Abstract
The use of service (cover) crops is widely practiced in soil agriculture due to their many benefits, including enhanced nutrient supply and improved soil health. Bacteria, as major decomposers of plant residues in the soil, play essential roles in nutrient cycling. This study examined the impact of various almond orchard management practices on the soil microbial community composition in a hyper-arid ecosystem. High-throughput sequencing was used to compare the microbial communities in two adjacent almond orchards managed with either organic (ORG) or regenerative agriculture (RA) practices, alongside an uncultivated (UC) site. Notably, little is known about the responses of soil bacterial communities in hyper-arid regions to intercrop mulch from service crops. This study may offer insights into the ecological limits of the benefits of service crops in promoting soil health under extreme conditions. Our findings demonstrate that RA management can alter soil organic carbon levels and reshape microbial communities by increasing overall bacterial abundance and enriching specific keystone taxa. These changes may have significant implications for nutrient cycling processes in hyper-arid agroecosystems.
1. Introduction
Given the urgency of ongoing climate change, population growth, and land degradation, immediate action and adaptation to increasingly harsh climatic conditions are imperative [1]. To better prepare for an uncertain future, it is essential to investigate the effects of agricultural activities and management practices in hyper-arid zones through a comprehensive examination of their impact on the soil microbiome. The soil microbiome plays a pivotal role in the multifunctionality of terrestrial ecosystems with its significant influence on plant health and agricultural productivity, representing a critical area of research. Certain soil microorganisms can initiate plant diseases, while others contribute to disease suppression, nutrient uptake, and the promotion of plant growth [2,3,4]. There is an urgent need to preserve soil microbial diversity and to implement agro-management programs focused on the tailored restoration of soil ecosystem functions [5,6]. This can be accomplished by adopting new or modified land management practices that go beyond merely preserving and supporting the existing taxonomic and functional microbial diversity in soils.
The emergent movement of regenerative agriculture focuses on (1) minimizing soil disturbance; (2) keeping soil protected; (3) maintaining living roots in the soil; (4) maximizing plant diversity; and (5) reintroducing livestock activity. Restoring soil profiles and biomes requires diverse strategies to enhance field biodiversity. These include diversifying cropping systems, minimizing soil disturbance through reduced or no-till practices, and boosting soil organic matter through organic amendments or the establishment of service crops [7,8]. While the definitions and objectives of the regenerative agriculture movement vary among sources, common goals include carbon sequestration and increased biodiversity [7].
As a movement rather than a standardized set of agricultural practices, regenerative agriculture requires further research to identify which practices most effectively achieve these goals and in which ecological contexts they are most beneficial. One promising practice within regenerative agriculture is the use of service crops [9]. Regarding the soil microbiome, service crops have been shown to enhance bacterial abundance and activity, as well as modestly increase bacterial biodiversity [10]. Additionally, service crops are suggested to mitigate the decline in perennial productivity often caused by the accumulation of soil pathogens [2].
Increased biodiversity resulting from regenerative agricultural practices has been documented in at least 26 studies [7]. However, there is a lack of research on the effects of regenerative agriculture on soil bacterial communities and limited evidence of successfully implementing sustainable cropping systems in hyper-arid regions [11,12].
Metabarcoding analysis utilizing next-generation high-throughput sequencing of soil DNA samples is an effective and essential tool for determining the soil microbiome composition. This technique delivers comprehensive insights into the diversity, abundance, and presence of specific phyla, genera, and species within samples [13,14], as well as the functional profile of the soil microbiome [15].
This study investigates the impact of different almond orchard management practices on the soil bacterial community in an organic almond orchard located in a hyper-arid region. Specifically, it examines whether providing irrigation during the dry winter time of a hyper-arid region, enabling the growth of winter service crops under such conditions, can enhance soil organic carbon content and soil biodiversity. To address this, we compared the bacterial community structures of soils from two almond orchard plots managed using organic (ORG) and regenerative (RA) practices, as well as a nearby uncultivated (UC) zone. We hypothesize that regenerative agriculture will lead to the highest organic matter content and the greatest bacterial biodiversity, with distinct differences in bacterial community compositions associated with each management approach.
2. Materials and Methods
2.1. Study Site
This study took place at a ten-year-old almond orchard owned by Kibbutz Neot Smadar, located in the southern part of the Negev Desert of Israel (east of the Ramon craters and about 60 km north of Eilat). The orchard was planted in 2010 on Fluvents (coarse desert alluvium), according to [16], and managed as certified organic crops. The field had been prepared for agricultural activity (leveling and boulder removal by bulldozers) two years before the orchard establishment. This site represents the border of climatic conditions for growing productive and economic almond orchards. The average annual precipitation measured in the last 25 years at Neot Smadar Meteorological Station is less than 34 mm; the average daily temperature in the last 15 years ranges from 28.5 °C to 13.8 °C (https://ims.gov.il/he/data_gov). The orchard was irrigated by an average of 650 mm yr−1 along the almond growing season (April–October) with drip irrigation laid out along the tree rows. The orchard was managed as certified organic crops and was divided since its establishment into two management zones: a regenerative agriculture (RA) zone, where service crops were grown between the tree rows during the winter months (November–March), and “common” organic zone (OR), where the soil between the tree rows was left bare. For control, we have a sample nearby area that was also prepared for agricultural activity, such as an orchard, but left uncultivated (UC). It is important to mention that the annual precipitation in the study area needs to be 34 mm for growing winter service crops (a mixture of Lolium rigidum and Bromus madritensis). The farmer applied supplementary irrigation of 500 mm with a separate micro sprinkler system during the winter season (November to March) only for the RA zone. As a result, each zone experiences different moisture regimes along the 10 years before sampling, i.e., RA: 1184 mm yr−1; ORG: 684 mm yr−1; and UC: 34 mm yr−1, as can be seen in the aerial photo (Figure 1).
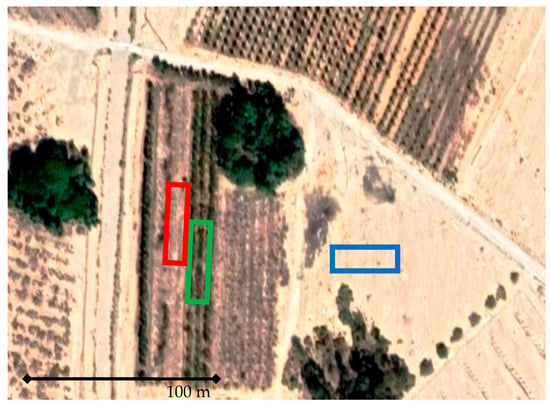
Figure 1.
Aerial photo from Google Maps showing study and sampling areas: green rectangle—RA; red rectangle—ORG; and blue rectangle—UC.
In August 2020, in each plot, four soil pits were excavated (0.6 m wide, 3 m long, and 1.2 m deep) with a backhoe, starting from the closest as possible to the tree trunk (without damaging the trees), diagonally toward the middle space between two tree rows. Soil samples of about 2 kg were collected along the pits to represent the in-layer orchard management from three layers: 0–10 cm, 10–30 cm, and 30–60 cm. For the biological analysis, about 1 kg of subsoil samples were sieved by a 2 mm sieve placed in a plastic bag, transported to the laboratory in an isolated container, and stored at 4 °C for the abiotic analysis. Subsamples of 1.5 mL were collected and stored at −20 °C for genetic analysis.
2.2. Abiotic Analysis
Soil moisture (SM) was measured gravimetrically by drying soil for 48 h at 105 °C. Organic matter content (OM) was measured gravimetrically by heating dried soil in a muffling furnace for 6 h at 400 °C. Soil pH was measured using a pH electrode from a filtered mixture of 1:2 soil and water. Soil electrical conductivity was used as a measurement for soil salinity, measured using an electrical conductivity/temperature electrode from a filtered mixture of 1:10 soil to distilled water [17].
2.3. Biotic Analysis
Soil DNA was extracted from 0.5 g soil using an Exgene soil DNA mini kit from GeneAll (Seoul, Republic of Korea), according to the producer’s protocol, using 50 µL elution buffer in the elution stage and storing the eluted DNA at −20 °C until DNA amplification. The eluted DNA was amplified twice using PCR. PCR1 was conducted by mixing 12.5 µL PCRBIO HS Taq Mix Red, 9.5 µL ultrapure water, 1 µL of primer CS1_515F (ACACTGACGACATGGTTCTACAGTGCCAGCMGCCGCGGT), 1 µL of primer CS2_806R (TACGGTAGCAGAGACTTGGTCTGGACTACHVGGGTWTCT), and 1 µL of the eluted DNA. The thermal cycling program was 95 °C for 3 min, 24 cycles of 98 °C for 10 s, 55 °C for 10 s, 72 °C for 20 s, and 72 °C for 1 min.
A 2 uL sample from the PCR1-amplified sample containing CS1/CS2 adaptors was amplified for 10 cycles in 10 µL using Fluidigm Access Array Barcode library according to the manufacturer’s protocol (2 uL barcode per reaction). DNA was purified using Kapa Pure Beads in a ratio of 0.65 x and quantified with Qubit using Denovix DsDNA (Wilmington, Delaware) high sensitivity assay. DNA size and integrity were quantified by Tapestation using Agilent DNA screen tape and reagents [18].
2.4. Sequencing
Samples were run on a dedicated MiSeq (Illumina, San Diego, CA, USA) machine with 30% Phix using the MiSeq Reagent Kit v2 500PE. Demultiplexing was performed using bcl2fastq, with default parameters allowing for 0 mismatches. Data were then mapped to PhiX using bowtie2 to remove PhiX control, and unmapped reads were quantified, collected, and examined using fast QC. Raw sequencing data were submitted to the NCBI under the accession number PRJNA1007432.
2.5. Data Analysis
Demultiplexed reads were uploaded into CLC genomics workbench (Qiagen, Hilden, Germany) and analyzed using the Microbial Genomics Module with the 16S microbiome pipeline. The analysis workflow consisted of quality filtration of the sequence data, and operational taxonomic unit (OTU) clustering was performed with default parameter settings. The adaptor sequence was removed, and the reads with a quality score lower than 25 or length < 150 were discarded. The maximum number of acceptable ambiguous nucleotides was set to 2, and the length of the reads was fixed at 200–500 bp. Chimeric sequences and singletons were detected and discarded. The remaining unique reads were used for OTU clustering, performed by alignment to the Greengenes database at 97% sequence similarity. For the redundancy analysis (RDA), we performed computations in R using the Vegan package [18], applying Bray–Curtis-transformed species abundance data to assess the relationships between bacterial community composition and environmental variables. The Shannon diversity index was calculated to assess alpha diversity within microbial communities. OTU (Operational Taxonomic Unit) abundance data were imported into R and processed using the Vegan package. The Shannon index, which accounts for both species richness and evenness, was computed for each sample using the diversity() function with the argument index = “Shannon”. This metric quantifies diversity by incorporating the proportional abundance of each OTU, with higher values indicating greater diversity. The input data_otu was a sample-by-OTU matrix, with counts representing the number of reads assigned to each OTU per sample.
Statistical analysis. Statistical analysis was conducted using XLSTAT. Two-way ANOVA was conducted, followed by Duncans MRT. Two-way PERMANOVA was conducted using the ADONIS function from the Vegan package in R [19]. Principle Component Analysis (PCA) was conducted using XLSTAT.
3. Results
3.1. Abiotic
3.1.1. Soil Moisture
As expected, the soil moisture varied between the different treatments and depths (Table 1). The RA samples had higher soil moisture in comparison with the UC and ORG samples in the 0–10 cm soil layer (p < 0.001, both comparisons), the 10–30 cm layer (p < 0.001 and p < 0.01, respectively), and the 30–60 cm layer (p < 0.001 and p < 0.05, respectively). The soil moisture was higher in the ORG than the UC samples in the 10–30 cm (p < 0.05) and 30–60 cm (p < 0.001) layers. Soil moisture differed between the soil layers in different patterns in the different treatments. In the UC and ORG treatments, we found an increasing moisture profile with depth; the 0–10 cm layer soil moisture was lower than both 10–30 cm and 30–60 cm (p < 0.05), while in the RA treatment, the 0–10 cm layer soil moisture was higher than in both the 10–30 cm and 30–60 cm layers, and the 10–30 cm soil layer had lower moisture than in the 30–60 cm layer but not significantly lower.

Table 1.
Mean (±SD) of abiotic soil parameters in different treatments and soil depths. (ORG—organic; RA—regenerative agriculture; UC—uncultivated).
3.1.2. Soil Organic Matter
In both the 0–10 cm and 10–30 cm soil layers, both the RA and ORG samples had higher organic matter content compared to the UC samples (p < 0.001 for all comparisons). In the 30–60 cm soil layer, the soil organic matter content was higher in both the RA and UC samples compared to the ORG samples (p < 0.005 and p < 0.05, respectively). The organic matter content was higher in the 0–10 cm layer compared to both the 10–30 cm and 30–60 cm soil layers in the UC (p < 0.01, both comparisons), ORG (p < 0.001, both comparisons), and RA (p < 0.001, both comparisons) treatments.
3.1.3. Soil Electrical Conductivity
In the 0–10 cm soil layer, the ORG samples showed higher soil electrical conductivity compared to both the UC and RA samples (p < 0.01 and p < 0.05, respectively), while in the 30–60 cm layer, the UC samples showed higher soil electrical conductivity than both the ORG and RA samples (p < 0.005 and p < 0.001, respectively). In the UC samples, both the 10–30 cm and 30–60 cm soil samples had higher electrical conductivity than the 0–10 cm sample (p < 0.001, both comparisons), while in the ORG samples, the 0–10 cm samples had higher electrical conductivity than both the 10–30 cm and 30–60 cm samples (p < 0.001 and 0.005, respectively) pH. The pH in the 0–10 cm soil layer samples was higher than in the 30–60 cm soil layer samples (p < 0.02). No further differences in pH between samples were found.
3.2. Biotic Components
Taxonomic analysis. In total, 255,747 reads (OTU) were obtained through sequencing: 23,386 belonged to Archaea, 11,559 were unidentified, and 220,802 belonged to Bacteria.
Effect of Regenerative Treatment of Bacteria Phyla Composition
A total of 34 phyla were identified in the soil samples. Proteobacteria was the dominant phylum in all samples, except in the 0–10 cm depth uncultivated (UC) sample (Figure 2). Actinobacteria had a relatively higher abundance in the 0–10 cm depth samples and was dominant in the 0–10 cm uncultivated sample. The phyla Bacteroidetes, Firmicutes, Gemmatimonadetes, and Acidobacteria, while present in significant amounts in the conservative and service crop treatments, were nearly absent in the uncultivated samples.
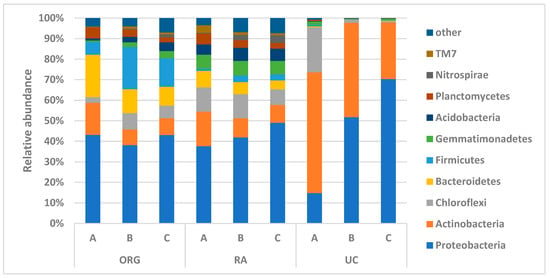
Figure 2.
Mean of phyla relative abundance. A. 0–10 cm, B. 10–30 cm, C. 30–60 cm. ORG—organic; RA—regenerative agriculture; UC—uncultivated.
3.3. Order
A total of 70 orders were detected, of which 31 orders were represented by more than 1–10% in at least one of the samples. Out of these 31 orders, seven were present across all three management systems, while seventeen orders were exclusive to ORG and RA, three were shared between RA and UC, and one was found only in ORG and UC (Table 2). Consequently, 27 and 28 orders were identified in ORG and RA, respectively, whereas the number of orders in UC was approximately 50% lower compared to the other two management systems.

Table 2.
Order present and absent in each of the three managements (ORG—organic; RA—regenerative agriculture; UC—uncultivated).
The most abundant orders consistently present across all three management systems were Actinomycetales, Bacillales, and Pseudomonadales. Each of these orders exhibits unique characteristics: Actinomycetales play a crucial role in breaking down recalcitrant organic matter, such as cellulose and lignin, and contribute to the stabilization of soil organic aggregates, functioning in some ways similarly to fungal communities. Members of the Bacillales order aid in nutrient acquisition and phytohormone production, promoting protection against soil pathogens and abiotic stressors. Pseudomonadales are common in the plant rhizosphere, where they act as non-pathogenic plant growth promoters and biocontrol agents.
In the 0–10 cm soil layer, the ORG sample exhibited a higher CHAO1 index compared to both the UC and RA samples (p < 0.05, both comparisons). In the 10–30 cm soil layer, the ORG sample had a higher CHAO1 index than both the UC and RA samples (p < 0.001 and p < 0.01, respectively), and the RA sample had a higher CHAO1 index than the UC sample (p < 0.001). In the 30–60 cm soil layer, both the ORG and RA samples had a higher CHAO1 index than the UC sample (p < 0.02 and p < 0.05, respectively). In the UC treatment, the 0–10 cm soil layer sample had a higher CHAO1 index than either the 10–30 cm and 30–60 cm soil layer samples (p < 0.01 and p < 0.001, respectively) (Table 3).

Table 3.
Mean diversity index (±SD) in different treatments and soil depths.
In the 10–30 cm soil layer, both the ORG and RA samples had a higher Shannon index than the UC sample (p < 0.001 for both comparisons), with the same pattern emerging in the 30–60 cm layer (p < 0.001 for both comparisons). In the UC treatment, the 0–10 cm soil layer had a higher Shannon index than both the 10–30 cm and 30–60 cm soil layer samples (p < 0.001 for both comparisons) (Table 3).
To assess the effects of the soil management practices on soil bacterial community, a redundancy analysis (RDA) function was performed (Figure 3). The results revealed distinct patterns among the three bacterial communities where the UC and the RA treatments are separated from each other while both showed partial overlap with the ORG treatment. The SM (soil moisture) and OM (organic matter) were significantly correlated with the bacterial community present in the RA site (the regenerative sampling site), whereas the bacterial community present in the ORG organic sampling site was correlated with EC., The bacterial community obtained from soil samples collected at the UC site showed no significant association with any of the measured soil physical–chemical data parameters.
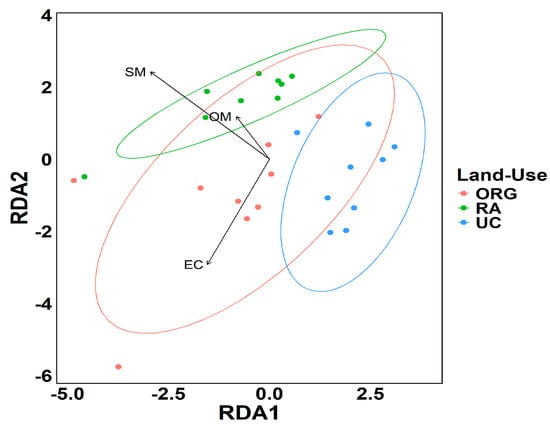
Figure 3.
Redundancy analysis for the different land uses. ORG—organic; RA—regenerative agriculture; UC—control.
PERMANOVA revealed that the bacterial community significantly differed between the soil depths, treatments, and interaction of soil depth and treatment (p < 0.001 for all the comparisons) (Table 4).

Table 4.
Permutational multivariate analysis of variance (PERMANOVA) for the bacterial community for different depths and treatments and interaction between them (Depth—soil sampling depth; Treat—treatments).
Characterization of Molecular Ecological Network of Bacteria Communities
The co-occurrence network characteristics of different bacterial co-occurrence networks were determined using Spearman correlations to explore differences in community structure among different soil treatments. The results indicated significant differences in the co-occurrence networks among the ORG, RA, and UC soils. ORG exhibited a higher number of nodes (103) and an average degree of 4.63 through abundance and correlation screening, whereas they were lower for RA (82 nodes, average degree of 3.88) and UC (52 nodes, average degree of 3.34), indicating a more complex microbial community structure in ORG (Figure 4). Moreover, for a low number of modularity, the number of edges and nodes was higher in ORG compared to RA and UC (Table 5), emphasizing the interconnection levels between the bacterial communities. By randomly removing nodes and observing changes in the average degree and natural connectivity, we demonstrated that the co-occurrence network of ORG was more stable. The total most connected taxa in each of the treatments were 13 for ORG and RA and 14 for UC.
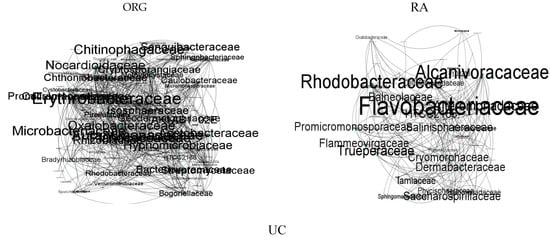
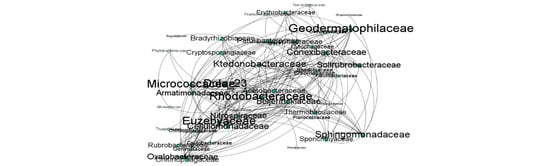
Figure 4.
The co-occurrence networks of bacterial communities at each one of the treatment sites; ORG—organic; RA—regenerative agriculture; UC—control. Note: In this visualization, each node represents an OTU categorized by family, with connections between nodes indicating statistically significant correlations (p < 0.01). The size of each node corresponds to its degree of connectivity, highlighting the centrality of OTUs within the network.

Table 5.
The co-occurrence networks of bacteria in three soil management types. The characteristics of network analysis of bacterial families (corr > 0.9; p ≤ 0.01).
4. Discussion
Soil microbial communities play a vital role in maintaining soil health, nutrient cycling, and plant productivity [20,21]. Irrigation is essential for supporting agricultural activity in the desert and, as a result, it can dramatically change the soil hydrological regime. In this study, the annual precipitation the zone received was from 34 to 684 up to 1184 mm yr−1 in the UN, ORG, and RA sites, respectively. The question raised in this study is whether applying extra irrigation to support living roots all year round in an almond orchard in hyper-arid regions will make a difference in the soil microbial community and activity. Organic (ORG) and regenerative agriculture (RA) management systems are designed to enhance soil quality, yet they differ in their principles and subsequent impacts on soil microbial communities [22]. Organic management tends to support greater organic microbial diversity and abundance particularly among taxa involved in the decomposition and nutrient cycling. However, certain practices, such as tillage, can disrupt microbial habitats and reduce the fungal-to-bacterial ratio. Regenerative agriculture expands upon organic principles by prioritizing soil health restoration through minimal disturbance, continuous plant cover, crop diversity, and livestock integration. These practices create a stable environment for soil microbes, promoting a balanced and resilient microbial community. Reduced tillage and permanent plant cover support fungal populations that are essential for building soil structure and enhancing nutrient exchange.
Our findings show that the organic matter content was significantly higher in the RA treatment compared to both the ORG treatment and the control (UC), suggesting that RA practices contributed to increased soil carbon stocks. Further research is needed to quantify the amount of carbon sequestered and to evaluate the overall carbon footprint of these activities. This observation is consistent with previous meta-analyses, although those studies did not focus on hyper-arid systems [23]. A high relative abundance of Actinobacteria in hyper-arid and arid ecosystems has been previously reported [24,25,26,27], particularly in the topsoil. This pattern was evident in our control plot but not in the other treatments, indicating a shift in the bacterial community composition. The analyses of phylum-level composition, PCA, and PERMANOVA further support that the treatments differ significantly in their bacterial community composition, especially in the 10–30 cm and 30–60 cm soil layers.
While our results align with the global trend of service crops enhancing bacterial biodiversity in comparison to bare fallow fields [10], they do not show an increase in bacterial biodiversity relative to ORG agriculture with the RA treatment. In a natural hyper-arid soil site, bacterial richness, but not diversity, has been shown to increase with simulated rainfall [28]. This may help explain the observed increases in soil bacterial richness in our study. Irrigation likely played a key role by significantly altering the hydrologic soil regime.
Regenerative management focuses on improving soil ecosystem services, such as carbon sequestration, soil health, and biodiversity restoration. This can be achieved by practices like intercropping, leaving crop residues on the soil surface, using organic amendments, avoiding pesticides, and reducing the use of chemical fertilizers [29].
Although network analysis does not always capture all existing interconnections within the bacterial community, it can still be useful for identifying key indicators that play crucial roles in shaping the network. Specifically, metrics such as edges, nodes, and average degree help elucidate the complex ecological relationships that are influenced by resource availability and environmental variations [28,29]. In our study, the number of nodes and edges in the bacteria network decreased from ORG to UC. This result supports our hypothesis that reduced organic matter leads to decreased microbial community complexity, aligning with previous studies [30,31] that reported increased aridity reduces not only bacterial diversity but also the overall biota community structure.
5. Conclusions
We hypothesize that regenerative agriculture will result in the highest organic matter content and the greatest bacterial biodiversity, with distinct differences in bacterial community compositions associated with each management approach. In summary, our results indicate that, although service crops are considered key components of the regenerative agriculture (RA) toolbox, their presence alone does not necessarily enhance soil bacterial biodiversity, at least not under the hyper-arid conditions studied here. This suggests that the effectiveness of applying service crops in a hyper-arid may be context-dependent and influenced by environmental factors such as climate, soil type, and management history. This study may provide evidence of an ecological threshold for the benefits of service crops in promoting soil health under extreme conditions. Further research is needed to disentangle the individual contributions of specific RA practices and to determine the conditions under which they are most effective. Such studies will be essential for optimizing RA strategies to enhance soil health and support sustainable agricultural productivity across diverse ecosystems.
Author Contributions
First draft, lab work, sequencing, methodology, software, I.A.; field sampling, lab work, final draft, conceptualization, G.E.; sequencing, data analysis, first draft, final draft, software, T.D.; conceptualizationsampling, lab work, data analysis, first draft, final draft, and submission, Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data will be available by request. The data were submitted to the NCBI under the accession number PRJNA1007432.
Acknowledgments
We would like to thank Yuval Shaul from Organic Ecologic Farming at the Neot Smadar community for providing the opportunity, sharing valuable information, and allowing us to excavate pits for soil sampling in their almond orchard.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Singh, P.K.; Chudasama, H. Pathways for climate change adaptations in arid and semi-arid regions. J. Clean. Prod. 2021, 284, 124744. [Google Scholar] [CrossRef]
- Vukicevich, E.; Lowery, T.; Bowen, P.; Úrbez-Torres, J.R.; Hart, M. Cover crops to increase soil microbial diversity and mitigate decline in perennial agriculture. A review. Agron. Sustain. Dev. 2016, 36, 48. Available online: https://link.springer.com/article/10.1007/s13593-016-0385-7 (accessed on 14 December 2023). [CrossRef]
- Smith, S.; Read, D. Mycorrhizal Symbiosis; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. Available online: https://link.springer.com/article/10.1007/s11274-017-2364-9 (accessed on 14 December 2023). [CrossRef] [PubMed]
- Fierer, N.; Walsh, C.M. Can we manipulate the soil microbiome to promote carbon sequestration in croplands? PLoS Biol. 2023, 21, e3002207. Available online: https://pubmed.ncbi.nlm.nih.gov/37437031/ (accessed on 23 April 2025). [CrossRef]
- Guerra, C.A.; Bardgett, R.D.; Caon, L.; Crowther, T.W.; Delgado-Baquerizo, M.; Montanarella, L.; Navarro, L.M.; Orgiazzi, A.; Singh, B.K.; Tedersoo, L.; et al. Tracking, targeting, and conserving soil biodiversity: A monitoring and indicator system can inform policy. Science 2021, 371, 239–241. Available online: https://www.science.org/doi/10.1126/science.abd7926 (accessed on 23 April 2025). [CrossRef]
- Newton, P.; Civita, N.; Frankel-Goldwater, L.; Bartel, K.; Johns, C. What Is Regenerative Agriculture? A Review of Scholar and Practitioner Definitions Based on Processes and Outcomes. Front. Sustain. Food Syst. 2020, 4, 577723. [Google Scholar] [CrossRef]
- Giller, K.E.; Hijbeek, R.; Andersson, J.A.; Sumberg, J. Regenerative Agriculture: An agronomic perspective. Outlook Agric. 2021, 50, 13–25. Available online: https://journals.sagepub.com/doi/full/10.1177/0030727021998063 (accessed on 3 December 2023). [CrossRef]
- Garcia, L.; Celette, F.; Gary, C.; Ripoche, A.; Valdés-Gómez, H.; Metay, A. Management of service crops for the provision of ecosystem services in vineyards: A review. Agric. Ecosyst. Environ. 2018, 251, 158–170. [Google Scholar] [CrossRef]
- Kim, N.; Zabaloy, M.C.; Guan, K.; Villamil, M.B. Do cover crops benefit soil microbiome? A meta-analysis of current research. Soil Biol. Biochem. 2020, 142, 107701. [Google Scholar] [CrossRef]
- Hermans, S.M.; Lear, G.; Case, B.S.; Buckley, H.L. The soil microbiome: An essential, but neglected, component of regenerative agroecosystems. Iscience 2023, 26, 106028. [Google Scholar] [CrossRef]
- Khangura, R.; Ferris, D.; Wagg, C.; Bowyer, J. Regenerative Agriculture—A Literature Review on the Practices and Mechanisms Used to Improve Soil Health. Sustainability 2023, 15, 2338. [Google Scholar] [CrossRef]
- Zhang, X.C.; Myrold, D.D.; Shi, L.L.; Kuzyakov, Y.; Dai, H.C.; Hoang, D.T.T.; Dippold, M.A.; Meng, X.T.; Song, X.N.; Li, Z.Y.; et al. Resistance of microbial community and its functional sensitivity in the rhizosphere hotspots to drought. Soil Biol. Biochem. 2021, 161, 108360. [Google Scholar] [CrossRef]
- Dhananjay, S.; Mittal, N.; Verma, S.; Singh, A.; Siddiqui, M.H. Applications of some advanced sequencing, analytical, and computational approaches in medicinal plant research: A review. Mol. Biol. Rep. 2024, 51, 23. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2: An improved and customizable approach for metagenome inference. BioRxiv 2020, v2 672295. Available online: https://www.biorxiv.org/content/10.1101/672295v2 (accessed on 2 April 2023).
- Itkin, D.; Ronen, A.; Needelman, B.; Crouvi, O.; Eshel, G. Israel Soil Taxonomy GIS (Online Version 2-1/2025); Environmental Resources Management Division and Digital Informavon Technologies Division, Ministry of Agriculture and Food Security: Beit Dagan, Israel, 2025. [Google Scholar]
- Whitford, W.G.; Stinnets, K.; Steinberger, Y. Effects of rainfall supplementation on microarthropods on decomposition roots in the Chihuahuan Desert. Pedobiologia 1988, 31, 147–156. [Google Scholar] [CrossRef]
- Yosef, S.; Doniger, T.; Appelbaum, I.; Sherman, C.; Rotbart, N. Effects of Vineyard Agro-management Practices on Soil Bacterial Community Composition, and Diversity. Microb. Ecol. 2024, 87, 17. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’hara, R.B.; Simpson, G.L.; Solymos, P. Vegan: Community Ecology Package. 2025. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 2 April 2023).
- Semenov, A.M. The Role of Microbial Communities in Soil Formation and Soil Ecosystem Health. Paleontol. J. 2020, 54, 843–852. [Google Scholar] [CrossRef]
- Sarkar, S.; Kumar, R.; Kumar, A.; Kumar, U.; Singh, D.K.; Mondal, S.; Kumawat, N.; Singh, A.K.; Raman, R.K.; Sundaram, P.K.; et al. Role of Soil Microbes to Assess Soil Health. In Structure and Functions of Pedosphere; Springer: Singapore, 2022; pp. 339–363. Available online: https://link.springer.com/chapter/10.1007/978-981-16-8770-9_14 (accessed on 23 April 2025).
- Kabenomuhangi, R. International Journal of Research Publication and Reviews Regenerative Agriculture and Soil Health: Enhancing Biodiversity through Sustainable Farming Practices. Int. J. Res. Publ. Rev. 2024, 5, 3203–3215. Available online: www.ijrpr.com (accessed on 23 April 2025).
- Poeplau, C.; Don, A. Carbon sequestration in agricultural soils via cultivation of cover crops—A meta-analysis. Agric. Ecosyst. Environ. 2015, 200, 33–41. [Google Scholar] [CrossRef]
- Neilson, J.W.; Quade, J.; Ortiz, M.; Nelson, W.M.; Legatzki, A.; Tian, F.; LaComb, M.; Betancourt, J.L.; Wing, R.A.; Soderlund, C.A.; et al. Life at the hyperarid margin: Novel bacterial diversity in arid soils of the Atacama Desert, Chile. Extremophiles 2012, 16, 553–566. Available online: https://link.springer.com/article/10.1007/s00792-012-0454-z (accessed on 5 December 2023). [CrossRef]
- Feng, W.; Zhang, Y.; Yan, R.; Lai, Z.; Qin, S.; Sun, Y.; She, W.; Liu, Z. Dominant soil bacteria and their ecological attributes across the deserts in northern China. Eur. J. Soil Sci. 2020, 71, 524–535. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/ejss.12866 (accessed on 5 December 2023). [CrossRef]
- Naidoo, Y.; Valverde, A.; Pierneef, R.E.; Cowan, D.A. Differences in Precipitation Regime Shape Microbial Community Composition and Functional Potential in Namib Desert Soils. Microb. Ecol. 2022, 83, 689–701. Available online: https://link.springer.com/article/10.1007/s00248-021-01785-w (accessed on 5 December 2023). [CrossRef] [PubMed]
- Demergasso, C.; Neilson, J.W.; Tebes-Cayo, C.; Véliz, R.; Ayma, D.; Laubitz, D.; Barberán, A.; Chong-Díaz, G.; Maier, R.M. Hyperarid soil microbial community response to simulated rainfall. Front. Microbiol. 2023, 14, 1202266. [Google Scholar] [CrossRef] [PubMed]
- Mącik, M.; Gryta, A.; Sas-Paszt, L.; Frąc, M. New insight into the soil bacterial and fungal microbiome after phosphorus biofertilizer application as an important driver of regenerative agriculture including biodiversity loss reversal and soil health restoration. Appl. Soil Ecol. 2023, 189, 104941. [Google Scholar] [CrossRef]
- Goberna, M.; Verdú, M. Cautionary notes on the use of co-occurrence networks in soil ecology. Soil Biol. Biochem. 2022, 166, 108534. [Google Scholar] [CrossRef]
- Jiao, S.; Chu, H.; Zhang, B.; Wei, X.; Chen, W.; Wei, G. Linking soil fungi to bacterial community assembly in arid ecosystems. iMeta 2022, 1, e2. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/imt2.2 (accessed on 23 April 2025). [CrossRef]
- Wang, X.; Zeng, J.; Chen, F.; Wang, Z.; Liu, H.; Zhang, Q.; Liu, W.; Wang, W.; Guo, Y.; Niu, Y.; et al. Aridity shapes distinct biogeographic and assembly patterns of forest soil bacterial and fungal communities at the regional scale. Sci. Total Environ. 2024, 948, 174812. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).