An Evaluation of Mercury Accumulation Dynamics in Tree Leaves Growing in a Contaminated Area as Part of the Ecosystem Services: A Case Study of Turda, Romania
Abstract
1. Introduction
2. Materials and Methods
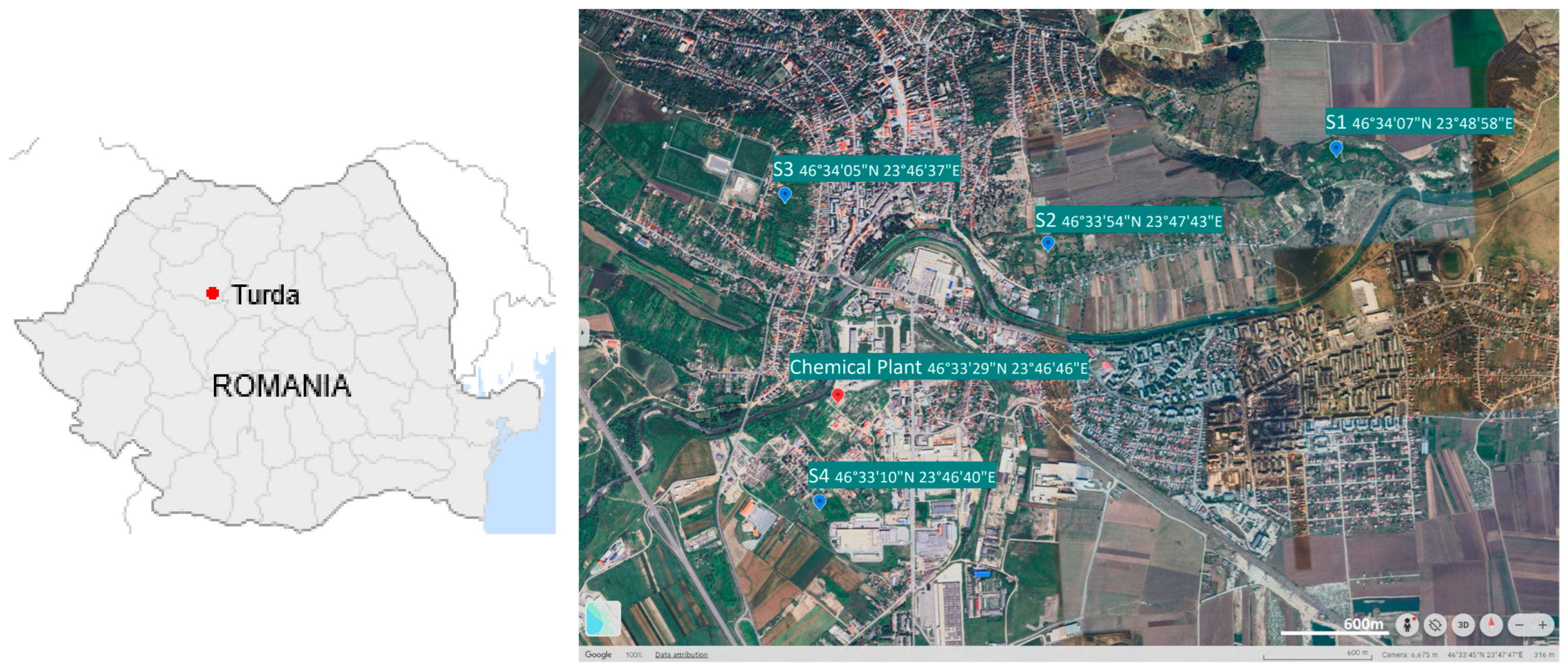
2.1. Study Area Description and Sampling
2.2. Sample Analysis
2.3. Geo-Accumulation Index (Igeo)
2.4. Mercury Accumulation Percentage
2.5. Statistics
3. Results and Discussion
3.1. Concentration Levels of Mercury in the Soil and Geo-Accumulation Index
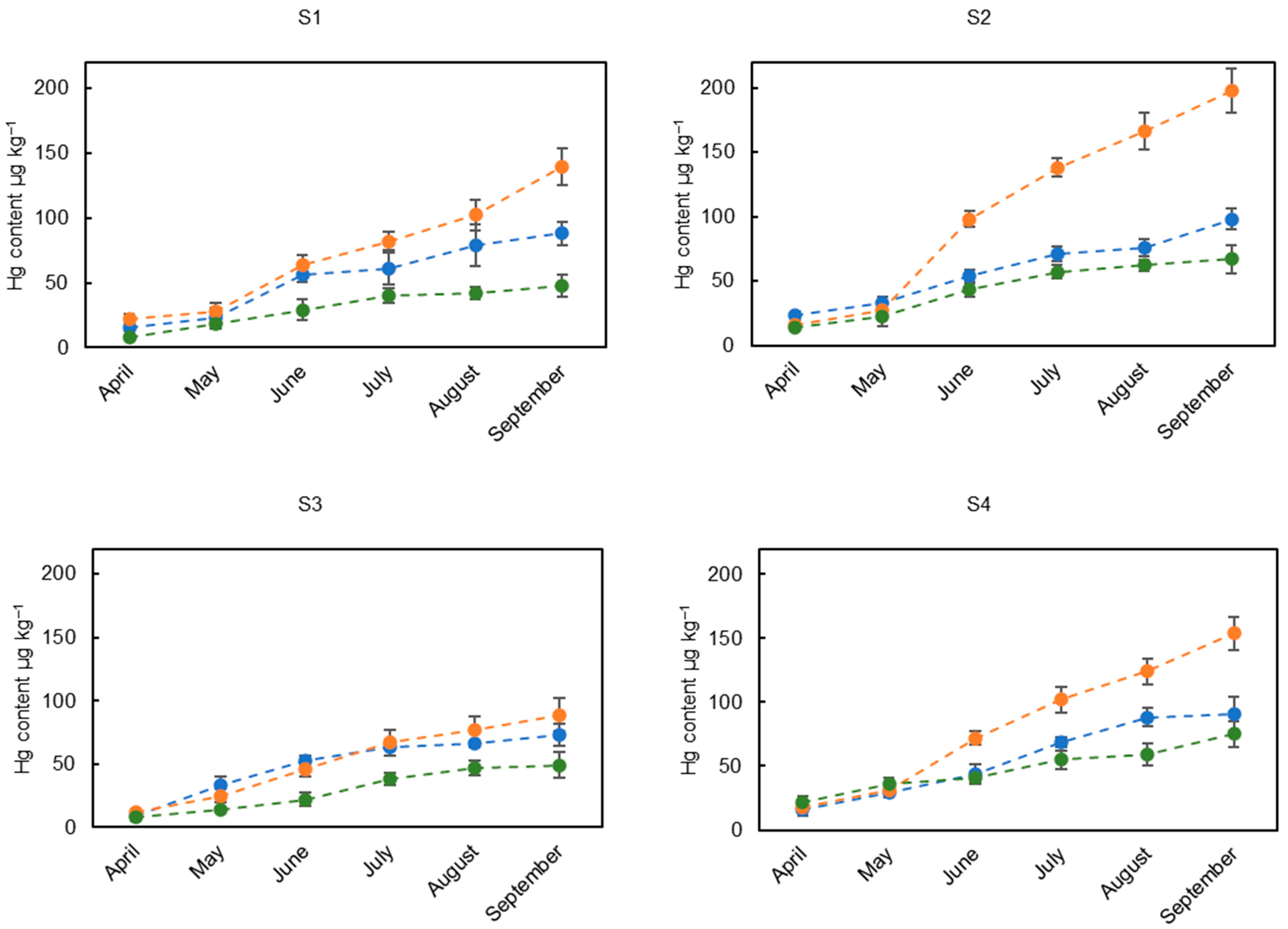
3.2. Mercury Concentrations in the Leaves of the Different Tree Species
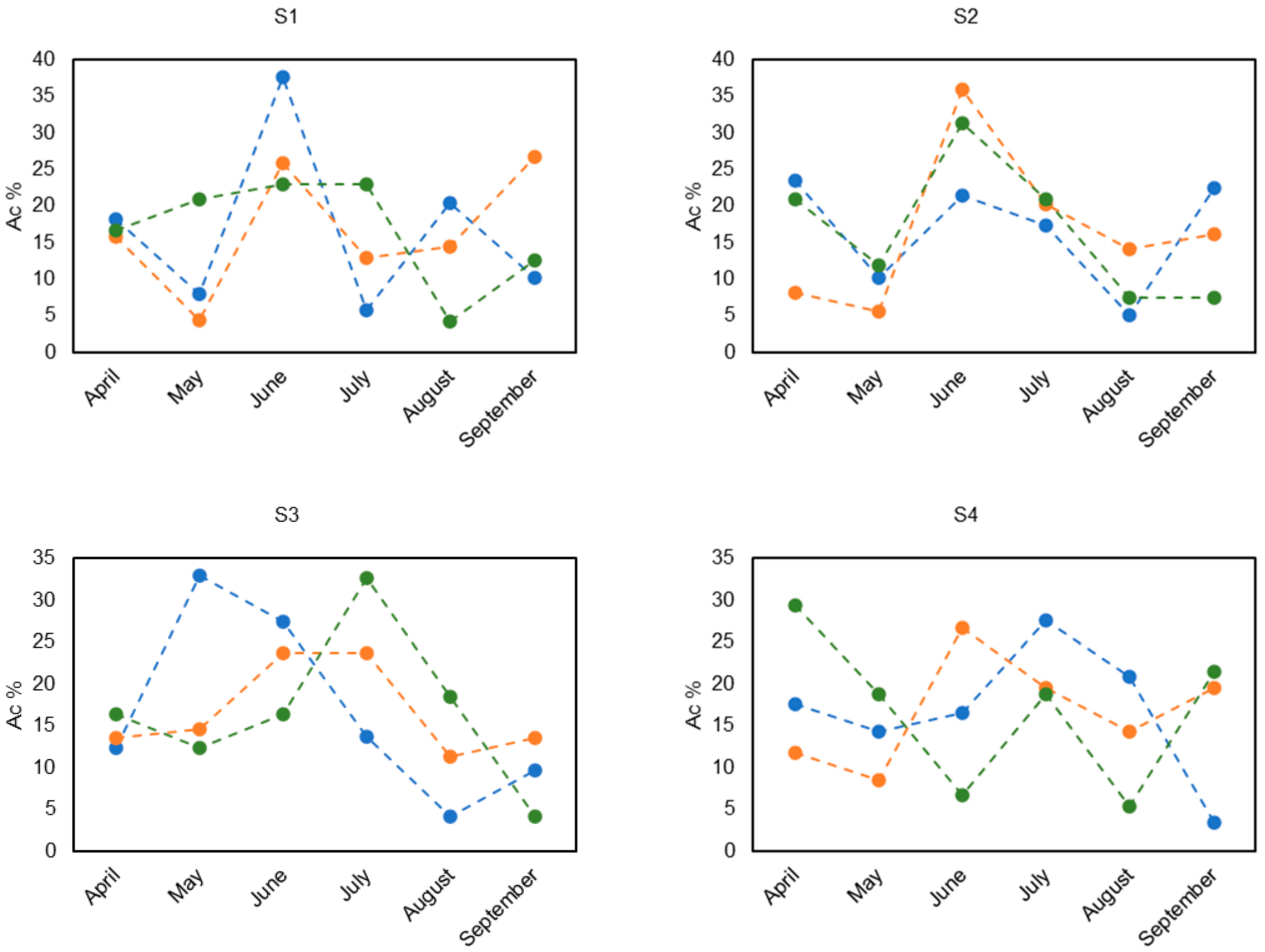
3.3. Mercury Accumulation Percentage over the Growing Season
3.4. Assessment of Hg Accumulation by Tree Species over the Growing Season
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahn, Y.S.; Jung, R.; Moon, J.-H. Approaches to understand historical changes of mercury in tree rings of Japanese cypress in industrial areas. Forests 2020, 11, 800. [Google Scholar] [CrossRef]
- Li, Y.; Ma, L.; Wang, Q.; Liu, W.; Saparov, G.; Abuduwaili, J. Unveiling mercury’s hidden threat: An integrated methodology for soil mercury risk assessment in Syr Darya River Basin, Central Asia. J. Hazard. Mater. 2025, 483, 136690. [Google Scholar] [CrossRef] [PubMed]
- Smical, I.; Muntean, A.; Micle, V.; Sur, I.M.; Moldovan, A.C. Study on human health risks associated with consuming vegetables grown in industrially polluted soil in Sasar area, NW Romania, in the context of sustainable development. Sustainability 2025, 17, 4072. [Google Scholar] [CrossRef]
- Senila, M.; Levei, E.; Miclean, M.; Senila, L.; Stefanescu, L.; Mărginean, S.; Ozunu, A.; Roman, C. Influence of pollution level on heavy metals mobility in soil from NW Romania. Environ. Eng. Manag. J. 2011, 10, 59–64. [Google Scholar] [CrossRef]
- Jeon, B.; Cizdziel, J.V.; Brewer, J.S.; Luke, W.T.; Cohen, M.D.; Ren, X.; Kelley, P. Gaseous Elemental Mercury Concentrations along the Northern Gulf of Mexico Using Passive Air Sampling, with a Comparison to Active Sampling. Atmosphere 2020, 11, 1034. [Google Scholar] [CrossRef]
- Bauštein, M.; Száková, J.; Stefanović, L.; Najmanová, J.; Sysalová, J.; Tlustoš, P. Assessment of Mercury Uptake by Plants in Former Cinnabar Mining Areas. Minerals 2024, 14, 1211. [Google Scholar] [CrossRef]
- Aragão, W.A.B.; Teixeira, F.B.; Fagundes, N.C.F.; Fernandes, R.M.; Fernandes, L.M.P.; da Silva, M.C.F.; Amado, L.L.; Sagica, F.E.S.; Oliveira, E.H.C.; Crespo-Lopez, M.E.; et al. Hippocampal dysfunction provoked by mercury chloride exposure: Evaluation of cognitive impairment, oxidative stress, tissue injury and nature of cell death. Oxid. Med. Cell. Longev. 2018, 2018, 7878050. [Google Scholar] [CrossRef] [PubMed]
- Adriano, D.C. Trace Elements in Terrestrial Environments: Biogeochemistry, Bioavailability and Risks of Metals, 2nd ed.; Springer: New York, NY, USA, 2001. [Google Scholar]
- Hlodák, M.; Matúš, P.; Urík, M.; Korenkova, L.; Mikusova, P.; Senila, M.; Divis, P. Evaluation of various inorganic and biological extraction techniques suitability for soil mercury phytoavailable fraction assessment. Water Air Soil Pollut. 2015, 226, 198. [Google Scholar] [CrossRef]
- Guédron, S.; Grangeon, S.; Jouravel, G.; Charlet, L.; Sarret, G. Atmospheric mercury incorporation in soils of an area impacted by a chlor-alkali plant (Grenoble, France): Contribution of canopy uptake. Sci. Total Environ. 2013, 445–446, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Senilă, M.; Levei, E.A.; Senilă, L.R.; Oprea, G.M.; Roman, C.M. Mercury in soil and perennial plants in a mining-affected urban area from Northwestern Romania. J. Environ. Sci. Health. A Tox. Hazard. Subst. Environ. Eng. 2012, 47, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sanz, A.; Millán, R.; Sierra, M.J.; Schmid, T.; García, G. Use of genus cistus in phytotechnologies: Application in a closed mercury mine. Land 2023, 12, 1533. [Google Scholar] [CrossRef]
- Wilkening, J.L.; Kurthen, A.L.; Guilbeau, K.; Libera, D.A.; Nelson, S.J.; Ming, J. Climate-driven alterations in the mercury cycle: Implications for wildlife managers through a one health lens. Land 2025, 14, 856. [Google Scholar] [CrossRef]
- Obrist, D.; Kirk, J.L.; Zhang, L.; Sunderland, E.M.; Jiskra, M.; Selin, N.E. A review of global environmental mercury processes in response to human and natural perturbations: Changes of emissions, climate, and land use. Ambio 2018, 47, 116–140. [Google Scholar] [CrossRef] [PubMed]
- Laacouri, A.; Nater, E.A.; Kolka, R.K. Distribution and uptake dynamics of mercury in leaves of common deciduous tree species in Minnesota, U.S.A. Environ. Sci. Technol. 2013, 47, 10462–10470. [Google Scholar] [CrossRef] [PubMed]
- Osterwalder, S.; Schibler, R.; Hüglin, C.; Schwarzenbach, B.; Stupple, G.; MacSween, K.; Bishop, K.; Alewell, C.; Buchmann, N. Spatial and seasonal dynamics of gaseous elemental mercury concentrations over Switzerland observed by a passive air sampler network. Environ. Sci. Atmos. 2024, 4, 848–860. [Google Scholar] [CrossRef]
- Pleijel, H.; Klingberg, J.; Nerentorp, M.; Broberg, M.C.; Nyirambangutse, B.; Munthe, J.; Wallin, G. Mercury accumulation in leaves of different plant types—the significance of tissue age and specific leaf area. Biogeosciences 2021, 18, 6313–6328. [Google Scholar] [CrossRef]
- Pan, J.; Chen, M.; Zhang, Z.; Zhang, H.; Zong, J.; Wang, Z.; Zhang, G. Risk assessments of plant leaf and soil mercury pollution in different functional areas of Changchun city. Forests 2023, 14, 1108. [Google Scholar] [CrossRef]
- Gustin, M.S.; Lindberg, S.E.; Weisberg, P.J. An update on the natural sources and sinks of atmospheric mercury. Appl. Geochem. 2008, 23, 482–493. [Google Scholar] [CrossRef]
- Bushey, J.T.; Nallana, A.G.; Montesdeoca, M.R.; Driscoll, C.T. Mercury dynamics of a northern hardwood canopy. Atmos. Environ. 2008, 42, 6905–6914. [Google Scholar] [CrossRef]
- Senila, M.; Levei, E.A.; Frentiu, T.; Mihali, C.; Angyus, S.B. Assessment of mercury bioavailability in garden soils around a former nonferrous metal mining area using DGT, accumulation in vegetables, and implications for health risk. Environ. Monit. Assess. 2023, 195, 1554. [Google Scholar] [CrossRef] [PubMed]
- Franzaring, J.; Haneke, J.; Sannino, A.; Radermacher, G.; Schweiger, A. Effects of legacy mining on mercury concentrations in conifer needles and mushrooms in northern Palatinate, Germany. Environ. Pollut. 2024, 357, 124406. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.; Gustin, M.S.; Weisberg, P.J. Evidence for nonstomatal uptake of Hg by aspen and translocation of hg from foliage to tree rings in Austrian pine. Environ. Sci. Technol. 2018, 52, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Chiarantini, L.; Rimondi, V.; Benvenuti, M.; Beutel, M.W.; Costagliola, P.; Gonnelli, C.; Lattanzi, P.; Paolieri, M. Black pine (Pinus nigra) barks as biomonitors of airborne mercury pollution. Sci. Total Environ. 2016, 569–570, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Esbrí, J.M.; Cacovean, H.; Higueras, P. Usage Proposal of a common urban decorative tree (Salix alba L.) to monitor the dispersion of gaseous mercury: A case study from Turda (Romania). Chemosphere 2018, 193, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Gustin, M.S.; Dunham-Cheatham, S.M.; Harper, J.F.; Choi, W.G.; Blum, J.D.; Johnson, M.W. Investigation of the biochemical controls on mercury uptake and mobility in trees. Sci. Total Environ. 2022, 851, 158101. [Google Scholar] [CrossRef] [PubMed]
- Higueras, P.; Amorós, J.A.; Esbrí, J.M.; García-Navarro, F.J.; Pérez de los Reyes, C.; Moreno, G. Time and space variations in mercury and other trace element contents in olive tree leaves from the Almadén Hg-mining district. J. Geochem. Explor. 2012, 123, 143–151. [Google Scholar] [CrossRef]
- Rodríguez Martin, J.A.; Gutiérrez, C.; Torrijos, M.; Nanos, N. Wood and bark of Pinus halepensis as archives of heavy metal pollution in the Mediterranean Region. Environ. Pollut. 2018, 239, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, H.; Zong, J.; Bai, X.; Yang, K.; Li, X.; Huang, X.; Hu, Y.; Bao, Y.; Razzaq, A.; et al. Mercury accumulation in urban forest leaves: Dynamics, driving factors, and potential economic value. J. Hazard. Mater. 2025, 490, 137763. [Google Scholar] [CrossRef] [PubMed]
- Tiodar, E.D.; Chiriac, C.M.; Pošćić, F.; Văcar, C.L.; Balázs, Z.R.; Coman, C.; Weindorf, D.C.; Banciu, M.; Krämer, U.; Podar, D. Plant colonizers of a mercury contaminated site: Trace metals and associated rhizosphere bacteria. Plant Soil 2024, 502, 373–396. [Google Scholar] [CrossRef]
- Craciun, A.I.; Ozunu, A. Comprehensive analysis and review of soil contamination and the associated risks at the Poșta-Rât Industrial Site in Turda, Cluj, Romania. J. Eng. Sci. Innov. 2024, 9, 383–392. [Google Scholar] [CrossRef]
- Frentiu, T.; Pintican, B.P.; Butaciu, S.; Mihaltan, A.I.; Ponta, M.; Frentiu, M. Determination, speciation and distribution of mercury in soil in the surroundings of a former chlor-alkali plant: Assessment of sequential extraction procedure and analytical technique. Chem. Cent. J. 2013, 7, 178. [Google Scholar] [CrossRef] [PubMed]
- Ministerial Order 956:1997; 303/bis/06.11.1997; Official Gazette of Romania: Bucharest, Romania, 1997.
- Senila, M.; Covaci, E.; Cadar, O.; Ponta, M.; Frentiu, M.; Frentiu, T. Mercury speciation in fish tissue by eco-scale thermal decomposition atomic absorption spectrometry: Method validation and risk exposure to methylmercury. Chem. Pap. 2018, 72, 441–448. [Google Scholar] [CrossRef]
- U.S. EPA. Method 7473 (SW-846): Mercury in Solids and Solutions by Thermal Decomposition, Amalgamation, and Atomic Absorption Spectrophotometry. 1998. Available online: https://www.epa.gov/sites/default/files/2015-07/documents/epa-7473.pdf (accessed on 15 June 2025).
- Senila, M.; Cadar, O.; Senila, L.; Hoaghia, A.; Miu, I. Mercury determination in natural zeolites by thermal decomposition atomic absorption spectrometry: Method validation in compliance with requirements for use as dietary supplements. Molecules 2019, 24, 4023. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Sanchez, A.; Murciego, A.; Alvarez-Ayusoa, E.; Santa Regina, I.; Rodriguez-Gonzalez, M.A. Mercury in soils and plants in an abandoned cinnabar mining area (SWSpain). J. Hazard. Mater. 2009, 168, 1319–1324. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Jimenez, E.; Gamarra, R.; Carpena-Ruiz, R.O.; Millan, R.; Penalosa, J.M.; Esteban, E. Mercury bioaccumulation and phytotoxicity in two wild plant species of Almaden area. Chemosphere 2006, 63, 1969–1973. [Google Scholar] [CrossRef] [PubMed]
- Higueras, P.L.; Amorós, J.Á.; Esbrí, J.M.; Pérez-de-los-Reyes, C.; López-Berdonces, M.A.; Garcia-Navvaro, F.J. Mercury transfer from soil to olive trees. A comparison of three different contaminated sites. Environ. Sci. Pollut. Res. 2016, 23, 6055–6061. [Google Scholar] [CrossRef] [PubMed]
- Fernández, S.; Poschenrieder, C.; Marcenò, C.; Gallego, J.R.; Jiménez-Gámez, D.; Bueno, A.; Afif, E. Phytoremediation capability of native plant species living on Pb-Zn and Hg-As mining wastes in the Cantabrian range, north of Spain. J. Geochem. Explor. 2017, 174, 10–20. [Google Scholar] [CrossRef]
- Millhollen, A.; Gustin, M.; Obrist, D. Foliar mercury accumulation and exchange for three tree species. Environ. Sci. Technol. 2006, 40, 6001–6006. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.W.; Castro, M.S. Investigation of factors affecting gaseous mercury concentrations in soils. Sci. Total Environ. 2012, 419, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Assad, M.; Parelle, J.; Cazaux, D.; Gimbert, F.; Chalot, M.; Tatin-Froux, F. Mercury uptake into poplar leaves. Chemosphere 2016, 146, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Poissant, L.; Pilote, M.; Yumvihoze, E.; Lean, D. Mercury concentrations and foliage/atmosphere fluxes in a maple forest ecosystem in Quebec, Canada. J. Geophys. Res. Atmos. 2008, 10, 307–319. [Google Scholar] [CrossRef]
- USDA Forest Service. Understanding i-Tree—Appendix 4: Conversion Factors for Leaf Area to Biomass. Available online: https://www.fs.usda.gov/nrs/pubs/gtr/gtrnrs200_appendixes/gtr_nrs200_appendix4.pdf (accessed on 15 June 2025).
- Morelli, G.; Ciani, F.; Cocozza, C.; Costagliola, P.; Fagotti, C.; Friani, R.; Lattanzi, P.; Manca, R.; Monnanni, A.; Nannoni, A.; et al. Riparian trees in mercury contaminated riverbanks: An important resource for sustainable remediation management. Environ. Res. 2024, 257, 119373. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Liu, X.; Zhang, X.; Guo, J.; Huang, J.; Ying, X.; Wang, Y.; Zhang, Q.; Kang, S. Important accumulated mercury pool in a remote alpine forest and dynamic accumulation revealed by tree rings in China’s Qilian Mountains. Sci. Total. Environ. 2024, 951, 175441. [Google Scholar] [CrossRef] [PubMed]


| Sampling Location | Hg Concentration (mg kg−1 dw) | Igeo |
|---|---|---|
| S1 | 7.3 ± 0.84 | 6.6 |
| S2 | 4.6 ± 0.25 | 5.9 |
| S3 | 2.4 ± 0.20 | 5.0 |
| S4 | 5.5 ± 0.38 | 6.2 |
| Sampling Location | Tree Species | Hg/Day ng g−1 | Hg/Season ng g−1 |
|---|---|---|---|
| S1 | white poplar | 0.48 | 72 |
| linden | 0.78 | 117 | |
| cherry plum | 0.27 | 40 | |
| S2 | white poplar | 0.50 | 75 |
| linden | 1.21 | 182 | |
| cherry plum | 0.35 | 53 | |
| S3 | white poplar | 0.43 | 64 |
| linden | 0.51 | 77 | |
| cherry plum | 0.27 | 41 | |
| S4 | white poplar | 0.50 | 75 |
| linden | 0.91 | 136 | |
| cherry plum | 0.35 | 53 | |
| * Average ± SD | white poplar | 0.48 b ± 0.03 | 75 b ± 5 |
| linden | 0.78 a ± 0.29 | 136 a ± 44 | |
| cherry plum | 0.31 b ± 0.05 | 53 b ± 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senila, M.; Varaticeanu, C.; Costiug, S.; Todor-Boer, O. An Evaluation of Mercury Accumulation Dynamics in Tree Leaves Growing in a Contaminated Area as Part of the Ecosystem Services: A Case Study of Turda, Romania. Land 2025, 14, 1529. https://doi.org/10.3390/land14081529
Senila M, Varaticeanu C, Costiug S, Todor-Boer O. An Evaluation of Mercury Accumulation Dynamics in Tree Leaves Growing in a Contaminated Area as Part of the Ecosystem Services: A Case Study of Turda, Romania. Land. 2025; 14(8):1529. https://doi.org/10.3390/land14081529
Chicago/Turabian StyleSenila, Marin, Cerasel Varaticeanu, Simona Costiug, and Otto Todor-Boer. 2025. "An Evaluation of Mercury Accumulation Dynamics in Tree Leaves Growing in a Contaminated Area as Part of the Ecosystem Services: A Case Study of Turda, Romania" Land 14, no. 8: 1529. https://doi.org/10.3390/land14081529
APA StyleSenila, M., Varaticeanu, C., Costiug, S., & Todor-Boer, O. (2025). An Evaluation of Mercury Accumulation Dynamics in Tree Leaves Growing in a Contaminated Area as Part of the Ecosystem Services: A Case Study of Turda, Romania. Land, 14(8), 1529. https://doi.org/10.3390/land14081529










