Response Mechanism of Soil Microbial Characteristics to Different Land-Use Types in China
Abstract
1. Introduction
2. Materials and Methods
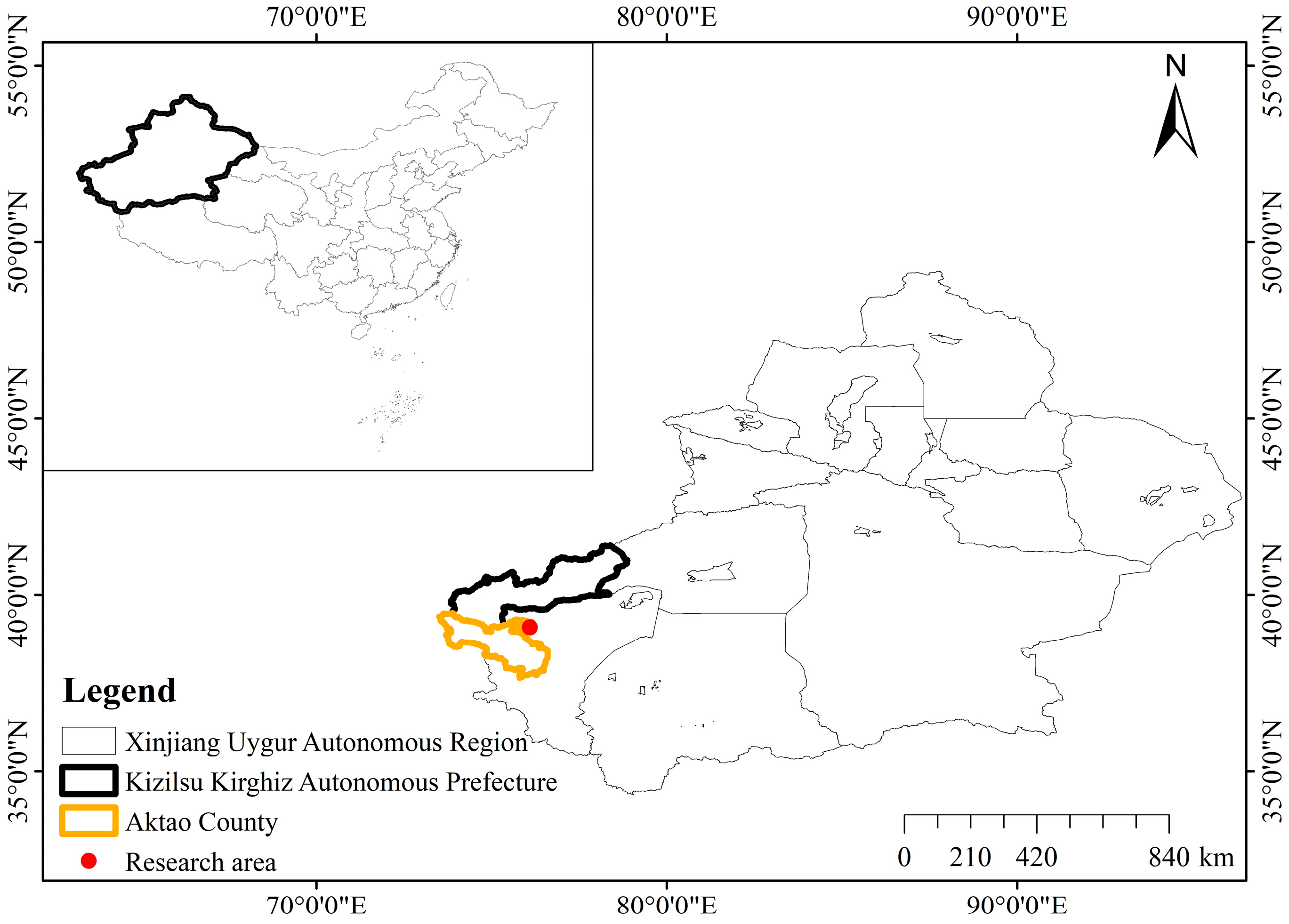
2.1. Study Area
2.2. Experimental Design and Soil Sampling
2.3. Determination of Soil Physicochemical Properties
2.4. Determination and Analysis of Soil Microbial Community
2.5. Statistical Analyses and Visualization
3. Results
3.1. Analysis of the Soil Physicochemical Properties in Different Land-Use Types
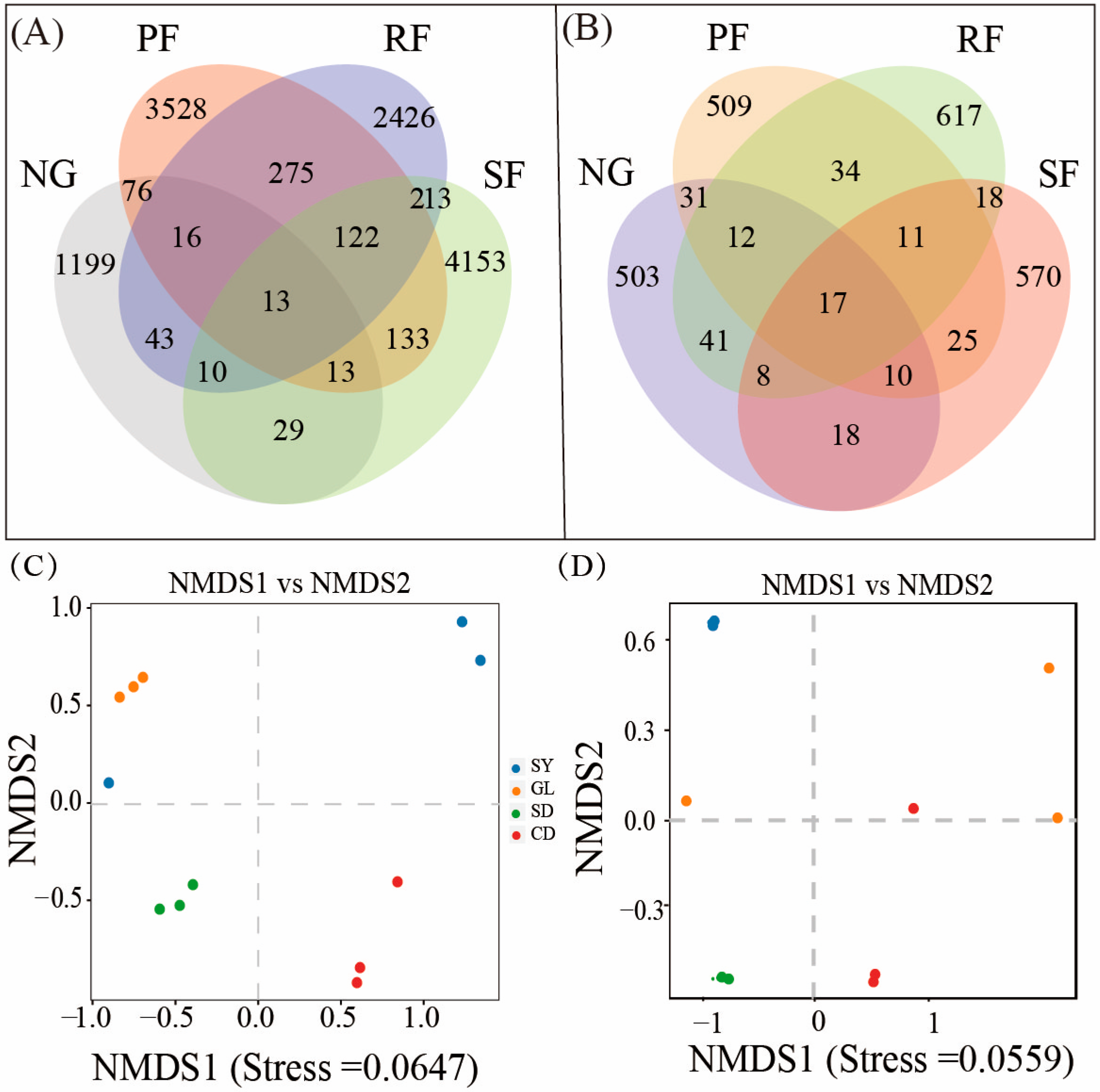
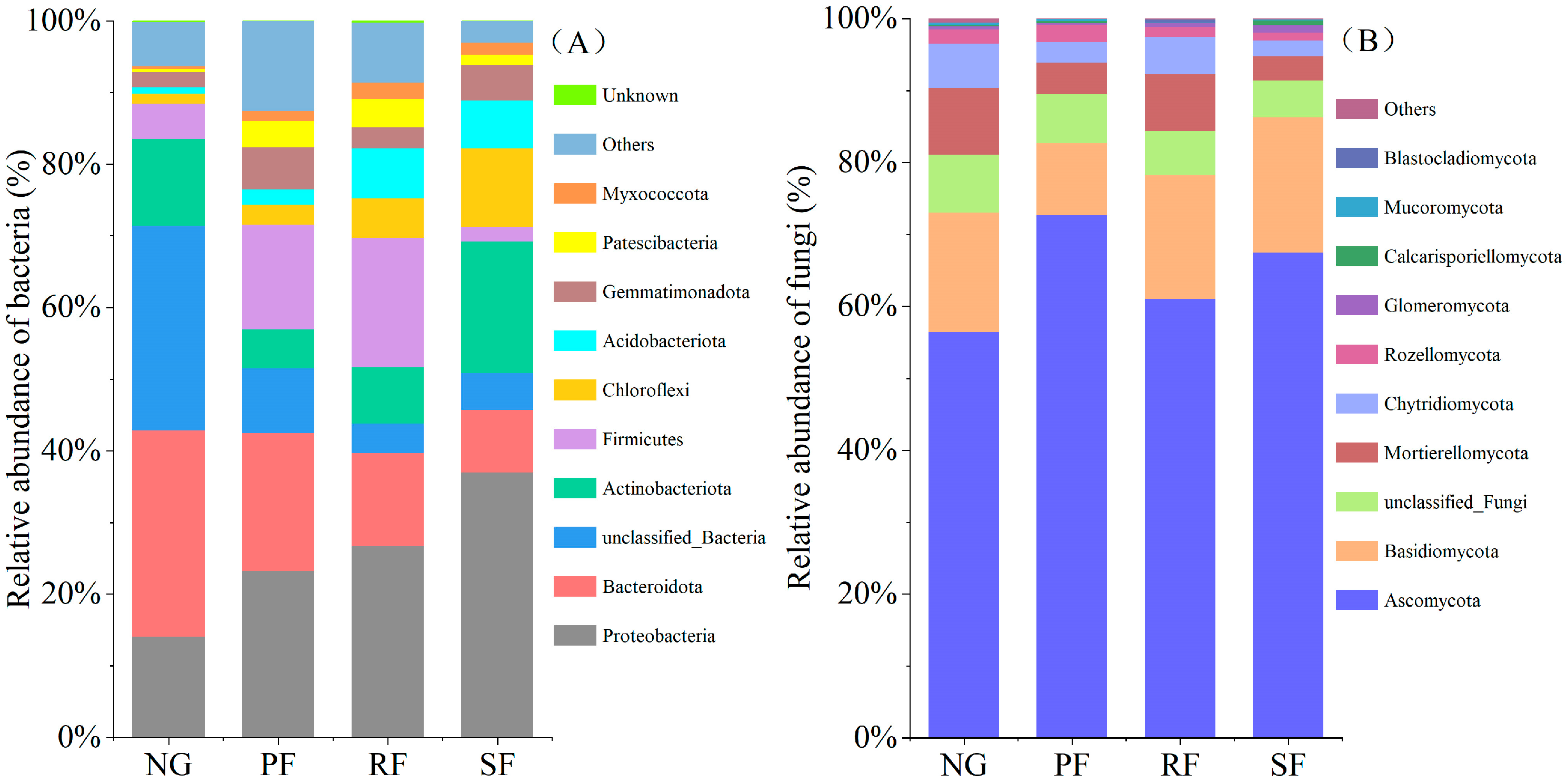
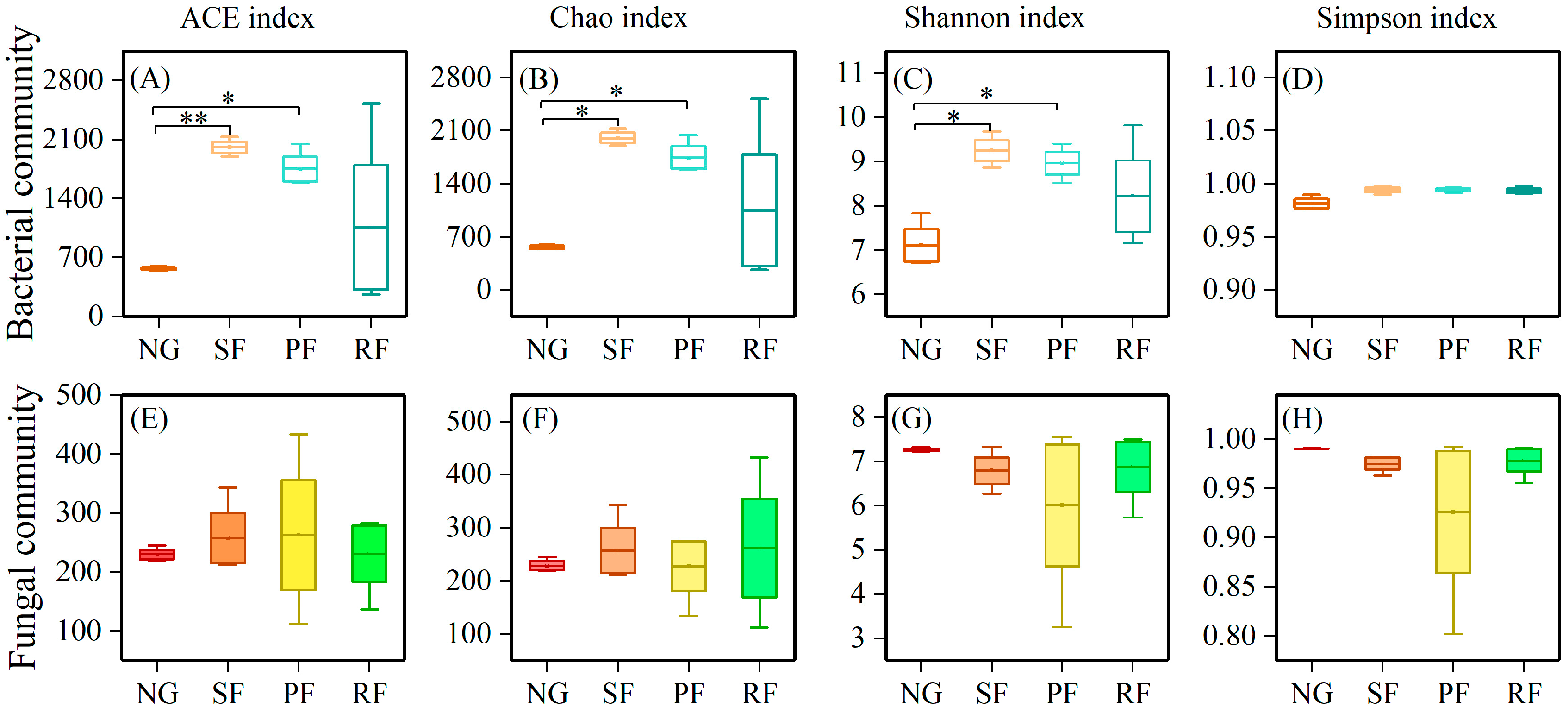
3.2. Changes of the Soil Microbial Community in Different Land-Use Types
3.3. Land-Use-Driven Interplay Between Soil Physicochemical Properties and Microbiota
3.4. Driving Factors of Soil Microbial Biodiversity in Different Land-Use Types
4. Discussion
4.1. Characteristics of the Soil Physicochemical Properties in Different Land-Use Types
4.2. Characteristics of the Soil Microbial Community Structure in Different Land-Use Types
4.3. The Interaction Between Soil Physicochemical Properties and Soil Microbial Community Under Different Land-Use Types
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| NG | native grassland |
| PF | two-year paddy field |
| RF | one-year corn-rice rotation field |
| SF | two-year sorghum field |
| OTUs | operational taxonomic unit counts |
| NMDS | nonmetric multidimensional scaling |
| SEMs | structural equation models |
| TCC | soil total carbon content |
| TNC | soil total nitrogen content |
| TPC | soil total phosphorus content |
| SOC | soil organic carbon |
| AN | soil available nitrogen content |
| AP | soil available phosphorus content |
| AK | soil available potassium content |
References
- Adhikari, K.; Hartemink, A.E. Linking soils to ecosystem services—A global review. Geoderma 2016, 262, 101–111. [Google Scholar] [CrossRef]
- Yadav, A.N.; Kour, D.; Kaur, T.; Devi, R.; Yadav, A.; Dikilitas, M.; Abdel-Azeem, A.M.; Ahluwalia, A.S.; Saxena, A.K. Bio-diversity, and biotechnological contribution of beneficial soil microbiomes for nutrient cycling, plant growth improvement and nutrient uptake. Biocatal. Agric. Biotechnol. 2021, 33, 102009. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef]
- Wagg, C.; Schlaeppi, K.; Banerjee, S.; Kuramae, E.E.; van der Heijden, M.G.A. Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nat. Commun. 2019, 10, 4841. [Google Scholar] [CrossRef]
- Lavallee, J.M.; Chomel, M.; Alvarez Segura, N.; Baggs, E.M.; Johnson, D. Land management shapes drought responses of dominant soil microbial taxa across grasslands. Nat. Commun. 2024, 15, 29. [Google Scholar] [CrossRef]
- Liao, J.; Dou, Y.; Yang, X.; An, S. Soil microbial community and their functional genes during grassland restoration. J. Environ. Manag. 2023, 325, 116488. [Google Scholar] [CrossRef]
- Yang, P.; Yuan, M.; Qu, C.; Liu, Y.; Li, X. Metagenomic insight into the soil microbial functions across land uses. J. Soils Sediments 2024, 24, 3684–3693. [Google Scholar] [CrossRef]
- Perezrodriguez, J.; Maqueda, C.; Carretero, M.I. Effect of some clay minerals on the growing of sulphate-reducing bacteria in anaerobic reactors. Appl. Clay Sci. 1989, 4, 449–459. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, H.L.; Yao, J.; He, M.Y.; Si, Y.; Feng, L.; Wang, F.; Wang, G.; Martin, M.F. Influence of clay minerals on the Bacillus halophilus Y38 activity under anaerobic condition. Appl. Clay Sci. 2010, 50, 533–537. [Google Scholar] [CrossRef]
- Ding, J.J.; Zhang, Y.G.; Wang, M.M.; Sun, X.; Cong, J. Soil organic matter quantity and quality shape microbial community compositions of subtropical broadleaved forests. Mol. Ecol. 2015, 24, 5175–5185. [Google Scholar] [CrossRef] [PubMed]
- Lauber, C.L.; Ramirez, K.S.; Aanderud, Z.; Lennon, J.T.; Fierer, N. Temporal variability in soil microbial communities across land-use types. ISME J. 2013, 7, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Huang, Y.; An, S.; Sun, H.; Bhople, P. Soil physicochemical and microbial characteristics of contrasting land-use types along soil depth gradients. Catena 2018, 162, 345–353. [Google Scholar] [CrossRef]
- Huang, J.; Liu, X.; Liu, J.; Li, Z.; Jiang, L.; Tang, C. Changes of soil bacterial community, network structure, and carbon, nitrogen and sulfur functional genes under different land use types. Catena 2023, 231, 107385. [Google Scholar] [CrossRef]
- Bahram, M.; Hildebrand, F.; Forslund, S.K.; Anderson, J.L.; Soudzilovskaia, N.A.; Bodegom, P.M.; Bengtsson-Palme, J.; Anslan, S.; Coelho, L.P.; Harend, H.; et al. Structure and function of the global topsoil microbiome. Nature 2018, 560, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef]
- Xiong, W.; Li, R.; Ren, Y.; Liu, C.; Zhao, Q.; Wu, H.; Jousset, A.; Shen, Q. Distinct roles for soil fungal and bacterial communities associated with the suppression of vanilla fusarium wilt disease. Soil Biol. Biochem. 2017, 107, 198–207. [Google Scholar] [CrossRef]
- Yao, Q.; Liu, J.; Yu, Z.; Li, Y.; Jin, J.; Liu, X.; Wang, G. Three years of biochar amendment alters soil physiochemical properties and fungal community composition in a black soil of northeast China. Soil Biol. Biochem. 2017, 110, 56–67. [Google Scholar] [CrossRef]
- Hasan, S.S.; Zhen, L.; Miah, M.G.; Ahamed, T.; Samie, A. Impact of land use change on ecosystem services: A review. Environ. Dev. 2020, 34, 100527. [Google Scholar] [CrossRef]
- Wall, D.H.; Bardgett, R.D. Soil Ecology and Ecosystem Services; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Burst, M.; Chauchard, S.; Dambrine, E.; Dupouey, J.-L.; Amiaud, B. Distribution of soil properties along forest-grassland interfaces: Influence of permanent environmental factors or land-use after-effects? Agric. Ecosyst. Environ. 2020, 289, 106739. [Google Scholar] [CrossRef]
- Francioli, D.; Cid, G.; Kanukollu, S.; Ulrich, A.; Hajirezaei, M.-R.; Kolb, S. Flooding causes dramatic compositional shifts and depletion of putative beneficial bacteria on the spring wheat microbiota. Front. Microbiol. 2021, 12, 773116. [Google Scholar] [CrossRef]
- Liu, S.; Plaza, C.; Ochoa-Hueso, R.; Trivedi, C.; Wang, J.; Trivedi, P.; Zhou, G.; Piñeiro, J.; Martins, C.S.C.; Singh, B.K.; et al. Litter and soil biodiversity jointly drive ecosystem functions. Glob. Change Biol. 2023, 29, 6276–6285. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Cui, Q.; Li, D.; Liu, Y.; Liu, K.; Li, Z. Characteristics of soil microbial community structure in different land use types of the Huanghe alluvial plain. Microorganisms 2025, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; He, W.; Sanders, C.J.; Zhang, J.; Zhou, J.; Wu, J.; Lu, Z.; Yu, M.; Li, Y.; Li, Y.; et al. Contributions of plant- and microbial-derived residuals to mangrove soil carbon stocks: Implications for blue carbon sequestration. Funct. Ecol. 2024, 38, 573–585. [Google Scholar] [CrossRef]
- Zhang, C.; Ndungu, C.N.; Feng, L.; Huang, J.; Ba, S.; Liu, W.; Cai, M. Plant diversity is more important than soil microbial diversity in explaining soil multifunctionality in Qinghai-Tibetan plateau wetlands. J. Environ. Manag. 2024, 365, 121509. [Google Scholar] [CrossRef]
- Yue, Y.; Lai, L.; Zhou, J.; Wang, G.; Zhu, Y.; Chen, Q.; Zheng, Y. Decoupled response of aboveground and belowground ecosystem multifunctionality to shrub encroachment in a semiarid grassland. J. Environ. Manag. 2025, 379, 124827. [Google Scholar] [CrossRef]
- He, M.; Fang, K.; Chen, L.; Feng, X.; Qin, S.; Kou, D.; He, H.; Liang, C.; Yang, Y. Depth-dependent drivers of soil microbial necromass carbon across Tibetan alpine grasslands. Glob. Change Biol. 2022, 28, 936–949. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.r-project.org/ (accessed on 25 March 2025).
- Qiang, F.; Sheng, C.; Zhang, J.; Jiang, L.; Zhou, J. Soil quality variation under different land use types and its driving factors in beijing. Forests 2024, 15, 993. [Google Scholar] [CrossRef]
- Herrick, J.E. Soil quality: An indicator of sustainable land management? Appl. Soil Ecol. 2000, 15, 75–83. [Google Scholar] [CrossRef]
- Islam, K.R.; Weil, R.R. Land use effects on soil quality in a tropical forest ecosystem of Bangladesh. Agric. Ecosyst. Environ. 2000, 79, 9–16. [Google Scholar] [CrossRef]
- Negasa, T.; Ketema, H.; Legesse, A.; Sisay, M.; Temesgen, H. Variation in soil properties under different land use types managed by smallholder farmers along the toposequence in southern Ethiopia. Geoderma 2017, 290, 40–50. [Google Scholar] [CrossRef]
- Nabi, F.; Yang, G.; Sajid, S.; Khan, A.; Ahmed, I.; Li, X. Linking soil microbial community with the changes in soil physicochemical properties in response to long-term agricultural land use change of different chronosequences and depth layers. Ecol. Indic. 2022, 145, 109727. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, K.; Li, Y. Spatial variability of soil physicochemical properties under different land uses impacted by erosion on karst hillslopes in southwest China. Earth Surf. Process. Landf. 2024, 49, 2245–2259. [Google Scholar] [CrossRef]
- Rieder, Á.; Madarász, B.; Szabó, J.A.; Dyson, J.; Jakab, G. Soil organic matter alteration velocity due to land-use change: A case study under conservation agriculture. Sustainability 2018, 10, 943. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Hu, F.; Li, H.; Zhang, Q.; Wang, K. Soil organic matter and silt contents determine soil particle surface electrochemical properties across a long-term natural restoration grassland. Catena 2020, 190, 104526. [Google Scholar] [CrossRef]
- Robinson, D.A.; Thomas, A.; Reinsch, S.; Mooney, S.J.; Whitmore, A.P. Analytical modelling of soil porosity and bulk density across the soil organic matter and land-use continuum. Sci. Rep. 2022, 12, 7085. [Google Scholar] [CrossRef]
- Romdhane, S.; Spor, A.; Banerjee, S.; Gervaix, J.; Karimi, B.; Philippot, L. Land-use intensification differentially affects bacterial, fungal and protist communities and decreases microbiome network complexity. Environ. Microbiome 2022, 17, 1. [Google Scholar] [CrossRef]
- Spain, A.M.; Krumholz, L.R.; Elshahed, M.S. Abundance, composition, diversity and novelty of soil proteobacteria. ISME J. 2009, 3, 992–1000. [Google Scholar] [CrossRef]
- Schneider, F.; Don, A.; Hennings, I.; Schmittmann, O.; Seidel, S.J. The effect of deep tillage on crop yield—What do we really know? Soil Tillage Res. 2017, 174, 193–204. [Google Scholar] [CrossRef]
- Mollier, S.; Kunstler, G.; Dupouey, J.-L.; Bergès, L.; Corcket, E. Forest management and former land use have no effect on soil fungal diversity in uneven-aged mountain high forests. Ann. For. Sci. 2024, 81, 2. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, S.; Jiang, C.; Wu, C.; Gao, M.; Wang, Q. A review of root exudates and rhizosphere microbiome for crop production. Environ. Sci. Pollut. Res. 2021, 28, 54497–54510. [Google Scholar] [CrossRef]
- Taylor, D.L.; Sinsabaugh, R.L. Soil Microbiology, Ecology and Biochemistry. In Soil Microbiology, Ecology and Biochemistry; Elsevier: Amsterdam, The Netherlands, 2015; pp. 77–109. [Google Scholar]
- Banik, G.G.; Heath, C.A. Hybridoma growth and antibody production as a function of cell density and specific growth rate in perfusion culture. Biotechnol. Bioeng. 1995, 48, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Lee, S.-H.; Jo, H.Y.; Finneran, K.T.; Kwon, M.J. Diversity and composition of soil Acidobacteria and Proteobacteria communities as a bacterial indicator of past land-use change from forest to farmland. Sci. Total Environ. 2021, 797, 148944. [Google Scholar] [CrossRef]
- Huang, R.; Crowther, T.W.; Sui, Y.; Sun, B.; Liang, Y. High stability and metabolic capacity of bacterial community promote the rapid reduction of easily decomposing carbon in soil. Commun. Biol. 2021, 4, 1376. [Google Scholar] [CrossRef]
- Zheng, B.; Xiao, Z.; Liu, J.; Zhu, Y.; Shuai, K.; Chen, X.; Liu, Y.; Hu, R.; Peng, G.; Li, J.; et al. Vertical differences in carbon metabolic diversity and dominant flora of soil bacterial communities in farmlands. Sci. Rep. 2024, 14, 9445. [Google Scholar] [CrossRef]
- Leclerc, L.; Calderón-Sanou, I.; Martinez-Almoyna, C.; Paillet, Y.; Thuiller, W.; Vincenot, L.; Kunstler, G. Beyond the role of climate and soil conditions: Living and dead trees matter for soil biodiversity in mountain forests. Soil Biol. Biochem. 2023, 187, 109194. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, M. Effect of bio-organic fertilizers partially substituting chemical fertilizers on labile organic carbon and bacterial community of citrus orchard soils. Plant Soil 2023, 483, 255–272. [Google Scholar] [CrossRef]
- Xiao, H.B.; Li, Z.W.; Dong, Y.T.; Chang, X.F.; Deng, L.; Huang, J.Q.; Nie, X.D.; Liu, C.; Liu, L.; Wang, D.Y. Changes in microbial communities and respiration following the revegetation of eroded soil. Agric. Ecosyst. Environ. 2017, 246, 30–37. [Google Scholar] [CrossRef]
- Abadie, J.; Avon, C.; Dupouey, J.L.; Lopez, J.M.; Tatoni, T.; Bergès, L. Land use legacies on forest understory vegetation and soils in the Mediterranean region: Should we use historical maps or in situ land use remnants? For. Ecol. Manag. 2018, 427, 17–25. [Google Scholar] [CrossRef]
- Ren, C.; Wang, T.; Xu, Y.; Deng, J.; Zhao, F.; Yang, G.; Ren, G. Differential soil microbial community responses to the linkage of soil organic carbon fractions with respiration across land-use changes. For. Ecol. Manag. 2018, 409, 170–178. [Google Scholar] [CrossRef]


| Soil Properties | NG | PF | RF | SF |
|---|---|---|---|---|
| pH | 7.87 ± 0.06 a | 7.41 ± 0.22 b | 7.62 ± 0.05 ab | 7.8 ± 0.09 ab |
| Bulk density (g·cm−3) | 0.81 ± 0.03 c | 1.55 ± 0.02 a | 1.55 ± 0.03 a | 1.44 ± 0.01 b |
| Soil porosity (%) | 69.43 ± 1.21 a | 41.38 ± 0.67 c | 41.51 ± 1.15 c | 45.66 ± 0.44 b |
| Sand content (%) | 49.94 ± 3.17 c | 62.91 ± 2.89 b | 62.47 ± 0.69 b | 89.4 ± 2.47 a |
| Clay content (%) | 28.28 ± 4 a | 21.09 ± 0.97 a | 23.31 ± 2.9 a | 5.27 ± 2.13 b |
| Silt content (%) | 21.78 ± 2.35 a | 16.00 ± 2.31 ab | 14.22 ± 2.22 b | 5.33 ± 1.54 c |
| Soil Nutrient Content | NG | PF | RF | SF |
|---|---|---|---|---|
| TCC (g·kg−1) | 12.86 ± 0.92 c | 15.72 ± 0.5 b | 17.19 ± 0.28 ab | 18.75 ± 0.28 a |
| SOC (g·kg−1) | 8.47 ± 0.23 a | 4.81 ± 0.3 b | 4.27 ± 0.46 b | 2.74 ± 0.13 c |
| TNC (g·kg−1) | 0.78 ± 0.06 | 1.53 ± 0.36 | 0.92 ± 0.15 | 1.08 ± 0.36 |
| AN (mg·kg−1) | 47.91 ± 8.3 ab | 43.64 ± 9.27 a | 36.2 ± 3.64 ab | 18.11 ± 1.23 b |
| TPC (g·kg−1) | 1.66 ± 002 a | 1.72 ± 0.05 a | 1.6 ± 0.08 a | 1.31 ± 0.04 b |
| AP (mg·kg−1) | 24.91 ± 2.07 | 27.51 ± 4.92 | 30.9 ± 5.56 | 20.52 ± 3.99 |
| TKC (g·kg−1) | 0.27 ± 0.09 | 0.37 ± 0.15 | 0.3 ± 0.1 | 0.4 ± 0.21 |
| AK (mg·kg−1) | 91.67 ± 11.7 | 176 ± 18.18 | 149.67 ± 45.8 | 132.33 ± 30.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, G.; Hu, Y.; Zhang, Y.; Han, Y.; Li, K.; Jia, H.; Zhu, X. Response Mechanism of Soil Microbial Characteristics to Different Land-Use Types in China. Land 2025, 14, 1229. https://doi.org/10.3390/land14061229
Ma G, Hu Y, Zhang Y, Han Y, Li K, Jia H, Zhu X. Response Mechanism of Soil Microbial Characteristics to Different Land-Use Types in China. Land. 2025; 14(6):1229. https://doi.org/10.3390/land14061229
Chicago/Turabian StyleMa, Gang, Yantao Hu, Yangyang Zhang, Yaoguang Han, Keyi Li, Hongtao Jia, and Xinping Zhu. 2025. "Response Mechanism of Soil Microbial Characteristics to Different Land-Use Types in China" Land 14, no. 6: 1229. https://doi.org/10.3390/land14061229
APA StyleMa, G., Hu, Y., Zhang, Y., Han, Y., Li, K., Jia, H., & Zhu, X. (2025). Response Mechanism of Soil Microbial Characteristics to Different Land-Use Types in China. Land, 14(6), 1229. https://doi.org/10.3390/land14061229






