Abstract
Rocky desertification, a severe form of land degradation in tropical and subtropical regions driven by vegetation loss and soil erosion, poses significant ecological and economic challenges. Field trials in Fengshan County, Guangxi, China, evaluated the efficacy of NPK compound fertilizers, slow-release fertilizers, and bio-organic fertilizers on soil rehabilitation, microbial diversity, and the growth of Mahonia fortunei, a key species for ecological restoration and understory cash crop cultivation. The results demonstrated the bio-organic fertilizer’s superiority in soil regeneration, increasing organic matter by 30.4% (Bolin), 15.73% (Longlai), and 21.83% (Longlei) compared to NPK compound fertilizers, alongside elevating the total nitrogen (reaching 19.4 g/kg in Bolin) and phosphorus (85.45% higher in Bolin). Bio-organic fertilizer increased enzyme activities by 27–202% and enhanced microbial diversity, notably Proteobacteria and Actinobacteria. Slow-release fertilizers maximized micronutrient availability (e.g., Cu increased by 151.65% in Bolin) and improved plant growth, achieving peak Mahonia fortunei (Lindl.) Fedde height (3.62 cm, increasing 9.04%) and ground diameter (4.5 cm, increasing 18.42%) in Longlei compared to NPK compound fertilizers. Regional variability highlighted the bio-organic fertilizer’s dominance in soil fertility metrics, while slow-release formulations excelled in micronutrient enrichment and plant performance. NPK compound fertilizers exhibited the lowest efficacy, potentially exacerbating soil degradation. This study advocates integrating bio-organic fertilizers for soil regeneration with targeted slow-release applications for crop productivity, particularly in understory cash crop systems. Such a dual approach bridges ecological restoration with economic resilience in karst ecosystems, offering scalable solutions for global rocky desertification mitigation.
1. Introduction
Rocky desertification refers to the degraded terrain in the karst area, characterized by soil and vegetation loss due to widespread and imprudent land usage. This results in heightened soil erosion, exposed bedrock, diminished land productivity, and the emergence of a desert landscape [1,2,3]. Restoring and re-establishing vegetation in rocky desertification zones is a crucial component of ecological restoration aimed at mitigating soil degradation and improving soil quality in these areas, which result from reverse succession and vegetation extinction [4,5,6]. A significant karst region exists in Southwest China, predominantly in Guizhou, Guangxi, Yunnan, and Chongqing [7,8]. Many of these regions are economically disadvantaged as a result of rocky desertification. Vegetation restoration through Chinese medicinal plant cultivation has emerged as a dual-purpose solution, combatting soil degradation while generating income through the “non-timber understory economy” (NTUE), an agroforestry model where cash crops are cultivated beneath forest canopies [9,10]. Presently, an increasing number of researchers are concentrating on cultivating various medicinal herbs beneath forest canopies. For instance, Zhou et al. cultivated Panax notoginseng (Burkill) F.H. Chen in forested areas [11], while Zhou et al. investigated the cultivation model of economic plants in young Cunninghamia lanceolata forests [12]. Additionally, Li et al. assessed food cultivation beneath Styphnolobium japonicum forests [13]. The integrated cultivation strategies yield substantially greater socioeconomic returns than individual planting systems, particularly in ecologically constrained regions.
Within NTUE systems, Mahonia fortunei (Lindl.) Fedde, a small evergreen shrub of the Berberidaceae family endemic to Guangxi Province, is a medicinal species with significant ecological and socioeconomic value in China. It presents exceptional potential for rocky desertification mitigation. This endemic Berberidaceae shrub combines ecological resilience with high medicinal value, containing bioactive alkaloids like berberine and palmatine that demonstrate antimicrobial, antioxidant, and antitumor properties [14]. Ecologically, it thrives in degraded karst soils through its drought tolerance, shade adaptation, and temperature resilience. Economically, its low-input cultivation requirements and substantial market value for traditional Yao medicine make it ideal for income generation in impoverished regions [15,16]. These dual characteristics position M. fortunei as a keystone species for reconciling ecological restoration with sustainable development.
Fertilization strategies in these nutrient-poor soils require careful optimization. While conventional NPK fertilizers initially boost crop yields, their prolonged use exacerbates soil degradation through organic matter depletion, accelerated erosion, and reduced microbial diversity [17,18,19,20]. In contrast, slow-release fertilizers exhibit controlled nutrient release kinetics that enhances fertilizer utilization efficiency while reducing application frequency. This technology concurrently achieves two agricultural objectives: mitigating environmental contamination associated with conventional fertilizers and increasing crop productivity in staple species, including Oryza sativa, Zea mays, and Triticum aestivum [21,22,23]. Bio-organic fertilizers, incorporating functional microorganisms with organic substrates, show particular promise by improving soil structure and microbial activity critical for sustainable agriculture [24,25]. This creates two critical hypotheses:
- Bio-organic fertilizers will outperform NPK and slow-release types in improving karst soil parameters (organic matter and microbial diversity).
- Fertilizer-induced soil improvements will positively correlate with Mahonia fortunei (Lindl.) Fedde growth parameters.
To test these hypotheses, we conducted a two-year field trial across Fengshan County’s karst villages (Bolin, Longlai, and Longlei) comparing (1) NPK compound fertilizers, (2) slow-release fertilizers, and (3) bio-organic fertilizers. Systematic comparisons were conducted to assess differential impacts on two critical parameters: the pedological characteristics of degraded soils and growth performance of understory-cultivated Mahonia fortunei (Lindl.) Fedde. The dual-objective selection criteria required optimal fertilizers demonstrating both edaphic restoration capacity and socioeconomic enhancement potential, thereby establishing evidence-based recommendations for sustainable fertilization strategies in ecologically fragile karst regions.
2. Materials and Methods
2.1. Introduction of the Research Region
The karst ecosystems of Fengshan County (geographical location shown in Figure 1), Guangxi Zhuang Autonomous Region, China, were classified per the LY/T 1840–2009 geomorphological assessment protocol (State Forestry Administration of China, 2009) into three degradation categories: light (LRD), moderate (MRD), and intense rocky desertification (IRD) [26,27]. The study area encompasses three characteristic monitoring plots: Bolin township (30° slope), representing MRD conditions (24°28′24″–24°28′25″ N, 106°50′28″–106°50′43″ E), Longlai (30° slope, 24°32′33″–24°32′37″ N, 106°47′50″–106°47′53″ E), and Longlei (0° slope, 24°31′13″ N, 106°47′14″ E), demonstrating LRD characteristics. These experimental clusters exhibit a subtropical monsoon climate with a mean annual temperature of 19.2 °C (Jan: 10.5 °C; Jul: 26.0 °C) and 1564.0 mm of precipitation. Geospatial parameters include elevation gradients from 784.7 to 879.0 m and 362 frost-free days annually.
Figure 1.
Location of the study region.
2.2. Materials
The following fertilizers were used: NPK compound fertilizer (purchased from Guangxi Huawuot Group Co. (Liuzhou, China); N:P:K ratio = 15:6:9, with a total nutrient content ≥ 30%); slow-release fertilizer (produced by the Guangxi Academy of Forestry, Nanning, China), adding the five elements Ga, Mg, Fe, Borne, and Cu; N:P:K ratio = 15:6:9, with a total nutrient content ≥ 30%); and bio-organic fertilizer (purchased from Guangxi Huawuot Group Co. (Liuzhou, China); the effective viable bacterial count ≥ 2 × 10⁸ CFU/g; organic matter ≥ 45%; N:P:K ratio = 6:6:3, with a total nutrient content ≥ 15%).
Mahonia fortunei (Lindl.) Fedde: this is a kind of warm temperate zone plant that has strong cold resistance, is not strict on soil requirements, and grows best on loose, fertile, well-drained sandy loam, so it can be planted in Guangxi rocky desertification areas [15].
2.3. Experimental Design
At the experimental sites of Longlai, Longlei, and Bolin, Mahonia fortunei (Lindl.) Fedde was cultivated with a planting density of 0.5 m × 0.5 m. Three plots were established for differential fertilizer applications under equivalent total nutrient content conditions: 0.25 kg per plant for both the NPK compound fertilizer and slow-release fertilizer treatments versus 0.5 kg per plant for bio-organic fertilizer application, with annual fertilization implementation. The fertilizer application method involved digging a fertilization trench of length × width × depth: 12.5 × 12.5 × 12.5 cm on average at a position of 20–25 cm from each herb, putting in the fertilizer, and covering the soil in time.
After two years, samples were taken from five to six sites within each standard plot using the multi-point mixing method (inter-root and non-inter-root soils were taken separately, and then they were uniformly mixed). Humus and pollutants on the soil surface were removed first. At each location, after the soil samples were homogenized using the quartering method, one sample (weighing 1.0 kg) was placed into a self-sealing bag, air-dried, pulverized, and then analyzed for its soil organic matter, total nitrogen, and total phosphorus. Following the aforementioned collection procedure, roughly 15 g of each soil sample was placed in a test tube and placed in a bucket of dry ice before being returned to the lab for enzyme activity measurement and microbiological detection.
2.4. Measurements of Soil Physicochemical Properties and Enzyme Activities
Soil organic matter was determined using the external heating method of potassium dichromate; the soil total nitrogen content was determined using an element analyzer (Vario MACRO Cube, Elementar, Germany); the available nitrogen was determined using the alkaline diffusion method; the total nitrogen was determined using the semi-trace Kjeldahl method; the total phosphorus and available phosphorus were determined via UV–visible spectrophotometry; and the total potassium and available potassium were determined via flame spectrophotometry [28,29]. Quantitative determination of boron (B), iron (Fe), copper (Cu), and zinc (Zn) in soil matrices was carried out using Flame Atomic Absorption Spectrophotometry (FAAS) [30].
Soil urease activity was quantified using the indophenol blue method. Briefly, 1 g of soil was reacted with urea (50 mM) in borate buffer (pH 10.0) at 37 °C for 2 h. Following reaction termination with KCl (1 M) and filtration, the NH4+ concentration was determined colorimetrically at 690 nm via the sodium dichloroisocyanurate–salicylate/NaOH chromogenic reaction. Enzyme activity was expressed as μg NH4+-N produced per gram of dry soil per hour (μg NH4+-N g−1 h−1) [31]. Soil sucrase activity was determined by quantifying glucose release via 3,5-dinitrosalicylic acid (DNS) chromogen. Air-dried soil (5 g) was incubated with 15 mL of 8% (w/v) glucose, 5 mL of phosphate buffer (0.2 M, pH 5.5), and 5 drops of toluene at 37 °C for 24 h. The suspension was filtered through quantitative filter paper, and 1 mL of filtrate was reacted with 3 mL DNS reagent at 100 °C for 5 min. Absorbance of the cooled solution was measured at 550 nm using a UV–vis spectrophotometer. Enzyme activity was calculated as the glucose mass produced per gram of soil per day (mg glucose·g−1·24 h−1) [32]. Alkaline phosphatase activity [33] was determined using the method of Tabatabai and Bremner.
In each differentially fertilized plot, three Mahonia fortunei (Lindl.) Fedde individuals were randomly selected, excised at the soil–stem interface using sterilized pruning shears, and subjected to biometric measurements: (1) shoot height determination from the excision plane to apical bud using a calibrated rigid ruler (±1 mm accuracy) and (2) basal stem diameter quantification at 1 cm above the excision plane through triplicate circumferential measurements with digital vernier calipers (resolution 0.01 mm).
2.5. Detection of Soil Bacterial Diversity
Soil samples were aseptically collected and commercially outsourced for high-throughput sequencing (Majorbio Bio-Pharm Technology Co., Ltd., Shanghai, China). Total metagenomic DNA was extracted using the E.Z.N.A.® Soil DNA Kit (Omega Bio-tek, Norcross, GA, USA), followed by quality verification via 1% (w/v) agarose gel electrophoresis. Nucleic acid quantification was performed via NanoDrop 2000 spectrophotometric analysis (Thermo Fisher Scientific, Waltham, MA, USA).
The hypervariable V3–V4 region of bacterial 16S rRNA genes was amplified using the primer pair 338F/806R [28] under standardized thermal cycling conditions: initial denaturation at 95 °C for 5 min; 25 cycles of 94 °C (45 s), 50 °C (30 s), and 72 °C (30 s); and final extension at 72 °C for 6 min. The PCR reactions (20 μL) contained 4 μL of 5 × FastPfu Buffer, 2 μL of dNTPs (2.5 mM), 0.8 μL of primers (5 μM), 0.4 U of FastPfu Polymerase (TransGen Biotech, Beijing, China), and 10 ng DNA template.
Amplicons were purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA) after 2% agarose gel electrophoresis. Quantification using the QuantiFluor™-ST fluorometer (Promega, Madison, WI, USA) preceded library preparation according to Illumina MiSeq specifications. PE300 paired-end sequencing (Shanghai Meiji Biomedical Technology Co., Ltd., Shanghai, China) was conducted following (1) adapter ligation: Y-shaped adapters were ligated to amplified fragments; (2) size selection: magnetic bead-based removal of self-ligated products; (3) PCR enrichment: library amplification with index primers; and (4) denaturation: the generation of single-stranded DNA templates with 0.1 M NaOH.
2.6. Data Processing
Descriptive analysis in one-way ANOVA was performed using SPSS 20.0 software, and Pearson correlation analyses of soil nutrients, microbial counts, enzyme activities, and the development of the Mahonia fortunei (Lindl.) Fedde under various fertilizer applications were carried out.
3. Results
3.1. Effects of Different Fertilizer Applications on Soil Organic Matter and Nitrogen, Phosphorus, and Potassium Contents of Soil
The highest levels of soil organic matter, total nitrogen, and total phosphorus were observed in Bolin, Longlai, and Longlei when bio-organic fertilizer was applied. The maximum total potassium content occurred in Longlai with bio-organic fertilizer application, while in Bolin and Longlei, the highest total potassium content was found when NPK compound fertilizer was applied.
Bio-organic fertilizer application consistently produced the highest soil organic matter content across all regions. In Bolin, organic matter reached 37.03 g/kg, a 48.23 percent increase over NPK compound fertilizer, while Longlai and Longlei recorded 54.08 g/kg (35.83 percent higher than NPK) and 51.78 g/kg (23.45 percent higher than NPK), respectively (Figure 2A). Slow-release fertilizer showed moderate improvements, with organic matter levels in Bolin, Longlai, and Longlei rising by 11.65 percent, 17.37 percent, and 1.33 percent compared to NPK.
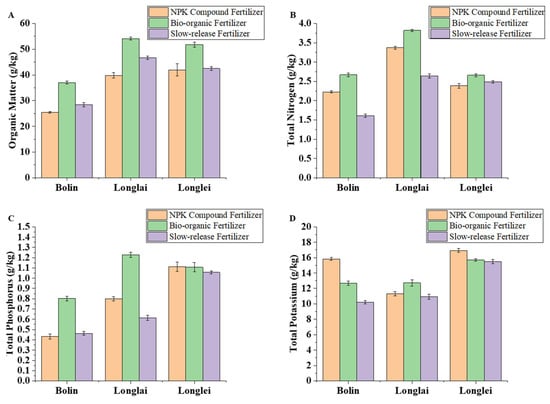
Figure 2.
Effects of application of different fertilizers in Bolin, Longlai, and Longlei on the contents of soil organic matter (A), total nitrogen (B), total phosphorus (C), and total potassium (D).
Total nitrogen concentrations were maximized under bio-organic fertilization, reaching 2.67 g/kg in Bolin, 3.82 g/kg in Longlai, and 2.66 g/kg in Longlei. These values exceeded those with NPK compound fertilizer by 19.89 percent, 13.35 percent, and 11.44 percent, respectively (Figure 2B). For phosphorus, the bio-organic fertilizer outperformed NPK in Bolin (0.803 g/kg under bio-organic compared to 0.433 g/kg under NPK) and Longlai (1.227 g/kg compared to 0.800 g/kg), but NPK achieved marginally higher phosphorus in Longlei (1.113 g/kg compared to 1.107 g/kg with bio-organic) (Figure 2C). The potassium trends differed regionally: the NPK compound fertilizer achieved the highest concentrations in Bolin (15.84 g/kg) and Longlei (16.93 g/kg), surpassing the bio-organic fertilizer by 15.84 percent and 7.25 percent, whereas the bio-organic fertilizer yielded the highest potassium in Longlai (12.72 g/kg, 12.56 percent above NPK) (Figure 2D).
In the regions of Bolin, Longlai, and Longlei, the application of bio-organic fertilizer resulted in the highest soil available nitrogen, available phosphorus, and available potassium contents, followed by slow-release fertilizer, while the NPK compound fertilizer exhibited the lowest values.
Bio-organic fertilizer also enhanced the available nitrogen, phosphorus, and potassium. The available nitrogen in Bolin, Longlai, and Longlei under bio-organic treatment measured 201.06 mg/kg (45.12 percent higher than NPK), 372.80 mg/kg (34.96 percent higher), and 301.56 mg/kg (22.90 percent higher), respectively. Slow-release fertilizer provided intermediate nitrogen levels, exceeding NPK by 29.53 percent, 11.51 percent, and 0.41 percent in these regions (Figure 3A). Available phosphorus with bio-organic fertilizer reached 18.77 mg/kg in Bolin (102.73 percent above NPK), 17.75 mg/kg in Longlai (58.87 percent higher), and 18.46 mg/kg in Longlei (42.47 percent higher). Slow-release fertilizer increased phosphorus by 14.15 percent, 7.37 percent, and 12.24 percent relative to NPK (Figure 3B). Similarly, bio-organic fertilization elevated the available potassium to 83.24 mg/kg in Bolin (173.80 percent higher than NPK), 147.13 mg/kg in Longlai (29.22 percent higher), and 216.58 mg/kg in Longlei (20.65 percent higher), with the slow-release fertilizer showing smaller yet significant improvements (Figure 3C).
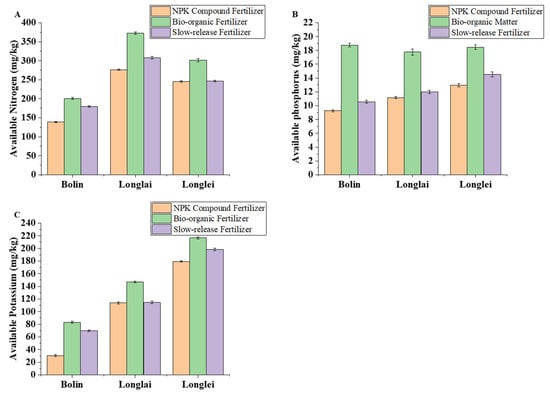
Figure 3.
Effects of application of different fertilizers in Bolin, Longlai, and Longlei on the contents of soil available nitrogen (A), available phosphorus (B), and available potassium (C).
3.2. Effects of Different Fertilizer Applications on Soil Micronutrient Content
In the regions of Bolin, Longlai, and Longlei, the application of slow-release fertilizer resulted in the highest soil B, Cu, Zn, and Fe contents. In Bolin, the soil B, Cu, Zn, and Fe contents under bio-organic fertilizer application were higher than those under NPK compound fertilizer application. In Longlai, the soil B, Cu, and Fe contents with bio-organic fertilizer exceeded those with NPK compound fertilizer, whereas the Zn content showed the opposite pattern. In Longlei, the soil B, Zn, and Fe contents were elevated with bio-organic fertilizer compared to NPK compound fertilizer application, while the Cu content demonstrated an inverse relationship.
Slow-release fertilizer generally increased the boron, copper, zinc, and iron contents, although the responses varied regionally. In Bolin, bio-organic fertilizer elevated boron by 56.16 percent over NPK, whereas slow-release fertilizer increased boron by 15.79 percent. In Longlai, slow-release fertilizer doubled the boron levels (102 percent increase over NPK), while bio-organic fertilizer provided minimal improvement (1.35 percent). Longlei saw a 25.82 percent boron increase with slow-release fertilizer and a 48.50 percent rise with bio-organic fertilizer (Figure 4A).
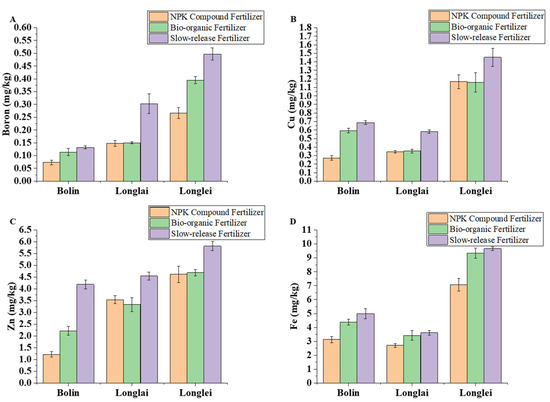
Figure 4.
Effects of application of different fertilizers in Bolin, Longlai, and Longlei on the contents of B (A), Cu (B), Zn (C), and Fe (D).
The copper content peaked under bio-organic fertilization, with Bolin, Longlai, and Longlei reaching 0.687 mg/kg (151.65 percent higher than NPK), 0.583 mg/kg (69.97 percent higher), and 1.453 mg/kg (24.51 percent higher), respectively (Figure 4B). The zinc levels followed similar trends: bio-organic fertilizer achieved 4.287 mg/kg in Bolin (242.35 percent above NPK), 4.547 mg/kg in Longlai (28.56 percent higher), and 5.813 mg/kg in Longlei (25.82 percent higher). Slow-release fertilizer consistently outperformed NPK but remained below the bio-organic results (Figure 4C). The iron content was highest with the bio-organic fertilizer (4.99–9.65 mg/kg), exceeding NPK by 33.54–59.37 percent, while the slow-release fertilizer provided intermediate concentrations (Figure 4D).
3.3. Effects of Different Fertilizer Applications on Soil Enzyme Activities
In the regions of Bolin, Longlai, and Longlei, the application of bio-organic fertilizer resulted in the highest activities of soil urease, sucrase, and alkaline phosphatase, followed by slow-release fertilizer, while the NPK compound fertilizer exhibited the lowest enzymatic activities.
Bio-organic fertilizer consistently maximized soil enzyme activities. Urease activity under bio-organic treatment reached 0.093 μg NH3-N/g in Bolin, 0.543 μg NH3-N/g in Longlai, and 0.570 μg NH3-N/g in Longlei, representing increases of 181.82 percent, 76.87 percent, and 30.43 percent compared to NPK compound fertilizer (Figure 5A). Slow-release fertilizer also enhanced urease activity, with the levels in Bolin, Longlai, and Longlei rising by 90.91 percent, 36.81 percent, and 3.66 percent relative to NPK.
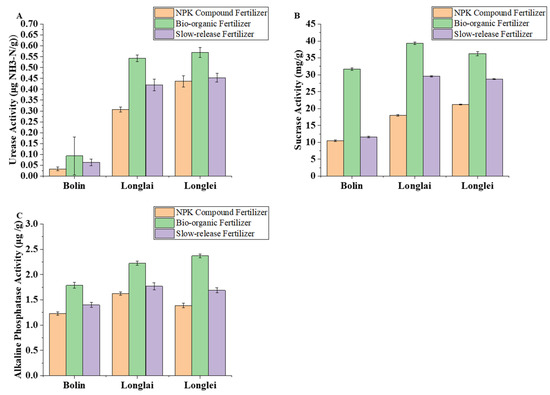
Figure 5.
Effects of application of different fertilizers in Bolin, Longlai, and Longlei on the activities of soil urease (A), sucrase (B), and alkaline phosphatase (C).
For sucrase activity, bio-organic fertilizer achieved the highest values across all regions: 31.69 mg/g in Bolin (201.52 percent above NPK), 39.30 mg/g in Longlai (118.46 percent higher), and 36.29 mg/g in Longlei (70.93 percent higher). Slow-release fertilizer showed secondary improvements, increasing sucrase activity by 10.09 percent, 64.09 percent, and 35.42 percent in these regions compared to NPK (Figure 5B).
Alkaline phosphatase activity followed similar trends. Bio-organic fertilizer produced peak activity in Bolin (1.786 μg/g), Longlai (2.223 μg/g), and Longlei (2.370 μg/g), exceeding NPK by 45.56 percent, 36.97 percent, and 70.87 percent, respectively. Slow-release fertilizer elevated the activity to 1.397 μg/g in Bolin (13.85 percent higher than NPK), 1.770 μg/g in Longlai (9.06 percent higher), and 1.687 μg/g in Longlei (21.63 percent higher) (Figure 5C).
3.4. Effects of Different Fertilizer Applications on the Growth of Mahonia fortunei (Lindl.) Fedde
In the regions of Bolin and Longlai, the application of bio-organic fertilizer resulted in the maximum plant height and basal diameter of Mahonia fortunei (Lindl.) Fedde, while in Longlei, slow-release fertilizer application produced the highest values for these growth parameters, followed by bio-organic fertilizer, with the NPK compound fertilizer demonstrating the lowest measurements.
Figure 6A illustrates that in the Bolin and Longlai regions, the maximum plant height of Mahonia fortunei (Lindl.) Fedde reached 1.807 cm and 2.23 cm, respectively, with the application of bio-organic fertilizer, representing increases of 20.22% and 22.33% compared to the use of the NPK compound fertilizer. In the Longlei region, the maximum plant height of Mahonia fortunei (Lindl.) Fedde reached 3.62 cm with the application of slow-release fertilizer, representing a 9.04% increase compared to the NPK compound fertilizer. This was followed by a height of 3.47 cm with bio-organic fertilizer, which was 4.52% higher than that achieved with the NPK compound fertilizer.
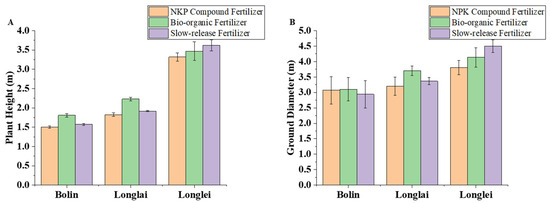
Figure 6.
Effects of application of different fertilizers in Bolin, Longlai, and Longlei on Mahonia fortunei (Lindl.) Fedde plant height (A) and ground diameter (B).
Figure 6B illustrates that in the Bolin and Longlai regions, the ground diameter of the Mahonia fortunei (Lindl.) Fedde was maximized with the application of bio-organic fertilizer, measuring 3.1 cm and 3.7 cm, respectively, representing increases of 1.08% and 20.64% compared to the application of NPK compound fertilizer. In the Longlai region, the maximum ground diameter of Mahonia fortunei (Lindl.) Fedde reached 4.5 cm with the application of slow-release fertilizer, representing an increase of 18.42% compared to NPK compound fertilizer. The diameter was 4.133 cm with bio-organic fertilizer, which was 11.1% greater than with NPK compound fertilizer.
3.5. Effects of Different Fertilizer Applications on the Soil Bacterial Communities in Different Regions
The diversity indexes of soil bacterial communities comprise the Shannon index, Simpson index, Chao1 index, and ACE index. A higher Shannon index signifies greater community diversity; a higher Simpson index (ranging from 0 to 1) denotes an uneven species distribution and a more significant ecological role of dominant organisms; the Chao1 index reflects the estimated species richness in the sample; and a higher ACE index indicates increased species complexity within the community. From Table 1, it can be seen that in the same area, the chao1 index, ACE index, and Shannon index of the soil were highest when bio-organic fertilizer was applied, followed by when slow-release fertilizer was applied.

Table 1.
Soil bacterial diversity indices in different regions under different fertilizer applications.
The species distribution of the top 10 bacteria at the gate level is shown in Figure 7, which shows that when NPK compound fertilizers were applied in the Bolin region, Proteobacteria, Acidobacteria, Firmicutes, and Verrucomicrobia predominated; when bio-organic fertilizers were applied, Proteobacteria, Acidobacteria, Chloroflexi, and Verrucomicrobia predominated; and when slow-release fertilizers were applied, Proteobacteria, Acidobacteria, Actinobacteria, and Bacteroidetes predominated. NPK compound fertilizers in the Longlai district were dominated by Proteobacteria, Acidobacteria, Actinobacteria, and Planctomycetes; bio-organic fertilizers were dominated by Proteobacteria, Acidobacteria, Actinobacteria, and Gemmatimonadetes; and slow-release fertilizers were dominated by Proteobacteria, Acidobacteria, Chloroflexi, Planctomycetes, Gemmatimonadetes, and Nitrospirae. When NPK compound fertilizers were used in Longlei, Proteobacteria, Acidobacteria, Firmicutes, Bacteroidetes, Chloroflexi, and Planctomycetes were present in greater numbers than when bio-organic fertilizers were used. Similarly, when slow-release fertilizers were used, Proteobacteria, Acidobacteria, Firmicutes, Bacteroidetes, Chloroflexi, and Planctomycetes were the most prevalent.
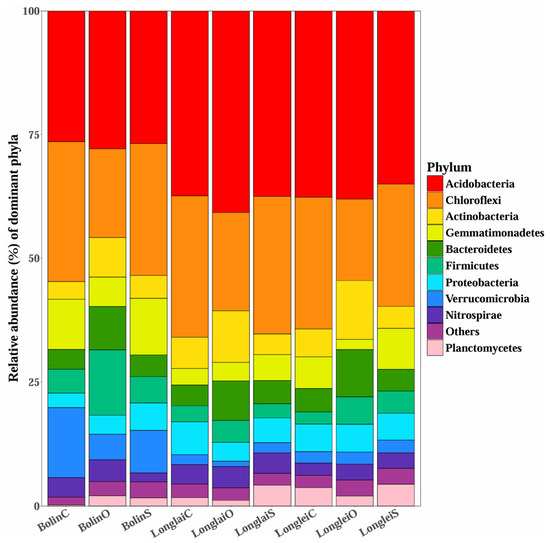
Figure 7.
The abundance of soil-dominant bacterial phyla in different regions with different fertilizers applied. Note: BolinC refers to the application of NPK compound fertilizer in the Bolin region; BolinO refers to the application of bio-organic fertilizer in the Bolin region; BolinS refers to the application of slow-release fertilizer in the Bolin region; LonglaiC refers to the application of NPK compound fertilizer in the Longlai region; LonglaiO refers to the application of bio-organic fertilizer in the Longlai region; LonglaiS refers to the application of slow-release fertilizer in the Longlai region; LongleiC refers to the application of NPK compound fertilizer in the Longlei region; LongleiO refers to the application of bio-organic fertilizer in the Longlei region; and LongleiS is slow-release fertilizer applied in the Longlei district.
3.6. Correlation Analysis of Soil Nutrients with Enzyme Activities and Plant Growth
Significant positive correlations (p < 0.01) were observed between organic matter and available nitrogen (r = 0.94), urease (r = 0.92), and sucrase (r = 0.88). Available potassium correlated strongly with plant height (r = 0.92), Cu (r = 0.77), and Fe (r = 0.78), while total phosphorus showed significant associations with available potassium (r = 0.85) and Zn (r = 0.46). Sucrase activity was positively correlated with acid phosphatase (r = 0.89), and urease correlated with both organic matter (r = 0.92) and available potassium (r = 0.88). Micronutrients Cu and Fe exhibited an exceptionally high correlation (r = 0.95). Negative correlations (p < 0.05) were detected between total nitrogen and Cu (r = −0.39) as well as Fe (r = −0.4) (Figure 8).
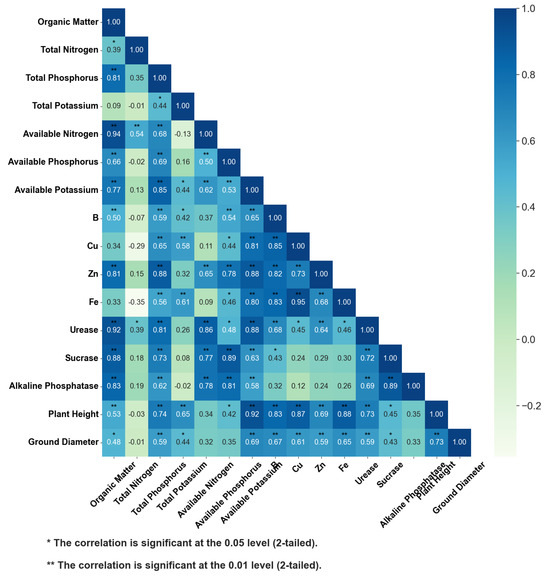
Figure 8.
Correlation between soil organic matter, total nitrogen, total phosphorus, total potassium, available nitrogen, available phosphorus, available potassium, trace elements, enzyme activities, and plant growth.
4. Discussion
4.1. Effects of Application of Different Fertilizers on Soil Fertility in Different Regions
Soil organic matter (SOM), a substantial carbon reservoir, plays a crucial role in the formation and stabilization of soil aggregates, affects soil fertility, and serves as a primary nutrition source for crops [29,34,35]. Our findings demonstrate that bio-organic fertilization significantly enhanced the soil organic matter content across all regions (Figure 2A; p < 0.01 for correlations with key nutrients), consistent with previous studies on organic amendment efficacy [36]. The regional variability in soil organic matter enhancement—peaking in Bolin (37.03 g/kg), followed by Longlai (54.08 g/kg) and Longlei (51.78 g/kg)—may reflect differential organic matter mineralization rates influenced by local pedoclimatic conditions and may also be associated with the degree of rocky desertification in these areas.
Nitrogen, phosphorus, and potassium are vital components influencing plant growth and development [37], with nitrogen specifically associated with photosynthesis, chlorophyll concentration, and crop output [38,39,40]. Bio-organic fertilizers maximized the total nitrogen (TN), total phosphorus (TP), available nitrogen (AN), available phosphorus (AP), and available potassium (AK) in Bolin, Longlai, and Longlei (Figure 2A–C and Figure 3; p < 0.01 for SOM-TN: r = 0.391; SOM-TP: r = 0.806; and SOM-AN: r = 0.937). These results align with those of Nardi et al. [41] and Liu et al. [24], who emphasized organic inputs’ superiority over synthetic fertilizers in sustaining soil health. While the NPK compound fertilizer achieved higher total potassium in Bolin and Longlei (Figure 2D), this localized advantage may mask critical long-term risks. Notably, the highest total potassium under bio-organic fertilization in Longlai (12.72 g/kg) coincided with severe rocky desertification, suggesting a potential linkage between NPK overuse and soil degradation.
This paradox—higher potassium levels in degraded soils—mirrors the findings of Ali et al. [42], who demonstrated that prolonged NPK compound fertilizer application directly contributes to soil degradation by disrupting nutrient equilibrium, accelerating organic matter depletion (SOM-TK correlation: r = 0.09, nonsignificant), and destabilizing microbial communities. In rocky desertified areas, elevated total potassium (TK) often correlates with desertification severity [43,44], as nutrient leaching and organic matrix loss leave residual minerals like potassium disproportionately concentrated in barren soils. The transient potassium benefits of NPK fertilizers observed in our study may, thus, reflect not true soil enrichment but rather the accelerated depletion of organic matter and biodiversity, a hallmark of soil degradation. Over time, such practices risk exacerbating rocky desertification, particularly in fragile ecosystems like Longlai, where NPK-driven nutrient imbalances may irreversibly compromise soil structure and function.
4.2. Effects of Application of Different Fertilizers on Soil Micronutrient Content and the Growth of Mahonia fortunei (Lindl.) Fedde in Different Regions
B, Cu, Zn, and Fe, as vital micronutrients for plant development [45], are crucial to plant growth. B is essential for the integrity and functionality of the cell wall, and, in contrast to most other micronutrients, the plant root system necessitates a continual external supply of trace levels of boron; otherwise, membrane function deteriorates within minutes [46,47]. Cu can form stable complexes with numerous organic NPK compounds and engage in various redox events within the cell; it also plays a role in photosynthesis, respiration, and cell wall remodeling in plants [48,49]. Zn functions as a catalytic or structural cofactor in numerous enzymes and regulatory proteins [50] and is crucial for chlorophyll production, carbohydrate creation, tryptophan synthesis, and protein synthesis [51,52,53]. Fe significantly contributes to photosynthesis, chlorophyll biosynthesis, and several fundamental metabolic activities in chloroplasts, owing to its redox activity that facilitates the acceptance and donation of electrons [54,55]. The studies by Yaganoglu, E et al. [42] and Maqueda, C et al. [43] indicate that the application of bio-organic fertilizers results in higher soil trace element content compared to chemical fertilizers, with a significant correlation between soil organic matter content and trace element levels (SOM-B: r = 0.505, p < 0.01; SOM-Zn: r = 0.490, p < 0.01). In the Longlai, Longlei, and Bolin regions, the application of slow-release fertilizers yielded the highest soil trace element content, which may be attributed to the trace elements added to this fertilizer. The soil trace element content under bio-organic fertilizer application was higher than that under NPK compound fertilizer application (Figure 4), consistent with previous research findings.
The maximum plant height and basal diameter of Mahonia fortunei (Lindl.) Fedde under slow-release fertilizer treatment were observed in the Longlei region (Figure 6), which may be attributed to the synergistic effects of local topographic gradients and microclimatic conditions characteristic of this specific karst terrain.
4.3. Effects of Application of Different Fertilizers on Soil Enzyme Activities in Different Regions
Soil enzymes are essential for soil energy conversion and nutrient cycling, and they serve as important indicators of microbial activity, soil fertility, and land quality changes [56,57]. A positive link between soil organic matter content and soil enzyme activity was identified [58]. The decomposition products of microorganisms, plant roots, and organic matter from plants and animals are the principal sources of soil enzymes. The creation and growth of soil humus are intricately connected to soil enzyme activity [59]. Bio-organic fertilizer, which is rich in microorganisms and plant and animal wastes, exhibits superior soil enzyme activity compared to chemical fertilizers. The utilization of bioorganic fertilizers in the Longlai, Longlei, and Bolin regions yielded the highest activities of soil urease, sucrase, and alkaline phosphatase in our research. These activities exhibited a positive correlation with the quantity of soil organic matter (Figure 5; urease: r = 0.918, p < 0.01; sucrase: r = 0.876, p < 0.01; acid phosphatase: r = 0.834, p < 0.01).
4.4. Effects of Applying Different Fertilizers on the Abundance, Diversity, and Structure of Soil Bacteria in Different Regions
Soil microorganisms play a pivotal role in ecosystems by regulating organic matter decomposition, nutrient cycling, plant growth, and soil quality, with fertilizer types exhibiting strong correlations with microbial diversity [60,61,62]. Previous studies indicate that prolonged application of chemical fertilizers reduces soil bacterial diversity [63,64]. In contrast, organic amendments such as animal manure and crop residues enhance bacterial diversity [65,66,67] and the co-application of crop residues with chemical fertilizers mitigates their negative impacts on microbial diversity [68]. This study demonstrates that in the Bolin, Longlei, and Longlai regions, soil bacterial diversity (Shannon index) and abundance (Chao1 and ACE indices) reached maximal levels under bio-organic fertilizer treatment. This outcome likely reflects the composition of bio-organic fertilizers, which utilize animal manure and crop residues as carriers, thereby outperforming NPK compound and slow-release fertilizers in promoting microbial richness.
Soil bacteria can be categorized into copiotrophs and oligotrophs based on carbon mineralization potential and growth rates [69]. Ecological strategies differentiate taxa such as Actinobacteria, Proteobacteria, and Bacteroidetes (copiotrophic taxa associated with nutrient-rich environments) from Acidobacteria and Chloroflexi (oligotrophic specialists adapted to low-nutrient conditions) [70]. In our study, bio-organic fertilization significantly enriched Proteobacteria, Actinobacteria, and Firmicutes compared to chemical or slow-release fertilizers. This shift likely reflects both the elevated nutrient availability from organic inputs and the direct introduction of functionally beneficial microbes through bio-organic amendments. Critically, these microbial changes have cascading impacts on soil functionality: Proteobacteria, pivotal drivers of carbon mineralization, nitrogen fixation, and sulfur oxidation [71], enhance nutrient turnover efficiency, directly supporting plant growth while reducing dependency on synthetic fertilizers. Their dominance under bio-organic treatment suggests improved soil fertility and resilience against nutrient depletion. Actinobacteria, renowned for decomposing recalcitrant polymers like cellulose and chitin [72], accelerate organic matter humification, thereby stabilizing soil carbon pools and improving aggregate formation.
In contrast, chemical fertilization favored oligotrophic taxa such as Chloroflexi and Acidobacteriota. Chloroflexi, relying on phototrophic metabolism under low-nutrient regimes [73,74], dominate in soils where organic inputs are scarce, reflecting a depauperate ecosystem with reduced biogeochemical activity. Acidobacteriota, thriving in acidic conditions [75], proliferate under chemical fertilization, corroborating the soil acidification caused by prolonged synthetic fertilizer use. Such shifts signal a decline in functional diversity, as these taxa exhibit limited metabolic versatility compared to copiotrophs, potentially impairing soil nutrient cycling and carbon sequestration over time.
Notably, slow-release fertilizers supported higher microbial diversity and abundance than conventional NPK fertilizers. While less impactful than bio-organic amendments, this suggests that controlled nutrient release partially mitigates the destabilizing effects of synthetic fertilizers on microbial communities, preserving critical functions like organic matter decomposition and disease suppression [76].
5. Conclusions
Fertilizer selection critically governs rocky desertification rehabilitation outcomes. Bio-organic fertilizers optimized soil health through enhanced organic matter (30.4% increase), macronutrients (N and P), micronutrients (Fe, Zn, and Cu), enzymatic activity (urease/sucrase >25% rise), and beneficial microbiota (Proteobacteria/Actinobacteria dominance). Slow-release fertilizers supported localized Mahonia fortunei (Lindl.) Fedde growth improvements (9–22% height increase) via controlled nutrient release, while NPK compound fertilizers risked soil degradation.
Spatial variability in the results underscores the need for context-specific strategies: bio-organic fertilizers are prioritized for soil rehabilitation, while slow-release variants may complement growth objectives in localized settings. The cultivation of Mahonia fortunei (Lindl.) Fedde under forest canopies further offers a sustainable agroforestry model, bridging ecological restoration with economic upliftment for impoverished communities. Future research should explore the long-term impacts of combined bio-organic and slow-release fertilization, alongside socioeconomic assessments of medicinal crop value chains. This integrated approach holds promise for scalable application in global karst ecosystems facing similar ecological and developmental challenges.
Author Contributions
Writing—original draft preparation, X.F.; validation, Y.S., X.H. and Z.L.; formal analysis Y.S.; data curation and investigation, B.P.; resources, Z.L.; writing—review and editing, Z.L. and H.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (42207394); Guangxi Science & Technology Innovation Base & Talent Development Special Program: “Canopy Bag-controlled Fertilization Technology for Forestry Applications and Research Team Competency Building” [GXKJ AD24010040]; Guangxi Key Research and Development Program of China (AB21196048); Forestry and Grassland Science and Technology Promotion Demonstration Project of the Central Government ([2023] TG17); and Key Laboratory Autonomous Projects (2021-A-02-01).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors are grateful to Guangxi Forest’s New Fertilizer Research and Development Center; the School of Civil Engineering, Southeast University; and the National and Local Unified Engineering Research Center for Basalt Fiber Production and Application Technology, Southeast University. Special thanks go to the anonymous reviewers for their constrictive comments in improving this manuscript.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Yuan, L.; Liu, W.D.; Su, S.; Chen, Z. The rocky desertification management in Guizhou province under the localized governance system. Front. Environ. Sci. 2022, 10, 1065663. [Google Scholar] [CrossRef]
- Wang, S.J.; Liu, Q.M.; Zhang, D.F. Karst rocky desertification in southwestern China: Geomorphology, landuse, impact and rehabilitation. Land Degrad. Dev. 2004, 15, 115–121. [Google Scholar] [CrossRef]
- Bai, X.Y.; Wang, S.J.; Xiong, K.N. Assessing spatial-temporal evolution processes of kaest rocky desertification land: Indications for restoration strategies. Land Degrad. Dev. 2013, 24, 47–56. [Google Scholar] [CrossRef]
- Fu, W.B.; Yan, Y.J.; Wang, K.; Hu, G.; Ling, Z.H. Effects of control measures on soil quality evolution in the Karst rocky desertification area in southwestern China. Res. Soil Water 2021, 28, 27–32. [Google Scholar]
- Jin, J.L.; Sheppard, S.R.J.; Jia, B.Q.; Wang, C. Planning to Practice: Impacts of Large-Scale and Rapid Urban Afforestation on Greenspace Patterns in the Beijing Plain Area. Forests 2021, 12, 316. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Wu, Y.H.; Chen, S.R.; Sui, M.Z.; Zhang, G.Q.; Liu, Q.F.; Chen, D.M.; He, Y.J.; Zang, L.P. Does the universal adaptive strategy theory apply to natural regeneration in heterogeneous subtropical karst forests? Ecol. Indic. 2024, 165, 112168. [Google Scholar] [CrossRef]
- Zhao, Y.; Han, Z.; Zhang, G.Q.; Chen, D.M.; Zang, L.P.; Liu, Q.F.; Guo, Y.; Xie, P.Y.; Chen, H.C.; He, Y.J. Variability of soil enzyme activities and nutrients with forest gap renewal interacting with soil depths in degraded karst forests. Ecol. Indic. 2024, 166, 112332. [Google Scholar] [CrossRef]
- Peng, J.; Xu, Y.Q.; Zhang, R.; Xiong, K.N.; Lan, A.J. Soil erosion monitoring and its implication in a limestone land suffering from rocky desertification in the Huajiang Canyon, Guizhou, Southwest China. Environ. Earth Sci. 2013, 69, 831–841. [Google Scholar] [CrossRef]
- Chen, X.L. The origin, development and propspect of non-timber forest-based economics. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2022, 46, 105–114. [Google Scholar]
- Somarriba, E. Revisiting the past-an essay on agroforestry definition. Agrofor. Syst. 1992, 19, 233–240. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, J.Q.; Gao, L.; Liu, F.Y.; Liu, Y.J. Effects of different management measures of cultivation Panax notoginseng under forest on runoff and sediment yield and soil characteristics on slope. J. Soil Water Conserv. 2024, 38, 20–30. [Google Scholar]
- Zhou, S.Q. The interplanting patterns of economic crops with young Chinese firplantations. J. Fujian Agric. For. Univ. (Nat. Sci. Ed.) 2005, 2, 234–238. [Google Scholar]
- Li, Q.S. Research and demonstration of planting grain crops under forest. Agric. Eng. 2020, 10, 112–119. [Google Scholar]
- Ouyang, Z.L.; Huang, D.A.; Teng, W.C.; Luo, X.H. Effects of Interplanting of Mahonia fortunei and Different Plants on Chemical Properties of Soil. Guangxi For. Sci. 2021, 50, 274–280. [Google Scholar]
- Lei, K.; Huang, D.A.; Teng, W.C.; Luo, X.H. Effects of Different Interplanting Models on Soil Chemical Properties. Guangxi For. Sci. 2021, 50, 60–65. [Google Scholar]
- Ma, T.S.; Deng, X.W.; Chen, L.; Xiang, W.H. The soil properties and their effects on plant diversity in different degrees of rocky desertification. Sci. Total Environ. 2020, 736, 139667. [Google Scholar] [CrossRef]
- Gomiero, T. Soil degradation, land scarcity and food security: Reviewing a complex challenge. Sustainability 2016, 8, 281. [Google Scholar] [CrossRef]
- Aziz, M.A.; Khan, K.S.; Khalid, R.; Shabaan, M.; Alghamdi, A.G.; Alasmary, Z.; Majrashi, M.A. Integrated application of biochar and chemical fertilizers improves wheat (Triticum aestivum) productivity by enhancing soil microbial activities. Plant Soil 2024, 502, 433–448. [Google Scholar] [CrossRef]
- Oldfield, E.E.; Bradford, M.A.; Wood, S.A. Global meta-analysis of the relationship between soil organic matter and crop yields. Soil 2019, 5, 15–32. [Google Scholar] [CrossRef]
- Fang, P.; Abler, D.; Lin, G.; Sher, A.; Quan, Q. Substituting Organic Fertilizer for Chemical Fertilizer: Evidence from Apple Growers in China. Land 2021, 10, 858. [Google Scholar] [CrossRef]
- Kassem, I.; Ablouh, E.; El Bouchtaoui, F.Z.; Jaouahar, M.; El Achaby, M. Polymer coated slow/controlled release granular fertilizers: Fundamentals and research trends. Prog. Mater. Sci. 2024, 144, 101269. [Google Scholar] [CrossRef]
- Sun, Y.; Mi, W.H.; Su, L.J.; Shan, Y.Y.; Wu, L.H. Controlled-release fertilizer enhances rice grain yield and N recovery efficiency in continuous non-flooding plastic film mulching cultivation system. Field Crops Res. 2019, 231, 122–129. [Google Scholar] [CrossRef]
- Guo, J.J.; Fan, J.L.; Zhang, F.C.; Yan, S.C.; Zheng, J.; Wu, Y.; Li, J.; Wang, Y.L.; Sun, X.; Liu, X.Q.; et al. Blending urea and slow-release nitrogen fertilizer increases dryland maize yield and nitrogen use efficiency while mitigating ammonia volatilization. Sci. Total Environ. 2021, 790, 148058. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Cui, S.; Wu, L.; Qi, W.; Chen, J.; Ye, Z.; Ma, J.; Liu, D. Effects of bio-organic fertilizer on soil fertility, yield, and quality of tea. J. Soil Sci. Plant Nutr. 2023, 23, 5109–5121. [Google Scholar] [CrossRef]
- Naher, U.A.; Biswas, J.C.; Maniruzzaman, M.; Khan, F.H.; Sarkar, M.I.U.; Jahan, A.; Hera, M.H.R.; Hossain, M.B.; Islam, A.; Islam, M.R. Bio-organic fertilizer: A green technology to reduce synthetic N and P fertilizer for rice production. Front. Plant Sci. 2021, 12, 602052. [Google Scholar] [CrossRef]
- Du, T.Y.; Hu, Y.C.; Liu, Y.S.; Wu, P.L.; Zhou, X.P. Rocky desertification in Guangxi karst mountainous area: Its tendency, formation causes and rehabilitation. Trans. CSAE 2008, 24, 96–101. [Google Scholar]
- Wei, X.C.; Deng, X.W.; Xiang, W.H.; Lei, P.F.; Ouyang, S.; Wen, H.F.; Chen, L. Calcium content and high calcium adaptation of plants in karst areas of southwestern Hunan, China. Biogeosciences 2018, 15, 2991–3002. [Google Scholar] [CrossRef]
- Huang, A.H.; Li, J.W.; Shen, Z.Q.; Wang, X.W.; Jin, M. High-throughput identification of clinical pathogenic fungi by hybridization to an oligonucleotide microarray. J. Clin. Microbiol. 2006, 44, 3299–3305. [Google Scholar] [CrossRef]
- De Clercq, T.; Merckx, R.; Elsen, A.; Vandendriessche, H. Impact of long-term compost amendments on soil fertility, soil organic matter fractions and nitrogen mineralization. In Proceedings of the 3rd International Symposium on Organic Matter Management and Compost Use in Horticulture, Murcia, Spain, 20–24 April 2015; pp. 79–86. [Google Scholar]
- Ernest, B.; Eltigani, A.; Yanda, P.Z.; Hansson, A.; Fridahl, M. Evaluation of selected organic fertilizers on conditioning soil health of smallholder households in Karagwe, Northwestern Tanzania. Heliyon 2024, 10, e26059. [Google Scholar] [CrossRef]
- Wan, X.; Qiu, S.M.; Zhou, R.Y.; Li, L.W.; Xing, W.; Yuan, Y.D. Urban forest soil properties and microbial characteristics: Seasonal and stand-specific variations. Appl. Soil Ecol. 2025, 209, 105995. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, X.W.; Laipan, M.; Wei, T.; Guo, J.K. Soil health improvement by inoculation of indigenous microalgae in saline soil. Environ. Geochem. Health 2024, 46, 23. [Google Scholar] [CrossRef] [PubMed]
- Burns, R.G. Enzyme-activity in soil-location and a possibile role in microbial ecology. Soil Biol. Biochem. 1982, 14, 423–427. [Google Scholar] [CrossRef]
- Johannes, A.; Sauzet, O.; Matter, A.; Boivin, P. Soil organic carbon content and soil structure quality of clayey cropland soils: A large-scale study in the Swiss Jura region. Soil Use Manag. 2023, 39, 707–716. [Google Scholar] [CrossRef]
- Reeves, D.W. The role of soil organic matter in maintaining soil quality in continuous cropping systems. Soil Tillage Res. 1997, 43, 131–167. [Google Scholar] [CrossRef]
- Li, J.; Xiao, X.M.; Lyu, J.; Gao, C.F.; Ali, M.; Zhang, G.B.; Feng, Z.; Yu, J.H. Integrating bio-organic fertilization increases twice-yearly cabbage crop production by modulating soil microbial community and biochemical properties in Northwest Plateau. Environ. Technol. Innov. 2024, 35, 103715. [Google Scholar] [CrossRef]
- Duncan, E.G.; O’Sullivan, C.A.; Roper, M.M.; Palta, J.; Whisson, K.; Peoples, M.B. Yield and nitrogen use efficiency of wheat increased with root length and biomass due to nitrogen, phosphorus, and potassium interactions. J. Plant Nutr. Soil Sci. 2018, 181, 364–373. [Google Scholar] [CrossRef]
- Bassi, D.; Menossi, M.; Mattiello, L. Nitrogen supply influences photosynthesis establishment along the sugarcane leaf. Sci. Rep. 2018, 8, 2327. [Google Scholar] [CrossRef]
- Xucheng, Z.; Zhouping, S. Effect of nitrogen fertilization on photosynthetic pigment and fluorescence characteristics in leaves of winter wheat cultivars on dryland. Acta Agric. Nucleatae Sin. 2007, 21, 299. [Google Scholar]
- Gu, J.; Zhou, Z.; Li, Z.; Dai, Q.; Kong, X.; Wang, Z.; Yang, J. Effects of the mutant with low chlorophyll content on photosynthesis and yield in rice. Acta Agron. Sin. 2016, 42, 553–562. [Google Scholar] [CrossRef]
- Nardi, S.; Morari, F.; Berti, A.; Tosoni, M.; Giardini, L. Soil organic matter properties after 40 years of different use of organic and mineral fertilisers. Eur. J. Agron. 2004, 21, 357–367. [Google Scholar] [CrossRef]
- Ali, A.; Liu, X.L.; Yang, W.P.; Li, W.G.; Chen, J.; Qiao, Y.J.; Gao, Z.Q.; Yang, Z.P. Impact of Bio-Organic Fertilizer Incorporation on Soil Nutrients, Enzymatic Activity, and Microbial Community in Wheat-Maize Rotation System. Agronomy 2024, 14, 1942. [Google Scholar] [CrossRef]
- Wang, L.J.; Wang, P.; Sheng, M.Y.; Tian, J. Ecological stoichiometry and environmental influencing factors of soil nutrients in the karst rocky desertification ecosystem, southwest China. Glob. Ecol. Conserv. 2018, 16, e00449. [Google Scholar] [CrossRef]
- Chen, F.; Zhou, D.Q.; Bai, X.Y.; Zeng, C.; Xiao, J.Y.; Qian, Q.H.; Luo, G.J. Responses of soil physical and chemical properties to karst rocky desertification evolution in typical karst valley area. In Proceedings of the 3rd International Conference on Environmental Science and Material Application (ESMA), Chongqing, China, 25–26 November 2017. [Google Scholar]
- Warman, P.R.; Termeer, W.C. Evaluation of sewage sludge, septic waste and sludge compost applications to corn and forage: Ca, Mg, S, Fe, Mn, Cu, Zn and B content of crops and soils. Bioresour. Technol. 2005, 96, 1029–1038. [Google Scholar] [CrossRef]
- Kot, F.S.; Farran, R.; Fujiwara, K.; Kharitonova, G.V.; Kochva, M.; Shaviv, A.; Sugo, T. On boron turnover in plant-litter-soil system. Geoderma 2016, 268, 139–146. [Google Scholar] [CrossRef]
- Takano, J.; Miwa, K.; Fujiwara, T. Boron transport mechanisms: Collaboration of channels and transporters. Trends Plant Sci. 2008, 13, 451–457. [Google Scholar] [CrossRef]
- Burkhead, J.L.; Reynolds, K.A.G.; Abdel-Ghany, S.E.; Cohu, C.M.; Pilon, M. Copper homeostasis. New Phytol. 2009, 182, 799–816. [Google Scholar] [CrossRef]
- Ravet, K.; Pilon, M. Copper and Iron Homeostasis in Plants: The Challenges of Oxidative Stress. Antioxid. Redox Signal. 2013, 19, 919–932. [Google Scholar] [CrossRef]
- Sinclair, S.A.; Krämer, U. The zinc homeostasis network of land plants. Biochim. Biophys. Acta-Mol. Cell Res. 2012, 1823, 1553–1567. [Google Scholar] [CrossRef] [PubMed]
- Palmgren, M.G.; Clemens, S.; Williams, L.E.; Kraemer, U.; Borg, S.; Schjorring, J.K.; Sanders, D. Zinc biofortification of cereals: Problems and solutions. Trends Plant Sci. 2008, 13, 464–473. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.A.; Lee, J.; Guerinot, M.L.; An, G. Zinc Deficiency-Inducible OsZIP8 Encodes a Plasma Membrane-Localized Zinc Transporter in Rice. Mol. Cells 2010, 29, 551–558. [Google Scholar] [CrossRef]
- Nadeem, F.; Abbas, S.; Waseem, F.; Ali, N.; Mahmood, R.; Bibi, S.; Deng, L.F.; Wang, R.F.; Zhong, Y.T.; Li, X.X. Phosphorus (P) and Zinc (Zn) nutrition constraints: A perspective of linking soil application with plant regulations. Environ. Exp. Bot. 2024, 226, 105875. [Google Scholar] [CrossRef]
- Liang, G. Iron uptake, signaling, and sensing in plants. Plant Commun. 2022, 3, 100349. [Google Scholar] [CrossRef] [PubMed]
- Finazzi, G.; Petroutsos, D.; Tomizioli, M.; Flori, S.; Sautron, E.; Villanova, V.; Rolland, N.; Seigneurin-Berny, D. Ions channels/transporters and chloroplast regulation. Cell Calcium 2015, 58, 86–97. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Wang, Y.S. Soil enzyme activities with greenhouse subsurface irrigation. Pedosphere 2006, 16, 512–518. [Google Scholar] [CrossRef]
- Demisie, W.; Liu, Z.Y.; Zhang, M.K. Effect of biochar on carbon fractions and enzyme activity of red soil. Catena 2014, 121, 214–221. [Google Scholar] [CrossRef]
- Wu, L.P.; Ma, H.; Zhao, Q.L.; Zhang, S.R.; Wei, W.L.; Ding, X.D. Changes in soil bacterial community and enzyme activity under five years straw returning in paddy soil. Eur. J. Soil Biol. 2020, 100, 103215. [Google Scholar] [CrossRef]
- Yang, F.; Tian, J.; Fang, H.J.; Gao, Y.; Xu, M.G.; Lou, Y.L.; Zhou, B.K.; Kuzyakov, Y. Functional soil organic matter fractions, microbial community, and enzyme activities in a Mollisol under 35 years manure and mineral fertilization. J. Soil Sci. Plant Nutr. 2019, 19, 430–439. [Google Scholar] [CrossRef]
- Bardgett, R.D.; van der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Rousk, J.; Brookes, P.C.; Bååth, E. Contrasting Soil pH Effects on Fungal and Bacterial Growth Suggest Functional Redundancy in Carbon Mineralization. Appl. Environ. Microbiol. 2009, 75, 1589–1596. [Google Scholar] [CrossRef]
- Xu, J.; Si, L.L.; Zhang, X.; Cao, K.; Wang, J.H. Various green manure-fertilizer combinations affect the soil microbial community and function in immature red soil. Front. Microbiol. 2023, 14, 1255056. [Google Scholar] [CrossRef]
- Ramirez, K.S.; Lauber, C.L.; Knight, R.; Bradford, M.A.; Fierer, N. Consistent effects of nitrogen fertilization on soil bacterial communities in contrasting systems. Ecology 2010, 91, 3463–3470. [Google Scholar] [CrossRef] [PubMed]
- Coolon, J.D.; Jones, K.L.; Todd, T.C.; Blair, J.M.; Herman, M.A. Long-term nitrogen amendment alters the diversity and assemblage of soil bacterial communities in tallgrass prairie. PLoS ONE 2013, 8, e67884. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.Y.; Deng, S.P.; Raun, W.R. Bacterial community structure and diversity in a century-old manure-treated agroecosystem. Appl. Environ. Microbiol. 2004, 70, 5868–5874. [Google Scholar] [CrossRef]
- Wang, G.; Liu, J.; Qi, X.; Jin, J.; Wang, Y.; Liu, X. Effects of fertilization on bacterial community structure and function in a black soil of Dehui region estimated by Biolog and PCR-DGGE methods. Acta Ecol. Sin. 2008, 28, 220–226. [Google Scholar]
- Chaudhry, V.; Rehman, A.; Mishra, A.; Chauhan, P.S.; Nautiyal, C.S. Changes in bacterial community structure of agricultural land due to long-term organic and chemical amendments. Microb. Ecol. 2012, 64, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Song, J.; Kim, B.Y.; Kim, M.S.; Joa, J.H.; Weon, H.Y. Characterization of the Bacterial and Archaeal Communities in Rice Field Soils Subjected to Long-Term Fertilization Practices. J. Microbiol. 2012, 50, 754–765. [Google Scholar] [CrossRef]
- Xiao, H.B.; Li, Z.W.; Chang, X.F.; Huang, J.Q.; Nie, X.D.; Liu, C.; Liu, L.; Wang, D.Y.; Dong, Y.T.; Jiang, J.Y. Soil erosion-related dynamics of soil bacterial communities and microbial respiration. Appl. Soil Ecol. 2017, 119, 205–213. [Google Scholar] [CrossRef]
- Liao, J.J.; Dou, Y.X.; Yang, X.; An, S.S. Soil microbial community and their functional genes during grassland restoration. J. Environ. Manag. 2023, 325, 116488. [Google Scholar] [CrossRef]
- Rampelotto, P.H.; Ferreira, A.D.; Barboza, A.D.M.; Roesch, L.F.W. Changes in Diversity, Abundance, and Structure of Soil Bacterial Communities in Brazilian Savanna Under Different Land Use Systems. Microb. Ecol. 2013, 66, 593–607. [Google Scholar] [CrossRef]
- Zeng, Q.C.; An, S.S.; Liu, Y. Soil bacterial community response to vegetation succession after fencing in the grassland of China. Sci. Total Environ. 2017, 609, 2–10. [Google Scholar] [CrossRef]
- Fullerton, H.; Moyer, C.L. Comparative Single-Cell Genomics of Chloroflexi from the Okinawa Trough Deep-Subsurface Biosphere. Appl. Environ. Microbiol. 2016, 82, 3000–3008. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.J.; Liu, W.B.; Zhu, C.; Luo, G.W.; Kong, Y.L.; Ling, N.; Wang, M.; Dai, J.Y.; Shen, Q.R.; Guo, S.W. Bacterial rather than fungal community composition is associated with microbial activities and nutrient-use efficiencies in a paddy soil with short-term organic amendments. Plant Soil 2018, 424, 335–349. [Google Scholar] [CrossRef]
- Wang, J.B.; Xie, J.H.; Li, L.L.; Effah, Z.; Xie, L.H.; Luo, Z.Z.; Zhou, Y.J.; Jiang, Y.J. Fertilization treatments affect soil CO2 emission through regulating soil bacterial community composition in the semiarid Loess Plateau. Sci. Rep. 2022, 12, 20123. [Google Scholar] [CrossRef] [PubMed]
- Niu, T.H.; Xie, J.M.; Li, J.; Zhang, J.; Zhang, X.D.; Ma, H.Y.; Wang, C. Response of rhizosphere microbial community of Chinese chives under different fertilization treatments. Front. Microbiol. 2022, 13, 1031624. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).