Eucalyptus-Biochar Application for Mitigating the Combined Effects of Metal Toxicity and Osmotic-Induced Drought in Casuarina glauca Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Soils, Plant Material, Experimental Design, and Growth Conditions
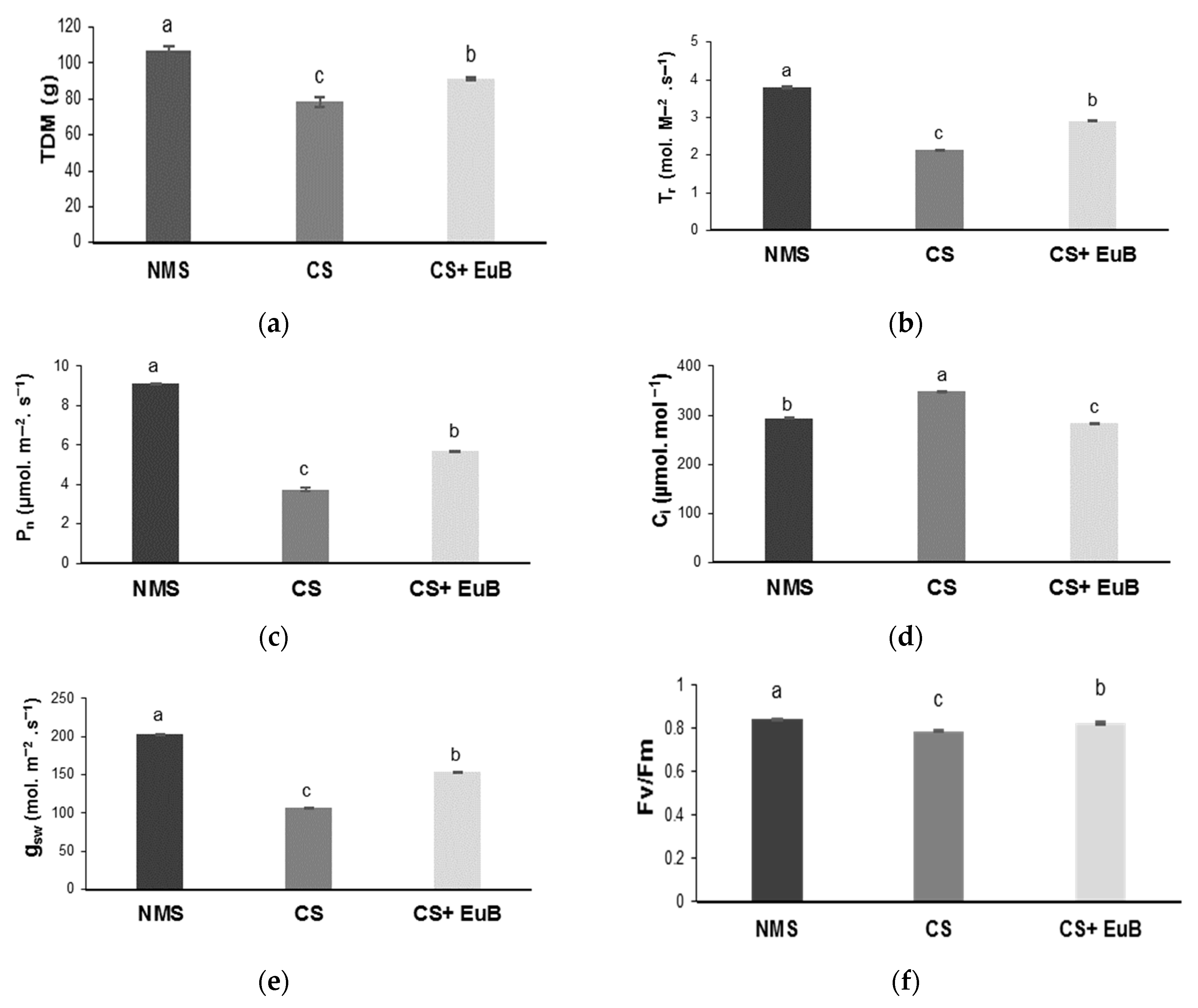
2.2. Determination of Photosynthetic Gas Exchange, Water Use Efficiency, Chlorophyll Fluorescence and Total Dry Mass
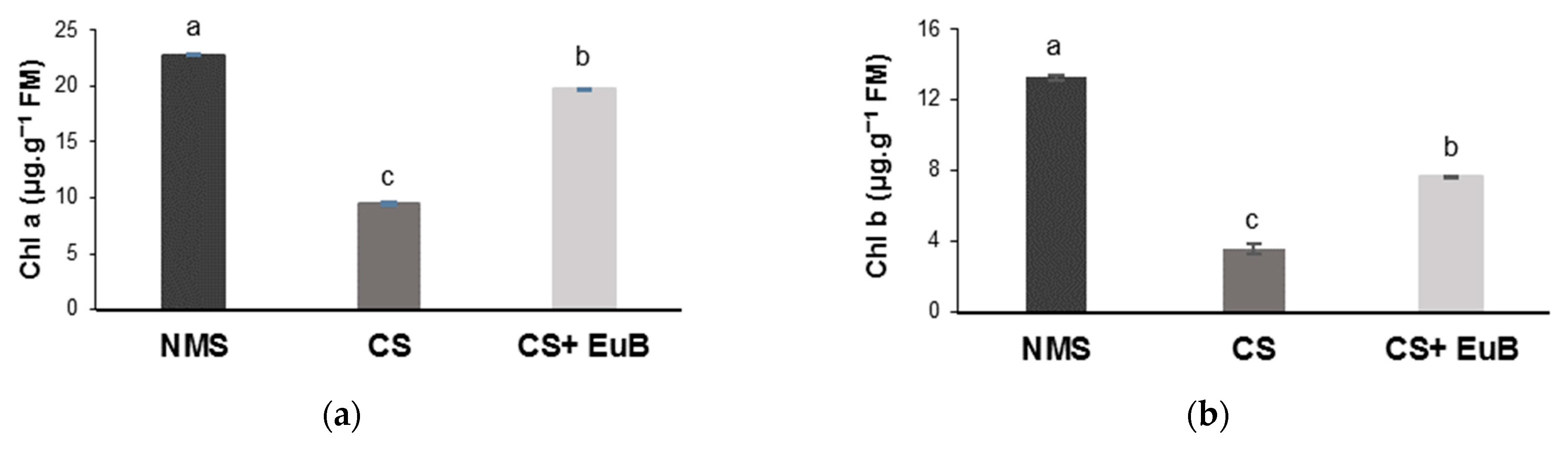
2.3. Determination of Chlorophylls and Carotenoids Contents
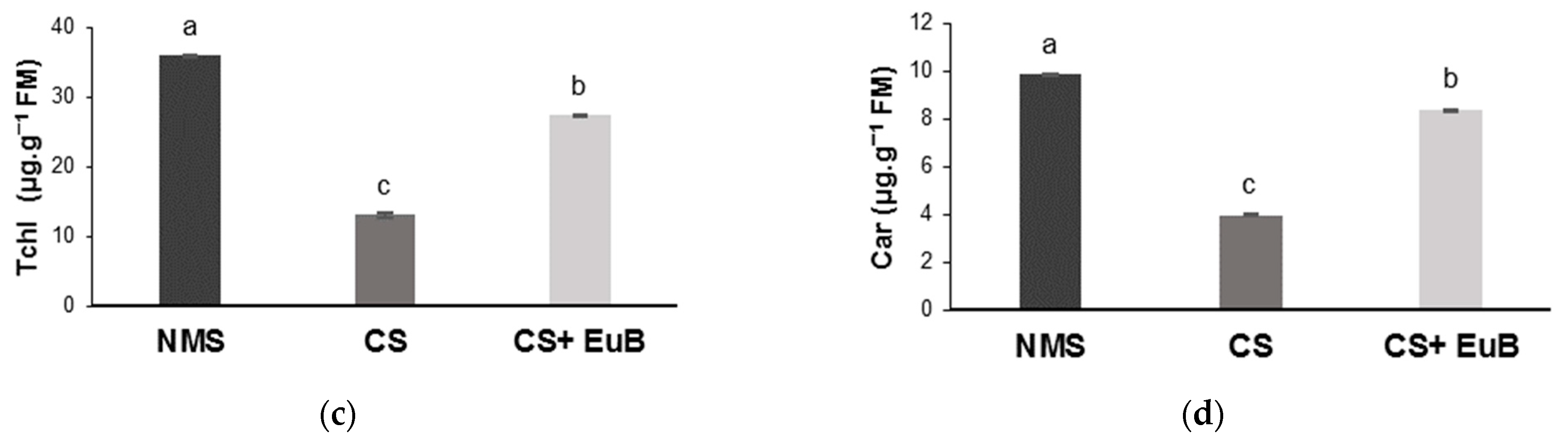
2.4. Determination of Water Status
2.5. Determination of Plant Biochemical Responses
2.6. Trace Metals Concentrations in Casuarina Glauca Organs and Phytoremediation Indices
2.7. Statistical Analysis
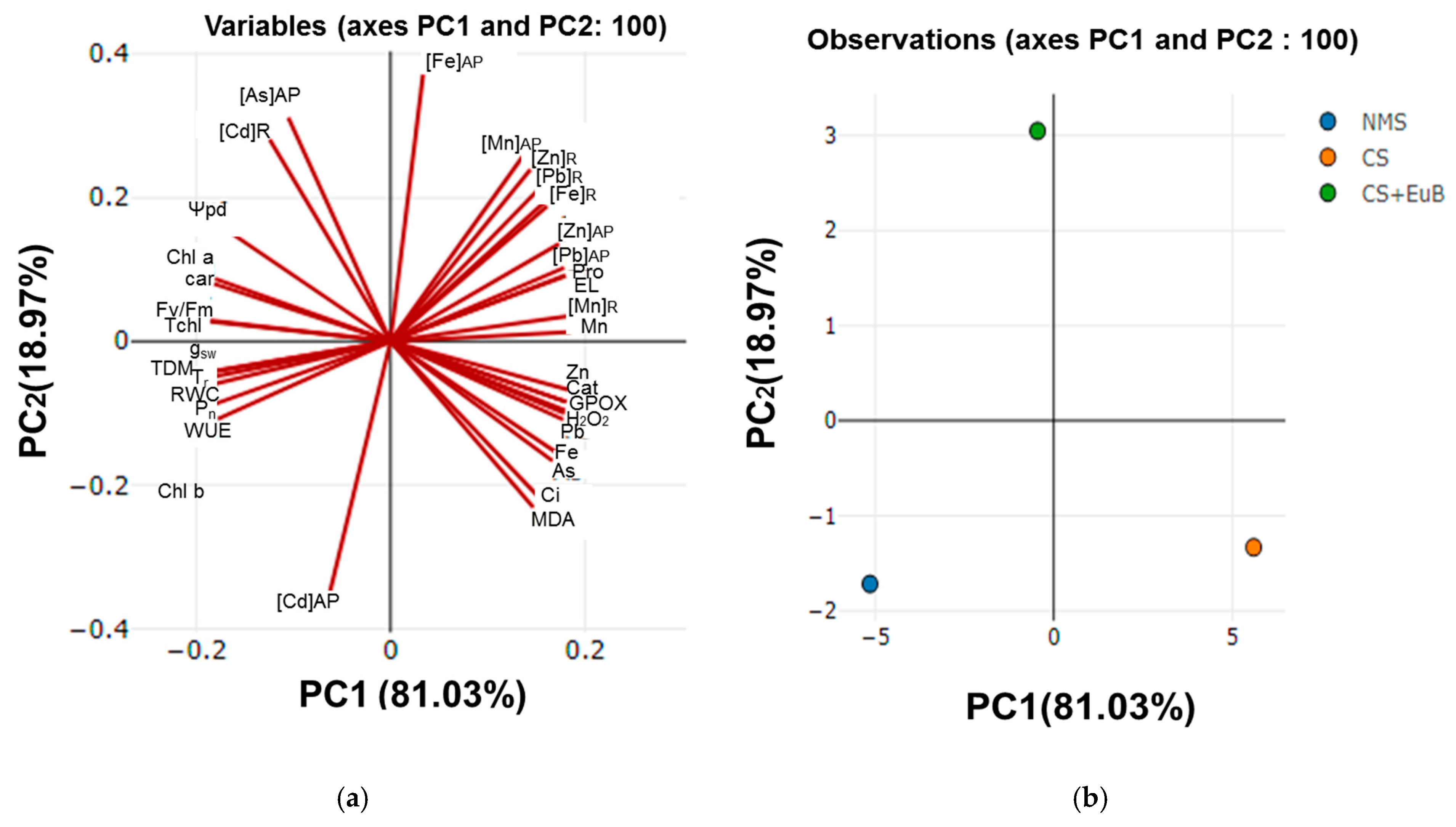
3. Results
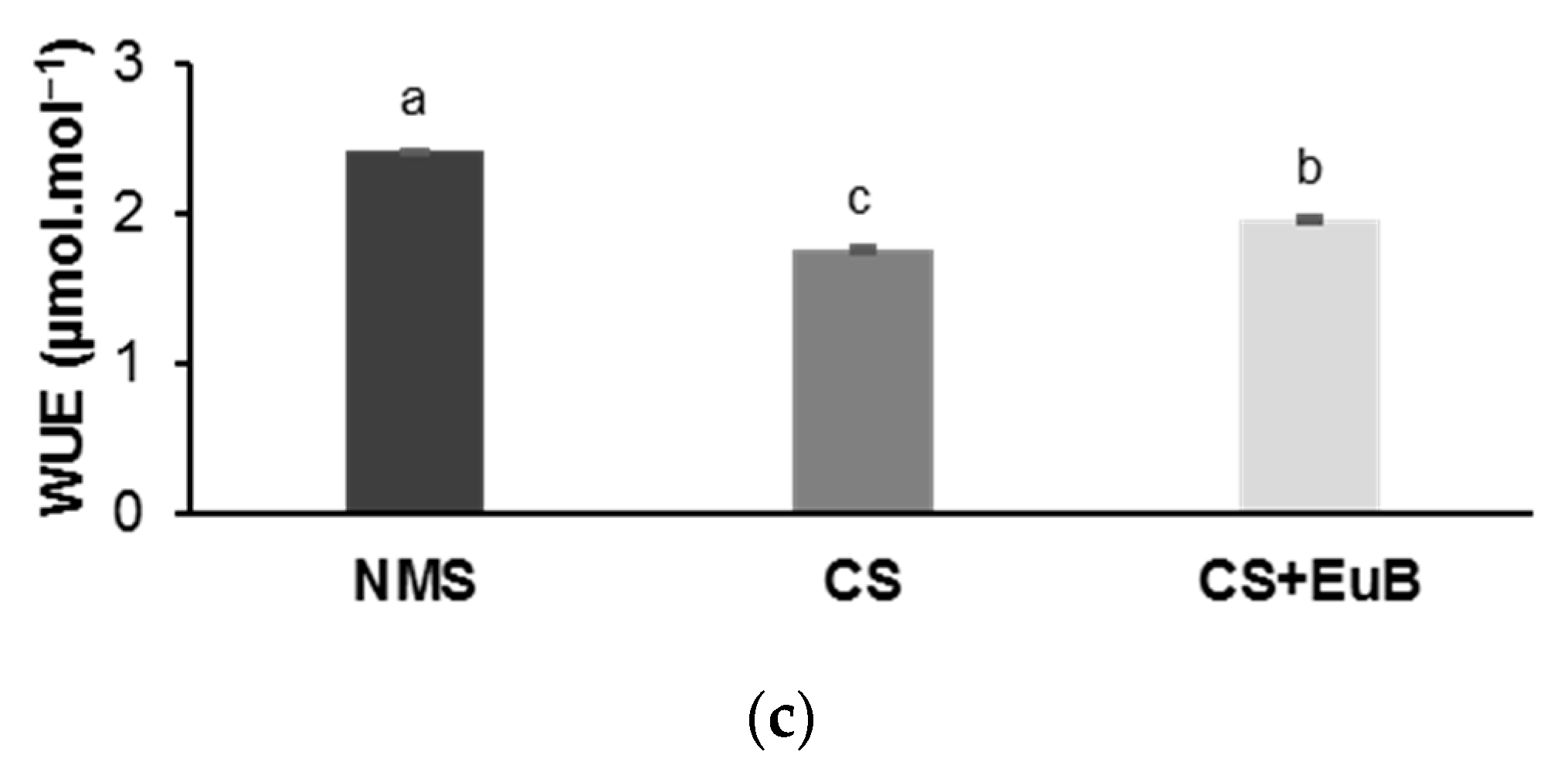
3.1. Total Dry Mass, Gas Exchanges, Chlorophyll Fluorescence and Chlorophyll Content
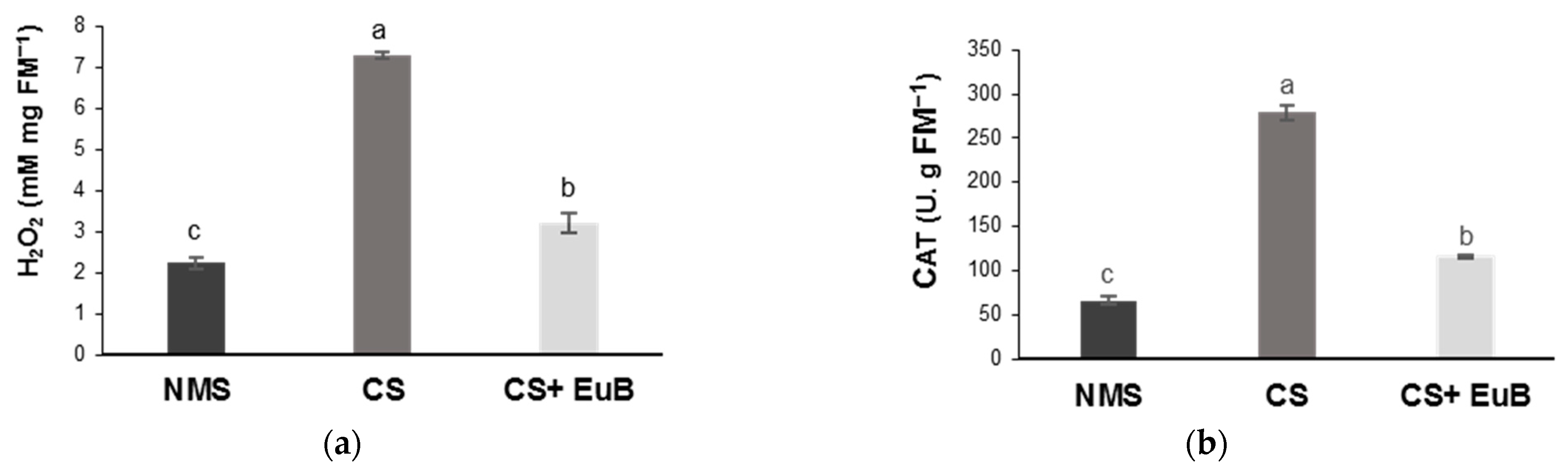
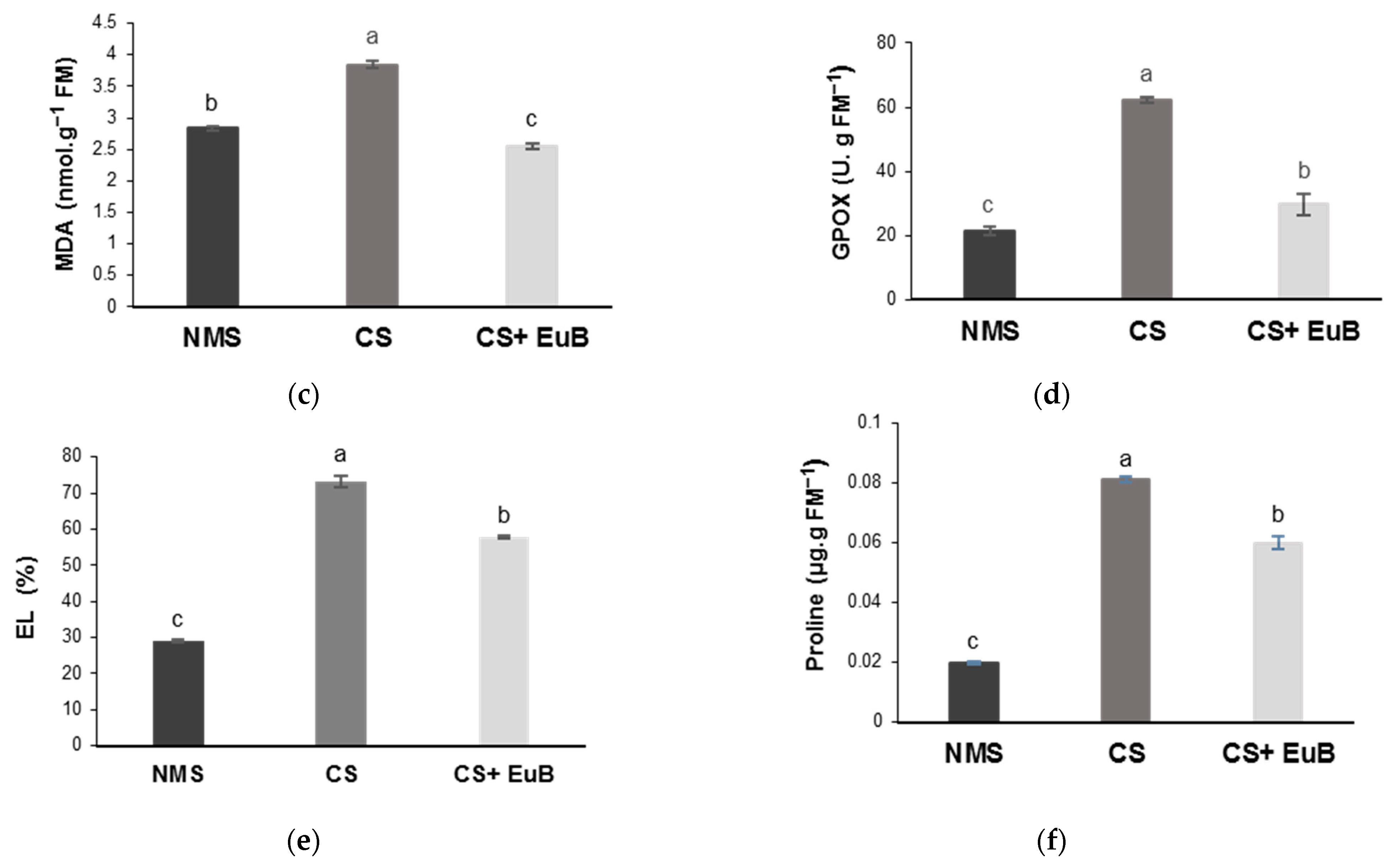
3.2. Water Status, Biochemical Compounds and Electrolyte Leakage
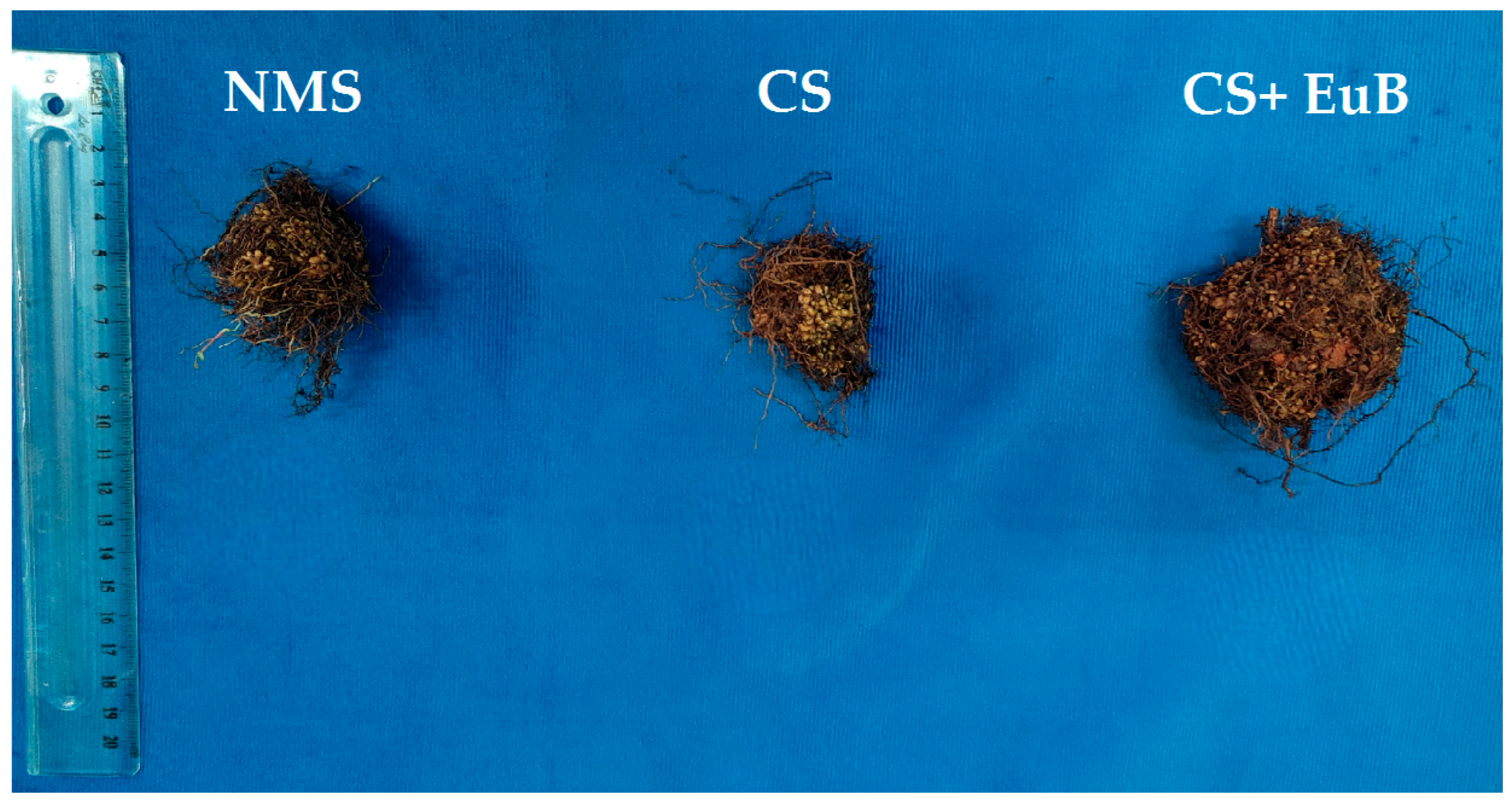
3.3. Accumulation and Phytoremediation Potentiality of Trace Metals
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NMS | Non-mining soil |
| CS | Contaminated soil |
| CS + EuB | Contaminated soil with biochar |
| EuB | Eucalyptus biochar |
| TM | Trace metals |
| TDM | Total dry mass |
| Fv/Fm | Chlorophyll fluorescence |
| Pn | Net photosynthetic rate |
| Tr | Transpiration rate |
| gsw | Stomatal conductance |
| Ci | Intercellular CO2 concentration |
| EL | Electrolyte Leakage |
| chl a | Chlorophyll a |
| chl b | Chlorophyll b |
| Tchl | Total chlorophyll |
| Car | Carotenoids content |
| Pro | Proline |
| Ψpd | Predawn water potential |
| WUE | Water use efficiency |
| RWC | Relative water content |
| MDA | Malondialdehyde |
| H2O2 | Hydrogen peroxide concentration |
| CAT | Catalase activity |
| GPOX | Guaiacol peroxidase activity |
References
- FAO. Statistical Yearbook 2024 Reveals Critical Insights on the Sustainability of Global Agriculture, Food Security, and the Importance of Agrifood Systems in Employment. Available online: https://www.fao.org/newsroom/detail/fao-statistical-yearbook-2024-reveals-critical-insights-on-the-sustainability-of-agriculture-food-security-and-the-importance-of-agrifood-in-employment/en (accessed on 24 May 2025).
- Rai, P.K.; Sonne, C.; Kim, K.-H. Heavy Metals and Arsenic Stress in Food Crops: Elucidating Antioxidative Defense Mechanisms in Hyperaccumulators for Food Security, Agricultural Sustainability, and Human Health. Sci. Total Environ. 2023, 874, 162327. [Google Scholar] [CrossRef] [PubMed]
- Focus, E.; Rwiza, M.J.; Mohammed, N.K.; Banzi, F.P. Health Risk Assessment of Trace Elements in Soil for People Living and Working in a Mining Area. J. Environ. Public Health 2021, 2021, 9976048. [Google Scholar] [CrossRef] [PubMed]
- Rashid, A.; Schutte, B.J.; Ulery, A.; Deyholos, M.K.; Sanogo, S.; Lehnhoff, E.A.; Beck, L. Heavy Metal Contamination in Agricultural Soil: Environmental Pollutants Affecting Crop Health. Agronomy 2023, 13, 1521. [Google Scholar] [CrossRef]
- Mesele, S.A.; Mechri, M.; Okon, M.A.; Isimikalu, T.O.; Wassif, O.M.; Asamoah, E.; Ahmad, H.A.; Moepi, P.I.; Gabasawa, A.I.; Bello, S.K.; et al. Current Problems Leading to Soil Degradation in Africa: Raising Awareness and Finding Potential Solutions. Eur. J. Soil Sci. 2025, 76, e70069. [Google Scholar] [CrossRef]
- Ghouma, A.; Aydi, A.; Martin, J.A.R.; Gasmi, M. Health Risk Assessment Associated to Heavy Metal Pollution Levels in Mediterranean Environment Soils: A Case Study in the Watershed of Sebkhet Ariana, Tunisia. Arab. J. Geosci. 2022, 15, 716. [Google Scholar] [CrossRef]
- Salhi, R.; Durães, N.; Dhaoui, M.; Patinha, C.; Ferreira da Silva, E.; Mlayah, A. Contamination Assessment and Availability of Potential Toxic Elements from the Sidi Driss Tailing Pile (NW Tunisia) Based on Geochemical and Geophysical Methods. J. Afr. Earth Sci. 2023, 202, 104921. [Google Scholar] [CrossRef]
- Ayari, J.; Barbieri, M.; Barhoumi, A.; Boschetti, T.; Braham, A.; Dhaha, F.; Charef, A. Trace Metal Element Pollution in Media from the Abandoned Pb and Zn Mine of Lakhouat, Northern Tunisia. J. Geochem. Explor. 2023, 247, 107180. [Google Scholar] [CrossRef]
- Xia, F.; Zhao, Z.; Niu, X.; Wang, Z. Integrated Pollution Analysis, Pollution Area Identification and Source Apportionment of Heavy Metal Contamination in Agricultural Soil. J. Hazard. Mater. 2024, 465, 133215. [Google Scholar] [CrossRef]
- Nouairi, J.; Baraud, F.; Leleyter, L.; Mefteh, S.; Rocha, F.; Medhioub, M. Spatial Distribution and Ecological Risk Assessment of Potentially Toxic Elements in Agricultural Soils, Stream Sediments, and Plants around Lakhouat Mine (Northwestern Tunisia). Arab. J. Geosci. 2021, 14, 130. [Google Scholar] [CrossRef]
- Mabrouk, L.; Mabrouk, W.; Mansour, H.B. High Leaf Fluctuating Asymmetry in Two Native Plants Growing in Heavy Metal-Contaminated Soil: The Case of Metlaoui Phosphate Mining Basin (Gafsa, Tunisia). Environ. Monit. Assess. 2020, 192, 406. [Google Scholar] [CrossRef]
- Achour, Y.; Souissi, R.; Tlil, H.; Heino, M.M.; Souissi, F. Heavy Metals (Pb, Zn, Cd) and Metalloids (Sb, As) in Carbonated Soils Contaminated by Mine Tailings (North Tunisia). In New Prospects in Environmental Geosciences and Hydrogeosciences; Chenchouni, H., Chaminé, H.I., Khan, M.F., Merkel, B.J., Zhang, Z., Li, P., Kallel, A., Khélifi, N., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 227–230. [Google Scholar]
- Álvarez-Rogel, J.; Peñalver-Alcalá, A.; González-Alcaraz, M.N. Spontaneous Vegetation Colonizing Abandoned Metal(Loid) Mine Tailings Consistently Modulates Climatic, Chemical and Biological Soil Conditions Throughout Seasons. Sci. Total Environ. 2022, 838, 155945. [Google Scholar] [CrossRef]
- Lohar, S.; Kumari, P.; Sharma, A.; Godara, V.; Nagar, V.; Prajapati, M.; Singhal, A.; Sharma, V.; Verma, R.; Singh Sankhla, M. Biochar Enhancing Soil Resilience: A Dual Strategy for Mitigating Heavy Metal Contamination and Drought Stress. Lett. Appl. NanoBioScience 2024, 13, 70. [Google Scholar] [CrossRef]
- Islam, M.; Sandhi, A. Heavy Metal and Drought Stress in Plants: The Role of Microbes—A Review. Gesunde Pflanz. 2023, 75, 695–708. [Google Scholar] [CrossRef]
- Qiao, P.; Wang, S.; Li, J.; Zhao, Q.; Wei, Y.; Lei, M.; Yang, J.; Zhang, Z. Process, Influencing Factors, and Simulation of the Lateral Transport of Heavy Metals in Surface Runoff in a Mining Area Driven by Rainfall: A Review. Sci. Total Environ. 2023, 857, 159119. [Google Scholar] [CrossRef]
- Machairas, E.; Varouchakis, E.A. Cost–Benefit Analysis and Risk Assessment for Mining Activities in Terms of Circular Economy and Their Environmental Impact. Geosciences 2023, 13, 318. [Google Scholar] [CrossRef]
- Mestanza-Ramón, C.; Cuenca-Cumbicus, J.; D’Orio, G.; Flores-Toala, J.; Segovia-Cáceres, S.; Bonilla-Bonilla, A.; Straface, S. Gold Mining in the Amazon Region of Ecuador: History and a Review of its Socio-Environmental Impacts. Land 2022, 11, 221. [Google Scholar] [CrossRef]
- Hachani, C.; Lamhamedi, M.S.; Abassi, M.; Sleimi, N.; Béjaoui, Z. Effects of Heavy Metal-Polluted Soil (Pb, Zn, and Cd) on Seed Emergence, Seedling Growth, and Antioxidant Activity in Four Fabaceae Species. Water. Air. Soil Pollut. 2022, 233, 263. [Google Scholar] [CrossRef]
- Rao, M.J.; Zheng, B. The Role of Polyphenols in Abiotic Stress Tolerance and Their Antioxidant Properties to Scavenge Reactive Oxygen Species and Free Radicals. Antioxidants 2025, 14, 74. [Google Scholar] [CrossRef] [PubMed]
- Farooq, T.H.; Rafay, M.; Basit, H.; Shakoor, A.; Shabbir, R.; Riaz, M.U.; Ali, B.; Kumar, U.; Qureshi, K.A.; Jaremko, M. Morpho-Physiological Growth Performance and Phytoremediation Capabilities of Selected Xerophyte Grass Species Toward Cr and Pb Stress. Front. Plant Sci. 2022, 13, 997120. [Google Scholar] [CrossRef]
- Kour, J.; Kohli, S.K.; Khanna, K.; Bakshi, P.; Sharma, P.; Singh, A.D.; Ibrahim, M.; Devi, K.; Sharma, N.; Ohri, P.; et al. Brassinosteroid Signaling, Crosstalk and, Physiological Functions in Plants Under Heavy Metal Stress. Front. Plant Sci. 2021, 12, 608061. [Google Scholar] [CrossRef]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent Developments in Enzymatic Antioxidant Defence Mechanism in Plants with Special Reference to Abiotic Stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Ashraf, M. Proline Alleviates Abiotic Stress Induced Oxidative Stress in Plants. J. Plant Growth Regul. 2023, 42, 4629–4651. [Google Scholar] [CrossRef]
- Gregory, P.J. RUSSELL REVIEW are Plant Roots Only “in” Soil or Are They “of” it? Roots, Soil Formation and Function. Eur. J. Soil Sci. 2022, 73, e13219. [Google Scholar] [CrossRef]
- Maity, P.J.; Pawlowski, K. Anthropogenic Influences on the Distribution of the Casuarina-Frankia Symbiosis. Symbiosis 2021, 84, 353–367. [Google Scholar] [CrossRef]
- Ghazouani, S.; Béjaoui, Z.; Michael, P.; Spiers, G.; Beckett, P.; Gtari, M.; Nkongolo, K. Rhizobioaugmentation of Casuarina glauca with N-Fixing Actinobacteria Frankia Decreases Enzymatic Activities in Wastewater Irrigated Soil: Effects of Frankia on C. glauca Growth. Ecotoxicology 2020, 29, 417–428. [Google Scholar] [CrossRef]
- Laamari, I.; Marques, I.; Ribeiro-Barros, A.I.; Béjaoui, Z.; Abassi, M. Can Saline Preconditioning Enhance Plant Survival in Degraded Soils? Physiological, Biochemical, and Molecular Responses in Casuarina Glauca Saplings. Plant Ecol. 2023, 224, 905–919. [Google Scholar] [CrossRef]
- Slaimi, R.; Abassi, M.; Béjaoui, Z. Assessment of Casuarina Glauca as Biofiltration Model of Secondary Treated Urban Wastewater: Effect on Growth Performances and Heavy Metals Tolerance. Environ. Monit. Assess. 2021, 193, 653. [Google Scholar] [CrossRef]
- Jorge, T.F.; Ramalho, J.C.; Alseekh, S.; Pais, I.P.; Leitão, A.E.; Rodrigues, A.P.; Scotti-Campos, P.; Ribeiro-Barros, A.I.; Fernie, A.R.; António, C. Will Casuarina Glauca Stress Resilience Be Maintained in the Face of Climate Change? Metabolites 2021, 11, 593. [Google Scholar] [CrossRef]
- Fernandes, I.; Paulo, O.S.; Marques, I.; Sarjkar, I.; Sen, A.; Graça, I.; Pawlowski, K.; Ramalho, J.C.; Ribeiro-Barros, A.I. Salt Stress Tolerance in Casuarina Glauca: Insights from the Branchlets Transcriptome. Plants 2022, 11, 2942. [Google Scholar] [CrossRef]
- Mansoor, S.; Kour, N.; Manhas, S.; Zahid, S.; Wani, O.A.; Sharma, V.; Wijaya, L.; Alyemeni, M.N.; Alsahli, A.A.; El-Serehy, H.A.; et al. Biochar as a Tool for Effective Management of Drought and Heavy Metal Toxicity. Chemosphere 2021, 271, 129458. [Google Scholar] [CrossRef]
- Kumar, A.; Bhattacharya, T.; Shaikh, W.A.; Roy, A. Sustainable Soil Management under Drought Stress Through Biochar Application: Immobilizing Arsenic, Ameliorating Soil Quality, and Augmenting Plant Growth. Environ. Res. 2024, 259, 119531. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, Y.-J.; Novak, A.; Yang, Y.; Wang, J. Role of Biochar in Improving Sandy Soil Water Retention and Resilience to Drought. Water 2021, 13, 407. [Google Scholar] [CrossRef]
- Ali, A.B.; Elshaikh, N.A. Review: Performance of Biochar under Diminish Water Stress in Plants. Commun. Soil Sci. Plant Anal. 2022, 53, 1–16. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, Q.; Chen, Y.; Lu, H. Effects of Biochar on Plant Growth and Hydro-Chemical Properties of Recycled Concrete Aggregate. Sci. Total Environ. 2023, 882, 163557. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Jabeen, N.; Chachar, Z.; Chachar, S.; Ahmed, S.; Ahmed, N.; Laghari, A.A.; Sahito, Z.A.; Farruhbek, R.; Yang, Z. The Role of Biochar in Enhancing Soil Health & Interactions with Rhizosphere Properties and Enzyme Activities in Organic Fertilizer Substitution. Front. Plant Sci. 2025, 16, 1595208. [Google Scholar] [CrossRef]
- Karimi, A.; Moezzi, A.; Chorom, M.; Enayatizamir, N. Application of Biochar Changed the Status of Nutrients and Biological Activity in a Calcareous Soil. J. Soil Sci. Plant Nutr. 2020, 20, 450–459. [Google Scholar] [CrossRef]
- Yuan, C.; Gao, B.; Peng, Y.; Gao, X.; Fan, B.; Chen, Q. A Meta-Analysis of Heavy Metal Bioavailability Response to Biochar Aging: Importance of Soil and Biochar Properties. Sci. Total Environ. 2021, 756, 144058. [Google Scholar] [CrossRef]
- Conte, P.; Librici, C.; Nicosia, A.; Palmeri, V.; Pampalone, V.; Ferro, V. Wood-Biochar Influence on Rill Erosion Processes and Hydrological Connectivity in Amended Soils. Hydrol. Process. 2025, 39, e70093. [Google Scholar] [CrossRef]
- Huang, X.; Cai, W.; Bordoloi, S. Measurement and Prediction of Gas Permeability Function for Biochar-Amended Rooted Soils. Geoderma 2024, 445, 116882. [Google Scholar] [CrossRef]
- Ceriani, A.; Dalle Fratte, M.; Agosto, G.; Beatrice, P.; Reguzzoni, M.; Bettucci, L.; Casini, D.; Cerabolini, B.E.L.; Montagnoli, A. Woody and Herbaceous Invasive Alien Plant Species-derived Biochars Are Potentially Optimal for Soil Amendment, Soil Remediation, and Carbon Storage. GCB Bioenergy 2024, 16, e13117. [Google Scholar] [CrossRef]
- Chandra, S.; Medha, I.; Bhattacharya, J.; Vanapalli, K.R.; Samal, B. Effect of the Co-Application of Eucalyptus Wood Biochar and Chemical Fertilizer for the Remediation of Multimetal (Cr, Zn, Ni, and Co) Contaminated Soil. Sustainability 2022, 14, 7266. [Google Scholar] [CrossRef]
- Shafiq, M.; Alazba, A.A.; Amin, M.T. Kinetic and Isotherm Studies of Ni2+ and Pb2+ Adsorption from Synthetic Wastewater Using Eucalyptus camdulensis—Derived Biochar. Sustainability 2021, 13, 3785. [Google Scholar] [CrossRef]
- Muhiwa, A.B.; Fonteh, M.F.; Njoyim, E.B.T.; Gapgue, F.N. Physicochemical Characterization of Biochars from Eucalyptus maiden Entandrophragma cylindricum (Liboyo), Milicia excelsa (Muvula.) and Ocotea michelsonie (Licheche) Used in Goma City, DR Congo for Water Treatment Potentials. Int. J. Eng. Sci. Technol. 2021, 13, 15–24. [Google Scholar] [CrossRef]
- Kalina, M.; Sovova, S.; Hajzler, J.; Kubikova, L.; Trudicova, M.; Smilek, J.; Enev, V. Biochar Texture—A Parameter Influencing Physicochemical Properties, Morphology, and Agronomical Potential. Agronomy 2022, 12, 1768. [Google Scholar] [CrossRef]
- Butphu, S.; Rasche, F.; Cadisch, G.; Kaewpradit, W. Eucalyptus Biochar Application Enhances Ca Uptake of Upland Rice, Soil Available P, Exchangeable K, Yield, and N Use Efficiency of Sugarcane in a Crop Rotation System. J. Plant Nutr. Soil Sci. 2020, 183, 58–68. [Google Scholar] [CrossRef]
- Ullah, A.; Ren, W.-L.; Tian, P.; Yu, X.-Z. Biochar as a Green Strategy in Alleviating Cd Mobility in Soil and Uptake in Plants: A Step towards Cd-Free Food. Int. Biodeterior. Biodegrad. 2024, 190, 105787. [Google Scholar] [CrossRef]
- Trifi, M.; Dermech, M.; Abdelkrim, C.; Azouzi, R.; Hjiri, B. Extraction Procedures of Toxic and Mobile Heavy Metal Fraction from Complex Mineralogical Tailings Affected by Acid Mine Drainage. Arab. J. Geosci. 2018, 11, 328. [Google Scholar] [CrossRef]
- Singh, B.; Singh, B.P.; Cowie, A.L. Characterisation and Evaluation of Biochars for Their Application as a Soil Amendment. Soil Res. 2010, 48, 516–525. [Google Scholar] [CrossRef]
- Béjaoui, Z. Tolérance de Divers Clones de Peuplier à L′hydromorphie: Aspects Morphologiques, Écophysiologiques et Métaboliques. Ph.D. Thesis, University of Carthage, Tunis, Tunisia, 2006. [Google Scholar]
- Hachani, C.; Lamhamedi, M.S.; Zine El Abidine, A.; Abassi, M.; Khasa, D.P.; Béjaoui, Z. Water Relations, Gas Exchange, Chlorophyll Fluorescence and Electrolyte Leakage of Ectomycorrhizal Pinus Halepensis Seedlings in Response to Multi-Heavy Metal Stresses (Pb, Zn, Cd). Microorganisms 2021, 10, 57. [Google Scholar] [CrossRef]
- de Santana, T.A.; Oliveira, P.S.; Silva, L.D.; Laviola, B.G.; de Almeida, A.-A.F.; Gomes, F.P. Water Use Efficiency and Consumption in Different Brazilian Genotypes of Jatropha curcas L. Subjected to Soil Water Deficit. Biomass Bioenergy 2015, 75, 119–125. [Google Scholar] [CrossRef]
- Denden, M.; Bettaieb, T.; Salhi, A.; Mathlouthi, M. Effet de la salinité sur la fluorescence chlorophyllienne, la teneur en proline et la production florale de trois espèces ornementales. Tropicultura 2005, 23, 220–225. [Google Scholar]
- Portillo-Estrada, M.; Copolovici, L.; Niinemets, Ü. Bias in Leaf Dry Mass Estimation After Oven-Drying Isoprenoid-Storing Leaves. Trees 2015, 29, 1805–1816. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. [34] Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Methods in Enzymology; Plant Cell Membranes; Academic Press: Cambridge, MA, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Shabala, S.N.; Shabala, S.I.; Martynenko, A.I.; Babourina, O.; Newman, I.A. Salinity Effect on Bioelectric Activity, Growth, Na+ Accumulation and Chlorophyll Fluorescence of Maize Leaves: A Comparative Survey and Prospects for Screening. Funct. Plant Biol. 1998, 25, 609–616. [Google Scholar] [CrossRef]
- Loutfy, N.; El-Tayeb, M.A.; Hassanen, A.M.; Moustafa, M.F.M.; Sakuma, Y.; Inouhe, M. Changes in the Water Status and Osmotic Solute Contents in Response to Drought and Salicylic Acid Treatments in Four Different Cultivars of Wheat (Triticum aestivum). J. Plant Res. 2012, 125, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Barrs, H.D.; Weatherley, P.E. A Re-Examination of the Relative Turgidity Technique for Estimating Water Deficits in Leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Singh, N.; Ma, L.Q.; Srivastava, M.; Rathinasabapathi, B. Metabolic Adaptations to Arsenic-Induced Oxidative Stress in Pteris vittata L. and Pteris ensiformis L. Plant Sci. 2006, 170, 274–282. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the Thiobarbituric Acid-Reactive-Substances Assay for Estimating Lipid Peroxidation in Plant Tissues Containing Anthocyanin and Other Interfering Compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts: I. Kinetics and Stoichiometry of Fatty Acid Peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Aebi, H. [13] Catalase In Vitro. In Methods in Enzymology; Oxygen Radicals in Biological Systems; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Anderson, J.M.; Chow, W.S.; Park, Y.-I. The Grand Design of Photosynthesis: Acclimation of the Photosynthetic Apparatus to Environmental Cues. Photosynth. Res. 1995, 46, 129–139. [Google Scholar] [CrossRef]
- Mika, A.; Lüthje, S. Properties of Guaiacol Peroxidase Activities Isolated from Corn Root Plasma Membranes. Plant Physiol. 2003, 132, 1489–1498. [Google Scholar] [CrossRef]
- Monneveux, P.; Nemmar, M. Contribution à l’étude de La Résistance à La Sécheresse Chez Le Blé Tendre (Triticum aestivum L.) et Chez Le Blé Dur (Triticum durum Desf.): Étude de l’accumulation de La Proline Au Cours Du Cycle de Développement. Agronomie 1986, 6, 583–590. [Google Scholar] [CrossRef]
- Blum, A.; Ebercon, A. Cell Membrane Stability as a Measure of Drought and Heat Tolerance in Wheat1. Crop Sci. 1981, 21, 43–47. [Google Scholar] [CrossRef]
- Nandillon, R.; Lebrun, M.; Miard, F.; Gaillard, M.; Sabatier, S.; Morabito, D.; Bourgerie, S. Contrasted Tolerance of Agrostis capillaris metallicolous and Non-Metallicolous Ecotypes in the Context of a Mining Technosol Amended by Biochar, Compost and Iron Sulfate. Environ. Geochem. Health 2021, 43, 1457–1475. [Google Scholar] [CrossRef]
- Pérez-Cid, B.; Lavilla, I.; Bendicho, C. Speeding up of a Three-Stage Sequential Extraction Method for Metal Speciation Using Focused Ultrasound. Anal. Chim. Acta 1998, 360, 35–41. [Google Scholar] [CrossRef]
- Malik, R.N.; Husain, S.Z.; Nazir, I. Heavy Metal Contamination and Accumulation in Soil and Wild Plant Species from Industrial Area of Islamabad, Pakistan. Pak. J. Bot. 2010, 42, 291–301. [Google Scholar]
- Padmavathiamma, P.K.; Li, L.Y. Phytoavailability and Fractionation of Lead and Manganese in a Contaminated Soil after Application of Three Amendments. Bioresour. Technol. 2010, 101, 5667–5676. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Zhang, M.; Zhang, L.; Li, J.; Liu, J. Physiological and Molecular Responses of Tropical Seagrass Enhalus acoroides Exposed to Simultaneous High Temperature and Hypoxia Stress. Mar. Environ. Res. 2025, 205, 106997. [Google Scholar] [CrossRef]
- Croce, R.; Carmo-Silva, E.; Cho, Y.B.; Ermakova, M.; Harbinson, J.; Lawson, T.; McCormick, A.J.; Niyogi, K.K.; Ort, D.R.; Patel-Tupper, D. Perspectives on Improving Photosynthesis to Increase Crop Yield. Plant Cell 2024, 36, 3944–3973. [Google Scholar] [CrossRef]
- Rizvi, A.; Zaidi, A.; Ameen, F.; Ahmed, B.; AlKahtani, M.D.; Khan, M.S. Heavy Metal Induced Stress on Wheat: Phytotoxicity and Microbiological Management. RSC Adv. 2020, 10, 38379–38403. [Google Scholar] [CrossRef]
- Wang, L.; Rinklebe, J.; Tack, F.M.G.; Hou, D. A Review of Green Remediation Strategies for Heavy Metal Contaminated Soil. Soil Use Manag. 2021, 37, 936–963. [Google Scholar] [CrossRef]
- Zhang, W.; Niu, W.; Luo, H. Effect of Biochar Amendment on the Growth and Photosynthetic Traits of Plants Under Drought Stress: A Meta-Analysis. Agronomy 2024, 14, 2952. [Google Scholar] [CrossRef]
- Mahmood, S.; Al-Solaimani, S.G.; Shams, S.; Naveed, S.; Haider, B.; Naveed, M.; Ali, R.; Waqas, M. Silicon and Biochar Synergistically Stimulate Nutrients Uptake, Photosynthetic Pigments, Gaseous Exchange and Oxidative Defense to Improve Maize Growth Under Salinity. Water. Air. Soil Pollut. 2024, 235, 413. [Google Scholar] [CrossRef]
- Hasnain, M.; Munir, N.; Abideen, Z.; Zulfiqar, F.; Koyro, H.W.; El-Naggar, A.; Caçador, I.; Duarte, B.; Rinklebe, J.; Yong, J.W.H. Biochar-Plant Interaction and Detoxification Strategies under Abiotic Stresses for Achieving Agricultural Resilience: A Critical Review. Ecotoxicol. Environ. Saf. 2023, 249, 114408. [Google Scholar] [CrossRef]
- Kumar, S.; Shah, S.H.; Vimala, Y.; Jatav, H.S.; Ahmad, P.; Chen, Y.; Siddique, K.H. Abscisic Acid: Metabolism, Transport, Crosstalk with Other Plant Growth Regulators, and its Role in Heavy Metal Stress Mitigation. Front. Plant Sci. 2022, 13, 972856. [Google Scholar] [CrossRef] [PubMed]
- Mashabela, M.D.; Masamba, P.; Kappo, A.P. Applications of Metabolomics for the Elucidation of Abiotic Stress Tolerance in Plants: A Special Focus on Osmotic Stress and Heavy Metal Toxicity. Plants 2023, 12, 269. [Google Scholar] [CrossRef]
- Sabir, A.; Naveed, M.; Bashir, M.A.; Hussain, A.; Mustafa, A.; Zahir, Z.A.; Kamran, M.; Ditta, A.; Núñez-Delgado, A.; Saeed, Q.; et al. Cadmium Mediated Phytotoxic Impacts in Brassica napus: Managing Growth, Physiological and Oxidative Disturbances through Combined Use of Biochar and Enterobacter sp. MN17. J. Environ. Manag. 2020, 265, 110522. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Lin, H.; Chen, Y.; Wang, H.; Ritenour, M.A.; Lin, Y. Hydrogen Peroxide-Induced Changes in Activities of Membrane Lipids-Degrading Enzymes and Contents of Membrane Lipids Composition in Relation to Pulp Breakdown of Longan Fruit during Storage. Food Chem. 2019, 297, 124955. [Google Scholar] [CrossRef]
- Altaf, M.A.; Hao, Y.; Shu, H.; Mumtaz, M.A.; Cheng, S.; Alyemeni, M.N.; Ahmad, P.; Wang, Z. Melatonin Enhanced the Heavy Metal-Stress Tolerance of Pepper by Mitigating the Oxidative Damage and Reducing the Heavy Metal Accumulation. J. Hazard. Mater. 2023, 454, 131468. [Google Scholar] [CrossRef]
- Anisimov, V.; Anisimova, L.; Krylenkin, D.; Dikarev, D.; Sanzharov, A.; Korneev, Y.N.; Kostyukov, I.; Kolyagin, Y.G. A Study on the Behavior of Cadmium in the Soil Solution–Plant System by the Lysimeter Method Using the 109Cd Radioactive Tracer. Plants 2023, 12, 649. [Google Scholar] [CrossRef]
- Graber, E.R.; Tsechansky, L.; Lew, B.; Cohen, E. Reducing Capacity of Water Extracts of Biochars and Their Solubilization of Soil Mn and Fe. Eur. J. Soil Sci. 2014, 65, 162–172. [Google Scholar] [CrossRef]
- Lu, H.; Li, Z.; Fu, S.; Méndez, A.; Gascó, G.; Paz-Ferreiro, J. Combining Phytoextraction and Biochar Addition Improves Soil Biochemical Properties in a Soil Contaminated with Cd. Chemosphere 2015, 119, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Ansari, M.W.; Gill, R.; Tuteja, N.; Gill, S.S. Arsenic Transport, Detoxification, and Recent Technologies for Mitigation: A Systemic Review. Plant Physiol. Biochem. 2024, 213, 108848. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, A.M.; Galyamova, M.R.; Sedykh, S.E. Bacterial Siderophores: Classification, Biosynthesis, Perspectives of Use in Agriculture. Plants 2022, 11, 3065. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Quan, G.; Yan, J.; Sui, F.; Wang, H.; Hina, K.; Abro, S.A.; Kitajima, N.; Kubota, H.; Tong, A. Synergistic Effects of Biochar and Arabidopsis helleri on Soil Cd and Pb Bioavailability and Uptake and Disposition. BioResources 2024, 19, 8324–8338. [Google Scholar] [CrossRef]
- Anne, O.; Mockevičienė, I.; Karčauskienė, D.; Repšienė, R.; Šiaudinis, G.; Barčauskaitė, K.; Žilė, G. Biochar-Assisted Phytoremediation Potential of Sewage Sludge Contaminated Soil. Sustainability 2024, 16, 183. [Google Scholar] [CrossRef]
- Gholizadeh, M.; Hu, X. Removal of Heavy Metals from Soil with Biochar Composite: A Critical Review of the Mechanism. J. Environ. Chem. Eng. 2021, 9, 105830. [Google Scholar] [CrossRef]
- Mohtadi, A.; Schat, H. A Comparison of Nickel and Zinc Uptake and Translocation in Three Species of Brassicaceae: The Ni Hyperaccumulator Odontarrhena corsica and Two Non-hyperaccumulators, Aurinia saxatilis and Lobularia maritima. Ecol. Res. 2024, 39, 596–604. [Google Scholar] [CrossRef]
- Van Der Ent, A.; Rylott, E.L. Inventing Hyperaccumulator Plants: Improving Practice in Phytoextraction Research and Terminology. Int. J. Phytoremediat. 2024, 26, 1379–1382. [Google Scholar] [CrossRef]

| Parameter | NMS | CS + EuB | CS | |
|---|---|---|---|---|
| pH (H2O) | 7.81 ± 0.01 b | 8.25 ± 0.005 a | 7.41 ± 0.012 c | |
| EC (µS·cm−1) | 53.50 ± 0.389 c | 133.80 ± 0.060 a | 70.80 ± 0.120 b | |
| P (%) | 30.96 ± 0.016 c | 36.51 ± 1.723 b | 40.33 ± 0.007 a | |
| K (cm·h−1) | 0.84 ± 0.008 a | 0.34 ± 0.018 c | 0.39 ± 0.004 b | |
| Ss (m2·g−1) | 1 ± 0.057 c | 20.36 ± 0.023 a | 18.13 ± 0.069 b | |
| Particle size distribution (%) | Sand | 22.40 | nd | 65.45 |
| Silt | 21.06 | nd | 14.80 | |
| Clay | 52.67 | nd | 19.45 | |
| Pseudototal concentration (mg/kg; DW) | Fe | 7605.14 ± 328.12 b | 164,242.66 ± 4776.18 a | 173,010.44 ± 1648.42 a |
| Zn | 48.75 ± 4.81 b | 4037.46 ± 47.97 a | 4028.82 ± 72.47 a | |
| Mn | 221.39 ± 22.87 d | 17,848.09 ± 521.02 b | 14,742.55 ± 384.35 c | |
| Pb | 55.30 ± 3.27 b | 4480.19 ± 307.08 a | 4402.05 ± 89.66 a | |
| Cd | 5.04 ± 0.09 b | 8.84 ± 0.06 a | 8.41 ± 0.23 a | |
| As | 60.86 ± 1.84 b | 476 ± 2.96 a | 447.50 ± 14.98 a | |
| Treatments | Fe | Zn | Mn | Pb | Cd | As |
|---|---|---|---|---|---|---|
| TF | ||||||
| NMS | 0.328 ± 0.073 a | 0.615 ± 0.010 a | 0.602 ± 0.140 a | 0.482 ± 0.007 a | 0.506 ± 0.017 a | 0.967 ± 0.046 a |
| CS | 0.025 ± 0.002 c | 0.137 ± 0.014 b | 0.083 ± 0.006 c | 0.034 ± 0.007 b | 0.593 ± 0.046 a | 0.195 ± 0.015 c |
| CS + EuB | 0.056 ± 0.021 b | 0.108 ± 0.023 b | 0.177 ± 0.033 b | 0.029 ± 0.001 c | 0.397 ± 0.009 b | 0.334 ± 0.044 b |
| BCF | ||||||
| NMS | 4.421 ± 0.552 c | 0.760 ± 0.036 b | 0.152 ± 0.038 c | 0.259 ± 0.010 b | _ | 1.805 ± 0.045 c |
| CS | 30.164 ± 2.147 b | 2.339 ± 0.151 a | 1.562 ± 0.130 a | 0.571 ± 0.046 a | _ | 5.267 ± 0.201 a |
| CS + EuB | 102.057 ± 0.574 a | 0.248 ± 0.001 c | 0.398 ± 0.013 b | 0.555 ± 0.018 a | 7.395 ± 0.150 a | 4.026 ± 0.119 b |
| FBC (mg·kg−1) | ||||||
| NMS | 24.560 ± 0.672 b | 10.618 ± 0.309 c | 67.564 ± 0.409 c | 13.677 ± 0.458 c | ˂QL | 36.046 ± 0.330 c |
| CS | 55.195 ± 0.918 a | 24.816 ± 0.136 b | 108.848 ± 0.040 b | 92.603 ± 0.585 b | ˂QL | 37.286 ± 0.032 b |
| CS + EuB | 15.844 ± 0.053 c | 249.922 ± 0.765 a | 245.230 ± 1.639 a | 161.233 ± 0.681 a | 0.078 ± 0.001 a | 37.899 ± 0.201 a |
| CFN (mg·g−1) | ||||||
| NMS | 2.759 ± 0.159 b | 0.058 ± 0.007 c | 0.046 ± 0.003 c | 0.027 ± 0.005 c | - | 0.068 ± 0.008 b |
| CS | 116.638 ± 3.261 a | 1.657 ± 0.061 b | 4.898 ± 0.170 b | 1.834 ± 0.047 b | 0.001 ± 0.0001 b | 0.530 ± 0.010 a |
| CS + EuB | 119.498 ± 5.680 a | 2.447 ± 0.123 a | 12.164 ± 0.317 a | 2.798 ± 0.103 a | 0.002 ± 0.00009 a | 0.543 ± 0.057 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayadi, O.; Tlili, K.; Bourgerie, S.; Bejaoui, Z. Eucalyptus-Biochar Application for Mitigating the Combined Effects of Metal Toxicity and Osmotic-Induced Drought in Casuarina glauca Seedlings. Land 2025, 14, 1423. https://doi.org/10.3390/land14071423
Ayadi O, Tlili K, Bourgerie S, Bejaoui Z. Eucalyptus-Biochar Application for Mitigating the Combined Effects of Metal Toxicity and Osmotic-Induced Drought in Casuarina glauca Seedlings. Land. 2025; 14(7):1423. https://doi.org/10.3390/land14071423
Chicago/Turabian StyleAyadi, Oumaima, Khawla Tlili, Sylvain Bourgerie, and Zoubeir Bejaoui. 2025. "Eucalyptus-Biochar Application for Mitigating the Combined Effects of Metal Toxicity and Osmotic-Induced Drought in Casuarina glauca Seedlings" Land 14, no. 7: 1423. https://doi.org/10.3390/land14071423
APA StyleAyadi, O., Tlili, K., Bourgerie, S., & Bejaoui, Z. (2025). Eucalyptus-Biochar Application for Mitigating the Combined Effects of Metal Toxicity and Osmotic-Induced Drought in Casuarina glauca Seedlings. Land, 14(7), 1423. https://doi.org/10.3390/land14071423











