Ecological Dynamics of Forest Stands with Castanopsis argentea (Blume) A.DC. in a Mountain Ecosystem: Vegetation Structure, Diversity, and Carbon Stock Under Tourism Pressure
Abstract
1. Introduction
2. Materials and Methods
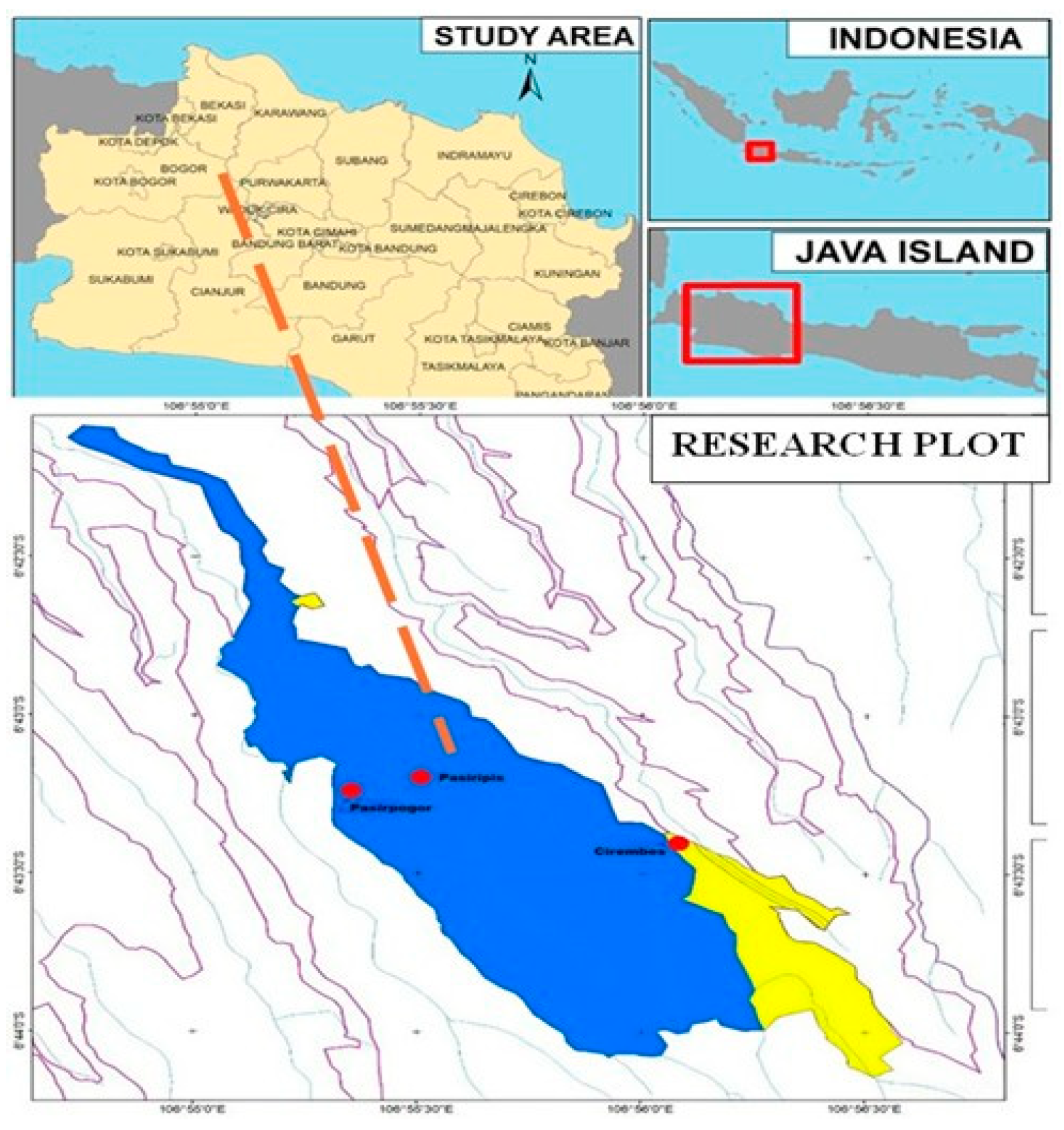
2.1. Experimental Site
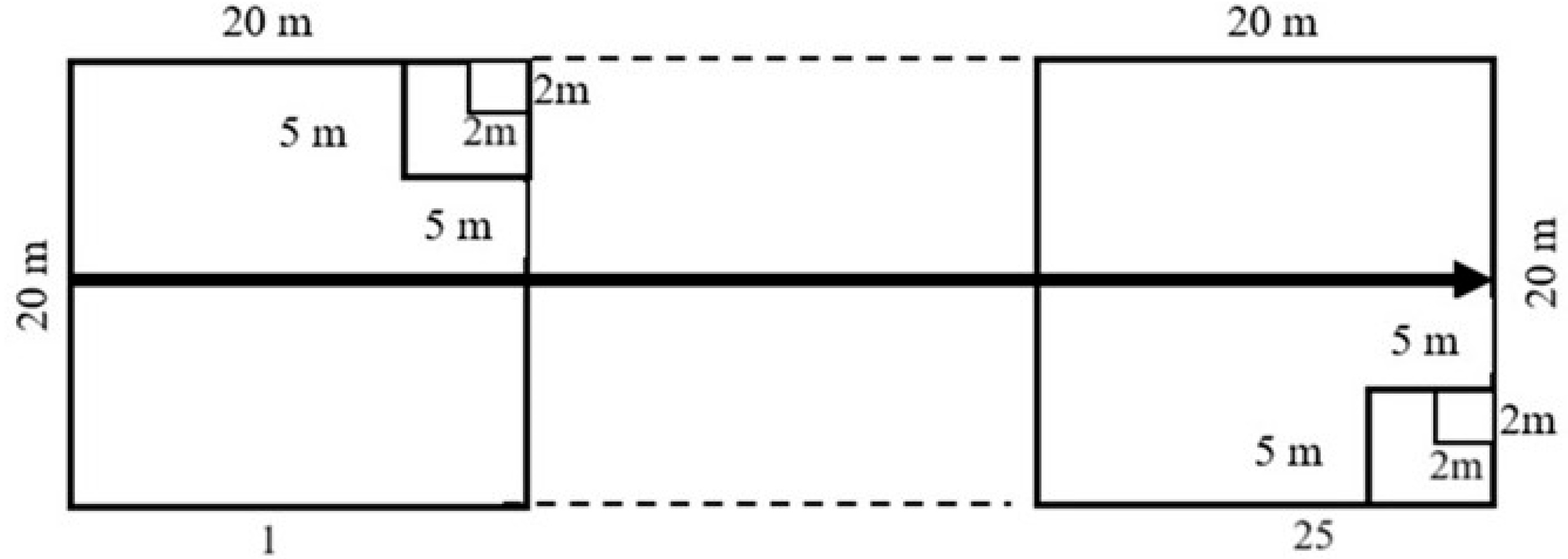
2.2. Sampling
- Tree Measurements: 20 m × 20 m subplots for measuring tree species.
- Belt Transects: 5 m × 5 m subplots for assessing saplings.
- Seedling Measurements: 2 m × 2 m subplots for recording seedlings.
2.3. Data Collection
- Soil samples: Soil samples were collected from a depth of approximately 0–20 cm in three sampling plot locations (CC2, CC3, and Cirembes forest). Samples were collected from five points in each plot (20 m × 20 m) and combined to obtain a composite sample. This work was undertaken in three replications for each plot; thus, each plot produced three composite soil samples. The samples were sealed in plastic bags and transported to the laboratory. The soil samples were analyzed in the laboratory of Bogor Agricultural University (IPB University). Not all soil properties were analyzed, but the analysis was carried out based on the most important soil nutrient properties that influence plant growth, the limitations of the materials, and the practicality of the analysis. The analyzed properties included water content measured using gravimetry, pH measured using a pH meter, total C measured using the colorimetric method, total N measured using the Kjeldahl method, available P measured using the Bray II method, base cations (K, Mg, Ca, and Na), cation exchange capacity measured using the NH4 OAc (pH 7.0) extraction method, and Texture 3 fractions (sand, ash, clay; %).
- Species Identification: All species names of trees, saplings, and seedlings were recorded. Samples of unidentified materials were collected and identified at the Forest Research and Development Laboratory, Bogor, using the World Flora Online database [25] for nomenclature reference.
- Tree Measurements: The heights and stem diameter at breast height (DBH) of trees were measured. For trees with buttresses, diameters were measured 20 cm above the buttress.
- Growth Stage Criteria: The trees, saplings, and seedlings were classified according to the following criteria [26,27], which are suitable for use in tropical vegetation to assess tree stand structure in conservation areas.
- Trees: Diameter at breast height (DBH) ≥ 10 cm at 1.3 m above the ground level.
- Saplings: Diameter < 10 cm and height > 1.5 m.
- Seedlings: Height < 1.5 m, including sprout.
2.4. Data Analysis
3. Results
3.1. Soil Characteristics
3.2. Plant Species Diversity
3.3. Species Composition
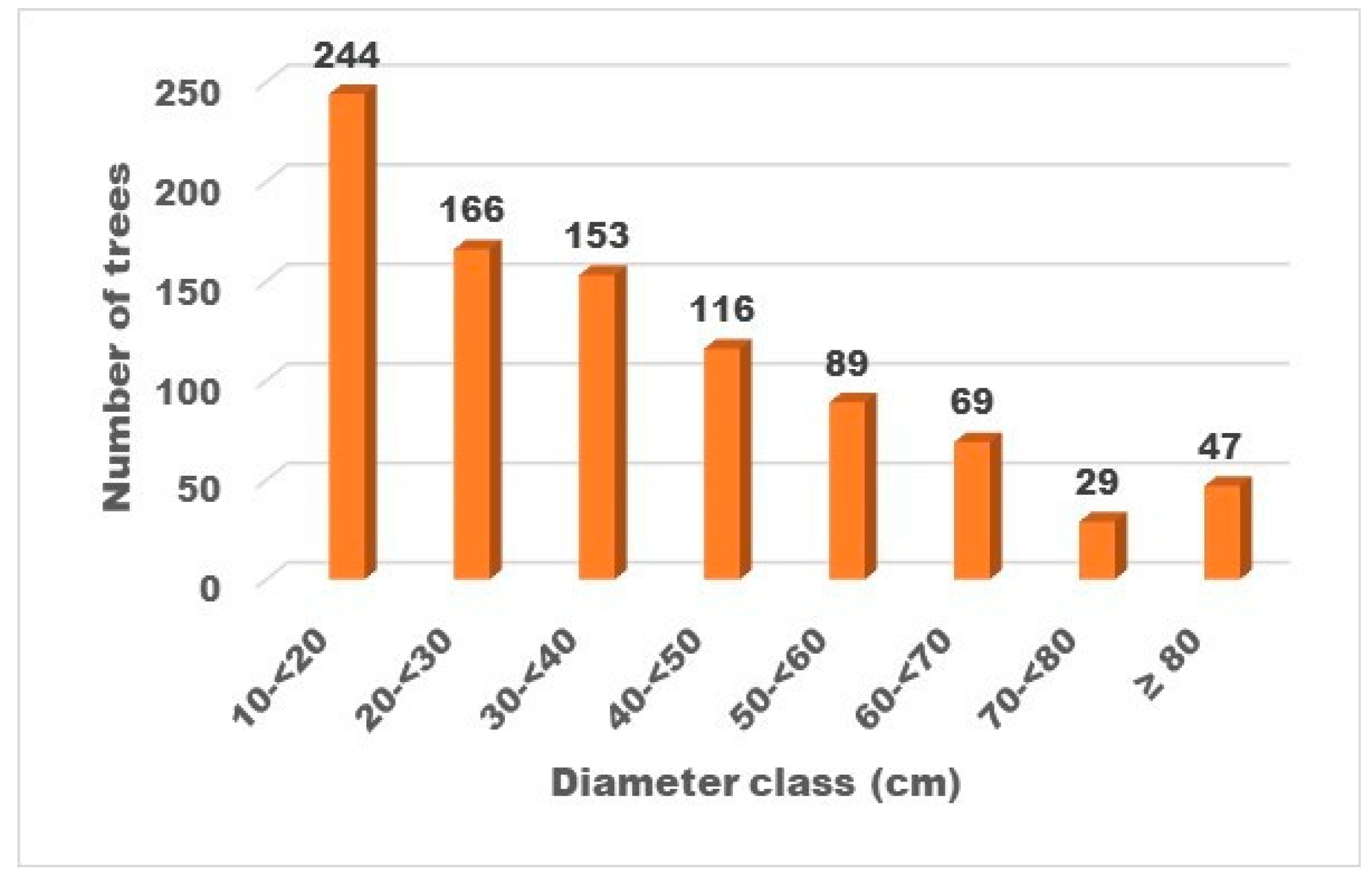
3.4. Forest Structure and Regeneration
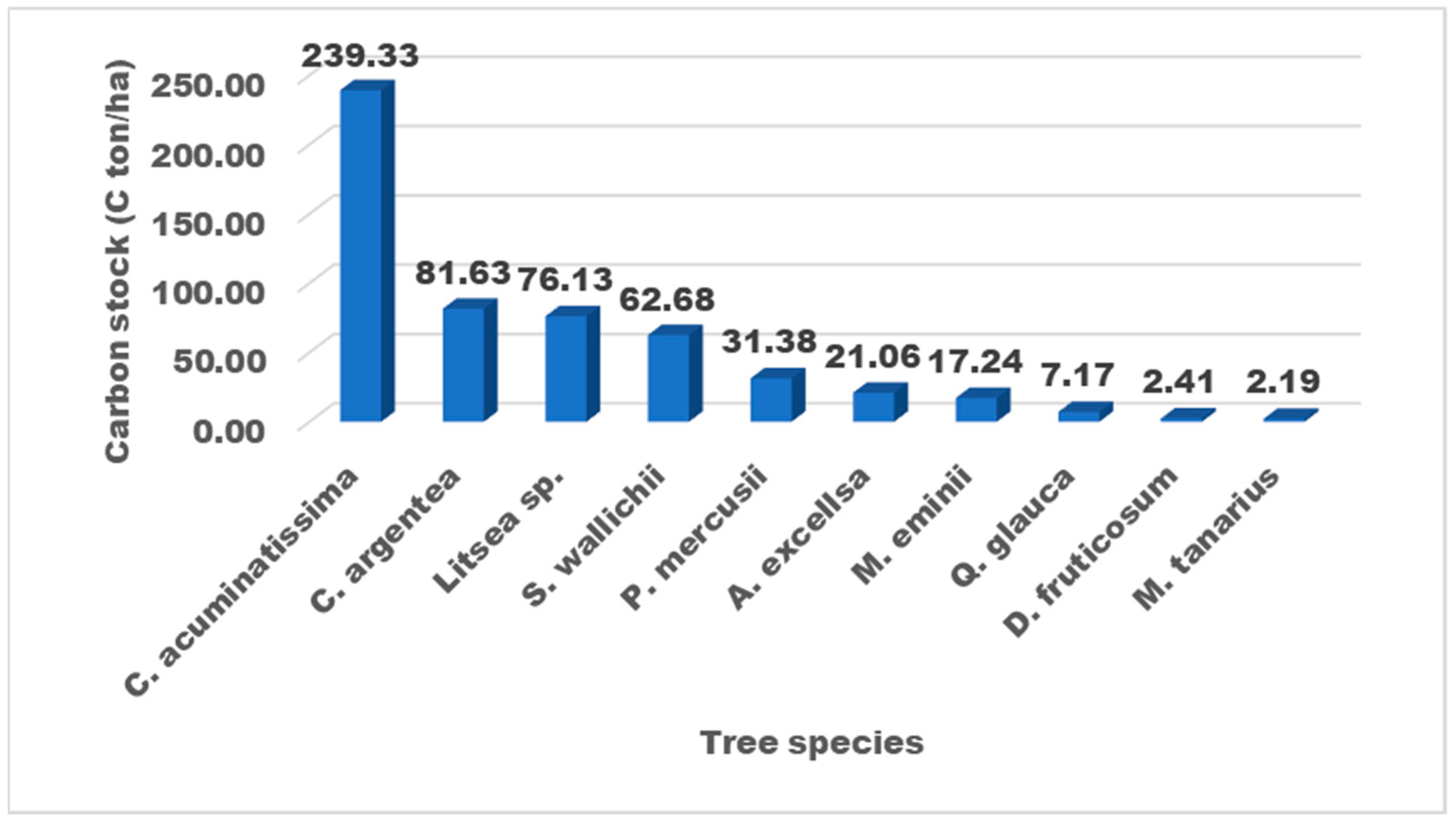
3.5. Carbon Stock
4. Discussion
4.1. Soil Characteristics
4.2. Plant Species Diversity
4.3. Species Composition
4.4. Forest Structure and Regeneration
4.5. Carbon Stock
4.6. Ecotourism Development in Study Site as Potential Threat to the Habitat of C. argentea
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barstow, M.; Kartawinata, K. Castanopsis Argentea. IUCN Red List. Threat. Species 2017. Available online: https://www.iucnredlist.org/species/62004506/62004510 (accessed on 13 February 2024).
- POWO. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Published on the Internet. Available online: http://www.plantsoftheworldonline.org/ (accessed on 2 February 2025).
- Fern, K. Useful Tropical Plants Database Last Update on 13 October 2024. Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. Available online: http://tropical.theferns.info/ (accessed on 2 February 2025).
- Sunarno, B.; Sosef, M.S.M. Castanopsis Argentea (Blume) A.DC. In Plant Resources of South-East Asia No 5(2): Timber Trees; Minor Commercial Timbers. Database Record: Prota4u.org/Prosea; Lemmens, R.H.M.J., Soerianegara, I., Wong, W.C., Eds.; PROSEA: Bogor, Indonesia, 1995. [Google Scholar]
- Wibowo, C. Hubungan Antara Keberadaan Saninten (Castanopsis Argentea Blume) Dengan Beberapa Sifat Tanah: Kasus Di Taman Nasional Gunung Gede Pangrango, Jawa Barat. Ph.D. Dissertation, Institut Pertanian, Bogor, Indonesia, 2006. [Google Scholar]
- Wardani, M.; Heriyanto, N.M. Autecological of Damar Asam [Shorea Hopeifolia (F. Heim)] Symington in National Park of South Bukit Barisan, Lampung. Bul. Plasma Nutfah 2016, 21, 89–98. [Google Scholar] [CrossRef]
- Mutaqien, Z.; Zuhri, M. Establishing a Long-Term Permanent Plot in Remnant Forest of Cibodas Botanic Garden, West Java. Biodiversitas 2011, 12, 218–224. [Google Scholar] [CrossRef]
- Yamada, I. Forest Ecological Studies of the Montane Forest of Mt. Pangrango, West Java IV. Floristic Composition along the Altitude. Jpn. J. Southeast Asian Stud. 1977, 15, 226–254. [Google Scholar] [CrossRef]
- Nurdiana, D.R.; Buot, I.E. Vegetation Community and Species Association of Castanopsis Spp. at Its Habitat in the Remnant Forest of Cibodas Botanical Garden, Indonesia. Biodiversitas 2021, 22, 4799–4807. [Google Scholar] [CrossRef]
- Galván-Cisneros, C.M.; Villa, P.M.; Coelho, A.J.P.; Campos, P.V.; Meira-Neto, J.A.A. Altitude as Environmental Filtering Influencing Phylogenetic Diversity and Species Richness of Plants in Tropical Mountains. J. Mt. Sci. 2023, 20, 285–298. [Google Scholar] [CrossRef]
- Hilwan, I.; Irfani, E. Distribution Pattern and Regeneration of Saninten (Castanopsis Argantea Blume) in Selabintana Resort, Gunung Gede Pangrango National Park. J. Trop. Silvic. 2018, 9, 53–59. [Google Scholar] [CrossRef]
- Dendang, B.; Handayani, W. Struktur Dan Komposisi Tegakan Hutan Di Taman Nasional Gunung Gede Pangrango, Jawa Barat. In Prosiding Seminar Nasasional Masyarakat Biodivsitas Indonesia; Smujo International: Surakarta, Indonesia, 2015; pp. 691–695. [Google Scholar] [CrossRef]
- Susanto, H.; Irnawati, I.; Akmal, H.; Abbas, E.W. Media Film Dokumenter Dan Pengaruhnya Terhadap Keterampilan Berpikir Kritis Siswa. Hist. J. Program Studi Pendidik. Sej. 2021, 9, 65–78. [Google Scholar] [CrossRef]
- Stronza, A.L.; Hunt, C.A.; Fitzgerald, L.A. Ecotourism for Conservation? Annu. Rev. Environ. Resour. 2019, 44, 229–253. [Google Scholar] [CrossRef]
- Das, M.; Chatterjee, B. Ecotourism: A Panacea or a Predicament? Tour Manag. Perspect. 2015, 14, 3–16. [Google Scholar] [CrossRef]
- Suwena, I.K.; Widyatmaja, I.G.N. Pengetahuan Dasar Ilmu Pariwisata 2017|PDF|Ilmu Sosial|Perjalanan; Pustaka Larasan: Denpasar, Indonesia, 2017. [Google Scholar]
- Sofiyudin, A.; Rosadi, R.; Priatna, D. Carrying Capacity Analysis of Nature Tourism at Selabintana, Gunung Gede Pangrango National Park, West Java. Indones. J. Appl. Environ. Stud. 2021, 2, 113–117. [Google Scholar] [CrossRef]
- Rezki, A.; Lidiawati, I.; Supriono, B. Nilai Ekonomi Kegunaan Wisata Alam Situgunung Taman Nasional Gunung Gede Pangrango. J. Nusa Sylva 2021, 21, 48–55. [Google Scholar] [CrossRef]
- Mokodongan, T. Analysis of The Application of Ecotourism Principles In Indonesian Natural Tourism Destinations: A Literature Review. J. Indones. Tour. Hosp. Recreat. 2024, 7, 155–164. [Google Scholar] [CrossRef]
- Butarbutar, R.; Soemarno, S. Environmental Effects of Ecotourism in Indonesia. J. Indones. Tour. Dev. Stud. 2013, 1, 97–107. [Google Scholar] [CrossRef]
- Brandt, J.S.; Buckley, R.C. A Global Systematic Review of Empirical Evidence of Ecotourism Impacts on Forests in Biodiversity Hotspots. Curr. Opin. Environ. Sustain. 2018, 32, 112–118. [Google Scholar] [CrossRef]
- Balai Penelitian Tanah. Peta Sumberdaya Tanah Eksplorasi Pulau Jawa; Balai Penelitian Tanah: Bogor, Indonesia, 2011; Available online: https://abuzadan.staff.uns.ac.id/2011/04/04/peta-sumberdaya-tanah-eksplorasi-pulau-jawa-dan-madura-re-digitize/ (accessed on 13 February 2024).
- Soil Survey Staff. Keys to Soil Taxonomy; USDA: Washington, DC, USA, 2022. Available online: https://www.nrcs.usda.gov/sites/default/files/2022-09/Keys-to-Soil-Taxonomy.pdf (accessed on 13 February 2024).
- Badan Pusat Statistik. Bogor Regency in Figures. Statistic Bogor Regency. West Java Province. 2024. Available online: https://bogorkab.bps.go.id/id/publication/2024/02/28/84386f87ea16ddb737175f38/kabupaten-bogor-dalam-angka-2024.html (accessed on 13 February 2024).
- WFO. World Flora Online. Available online: http://www.worldfloraonline.org (accessed on 12 March 2024).
- Heriyanto, N.M.; Gunawan, H. Keanekaragaman Jenis Pohon Dan Potensi Serapan Karbon Taman Kehati Bumi Patra, Indramayu, Jawa Barat. Bul. Kebun Raya 2020, 23, 210–219. [Google Scholar] [CrossRef]
- Wardani, M.; Astuti, I.P.; Heriyanto, N.M. Analisis Vegetasi Jenis-Jenis Dipterocarpaceae Di Kawasan Hutan Seksi I Way Kanan, Taman Nasional Way Kambas, Lampung. Bul. Kebun Raya 2017, 20, 51–64. [Google Scholar]
- Kindt, R. WorldFlora: An R Package for Exact and Fuzzy Matching of Plant Names against the World Flora Online Taxonomic Backbone Data. Appl. Plant Sci. 2020, 8, e11388. [Google Scholar] [CrossRef]
- BGCI. GlobalTree Portal. Botanic Gardens Conservation International. Available online: https://www.bgci.org/resources/bgci-databases/globaltree-portal/ (accessed on 12 March 2024).
- Kusmana, C.; Susanti, S. Species Composition and Stand Structure of Natural Forest in Hutan Pendidikan Gunung Walat, Sukabumi. J. Trop. Silvic. 2015, 6, 210–217. [Google Scholar]
- Heriyanto, N.M.; Samsoedin, I.; Kartawinata, K. Tree Species Diversity, Structural Characteristics and Carbon Stock in a One-Hectare Plot of the Protection Forest Area in West Lampung Regency, Indonesia. Reinwardtia 2019, 18, 1–8. [Google Scholar] [CrossRef]
- Mansur, M.; Kartawinata, K. Phytosociology of a Lower Montane Forest on Mt. Batulanteh, Sumbawa, Indonesia. Reinwardtia 2017, 16, 77–92. [Google Scholar] [CrossRef]
- Sadili, A.; Kartawinata, K.; Soedjito, H.; Sambas, E. Tree Species Diversity in a Pristine Montane Forest Previously Untouched by Human Activities in Foja Mountains, Papua, Indonesia. Reinwardtia 2018, 17, 133–154. [Google Scholar] [CrossRef]
- Misra, K.C. Manual of Plant Ecology, 2nd ed.; New Delhi Oxford and IBH Publishing: New Delhi, India, 1980. [Google Scholar]
- Chave, J.; Réjou-Méchain, M.; Búrquez, A.; Chidumayo, E.; Colgan, M.S.; Delitti, W.B.C.; Duque, A.; Eid, T.; Fearnside, P.M.; Goodman, R.C.; et al. Improved Allometric Models to Estimate the Aboveground Biomass of Tropical Trees. Glob./Chang. Biol. 2014, 20, 3177–3190. [Google Scholar] [CrossRef] [PubMed]
- Muslich, M.; Wardani, M.; Kalima, T.; Rulliyati, S.; Damayanti, R.; Hadjib, N.; Pari, G.; Suprapti, S.; Iskandar, M.I.; Abdurachman; et al. Atlas Kayu Indonesia Jilid IV. Badan Penelitian Dan Pengembangan Kehutanan. IV; Kemeterian Kehutanan, Badan Penelitian dan Pengembangan Kehutanan, Pusat Penelitian dan Pengembangan Keteknikan Kehutanan dan Pengolahan Hasil Hutan (Pustekolah): Bogor, Indonesia, 2013; Available online: https://id.scribd.com/document/544884899/Atlas-Kayu-Jilid-IV (accessed on 13 February 2024).
- International Center Research in Agroforestry/ICRAF. DWood Density Database. ICRAF. www.worldagroforestry.org.: Bogor, Indonesia 2017. Available online: https://apps.worldagroforestry.org/sea/Products/AFDbases/WD/Index.htm (accessed on 13 February 2024).
- International Panel on Climate Change. Climate Change 2013: The Physical Science Basis. 2013. Available online: https://www.ipcc.ch/report/ar5/wg1/ (accessed on 13 February 2024).
- Martin, A.R.; Thomas, S.C. A Reassessment of Carbon Content in Tropical Trees. PLoS ONE 2011, 6, e23533. [Google Scholar] [CrossRef] [PubMed]
- Microsoft Office Excel. Microsoft Inc. United States of America. 2013. Available online: https://www.microsoft.com/id-id/ (accessed on 13 February 2024).
- Mawazin, M.; Pamoengkas, P.; Darwo, D.; Heriansyah, I.; Dewi, R. Restoration of 15 Years: Analysing Species Composition, Potential, and Diversity. J. Penelit. Hutan Tanam. 2024, 21, 115–124. [Google Scholar]
- Yudaputra, A.; Rahardjo, P. Plant Species Richness and Diversity in Karangsambung-Karangbolong National Geopark, Indonesia. Biodiversitas 2020, 21, 1735–1742. [Google Scholar] [CrossRef]
- Brindis-Badillo, D.A.; Arroyo-Rodríguez, V.; Mendoza, E.; Wies, G.; Martínez-Ramos, M.; Brindis-Badillo, D.A.; Arroyo-Rodríguez, V.; Mendoza, E.; Wies, G.; Martínez-Ramos, M. Conserving Dominant Trees in Human-Modified Landscapes at the Lacandon Tropical Rainforest. BCons 2022, 270, 109548. [Google Scholar] [CrossRef]
- Kessler, M.; Keßler, P.J.A.; Gradstein, S.R.; Bach, K.; Schmull, M.; Pitopang, R. Tree Diversity in Primary Forest and Different Land Use Systems in Central Sulawesi, Indonesia. Biodivers. Conserv. 2005, 14, 547–560. [Google Scholar] [CrossRef]
- Rezekiah, A.; Ruslan, M.; Kadir, S.; Mahmud, M. Vegetation Composition and Structure across Land Use Types in a Rotational Cultivation System in Meratus Mountain, South Kalimantan, Indonesia. Biodiversitas 2022, 23, 4234–4242. [Google Scholar] [CrossRef]
- Angst, G.; Potapov, A.; Joly, F.X.; Angst, Š.; Frouz, J.; Ganault, P.; Eisenhauer, N. Conceptualizing Soil Fauna Effects on Labile and Stabilized Soil Organic Matter. Nat. Commun. 2024, 15, 5005. [Google Scholar] [CrossRef]
- Schvezov, N.; Caffetti, J.; Silva, C.; Boeris, J.; Baldo, D.; Lajmanovich, R. Impact of Soil from Monoculture Pine Plantations on Two Anuran Species from the Atlantic Forest: Odontophrynus Reigi and Leptodactylus Luctator. Sci. Total Environ. 2023, 869, 161769. [Google Scholar] [CrossRef]
- Franco, A.L.C.; Bartz, M.L.C.; Cherubin, M.R.; Baretta, D.; Cerri, C.E.P.; Feigl, B.J.; Wall, D.H.; Davies, C.A.; Cerri, C.C. Loss of Soil (Macro)Fauna Due to the Expansion of Brazilian Sugarcane Acreage. Sci. Total Environ. 2016, 563–564, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Rosalina, F.; Maipauw, N.J. Sifat Kimia Tanah Pada Beberapa Tipe Vegetasi. Median 2019, 11, 1–9. [Google Scholar] [CrossRef]
- Salam, H. Mengulas Sejarah Erupsi Gunung Gede, Tertidur 66 Tahun Sejak Letusan Terakhir 1957; Radar: Sukabumi, Indonesia, 2023; Available online: https://radarsukabumi.com/berita-utama/mengulas-sejarah-erupsi-gunung-gede-tertidur-66-tahun-sejak-letusan-terakhir-1957/ (accessed on 4 March 2025).
- Yu, C.; Mawodza, T.; Atkinson, B.S.; Atkinson, J.A.; Sturrock, C.J.; Whalley, R.; Hawkesford, M.J.; Cooper, H.; Zhang, X.; Zhou, H.; et al. The Effects of Soil Compaction on Wheat Seedling Root Growth are Specific to Soil Texture and Soil Moisture Status. Rhizosphere 2024, 29, 100838. [Google Scholar] [CrossRef]
- Mora-Motta, D.; Chavarro-Bermeo, D.; Olaya-Montes, J.P.; Vargas-Garcia, A.; Bonilla, C.; Estrada-Bonilla, R.; Torres-Cuesta, D.; Mora-Motta, D.; Chavarro-Bermeo, J.P.; Olaya-Montes, A.; et al. Phosphate-Solubilizing Bacteria with Low-Solubility Fertilizer Improve Soil P Availability and Yield of Kikuyu Grass. Microorganisms 2023, 11, 1748. [Google Scholar] [CrossRef]
- Bongiorno, G. Novel Soil Quality Indicators for the Evaluation of Agricultural Management Practices: A Biological Perspective. Front. Agric. Sci. Eng. 2020, 7, 257–274. [Google Scholar] [CrossRef]
- Panda, N.D.; Jawang, U.P.; Lewu, L.D. Pengaruh Bahan Organik Terhadap Daya Ikat Air Pada Ultisol Lahan Kering. J. Tanah Dan Sumberd. Lahan 2021, 8, 327–332. [Google Scholar] [CrossRef]
- Nurhartanto, N.; Zulkarnain, Z.; Wicaksono, A.A. Analisis Beberapa Sifat Fisik Tanah Sebagai Indikator Kerusakan Tanah Pada Lahan Kering. J. Agroekoteknologi Trop. Lembab 2022, 4, 107–112. [Google Scholar] [CrossRef]
- Cortijos-López, M.; Sánchez-Navarrete, P.; Lasanta, T.; Nadal-Romero, E. How Do Acid or Alkaline Soil Environments Affect Soil Organic Carbon Stocks in a Post-Abandonment Secondary Succession Process in Mediterranean Mountain Areas? Catena 2023, 232, 107384. [Google Scholar] [CrossRef]
- Yaseen, M.; Fan, G.; Zhou, X.; Long, W.; Feng, G. Plant Diversity and Soil Nutrients in a Tropical Coastal Secondary Forest: Association Ordination and Sampling Year Differences. Forests 2022, 13, 376. [Google Scholar] [CrossRef]
- Hardiansyah, G.; Prasetyo, L.B.; Darusman, D.; Yulianda, F. Land Suitability and Ecosystem Services for Ecotourism Development. J. Environ. Manag. 2021. [Google Scholar]
- Hairiah, K.; Rahayu, S.; van Noordwijk, M.; Cadisch, G.; Palm, C. Carbon Stock Assessment for Land Use Systems; World Agroforestry Centre (ICRAF) Southeast Asia Regional Office: Bogor, Indonesia, 2011. [Google Scholar]
- Lal, R. Soil Carbon Sequestration to Mitigate Climate Change. Geoderma 2004, 123, 1–22. [Google Scholar] [CrossRef]
- IPCC. Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2019. [Google Scholar]
- Wang, Y.; Chen, S.; He, W.; Ren, J.; Wen, X.; Wang, Y.; Li, X.; Chen, G.; Feng, M.; Fan, C. Shrub Diversity and Niche Characteristics in the Initial Stage of Reconstruction of Low-Efficiency Cupressus Funebris Stands. Forests 2021, 12, 1492. [Google Scholar] [CrossRef]
- Supartono, T.; Adhya, I.; Kosasih, D.; Wildani, W. Tree Species Diversity Adapted to Pinus Merkusii Forests in Gunung Ciremai National Park, West Java, Indonesia. Biodiversitas 2023, 24, 4314–4323. [Google Scholar] [CrossRef]
- Liu, F.; Tan, C.; Yang, Z.; Li, J.; Xiao, H.; Tong, Y. Regeneration and Growth of Tree Seedlings and Saplings in Created Gaps of Different Sizes in a Subtropical Secondary Forest in Southern China. For. Ecol. Manag. 2022, 511, 120143. [Google Scholar] [CrossRef]
- Zébazé, D.; Gorel, A.; Gillet, J.F.; Houngbégnon, F.; Barbier, N.; Ligot, G.; Lhoest, S.; Kamdem, G.; Libalah, M.; Droissart, V.; et al. Natural Regeneration in Tropical Forests along a Disturbance Gradient in South-East Cameroon. For. Ecol. Manag. 2023, 547, 121402. [Google Scholar] [CrossRef]
- Norden, N.; Chazdon, R.L.; Chao, A.; Jiang, Y.H.; Vílchez-Alvarado, B. Resilience of Tropical Rain Forests: Tree Community Reassembly in Secondary Forests. Ecol. Lett. 2009, 12, 385–394. [Google Scholar] [CrossRef]
- Imanuddin, R.; Hidayat, A.; Rachmat, H.H.; Turjaman, M.; Pratiwi; Nurfatriani, F.; Indrajaya, Y.; Susilowati, A. Reforestation and Sustainable Management of Pinus Merkusii Forest Plantation in Indonesia: A Review. Forests 2020, 11, 1235. [Google Scholar] [CrossRef]
- Setiawan, N.N.; Sulistyawati, E. A Seed Rain Community in a Reforested Post-Agricultural Field and Adjacent Secondary Forest of Mount Papandayan Nature Reserve, West Java, Indonesia. J. For. Res. 2021, 32, 1013–1023. [Google Scholar] [CrossRef]
- Hidayah, N. Survivorship and Growth Rate of Rasamala (Altingia Excelsa Noronha), Puspa (Schima Wallichii (DC.) Korth.) and Jamuju (Dacrycarpus Imbricatus (Blume) de Laub.); Institut Pertanian: Bogor, Indonesia, 2011; Available online: https://repository.ipb.ac.id/handle/123456789/52131 (accessed on 5 March 2025).
- CABI. Maesopsis Eminii (Umbrella Tree). CABI Compend. 2019, 2019, 32199. [Google Scholar] [CrossRef]
- Karlinasari, L.; Andini, S.; Worabai, D.; Pamungkas, P.; Budi, S.W.; Siregar, I.Z. Tree Growth Performance and Estimation of Wood Quality in Plantation Trials for Maesopsis eminii and Shorea spp. J. For. Res. 2018, 29, 1157–1166. [Google Scholar] [CrossRef]
- Dani, R.S.; Baniya, C.B. Seedling Potential Attributing Ecological Variables: Trees Species Diversity along an Elevational Gradient in the Temperate Hill Forest, Central Nepal, Version 1; PREPRINT: Kathmandu, Nepal, 2023. [Google Scholar] [CrossRef]
- Cahyanti, L.D.; Sumarni, T.; Widaryanto, E. Potensi Alelopat Daun Pinus (Pinus Spp.) Sebagai Bioherbisida Pra Tumbuh Pada Gulma Krokot (Portulaca Oleracea). Gontor. Agrotech. Sci. J. 2015, 1, 21–31. [Google Scholar] [CrossRef]
- Lucini, F.A.; Morone, F.; Tomassone, M.S.; Makse, H.A. Diversity Increases the Stability of Ecosystems. PLoS ONE 2020, 15, e0228692. [Google Scholar] [CrossRef]
- Suzuki, K.F.; Kobayashi, Y.; Seidl, R.; Senf, C.; Tatsumi, S.; Koide, D.; Azuma, W.A.; Higa, M.; Koyanagi, T.F.; Qian, S.; et al. The Potential Role of an Alien Tree Species in Supporting Forest Restoration: Lessons from Shiretoko National Park, Japan. For. Ecol. Manag. 2021, 493, 119253. [Google Scholar] [CrossRef]
- Richardson, D.M.; Rejmánek, M. Trees and Shrubs as Invasive Alien Species—A Global Review. Divers. Distrib. 2011, 17, 788–809. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Jagodziński, A.M. Impact of Invasive Tree Species on Natural Regeneration Species Composition, Diversity, and Density. Forests 2020, 11, 456. [Google Scholar] [CrossRef]
- Giles, A.L.; Schietti, J.; Rosenfield, M.F.; Mesquita, R.C.; Vieira, D.L.M.; Vieira, I.C.G.; Poorter, L.; Brancalion, P.H.S.; Peña-Claros, M.; Siqueira, J.; et al. Simple Ecological Indicators Benchmark Regeneration Success of Amazonian Forests. Commun. Earth Environ. 2024, 5, 780. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.; Zhou, M.; Ai, D.; Wang, G.; Wang, Y.; Chu, C.; Lundholm, J.T. The Effect of Environmental Heterogeneity on Species Richness Depends on Community Position along the Environmental Gradient. Sci. Rep. 2015, 5, 15723. [Google Scholar] [CrossRef]
- Chapagain, U.; Chapagain, B.P.; Nepal, S.; Manthey, M. Impact of Disturbances on Species Diversity and Regeneration of Nepalese Sal (Shorea Robusta) Forests Managed under Different Management Regimes. Earth 2021, 2, 826–844. [Google Scholar] [CrossRef]
- Mildrexler, D.J.; Berner, L.T.; Law, B.E.; Birdsey, R.A.; Moomaw, W.R. Protect Large Trees for Climate Mitigation, Biodiversity, and Forest Resilience. Conserv. Sci. Pract. 2023, 5, e12944. [Google Scholar] [CrossRef]
- Chazdon, R.L. Tropical Forest Recovery: Legacies of Human Impact and Natural Disturbances. Perspect. Plant Ecol. Evol. Syst. 2003, 6, 51–71. [Google Scholar] [CrossRef]
- Finegan, B. Pattern and Process in Neotropical Secondary Rain Forests: The First 100 Years of Succession. Trends Ecol. Evol. 1996, 11, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Lamb, D.; Erskine, P.D.; Parrotta, J.A. Restoration of Degraded Tropical Forest Landscapes. Science 2005, 310, 1628–1632. [Google Scholar] [CrossRef] [PubMed]
- Ashton, M.S.; Gunatilleke, C.V.S.; Singhakumara, B.M.P.; Gunatilleke, I.A.U.N. Restoration Pathways for Rain Forest in Southwest Sri Lanka: A Review of Concepts and Models. For. Ecol. Manag. 2001, 154, 409–430. [Google Scholar] [CrossRef]
- Rosalina, Y.; Kartawinata, K.; Nisyawati, N.; Nurdin, E.; Supriatna, J. Floristic Composition and Structure of a Peat Swamp Forest in the Conservation Area of the PT National Sago Prima, Selat Panjang, Riau, Indonesia. Reinwardtia 2014, 14, 193–210. [Google Scholar] [CrossRef]
- Heriyanto, N.M.; Samsoedin, I.; Bismark, M. Biodiversity Ffora and Fauna in the Region Forest Bukit Datuk Dumai Riau Province. J. Sylva Lestari 2019, 7, 82–94. [Google Scholar] [CrossRef]
- Samsoedin, I.; Heriyanto, N.M. Structureand Species Composition of Lowland Disturbed Forest at Lepan River Forest Complex, Sei Serdang, Gunung Leuser National Park, North Sumatra. J. Penelit. Hutan Dan Konserv. Alam 2010, 7, 299–314. [Google Scholar] [CrossRef][Green Version]
- Brown, S. Estimating Biomass and Biomass Change of Tropical Forest. A Primer, FAO. 1997. Available online: https://www.fao.org/sustainable-forest-management/toolbox/tools/tool-detail/en/c/217911/ (accessed on 13 February 2024).
- Dharmawan, I.W.S.; Heriyanto, N.M.; Garsetiasih, R.; Kwatrina, R.T.; Sawitri, R.; Denny; Setyawati, T.; Pratiwi; Narendra, B.H.; Siregar, C.A.; et al. The Dynamics of Vegetation Structure, Composition and Carbon Stock in Peatland Ecosystem of Old Secondary Forest in Riau and South Sumatra Provinces. Land 2024, 13, 663. [Google Scholar] [CrossRef]
- Houghton, R.A.; Byers, B.; Nassikas, A.A. A Role for Tropical Forests in Stabilizing Atmospheric CO2. Nat. Clim. Chang. 2015, 5, 1022–1023. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef]
- Rahman, A.A.; Zainol, N.; Ramli, A.; Manzoor, H. Challenges in Creating Ecotourism in Rural Area: A Case of RK Eco Farm Business Venturing. IOP Conf. Ser. Earth Environ. Sci. 2020, 596, 012050. [Google Scholar] [CrossRef]
- Labonda, K.C.; Wipranata, B.I.; Wirawati, S. Rencana Pengelolaan Danau Tambing Sebagai Kawasan Ekowisata. J. Sains Teknol. Urban Peranc. Arsit. 2021, 3, 3387–3400. [Google Scholar] [CrossRef]
- Beech, E.; Crowley, D.; Wilson, B. Liquidambar Excelsa. IUCN Red List. Threat. Species 2019, 2019, e.T60761029A60761083. [Google Scholar] [CrossRef]
- Sadili, A.; Salamah, A.; Mirmanto, E.; Kartawinata, K. Variation in the Composition and Structure of Natural Lowland Forest at Bodogol, Gunung Gede Pangrango National Park, West Java, Indonesia. Reinwardtia 2023, 22, 1–25. [Google Scholar] [CrossRef]
- De Kok, R.P.J.; Sengun, S. A Revision of Cinnadenia Kosterm. (Lauraceae). Adansonia 2020, 42, 105–112. [Google Scholar] [CrossRef]
- de Kok, R.P.J. A Revision of Litsea (Lauraceae) in Peninsular Malaysia and Singapore. Gard. Bull. Singap. 2021, 73, 81–178. [Google Scholar] [CrossRef]
- Sihombing, V.S.; Karlina, E.; Garsetiasih, R.; Rianti, A.; Sawitri, R. Environment Carrying Capacity of Ecotourism in Aek Nauli Research Forest, Simalungun Regency, North Sumatera. Indones. J. For. Res. 2022, 9, 147–163. [Google Scholar] [CrossRef]
- Hayward, M.W. Using the IUCN Red List to Determine Effective Conservation Strategies. Biodivers. Conserv. 2011, 20, 2563–2573. [Google Scholar] [CrossRef]
- Curovic, Ž.; Čurović, M.; Spalević, V.; Janic, M.; Sestras, P.; Popovíc, S.G. Identification and Evaluation of Landscape as a Precondition for Planning Revitalization and Development of Mediterranean Rural Settlements—Case Study: Mrkovi Village, Bay of Kotor, Montenegro. Sustainability 2019, 11, 2039. [Google Scholar] [CrossRef]
- Sadikin, P.N.; Arifin, H.S.; Pramudya, B.; Mulatsih, S. Carrying Capacity to Preserve Biodiversity on Ecotourism in Mount Rinjani National Park, Indonesia. Biodiversitas 2017, 18, 978–989. [Google Scholar] [CrossRef]
- Rahayuningsih, T.; Muntasib, E.K.S.H.; Prasetyo, L.B. Nature Based Tourism Resources Assessment Using Geographic Information System (GIS): Case Study in Bogor. Procedia Environ. Sci. 2016, 33, 365–375. [Google Scholar] [CrossRef]
- Lanier, P. The Positive impacts of ecotourism in protected areas. WIT Trans. Ecol. Environ. 2014, 187, 11. [Google Scholar] [CrossRef]
- Anup, K.C. Tourism and Its Role in Environmental Conservation. J. Tour. Hosp. Educ. 2018, 8, 30–47. [Google Scholar] [CrossRef]
- Wondirad, A.; Tolkach, D.; King, B. Stakeholder Collaboration as a Major Factor for Sustainable Ecotourism Development in Developing Countries. Tour. Manag. 2020, 78, 104024. [Google Scholar] [CrossRef]
- Sahani, N. Application of Hybrid SWOT-AHP-FuzzyAHP Model for Formulation and Prioritization of Ecotourism Strategies in Western Himalaya, India. Int. J. Geoheritage Parks 2021, 9, 349–362. [Google Scholar] [CrossRef]

| No | Location | Area (ha) | Sample Plot (ha) |
|---|---|---|---|
| 1 | Cable Car Station 2/CC 2 | 3 | 1 |
| 2 | Cable Car Station 3/CC 3 | 3 | 1 |
| 3 | Cirembes Disturbed Primary Forest | 2 | 1 |
| Plot | Parameter | Type of Analysis |
|---|---|---|
| 20 m × 20 m | Soil Analysis | pH (H2O), Water Content, C, N, P2O5, CEC & Texture |
| 20 m × 20 m | Name of species | Species composition Endemism Conservation status |
| Number of species | Species density | |
| Frequency of species | Importance value index Shannon index | |
| DBH and height | Stand biomass Carbon stock | |
| 5 m × 5 m | Name of species | Species composition Endemism Conservation status |
| Number of species | Species density | |
| Frequency of species | Importance value index Shannon index | |
| DBH and height | Stand biomass Carbon stock | |
| 2 m × 2 m | Name of species | Species composition Endemism Conservation status |
| Number of species | Species density | |
| Frequency of species | Importance value index Shannon index |
| Location | pH (H2O) | Water Content (%) | C Organic (%) | N Total (%) | P2O5 (mg/kg) | CEC (Cmol (+)/kg) | Texture 3 Fractions (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| Sand | Silt | Clay | |||||||
| CC2 (disturbed primary forest dominated by saninten, earmarked for cable car 2) | 5.5/a | 32.30 | 4.54 h | 0.36 m | <1.79 l | 28.30 h | 97 | 3 | 0 |
| CC3 (disturbed primary forest earmarked for cable car station 3) | 5.3/a | 29.21 | 6.08 vh | 0.50 m | <1.79 l | 22.90 h | 98 | 2 | 0 |
| Cirembes disturbed primary forest | 4.9/va | 43.90 | 8.11 vh | 0.67 h | <1.79 l | 33.34 h | 97 | 2 | 1 |
| No. | Sample Site | Stages | Shannon–Wiener Species Diversity Index (H′) |
|---|---|---|---|
| 1 | CC2 | Tree | 2.16 ** |
| CC3 | Tree | 1.93 ** | |
| Cirembes | Tree | 1.91 ** | |
| All sites combined | Tree | 2.11 *** | |
| 2 | CC2 | Sapling | 2.11 ** |
| CC3 | Sapling | 4.39 *** | |
| Cirembes | Sapling | 2.23 ** | |
| All sites combined | Sapling | 2.31 *** | |
| 3 | CC2 | Seedling | 4.84 *** |
| CC3 | Seedling | 2.27 ** | |
| Cirembes | Seedling | 1.98 ** | |
| All sites combined | Seedling | 2.43 *** |
| No. | Sample Site and Species | IUCN | Density (N/ha) | Importance Value Index (IVI) (%) |
|---|---|---|---|---|
| A. | CC2 | |||
| 1 | Castanopsis acuminatissima (Riung anak) | Least concern/LC | 132 | 85.95 |
| 2 | Litsea sp./Huru | Least concern/LC | 93 | 55.07 |
| 3 | Schima wallichii (Puspa) | Least concern/LC | 59 | 36.95 |
| 4 | Castanopsis argentea (Saninten) | Endangered under criteria A2c./EN | 47 | 32.86 |
| 5 | Altingia excellsa (Rasamala) | Least concern/LC | 26 | 18.27 |
| 6 | Decaspermum fruticosum (Kisireum) | Least concern/LC | 19 | 12.41 |
| 7 | Maesopsis eminii (ky afrika) | Least concern/LC | 9 | 10.33 |
| B. | CC3 | |||
| 1. | Pinus merkusii (Pinus) | Vulnerable under criteria B2ab(ii,iii,v). /VU | 32 | 84.60 |
| 2. | Castanopsis argentea (Saninten) | Endangered under criteria A2c./EN | 26 | 74.63 |
| 3. | Schima wallichii (Puspa) | Least concern/LC | 12 | 38.16 |
| 4. | Castanopsis acuminatissima (Riung anak) | Least concern/LC | 13 | 38.09 |
| 5. | Litsea sp. (Huru) | Least concern/LC | 5 | 15.64 |
| 6. | Altingia excellsa (Rasamala) | Least concern/LC | 5 | 15.31 |
| C. | Cirembes | |||
| 1. | Castanopsis acuminatissima (Riung anak) | Least concern/LC | 88 | 102.82 |
| 2. | Castanopsis argentea (Saninten) | Endangered under criteria a2c./EN | 43 | 55.33 |
| 3. | Schima wallichii (Puspa) | Least concern/lc | 38 | 42.78 |
| 4. | Litsea sp. (Huru) | Least concern/LC | 23 | 28.82 |
| 5. | Pinus merkusii (Pinus) | Vulnerable under criteria B2ab(ii,iii,v). /VU | 25 | 27.01 |
| No. | Sample Sites and Species | IUCN | IVI (%) | ||
|---|---|---|---|---|---|
| Seedling | Sapling | Tree | |||
| A. | CC2 | ||||
| 1 | Castanopsis acuminatissima/Riung anak | Least concern/LC | 21.32 | 20.39 | 85.95 |
| 2 | Castanopsis argentea/Saninten | Endangered under criteria A2c./EN | 17.33 | 14.55 | 32.86 |
| 3 | Decaspermum fruticosum/Kisireum | Least concern/LC | 17.95 | 71.15 | 12.41 |
| 4 | Syzygium nervosum DC. = Eugernia overculata/Salam anjing | Least concern/LC | 14.31 | 15.92 | 6.27 |
| 5 | Litsea sp./Huru Litsea angulata/Huru Litsea tomentosa/Huru meuhmal | Least concern/LC Least concern/LC | 47.33 | 73.82 | 55.07 |
| 6 | Macaranga tanarius/Mara | Least concern/LC | 6.80 | 5.85 | 7.47 |
| 7 | Schima wallichii/Puspa | Least concern/LC | 26.04 | 16.41 | 36.95 |
| 8 | Sterculia oblongata/Hantap | Least concern/LC | 3.03 | 41.30 | 1.95 |
| 9 | Symplocos fasciculata/Jirak | Least concern/LC | 15.38 | 12.24 | 1.25 |
| B. | CC3 | ||||
| 1. | Calliandra calothyrsus/Kaliandra | - | 34.97 | 202.48 | 7.80 |
| 2. | Castanopsis argentea/Saninten | Endangered under criteria A2c./EN | 5.41 | 11.74 | 74.63 |
| C. | Cirembes | ||||
| 1. | Castanopsis acuminatissima/Riung anak | Least concern/LC | 70.30 | 19.44 | 102.82 |
| 2. | Castanopsis argentea/Saninten | Endangered under criteria A2c./EN | 31.22 | 7.05 | 55.33 |
| 3. | Syzygium nervosum DC. = Eugernia overculata/Salam anjing | Least concern/LC | 7.00 | 28.62 | 10.69 |
| 4. | Litsea sp./Huru | - | 56.30 | 75.71 | 28.82 |
| 5. | Schima wallichii/Puspa | Least concern/LC | 51.43 | 31.87 | 42.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawitri, R.; Heriyanto, N.M.; Dharmawan, I.W.S.; Kwatrina, R.T.; Gunawan, H.; Garsetiasih, R.; Takandjandji, M.; Rianti, A.; Sihombing, V.S.; Mindawati, N.; et al. Ecological Dynamics of Forest Stands with Castanopsis argentea (Blume) A.DC. in a Mountain Ecosystem: Vegetation Structure, Diversity, and Carbon Stock Under Tourism Pressure. Land 2025, 14, 1187. https://doi.org/10.3390/land14061187
Sawitri R, Heriyanto NM, Dharmawan IWS, Kwatrina RT, Gunawan H, Garsetiasih R, Takandjandji M, Rianti A, Sihombing VS, Mindawati N, et al. Ecological Dynamics of Forest Stands with Castanopsis argentea (Blume) A.DC. in a Mountain Ecosystem: Vegetation Structure, Diversity, and Carbon Stock Under Tourism Pressure. Land. 2025; 14(6):1187. https://doi.org/10.3390/land14061187
Chicago/Turabian StyleSawitri, Reny, Nur Muhammad Heriyanto, I Wayan Susi Dharmawan, Rozza Tri Kwatrina, Hendra Gunawan, Raden Garsetiasih, Mariana Takandjandji, Anita Rianti, Vivin Silvaliandra Sihombing, Nina Mindawati, and et al. 2025. "Ecological Dynamics of Forest Stands with Castanopsis argentea (Blume) A.DC. in a Mountain Ecosystem: Vegetation Structure, Diversity, and Carbon Stock Under Tourism Pressure" Land 14, no. 6: 1187. https://doi.org/10.3390/land14061187
APA StyleSawitri, R., Heriyanto, N. M., Dharmawan, I. W. S., Kwatrina, R. T., Gunawan, H., Garsetiasih, R., Takandjandji, M., Rianti, A., Sihombing, V. S., Mindawati, N., Pratiwi, Kalima, T., Marsandi, F., Wardani, M., Denny, & Dodo. (2025). Ecological Dynamics of Forest Stands with Castanopsis argentea (Blume) A.DC. in a Mountain Ecosystem: Vegetation Structure, Diversity, and Carbon Stock Under Tourism Pressure. Land, 14(6), 1187. https://doi.org/10.3390/land14061187







