A Review of Plant-Mediated and Fertilization-Induced Shifts in Ammonia Oxidizers: Implications for Nitrogen Cycling in Agroecosystems
Abstract
1. Introduction
2. Above Ground Plant Community Composition’s Influence on Ammonia Oxidizers
2.1. Differences in Ammonia Oxidizers Due to Plant Composition
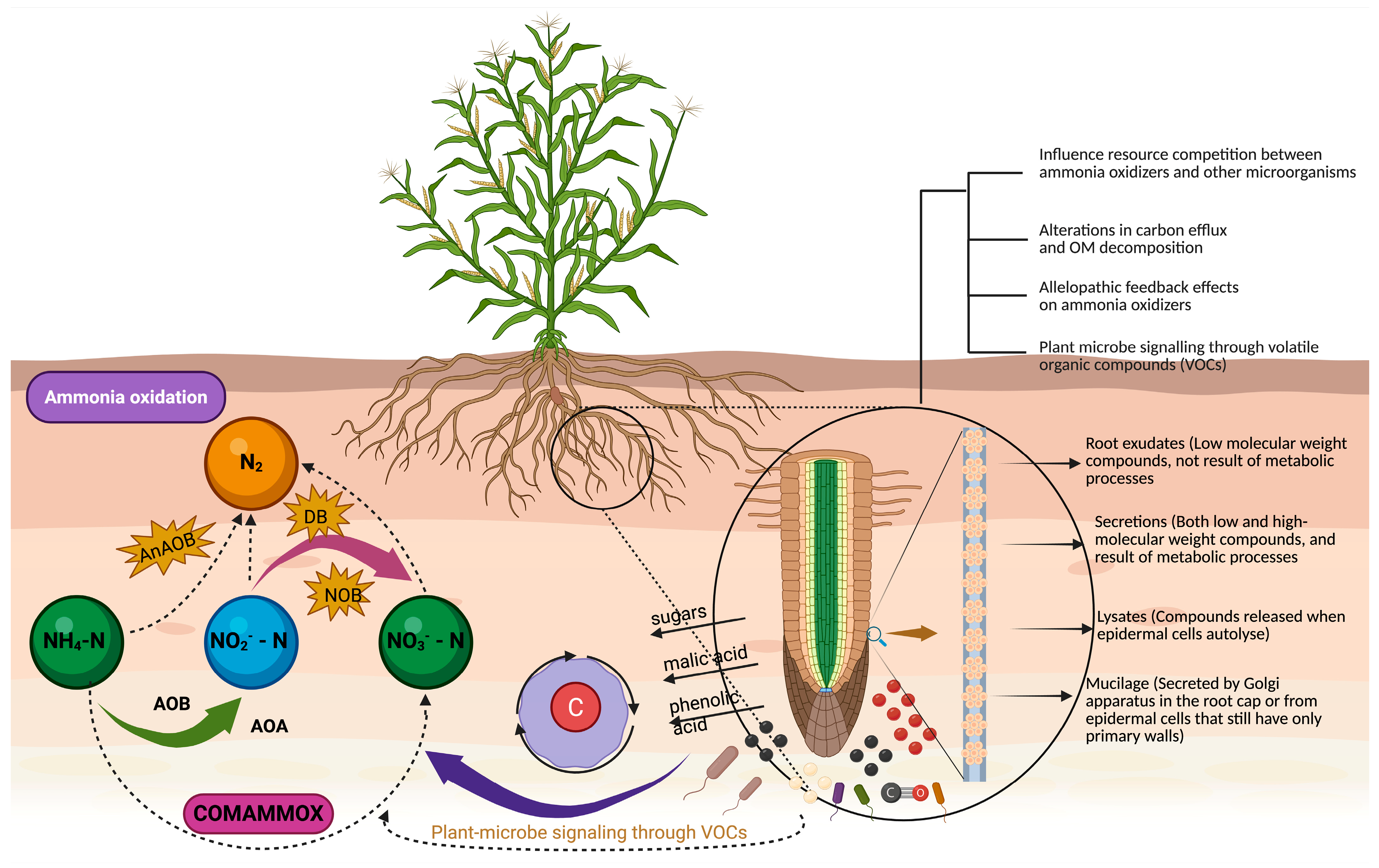
2.2. Root Exudates and Rhizosphere Dynamics
2.3. Allelopathic Compounds
2.4. Plant–Microbiome Signaling
2.5. Plant Nitrophily Gradients
2.6. Photosynthetic Pathways
2.7. Plant Resource Use Strategies Influence on AOA/AOB
2.8. Plant-Nitrifier Competition for Substrate
3. Fertilization Influence on Community Structure of amoA
3.1. Substrate Concentrations (NH3+, NH4⁺ and NO3⁻)
3.2. Soil pH
3.3. Differential Responses of AOA and AOB to Fertilization
3.4. Microbial Adaptation and Ammonia Tolerance
4. Agronomic Strategies for Improving Nitrogen Fertilization in Agroecosystems
4.1. Agronomic–Ecological Trade Offs in N Fertilization: Yield Gains vs. Environmental Costs
4.2. Emphasize on Measuring Gross N Transformation Rates
- What proportion of added fertilizer N is quickly immobilized or lost via denitrification?
- How do microbial communities respond to different fertilizer sources or management practices?
- Which pathways dominate under different environmental or cropping conditions?
4.3. Usage of Nitrification Inhibitors
4.4. Biological Nitrification Inhibition (BNI)
4.5. Optimizing Irrigation and Fertilizer Inputs for Enhanced Agroecosystem Productivity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AOA | Ammonia-Oxidizing Archaea |
| AOB | Ammonia-Oxidizing Bacteria |
| AMO | Ammonia Monooxygenase |
| amoA | Ammonia monooxygenase subunit A gene |
| BNI | Biological Nitrification Inhibition |
| C | Carbon |
| C/N | Carbon to Nitrogen Ratio |
| DCD | Dicyandiamide |
| DMPP | 3,4-Dimethylpyrazole Phosphate |
| DOC | Dissolved Organic Carbon |
| EC | Electrical Conductivity |
| HAO | Hydroxylamine Oxidoreductase |
| Km | Michaelis-Menten constant |
| MCO | Multi-Copper Oxidase |
| N | Nitrogen |
| NH3 | Ammonia |
| NH4⁺ | Ammonium |
| NO2⁻ | Nitrite |
| NO3⁻ | Nitrate |
| N2O | Nitrous Oxide |
| NI | Nitrification Inhibitor |
| NUE | Nitrogen Use Efficiency |
| PSM | Plant Secondary Metabolite |
| SNIs | Synthetic Nitrification Inhibitors |
| VOC | Volatile Organic Compound |
| Vm | Maximum rate of enzymatic activity |
References
- Fowler, D.; Coyle, M.; Skiba, U.; Sutton, M.A.; Cape, J.N.; Reis, S.; Sheppard, L.J.; Jenkins, A.; Grizzetti, B.; Galloway, J.N.; et al. The Global Nitrogen Cycle in the Twenty-First Century. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20130164. [Google Scholar] [CrossRef]
- Zhang, X.; Ward, B.B.; Sigman, D.M. Global Nitrogen Cycle: Critical Enzymes, Organisms, and Processes for Nitrogen Budgets and Dynamics. Chem. Rev. 2020, 120, 5308–5351. [Google Scholar] [CrossRef] [PubMed]
- Galloway, J.N.; Cowling, E.B. Reactive Nitrogen and The World: 200 Years of Change. AMBIO J. Human Environ. 2002, 31, 64–71. [Google Scholar] [CrossRef]
- Cavigelli, M.A.; Grosso, S.J.D.; Liebig, M.A.; Snyder, C.S.; Fixen, P.E.; Venterea, R.T.; Leytem, A.B.; McLain, J.E.; Watts, D.B. US Agricultural Nitrous Oxide Emissions: Context, Status, and Trends. Front. Ecol. Environ. 2012, 10, 537–546. [Google Scholar] [CrossRef]
- Meisinger, J.J.; Schepers, J.S.; Raun, W.R. Crop Nitrogen Requirement and Fertilization. In Nitrogen in Agricultural Systems; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; pp. 563–612. ISBN 978-0-89118-191-0. [Google Scholar]
- Cavicchioli, R.; Ripple, W.J.; Timmis, K.N.; Azam, F.; Bakken, L.R.; Baylis, M.; Behrenfeld, M.J.; Boetius, A.; Boyd, P.W.; Classen, A.T.; et al. Scientists’ Warning to Humanity: Microorganisms and Climate Change. Nat. Rev. Microbiol. 2019, 17, 569–586. [Google Scholar] [CrossRef]
- Kumari, S.; Devi, M.; Thakur, K.; Minhas, B.; Bhatt, A.K.; Kaushik, N. Impact of Anthropogenic Activities on Microbial Diversity and Soil Health. In Advancements in Microbial Biotechnology for Soil Health; Bhatia, R.K., Walia, A., Eds.; Springer Nature: Singapore, 2024; pp. 227–248. ISBN 978-981-99-9482-3. [Google Scholar]
- Canfield, D.E.; Glazer, A.N.; Falkowski, P.G. The Evolution and Future of Earth’s Nitrogen Cycle. Science 2010, 330, 192–196. [Google Scholar] [CrossRef]
- Firestone, M.; Davidson, E. Microbiological Basis of NO and N2O Production and Consumption in Soil. Exchange of Trace Gases between terrestrial Ecosystems and the Atmosphere; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1989; Volume 47. [Google Scholar]
- Burris, R.H.; Roberts, G.P. Biological Nitrogen Fixation. Annu. Rev. Nutr. 1993, 13, 317–335. [Google Scholar] [CrossRef]
- Kox, M.A.R.; Jetten, M.S.M. The Nitrogen Cycle. In Principles of Plant-Microbe Interactions: Microbes for Sustainable Agriculture; Lugtenberg, B., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 205–214. ISBN 978-3-319-08575-3. [Google Scholar]
- Akiyama, H.; Yan, X.; Yagi, K. Estimations of Emission Factors for Fertilizer-Induced Direct N2O Emissions from Agricultural Soils in Japan: Summary of Available Data. Soil Sci. Plant Nutr. 2006, 52, 774–787. [Google Scholar] [CrossRef]
- Grzyb, A.; Wolna-Maruwka, A.; Niewiadomska, A. The Significance of Microbial Transformation of Nitrogen Compounds in the Light of Integrated Crop Management. Agronomy 2021, 11, 1415. [Google Scholar] [CrossRef]
- Rotthauwe, J.H.; Witzel, K.P.; Liesack, W. The Ammonia Monooxygenase Structural Gene amoA as a Functional Marker: Molecular Fine-Scale Analysis of Natural Ammonia-Oxidizing Populations. Appl. Environ. Microbiol. 1997, 63, 4704–4712. [Google Scholar] [CrossRef]
- Daims, H.; Lebedeva, E.V.; Pjevac, P.; Han, P.; Herbold, C.; Albertsen, M.; Jehmlich, N.; Palatinszky, M.; Vierheilig, J.; Bulaev, A.; et al. Complete Nitrification by Nitrospira Bacteria. Nature 2015, 528, 504–509. [Google Scholar] [CrossRef] [PubMed]
- van Kessel, M.A.H.J.; Speth, D.R.; Albertsen, M.; Nielsen, P.H.; Op den Camp, H.J.M.; Kartal, B.; Jetten, M.S.M.; Lücker, S. Complete Nitrification by a Single Microorganism. Nature 2015, 528, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Norton, J.M.; Klotz, M.G.; Stein, L.Y.; Arp, D.J.; Bottomley, P.J.; Chain, P.S.G.; Hauser, L.J.; Land, M.L.; Larimer, F.W.; Shin, M.W.; et al. Complete Genome Sequence of Nitrosospira multiformis, an Ammonia-Oxidizing Bacterium from the Soil Environment. Appl. Environ. Microbiol. 2008, 74, 3559–3572. [Google Scholar] [CrossRef]
- Coleman, R.E.; Lancaster, K.M. Heme P460: A (Cross) Link to Nitric Oxide. Acc. Chem. Res. 2020, 53, 2925–2935. [Google Scholar] [CrossRef]
- Caranto, J.D.; Lancaster, K.M. Nitric Oxide Is an Obligate Bacterial Nitrification Intermediate Produced by Hydroxylamine Oxidoreductase. Proc. Natl. Acad. Sci. USA 2017, 114, 8217–8222. [Google Scholar] [CrossRef]
- Kozlowski, J.A.; Stieglmeier, M.; Schleper, C.; Klotz, M.G.; Stein, L.Y. Pathways and Key Intermediates Required for Obligate Aerobic Ammonia-Dependent Chemolithotrophy in Bacteria and Thaumarchaeota. ISME J. 2016, 10, 1836–1845. [Google Scholar] [CrossRef] [PubMed]
- Treusch, A.H.; Leininger, S.; Kletzin, A.; Schuster, S.C.; Klenk, H.; Schleper, C. Novel Genes for Nitrite Reductase and Amo-related Proteins Indicate a Role of Uncultivated Mesophilic Crenarchaeota in Nitrogen Cycling. Environ. Microbiol. 2005, 7, 1985–1995. [Google Scholar] [CrossRef]
- Vajrala, N.; Martens-Habbena, W.; Sayavedra-Soto, L.A.; Schauer, A.; Bottomley, P.J.; Stahl, D.A.; Arp, D.J. Hydroxylamine as an Intermediate in Ammonia Oxidation by Globally Abundant Marine Archaea. Proc. Natl. Acad. Sci. USA 2013, 110, 1006–1011. [Google Scholar] [CrossRef]
- Kerou, M.; Offre, P.; Valledor, L.; Abby, S.S.; Melcher, M.; Nagler, M.; Weckwerth, W.; Schleper, C. Proteomics and Comparative Genomics of Nitrososphaera Viennensis Reveal the Core Genome and Adaptations of Archaeal Ammonia Oxidizers. Proc. Natl. Acad. Sci. USA 2016, 113, E7937–E7946. [Google Scholar] [CrossRef]
- Spang, A.; Poehlein, A.; Offre, P.; Zumbrägel, S.; Haider, S.; Rychlik, N.; Nowka, B.; Schmeisser, C.; Lebedeva, E.V.; Rattei, T.; et al. The Genome of the Ammonia-oxidizing Candidatus Nitrososphaera Gargensis: Insights into Metabolic Versatility and Environmental Adaptations. Environ. Microbiol. 2012, 14, 3122–3145. [Google Scholar] [CrossRef]
- Norton, J.; Ouyang, Y. Controls and Adaptive Management of Nitrification in Agricultural Soils. Front. Microbiol. 2019, 10, 1931. [Google Scholar] [CrossRef] [PubMed]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; Da Rocha, U.N.; Shi, S.; Cho, H.; Karaoz, U.; Loqué, D.; Bowen, B.P.; et al. Dynamic Root Exudate Chemistry and Microbial Substrate Preferences Drive Patterns in Rhizosphere Microbial Community Assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Ai, C.; Liang, G.; Sun, J.; Wang, X.; He, P.; Zhou, W.; He, X. Reduced Dependence of Rhizosphere Microbiome on Plant-Derived Carbon in 32-Year Long-Term Inorganic and Organic Fertilized Soils. Soil Biol. Biochem. 2015, 80, 70–78. [Google Scholar] [CrossRef]
- Prosser, J.I.; Nicol, G.W. Archaeal and Bacterial Ammonia-Oxidisers in Soil: The Quest for Niche Specialisation and Differentiation. Trends Microbiol. 2012, 20, 523–531. [Google Scholar] [CrossRef]
- Nicol, G.W.; Leininger, S.; Schleper, C.; Prosser, J.I. The Influence of Soil pH on the Diversity, Abundance and Transcriptional Activity of Ammonia Oxidizing Archaea and Bacteria. Environ. Microbiol. 2008, 10, 2966–2978. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Sahrawat, K.L.; Nakahara, K.; Rao, I.M.; Ishitani, M.; Hash, C.T.; Kishii, M.; Bonnett, D.G.; Berry, W.L.; Lata, J.C. A Paradigm Shift towards Low-Nitrifying Production Systems: The Role of Biological Nitrification Inhibition (BNI). Ann. Bot. 2013, 112, 297–316. [Google Scholar] [CrossRef] [PubMed]
- Tesfamariam, T.; Yoshinaga, H.; Deshpande, S.P.; Srinivasa Rao, P.; Sahrawat, K.L.; Ando, Y.; Nakahara, K.; Hash, C.T.; Subbarao, G.V. Biological Nitrification Inhibition in Sorghum: The Role of Sorgoleone Production. Plant Soil 2014, 379, 325–335. [Google Scholar] [CrossRef]
- Norton, J. Diversity and Environmental Distribution of Ammonia-Oxidizing Bacteria. In Nitrification; American Society of Microbiology: Washington, DC, USA, 2011; pp. 39–55. ISBN 978-1-55581-481-6. [Google Scholar]
- Bardgett, R.D.; Mommer, L.; De Vries, F.T. Going Underground: Root Traits as Drivers of Ecosystem Processes. Trends Ecol. Evol. 2014, 29, 692–699. [Google Scholar] [CrossRef]
- Somers, E.; Vanderleyden, J.; Srinivasan, M. Rhizosphere Bacterial Signalling: A Love Parade beneath Our Feet. Crit. Rev. Microbiol. 2004, 30, 205–240. [Google Scholar] [CrossRef]
- Kowalchuk, G.A.; Stephen, J.R. Ammonia-Oxidizing Bacteria: A Model for Molecular Microbial Ecology. Annu. Rev. Microbiol. 2001, 55, 485–529. [Google Scholar] [CrossRef]
- Trivedi, C.; Reich, P.B.; Maestre, F.T.; Hu, H.-W.; Singh, B.K.; Delgado-Baquerizo, M. Plant-Driven Niche Differentiation of Ammonia-Oxidizing Bacteria and Archaea in Global Drylands. ISME J. 2019, 13, 2727. [Google Scholar] [CrossRef] [PubMed]
- Malchair, S.; De Boeck, H.J.; Lemmens, C.M.H.M.; Ceulemans, R.; Merckx, R.; Nijs, I.; Carnol, M. Diversity–Function Relationship of Ammonia-Oxidizing Bacteria in Soils among Functional Groups of Grassland Species under Climate Warming. Appl. Soil Ecol. 2010, 44, 15–23. [Google Scholar] [CrossRef]
- Thion, C.E.; Poirel, J.D.; Cornulier, T.; De Vries, F.T.; Bardgett, R.D.; Prosser, J.I. Plant Nitrogen-Use Strategy as a Driver of Rhizosphere Archaeal and Bacterial Ammonia Oxidiser Abundance. FEMS Microbiol. Ecol. 2016, 92, fiw091. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, J. Biochemistry & Molecular Biology of Plants; Buchanan, B.B., Gruissem, W., Jones, R.L., Eds.; American Society of Plant Physiologists: Rockville, MD, USA, 2000; ISBN 978-0-943088-37-2. [Google Scholar]
- Pitman, N.C.A.; Jørgensen, P.M. Estimating the Size of the World’s Threatened Flora. Science 2002, 298, 989. [Google Scholar] [CrossRef]
- Berg, G.; Smalla, K. Plant Species and Soil Type Cooperatively Shape the Structure and Function of Microbial Communities in the Rhizosphere: Plant Species, Soil Type and Rhizosphere Communities. FEMS Microbiol. Ecol. 2009, 68, 1–13. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, H.Y.H.; Ruan, H. Global Negative Effects of Nitrogen Deposition on Soil Microbes. ISME J. 2018, 12, 1817–1825. [Google Scholar] [CrossRef]
- Qiao, M.; Xiao, J.; Yin, H.; Pu, X.; Yue, B.; Liu, Q. Analysis of the Phenolic Compounds in Root Exudates Produced by a Subalpine Coniferous Species as Responses to Experimental Warming and Nitrogen Fertilisation. Chem. Ecol. 2014, 30, 555–565. [Google Scholar] [CrossRef]
- Girardi, J.; Korz, S.; Muñoz, K.; Jungkunst, H.; Brunn, M. Phenolic Compounds from Invasive Fallopia Japonica Inhibit Nitrification. In Proceedings of the EGU General Assembly Conference Abstracts, Vienna, Austria, 23–27 May 2022. [Google Scholar]
- He, W.; Yuan, Y.; Zhang, Z.; Xiao, J.; Liu, Q.; Laiho, R.; Yin, H. Effect of N Addition on Root Exudation and Associated Microbial N Transformation under Sibiraea Angustata in an Alpine Shrubland. Plant Soil 2021, 460, 469–481. [Google Scholar] [CrossRef]
- Liu, Y.; Evans, S.E.; Friesen, M.L.; Tiemann, L.K. Root Exudates Shift How N Mineralization and N Fixation Contribute to the Plant-Available N Supply in Low Fertility Soils. Soil Biol. Biochem. 2022, 165, 108541. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, W.; Zhang, Z.; Xiao, J.; Li, D.; Liu, Q.; Yin, H. Impacts of Oxalic Acid and Glucose Additions on N Transformation in Microcosms via Artificial Roots. Soil Biol. Biochem. 2018, 121, 16–23. [Google Scholar] [CrossRef]
- Huang, X.-F.; Chaparro, J.M.; Reardon, K.F.; Zhang, R.; Shen, Q.; Vivanco, J.M. Rhizosphere Interactions: Root Exudates, Microbes, and Microbial Communities. Botany 2014, 92, 267–275. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Kessler, A.; Kalske, A. Plant Secondary Metabolite Diversity and Species Interactions. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 115–138. [Google Scholar] [CrossRef]
- Zakir, H.A.K.M.; Subbarao, G.V.; Pearse, S.J.; Gopalakrishnan, S.; Ito, O.; Ishikawa, T.; Kawano, N.; Nakahara, K.; Yoshihashi, T.; Ono, H.; et al. Detection, Isolation and Characterization of a Root-Exuded Compound, Methyl 3-(4-Hydroxyphenyl) Propionate, Responsible for Biological Nitrification Inhibition by Sorghum (Sorghum Bicolor). New Phytol. 2008, 180, 442–451. [Google Scholar] [CrossRef]
- Schulz-Bohm, K.; Gerards, S.; Hundscheid, M.; Melenhorst, J.; de Boer, W.; Garbeva, P. Calling from Distance: Attraction of Soil Bacteria by Plant Root Volatiles. ISME J. 2018, 12, 1252–1262. [Google Scholar] [CrossRef] [PubMed]
- Peters, N.K.; Frost, J.W.; Long, S.R. A Plant Flavone, Luteolin, Induces Expression of Rhizobium meliloti Nodulation Genes. Science 1986, 233, 977–980. [Google Scholar] [CrossRef]
- Garbeva, P.; Weisskopf, L. Airborne Medicine: Bacterial Volatiles and Their Influence on Plant Health. New Phytol. 2020, 226, 32–43. [Google Scholar] [CrossRef]
- Wang, X.-L.; Si, Z.-Q.; Yu, H.; Qi, L.; Liu, W.; Shi, J.; Song, P. Unveiling the Dual Role of Heterotrophic Ammonia-Oxidizing Bacteria: Enhancing Plant Regrowth through Modulating Cytokinin Delivery. Front. Microbiol. 2023, 14, 1268442. [Google Scholar] [CrossRef]
- Moreau, D.; Pivato, B.; Bru, D.; Busset, H.; Deau, F.; Faivre, C.; Matejicek, A.; Strbik, F.; Philippot, L.; Mougel, C. Plant Traits Related to Nitrogen Uptake Influence Plant-Microbe Competition. Ecology 2015, 96, 2300–2310. [Google Scholar] [CrossRef]
- Moreau, D.; Milard, G.; Munier-Jolain, N. A Plant Nitrophily Index Based on Plant Leaf Area Response to Soil Nitrogen Availability. Agron. Sustain. Dev. 2014, 33, 809–815. [Google Scholar] [CrossRef]
- Chang, X.; Wang, W.; Zhou, H. Nitrogen Acquisition by Invasive Plants: Species Preferential N Uptake Matching with Soil N Dynamics Contribute to Its Fitness and Domination. Plants 2025, 14, 748. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, D.J.; Lemaire, G.; Gosse, G.; Cruz, P.; Draycott, A.; Neeteson, J.J. Decline in Percentage N of C3 and C4 Crops with Increasing Plant Mass. Ann. Bot. 1990, 66, 425–436. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Shi, W.; Kronzucker, H.J. Nitrogen Transformations in Modern Agriculture and the Role of Biological Nitrification Inhibition. Nat. Plants 2017, 3, 17074. [Google Scholar] [CrossRef]
- Wichern, F.; Eberhardt, E.; Mayer, J.; Joergensen, R.G.; Müller, T. Nitrogen Rhizodeposition in Agricultural Crops: Methods, Estimates and Future Prospects. Soil Biol. Biochem. 2008, 40, 30–48. [Google Scholar] [CrossRef]
- Carvalhais, L.C.; Dennis, P.G.; Fan, B.; Fedoseyenko, D.; Kierul, K.; Becker, A.; von Wiren, N.; Borriss, R. Linking Plant Nutritional Status to Plant-Microbe Interactions. PLoS ONE 2013, 8, e68555. [Google Scholar] [CrossRef]
- Yin, C.; Casa Vargas, J.M.; Schlatter, D.C.; Hagerty, C.H.; Hulbert, S.H.; Paulitz, T.C. Rhizosphere Community Selection Reveals Bacteria Associated with Reduced Root Disease. Microbiome 2021, 9, 86. [Google Scholar] [CrossRef]
- Liljeroth, E.; Kuikman, P.; Van Veen, J.A. Carbon Translocation to the Rhizosphere of Maize and Wheat and Influence on the Turnover of Native Soil Organic Matter at Different Soil Nitrogen Levels. Plant Soil 1994, 161, 233–240. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Ayangbenro, A.S.; Glick, B.R.; Babalola, O.O. Plant Health: Feedback Effect of Root Exudates-Rhizobiome Interactions. Appl. Microbiol. Biotechnol. 2019, 103, 1155–1166. [Google Scholar] [CrossRef]
- Molefe, R.R.; Amoo, A.E.; Babalola, O.O. Communication between Plant Roots and the Soil Microbiome; Involvement in Plant Growth and Development. Symbiosis 2023, 90, 231–239. [Google Scholar] [CrossRef]
- Marques, A.C.R.; de Oliveira, L.B.; Nicoloso, F.T.; Jacques, R.J.S.; Giacomini, S.J.; Quadros, F.L.F. de Biological Nitrogen Fixation in C4 Grasses of Different Growth Strategies of South America Natural Grasslands. Appl. Soil Ecol. 2017, 113, 54–62. [Google Scholar] [CrossRef]
- Lambers, H.; Raven, J.A.; Shaver, G.R.; Smith, S.E. Plant Nutrient-Acquisition Strategies Change with Soil Age. Trends Ecol. Evol. 2008, 23, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Mcgill, B.; Enquist, B.; Weiher, E.; Westoby, M. Rebuilding Community Ecology from Functional Traits. Trends Ecol. Evol. 2006, 21, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The Worldwide Leaf Economics Spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Violle, C.; Navas, M.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the Concept of Trait Be Functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Wardle, D.A. Aboveground-Belowground Linkages: Biotic Interactions, Ecosystem Processes, and Global Change; Oxford University Press: Oxford, UK, 2010; ISBN 978-0-19-954687-9. [Google Scholar]
- Garnier, E.; Cortez, J.; Billès, G.; Navas, M.-L.; Roumet, C.; Debussche, M.; Laurent, G.; Blanchard, A.; Aubry, D.; Bellmann, A.; et al. Plant Functional Markers Capture Ecosystem Properties During Secondary Succession. Ecology 2004, 85, 2630–2637. [Google Scholar] [CrossRef]
- Lavorel, S.; Grigulis, K.; Lamarque, P.; Colace, M.-P.; Garden, D.; Girel, J.; Pellet, G.; Douzet, R. Using Plant Functional Traits to Understand the Landscape Distribution of Multiple Ecosystem Services. J. Ecol. 2011, 99, 135–147. [Google Scholar] [CrossRef]
- Fortunel, C.; Garnier, E.; Joffre, R.; Kazakou, E.; Quested, H.; Grigulis, K.; Lavorel, S.; Ansquer, P.; Castro, H.; Cruz, P.; et al. Leaf Traits Capture the Effects of Land Use Changes and Climate on Litter Decomposability of Grasslands across Europe. Ecology 2009, 90, 598–611. [Google Scholar] [CrossRef]
- Orwin, K.H.; Buckland, S.M.; Johnson, D.; Turner, B.L.; Smart, S.; Oakley, S.; Bardgett, R.D. Linkages of Plant Traits to Soil Properties and the Functioning of Temperate Grassland. J. Ecol. 2010, 98, 1074–1083. [Google Scholar] [CrossRef]
- de Vries, F.T.; Manning, P.; Tallowin, J.R.B.; Mortimer, S.R.; Pilgrim, E.S.; Harrison, K.A.; Hobbs, P.J.; Quirk, H.; Shipley, B.; Cornelissen, J.H.C.; et al. Abiotic Drivers and Plant Traits Explain Landscape-Scale Patterns in Soil Microbial Communities. Ecol. Lett. 2012, 15, 1230–1239. [Google Scholar] [CrossRef]
- Grigulis, K.; Lavorel, S.; Krainer, U.; Legay, N.; Baxendale, C.; Dumont, M.; Kastl, E.; Arnoldi, C.; Bardgett, R.D.; Poly, F.; et al. Relative Contributions of Plant Traits and Soil Microbial Properties to Mountain Grassland Ecosystem Services. J. Ecol. 2013, 101, 47–57. [Google Scholar] [CrossRef]
- van der Heijden, M.G.A.; Bardgett, R.D.; van Straalen, N.M. The Unseen Majority: Soil Microbes as Drivers of Plant Diversity and Productivity in Terrestrial Ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setälä, H.; van der Putten, W.H.; Wall, D.H. Ecological Linkages between Aboveground and Belowground Biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.M.; Shugart, H.H.; Woodward, F.I.; Burton, P.J. Plant Functional Types. In Vegetation Dynamics & Global Change; Solomon, A.M., Shugart, H.H., Eds.; Springer: Boston, MA, USA, 1993; pp. 272–292. ISBN 978-1-4613-6217-3. [Google Scholar]
- Grassein, F.; Till-Bottraud, I.; Lavorel, S. Plant Resource-Use Strategies: The Importance of Phenotypic Plasticity in Response to a Productivity Gradient for Two Subalpine Species. Ann. Bot. 2010, 106, 637–645. [Google Scholar] [CrossRef]
- Metay, A.; Magnier, J.; Guilpart, N.; Christophe, A. Nitrogen Supply Controls Vegetative Growth, Biomass and Nitrogen Allocation for Grapevine (Cv. Shiraz) Grown in Pots. Funct. Plant Biol. 2014, 42, 105–114. [Google Scholar] [CrossRef]
- Elser, J.J.; Bracken, M.E.S.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global Analysis of Nitrogen and Phosphorus Limitation of Primary Producers in Freshwater, Marine and Terrestrial Ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef]
- Norby, R.J.; Warren, J.M.; Iversen, C.M.; Medlyn, B.E.; McMurtrie, R.E. CO2 Enhancement of Forest Productivity Constrained by Limited Nitrogen Availability. Proc. Natl. Acad. Sci. USA 2010, 107, 19368–19373. [Google Scholar] [CrossRef]
- Reich, P.B.; Hobbie, S.E. Decade-Long Soil Nitrogen Constraint on the CO2 Fertilization of Plant Biomass. Nat. Clim. Change 2013, 3, 278–282. [Google Scholar] [CrossRef]
- LeBauer, D.S.; Treseder, K.K. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 2008, 89, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Vitousek, P.M.; Naylor, R.; Crews, T.; David, M.B.; Drinkwater, L.E.; Holland, E.; Johnes, P.J.; Katzenberger, J.; Martinelli, L.A.; Matson, P.A.; et al. Nutrient Imbalances in Agricultural Development. Science 2009, 324, 1519–1520. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhuang, Q. Modeling the Effects of Organic Nitrogen Uptake by Plants on the Carbon Cycling of Boreal Forest and Tundra Ecosystems. Biogeosciences 2013, 10, 7943–7955. [Google Scholar] [CrossRef]
- Kaye, J.P.; Hart, S.C. Competition for Nitrogen between Plants and Soil Microorganisms. Trends Ecol. Evol. 1997, 12, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Chapin, F.S.; Firestone, M.K.; Field, C.B.; Chiariello, N.R. Nitrogen Limitation of Microbial Decomposition in a Grassland under Elevated CO2. Nature 2001, 409, 188–191. [Google Scholar] [CrossRef]
- Inselsbacher, E.; Hinko-Najera Umana, N.; Stange, F.C.; Gorfer, M.; Schüller, E.; Ripka, K.; Zechmeister-Boltenstern, S.; Hood-Novotny, R.; Strauss, J.; Wanek, W. Short-Term Competition between Crop Plants and Soil Microbes for Inorganic N Fertilizer. Soil Biol. Biochem. 2010, 42, 360–372. [Google Scholar] [CrossRef]
- Hodge, A.; Robinson, D.; Fitter, A. Are Microorganisms More Effective than Plants at Competing for Nitrogen? Trends Plant Sci. 2000, 5, 304–308. [Google Scholar] [CrossRef]
- Tate, R.L. Soil Microbiology; John Wiley & Sons, Incorporated: Hoboken, NJ, USA, 2020; ISBN 978-0-470-31110-3. [Google Scholar]
- Chinthalapudi, D.P.M.; Pokhrel, S.; Kingery, W.L.; Shankle, M.W.; Ganapathi Shanmugam, S. Exploring the Synergistic Impacts of Cover Crops and Fertilization on Soil Microbial Metabolic Diversity in Dryland Soybean Production Systems Using Biolog EcoPlates. Appl. Biosci. 2023, 2, 328–346. [Google Scholar] [CrossRef]
- Craine, J.M.; Morrow, C.; Fierer, N. Microbial nitrogen limitation increases decomposition. Ecology 2007, 88, 2105–2113. [Google Scholar] [CrossRef]
- Manzoni, S.; Schaeffer, S.M.; Katul, G.; Porporato, A.; Schimel, J.P. A Theoretical Analysis of Microbial Eco-Physiological and Diffusion Limitations to Carbon Cycling in Drying Soils. Soil Biol. Biochem. 2014, 73, 69–83. [Google Scholar] [CrossRef]
- Parton, W.; Silver, W.L.; Burke, I.C.; Grassens, L.; Harmon, M.E.; Currie, W.S.; King, J.Y.; Adair, E.C.; Brandt, L.A.; Hart, S.C.; et al. Global-Scale Similarities in Nitrogen Release Patterns During Long-Term Decomposition. Science 2007, 315, 361–364. [Google Scholar] [CrossRef]
- Di, H.J.; Cameron, K.C.; Shen, J.P.; Winefield, C.S.; O’Callaghan, M.; Bowatte, S.; He, J.Z. Nitrification Driven by Bacteria and Not Archaea in Nitrogen-Rich Grassland Soils. Nat. Geosci. 2009, 2, 621–624. [Google Scholar] [CrossRef]
- Winkler, K.; Fuchs, R.; Rounsevell, M.; Herold, M. Global Land Use Changes Are Four Times Greater than Previously Estimated. Nat. Commun. 2021, 12, 2501. [Google Scholar] [CrossRef]
- Verhamme, D.T.; Prosser, J.I.; Nicol, G.W. Ammonia Concentration Determines Differential Growth of Ammonia-Oxidising Archaea and Bacteria in Soil Microcosms. ISME J. 2011, 5, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Könneke, M.; Bernhard, A.E.; de la Torre, J.R.; Walker, C.B.; Waterbury, J.B.; Stahl, D.A. Isolation of an Autotrophic Ammonia-Oxidizing Marine Archaeon. Nature 2005, 437, 543–546. [Google Scholar] [CrossRef]
- Jung, M.-Y.; Park, S.-J.; Min, D.; Kim, J.-S.; Rijpstra, W.I.C.; Sinninghe Damsté, J.S.; Kim, G.-J.; Madsen, E.L.; Rhee, S.-K. Enrichment and Characterization of an Autotrophic Ammonia-Oxidizing Archaeon of Mesophilic Crenarchaeal Group I.1a from an Agricultural Soil. Appl. Environ. Microbiol. 2011, 77, 8635–8647. [Google Scholar] [CrossRef]
- Martens-Habbena, W.; Berube, P.M.; Urakawa, H.; de la Torre, J.R.; Stahl, D.A. Ammonia Oxidation Kinetics Determine Niche Separation of Nitrifying Archaea and Bacteria. Nature 2009, 461, 976–979. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Tan, C.; Chen, H.; Ye, M.; Fan, X.; Zheng, W.; Gao, Z.; Peng, H.; Liang, Y. Insight into the Role of Competition in Niche Differentiation between Ammonia-Oxidizing Archaea and Bacteria in Ammonium-Rich Alkaline Soil: A Network-Based Study. Soil Biol. Biochem. 2022, 168, 108638. [Google Scholar] [CrossRef]
- Leininger, S.; Urich, T.; Schloter, M.; Schwark, L.; Qi, J.; Nicol, G.W.; Prosser, J.I.; Schuster, S.C.; Schleper, C. Archaea Predominate among Ammonia-Oxidizing Prokaryotes in Soils. Nature 2006, 442, 806–809. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Bai, T.; Xiao, R.; Wang, P.; Wang, F.; Duryee, A.M.; Wang, Y.; Zhang, Y.; Hu, S. Vertical Distribution of Ammonia-Oxidizing Microorganisms across a Soil Profile of the Chinese Loess Plateau and Their Responses to Nitrogen Inputs. Sci. Total Environ. 2018, 635, 240–248. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Lu, Q.; Raza, W.; Huang, Q.; Shen, Q. Ammonia Oxidizer Abundance in Paddy Soil Profile with Different Fertilizer Regimes. Appl. Soil Ecol. 2014, 84, 38–44. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Zhang, Y.; Lang, M.; Christie, P.; Bei, S.; Zhang, J. Dynamics of Ammonia Oxidizers in Response to Different Fertilization Inputs in Intensively Managed Agricultural Soils. Appl. Soil Ecol. 2021, 157, 103729. [Google Scholar] [CrossRef]
- Chu, M.; Jagadamma, S.; Walker, F.R.; Eash, N.S.; Buschermohle, M.J.; Duncan, L.A. Effect of Multispecies Cover Crop Mixture on Soil Properties and Crop Yield. Agric. Environ. Lett. 2017, 2, 170030. [Google Scholar] [CrossRef]
- Shen, B.; Guo, J.; Chen, P. A Survey of P2P Virtual World Infrastructure. In Proceedings of the 2012 IEEE Ninth International Conference on e-Business Engineering, Hangzhou, China, 9–11 September 2012; IEEE: Piscataway, NJ, USA, 2012; pp. 296–303. [Google Scholar]
- Fierer, N. Embracing the Unknown: Disentangling the Complexities of the Soil Microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Carey, C.J.; Dove, N.C.; Beman, J.M.; Hart, S.C.; Aronson, E.L. Meta-Analysis Reveals Ammonia-Oxidizing Bacteria Respond More Strongly to Nitrogen Addition than Ammonia-Oxidizing Archaea. Soil Biol. Biochem. 2016, 99, 158–166. [Google Scholar] [CrossRef]
- Ouyang, Y.; Evans, S.E.; Friesen, M.L.; Tiemann, L.K. Effect of Nitrogen Fertilization on the Abundance of Nitrogen Cycling Genes in Agricultural Soils: A Meta-Analysis of Field Studies. Soil Biol. Biochem. 2018, 127, 71–78. [Google Scholar] [CrossRef]
- Zhong, Y.; Yan, W.; Canisares, L.P.; Wang, S.; Brodie, E.L. Alterations in Soil pH Emerge as a Key Driver of the Impact of Global Change on Soil Microbial Nitrogen Cycling: Evidence from a Global Meta-Analysis. Glob. Ecol. Biogeogr. 2023, 32, 145–165. [Google Scholar] [CrossRef]
- Hatzenpichler, R. Diversity, Physiology, and Niche Differentiation of Ammonia-Oxidizing Archaea. Appl. Environ. Microbiol. 2012, 78, 7501–7510. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, C.; Su, Y.; Peng, W.; Lu, R.; Liu, Y.; Huang, H.; He, X.; Yang, M.; Zhu, S. Soil Acidification Caused by Excessive Application of Nitrogen Fertilizer Aggravates Soil-Borne Diseases: Evidence from Literature Review and Field Trials. Agric. Ecosyst. Environ. 2022, 340, 108176. [Google Scholar] [CrossRef]
- Goulding, K.W.T. Soil Acidification and the Importance of Liming Agricultural Soils with Particular Reference to the United Kingdom. Soil Use Manag. 2016, 32, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Niu, S. A Global Analysis of Soil Acidification Caused by Nitrogen Addition. Environ. Res. Lett. 2015, 10, 024019. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Meng, T.; Zhu, T.; Müller, C.; Cai, Z. Heterotrophic Nitrification Is the Predominant NO3—Production Pathway in Acid Coniferous Forest Soil in Subtropical China. Biol. Fertil. Soils 2013, 49, 955–957. [Google Scholar] [CrossRef]
- Norton, J.M.; Stark, J.M. Regulation and Measurement of Nitrification in Terrestrial Systems. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2011; Volume 486, pp. 343–368. ISBN 978-0-12-381294-0. [Google Scholar]
- Cheng, Y.; Wang, J.; Mary, B.; Zhang, J.; Cai, Z.; Chang, S.X. Soil pH Has Contrasting Effects on Gross and Net Nitrogen Mineralizations in Adjacent Forest and Grassland Soils in Central Alberta, Canada. Soil Biol. Biochem. 2013, 57, 848–857. [Google Scholar] [CrossRef]
- Ste-Marie, C.; Paré, D. Soil, pH and N Availability Effects on Net Nitrification in the Forest Floors of a Range of Boreal Forest Stands. Soil Biol. Biochem. 1999, 31, 1579–1589. [Google Scholar] [CrossRef]
- De Boer, W.; Laanbroek, H.J. Ureolytic Nitrification at Low pH by Nitrosospira Spec. Arch. Microbiol. 1989, 152, 178–181. [Google Scholar] [CrossRef]
- Pereira e Silva, M.C.; Poly, F.; Guillaumaud, N.; van Elsas, J.D.; Falcão Salles, J. Fluctuations in Ammonia Oxidizing Communities Across Agricultural Soils Are Driven by Soil Structure and pH. Front. Microbiol. 2012, 3, 77. [Google Scholar] [CrossRef] [PubMed]
- Pommerening-Röser, A.; Koops, H.-P. Environmental pH as an Important Factor for the Distribution of Urease Positive Ammonia-Oxidizing Bacteria. Microbiol. Res. 2005, 160, 27–35. [Google Scholar] [CrossRef]
- Yao, H.; Gao, Y.; Nicol, G.W.; Campbell, C.D.; Prosser, J.I.; Zhang, L.; Han, W.; Singh, B.K. Links between Ammonia Oxidizer Community Structure, Abundance, and Nitrification Potential in Acidic Soils. Appl. Environ. Microbiol. 2011, 77, 4618–4625. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.T.; Berg-Lyons, D.; Caporaso, J.G.; Walters, W.A.; Knight, R.; Fierer, N. Examining the Global Distribution of Dominant Archaeal Populations in Soil. ISME J. 2011, 5, 908–917. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, L.; Zhu, Y.; Zhang, J.; He, J. Abundance and Composition of Ammonia-oxidizing Bacteria and Ammonia-oxidizing Archaea Communities of an Alkaline Sandy Loam. Environ. Microbiol. 2008, 10, 1601–1611. [Google Scholar] [CrossRef]
- Zhang, L.-M.; Offre, P.R.; He, J.-Z.; Verhamme, D.T.; Nicol, G.W.; Prosser, J.I. Autotrophic Ammonia Oxidation by Soil Thaumarchaea. Proc. Natl. Acad. Sci. USA 2010, 107, 17240–17245. [Google Scholar] [CrossRef]
- Wessén, E.; Nyberg, K.; Jansson, J.K.; Hallin, S. Responses of Bacterial and Archaeal Ammonia Oxidizers to Soil Organic and Fertilizer Amendments under Long-Term Management. Appl. Soil Ecol. 2010, 45, 193–200. [Google Scholar] [CrossRef]
- Muema, E.K.; Cadisch, G.; Rasche, F. Soil Texture Modulates the Response of Ammonia-Oxidizing Prokaryotes to Biochemical Quality of Organic Inputs in Tropical Agricultural Soils. Soil Biol. Biochem. 2016, 100, 218–228. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, L.; Wang, B.; Lin, X.; Zhu, J.; Cai, Z.; Yan, X.; Jia, Z. Long-Term Field Fertilization Significantly Alters Community Structure of Ammonia-Oxidizing Bacteria Rather than Archaea in a Paddy Soil. Soil Sci. Soc. Am. J. 2011, 75, 1431–1439. [Google Scholar] [CrossRef]
- Fan, F.; Yang, Q.; Li, Z.; Wei, D.; Cui, X.; Liang, Y. Impacts of Organic and Inorganic Fertilizers on Nitrification in a Cold Climate Soil Are Linked to the Bacterial Ammonia Oxidizer Community. Microb. Ecol. 2011, 62, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Palomo, A.; Pedersen, A.G.; Fowler, S.J.; Dechesne, A.; Sicheritz-Pontén, T.; Smets, B.F. Comparative Genomics Sheds Light on Niche Differentiation and the Evolutionary History of Comammox Nitrospira. ISME J. 2018, 12, 1779–1793. [Google Scholar] [CrossRef] [PubMed]
- Kits, K.D.; Sedlacek, C.J.; Lebedeva, E.V.; Han, P.; Bulaev, A.; Pjevac, P.; Daebeler, A.; Romano, S.; Albertsen, M.; Stein, L.Y.; et al. Kinetic Analysis of a Complete Nitrifier Reveals an Oligotrophic Lifestyle. Nature 2017, 549, 269–272. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Z.; Wang, S.; Zhao, Y.; Wright, A.L.; Jiang, X. Responses of Ammonia-Oxidizers and Comammox to Different Long-Term Fertilization Regimes in a Subtropical Paddy Soil. Eur. J. Soil Biol. 2019, 93, 103087. [Google Scholar] [CrossRef]
- Zhao, Z.; Huang, G.; He, S.; Zhou, N.; Wang, M.; Dang, C.; Wang, J.; Zheng, M. Abundance and Community Composition of Comammox Bacteria in Different Ecosystems by a Universal Primer Set. Sci. Total Environ. 2019, 691, 146–155. [Google Scholar] [CrossRef]
- Stopnišek, N.; Gubry-Rangin, C.; Höfferle, Š.; Nicol, G.W.; Mandič-Mulec, I.; Prosser, J.I. Thaumarchaeal Ammonia Oxidation in an Acidic Forest Peat Soil Is Not Influenced by Ammonium Amendment. Appl. Environ. Microbiol. 2010, 76, 7626–7634. [Google Scholar] [CrossRef]
- Levičnik-Höfferle, Š.; Nicol, G.W.; Ausec, L.; Mandić-Mulec, I.; Prosser, J.I. Stimulation of Thaumarchaeal Ammonia Oxidation by Ammonia Derived from Organic Nitrogen but Not Added Inorganic Nitrogen. FEMS Microbiol. Ecol. 2012, 80, 114–123. [Google Scholar] [CrossRef]
- Prosser, J.I.; Nicol, G.W. Relative Contributions of Archaea and Bacteria to Aerobic Ammonia Oxidation in the Environment. Environ. Microbiol. 2008, 10, 2931–2941. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, J.; Bah, H.; Müller, C.; Cai, Z.; Zhu, B. Soil Gross Nitrogen Transformations in Forestland and Cropland of Regosols. Sci. Rep. 2021, 11, 223. [Google Scholar] [CrossRef]
- Lassaletta, L.; Billen, G.; Grizzetti, B.; Anglade, J.; Garnier, J. 50 Year Trends in Nitrogen Use Efficiency of World Cropping Systems: The Relationship between Yield and Nitrogen Input to Cropland. Environ. Res. Lett. 2014, 9, 105011. [Google Scholar] [CrossRef]
- Zhang, X.; Davidson, E.A.; Mauzerall, D.L.; Searchinger, T.D.; Dumas, P.; Shen, Y. Managing Nitrogen for Sustainable Development. Nature 2015, 528, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Shcherbak, I.; Millar, N.; Robertson, G.P. Global Metaanalysis of the Nonlinear Response of Soil Nitrous Oxide (N2O) Emissions to Fertilizer Nitrogen. Proc. Natl. Acad. Sci. USA 2014, 111, 9199–9204. [Google Scholar] [CrossRef] [PubMed]
- Davidson, E.A.; Kanter, D. Inventories and Scenarios of Nitrous Oxide Emissions. Environ. Res. Lett. 2014, 9, 105012. [Google Scholar] [CrossRef]
- Huang, T.; Ju, X.; Yang, H. Nitrate Leaching in a Winter Wheat-Summer Maize Rotation on a Calcareous Soil as Affected by Nitrogen and Straw Management. Sci. Rep. 2017, 7, 42247. [Google Scholar] [CrossRef]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant Acidification in Major Chinese Croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef]
- Booth, M.S.; Stark, J.M.; Rastetter, E. Controls on Nitrogen Cycling in Terrestrial Ecosystems: A Synthetic Analysis of Literature Data. Ecol. Monogr. 2005, 75, 139–157. [Google Scholar] [CrossRef]
- Byun, E.; Müller, C.; Parisse, B.; Napoli, R.; Zhang, J.-B.; Rezanezhad, F.; Van Cappellen, P.; Moser, G.; Jansen-Willems, A.B.; Yang, W.H.; et al. A Global Dataset of Gross Nitrogen Transformation Rates across Terrestrial Ecosystems. Sci. Data 2024, 11, 1022. [Google Scholar] [CrossRef]
- Jones, J.M.; Richards, B.N. Effect of Reforestation on Turnover of 15N-Labelled Nitrate and Ammonium in Relation to Changes in Soil Microflora. Soil Biol. Biochem. 1977, 9, 383–392. [Google Scholar] [CrossRef]
- Ayiti, O.E.; Babalola, O.O. Factors Influencing Soil Nitrification Process and the Effect on Environment and Health. Front. Sustain. Food Syst. 2022, 6, 821994. [Google Scholar] [CrossRef]
- Cui, L.; Li, D.; Wu, Z.; Xue, Y.; Xiao, F.; Gong, P.; Zhang, L.; Song, Y.; Yu, C.; Du, Y.; et al. Effects of Combined Nitrification Inhibitors on Soil Nitrification, Maize Yield and Nitrogen Use Efficiency in Three Agricultural Soils. PLoS ONE 2022, 17, e0272935. [Google Scholar] [CrossRef] [PubMed]
- Borzouei, A.; Karimzadeh, H.; Müller, C.; Sanz-Cobena, A.; Zaman, M.; Kim, D.-G.; Ding, W. Relationship between Nitrapyrin and Varying Nitrogen Application Rates with Nitrous Oxide Emissions and Nitrogen Use Efficiency in a Maize Field. Sci. Rep. 2022, 12, 18424. [Google Scholar] [CrossRef]
- Yang, M.; Fang, Y.; Sun, D.; Shi, Y. Efficiency of Two Nitrification Inhibitors (Dicyandiamide and 3, 4-Dimethypyrazole Phosphate) on Soil Nitrogen Transformations and Plant Productivity: A Meta-Analysis. Sci. Rep. 2016, 6, 22075. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, Z.; Zhou, Q. Dicyandiamide Sorption-Desorption Behavior on Soils and Peat Humus. Pedosphere 2004, 18, 378–385. [Google Scholar]
- Abalos, D.; Jeffery, S.; Sanz-Cobena, A.; Guardia, G.; Vallejo, A. Meta-Analysis of the Effect of Urease and Nitrification Inhibitors on Crop Productivity and Nitrogen Use Efficiency. Agric. Ecosyst. Environ. 2014, 189, 136–144. [Google Scholar] [CrossRef]
- Goring, C.A.I. Control of nitrification by 2-chloro-6-(trichloro-methyl) pyridine. Soil Sci. 1962, 93, 211–218. [Google Scholar] [CrossRef]
- Huang, L.; Chakrabarti, S.; Cooper, J.; Perez, A.; John, S.M.; Daroub, S.H.; Martens-Habbena, W. Ammonia-Oxidizing Archaea Are Integral to Nitrogen Cycling in a Highly Fertile Agricultural Soil. ISME Commun. 2021, 1, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bozal-Leorri, A.; Subbarao, G.V.; Kishii, M.; Urmeneta, L.; Kommerell, V.; Karwat, H.; Braun, H.-J.; Aparicio-Tejo, P.M.; Ortiz-Monasterio, I.; González-Murua, C.; et al. Biological Nitrification Inhibitor-Trait Enhances Nitrogen Uptake by Suppressing Nitrifier Activity and Improves Ammonium Assimilation in Two Elite Wheat Varieties. Front. Plant Sci. 2022, 13, 1034219. [Google Scholar] [CrossRef]
- Frelih-Larsen, D.A.; Riedel, A.; Gattinger, P.D.A.; Niether, D.W. Nitrification Inhibitors: Biological and Synthetic; German Environment Agency: Dessau-Roßlau, Germany, 2022. [Google Scholar]
- Xie, L.; Liu, D.; Chen, Z.; Niu, Y.; Meng, L.; Ding, W. Non-Native Brachiaria Humidicola with Biological Nitrification Inhibition Capacity Stimulates in Situ Grassland N2O Emissions. Front. Microbiol. 2023, 14, 1127179. [Google Scholar] [CrossRef]
- Kuppe, C.W.; Postma, J.A. Benefits and Limits of Biological Nitrification Inhibitors for Plant Nitrogen Uptake and the Environment. Sci. Rep. 2024, 14, 15027. [Google Scholar] [CrossRef]
- Rojas-Pinzon, P.A.; Prommer, J.; Sedlacek, C.J.; Sandén, T.; Spiegel, H.; Pjevac, P.; Fuchslueger, L.; Giguere, A.T. Inhibition Profile of Three Biological Nitrification Inhibitors and Their Response to Soil pH Modification in Two Contrasting Soils. FEMS Microbiol. Ecol. 2024, 100, fiae072. [Google Scholar] [CrossRef] [PubMed]
- Saud, S.; Wang, D.; Fahad, S. Improved Nitrogen Use Efficiency and Greenhouse Gas Emissions in Agricultural Soils as Producers of Biological Nitrification Inhibitors. Front. Plant Sci. 2022, 13, 854195. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, G.V.; Rao, I.M.; Nakahara, K.; Sahrawat, K.L.; Ando, Y.; Kawashima, T. Potential for Biological Nitrification Inhibition to Reduce Nitrification and N2O Emissions in Pasture Crop–Livestock Systems. Animal 2013, 7, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Liu, G.; Chen, Q. Research Hotspots and Trends of Nitrification Inhibitors: A Bibliometric Review from 2004–2023. Sustainability 2024, 16, 3906. [Google Scholar] [CrossRef]
- Mueller, N.D.; Gerber, J.S.; Johnston, M.; Ray, D.K.; Ramankutty, N.; Foley, J.A. Closing Yield Gaps through Nutrient and Water Management. Nature 2012, 490, 254–257. [Google Scholar] [CrossRef]
- Messiga, A.J.; Nyamaizi, S.; Yu, S.; Dorais, M. Blueberry Yield and Soil Mineral Nitrogen Response to Nitrogen Fertilizer and Nitrification Inhibitors under Drip-Fertigation Systems. Agronomy 2021, 11, 2144. [Google Scholar] [CrossRef]
- Chang, J.; Havlík, P.; Leclère, D.; De Vries, W.; Valin, H.; Deppermann, A.; Hasegawa, T.; Obersteiner, M. Reconciling Regional Nitrogen Boundaries with Global Food Security. Nat. Food 2021, 2, 700–711. [Google Scholar] [CrossRef]
- Morris, T.F.; Murrell, T.S.; Beegle, D.B.; Camberato, J.J.; Ferguson, R.B.; Grove, J.; Ketterings, Q.; Kyveryga, P.M.; Laboski, C.A.M.; McGrath, J.M.; et al. Strengths and Limitations of Nitrogen Rate Recommendations for Corn and Opportunities for Improvement. Agron. J. 2018, 110, 1–37. [Google Scholar] [CrossRef]
- Guardia, G.; Marsden, K.A.; Vallejo, A.; Jones, D.L.; Chadwick, D.R. Determining the Influence of Environmental and Edaphic Factors on the Fate of the Nitrification Inhibitors DCD and DMPP in Soil. Sci. Total Environ. 2018, 624, 1202–1212. [Google Scholar] [CrossRef]
- Yao, R.; Li, H.; Yang, J.; Zhu, W.; Yin, C.; Wang, X.; Xie, W.; Zhang, X. Combined Application of Biochar and N Fertilizer Shifted Nitrification Rate and amoA Gene Abundance of Ammonia-Oxidizing Microorganisms in Salt-Affected Anthropogenic-Alluvial Soil. Appl. Soil Ecol. 2022, 171, 104348. [Google Scholar] [CrossRef]
- Guo, J.; Ling, N.; Chen, H.; Zhu, C.; Kong, Y.; Wang, M.; Shen, Q.; Guo, S. Distinct Drivers of Activity, Abundance, Diversity and Composition of Ammonia-Oxidizers: Evidence from a Long-Term Field Experiment. Soil Biol. Biochem. 2017, 115, 403–414. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, F.; Jia, X.; Chen, J. Distinct Responses of Ammonia-Oxidizing Bacteria and Archaea to Green Manure Combined with Reduced Chemical Fertilizer in a Paddy Soil. J. Soils Sediments 2019, 19, 1613–1623. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chinthalapudi, D.P.M.; Kingery, W.; Ganapathi Shanmugam, S. A Review of Plant-Mediated and Fertilization-Induced Shifts in Ammonia Oxidizers: Implications for Nitrogen Cycling in Agroecosystems. Land 2025, 14, 1182. https://doi.org/10.3390/land14061182
Chinthalapudi DPM, Kingery W, Ganapathi Shanmugam S. A Review of Plant-Mediated and Fertilization-Induced Shifts in Ammonia Oxidizers: Implications for Nitrogen Cycling in Agroecosystems. Land. 2025; 14(6):1182. https://doi.org/10.3390/land14061182
Chicago/Turabian StyleChinthalapudi, Durga P. M., William Kingery, and Shankar Ganapathi Shanmugam. 2025. "A Review of Plant-Mediated and Fertilization-Induced Shifts in Ammonia Oxidizers: Implications for Nitrogen Cycling in Agroecosystems" Land 14, no. 6: 1182. https://doi.org/10.3390/land14061182
APA StyleChinthalapudi, D. P. M., Kingery, W., & Ganapathi Shanmugam, S. (2025). A Review of Plant-Mediated and Fertilization-Induced Shifts in Ammonia Oxidizers: Implications for Nitrogen Cycling in Agroecosystems. Land, 14(6), 1182. https://doi.org/10.3390/land14061182






