Understory Forage Quality for Grazing Animals in Chilean Patagonian Forests
Abstract
1. Introduction
2. Materials and Methods
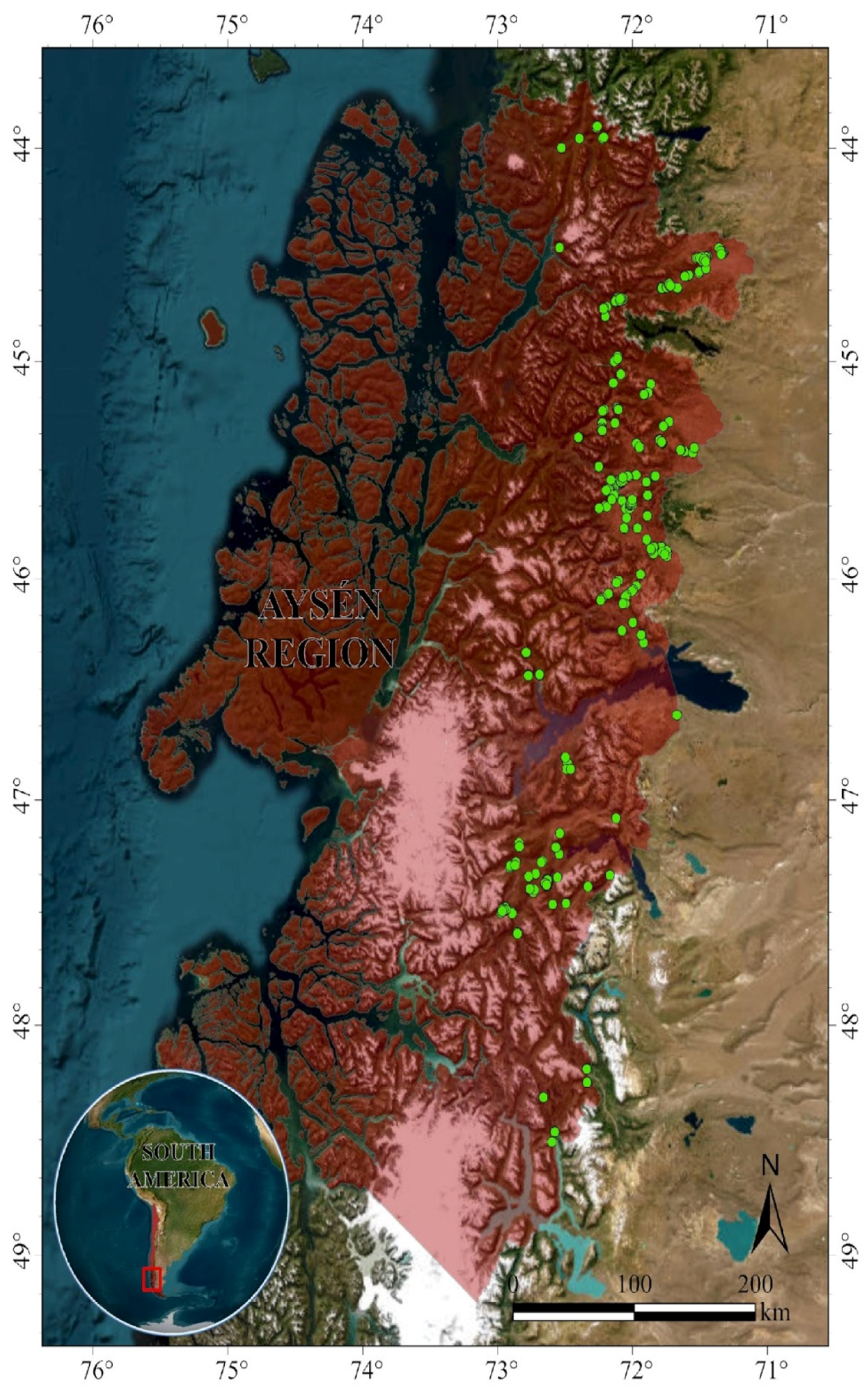
2.1. Study Area
2.2. Sampling Design and Data Taking
2.3. Plant Nutritional Data Approach
- (i)
- Crude protein index (CPI) based on average CP levels (Figure 2C) as follows: 0–10% (very low CP), 10–12% (low CP), 12–15% (medium CP), 15–18% (high CP), and >18% (very high CP).
- (ii)
- Phenological index (PHI) (Figure 2D), where plant phenological stages were classified and rated (rPHI) as follows: absent (rPHI = 0.00) when the plant species was not available, 0 (rPHI = 0.25) when the plant species occurred at the beginning or the end of the growing phase, intermediate stage (rPHI = 0.50) when the plant species was fruiting or drying; near-complete development (rPHI = 0.75) when the plant species was near to or continuing flowering; and complete development (rPHI = 1.00) when the plant species was at maximum flowering expression.
- (iii)
- Specific quality index (SQI) was calculated following Daget and Poissonet [40] (Figure 2D). This index integrates phenological stage, species palatability, and digestibility for ruminants. Each species was assigned a specific quality value (SQ) from 0 (no zootechnical interest) to 10 (maximum interest). For validation, our SQI values were compared with existing literature for the region [5,40,41].
2.4. Data Analyses
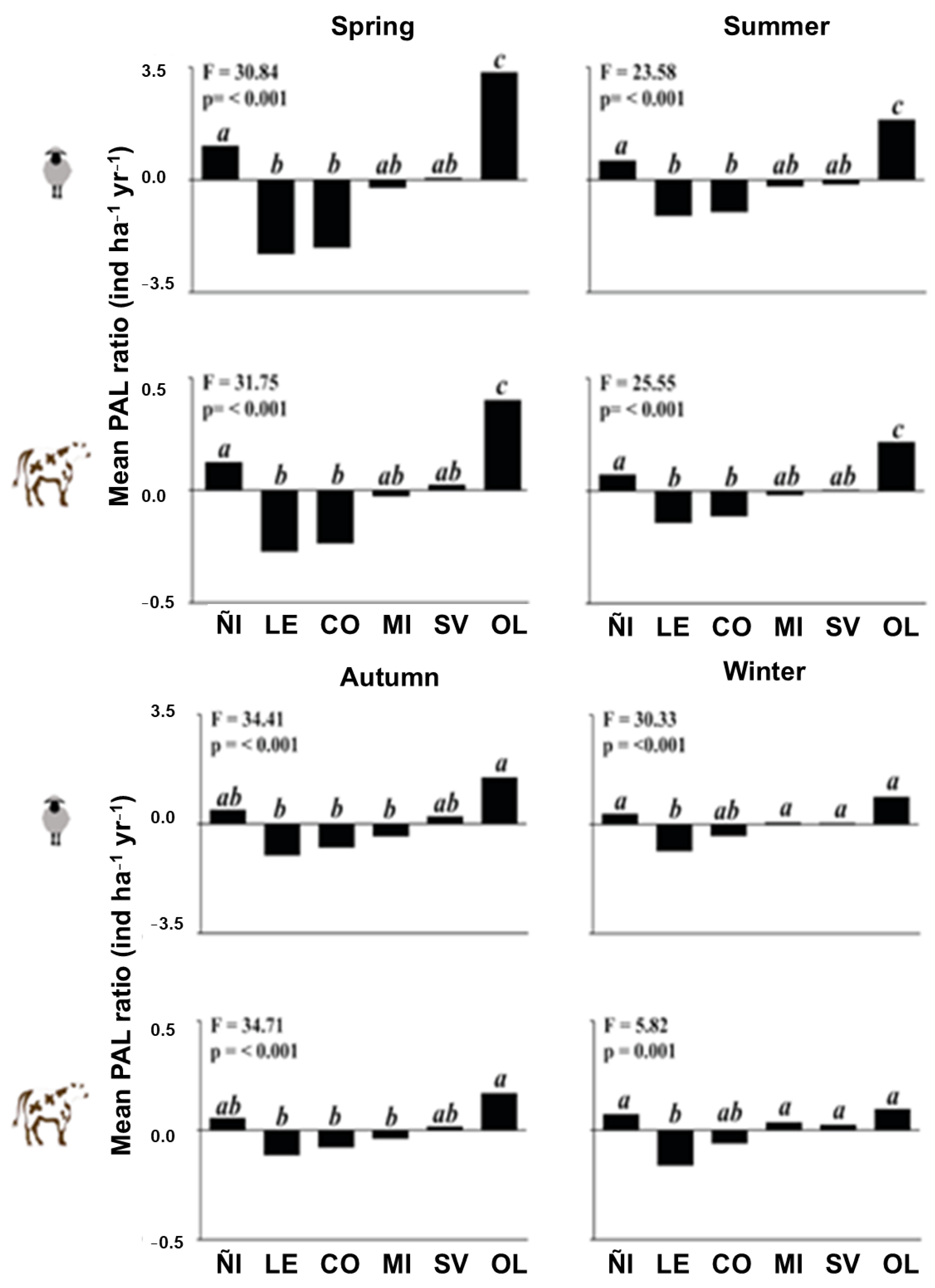
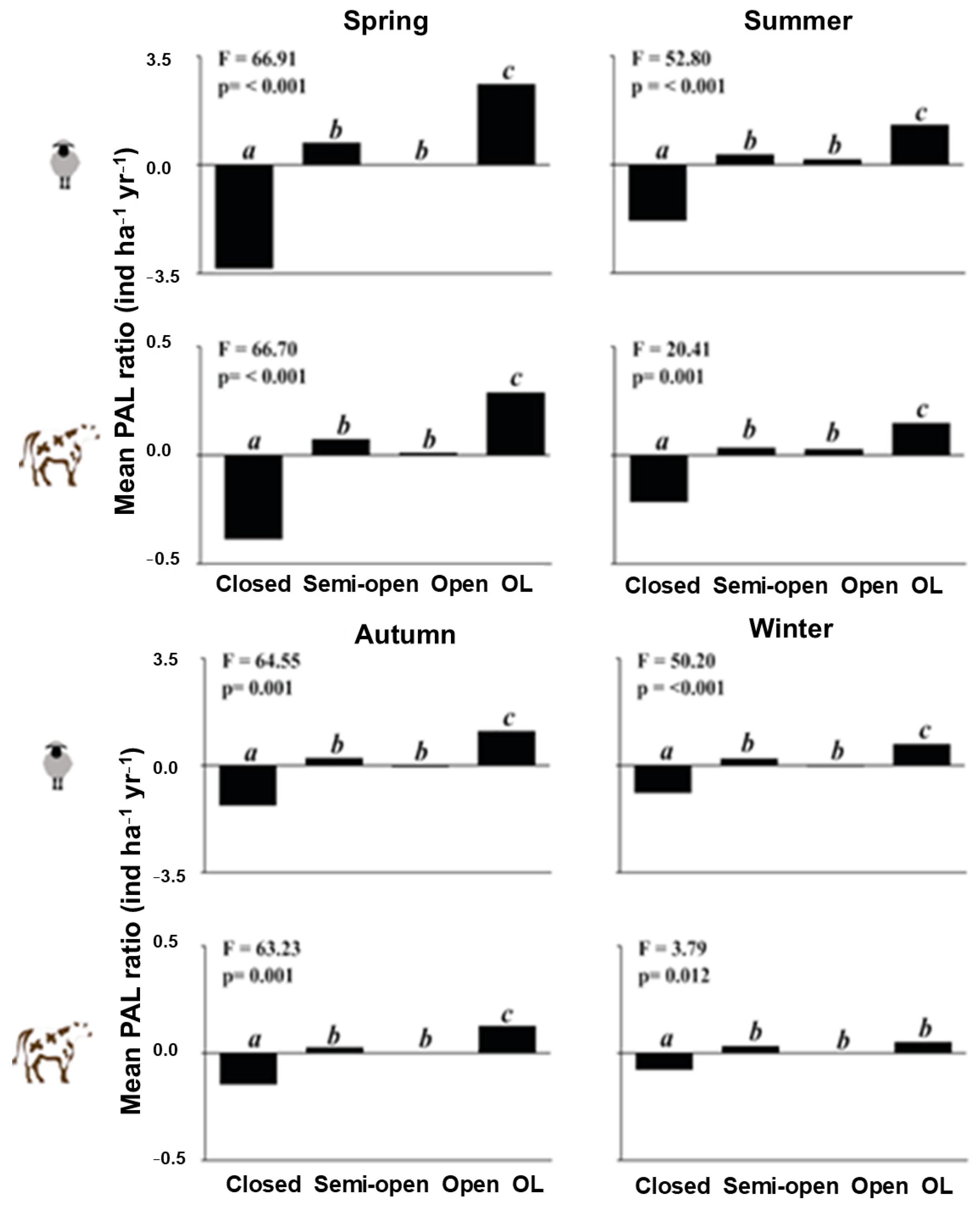
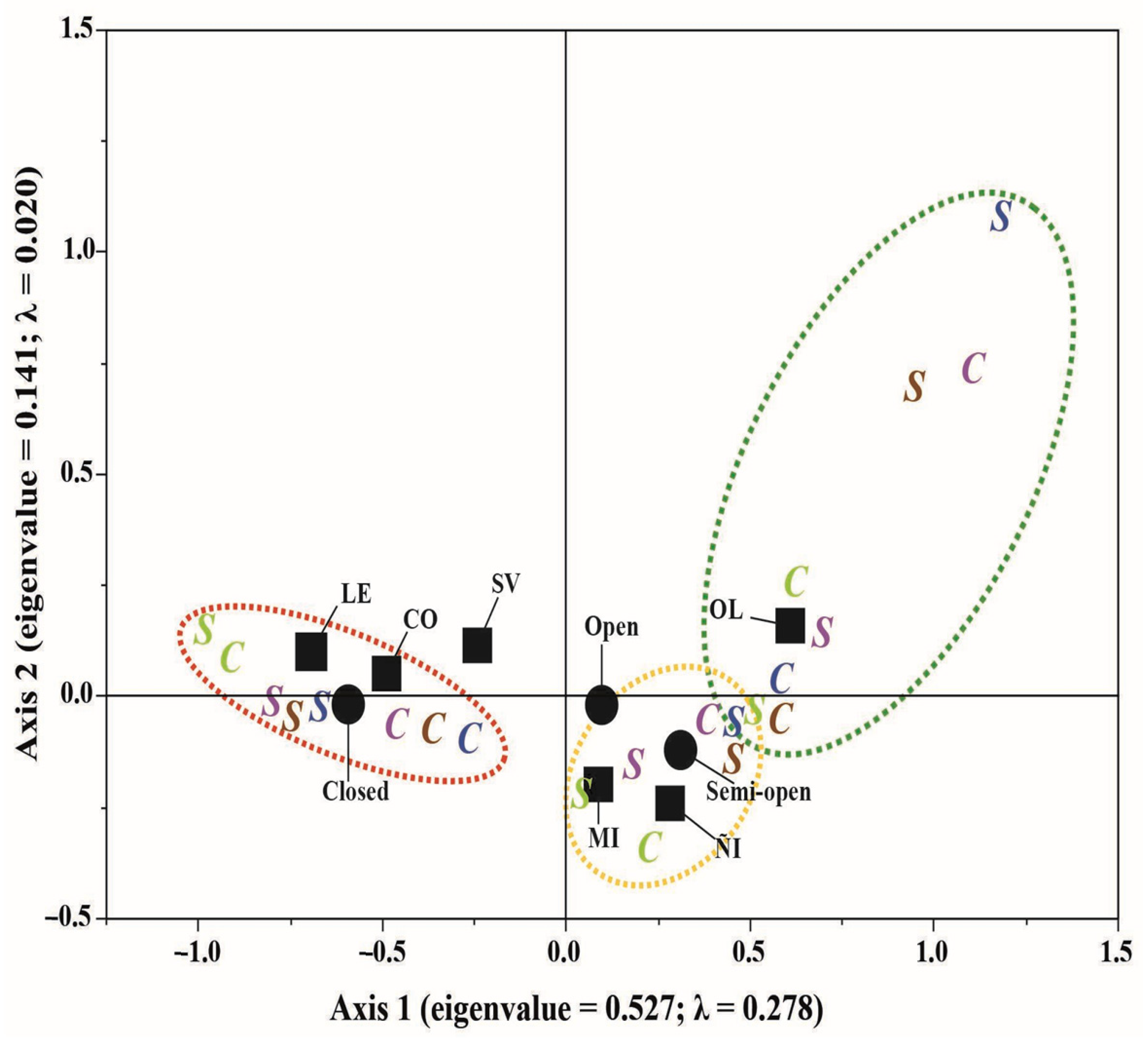
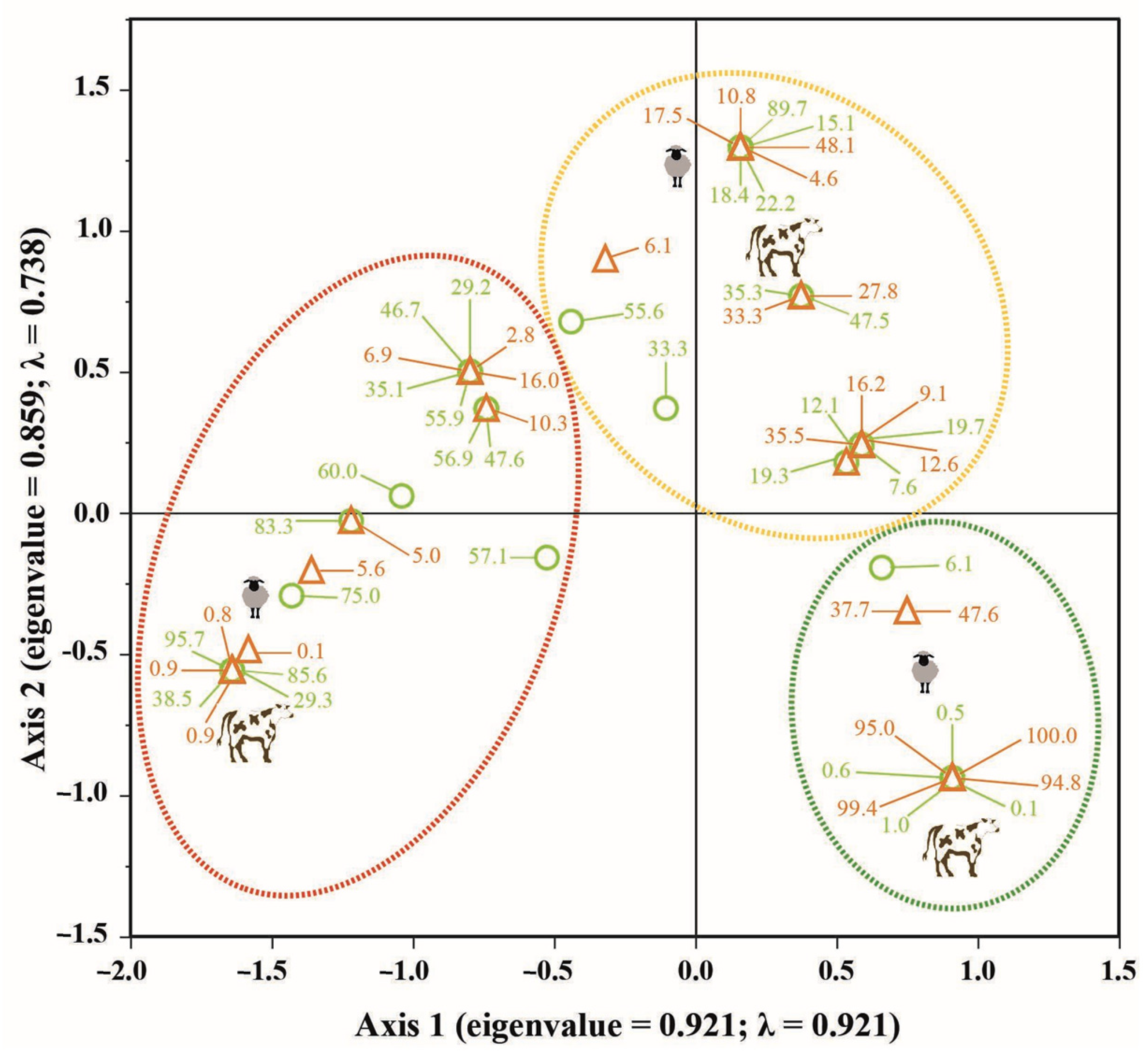
3. Results
4. Discussion
4.1. Plant Origins and Growth Habits
4.2. Nutritional Subsidies for Grazing Animals
4.3. Limitations of the Present Study
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Code | Species | Origin | Growth Habit | Environment Type | Total | Canopy Cover | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CO | LE | MI | ÑI | SV | OL | Closed | Semi-Open | Open | ||||||
| ACIN | Acaena integerrima | Native | Forb | 0.0 | 0.5 | 0.0 | 1.3 | 0.3 | 1.3 | 3.5 | 0.4 | 1.8 | 0.7 | 2.9 |
| ACMA | Acaena magellanica | Native | Forb | 0.0 | 1.1 | 0.0 | 0.0 | 0.0 | 0.8 | 1.9 | 1.1 | 0.4 | 0.0 | 1.4 |
| ACMI | Achillea millefolium | Exotic | Forb | 0.0 | 1.6 | 0.3 | 5.1 | 0.0 | 7.5 | 14.4 | 3.6 | 5.4 | 0.4 | 9.4 |
| ACOV | Acaena ovalifolia | Native | Forb | 2.7 | 15.2 | 3.2 | 8.8 | 2.4 | 5.6 | 38.0 | 30.1 | 12.7 | 1.1 | 43.8 |
| ACPI | Acaena pinnatifida | Native | Forb | 1.3 | 6.4 | 1.1 | 9.1 | 0.0 | 2.7 | 20.6 | 9.4 | 13.0 | 1.8 | 24.3 |
| ADCH | Adenocaulon chilense | Native | Forb | 2.4 | 17.6 | 1.9 | 4.3 | 0.0 | 0.5 | 26.7 | 29.7 | 5.4 | 0.4 | 35.5 |
| AGCA | Agrostis capillaris | Exotic | Graminoid | 0.5 | 6.1 | 0.0 | 5.9 | 0.0 | 12.6 | 25.1 | 8.0 | 8.3 | 0.7 | 17.0 |
| AGLE | Agrostis leptotricha | Native | Graminoid | 0.0 | 0.0 | 0.0 | 0.3 | 0.0 | 0.0 | 0.3 | 0.0 | 0.4 | 0.0 | 0.4 |
| AGST | Agrostis stolonifera | Exotic | Graminoid | 0.0 | 0.3 | 0.3 | 2.9 | 0.5 | 1.1 | 5.1 | 1.8 | 3.3 | 0.4 | 5.4 |
| AICA | Aira caryophyllea | Exotic | Graminoid | 0.3 | 0.3 | 0.0 | 1.1 | 0.0 | 4.3 | 5.9 | 0.0 | 1.8 | 0.4 | 2.2 |
| AMLU | Amomyrtus luma | Native | Tree | 0.5 | 0.0 | 0.3 | 0.0 | 0.8 | 0.0 | 1.6 | 1.4 | 0.7 | 0.0 | 2.2 |
| ANOD | Anthoxanthum odoratum | Exotic | Graminoid | 0.0 | 0.0 | 0.0 | 2.4 | 0.0 | 0.0 | 2.4 | 1.8 | 1.4 | 0.0 | 3.3 |
| ANMU | Anemone multifida | Native | Forb | 0.0 | 4.0 | 0.8 | 10.2 | 0.3 | 1.1 | 16.3 | 6.9 | 12.3 | 1.4 | 20.7 |
| ARCH | Aristotelia chilensis | Native | Tree | 0.0 | 0.3 | 0.0 | 0.0 | 0.3 | 0.5 | 1.1 | 0.7 | 0.0 | 0.0 | 0.7 |
| ARMA | Armeria maritima | Native | Forb | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 |
| AZLA | Azara lanceolata | Native | Tree | 2.7 | 0.3 | 0.5 | 0.0 | 2.1 | 0.0 | 5.6 | 5.8 | 1.8 | 0.0 | 7.6 |
| BAMA | Baccharis magellanica | Native | Shrub | 0.0 | 1.3 | 0.8 | 5.3 | 0.0 | 0.8 | 8.3 | 3.6 | 5.8 | 0.7 | 10.1 |
| BEDA | Berberis darwinii | Native | Shrub | 2.1 | 5.1 | 1.6 | 4.0 | 1.6 | 7.2 | 21.7 | 12.7 | 6.2 | 0.7 | 19.6 |
| BEEM | Berberis empetrifollia | Native | Shrub | 0.0 | 0.3 | 0.0 | 0.3 | 0.0 | 0.0 | 0.5 | 0.0 | 0.4 | 0.4 | 0.7 |
| BEMI | Berberis microphylla | Native | Shrub | 1.9 | 6.1 | 0.8 | 17.6 | 0.8 | 5.3 | 32.6 | 18.8 | 16.7 | 1.4 | 37.0 |
| BESE | Berberis serratodentata | Native | Shrub | 0.3 | 3.7 | 0.5 | 0.3 | 0.3 | 0.0 | 5.1 | 6.5 | 0.4 | 0.0 | 6.9 |
| BLMO | Blechnum mochaenum | Native | Fern | 0.3 | 0.0 | 0.0 | 0.0 | 0.3 | 0.0 | 0.5 | 0.7 | 0.0 | 0.0 | 0.7 |
| BLCL | Blechnum chilense | Native | Fern | 0.3 | 0.0 | 0.0 | 0.0 | 0.5 | 0.0 | 0.8 | 1.1 | 0.0 | 0.0 | 1.1 |
| BLPE | Blechnum pennamarina | Native | Fern | 4.5 | 12.3 | 2.4 | 13.9 | 2.1 | 9.1 | 44.4 | 33.3 | 14.1 | 0.4 | 47.8 |
| BRSE | Bromus setifolius | Native | Graminoid | 0.3 | 5.9 | 0.3 | 0.5 | 0.0 | 0.3 | 7.2 | 7.2 | 2.2 | 0.0 | 9.4 |
| BRUN | Bromus unioloides | Native | Graminoid | 0.0 | 0.8 | 0.0 | 0.0 | 0.0 | 0.5 | 1.3 | 1.1 | 0.0 | 0.0 | 1.1 |
| CABI | Calceolaria biflora | Native | Forb | 0.0 | 2.1 | 0.0 | 2.9 | 0.0 | 0.0 | 5.1 | 2.2 | 4.0 | 0.7 | 6.9 |
| CAVA | Capsidium valdivianum | Native | Liana | 0.3 | 0.0 | 0.0 | 0.0 | 0.5 | 0.0 | 0.8 | 1.1 | 0.0 | 0.0 | 1.1 |
| CABA | Carex banksii | Native | Graminoid | 0.0 | 0.0 | 0.0 | 2.7 | 0.0 | 0.5 | 3.2 | 2.2 | 1.4 | 0.0 | 3.6 |
| CADA | Carex darwinii | Native | Graminoid | 0.3 | 0.3 | 0.0 | 0.3 | 0.0 | 2.4 | 3.2 | 0.4 | 0.7 | 0.0 | 1.1 |
| CAFU | Carex fuscula | Native | Graminoid | 0.0 | 0.0 | 0.0 | 1.1 | 0.0 | 0.3 | 1.3 | 0.4 | 1.1 | 0.0 | 1.4 |
| CAGA | Carex gayana | Native | Graminoid | 0.0 | 0.0 | 0.0 | 0.5 | 0.0 | 0.5 | 1.1 | 0.0 | 0.7 | 0.0 | 0.7 |
| CEAR | Cerastium arvense | Exotic | Forb | 0.0 | 4.0 | 0.8 | 8.3 | 0.0 | 1.9 | 15.0 | 5.4 | 10.5 | 1.8 | 17.8 |
| CEGL | Cerastium glomeratum | Exotic | Forb | 0.3 | 1.6 | 0.3 | 4.0 | 1.1 | 3.5 | 10.7 | 4.7 | 5.1 | 0.0 | 9.8 |
| CHCU | Chusquea culeou | Native | Shrub | 2.4 | 1.9 | 0.3 | 1.1 | 2.4 | 0.5 | 8.6 | 8.0 | 2.9 | 0.0 | 10.9 |
| CHDI | Chiliotrichum diffusum | Native | Shrub | 0.0 | 4.3 | 0.0 | 3.2 | 0.0 | 1.1 | 8.6 | 5.8 | 4.0 | 0.4 | 10.1 |
| CIVU | Cirsium vulgare | Exotic | Shrub | 0.5 | 1.1 | 0.0 | 2.1 | 0.5 | 0.5 | 4.8 | 2.5 | 3.3 | 0.0 | 5.8 |
| COBI | Collomia biflora | Native | Forb | 0.0 | 0.0 | 0.0 | 1.1 | 0.0 | 0.0 | 1.1 | 0.0 | 1.4 | 0.0 | 1.4 |
| COES | Colletia spinosa | Native | Shrub | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.0 | 0.3 | 0.0 | 0.4 | 0.0 | 0.4 |
| COHY | Colletia hystrix | Native | Shrub | 0.0 | 0.0 | 0.0 | 1.1 | 0.0 | 0.0 | 1.1 | 0.0 | 1.1 | 0.4 | 1.4 |
| COIN | Colliguaja integerrima | Native | Shrub | 0.0 | 0.0 | 0.0 | 0.0 | 0.5 | 0.0 | 0.5 | 0.0 | 0.4 | 0.4 | 0.7 |
| COLE | Codonorchis lessonii | Native | Forb | 0.3 | 4.8 | 0.3 | 0.0 | 0.0 | 0.3 | 5.6 | 6.9 | 0.4 | 0.0 | 7.2 |
| CRCA | Crepis capillaris | Exotic | Forb | 1.1 | 0.8 | 0.0 | 3.5 | 0.0 | 3.5 | 8.8 | 2.5 | 4.3 | 0.4 | 7.2 |
| DAGL | Dactylis glomerata | Exotic | Graminoid | 0.5 | 7.2 | 0.8 | 8.3 | 2.1 | 13.9 | 32.9 | 9.8 | 14.5 | 1.4 | 25.7 |
| DEDE | Dendroligotrichum dendroides | Native | Bryophyte | 0.0 | 0.0 | 0.3 | 0.3 | 0.3 | 0.0 | 0.8 | 0.7 | 0.0 | 0.4 | 1.1 |
| DICH | Discaria chacaye | Native | Shrub | 0.0 | 0.0 | 0.0 | 3.5 | 0.0 | 0.0 | 3.5 | 1.8 | 2.9 | 0.0 | 4.7 |
| DIPA | Diplolepis pachyphylla | Native | Liana | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.4 | 0.0 | 0.0 | 0.4 |
| DIPU | Digitalis purpurea | Exotic | Forb | 0.5 | 0.0 | 0.0 | 0.0 | 0.3 | 0.0 | 0.8 | 0.7 | 0.4 | 0.0 | 1.1 |
| DRVE | Draba verna | Exotic | Forb | 0.0 | 0.0 | 0.0 | 1.6 | 0.0 | 0.0 | 1.6 | 0.7 | 1.4 | 0.0 | 2.2 |
| DRWI | Drimys winteri | Native | Tree | 0.3 | 0.0 | 0.0 | 0.0 | 0.5 | 0.0 | 0.8 | 1.1 | 0.0 | 0.0 | 1.1 |
| DYGL | Dysopsis glechomoides | Native | Forb | 0.5 | 1.6 | 0.3 | 0.8 | 0.5 | 0.3 | 4.0 | 2.9 | 1.8 | 0.4 | 5.1 |
| ECVU | Echium vulgare | Exotic | Forb | 0.0 | 0.0 | 0.3 | 0.3 | 0.0 | 0.8 | 1.3 | 0.4 | 0.4 | 0.0 | 0.7 |
| ELAN | Elymus angulatus | Native | Graminoid | 0.0 | 0.0 | 0.0 | 1.3 | 0.0 | 0.0 | 1.3 | 1.1 | 0.7 | 0.0 | 1.8 |
| ELPA | Eleocharis pachycarpa | Native | Graminoid | 0.0 | 0.8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.8 | 1.1 | 0.0 | 0.0 | 1.1 |
| ELRE | Elymus repens | Exotic | Graminoid | 0.0 | 0.0 | 0.0 | 0.3 | 0.0 | 0.0 | 0.3 | 0.0 | 0.4 | 0.0 | 0.4 |
| EMCO | Embothrium coccineum | Native | Tree | 0.8 | 0.3 | 0.0 | 0.3 | 0.0 | 0.0 | 1.3 | 0.7 | 1.1 | 0.0 | 1.8 |
| EMRU | Empetrum rubrum | Native | Shrub | 0.0 | 0.8 | 0.0 | 2.7 | 0.0 | 0.5 | 4.0 | 2.9 | 1.8 | 0.0 | 4.7 |
| ESAL | Escallonia alpina | Native | Shrub | 0.0 | 0.0 | 0.0 | 0.5 | 0.0 | 0.3 | 0.8 | 0.4 | 0.4 | 0.0 | 0.7 |
| ESRU | Escallonia rubra | Native | Shrub | 0.3 | 0.0 | 0.0 | 0.3 | 0.0 | 0.0 | 0.5 | 0.4 | 0.4 | 0.0 | 0.7 |
| ESVI | Escallonia virgata | Native | Shrub | 0.0 | 0.0 | 0.0 | 3.2 | 0.0 | 1.6 | 4.8 | 2.5 | 1.8 | 0.0 | 4.3 |
| FEMA | Festuca magellanica | Native | Graminoid | 0.3 | 4.5 | 0.3 | 1.3 | 0.0 | 0.3 | 6.7 | 6.2 | 2.5 | 0.0 | 8.7 |
| FEPA | Festuca pallescens | Native | Graminoid | 0.0 | 2.7 | 0.0 | 5.6 | 0.0 | 1.1 | 9.4 | 1.8 | 7.2 | 2.2 | 11.2 |
| FERU | Festuca rubra | Native | Graminoid | 0.0 | 0.3 | 0.0 | 2.1 | 0.0 | 5.3 | 7.8 | 0.4 | 2.9 | 0.0 | 3.3 |
| FRCH | Fragaria chiloensis | Native | Forb | 0.3 | 7.8 | 0.3 | 11.5 | 0.0 | 2.1 | 21.9 | 12.7 | 13.0 | 1.1 | 26.8 |
| FUMA | Fuchsia magellanica | Native | Shrub | 1.3 | 0.8 | 0.0 | 0.0 | 2.1 | 3.5 | 7.8 | 4.3 | 1.4 | 0.0 | 5.8 |
| GAAP | Galium aparine | Exotic | Forb | 0.0 | 0.3 | 0.0 | 1.3 | 0.0 | 0.0 | 1.6 | 1.4 | 0.7 | 0.0 | 2.2 |
| GAHY | Galium hypocarpium | Native | Forb | 0.3 | 1.6 | 0.3 | 0.8 | 0.0 | 2.4 | 5.3 | 1.4 | 2.5 | 0.0 | 4.0 |
| GALU | Gavilea lutea | Native | Forb | 0.3 | 4.3 | 0.0 | 1.3 | 0.0 | 0.3 | 6.1 | 6.5 | 1.4 | 0.0 | 8.0 |
| GAMU | Gaultheria mucronata | Native | Shrub | 2.4 | 9.9 | 0.8 | 4.8 | 0.0 | 7.5 | 25.4 | 17.4 | 6.2 | 0.7 | 24.3 |
| GERMA | Geranium magallanicum | Native | Forb | 1.1 | 5.1 | 0.0 | 5.6 | 0.0 | 5.1 | 16.8 | 9.1 | 6.5 | 0.4 | 15.9 |
| GEMO | Geranium molle | Exotic | Forb | 0.0 | 0.0 | 0.0 | 1.1 | 0.0 | 2.1 | 3.2 | 0.4 | 1.1 | 0.0 | 1.4 |
| GEUMA | Geum magellanicum | Native | Forb | 0.3 | 2.4 | 0.3 | 0.8 | 0.0 | 0.0 | 3.7 | 3.3 | 1.8 | 0.0 | 5.1 |
| GRRU | Griselinia ruscifolia | Native | Shrub | 1.1 | 0.0 | 0.0 | 0.0 | 0.8 | 0.0 | 1.9 | 2.2 | 0.4 | 0.0 | 2.5 |
| GRSP | Greigia sphacelata | Native | Forb | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 |
| GUMA | Gunnera magellanica | Native | Forb | 0.0 | 2.1 | 0.8 | 0.3 | 0.0 | 0.0 | 3.2 | 3.6 | 0.7 | 0.0 | 4.3 |
| GUTI | Gunnera tinctoria | Native | Forb | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.4 | 0.0 | 0.0 | 0.4 |
| HOLA | Holcus lanatus | Exotic | Graminoid | 2.1 | 8.0 | 3.2 | 21.7 | 1.6 | 21.9 | 58.6 | 22.1 | 26.4 | 1.1 | 49.6 |
| HYDE | Hymenophyllum dentatum | Native | Forb | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.0 | 0.3 | 0.4 | 0.0 | 0.0 | 0.4 |
| HYRA | Hypochaeris radicata | Exotic | Forb | 3.2 | 4.0 | 0.5 | 8.8 | 1.1 | 16.3 | 34.0 | 8.3 | 14.9 | 0.7 | 23.9 |
| HYSE | Hydrangea serratifolia | Native | Liana | 0.3 | 0.0 | 0.0 | 0.0 | 0.5 | 0.0 | 0.8 | 1.1 | 0.0 | 0.0 | 1.1 |
| JUPR | Juncus procerus | Native | Graminoid | 0.5 | 0.3 | 0.0 | 1.1 | 0.0 | 1.6 | 3.5 | 0.4 | 2.2 | 0.0 | 2.5 |
| LAHA | Lagenophora harioti | Native | Forb | 0.3 | 2.1 | 0.3 | 0.0 | 0.8 | 0.0 | 3.5 | 4.0 | 0.7 | 0.0 | 4.7 |
| LAMA | Lathyrus magellanicus | Native | Forb | 0.0 | 0.0 | 0.0 | 2.1 | 0.3 | 0.0 | 2.4 | 1.1 | 2.2 | 0.0 | 3.3 |
| LAPH | Laureliopsis philippiana | Native | Tree | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.4 | 0.0 | 0.0 | 0.4 |
| LESC | Leptinella scariosa | Native | Forb | 0.5 | 0.5 | 0.0 | 0.0 | 0.8 | 4.8 | 6.7 | 1.1 | 1.4 | 0.0 | 2.5 |
| LETH | Leucheria thermarum | Native | Forb | 0.0 | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.4 | 0.0 | 0.0 | 0.4 |
| LEVU | Leucanthemum vulgare | Exotic | Forb | 0.0 | 0.5 | 0.0 | 0.0 | 0.0 | 3.5 | 4.0 | 0.0 | 0.7 | 0.0 | 0.7 |
| LOFE | Lomatia ferruginea | Native | Tree | 1.1 | 0.0 | 0.3 | 0.3 | 0.3 | 0.0 | 1.9 | 2.2 | 0.4 | 0.0 | 2.5 |
| LOPE | Lotus pedunculatus | Exotic | Forb | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.0 | 0.3 | 0.0 | 0.4 | 0.0 | 0.4 |
| LURA | Luzula racemosa | Native | Forb | 0.3 | 0.3 | 0.0 | 0.0 | 0.5 | 0.0 | 1.1 | 1.1 | 0.4 | 0.0 | 1.4 |
| LUPO | Lupinus polyphyllus | Exotic | Forb | 0.0 | 0.5 | 0.0 | 1.1 | 0.0 | 1.9 | 3.5 | 0.0 | 2.2 | 0.0 | 2.2 |
| MADI | Maytenus disticha | Native | Shrub | 0.5 | 10.2 | 0.5 | 5.3 | 0.0 | 0.0 | 16.6 | 15.2 | 6.9 | 0.4 | 22.5 |
| MAGR | Macrachaenium gracile | Native | Forb | 0.3 | 2.7 | 0.3 | 0.0 | 0.0 | 0.0 | 3.2 | 4.3 | 0.0 | 0.0 | 4.3 |
| MESA | Medicago sativa | Exotic | Forb | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 |
| MICO | Mitraria coccinea | Native | Liana | 0.3 | 0.0 | 0.0 | 0.0 | 1.6 | 0.0 | 1.9 | 1.8 | 0.7 | 0.0 | 2.5 |
| MUDE | Mutisia decurrens | Native | Liana | 0.0 | 0.0 | 0.0 | 0.5 | 0.0 | 0.0 | 0.5 | 0.4 | 0.4 | 0.0 | 0.7 |
| MUSP | Mulinum spinosum | Native | Shrub | 0.0 | 1.6 | 0.3 | 3.5 | 0.3 | 1.9 | 7.5 | 1.1 | 4.7 | 1.8 | 7.6 |
| MUSPI | Mutisia spinosa | Native | Liana | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| MYCH | Myrceugenia chrysocarpa | Native | Shrub | 0.3 | 0.3 | 0.0 | 0.3 | 0.0 | 0.0 | 0.8 | 0.0 | 1.1 | 0.0 | 1.1 |
| MYOP | Myoschilos oblonga | Native | Shrub | 0.0 | 0.5 | 0.3 | 0.8 | 0.0 | 0.0 | 1.6 | 1.1 | 0.7 | 0.4 | 2.2 |
| MYPL | Myrceugenia planipes | Native | Shrub | 0.5 | 0.0 | 0.0 | 0.0 | 0.5 | 0.0 | 1.1 | 1.1 | 0.4 | 0.0 | 1.4 |
| NEGR | Nertera granadensis | Native | Forb | 1.1 | 0.5 | 0.8 | 0.0 | 1.9 | 0.0 | 4.3 | 3.6 | 2.2 | 0.0 | 5.8 |
| NOAN | Nothofagus antarctica | Native | Tree | 0.0 | 0.0 | 0.0 | 13.9 | 0.0 | 0.3 | 14.2 | 6.5 | 11.2 | 1.1 | 18.8 |
| NOBE | Nothofagus betuloides | Native | Tree | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.0 | 0.3 | 0.4 | 0.0 | 0.0 | 0.4 |
| NODO | Nothofagus dombeyi | Native | Tree | 1.9 | 0.3 | 0.0 | 0.0 | 0.8 | 0.8 | 3.7 | 2.5 | 1.4 | 0.0 | 4.0 |
| NOPU | Nothofagus pumilio | Native | Tree | 0.0 | 18.2 | 0.5 | 1.1 | 0.0 | 0.8 | 20.6 | 21.7 | 4.3 | 0.7 | 26.8 |
| OLJU | Olsynium junceum | Native | Forb | 0.0 | 0.0 | 0.0 | 0.5 | 0.0 | 0.0 | 0.5 | 0.0 | 0.7 | 0.0 | 0.7 |
| OSCH | Osmorrhiza chilensis | Native | Forb | 1.6 | 20.6 | 1.1 | 9.1 | 1.1 | 0.5 | 34.0 | 32.2 | 12.3 | 0.7 | 45.3 |
| OSDE | Osmorhiza depauperata | Native | Forb | 0.0 | 2.1 | 0.0 | 0.0 | 0.0 | 0.3 | 2.4 | 2.9 | 0.0 | 0.0 | 2.9 |
| OVAN | Ovidia andina | Native | Shrub | 0.3 | 0.3 | 0.0 | 1.1 | 0.0 | 0.3 | 1.9 | 0.0 | 2.2 | 0.0 | 2.2 |
| OVPI | Ovidia pillopillo | Native | Shrub | 0.0 | 0.0 | 0.5 | 0.3 | 0.0 | 0.0 | 0.8 | 0.7 | 0.4 | 0.0 | 1.1 |
| OXEN | Oxalis enneaphylla | Native | Forb | 0.0 | 0.0 | 0.0 | 2.1 | 0.0 | 0.0 | 2.1 | 0.4 | 2.5 | 0.0 | 2.9 |
| PAVI | Parentucellia viscosa | Exotic | Forb | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.0 | 0.3 | 0.0 | 0.4 | 0.0 | 0.4 |
| PHAL | Phleum alpinum | Native | Graminoid | 0.0 | 0.5 | 0.0 | 2.4 | 0.0 | 2.1 | 5.1 | 1.1 | 2.2 | 0.7 | 4.0 |
| PHPR | Phleum pratense | Exotic | Graminoid | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| PHSE | Phacelia secunda | Native | Forb | 0.0 | 1.9 | 0.0 | 1.1 | 0.0 | 0.0 | 2.9 | 2.5 | 1.4 | 0.0 | 4.0 |
| PIPO | Pinus ponderosa | Exotic | Tree | 0.0 | 0.0 | 0.3 | 0.0 | 0.0 | 0.5 | 0.8 | 0.0 | 0.0 | 0.4 | 0.4 |
| PLLA | Plantago lanceolata | Exotic | Forb | 0.3 | 1.3 | 0.3 | 0.8 | 1.3 | 6.7 | 10.7 | 1.4 | 3.6 | 0.4 | 5.4 |
| PLMA | Plantago major | Exotic | Forb | 0.5 | 0.0 | 0.8 | 1.9 | 0.5 | 0.8 | 4.5 | 3.3 | 1.8 | 0.0 | 5.1 |
| POAL | Poa alopecurus | Native | Graminoid | 0.0 | 0.0 | 0.0 | 0.5 | 0.0 | 0.0 | 0.5 | 0.4 | 0.4 | 0.0 | 0.7 |
| POAN | Poa annua | Exotic | Graminoid | 0.0 | 0.0 | 0.0 | 1.1 | 0.0 | 0.3 | 1.3 | 0.7 | 0.7 | 0.0 | 1.4 |
| POAU | Polypogon australis | Native | Forb | 0.0 | 0.0 | 0.0 | 0.3 | 0.0 | 0.0 | 0.3 | 0.0 | 0.0 | 0.4 | 0.4 |
| POBU | Poa bulbosa | Exotic | Graminoid | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 |
| POPR | Poa pratensis | Exotic | Graminoid | 1.3 | 5.1 | 1.3 | 13.6 | 1.1 | 11.0 | 33.4 | 10.5 | 18.1 | 1.8 | 30.4 |
| POMU | Polystichum multifidum | Native | Fern | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.0 | 0.3 | 0.4 | 0.0 | 0.0 | 0.4 |
| PONU | Podocarpus nubigenus | Native | Tree | 0.3 | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.5 | 0.0 | 0.7 | 0.0 | 0.7 |
| PRMA | Protousnea magellanica | Native | Lichens | 0.5 | 5.9 | 0.0 | 2.1 | 0.3 | 0.8 | 9.6 | 6.9 | 5.1 | 0.0 | 12.0 |
| PRVU | Prunella vulgaris | Exotic | Fern | 1.1 | 0.5 | 0.5 | 4.3 | 1.6 | 1.3 | 9.4 | 5.8 | 5.1 | 0.0 | 10.9 |
| QUCH | Quinchamalium chilense | Native | Forb | 0.0 | 0.0 | 0.0 | 0.8 | 0.0 | 0.5 | 1.3 | 0.7 | 0.4 | 0.0 | 1.1 |
| RAMI | Ranunculus minutiflorus | Native | Forb | 0.5 | 0.0 | 0.0 | 0.5 | 0.0 | 0.0 | 1.1 | 1.1 | 0.4 | 0.0 | 1.4 |
| RALA | Raukaua laetevirens | Native | Shrub | 1.6 | 0.0 | 0.3 | 0.0 | 0.5 | 0.3 | 2.7 | 2.9 | 0.4 | 0.0 | 3.3 |
| RARE | Ranunculus repens | Exotic | Forb | 0.8 | 1.1 | 1.3 | 0.5 | 2.1 | 0.5 | 6.4 | 4.3 | 3.6 | 0.0 | 8.0 |
| RHSP | Rhaphithamnus spinosus | Native | Shrub | 0.5 | 0.0 | 0.0 | 0.0 | 0.8 | 0.0 | 1.3 | 1.1 | 0.7 | 0.0 | 1.8 |
| RIRU | Ribes rubrum | Exotic | Shrub | 0.0 | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.4 | 0.0 | 0.0 | 0.4 |
| RICU | Ribes cucullatum | Native | Shrub | 0.0 | 2.9 | 0.0 | 10.2 | 0.0 | 0.5 | 13.6 | 6.5 | 9.8 | 1.4 | 17.8 |
| RIMA | Ribes magellanicum | Native | Shrub | 0.5 | 4.8 | 0.8 | 1.9 | 0.5 | 0.0 | 8.6 | 8.7 | 2.2 | 0.7 | 11.6 |
| ROEG | Rosa eglanteria | Exotic | Shrub | 0.0 | 0.0 | 0.3 | 0.5 | 0.0 | 0.3 | 1.1 | 0.4 | 0.4 | 0.4 | 1.1 |
| RUAC | Rumex acetocella | Exotic | Forb | 0.0 | 4.3 | 0.8 | 15.2 | 0.8 | 10.7 | 31.8 | 9.1 | 18.1 | 1.4 | 28.6 |
| RUCR | Rumex crispus | Exotic | Forb | 0.0 | 0.0 | 0.0 | 0.8 | 0.5 | 2.1 | 3.5 | 0.4 | 1.1 | 0.4 | 1.8 |
| RUGE | Rubus geoides | Native | Forb | 0.8 | 1.6 | 1.3 | 1.1 | 1.3 | 1.9 | 8.0 | 6.2 | 2.2 | 0.0 | 8.3 |
| RURA | Rubus radicans | Native | Forb | 0.8 | 0.0 | 0.0 | 0.0 | 0.8 | 0.0 | 1.6 | 2.2 | 0.0 | 0.0 | 2.2 |
| SACO | Saxegothaea conspicua | Native | Tree | 0.5 | 0.0 | 0.0 | 0.0 | 0.5 | 0.0 | 1.1 | 1.1 | 0.4 | 0.0 | 1.4 |
| SAGR | Sanicula graveolens | Native | Forb | 0.0 | 0.0 | 0.0 | 1.1 | 0.0 | 0.0 | 1.1 | 0.0 | 1.4 | 0.0 | 1.4 |
| SARE | Sarmienta repens | Native | Liana | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.4 | 0.0 | 0.0 | 0.4 |
| SCAN | Schoenus andinus | Native | Forb | 0.0 | 0.5 | 0.0 | 0.0 | 0.0 | 0.5 | 1.1 | 0.7 | 0.0 | 0.0 | 0.7 |
| SCPA | Schinus patagonicus | Native | Shrub | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.5 | 0.8 | 0.0 | 0.0 | 0.4 | 0.4 |
| SEFI | Senecio filaginoides | Native | Shrub | 0.0 | 1.9 | 0.5 | 0.5 | 0.0 | 0.5 | 3.5 | 2.5 | 1.1 | 0.4 | 4.0 |
| SEPA | Senecio patagonico | Native | Shrub | 0.0 | 0.0 | 0.0 | 1.1 | 0.0 | 0.5 | 1.6 | 0.4 | 0.7 | 0.4 | 1.4 |
| STME | Stellaria media | Exotic | Forb | 0.0 | 0.5 | 0.0 | 0.5 | 0.0 | 0.0 | 1.1 | 0.4 | 0.7 | 0.4 | 1.4 |
| TAOF | Taraxacum officinale | Exotic | Forb | 2.7 | 12.6 | 0.8 | 26.2 | 1.3 | 16.8 | 60.4 | 25.0 | 31.2 | 2.9 | 59.1 |
| TRPR | Trifollium pratense | Exotic | Forb | 0.8 | 1.3 | 0.8 | 5.1 | 0.0 | 13.9 | 21.9 | 1.8 | 8.7 | 0.4 | 10.9 |
| TRRE | Trifolium repens | Exotic | Forb | 2.1 | 6.4 | 1.6 | 18.7 | 2.1 | 16.8 | 47.9 | 16.7 | 23.9 | 1.4 | 42.0 |
| UNTE | Uncinia tenuis | Native | Graminoid | 0.0 | 0.5 | 0.0 | 0.0 | 0.3 | 0.0 | 0.8 | 1.1 | 0.0 | 0.0 | 1.1 |
| URDI | Urtica dioica | Exotic | Forb | 0.0 | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.4 | 0.0 | 0.0 | 0.4 |
| VACA | Valeriana carnosa | Native | Forb | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 |
| VAFO | Valeriana fonckii | Native | Forb | 0.0 | 0.5 | 0.0 | 0.0 | 0.0 | 0.3 | 0.8 | 0.0 | 0.0 | 0.7 | 0.7 |
| VALA | Valeriana lapathifolia | Native | Forb | 0.0 | 1.3 | 0.0 | 0.0 | 0.0 | 0.0 | 1.3 | 1.8 | 0.0 | 0.0 | 1.8 |
| VEPE | Veronica peregrina | Exotic | Forb | 0.0 | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.4 | 0.0 | 0.0 | 0.4 |
| VESE | Veronica serpyllifolia | Exotic | Forb | 0.0 | 0.5 | 0.0 | 1.9 | 0.0 | 0.0 | 2.4 | 2.5 | 0.7 | 0.0 | 3.3 |
| VIBI | Vicia bijuga | Native | Forb | 0.0 | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.0 | 0.0 | 0.4 | 0.4 |
| VIHI | Vicia hirsuta | Exotic | Forb | 0.0 | 1.1 | 0.0 | 0.0 | 0.0 | 0.0 | 1.1 | 1.4 | 0.0 | 0.0 | 1.4 |
| VINI | Vicia nigricans | Native | Forb | 0.0 | 1.3 | 0.0 | 0.0 | 0.0 | 0.0 | 1.3 | 1.8 | 0.0 | 0.0 | 1.8 |
| VIRE | Viola reichei | Native | Forb | 0.0 | 11.8 | 0.5 | 2.9 | 0.0 | 0.3 | 15.5 | 15.6 | 4.7 | 0.4 | 20.7 |
References
- Phalan, B.; Onial, M.; Balmford, A.; Green, R.E. Reconciling food production and biodiversity conservation: Land sharing and land sparing compared. Science 2011, 333, 1289–1291. [Google Scholar] [CrossRef] [PubMed]
- Gamfeldt, L.; Snäll, T.; Bagchi, R.; Jonsson, M.; Gustafsson, L.; Kjellander, P.; Ruiz-Jaen, M.C.; Fröberg, M.; Stendahl, J.; Philipson, C.D. Higher levels of multiple ecosystem services are found in forests with more tree species. Nat. Comm. 2013, 4, e1340. [Google Scholar] [CrossRef] [PubMed]
- Tittonell, P.; Hara, S.; Bruzzone, O.; Álvarez, V.E.; Easdale, M.; Aramayo, V.; Enriquez, A.; Laborda, L.; Trinco, F.; Villagra, S.; et al. Ecosystem services and disservices associated with pastoral systems from Patagonia, Argentina: A review. Cah. Agric. 2021, 30, e43. [Google Scholar] [CrossRef]
- Gargaglione, V.; Gonzalez Polo, M.; Birgi, J.; Toledo, S.; Peri, P.L. Silvopastoral use of Nothofagus antarctica forests in Patagonia: Impact on soil microorganisms. Agrofor. Syst. 2022, 96, 957–968. [Google Scholar] [CrossRef]
- Varela, S.; Diez, J.P.; Gazotti, J.I.; Valiña, P.; Furlan, N.; Cardozo, A.; Cancino, A.; Fariña, C.; Castillo, D.; Umaña, F.; et al. Manejo de bosques con ganadería integrada en Patagonia argentina: Ajuste metodológico para la determinación de la línea de base en ecosistemas complejos y paisajes heterogéneos. Bosque 2023, 44, 255–261. [Google Scholar] [CrossRef]
- Gilliam, F.S. The ecological significance of the herbaceous layer in temperate forest ecosystems. Bioscience 2007, 57, 845–858. [Google Scholar] [CrossRef]
- Huertas Herrera, A.; Toro-Manríquez, M.D.R.; Villagrán, S.; Martínez Pastur, G.; Llobat, L.; Marín-García, P.J. A pivotal nutritional potential of understory vascular plants in Patagonian forests. Trees For. People 2024, 17, e100622. [Google Scholar] [CrossRef]
- Török, P.; Valkó, O.; Deák, B.; Kelemen, A.; Tóthmérész, B. Traditional cattle grazing in a mosaic alkali landscape: Effects on grassland biodiversity along a moisture gradient. PLoS ONE 2014, 9, e97095. [Google Scholar] [CrossRef]
- Török, P.; Hölzel, N.; van Diggelen, R.; Tischew, S. Grazing in European open landscapes: How to reconcile sustainable land management and biodiversity conservation? Agric. Ecosyst. Environ. 2016, 234, 1–4. [Google Scholar] [CrossRef]
- McKinney, M.L.; Lockwood, J.L. Biotic homogenization: A few winners replacing many losers in the next mass extinction. Trends Ecol. Evol. 1999, 14, 450–453. [Google Scholar] [CrossRef]
- Marconi, L.; Armengot, L. Complex agroforestry systems against biotic homogenization: The case of plants in the herbaceous stratum of cocoa production systems. Agric. Ecosyst. Environ. 2020, 287, e106664. [Google Scholar] [CrossRef]
- Öllerer, K.; Varga, A.; Kirby, K.; Demeter, L.; Biró, M.; Bölöni, J.; Molnár, Z. Beyond the obvious impact of domestic livestock grazing on temperate forest vegetation: A global review. Biol. Conserv. 2019, 237, 209–219. [Google Scholar] [CrossRef]
- Martínez Pastur, G.; Rosas, Y.M.; Chaves, J.; Cellini, J.M.; Barrera, M.D.; Favoretti, S.; Lencinas, M.V.; Peri, P.L. Changes in forest structure values along the natural cycle and different management strategies in Nothofagus antarctica forests. For. Ecol. Manag. 2021, 486, e118973. [Google Scholar] [CrossRef]
- Deng, J.; Fang, S.; Fang, X.; Jin, Y.; Kuang, Y.; Lin, F.; Liu, J.; Ma, J.; Nie, Y.; Ouyang, S.; et al. Forest understory vegetation study: Current status and future trends. For. Res. 2023, 3, e6. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Thakur, M.; Jia, Z.; Hong, Y.; Yang, W.; An, S.; Zhou, X. Light effects on seedling growth in simulated forest canopy gaps vary across species from different successional stages. Front. For. Glob. Change 2023, 5, e1088291. [Google Scholar] [CrossRef]
- Jin, P.; Xu, M.; Yang, Q.; Zhang, J. The influence of stand composition and season on canopy structure and understory light environment in different subtropical montane Pinus massoniana forests. PeerJ 2024, 12, e17067. [Google Scholar] [CrossRef]
- Martínez Pastur, G.; Rosas, Y.M.; Cellini, J.M.; Barrera, M.D.; Toro-Manríquez, M.; Huertas Herrera, A.; Favoretti, S.; Lencinas, M.V.; Peri, P.L. Conservation values of understory vascular plants in even- and uneven-aged Nothofagus antarctica forests. Biodiv. Conserv. 2020, 29, 3783–3805. [Google Scholar] [CrossRef]
- Rybar, J.; Bosela, M.; Marcis, P.; Ujházyová, M.; Polťák, D.; Hederová, L.; Ujházy, K. Effects of tree canopy on herbaceous understorey throughout the developmental cycle of a temperate mountain primary forest. For. Ecol. Manag. 2023, 546, e121353. [Google Scholar] [CrossRef]
- Vázquez, D.P. Multiple effects of introduced mammalian herbivores in a temperate forest. Biol. Invasions 2002, 4, 175–191. [Google Scholar] [CrossRef]
- Gilhaus, K.; Stelzner, F.; Hölzel, N. Cattle foraging habits shape vegetation patterns of alluvial year-round grazing systems. Plant Ecol. 2014, 215, 169–179. [Google Scholar] [CrossRef]
- Rhodes, A.C.; Larsen, R.T.; Clair, S. Differential effects of cattle, mule deer, and elk herbivory on aspen forest regeneration and recruitment. For. Ecol. Manag. 2018, 422, 273–280. [Google Scholar] [CrossRef]
- Salinas, J. Ganadería Integrada al Manejo de Los Bosques de Ñirre de Aysén; Documento de Divulgación 53; INFOR: Santiago, Chile, 2021. [Google Scholar]
- Gomez, F.A.; Tarabini, M.M.; La Manna, L.A.; von Muller, A. Effects of livestock on the quality of the riparian forest, soil and water in Nothofagus silvopastoral systems. Agrofor. Syst. 2024, 98, 2293–2308. [Google Scholar] [CrossRef]
- Quinteros, P.; Hansen, N.; Kutschker, A. Composición y diversidad del sotobosque de ñire (Nothofagus antarctica) en función de la estructura del bosque. Ecol. Austral 2010, 20, 225–234. Available online: https://ojs.ecologiaaustral.com.ar/index.php/Ecologia_Austral/article/view/1302 (accessed on 15 November 2024).
- Martínez Pastur, G.; Cellini, J.M.; Chaves, J.E.; Rodríguez-Souilla, J.; Benítez, J.; Rosas, Y.M.; Soler, R.; Lencinas, M.V.; Peri, P.L. Changes in forest structure modify understory and livestock occurrence along the natural cycle and different management strategies in Nothofagus antarctica forests. Agrofor. Syst. 2022, 96, 1039–1052. [Google Scholar] [CrossRef]
- Quinteros, C.P.; Bava, J.O.; López Bernal, P.M.; Gobbi, M.E.; Defossé, G.E. Competition effects of grazing-modified herbaceous vegetation on growth, survival and water relations of lenga (Nothofagus pumilio) seedlings in a temperate forest of Patagonia, Argentina. Agrofor. Syst. 2017, 91, 597–611. [Google Scholar] [CrossRef]
- Huertas Herrera, A.; Cellini, J.M.; Barrera, M.D.; Lencinas, M.V.; Martínez Pastur, G. Environment and anthropogenic impacts as main drivers of plant assemblages in forest mountain landscapes of Southern Patagonia. For. Ecol. Manag. 2018, 430, 380–393. [Google Scholar] [CrossRef]
- Vázquez, D.P.; Simberloff, D. Changes in interaction biodiversity induced by an introduced ungulate. Ecol. Lett. 2003, 6, 1077–1083. [Google Scholar] [CrossRef]
- de Paz, M.; Raffaele, E. Cattle change plant reproductive phenology, promoting community changes in a post-fire Nothofagus forest in northern Patagonia, Argentina. J. Plant Ecol. 2013, 6, 459–467. [Google Scholar] [CrossRef][Green Version]
- Velamazán, M.; Sánchez-Zapata, J.A.; Moral-Herrero, R.; Jacquemin, E.; Sáez-Tovar, J.; Barbosa, J. Contrasting effects of wild and domestic ungulates on fine-scale responses of vegetation to climate and herbivory. Land. Ecol. 2023, 38, 3463–3478. [Google Scholar] [CrossRef]
- Soler, R.; Martínez Pastur, G.; Lencinas, M.V.; Borrelli, L. Differential forage use between large native and domestic herbivores in Southern Patagonian Nothofagus forests. Agrofor. Syst. 2012, 85, 397–409. [Google Scholar] [CrossRef]
- Huertas Herrera, A.; Toro-Manríquez, M.; Salinas, J.; Rivas Guíñez, F.; Lencinas, M.V.; Martínez Pastur, G. Relationships among livestock. structure. and regeneration in Chilean Austral Macrozone temperate forests. Trees For. People 2023, 13, e100426. [Google Scholar] [CrossRef]
- AGRIMED. Atlas Agroclimático de Chile: Estado Actual y Tendencias del Clima; Tomo VI: Regiones de Aysén y Magallanes; Gobierno de Chile: Santiago, Chile, 2017. [Google Scholar]
- ESRI. ArcGIS Desktop: Release 10; Environmental Systems Research Institute Inc.: Redlands, CA, USA, 2011. [Google Scholar]
- CONAF. Catastro de Los Recursos Vegetacionales Nativos de Chile al Año 2020; Departamento de Monitoreo de Ecosistemas Forestales, CONAF: Santiago, Chile, 2021; 76p. [Google Scholar]
- Frazer, G.W.; Fournier, R.A.; Trofymow, J.A.; Hall, R.J. A comparison of digital and film fisheye photography for analysis of forest canopy structure and gap light transmission. Agric. For. Meteorol. 2001, 109, 249–263. [Google Scholar] [CrossRef]
- Levy, E.G.; Madden, E.A. The point method of pasture analyses. N. Z. J. Agric. Res. 1933, 46, 267–379. [Google Scholar]
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemist: New York, NY, USA, 2000. [Google Scholar]
- INRA. Alimentation des Ruminants; Éditions Quæ: Versailles, France, 2018; 728p. [Google Scholar] [CrossRef]
- Daget, P.; Poissonet, J. Un procédé d’estimation de la valeur pastorale des pâturages. Rev. Fourrages 1972, 49, 31–39. [Google Scholar]
- Lara, A.; Cruz, G. Evaluación del Potencial de Pastoreo del Área de Uso Agropecuario de la XII Región, Magallanes y de la Antártica Chilena; Universidad de Chile: Santiago, Chile, 1987. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Statist. Soft. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Coverdale, T.C.; Davies, A. Unravelling the relationship between plant diversity and vegetation structural complexity: A review and theoretical framework. J. Ecol. 2023, 111, 1378–1395. [Google Scholar] [CrossRef]
- O’Brien, M.J.; O’Hara, K.L.; Erbilgin, N.; Wood, D.L. Overstory and shrub effects on natural regeneration processes in native Pinus radiata stands. For. Ecol. Manag. 2007, 240, 178–185. [Google Scholar] [CrossRef]
- Warner, J.H.; Harper, K.T. Understory characteristics related to site quality for aspen in Utah. Brigham Young Univ. Sci. Bull. 1972, 16, 1–11. Available online: https://scholarsarchive.byu.edu/byuscib/vol16/iss2/1 (accessed on 15 November 2024).
- Veblen, T.; Veblen, A.; Schlegel, F. Understorey patterns in mixed evergreen-deciduous Nothofagus forests in Chile. J. Ecol. 1979, 67, 809–823. [Google Scholar] [CrossRef]
- Damascos, M.A.; Raport, E.H. Diferencias en la flora herbácea y arbustiva entre claros y áreas bajo dosel en un bosque de Nothofagus pumilio en Argentina. Rev. Chil. Hist. Nat. 2002, 75, 465–472. [Google Scholar] [CrossRef]
- Bílek, L.; Remeš, J.; Podrázský, V.; Rozenbergar, D.; Diaci, J.; Zahradník, D. Gap regeneration in near-natural European beech forest stands in Central Bohemia: The role of heterogeneity and micro-habitat factors. Dendrobiology 2014, 71, 59–71. [Google Scholar] [CrossRef]
- Frei, K.; E-Vojtkó, A.; Tölgyesi, C.; Vojtkó, A.; Farkas, T.; Erdős, L.; Li, G.; Lőrincz, A.; Bátori, Z. Topographic complexity drives trait composition as well as functional and phylogenetic diversity of understory plant communities in microrefugia: New insights for conservation. For. Ecosyst. 2025, 12, e100278. [Google Scholar] [CrossRef]
- Noualhaguet, M.; Work, T.; Nock, C.; Macdonald, S.E.; Aubin, I.; Fenton, N. Functional responses of understory plants to natural disturbance-based management in eastern and western Canada. Ecol. Appl. 2024, 34, e3011. [Google Scholar] [CrossRef] [PubMed]
- Salinas, J.; Acuña Aroca, B.; Hepp, K.; Little Cárdenas, C.; Moya Navarro, I.; Peri, P.L.; Sotomayor Garretón, A. Sistemas Silvopastorales una Alternativa de Manejo Sostenible Para Bosques de Ñirre (Nothofagus antarctica (G. Forst.) Oerst.) Región de Aysén; INFOR: Coyhaique, Chile, 2017. [Google Scholar] [CrossRef]
- Alonso, M.F.; Wentzel, H.; Schmidt, A.; Balocchi, O. Plant community shifts along tree canopy cover gradients in grazed Patagonian Nothofagus antarctica forests and grasslands. Agrofor. Syst. 2020, 94, 651–661. [Google Scholar] [CrossRef]
- Soler, R.; Martínez Pastur, G.; Lencinas, M.V.; Borrelli, L. Seasonal diet of Lama guanicoe (Camelidae: Artiodactyla) in a heterogeneous landscape of South Patagonia. Bosque 2013, 34, 129–141. [Google Scholar] [CrossRef]


| Variables | ÑI | LE | CO | MI | SV | OL | F(p) |
|---|---|---|---|---|---|---|---|
| Native species | 38.0 b | 58.4 c | 46.1 b | 42.2 b | 40.5 b | 21.6 a | 27.44 (<0.01) |
| Exotic species | 53.4 b | 16.4 a | 22.6 a | 34.4 ab | 33.2 ab | 68.6 c | 52.85 (<0.01) |
| Trees | 2.7 b | 6.7 c | 6.3 c | 2.1 b | 2.8 ab | 0.3 a | 12.38 (<0.01) |
| Shrubs | 15.3 c | 12.8 b | 13.4 b | 11.1 ab | 11.5 ab | 6.4 a | 5.98 (<0.01) |
| Forbs | 35.5 b | 34.7 b | 23.1 a | 28.8 ab | 28.1 ab | 31.9 b | 2.83 (0.02) |
| Graminoids | 30.1 b | 11.9 a | 10.7 a | 21.5 ab | 16.9 ab | 44.8 c | 37.20 (<0.01) |
| Ferns | 5.4 | 3.5 | 6.5 | 7.1 | 5.9 | 3.3 | 2.06 (0.07) |
| Lianas | 0.1 a | - | 1.0 b | - | 1.1 b | - | 14.08 (0.01) |
| Lichens | 0.4 b | 0.8 c | 0.1 a | - | 0.2 b | 0.1 a | 3.03 (0.01) |
| Bryophytes | 1.9 a | 4.4 b | 7.6 c | 6.0 bc | 7.2 c | 3.4 ab | 5.16 (0.01) |
| Total | 91.4 b | 74.8 a | 68.7 a | 76.6 a | 73.7 a | 90.2 a | 29.57 (<0.01) |
| Coarse-woody debris | 4.1 a | 21.0 c | 27.4 c | 17.4 bc | 14.0 b | 3.7 a | 34.05 (<0.01) |
| Bare soil | 4.5 a | 4.2 a | 3.9 a | 6.0 a | 12.3 b | 6.1 a | 2.80 (0.02) |
| Variables | Closed | Semi-Open | Open | OL | F(p) |
|---|---|---|---|---|---|
| Native species | 56.6 c | 36.2 b | 33.3 ab | 21.5 a | 51.70 (<0.01) |
| Exotic species | 20.4 a | 51.9 c | 39.6 b | 68.6 d | 77.84 (<0.01) |
| Trees | 6.1 c | 2.7 b | 1.2 a | 0.2 a | 18.63 (<0.01) |
| Shrubs | 15.1 c | 11.8 b | 11.8 b | 6.2 a | 10.45 (<0.01) |
| Forbs | 31.4 a | 37.0 b | 31.0 a | 31.1 a | 2.73 (0.04) |
| Graminoids | 12.6 a | 30.1 b | 27.6 b | 45.8 c | 61.33 (<0.01) |
| Ferns | 6.1 c | 3.3 b | 0.3 a | 3.3 b | 5.77 (0.01) |
| Lianas | 0.2 | 0.1 | - | - | 2.17 (0.09) |
| Lichens | 0.6 | 0.5 | - | 0.1 | 2.26 (0.08) |
| Bryophytes | 4.9 c | 2.6 b | 1.0 a | 3.4 b | 3.94 (0.01) |
| Total | 77.0 a | 88.1 b | 72.9 a | 90.1 b | 22.91 (<0.01) |
| Coarse-woody debris | 20.6 c | 5.9 b | 0.8 a | 3.7 b | 44.05 (<0.01) |
| Bare soil | 2.4 a | 6.0 b | 26.3 c | 6.2 b | 40.06 (<0.01) |
| Variable | Estimate | Standard Error | Z-Value | p-Value |
|---|---|---|---|---|
| Intercept | −11.79 | 0.142 | −8.32 | <0.001 |
| Environment types (LE) | −0.29 | 0.135 | −2.18 | 0.030 |
| Environment types (MI) | 0.22 | 0.192 | 1.13 | 0.259 |
| Environment types (OL) | 0.31 | 0.221 | 1.43 | 0.157 |
| Environment types (EV) | −0.06 | 0.198 | −0.29 | 0.766 |
| Environment types (ÑI) | 0.69 | 0.140 | 4.93 | <0.001 |
| Canopy cover (open) | 0.62 | 0.174 | 3.58 | <0.001 |
| Season (spring) | 1.27 | 0.083 | 15.21 | <0.001 |
| Season (summer) | 0.64 | 0.083 | 7.62 | <0.001 |
| Season (winter) | −0.16 | 0.083 | −1.96 | 0.050 |
| Forbs | 0.01 | 0.001 | 5.54 | <0.001 |
| Graminoid | 0.01 | 0.001 | 13.75 | <0.001 |
| Shrubs | −0.01 | 0.003 | −4.81 | <0.001 |
| Trees | −0.03 | 0.005 | −6.07 | <0.001 |
| Variables | Estimate | Standard Error | Z-Value | p-Value |
|---|---|---|---|---|
| Intercept | −26.28 | 0.094 | −28.07 | <0.001 |
| Environment types (LE) | −0.24 | 0.089 | −2.66 | 0.008 |
| Environment types (MI) | 0.21 | 0.127 | 1.62 | 0.105 |
| Environment types (OL) | 0.17 | 0.146 | 1.14 | 0.257 |
| Environment types (EV) | 0.05 | 0.131 | 0.41 | 0.681 |
| Environment types (ÑI) | 0.45 | 0.093 | 4.85 | <0.001 |
| Canopy cover (open) | 0.51 | 0.115 | 4.39 | <0.001 |
| Season (spring) | 0.89 | 0.055 | 16.13 | <0.001 |
| Season (summer) | 0.25 | 0.055 | 4.60 | <0.001 |
| Season (winter) | −0.17 | 0.055 | −3.06 | 0.002 |
| Forbs | 0.01 | 0.001 | 5.35 | <0.001 |
| Graminoid | 0.01 | 0.001 | 18.22 | <0.001 |
| Shrubs | −0.01 | 0.002 | −4.26 | <0.001 |
| Trees | −0.04 | 0.003 | −14.14 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brisard, T.; Brisard, A.; Toro-Manríquez, M.D.R.; Villagrán Chacón, S.; Marín-García, P.J.; Llobat, L.; Martínez Pastur, G.; Maluenda, S.M.; Huertas Herrera, A. Understory Forage Quality for Grazing Animals in Chilean Patagonian Forests. Land 2025, 14, 1081. https://doi.org/10.3390/land14051081
Brisard T, Brisard A, Toro-Manríquez MDR, Villagrán Chacón S, Marín-García PJ, Llobat L, Martínez Pastur G, Maluenda SM, Huertas Herrera A. Understory Forage Quality for Grazing Animals in Chilean Patagonian Forests. Land. 2025; 14(5):1081. https://doi.org/10.3390/land14051081
Chicago/Turabian StyleBrisard, Thomas, Amelie Brisard, Mónica D. R. Toro-Manríquez, Soraya Villagrán Chacón, Pablo Jesús Marín-García, Lola Llobat, Guillermo Martínez Pastur, Sabina Miguel Maluenda, and Alejandro Huertas Herrera. 2025. "Understory Forage Quality for Grazing Animals in Chilean Patagonian Forests" Land 14, no. 5: 1081. https://doi.org/10.3390/land14051081
APA StyleBrisard, T., Brisard, A., Toro-Manríquez, M. D. R., Villagrán Chacón, S., Marín-García, P. J., Llobat, L., Martínez Pastur, G., Maluenda, S. M., & Huertas Herrera, A. (2025). Understory Forage Quality for Grazing Animals in Chilean Patagonian Forests. Land, 14(5), 1081. https://doi.org/10.3390/land14051081









