- Walls, S.C.; Barichivich, W.J.; Brown, M.E. Drought, Deluge and Declines: The Impact of Precipitation Extremes on Amphibians in a Changing Climate. Biology 2013, 2, 399–418. [Google Scholar] [CrossRef]
- Twomey, E.; Sylvester, F.; Jourdan, J.; Hollert, H.; Schulte, L.M. Quantifying exposure of amphibian species to heat waves, cold spells, and droughts. Conserv. Biol. 2025, e70074. [Google Scholar] [CrossRef]
- Wu, N.C.; Bovo, R.P.; Enriquez-Urzelai, U.; Clusella-Trullàs, S.; Kearney, M.R.; Navas, C.A.; Kong, J.D. Global exposure risk of frogs to increasing environmental dryness. Nat. Clim. Chang. 2024, 14, 1314–1322. [Google Scholar] [CrossRef]
- Parmesan, C.; Root, T.L.; Willig, M.R. Impacts of extreme weather and climate on terrestrial biota. Bull. Am. Meteorol. Soc. 2000, 81, 443–450. [Google Scholar] [CrossRef]
- Fink, A.H.; Brücher, T.; Krüger, A.; Leckebusch, G.C.; Pinto, J.G.; Ulbrich, U. The 2003 European summer heatwaves and drought–synoptic diagnosis and impacts. Weather 2004, 59, 209–216. [Google Scholar] [CrossRef]
- Vautard, R.; Yiou, P.; D’Andrea, F.; de Noblet, N.; Viovy, N.; Cassou, C.; Polcher, J.; Ciais, P.; Kageyama, M.; Fan, Y. Summertime European heat and drought waves induced by wintertime Mediterranean rainfall deficit. Geophys. Res. Lett. 2007, 34, 1–5. [Google Scholar] [CrossRef]
- Pottier, P.; Kearney, M.R.; Wu, N.C.; Gunderson, A.R.; Rej, J.E.; Rivera-Villanueva, A.N.; Pollo, P.; Burke, S.; Drobniak, S.M.; Nakagawa, S. Vulnerability of amphibians to global warming. Nature 2025, 639, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Alexander, L.V. Global observed long-term changes in temperature and precipitation extremes: A review of progress and limitations in IPCC assessments and beyond. Weather. Clim. Extrem. 2016, 11, 4–16. [Google Scholar] [CrossRef]
- Beck, H.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Lutsko, N.J.; Dufour, A.; Zeng, Z.; Jiang, X.; Van Dijk, A.I.J.M.; Miralles, D.G. High-resolution (1 km) Köppen-Geiger maps for 1901–2099 based on constrained CMIP6 projections. Sci. Data 2023, 10, 724. [Google Scholar] [CrossRef]
- Peñuelas, J.; Boada, M. A global change-induced biome shift in the Montseny mountains (NE Spain). Glob. Change Biol. 2003, 9, 131–140. [Google Scholar] [CrossRef]
- Enriquez-Urzelai, U.; Bernardo, N.; Moreno-Rueda, G.; Montori, A.; Llorente, G.A. Are amphibians tracking their climatic niches in response to climate warming? A test with Iberian amphibians. Clim. Chang. 2019, 154, 289–301. [Google Scholar] [CrossRef]
- Díaz-Paniagua, C. Variability in timing of larval season in an amphibian community in SW Spain. Ecography 1992, 15, 267–272. Available online: https://www.jstor.org/stable/3683156 (accessed on 21 November 2024.). [CrossRef]
- Díaz-Paniagua, C.; Gómez-Rodríguez, C.; Portheault, A.; Florencio, M. Why a system of heterogeneous temporary ponds favours amphibian communities? Amphibians in Doñana National Park, an example of preserved breeding habitats. In International Conference On Mediterranean Temporary Ponds, Maó Menorca, Spain, 5–8 May 2009; Fraga, I., Arguimbau, P., Eds.; Consell Insular Menorca, Recerca: Barcelona, Spain, 2009; pp. 245–254. Available online: https://www.menorcabiosfera.org/documents/documents/2034doc2.pdf (accessed on 20 March 2025).
- Richter-Boix, A.; Llorente, G.A.; Montori, A. A comparative study of predator-induced phenotype in tadpoles across a pond permanency gradient. Hydrobiologia 2007, 583, 43–56. [Google Scholar] [CrossRef]
- Scheele, B.C.; Driscoll, D.A.; Fischer, J.; Hunter, D.A. Decline of an endangered amphibian during an extreme climatic event. Ecosphere 2012, 3, 101. [Google Scholar] [CrossRef]
- Cayuela, H.; Arsovski, D.; Bonnaire, E.; Duguet, R.; Joly, P.; Besnard, A. The impact of severe drought on survival, fecundity, and population persistence in an endangered amphibian. Ecosphere 2016, 7, e01246. [Google Scholar] [CrossRef]
- Berven, K.A. Factors affecting population fluctuations in larval and adult stages of the wood frog (Rana sylvatica). Ecology 1990, 71, 1599–1608. [Google Scholar] [CrossRef]
- Tejedo, M.; Marangoni, F.; Pertoldi, C.; Richter-Boix, A.; Laurila, A.; Orizaola, G.; Nicieza, A.G.; Alvarez, D.; Gomez-Mestre, I. Contrasting effects of environmental factors during larval stage on morphological plasticity in post-metamorphic frogs. Clim. Res. 2010, 43, 31–39. [Google Scholar] [CrossRef]
- Kohli, A.K.; Lindauer, A.L.; Brannelly, L.A.; Ohmer, M.E.B.; Richards-Zawacki, C.; Rollins-Smith, L.; Voyles, J. Disease and the Drying Pond: Examining Possible Links among Drought, Immune Function, and Disease. Development in Amphibians. Physiol. Biochem. Zool. 2019, 92, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Sinsch, U. Postmetamorphic dispersal and recruitment of first breeders in a Bufo calamita metapopulation. Oecologia 1997, 112, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Sinsch, U.; Oromi, N.; Miaud, C.; Denton, J.; Sanuy, D. Spatial ecology of natterjack toads. Anim. Conserv. 2012, 15, 388–396. [Google Scholar] [CrossRef]
- Martínez-Gil, H.; Sánchez-Montes, G.; Montes-Gavilán, P.; Ugarte, G.; Martínez-Solano, I. Fine-scale functional connectivity of two syntopic pond-breeding amphibians with contrasting life-history traits: An integrative assessment of direct and indirect estimates of dispersal. Conserv. Genet. 2023, 24, 361–374. [Google Scholar] [CrossRef]
- Capellà-Marzo, B.; Sánchez-Montes, G.; Martínez-Solano, I. Contrasting demographic trends and asymmetric migration rates in a spatially structured amphibian population. Integr. Zool. 2020, 15, 482–497. [Google Scholar] [CrossRef]
- Price, S.J.; Browne, R.A.; Dorcas, M.E. Resistance and Resilience of A Stream Salamander To Supraseasonal Drought. Herpetologica 2012, 68, 312–323. [Google Scholar] [CrossRef]
- Taylor, B.E.; Scott, D.E.; Gibbons, J.W. Catastrophic reproductive failure, terrestrial survival, and persistence of the marbled salamander. Conserv. Biol. 2006, 20, 792–801. [Google Scholar] [CrossRef]
- Montori, A.; Herrero, P. Caudata. In Amphibia-Lissamphibia; Ramos, M.A., Alba, J., Bellés, X., Gosálbez, J., Guera, Á., Macpherson, E., Serrano, J., Templado, J., Eds.; Museo Nacional de Ciencias Naturales; Fauna Ibérica; CSIC: Madrid, Spain, 2004; Volume 24, pp. 43–275. ISBN 84-00-08292-3. [Google Scholar]
- Grossenbacher, K.; Thiesmeier, B.; Böhme, W. (Eds.) Handbuch der Reptilien und Amphibien Europas. Band 4/I: Schwanzlurche (Urodela) I; Aula-Verlag: Wiesbaden, Germany, 1999; 409p, ISBN 9783891040058. [Google Scholar]
- Grossenbacher, K.; Thiesmeier, B.; Böhme, W. (Eds.) Handbuch der Reptilien und Amphibien Europas. Band 4/IIa Schwanzlurche (Urodela) II; Aula-Verlag: Wiesbaden, Germany, 2003; 368p, ISBN 9783891046739. [Google Scholar]
- Thiesmeier, B.; Grossenbacher, K. (Eds.) Handbuch der Reptilien und Amphibien Europas. Band 4/IIb Schwanzlurche (Urodela) III; Aula-Verlag: Wiesbaden, Germany, 2004; 391p, ISBN 9783891046746. [Google Scholar]
- Sánchez-Montes, G.; Wang, J.; Ariño, A.H.; Martínez-Solano, Í. Mountains as barriers to gene flow in amphibians: Quantifying the differential effect of a major mountain ridge on the genetic structure of four sympatric species with different life history traits. J. Biogeogr. 2018, 45, 318–331. [Google Scholar] [CrossRef]
- Cayuela, H.; Valenzuela-Sánchez, A.; Teulier, L.; Martínez-Solano, I.; Léna, J.-P.; Merilä, J.; Muths, E.; Shine, R.; Quay, L.; Denoël, M.; et al. Determinants and Consequences of Dispersal in Vertebrates with Complex Life Cycles: A Review of Pond-Breeding Amphibians. Q. Rev. Biol. 2020, 95, 1–36. [Google Scholar] [CrossRef]
- Taylor, P.D.; Fahrig, L.; Henein, K.; Merriam, G. Connectivity Is a Vital Element of Landscape Structure. Oikos 1993, 68, 571. [Google Scholar] [CrossRef]
- Vogt, P.; Ferrari, J.R.; Lookingbill, T.R.; Gardner, R.H.; Riitters, K.H.; Ostapowicz, K. Mapping functional connectivity. Ecol. Indic. 2009, 9, 64–71. [Google Scholar] [CrossRef]
- Blaustein, A.R.; Walls, S.C.; Bancroft, B.A.; Lawler, J.J.; Searle, C.L.; Gervasi, S.S. Direct and Indirect Effects of Climate Change on Amphibian Populations. Diversity 2010, 2, 281–313. [Google Scholar] [CrossRef]
- Beebee, T.J.C. Amphibian breeding and climate. Nature 1995, 74, 219–220. [Google Scholar] [CrossRef]
- Tryjanowski, P.; Mariusz, R.; Sparks, T. Changes in spawning dates of Common Frogs and Common Toads in western Poland in 1978–2002. Ann. Zool. Fenn. 2003, 40, 459–464. Available online: http://www.jstor.org/stable/23735858 (accessed on 21 November 2024).
- Scott, A.W.; Pithart, D.; Adamson, J.K. Long-term United Kingdom trends in the breeding phenology of the common frog, Rana temporaria. J. Herpetol. 2008, 42, 89–96. [Google Scholar] [CrossRef]
- Prodon, R.; Geniez, P.; Cheylan, M.; Devers, F.; Chuine, I.; Besnard, A. A reversal of the shift towards earlier spring phenology in several Mediterranean reptiles and amphibians during the 1998–2013 warming slowdown. Glob. Change Biol. 2017, 23, 5481–5491. [Google Scholar] [CrossRef]
- Montori, A.; Amat, F. Surviving on the edge: Present and future effects of climate warming on the common frog (Rana temporaria) population in the Montseny massif (NE Iberia). PeerJ 2023, 11, e14527. [Google Scholar] [CrossRef]
- Ficetola, G.F.; Maiorano, L. Contrasting effects of temperature and precipitation change on amphibian phenology, abundance and performance. Oecologia 2016, 181, 683–693. [Google Scholar] [CrossRef]
- Wellborn, G.A.; Skelly, D.K.; Werner, E.E. Mechanisms creating community structure across a freshwater habitat gradient. Ann. Rev. Ecol. Evol. Syst. 1996, 27, 337–363. [Google Scholar] [CrossRef]
- Babbitt, K.J.; Baber, M.J.; Tarr, T.L. Patterns of larval amphibian distribution along a wetland hydroperiod gradient. Can. J. Zool. 2003, 81, 1539–1552. [Google Scholar] [CrossRef]
- Van Buskirk, J. Habitat partitioning in European and North American pond-breeding frogs and toads. Div. Distrib. 2003, 9, 399–410. [Google Scholar] [CrossRef]
- Richter-Boix, A.; Llorente, G.A.; Montori, A. A comparative analysis of the adaptive developmental plasticity hypothesis in six Mediterranean anuran species along a pond permanency gradient. Evol. Ecol. Res. 2006, 8, 1139–1154. Available online: https://www.evolutionary-ecology.com/issues/v08n06/ppar1922.pdf (accessed on 21 November 2024).
- Richter-Boix, A.; Llorente, G.A.; Montori, A. Segregación espacial y temporal de una comunidad de anfibios en una región mediterránea. Munibe (Supl./Gehigarria) 2007, 25, 120–128. [Google Scholar]
- Jakob, C.; Poizat, G.; Veith, M.; Seitz, A.; Crivelli, A. Breeding phenology and larval distribution of amphibians in a Mediterranean pond network with unpredictable hydrology. Hydrobiologia 2003, 499, 51–61. [Google Scholar] [CrossRef]
- Blondel, J.; Aronson, J. Biology and Wildlife of the Mediterranean Region; Oxford University Press: Oxford, UK, 1999; 318p, ISBN 9780198500353. [Google Scholar]
- Directive 92/43/EEC-Conservation of Natural Habitats and of Wild Fauna and Flora-Habitats Directive (21.05.1992). Available online: https://eur-lex.europa.eu/eli/dir/1992/43/oj/eng (accessed on 16 August 2025).
- Resetarits, W.J., Jr.; Wilbur, H.M. Choice of oviposition site by Hyla chrysoscelis: Role of predators and competitors. Ecology 1989, 70, 220–228. [Google Scholar] [CrossRef]
- Morand, A.; Joly, P. Habitat variability and space utilization by the amphibian communities of the French Upper-Rhone floodplain. Hydrobiologia 1995, 300/301, 249–257. [Google Scholar] [CrossRef]
- Morand, A.; Joly, P.; Grolet, O. Phenotypic variation in metamorphosis in five anuran species along a gradient of stream influence. C.R. Acad. Sci. Paris 1997, 320, 645–652. [Google Scholar] [CrossRef]
- Cayuela, H.; Cheylan, M.; Joly, P. The best of a harsh lot in a specialized species: Breeding habitat use by the yellow-bellied toad (Bombina variegata) on rocky riverbanks. Amphib.-Reptil. 2011, 32, 533–539. [Google Scholar] [CrossRef]
- Pujol-Buxó, E.; Riaño, G.M.; Llorente, G.A. Mild segregation in the breeding preferences of an invasive anuran (Discoglossus pictus) and its main native competitor (Epidalea calamita) in ephemeral ponds. Amphib.-Reptil. 2019, 40, 425–435. [Google Scholar] [CrossRef]
- Relyea, R.A.; Hoverman, J.T. The impact of larval predators and competitors on the morphology and fitness of juvenile treefrogs. Oecologia 2003, 134, 596–604. [Google Scholar] [CrossRef]
- Gervasi, S.S.; Foufopoulos, J. Costs of plasticity: Responses to desiccation decrease post-metamorphic immune function in a pond-breeding amphibian. Funct. Ecol. 2008, 22, 100–108. [Google Scholar] [CrossRef]
- Richter-Boix, A.; Tejedo, M.; Rezende, E.L. Evolution and plasticity of anuran larval development in response to desiccation. A comparative analysis. Ecol. Evol. 2011, 1, 15–25. [Google Scholar] [CrossRef]
- Gómez-Rodríguez, C.; Díaz-Paniagua, C.; Bustamante, J.; Portheault, A.; Florencio, M. Inter-annual variability in amphibian assemblages: Implications for diversity assessment and conservation in temporary ponds. Aquat. Conserv. Mar. Freshw. Ecosyst. 2010, 20, 668–677. [Google Scholar] [CrossRef]
- Gómez-Rodríguez, C.; Díaz-Paniagua, C.; Bustamante, J.; Serrano, L.; Portheault, A. Relative importance of dynamic and static environmental variables as predictors of amphibian diversity patterns. Acta Oecol. 2010, 36, 650–658. [Google Scholar] [CrossRef]
- Noss, R.F.; Carroll, C.; Vance-Borland, K.; Wuerthner, G. A multicriteria assessment of the irreplaceability and vulnerability of sites in the Greater Yellowstone Ecosystem. Conserv Biol 2002, 16, 895. [Google Scholar] [CrossRef]
- Mac Nally, R.; Horrocks, G.F.B.; Lada, H. Anuran responses to pressures from high-amplitude drought–flood–drought sequences under climate change. Clim. Chang. 2017, 141, 243–257. [Google Scholar] [CrossRef]
- Pollard, C.J.; Stockwell, M.P.; Bower, D.S.; Garnham, J.I.; Pickett, E.J.; Darcovich, K.; O’meara, J.; Clulow, J.; Mahony, M.J. Removal of an exotic fish influences amphibian breeding site selection. J. Wildl. Manag. 2017, 81, 720–727. [Google Scholar] [CrossRef]
- Colomer, M.A.; Margalida, A.; Sanuy, I.; Llorente, G.A.; Sanuy, D.; Pujol-Buxó, E. A computational model approach to assess the effect of climate change on the growth and development of tadpoles. Ecol. Model. 2021, 461, 109763. [Google Scholar] [CrossRef]
- Pujol-Buxó, E.; García-Salmerón, A.; Valera-Florensa, J.; Loras-Ortí, F.; Baena-Crespo, O.; Maluquer-Margalef, J.; Mora-Rueda, C.; Martínez-Silvestre, A. Seguiment i Diagnosi de les Poblacions D’amfibis a Diferents Punts D’aigua de la Xarxa de Parcs Naturals de la Diputació de Barcelona; Unpublished Report; Diputació de Barcelona: Barcelona, Spain, 2024; 58p. [Google Scholar]
- Zylstra, E.R.; Swann, D.E.; Hossack, B.R.; Muths, E.; Steidl, R.J. Drought-mediated extinction of an arid-land amphibian: Insights from a spatially explicit dynamic occupancy model. Ecol. Appl. 2019, 29, e01859. [Google Scholar] [CrossRef] [PubMed]
- Blaustein, A.R.; Urbina, J.; Snyder, P.W.; Reynolds, E.; Dang, T.; Hoverman, J.T.; Han, B.; Olson, D.H.; Searle, C.; Hambalek, N.M. Effects of Emerging Infectious Diseases on Amphibians: A Review of Experimental Studies. Diversity 2018, 10, 81. [Google Scholar] [CrossRef]
- Székely, D.; Cogălniceanu, D.; Székely, P.; Armijos-Ojeda, D.; Espinosa-Mogrovejo, V.; Denoël, M. How to recover from a bad start: Size at metamorphosis affects growth and survival in a tropical amphibian. BMC Ecol. 2020, 20, 24. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.L.; Rowland, F.E.; Semlitsch, R.D. Variation in phenology and density differentially affects predator–prey interactions between salamanders. Oecologia 2017, 185, 475–486. [Google Scholar] [CrossRef]
- Jara, F.G.; Thurman, L.L.; Montiglio, P.O.; Sih, A.; Garcia, T.S. Warming-induced shifts in amphibian phenology and behavior lead to altered predator–prey dynamics. Oecologia 2019, 189, 803–813. [Google Scholar] [CrossRef]
- Díaz-Paniagua, C. Facteurs associés à la reproduction des amphibiens de Doñana. Détermination de l’habitat. Bull. De La Société Herpétologique De Fr. 1982, 22, 24–26. [Google Scholar]
- Lorrain-Soligon, L.; Robin, F.; Bertin, X.; Jankovic, M.; Rousseau, P.; Lelong, V.; Brischoux, F. Long-term trends of salinity in coastal wetlands: Effects of climate, extreme weather events, and sea water level. Environ. Res. 2023, 237, 116937. [Google Scholar] [CrossRef]
- Lorrain-Soligon, L.; Boudard, L.; Sebastiano, M.; Costantini, D.; Angelier, F.; Ribout, C.; Leclerc, M.; Kato, A.; Robin, F.; Brischoux, F. Salty surprises: Developmental and behavioral responses to environmental salinity reveal higher tolerance of inland rather than coastal Bufo spinosus tadpoles. Environ. Res. 2025, 264, 120401. [Google Scholar] [CrossRef]
- Herbert, E.R.; Boon, P.; Burgin, A.J.; Neubauer, S.C.; Franklin, R.B.; Ardón, M.; Hopfensperger, K.N.; Lamers, L.P.M.; Gell, P. A global perspective on wetland salinization: Ecological consequences of agrowing threat to freshwater wetlands. Ecosphere 2015, 6, art206–art243. [Google Scholar] [CrossRef]
- Relyea, R.; Mattes, B.; Schermerhorn, C.; Shepard, I. Freshwater salinization and the evolved tolerance of amphibians. Ecol. Evol. 2024, 14, e11069. [Google Scholar] [CrossRef]
- Lorrain-Soligon, L.; Brischoux, F.; Pétillon, J. The interactive effects of salt and heat on coastal ectotherms. Trends Ecol. Evol. 2024, 39, 1076–1079. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Mestre, I.; Tejedo, M.; Ramayo, E.; Estepa, J. Developmental alterations and osmoregulatory physiology of a larval anuran under osmotic stress. Physiol. Biochem. Zool. 2004, 77, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Hart, B.T.; Lake, P.S.; Webb, J.A.; Grace, M.R. Ecological risk to aquatic systems from salinity increases. Aust. J. Bot. 2003, 51, 689–702. [Google Scholar] [CrossRef]
- Walker, R.H.; Belvin, A.C.; Mouser, J.B.; Pennino, A.; Plont, S.; Robinson, C.D.; Smith, L.B.; Thapa, J.; Zipper, C.E.; Angermeier, P.L.; et al. Global review reveals how disparate study motivations, analytical designs, and focal ions limit understanding of salinization effects on freshwater animals. Sci. Total Environ. 2023, 892, 164061. [Google Scholar] [CrossRef]
- Burraco, P.; Gomez-Mestre, I. Physiological stress responses in amphibian larvae to multiple stressors reveal marked anthropogenic effects even below lethal levels. Physiol. Biochem. Zool. 2016, 89, 462–472. [Google Scholar] [CrossRef]
- Tornabene, B.J.; Hossack, B.R.; Crespi, E.J.; Breuner, C.W. Corticosterone mediates a growth-survival tradeoff for an amphibian exposed to increased salinity. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2021, 335, 703–715. [Google Scholar] [CrossRef]
- Tornabene, B.J.; Crespi, E.J.; Breuner, C.W.; Hossack, B.R. Testing whether adrenal steroids mediate phenotypic and physiologic effects of elevated salinity on larval tiger salamanders. Integr. Zool. 2022, 18, 27–44. [Google Scholar] [CrossRef]
- Gomez-Mestre, I.; Tejedo, M. Local adaptation of an anuran amphibian to osmotically stressful environments. Evolution 2003, 57, 1889–1899. [Google Scholar] [CrossRef]
- Gomez-Mestre, I.; Tejedo, M. Adaptation or exaptation? An experimental test of hypotheses on the origin of salinity tolerance in Bufo calamita. J. Evol. Biol. 2005, 18, 847–855. [Google Scholar] [CrossRef]
- Wu, C.-S.; Kam, Y.C. Effects of salinity on the survival, growth, development, and metamorphosis of Fejervarya limnocharis tadpoles living in brackish water. Zool. Sci. 2009, 26, 476–482. [Google Scholar] [CrossRef]
- Bernabò, I.; Bonacci, A.; Coscarelli, F.; Tripepi, M.; Brunelli, E. Effects of salinity stress on Bufo balearicus and Bufo bufo tadpoles: Tolerance, morphological gill alterations and Na+/K+-ATPase localization. Aquat. Toxicol. 2013, 132–133C, 119–133. [Google Scholar] [CrossRef]
- Wood, L.; Welch, A.M. Assessment of interactive effects of elevated salinity and three pesticides on life history and behavior of southern toad (Anaxyrus terrestris) tadpoles. Environ. Toxicol. Chem. 2015, 34, 667–676. [Google Scholar] [CrossRef]
- Lukens, E.; Wilcoxen, T.E. Effects of elevated salinity on Cuban treefrog Osteopilus septontrionalis aldosterone levels, growth, and development. Mar. Freshw. Behav. Physiol. 2020, 53, 99–111. [Google Scholar] [CrossRef]
- Tornabene, B.J.; Breuner, C.W.; Hossack, B.R. Comparative effects of energy-related saline wastewaters and sodium chloride on hatching, survival, and fitness-associated traits of two amphibian species. Environ. Toxicol. Chem. 2021, 40, 3137–3147. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.M.; Brady, S.P.; Mattheus, N.M.; Earley, R.L.; Diamond, M.; Crespi, E.J. Physiological consequences of exposure to salinized roadside ponds on wood frog larvae and adults. Biol. Conserv. 2017, 209, 98–106. [Google Scholar] [CrossRef]
- Haramura, T. Hatching plasticity in response to salinity levels in a rhacophorid frog inhabiting a coastal area. J. Zool. 2016, 299, 125–131. [Google Scholar] [CrossRef]
- Denoël, M.; Bichot, M.; Ficetola, G.F.; Delcourt, J.; Ylieff, M.; Kestemont, P.; Poncin, P. Cumulative effects of road de-icing salt on amphibian behavior. Aquat. Toxicol. 2010, 99, 275–280. [Google Scholar] [CrossRef]
- Beebee, T.J.C. Salt tolerance of natterjack toad (Bufo calamita) eggs and larvae from coastal and inland populations in Britain. J. Herpetol. 1985, 1, 14–16. [Google Scholar]
- Ortiz-Santaliestra, M.E.; Fernández-Benéitez, M.J.; Lizana, M.; Marco, A. Adaptation to osmotic stress provides protection against ammonium nitrate in Pelophylax perezi embryos. Environ. Pollut. 2010, 158, 934–940. [Google Scholar] [CrossRef]
- Thirion, J.M. Salinity of the reproductive habitats of the Western Spadefoot Toad Pelobates cultripes (Cuvier, 1829), along the Atlantic coast of France (Anura: Pelobatidae). Herpetozoa 2014, 27, 13–20. [Google Scholar]
- Galán, P.; Rodríguez-Fernández, S. Efecto de los temporales atlánticos invernales sobre la población de Discoglossus galganoi de los acantilados costeros de Galicia. Boletín Asoc. Herpetol. Española 2018, 29, 70–75. [Google Scholar]
- Belmar, O.; Velasco, J.; Martinez-Capel, F. Hydrological Classification of Natural Flow Regimes to Support Environmental Flow Assessments in Intensively Regulated Mediterranean Rivers, Segura River Basin (Spain). Environ. Manag. 2011, 47, 992–1004. [Google Scholar] [CrossRef]
- Bonada, N.; Cañedo-Argüelles, M.; Gallart, F.; von Schiller, D.; Fortuño, P.; Latron, J.; Llorens, P.; Múrria, C.; Soria, M.; Vinyoles, D.; et al. Conservation and management of isolated pools in temporary rivers. Water 2020, 12, 2870. [Google Scholar] [CrossRef]
- Magand, C.; Alves, M.H.; Calleja, E.; Datry, T.; Dörflinger, G.; England, J.; Gallart, F.; Gómez, R.; Jorda-Capdevila, D.; Marti, E.; et al. Intermittent Rivers and Ephemeral Streams: What Water Managers Need to Know; Technical report–Cost ACTION CA 15113; European Cooperation in Science and Technology: Brussels, Belgium, 2020. [Google Scholar] [CrossRef]
- Datry, T.; Larned, S.T.; Tockner, K. Intermittent Rivers: A Challenge for Freshwater Ecology. Bioscience 2014, 64, 229–235. [Google Scholar] [CrossRef]
- Leigh, C.; Boulton, A.J.; Courtwright, J.L.; Fritz, K.; May, C.L.; Walker, R.H.; Datry, T. Ecological research and management of intermittent rivers: An historical review and future directions. Freshw. Biol. 2016, 61, 1181–1199. [Google Scholar] [CrossRef]
- Munné, A.; Bonada, N.; Cid, N.; Gallart, F.; Solà, C.; Bardina, M.; Rovira, A.; Sierra, C.; Soria, M.; Fortuño, P.; et al. A Proposal to Classify and Assess Ecological Status in Mediterranean Temporary Rivers: Research Insights to Solve Management Needs. Water 2021, 13, 767. [Google Scholar] [CrossRef]
- Soria, M.; Leigh, C.; Datry, T.; Bini, L.M.; Bonada, N. Biodiversity in perennial and intermittent rivers: A meta-analysis. Oikos 2017, 126, 1078–1089. [Google Scholar] [CrossRef]
- Acuña, V.; Hunter, M.; Ruhí, A. Managing temporary streams and rivers as unique rather than second-class ecosystems. Biol. Conserv. 2017, 211, 12–19. [Google Scholar] [CrossRef]
- Bernardo, J.M.; Ilhéu, M.; Matono, P.; Costa, A.M. Interannual variation of fish assemblage structure in a Mediterranean river: Implications of streamflow on the dominance of native or exotic species. River Res. Appl. 2003, 19, 521–532. [Google Scholar] [CrossRef]
- Muñoz, I.; García-Berthou, E.; Sabater, S. The Effect of Multiple Stressors on Biological Communities in the Llobregat. In The Llobregat. The Handbook of Environmental Chemistry; Sabater, S., Ginebreda, A., Barceló, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 21. [Google Scholar] [CrossRef]
- Matono, P.; Da Silva, J.; Ilhéu, M. How Does an Invasive Cyprinid Benefit from the Hydrological Disturbance of Mediterranean Temporary Streams? Diversity 2018, 10, 47. [Google Scholar] [CrossRef]
- Guareschi, S.; South, J. Biological invasions in intermittent rivers and streams: Current knowledge, and future frontiers. Ecosistemas 2024, 33, 2600. [Google Scholar] [CrossRef]
- King, A.J.; Townsend, S.A.; Douglas, M.M.; Kennard, M.J. Implications of water extraction on the low-flow hydrology and ecology of tropical savannah rivers: An appraisal for northern Australia. Freshw. Sci. 2015, 34, 741–758. [Google Scholar] [CrossRef]
- Stefanidis, K.; Panagopoulos, Y.; Psomas, A.; Mimikou, M. Assessment of the natural flow regime in a Mediterranean river impacted from irrigated agriculture. Sci. Total Environ. 2016, 573, 1492–1502. [Google Scholar] [CrossRef]
- Skoulikidis, N.T.; Vardakas, L.; Karaouzas, I.; Economou, A.N.; Dimitrou, E.; Zogaris, S. Assessing water stress in Mediterranean lotic systems: Insights from an artificially intermittent river in Greece. Aquat. Sci. 2011, 73, 581–597. [Google Scholar] [CrossRef]
- Sabo, J.L. Predicting the river’s blue line for fish conservation. Proc. Natl. Acad. Sci. USA 2014, 111, 13686–13687. [Google Scholar] [CrossRef] [PubMed]
- Ruhí, A.; Olden, J.D.; Sabo, J.L. Declining streamflow induces collapse and replacement of native fish in the American Southwest. Front. Ecol. Environ. 2016, 14, 465–472. [Google Scholar] [CrossRef]
- Jaeger, K.L.; Olden, J.D.; Pelland, N.A. Climate change poised to threaten hydrologic connectivity and endemic fishes in dryland streams. Proc. Natl. Acad. Sci. USA 2014, 111, 13894–13899. [Google Scholar] [CrossRef]
- Lytle, D.A.; Poff, N.L. Adaptation to natural flow regimes. Trends Ecol. Evol. 2004, 19, 94–100. [Google Scholar] [CrossRef]
- Chessman, B.C. Relationships between lotic macroinvertebrate traits and responses to extreme drought. Freshw. Biol. 2015, 60, 50–63. [Google Scholar] [CrossRef]
- Serra-Cobo, J.; Marques, T.; Martínez-Rica, J.P. Ecological segregation between Rana pyrenaica and Rana temporaria, and differential predation of Euproctus asper on their tadpoles. Netherland J. Zool. 2000, 50, 65–73. [Google Scholar] [CrossRef]
- Colomer, M.À.; Montori, A.; García, E.; Fondevilla, C. Using a bioinspired model to determine the extinction risk of Calotriton asper populations as a result of an increase in extreme rainfall in a scenario of climatic change. Ecol. Model. 2014, 281, 1–14. [Google Scholar] [CrossRef]
- Guinart, D.; Solórzano, S.; Amat, F.; Grau, J.; Fernández-Guiberteau, D.; Montori, A. Habitat Management of the Endemic and Critical Endangered Montseny Brook Newt (Calotriton arnoldi). Land 2022, 11, 449. [Google Scholar] [CrossRef]
- Ball, S.E.; Bovero, S.; Sotgiu, G.; Tessa, G.; Angelini, C.; Bielby, J.; Durrant, C.; Favelli, M.; Gazzaniga, E.; Garner, T.W.J. Islands within an island: Population genetic structure of the endemic Sardinian newt, Euproctus platycephalus. Ecol. Evol. 2017, 7, 1190–1211. [Google Scholar] [CrossRef] [PubMed]
- Montori, A.; Llorente, G.A.; Richter-Boix, À. Habitat features affecting the small-scale distribution and longitudinal migration patterns of Calotriton asper in a Pre-Pyrenean population. Amphib.-Reptil. 2008, 29, 371–381. [Google Scholar] [CrossRef]
- Ribera, I.; Vogler, A. Habitat type as a determinant of species range sizes: The example of lotic-lentic differences in aquatic Coleoptera. Biol. J. Linn. Soc. 2000, 71, 33–52. [Google Scholar] [CrossRef]
- Ribera, I.; Foster, G.N.; Vogler, A.P. Does habitat use explain large scale species richness patterns of aquatic beetles in Europe? Ecography 2003, 26, 145–152. Available online: https://www.jstor.org/stable/3683429 (accessed on 21 November 2024). [CrossRef]
- Abellán, P.; Ribera, I. Geographic location and phylogeny are the main determinants of the size of the geographical range in aquatic beetles. BMC Evol. Biol. 2011, 11, 344. Available online: http://www.biomedcentral.com/1471-2148/11/344 (accessed on 5 August 2025). [CrossRef]
- Talavera, A.; Palmada-Flores, M.; Burriel-Carranza, B.; Valbuena-Ureña, E.; Mochales-Riaño, G.; Adams, D.C.; Tejero-Cicuentes, H.; Soler-Membrives, A.; Amat, F.; Guinart, D.; et al. Genomic insights into the montseny brook newt (Calotriton arnoldi), a critically endangered glacial relict. iScience 2024, 27, 108665. [Google Scholar] [CrossRef]
- Puig-Gironès, R.; Bel, G.; Cid, N.; Cañedo-Argüelles, M.; Fernández-Calero, J.M.; Quevedo-Ortiz, G.; Fortuño, P.; Vinyoles, D.; Real, J.; Pujol-Buxó, E.; et al. Water availability and biological interactions shape amphibian abundance and diversity in Mediterranean temporary rivers. Sci. Total Environ. 2024, 953, 175917. [Google Scholar] [CrossRef]
- Kerezsy, A.; Gido, K.; Magalhães, M.F.; Skelton, P.H. The Biota of Intermittent Rivers and Ephemeral Streams: Fishes. In Intermittent Rivers and Ephemeral Streams; Academic Press: Cambridge, MA, USA, 2017; pp. 273–298. [Google Scholar] [CrossRef]
- Huxter, E.H.H.; Van Meerveld, H.J. Intermittent and perennial streamflow regime characteristics in the Okanagan. Can. Water Resour. J. 2012, 37, 391–414. [Google Scholar] [CrossRef]
- Fraser, N.; Schumer, R. Intermittency in dust deposition rates around the world. In Proceedings of the AGU Fall Meeting, San Francisco, CA, USA, 5–9 December 2011; Available online: https://ui.adsabs.harvard.edu/abs/2011AGUFMPP23B1850F (accessed on 5 August 2025).
- Camarasa-Belmonte, A.M. Flash-flooding of Ephemeral Streams in the Context of Climate Change. CIG 2021, 47, 121–142. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, Z.; Zhang, Z.; Wu, X.; Lai, C.; Zeng, Z.; Chen, X. Extreme drought-heatwave exacerbates water quality deterioration in China. Ecol. Indic. 2025, 170, 113008. [Google Scholar] [CrossRef]
- Corominas, L.; Foley, J.; Guest, J.S.; Hospido, A.; Larsen, H.F.; Morera, S.; Shaw, A. Life cycle assessment applied to wastewater treatment: State of the art. Water Res. 2013, 47, 5480–5492. [Google Scholar] [CrossRef] [PubMed]
- Wagner, N.; Reichenbecher, W.; Teichmann, H.; Tappeser, B.; Lötters, S. Questions concerning the potential impact of glyphosate-based herbicides on amphibians. Environ. Toxicol. Chem. 2013, 32, 1688–1700. [Google Scholar] [CrossRef] [PubMed]
- Chow, R.; Curchod, L.; Davies, E.; Veludo, A.F.; Oltramare, C.; Dalvie, M.A.; Stamm, C.; Röösli, M.; Fuhrimann, S. Seasonal drivers and risks of aquatic pesticide pollution in drought and post-drought conditions in three Mediterranean watersheds. Sci. Total Environ. 2023, 858, 159784. [Google Scholar] [CrossRef]
- Curchod, L.; Oltramare, C.; Junghan, M.; Stamm, C.; Dalvie, M.A.; Röösli, M.; Fuhrimann, S. Temporal variation of pesticide mixtures in rivers of three agricultural watersheds during a major drought in the Western Cape, South Africa. Water Res. X 2020, 6, 100039. [Google Scholar] [CrossRef]
- Church, D.R.; Bailey, L.L.; Wilbur, H.M.; Kendall, W.L.; Hines, J.E. Iteroparity in the variable environment of the salamander Ambystoma tigrinum. Ecology 2007, 88, 891–903. [Google Scholar] [CrossRef]
- Carnicer, J.; Coll, M.; Ninyerola, M.; Pons, X.; Sanchez, X.; Peñuelas, J. Widespread crown condition decline, food web disruption, and amplified tree mortality with increased climate change-type drought. Proc. Natl. Acad. Sci. USA 2011, 108, 1474–1478. [Google Scholar] [CrossRef]
- Carnicer, J.; Vives-Ingla, M.; Blanquer, L.; Méndez-Camps, X.; Rosell, C.; Sabaté, S.; Gutiérrez, E.; Sauras, T.; Peñuelas, J.; Barbeta, A. Forest resilience to global warming is strongly modulated by local-scale topographic, microclimatic and biotic conditions. J. Ecol. 2021, 109, 3322–3339. [Google Scholar] [CrossRef]
- Reading, C.J. Linking global warming to amphibian declines through its effects on female body condition and survivorship. Oecologia 2007, 151, 125–131. [Google Scholar] [CrossRef]
- Daszak, P.; Scott, D.E.; Kilpatrick, A.M.; Faggioni, C.; Gibbons, J.W.; Porter, D. Amphibian population declines at savannah river site are linked to climate, not chytridiomycosis. Ecology 2005, 86, 3232–3237. [Google Scholar] [CrossRef]
- Hossack, B.R.; Lowe, W.H.; Corn, P.S. Rapid increases and time-lagged declines in amphibian occupancy after wildfire. Conserv. Biol. 2013, 27, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, F.; Lasserre, B.; Chirici, G.; Tognetti, R.; Marchetti, M. Deadwood occurrence and forest structure as indicators of old-growth forest conditions in Mediterranean mountainous ecosystems. Ecoscience 2012, 19, 344–355. [Google Scholar] [CrossRef]
- Badalamenti, E.; La Mantia, T.; La Mantia, G.; Cairone, A.; La Mela Veca, D.S. Living and dead aboveground biomass in Mediterranean forests: Evidence of old-growth traits in a Quercus pubescens Willd. sl stand. Forests 2017, 8, 187. [Google Scholar] [CrossRef]
- Parisi, F.; Pioli, S.; Lombardi, F.; Fravolini, G.; Marchetti, M.; Tognetti, R. Linking deadwood traits with saproxylic invertebrates and fungi in European forests-a review. iForest-Biogeosci. For. 2018, 11, 423. [Google Scholar] [CrossRef]
- Batllori, E.; De Cáceres, M.; Brotons, L.; Ackerly, D.D.; Moritz, M.A.; Lloret, F. Cumulative effects of fire and drought in Mediterranean ecosystems. Ecosphere 2017, 8, e01906. [Google Scholar] [CrossRef]
- Karavani, A.; Boer, M.M.; Baudena, M.; Colinas, C.; Díaz-Sierra, R.; Pemán, J.; de Luis, M.; Enríquez-de-Salamanca, Á.; Resco de Dios, V. Fire-induced deforestation in drought-prone Mediterranean forests: Drivers and unknowns from leaves to communities. Ecol. Monogr. 2018, 88, 141–169. [Google Scholar] [CrossRef]
- Ogaya, R.; Peñuelas, J. Climate change effects in a Mediterranean forest following 21 consecutive years of experimental drought. Forests 2021, 12, 306. [Google Scholar] [CrossRef]
- Naiman, R.J.; Bechtold, J.S.; Drake, D.C.; Latterell, J.J.; O’Keefe, T.C.; Balian, E.V. Origins, Patterns, and Importance of Heterogeneity in Riparian Systems. In Ecosystem Function in Heterogeneous Landscapes; Lovett, G.M., Turner, M.G., Jones, C.G., Weathers, K.C., Eds.; Springer: New York, NY, USA, 2005. [Google Scholar] [CrossRef]
- Naiman, R.J.; Decamps, H. The ecology of interfaces: Riparian zones. Annu. Rev. Ecol. Syst. 1997, 28, 621–658. [Google Scholar] [CrossRef]
- Burbrink, F.T.; Phillips, C.A.; Heske, E.J. A riparian zone in Southern Illinois as a potential dispersal corridor for reptiles and amphibians. Biol. Conserv. 1998, 86, 107–115. [Google Scholar] [CrossRef]
- Aguilar, F.F.; Velo-Antón, G.; Tarroso, P.; Segurado, P. Fine-scale habitat preferences of riparian ectotherms in a human-influenced landscape: Insights from two herptiles endemic to the Iberian Peninsula. Biodivers. Conserv. 2025, 34, 2227–2245. [Google Scholar] [CrossRef]
- Pausas, J.G.; Fernández-Muñoz, S. Fire regime changes in the Western Mediterranean Basin: From fuel-limited to drought-driven fire regime. Clim. Chang. 2012, 110, 215–226. [Google Scholar] [CrossRef]
- Wasserman, T.N.; Mueller, S.E. Climate influences on future fire severity: A synthesis of climate-fire interactions and impacts on fire regimes, high-severity fire, and forests in the western United States. Fire Ecol. 2023, 19, 43. [Google Scholar] [CrossRef]
- Pausas, J.G. Changes in Fire and Climate in the Eastern Iberian Peninsula (Mediterranean Basin). Clim. Chang. 2004, 63, 337–350. [Google Scholar] [CrossRef]
- Dunham, J.B.; Rosenberger, A.E.; Luce, C.H.; Rieman, B.E. Influences of wildfire and channel reorganization on spatial and temporal variation in stream temperature and the distribution of fish and amphibians. Ecosystems 2007, 10, 335–346. [Google Scholar] [CrossRef]
- Dos Anjos, A.G.; Solé, M.; Benchimol, M. Fire effects on anurans: What we know so far? For. Ecol. Manag. 2021, 495, 119338. [Google Scholar] [CrossRef]
- Montori, A.; Llorente, G.A.; Clivillé, S.; Santos, X.; Carretero, M.A. Efectes de l’incendi forestal de 1994 sobre les poblacions d’amfibis del Parc Natural del Garraf. Monogr. Diput. De Barc. 2000, 30, 105–108. Available online: https://parcs.diba.cat/documents/182160/f3fbe2c5-c98f-4498-b808-abd350256b69 (accessed on 21 December 2024).
- Montori, A. Estat de les Poblacions D’amfibis del Parc del Garraf 16 Anys Després de L’incendi Forestal de 1994 i Efectes dels Focs Repetitius Sobre les Comunitats D’amfibis; Unpublished Report; ACOM 2010; Department D’innovació, Universitats i Empreses, Generalitat de Catalunya: Catalona, Spain, 2010; 55p. [Google Scholar]
- Chergui, B.; Ayres, C.; Santos, X. Assessing the response of amphibians to wildfire according to forest type and bioregion affinity of species. Basic Appl. Herpetol. 2022, 36, 5–17. [Google Scholar] [CrossRef]
- Carretero, J.M.; Guzmán, E.; Martínez, M.; Úbeda, X. Els Incendis Forestals a L’àrea del Garraf-Castelldefels; II premi “Castelldefels ambit sostenible”; Diputacio Barcelona: Barcelona, Spain, 2003; 148p. [Google Scholar]
- Moretti, M.; Duelli, P.; Obrist, M.K. Biodiversity and resilience of arthropod communities after fire disturbance in temperate forests. Oecologia 2006, 149, 312–327. [Google Scholar] [CrossRef]
- Santos, X.; Bros, V.; Miño, A. Recolonization of a burned Mediterranean area by terrestrial gastropods. Biodivers. Conserv. 2009, 18, 3153–3165. [Google Scholar] [CrossRef]
- Moretti, M.; Legg, C. Combining plant and animal traits to assess community functional responses to disturbance. Ecography 2008, 31, 299–309. [Google Scholar] [CrossRef]
- Burrow, A.; Maerz, J. How plants affect amphibian populations. Biol. Rev. 2022, 97, 1749–1767. [Google Scholar] [CrossRef] [PubMed]
- Haggerty, C.J.; Crisman, T.L.; Rohr, J.R. Effects of forestry-driven changes to groundcover and soil moisture on amphibian desiccation, dispersal, and survival. Ecol. Appl. 2019, 29, e01870. [Google Scholar] [CrossRef] [PubMed]
- Haggerty, C.J.; Crisman, T.L.; Rohr, J.R. Direct and indirect effects of pine silviculture on the larval occupancy and breeding of declining amphibian species. J. Appl. Ecol. 2019, 56, 2652–2662. [Google Scholar] [CrossRef]
- Muñoz, A.; Felicisimo, A.M.; Santos, X. Assessing the resistance of a breeding amphibian community to a large wildfire. Acta Oecologica 2019, 99, 103439. [Google Scholar] [CrossRef]
- Martínez-Silvestre, A.; Garcia-Salmeron, A.; Pujol-Buxó, E.; Baena, O. Anàlisi de patògens emergents dels amfibis dins de 7 parcs de la Xarxa de Parcs Naturals de la Diputació de Barcelona. Butll. Soc. Catalana D’herpetologia 2022, 29, 44–55. [Google Scholar]
- Longcore, J.E.; Pessier, A.P.; Nichols, D.K. Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia 1999, 91, 219–227. [Google Scholar] [CrossRef]
- Martel, A.; Spitzen-van der Sluijs, A.; Blooi, M.; Bert, W.; Ducatelle, R.; Fisher, M.C.; Woeltjes, A.; Bosman, W.; Chiers, K.; Bossuyt, F.; et al. Batrachochytrium salamandrivorans sp. nov. causes lethal chytridiomycosis in amphibians. Proc. Natl. Acad. Sci. USA 2013, 110, 15325–15329. [Google Scholar] [CrossRef]
- Granoff, A.; Came, P.E.; Breeze, D.C. Viruses and renal carcinoma of Rana pipiens: I. The isolation and properties of virus from normal and tumor tissues. Virology 1966, 29, 133–148. [Google Scholar] [CrossRef]
- Bosch, J.; Martínez-Solano, I.; García-París, M. Evidence of a chytrid fungus infection involved in the decline of the common midwife toad (Alytes obstetricans) in protected areas of central Spain. Biol. Conserv. 2001, 97, 331–337. [Google Scholar] [CrossRef]
- Walker, S.F.; Bosch, J.; James, T.Y.; Litvintseva, A.P.; Valls, J.A.O.; Piña, S.; García, G.; Rosa, G.A.; Cunningham, A.A.; Hole, S.; et al. Invasive pathogens threaten species recovery programs. Curr. Biol. 2008, 18, R853–R854. [Google Scholar] [CrossRef] [PubMed]
- Martel, A.; Vila-Escale, M.; Fernández-Giberteau, D.; Martinez-Silvestre, A.; Canessa, S.; Van Praet, S.; Pannon, P.; Chiers, K.; Ferran, A.; Kelly, M.; et al. Integral chain management of wildlife diseases. Conserv. Lett. 2020, 13, e12707. [Google Scholar] [CrossRef]
- Price, S.J.; Garner, T.W.J.; Nichols, R.A.; Balloux, F.; Ayres, C.; Mora-Cabello de Alba, A.; Bosch, J. Collapse of amphibian communities due to an introduced Ranavirus. Curr. Biol. 2014, 24, 2586–2591. [Google Scholar] [CrossRef] [PubMed]
- Scheele, B.C.; Hunter, D.A.; Banks, S.C.; Pierson, J.C.; Skerratt, L.F.; Webb, R.; Driscoll, D.A. High adult mortality in disease-challenged frog populations increases vulnerability to drought. J. Anim. Ecol. 2016, 85, 1453–1460. [Google Scholar] [CrossRef]
- Buttimer, S.; Medina, D.; Martins, R.A.; da Silva, A.G.M.; Neely, W.J.; Haddad, C.F.B.; DiRenzo, G.V.; Catenazzi, A.; Bell, R.C.; Becker, C.G. Experimental Drought Suppresses Amphibian Pathogen Yet Intensifies Transmission and Disrupts Protective Skin Microbiome. Glob. Change Biol. 2025, 31, e70275. [Google Scholar] [CrossRef]
- Johnson, M.L.; Berger, L.; Philips, L.; Speare, R. Fungicidal effects Batrachochytrium dendrobatidis, UV light, desiccation and heat on the amphibian chytrid Batrachochytrium dendrobatidis. Dis. Aquat. Org. 2003, 57, 255–260. [Google Scholar] [CrossRef]
- Piotrowski, J.S.; Annis, S.L.; Longcore, J.E. Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians. Mycologia 2004, 96, 9–15. [Google Scholar] [CrossRef]
- Kilpatrick, A.M.; Briggs, C.J.; Daszak, P. The ecology and impact of chytridiomycosis: An emerging disease of amphibians. Trends Ecol. Evol. 2010, 25, 109–118. [Google Scholar] [CrossRef]
- Van Rooij, P.; Martel, A.; Haesebrouck, F.; Pasmans, F. Amphibian chytridiomycosis: A review with focus on fungus-host interactions. Vet. Res. 2015, 46, 137. [Google Scholar] [CrossRef]
- Global Invasive Species Database (GISD). Species profile Batrachochytrium dendrobatidis. 2015. Available online: http://www.iucngisd.org/gisd/species.php?sc=123 (accessed on 12 April 2025).
- Garmyn, A.; Van Rooij, P.; Pasmans, F.; Hellebuyck, T.; Van Den Broeck, W.; Haesebrouck, F.; Martel, A. Waterfowl: Potential environmental reservoirs of the chytrid fungus Batrachochytrium dendrobatidis. PLoS ONE 2012, 7, e35038. [Google Scholar] [CrossRef]
- Farrer, R.A.; Weinert, L.A.; Bielby, J.; Garner, T.W.; Balloux, F.; Clare, F.; Bosch, J.; Cunningham, A.A.; Weldon, C.; du Preez, L.H.; et al. Multiple emergences of genetically diverse amphibian-infecting chytrids include a globalized hypervirulent recombinant lineage. Proc. Natl. Acad. Sci. USA 2011, 108, 18732–18736. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.F.; Bosch, J.; Gomez, V.; Garner, T.W.; Cunningham, A.A.; Schmeller, D.S.; Ninyerola, M.; Henk, D.A.; Ginestet, C.; Arthur, C.P.; et al. Factors driving pathogenicity vs. prevalence of amphibian panzootic chytridiomycosis in Iberia. Ecol. Lett. 2010, 13, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, J.; Martins, A.G.D.S.; Domingos, A.H.R.; Santos, I.; Viroomal, I.B.; Toledo, L.F. Seasonal prevalence of the amphibian chytrid in a tropical pond-dwelling tadpole species. Dis. Aquat. Org. 2020, 142, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Kolby, J.E.; Ramirez, S.D.; Berger, L.; Richards-Hrdlicka, K.L.; Jocque, M.; Skerratt, L.F. Terrestrial dispersal and potential environmental transmission of the amphibian chytrid fungus (Batrachochytrium dendrobatidis). PLoS ONE 2015, 10, e0125386. [Google Scholar] [CrossRef]
- Prado, J.S.; Ernetti, J.R.; Pontes, M.R.; Toledo, L.F. Chytrid in the clouds: An alternative passive transport of a lethal pathogen for amphibians. Hydrobiologia 2023, 850, 2061–2073. [Google Scholar] [CrossRef]
- Padgett-Flohr, G.E.; Hopkins, R.L. Landscape epidemiology of Batrachochytrium dendrobatidis in central California. Ecography 2010, 33, 688–697. [Google Scholar] [CrossRef]
- Strauss, A.; Smith, K.G. Why does amphibian chytrid (Batrachochytrium dendrobatidis) not occur everywhere? An exploratory study in Missouri ponds. PLoS ONE 2013, 8, e76035. [Google Scholar] [CrossRef]
- Martínez-Silvestre, A.; Loras-Ortí, F.; Garcia-Salmeron, A.; Pujol-Buxó, E.; Pérez-Novo, I.; Maluquer-Margalef, J.; Poch, S.; Thumsová, B.; Bosch, J. Introduced Mediterranean painted frogs (Discoglossus pictus) are possible supershedders of the fungus Batrachochytrium dendrobatidis in Catalonia (NE Spain). Amphib.-Reptil. 2023, 44, 257–261. [Google Scholar] [CrossRef]
- Montori, A.; San Sebastián, O.; Franch, M.; Pujol-Buxó, E.; Llorente, G.A.; Fernández-Loras, A.; Richter-Boix, A.; Bosch, J. Observations on the intensity and prevalence of Batrachochytridium dendrobatidis in sympatric and allopatric Epidalea calamita (native) and Discoglossus pictus (invasive) populations. Basic Appl. Herpetol. 2019, 33, 5–17. [Google Scholar] [CrossRef]
- Ruggeri, J.; de Carvalho-e-Silva, S.P.; James, T.Y.; Toledo, L.F. Amphibian chytrid infection is influenced by rainfall seasonality and water availability. Dis. Aquat. Org. 2018, 127, 107–115. [Google Scholar] [CrossRef]
- Kupferberg, S.J.; Moidu, H.; Adams, A.J.; Catenazzi, A.; Grefsrud, M.; Bobzien, S.; Leidy, R.; Carlson, S.M. Seasonal drought and its effects on frog population dynamics and amphibian disease in intermittent streams. Ecohydrology 2022, 15, e2395. [Google Scholar] [CrossRef]
- Fernández, S. Factores Bióticos y Abióticos Responsables de la Distribución e Incidencia de Batrachochytrium dendrobatidis en Poblaciones de Anfibios de Zonas Templadas. Ph.D. Thesis, Facultad de Ciencias Biológicas, Universidad Complutense de Madrid, Madrid, Spain, 2018; 231p. Available online: https://hdl.handle.net/20.500.14352/16207 (accessed on 3 November 2024).
- Bosch, J.; Thumsová, B.; Puschendorf, R.; Bielby, J. Drivers of Batrachochytrium dendrobatidis infection load, with evidence of infection tolerance in adult male toads (Bufo spinosus). Oecologia 2023, 202, 165–174. [Google Scholar] [CrossRef]
- Buttimer, S.; Moura-Campos, D.; Greenspan, S.E.; Neely, W.J.; Ferrante, L.; Toledo, L.F.; Becker, C.G. Skin microbiome disturbance linked to drought-associated amphibian disease. Ecol. Lett. 2024, 27, e14372. [Google Scholar] [CrossRef]
- McDevitt-Galles, T.; Moss, W.E.; Calhoun, D.M.; Briggs, C.J.; Pieter, T.J. How extreme drought events, introduced species, and disease interact to influence threatened amphibian populations. Johns. Freshw. Sci. 2022, 41, 680–694. [Google Scholar] [CrossRef]
- More, S.; Miranda, A.; Bicout, M.; Bøtner, D.; Butterworth, A.; Calistri, P.; Depner, K.; Edwards, S.; Garin-Bastuji, B.; Good, M.; et al. Scientific Opinion on the risk of survival, establishment and spread of Batrachochytrium salamandrivorans (Bsal) in the EU. EFSA J. 2018, 16, 5259. [Google Scholar] [CrossRef]
- Malagon, D.A.; Melara, L.A.; Prosper, O.F.; Lenhart, S.; Carter, E.D.; Fordyce, J.A.; Peternon, A.E.; Miller, D.L.; Gray, M.J. Host density and habitat structure influence host contact rates and Batrachochytrium salamandrivorans transmission. Sci. Rep. 2020, 10, 5584. [Google Scholar] [CrossRef] [PubMed]
- Deiß, F.; Ginal, P.; Rödder, D. Microclimatic Growth Rates of Batrachochytrium salamandrivorans under Current and Future Climates: A Very High Spatial Resolution SDM for Bsal and Salamandra salamandra (Linnaeus, 1758) within Forest Habitats of the European Hotspot Area. Diversity 2024, 16, 510. [Google Scholar] [CrossRef]
- Stegen, G.; Pasmans, F.; Schmidt, B.R.; Rouffaer, L.O.; Van Praet, S.; Schaub, M.; Canessa, S.; Laudelout, A.; Kinet, T.; Adriaensen, C.; et al. Drivers of salamander extirpation mediated by Batrachochytrium salamandrivorans. Nature 2017, 544, 353–356. [Google Scholar] [CrossRef]
- Spitzen van der Sluijs, A.; Stegen, G.; Bogaerts, S.; Canesa, S.; Steinfarzt, S.; Jansen, N.; Bosman, W.; Pasmans, F.; Martel, A. Post-epizootic salamander persistence in a disease-free refugium suggests poor dispersal ability of Batrachochytrium salamandrivorans. Sci. Rep. 2018, 8, 3800. [Google Scholar] [CrossRef]
- Kelly, M.; Cuomo, C.A.; Beukema, W.; Carranza, S.; Erens, J.; Foubert, M.; Li, Z.; Lötters, S.; Schulz, V.; Steinfartz, S.; et al. High phenotypic diversity correlated with genomic variation across the European Batrachochytrium salamandrivorans epizootic. PLoS Pathog. 2024, 20, e1012579. [Google Scholar] [CrossRef]
- Jancovich, J.K.; Davidson, E.W.; Parameswaran, N.; Mao, J.; Chinchar, V.G.; Collins, J.P.; Jacobs, B.L.; Storfer, A. Evidence for emergence of an amphibian iridoviral disease because of human-enhanced spread. Mol. Ecol. 2005, 14, 213–224. [Google Scholar] [CrossRef]
- Jancovich, J.K.; Bremont, M.; Touchman, J.W.; Jacobs, B.L. Evidence for multiple recent host species shifts among the ranaviruses (family Iridoviridae). J. Virol. 2010, 84, 2636–2647. [Google Scholar] [CrossRef] [PubMed]
- Bandín, I.; Dopazo, C. Host range, host specificity and hypothesized host shift events among viruses of lower vertebrates. Vet. Res. 2011, 42, 67. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Green, D.E.; Fellers, G.; Chinchar, V.G. Molecular characterization of iridoviruses isolated from sympatric amphibians and fish. Virus Res. 1999, 63, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Bayley, A.E.; Hill, B.J.; Feist, S.W. Susceptibility of the European common frog Rana temporaria to a panel of ranavirus isolates from fish and amphibian hosts. Dis. Aquat. Org. 2013, 103, 171–183. [Google Scholar] [CrossRef]
- Thumsová, B.; Donaire-Barroso, D.; Mouden El, E.H.; Bosch, J. Fatal chytridiomycosis in the Moroccan midwife toad Alytes maurus and potential distribution of Batrachochytrium dendrobatidis across Morocco. Afr. J. Herpetol. 2022, 71, 72–82. [Google Scholar] [CrossRef]
- Altizer, S.; Ostfeld, R.S.; Johnson, P.T.; Kutz, S.; Harvell, C.D. Climate change and infectious diseases: From evidence to a predictive framework. Science 2013, 341, 514–519. [Google Scholar] [CrossRef]
- Price, S.J.; Leung, W.T.M.; Owen, C.J.; Puschendorf, R.; Sergeant, C.; Cunningham, A.A.; Balloux, F.; Garner, T.W.J.; Nichols, R.A. Effects of historic and projected climate change on the range and impacts of an emerging wildlife disease. Glob. Change Biol. 2019, 25, 2648–2660. [Google Scholar] [CrossRef]
- Allender, M.C.; Mitchell, M.A.; Torres, T.; Sekowska, J.; Driskell, E.A. Pathogenicity of Frog Virus 3-like Virus in Red-eared Slider Turtles (Trachemys scripta elegans) at Two Environmental Temperatures. J. Comp. Pathol. 2013, 149, 356–367. [Google Scholar] [CrossRef]
- Rojas, S.; Richards, K.; Jancovich, J.K.; Davidson, E.W. Influence of temperature on ranavirus infection in larval salamanders, Ambystoma tigrinum. Dis. Aquat. Org. 2005, 63, 95–100. [Google Scholar] [CrossRef]
- Brunner, J.L.; Storfer, A.; Le Sage, E.H.; Garner, T.W.J.; Gray, M.J.; Hoverman, J.T. Ranavirus Ecology: From Individual Infections to Population Epidemiology to Community Impacts. In Ranaviruses; Gray, M.J., Chinchar, V.G., Eds.; Springer: Cham, Switzerland, 2025. [Google Scholar] [CrossRef]
- Cohen, J.M.; Sauer, E.L.; Santiago, O.; Spencer, S.; Rohr, J.R. Divergent impacts of warming weather on wildlife disease risk across climates. Science 2020, 370, eabb1702. [Google Scholar] [CrossRef]
- Daszak, P.; Cunningham, A.A.; Hyatt, A.D. Anthropogenic environmental change and the emergence of infectious diseases in wildlife. Acta Trop. 2001, 78, 103–116. [Google Scholar] [CrossRef]
- Davis, D.R.; Ferguson, K.J.; Schwarz, M.S.; Kerby, J.L. Effects of agricultural pollutants on stress hormones and viral infection in larval salamanders. Wetlands 2020, 40, 577–586. [Google Scholar] [CrossRef]
- Rosa, G.; Sabino-Pinto, J.; Laurentino, T.; Martel, A.; Pasmans, F.; Rebelo, R.; Griffiths, R.A.; Stöhr, A.C.; Marsschang, R.E.; Price, S.J.; et al. Impact of asynchronous emergence of two lethal pathogens on amphibian assemblages. Sci. Rep. 2017, 7, 43260. [Google Scholar] [CrossRef] [PubMed]
- Humphries, J.E.; Lanctôt, C.M.; Robert, J.; McCallum, H.I.; Newell, D.A.; Grogan, L.F. Do immune system changes at metamorphosis predict vulnerability to chytridiomycosis? An update. Dev. Comp. Immunol. 2022, 136, 104510. [Google Scholar] [CrossRef] [PubMed]
- Harrison, X.A.; Price, S.J.; Hopkins, K.; Leung, W.T.M.; Sergeant, C.; Garner, T.W.J. Diversity-stability dynamics of the amphibian skin microbiome and susceptibility to a lethal viral pathogen. Front. Microbiol. 2019, 10, 2883. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.J.; Pawlik, A.H.; Harrison, X.A. Amphibian ranaviruses in Europe: Important directions for future research. Facets 2020, 5, 598–614. [Google Scholar] [CrossRef]
- Haislip, N.A.; Gray, M.J.; Hoverman, J.T.; Miller, D.L. Development and disease: How susceptibility to an emerging pathogen changes through anuran development. PLoS ONE 2011, 6, e22307. [Google Scholar] [CrossRef]
- Kwon, S.; Park, J.; Choi, W.J.; Koo, K.S.; Lee, J.G.; Park, D. First case of ranavirus-associated mass mortality in a natural population of the Huanren frog (Rana huanrenensis) tadpoles in South Korea. Anim. Cells Syst. 2017, 21, 358–364. [Google Scholar] [CrossRef]
- Teacher, A.G.F.; Cunningham, A.A.; Garner, T.W.J. Assessing the long-term impact of Ranavirus infection in wild common frog populations. Anim. Conserv. 2010, 13, 514–522. [Google Scholar] [CrossRef]
- De Necker, L.; Brendonck, L.; Vanschoenwinkel, B.; Florencio, M.; Rhazi, L.; Gołdyn, B. Lessons from pond creation and restoration projects in Europe. Restor. Ecol. 2025, 33, e14342. [Google Scholar] [CrossRef]
- Mathwin, R.; Wassens, S.; Young, J.; Ye, Q.; Bradshaw, C.J. Manipulating water for amphibian conservation. Conserv. Biol. 2021, 35, 24–34. [Google Scholar] [CrossRef]
- Shoo, L.P.; Olson, D.H.; McMenamin, S.K.; Murray, K.A.; Van Sluys, M.; Donnelly, M.A.; Stratford, D.; Terhivuo, J.; Merino-Viteri, A.; Herbert, S.M.; et al. Engineering a future for amphibians under climate change. J. Appl. Ecol. 2011, 48, 487–492. [Google Scholar] [CrossRef]
- Henao-Casas, J.D.; Fernández-Escalante, E.; Ayuga, F. Alleviating drought and water scarcity in the Mediterranean region through managed aquifer recharge. Hydrogeol. J. 2022, 30, 1685–1699. [Google Scholar] [CrossRef]
- Sanz, D.B.; Iribas, B.L.; Calvo, P.C. Proposal for analysis of minimum ecological flow regimes based on the achievement of technical and environmental objectives: Tagus River basin case study (Spain). Environ. Earth Sci. 2025, 84, 26. [Google Scholar] [CrossRef]
- Mezger, G.; del Tánago, M.G.; De Stefano, L. Environmental flows and the mitigation of hydrological alteration downstream from dams: The Spanish case. J. Hydrol. 2021, 598, 125732. [Google Scholar] [CrossRef]
- Tickner, D.; Opperman, J.J.; Abell, R.; Acreman, M.; Arthington, A.H.; Bunn, S.E.; Cooke, S.J.; Dalton, J.; Darwall, W.; Edwards, G.; et al. Bending the curve of global freshwater biodiversity loss: An emergency recovery plan. BioScience 2020, 70, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Rowe, J.C.; Pearl, C.A.; Duarte, A.; McCreary, B.; Adams, M.J. Population dynamics of the threatened Oregon spotted frog before and after drought mitigation. J. Wildl. Manag. 2024, 88, e22496. [Google Scholar] [CrossRef]
- Beranek, C.T.; Sanders, S.; Clulow, J.; Mahony, M. Factors influencing persistence of a threatened amphibian in restored wetlands despite severe population decline during climate change driven weather extremes. Biodivers. Conserv. 2022, 31, 1267–1287. [Google Scholar] [CrossRef]
- Baumberger, K.L.; Backlin, A.R.; Gallegos, E.A.; Hitchcock, C.J.; Fisher, R.N. Mitigation ponds offer drought resiliency for western spadefoot (Spea hammondii) populations. Bull. South. Calif. Acad. Sci. 2020, 119, 6–17. [Google Scholar] [CrossRef]
- Ruhí, A.; San Sebastian, O.; Feo, C.; Franch, M.; Gascon, S.; Richter-Boix, A.; Boix, D.; Llorente, G. Man-made Mediterranean temporary ponds as a tool for amphibian conservation. Ann. De Limnol.-Int. J. Limnol. 2012, 48, 81–93. [Google Scholar] [CrossRef]
- Evans, M.J.; Scheele, B.C.; Westgate, M.J.; Yebra, M.; Newport, J.S.; Manning, A.D. Beyond the pond: Terrestrial habitat use by frogs in a changing climate. Biol. Conserv. 2020, 249, 108712. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).