Distribution and Habitat Suitability of the Malabar Slender Loris (Loris lydekkerianus malabaricus) in the Aralam Wildlife Sanctuary, India
Abstract
1. Introduction
2. Materials and Methods
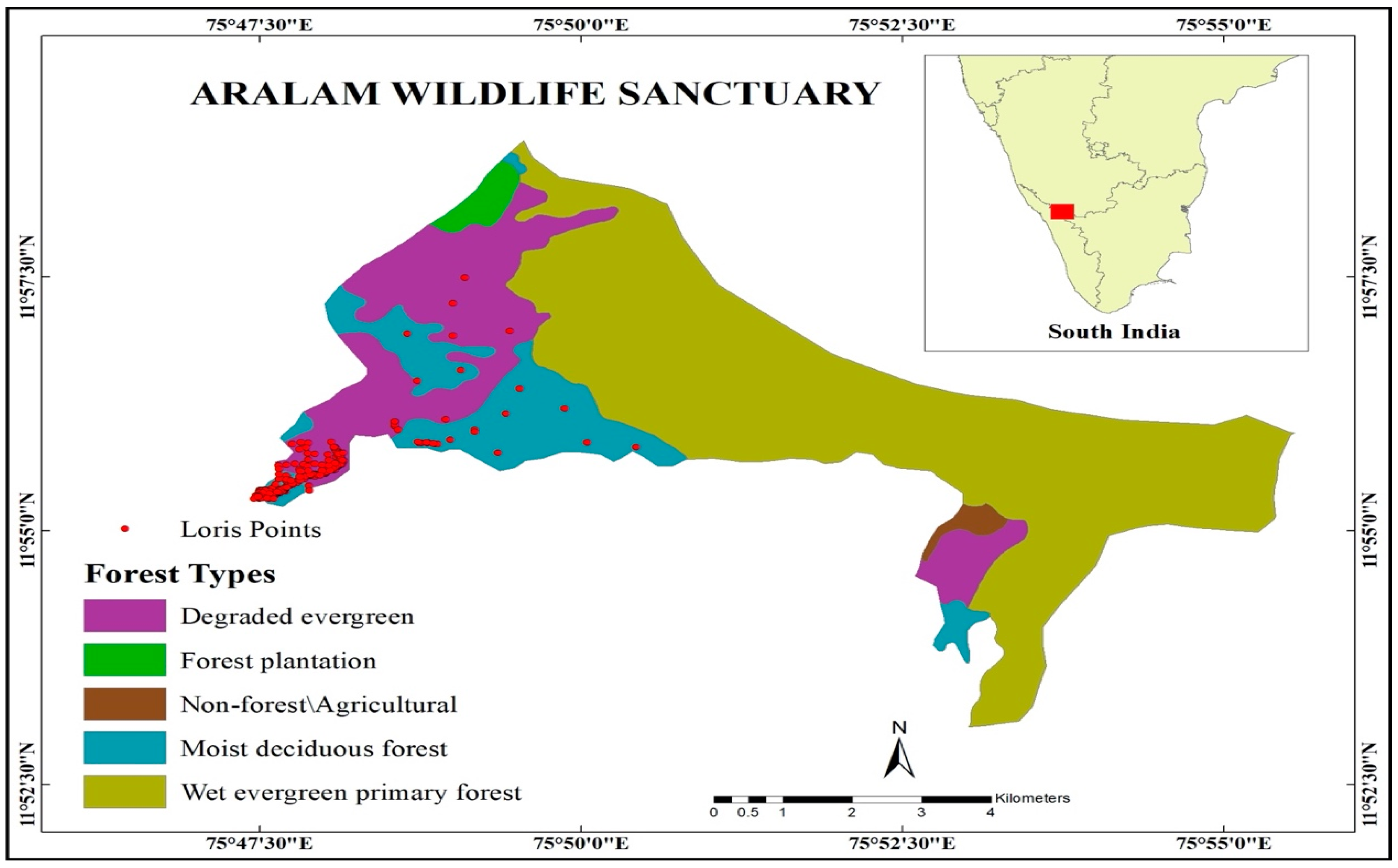
2.1. Study Site
2.2. Data Collection-Loris Surveys
2.3. Species Distribution Modeling
2.4. Downloading and Preparing Environmental Variable Layers
2.5. MaxEnt Modeling Analysis
2.6. Future Climatic Projections and Model Evaluations
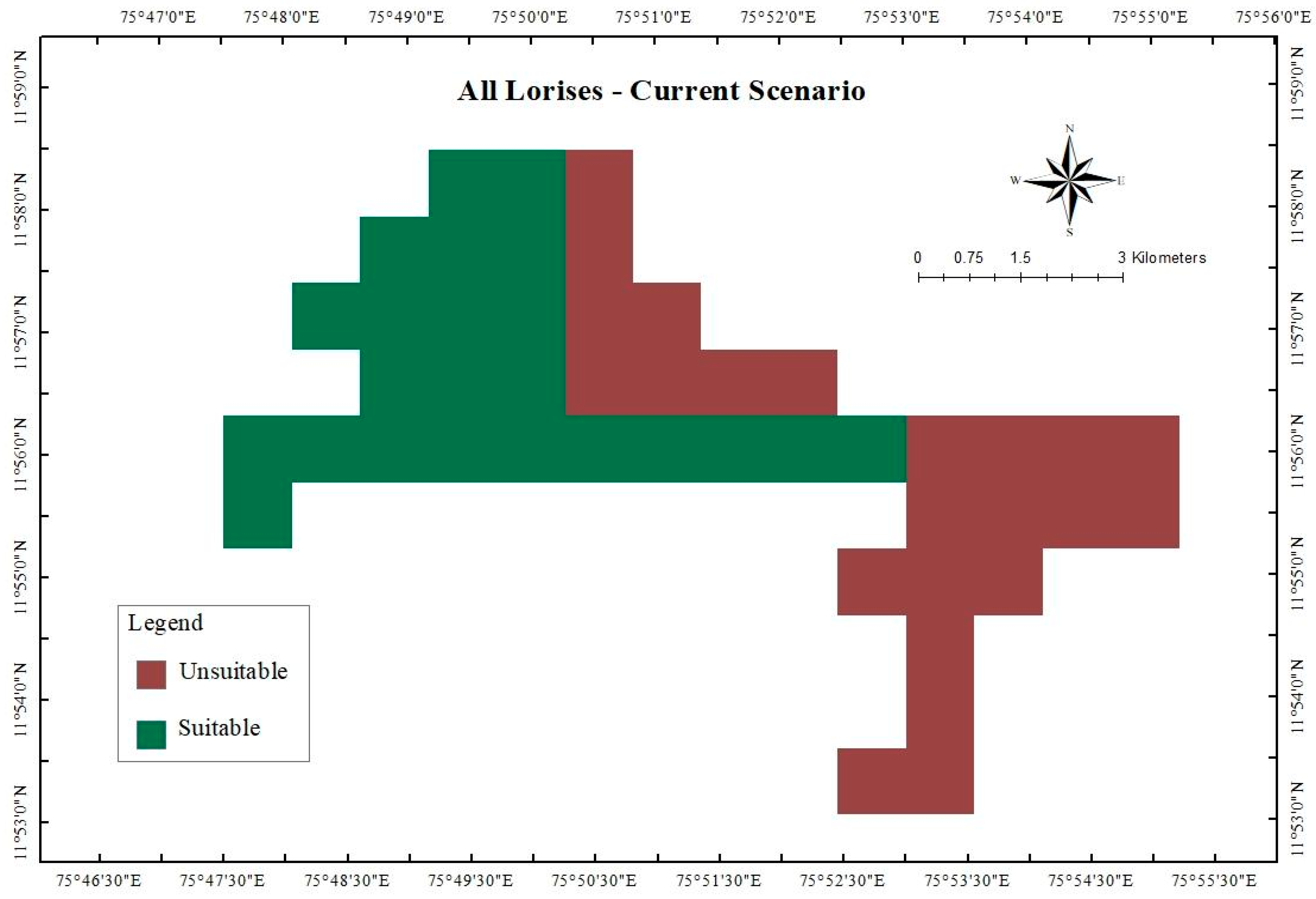
3. Results
3.1. Important Environmental Variables
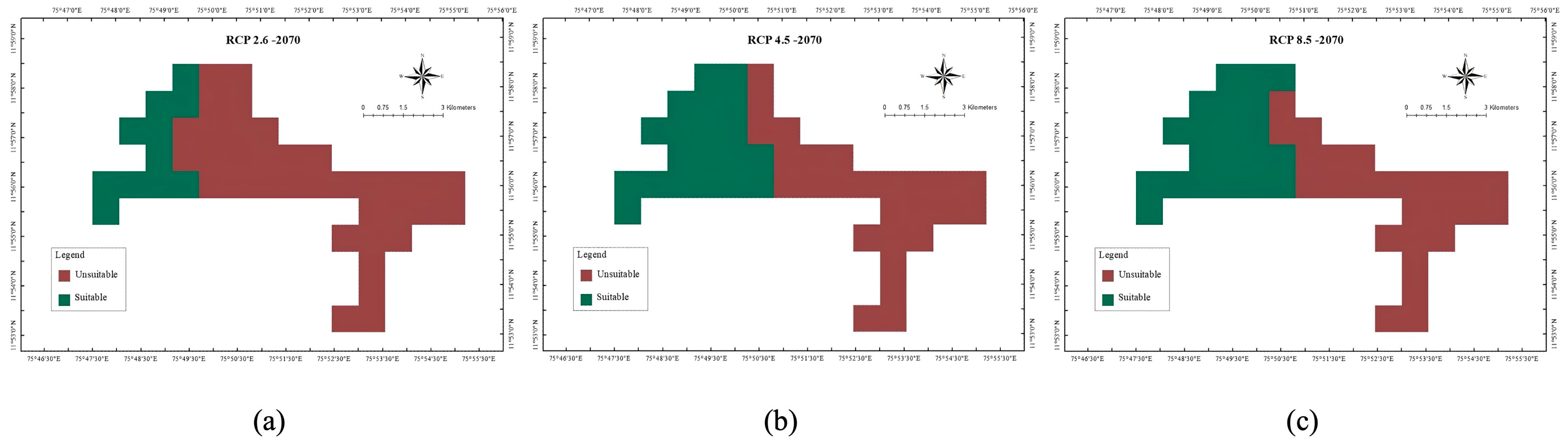
3.2. Projected Shifts in Habitat Suitability Under Future Climate Scenarios
4. Discussion
4.1. The Present Study and Key Findings
4.2. Habitat Preferences and Variations
4.3. Environmental Drivers and Anthropogenic Impacts
4.4. Projected Habitat Changes and Broader Implications
4.5. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC (Intergovernmental Panel on Climate Change). Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change Core Writing Team; Pachauri, R.K., Meyer, L.A., Eds.; Climate Change; IPCC: Geneva, Switzerland, 2014; 151p. [Google Scholar]
- Adhikari, D.; Barik, S.K.; Upadhaya, K. Habitat distribution modeling for reintroduction of Ilex khasiana Purk., a critically endangered tree species of northeastern India. Ecol. Eng. 2012, 40, 37–43. [Google Scholar] [CrossRef]
- Barnosky, A.D.; Matzke, N.; Tomiya, S.; Wogan, G.O.U.; Swartz, B.; Quental, T.B.; Marshall, C.; McGuire, J.L.; Lindsey, E.L.; Maguire, K.C.; et al. Has the Earth’s sixth mass extinction already arrived? Nature 2011, 471, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Worth, J.R.P.; Harrison, P.A.; Williamson, G.J.; Jordan, G.J. Whole range and regional-based ecological niche models predict differing exposure to 21st century climate change in the key cool temperate rainforest tree southern beech (N othofagus cunninghamii). Austral Ecol. 2015, 40, 126–138. [Google Scholar] [CrossRef]
- Waller, N.L.; Gynther, I.C.; Freeman, A.B.; Lavery, T.H.; Leung, L.K.-P. The Bramble Cay Melomys Melomys rubicola (Rodentia:Muridae): A first mammalian extinction caused by human-induced climate change? Wildl. Res. 2017, 44, 9–21. [Google Scholar] [CrossRef]
- Radchuk, V.; Reed, T.; Teplitsky, C.; van de Pol, M.; Charmantier, A.; Hassall, C.; Adamík, P.; Adriaensen, F.; Ahola, M.P.; Arcese, P.; et al. Adaptive responses of animals to climate change are most likely insufficient. Nat. Commun. 2019, 10, 3109. [Google Scholar] [CrossRef]
- Korstjens, A.H.; Hillyer, A. Primates and climate change: A review of current knowledge. In An Introduction to Primate Conservation; Oxford University: Oxford, UK, 2016. [Google Scholar]
- Gregory, S.D.; Brook, B.W.; Goossens, B.; Ancrenaz, M.; Alfred, R.; Ambu, L.N.; Fordham, D.A. Long-term field data and climate-habitat models show that orangutan persistence depends on effective forest management and greenhouse gas mitigation. PLoS ONE 2012, 7, e43846. [Google Scholar] [CrossRef]
- Tosi, A.J. Forest monkeys and Pleistocene refugia: A phylogeographic window onto the disjunct distribution of the Chlorocebus lhoesti species group. Zool. J. Linn. Soc. 2008, 154, 408–418. [Google Scholar] [CrossRef]
- Chandran, M.S.; Rao, G.R.; Gururaja, K.V.; Ramachandra, T.V. Ecology of the swampy relic forests of Kathalekan from central Western Ghats, India. Bioremediat. Biodivers. Bioavailab. 2010, 4, 54–68. [Google Scholar]
- Chetana, H.C.; Ganesh, T. Reconciling natural history and species ecology: Myristica beddomei (Myristicaceae) in the Western Ghats, India. Trop. Conserv. Sci. 2013, 6, 663–673. [Google Scholar] [CrossRef]
- Priti, H.; Aravind, N.A.; Uma Shaanker, R.U.; Ravikanth, G. Modeling impacts of future climate on the distribution of Myristicaceae species in the Western Ghats, India. Ecol. Engin. 2016, 89, 14–23. [Google Scholar] [CrossRef]
- Khanna, B.A. Reduction in Forest Area Has Led to Deficit Rainfall. Deccan Herald Newspaper, 19 August 2016; p. 6. [Google Scholar]
- Singh, M.; Singh, M.; Kumara, H.N.; Kumar, S.; Gnanaolivu, S.D.; Sasi, R. A review of research on the distribution, ecology, behaviour, and conservation of the Slender Loris Loris lydekkerianus (Mammalia: Primates: Lorisidae) in India. J. Threat. Taxa 2021, 13, 19540–19552. [Google Scholar] [CrossRef]
- Gnanaolivu, S.D.; Kumara, H.N.; Singh, M.; Sudarsanam, D. Ecological determinants of Malabar slender loris (Loris lydekkerianus malabaricus, Cabrera 1908) occupancy and abundance in Aralam Wildlife Sanctuary, Western Ghats, India. Int. J. Primatol. 2020, 41, 511–524. [Google Scholar] [CrossRef]
- Groves, C.P. Primate Taxonomy; Smithsonian Institution Press: Washington, DC, USA, 2001. [Google Scholar]
- Roonwal, M.L.; Mohnot, S.M. Primates of South Asia: Ecology, Sociobiology and Behavior; Harvard University Press: London, UK, 1977. [Google Scholar]
- Schulze, H.; Meier, B. The subspecies of Loris tardigradus and their conservation status: A review. In Creatures of the Dark: The Nocturnal Prosimians; Alterman, L., Doyle, G.A., Izard, M.K., Eds.; Plenum Press: New York, NY, USA, 1995; pp. 193–210. [Google Scholar]
- Kumara, H.N.; Nekaris, K.A.I.; Singh, M. Loris lydekkerianus ssp. malabaricus (amended version of 2020 assessment). IUCN Red List. Threat. Species 2022, e.T44720A217744540. [Google Scholar] [CrossRef]
- Gnanaolivu, S.D.; Campera, M.; Nekaris, K.A.I.; Nijman, V.; Satish, R.; Babu, S.; Singh, M. Medicine, black magic and supernatural beings: Cultural rituals as a significant threat to slender lorises in India. People Nat. 2022, 4, 734–746. [Google Scholar] [CrossRef]
- Nekaris, K.A.I. Social lives of adult Mysore slender lorises (Loris lydekkerianus lydekkerianus). Am. J. Primatol. 2006, 68, 1171–1182. [Google Scholar] [CrossRef]
- Sasi, R.; Kumara, H.N. Distribution and relative abundance of the slender loris Loris lydekkerianus in Southern Kerala, India. Prim. Conserv. 2014, 28, 165–170. [Google Scholar] [CrossRef]
- Gnanaolivu, S.D.; Singh, M.; Sudarsanam, D. Habitat structure and use of the Malabar slender loris (Loris lydekkerianus malabaricus, Cabrera 1908) in the Western Ghats, India. Department of Advanced Zoology and Biotechnology, Loyola College, University of Madras, Chennai, India, 2020, in preparation.
- Menon, A.R.R. Vegetation Mapping and Analysis of Aralam Wildlife Sanctuary Using Remote Sensing Techniques. KFRI Res. Rep. 1999, 168. Available online: https://docs.kfri.res.in/xxxxKFRI-RR/KFRI-RR168.pdf (accessed on 12 April 2025).
- Sterling, E.J.; Ramaroson, M.G. Rapid assessment of the primate fauna of the eastern slopes of the Réserve Naturelle Intégrale d’Andringitra, Madagascar. Fieldiana Zool. 1996, 85, 293–305. [Google Scholar]
- Charles-Dominique, P.; Bearder, S.K. Field studies of lorisoid behaviour: Methodological aspects. In The Study of Prosimian Behaviour; Doyle, G.A., Martin, R.D., Eds.; Academic Press: New York, NY, USA, 1979; pp. 567–629. [Google Scholar]
- Gamage, S.; Liyanage, W.; Weerakoon, D.; Gunwardena, A. Habitat quality and availability of the Sri Lanka red slender loris Loris tardigradus tardigradus (Mammalia: Primates: Lorisidae) in the Kottawa Arboretum. J. Threat. Taxa 2009, 1, 65–71. [Google Scholar] [CrossRef]
- Phillips, S.B.; Aneja, V.P.; Kang, D.; Arya, S.P. Modeling and analysis of the atmospheric nitrogen deposition in North Carolina. Int. J. Glob. Environ. Issues 2006, 6, 231–252. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M.; Schapire, R.E. A maximum entropy approach to species distribution modeling. In Proceedings of the 21st International Conference on Machine Learning, Banff, AB, Canada, 4–8 July 2004; p. 83. [Google Scholar] [CrossRef]
- Elith, J.; Graham, H.C.; Dudík, M.; Ferrier, S.; Guisan, A.; Ferrier, S.; Huettmann, F.R.; Leathwick, J.; Lehmann, A.; Li, J.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Hernandez, P.A.; Graham, C.H.; Master, L.L.; Albert, D.L. The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography 2006, 29, 773–785. [Google Scholar] [CrossRef]
- Jaynes, E.T. Information theory and statistical mechanics. Phys. Rev. 1957, 106, 620–630. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Steen, V.A.; Tingley, M.W.; Paton, P.W.C.; Elphick, C.S. Spatial thinning and class balancing: Key choices lead to variation in the performance of species distribution models with citizen science data. Methods Ecol. Evol. 2021, 12, 216–226. [Google Scholar] [CrossRef]
- Kass, J.M.; Vilela, B.; Aiello-Lammens, M.E.; Muscarella, R.; Merow, C.; Anderson, R.P. Wallace: A flexible platform for reproducible modeling of species niches and distributions built for community expansion. Methods Ecol. Evol. 2018, 9, 1151–1156. [Google Scholar] [CrossRef]
- Rowe, R.J.; Terry, R.C. Small mammal responses to environmental change: Integrating past and present dynamics. J. Mammal. 2014, 95, 1157–1174. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Esri. ArcGIS Desktop: Release 10.8; Environmental Systems Research Institute: Redlands, CA, USA, 2020. [Google Scholar]
- Fuller, G.; Raghanti, M.A.; Dennis, P.M.; Kuhar, C.W.; Willis, M.A.; Schook, M.W.; Lukas, K.E. A comparison of nocturnal primate behavior in exhibits illuminated with red and blue light. Appl. Anim. Behav. Sci. 2016, 184, 126–134. [Google Scholar] [CrossRef]
- Potapov, P.; Li, X.; Hernandez-Serna, A.; Tyukavina, A.; Hansen, M.C.; Kommareddy, A.; Pickens, A.; Turubanova, S.; Tang, H.; Silva, C.E.; et al. Mapping and monitoring global forest canopy height through integration of GEDI and Landsat data. Remote Sens. Environ. 2020, 253, 112165. [Google Scholar] [CrossRef]
- Gumma, M.K.; Mohammad, I.; Nedumaran, S.; Whitbread, A.; Lagerkvist, C.J. Urban sprawl and adverse impacts on agricultural land: A case study on Hyderabad, India. Remote Sens. 2017, 9, 1136. [Google Scholar] [CrossRef]
- De Bin, R.; Janitza, S.; Sauerbrei, W.; Boulesteix, A.L. Subsampling versus bootstrapping in resampling-based model selection for multivariable regression. Biometrics 2016, 72, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Veloz, S.D. Spatially auto correlated sampling falsely inflates measures of accuracy for presence-only niche models. J. Biogeogr. 2009, 36, 2290–2299. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. Environmental niche equivalency versus conservatism: Quantitative approaches to niche evolution. Evolution 2008, 62, 2868–2883. [Google Scholar] [CrossRef]
- Morales, N.S.; Fernández, I.C.; Baca-González, V. MaxEnt’s parameter configuration and small samples: Are we paying attention to recommendations? A systematic review. PeerJ 2017, 5, e3093. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef]
- Wordley, C.F.R.; Sankaran, M.; Mudappa, D.; Altringham, J.D. Landscape scale habitat suitability modeling of bats in the Western Ghats of India: Bats like something in their tea. Biol. Conserv. 2015, 191, 529–536. [Google Scholar] [CrossRef]
- Reineking, B. Constrain to perform: Regularization of habitat models. Ecol. Model. 2006, 193, 675–690. [Google Scholar] [CrossRef]
- Moss, R.H.; Edmonds, J.A.; Hibbard, K.A.; Manning, M.R.; Rose, S.K.; Van Vuuren, D.P.; Carter, T.R.; Emori, S.; Kainuma, M.; Kram, T.; et al. The next generation of scenarios for climate change research and assessment. Nature 2010, 463, 747–756. [Google Scholar] [CrossRef]
- Sharma, J.; Upgupta, S.; Jayaraman, M.; Chaturvedi, R.K.; Bala, G.; Ravindranath, N.H. Vulnerability of forests in India: A national scale assessment. Environ. Manag. 2017, 60, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.; Knutti, R.; Arblaster, J.; Dufresne, J.-L.; Fichefet, T.; Friedlingstein, P.; Gao, X.; Gutowski, W.J.; Johns, T.; Krinner, G.; et al. Long-term Climate Change: Projections, Commitments and Irreversibility. In Climate Change 2013—The Physical Science Basis: Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M.M.B., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK, 2013; pp. 1029–1136. [Google Scholar]
- Beaumont, L.J.; Hughes, L.; Poulsen, M. Predicting species distributions: Use of climatic parameters in BIOCLIM and its impact on predictions of species’ current and future distributions. Ecol. Model. 2007, 200, 347–359. [Google Scholar] [CrossRef]
- Kumar, S.; Stohlgren, T.J. MaxEnt modeling for predicting suitable habitat for threatened and endangered tree canopies of the US Great Plains. J. Ecol. Nat. Environ. 2009, 1, 94–98. [Google Scholar]
- Kumara, H.N.; Singh, M.; Kumar, S. Distribution, habitat correlates and conservation of slender loris (Loris lydekkerianus) in Karnataka, India. Int. J. Primatol. 2006, 27, 941–969. [Google Scholar] [CrossRef]
- Sukumar, R.; Suresh, H.S.; Ramesh, R. Climate change and its impact on tropical forest ecosystems in southern India. J. Biogeogr. 1995, 22, 533–544. [Google Scholar] [CrossRef]
- Sukumar, R.; Suresh, H.S.; Dattaraja, H.S. Monoculture plantations and their impact on biodiversity in the Western Ghats. Ambio 1998, 27, 579–585. [Google Scholar]
- Nekaris, K.A.I.; Stevens, N.J. All lorises are not slow: Rapid arboreal locomotion in the newly recognised red slender loris (Loris tardigradus tardigradus) of southwestern Sri Lanka. Am. J. Phys. Anthropol. 2005, 40, 156. [Google Scholar]
- Sherwin, H.A.; Montgomery, W.I.; Lundy, M.G. The impact and implications of climate change for bats. Mammal Rev. 2013, 43, 171–182. [Google Scholar] [CrossRef]
- Kalle, R.; Ramesh, T.; Qureshi, Q.; Sankar, K. Predicting the distribution pattern of small carnivores in response to environmental factors in the Western Ghats. PLoS ONE 2013, 8, e79295. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Mungi, N.A.; Kawamichi, T.; Rawat, G.S.; Adhikari, B.S.; Wilkening, J.L. Insights from present distribution of an alpine mammal Royle’s pika (Ochotona roylei) to predict future climate change impacts in the Himalaya. Reg. Environ. Change 2019, 19, 2423–2435. [Google Scholar] [CrossRef]
- Thibault, K.M.; Ernest, S.K.M.; White, E.P.; Brown, J.H.; Goheen, J.R. Long-term insights into the influence of precipitation on community dynamics in desert rodents. J. Mammal. 2010, 91, 787–797. [Google Scholar] [CrossRef]
- Goosem, M. Fragmentation impacts caused by roads through rainforests. Curr. Sci. 2007, 93, 1587–1595. [Google Scholar]
- Benítez-López, A.; Alkemade, R.; Verweij, P.A. The impacts of roads and other infrastructure on mammal and bird populations: A meta-analysis. Biol. Conserv. 2010, 143, 1307–1316. [Google Scholar] [CrossRef]
- Laurance, W.F.; Goosem, M.; Laurance, S.G. Impacts of roads and linear clearings on tropical forests. Trends Ecol. Evol. 2009, 24, 659–669. [Google Scholar] [CrossRef]
- Rich, C.; Longcore, T. Ecological Consequences of Artificial Night Lighting; Island Press: Washington, DC, USA, 2006. [Google Scholar]
- N’Goran, P.K.; Boesch, C.; Mundry, R.; N’Goran, E.K.; Herbinger, I.; Yapi, F.A.; Kuhl, H.S. Hunting, law enforcement, and African primate conservation. Conserv. Biol. 2012, 26, 565–571. [Google Scholar] [CrossRef]
- Rathore, A.; Bhatnagar, Y.V. Threats to wildlife in India: A review of illegal trade and poaching practices. Environ. Conserv. 2019, 46, 345–357. [Google Scholar]
- Williams, J.W.; Jackson, S.T.; Kutzbach, J.E. Projected distributions of novel and disappearing climates by 2100 AD. Proc. Nat. Acad. Sci. USA 2003, 104, 5738–5742. [Google Scholar] [CrossRef]
- Das, J.; De Silva, A. Conservation of Sri Lankan lorises: A review of conservation priorities. Prim. Conserv. 2005, 20, 67–72. [Google Scholar]
- Chavan, V.; Rane, S.; Patwardhan, A. Climate resilience strategies for the Western Ghats biodiversity hotspot. Environ. Res. Commun. 2020, 2, 045001. [Google Scholar]
- IPCC (Intergovernmental Panel on Climate Change). Climate Change 2021: The Physical Science Basis. In Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2021. [Google Scholar]
- Menon, V. Indian Mammals: A Field Guide; Hachette India: Delhi, India, 2020. [Google Scholar]

| Serial Number | Variables | Description |
|---|---|---|
| 1 | Bio1 | Annual mean temperature |
| 2 | Bio2 | Mean diurnal range (mean of monthly (max temp − min temp)) |
| 3 | Bio3 | Isothermality (BIO2/BIO7) (×100) |
| 4 | Bio4 | Temperature seasonality (standard deviation ×100) |
| 5 | Bio5 | Max temperature of warmest month |
| 6 | Bio6 | Min temperature of coldest month |
| 7 | Bio7 | Temperature annual range (BIO5-BIO6) |
| 8 | Bio8 | Mean temperature of wettest quarter |
| 9 | Bio9 | Mean temperature of driest quarter |
| 10 | Bio10 | Mean temperature of warmest quarter |
| 11 | Bio11 | Mean temperature of coldest quarter |
| 12 | Bio12 | Annual precipitation |
| 13 | Bio13 | Precipitation of wettest month |
| 14 | Bio14 | Precipitation of driest month |
| 15 | Bio15 | Precipitation seasonality (coefficient of variation) |
| 16 | Bio16 | Precipitation of wettest quarter |
| 17 | Bio17 | Precipitation of driest quarter |
| 18 | Bio18 | Precipitation of warmest quarter |
| 19 | Bio19 | Precipitation of coldest quarter |
| 20 | ASPECT | Derived continuous layer from DEM. Calculated as compass direction of the downslope direction using spatial analyst extension of ArcGIS 10.8 |
| 21 | SLOPE | Derived continuous layer from DEM. Calculated as degrees using spatial analyst extension of ArcGIS 10.8 |
| 22 | ELEVATION | Digital elevation model (DEM) generated from stereo images of Indian remote sensing satellite Cartosat-1 with 30 m resolution |
| 23 | ROAD | Distance from road; derived continuous layer created by calculating Euclidean distance from road using ArcGIS 10.8 |
| 24 | LANDUSE | Distance from croplands; derived continuous layer created by calculating Euclidean distance from road using ArcGIS 10.8 |
| 25 | TREECOVER | Layer showing the treecover of the different forest areas |
| 26 | LIGHT | Nocturnal light disturbance |
| 27 | VILLAGES | Distance from villages; derived continuous layer created by calculating Euclidean distance from villages using ArcGIS 10.8 |
| 28 | WATERBODIES | Distance from waterbodies; derived continuous layer created by calculating Euclidean distance from waterbodies using ArcGIS 10.8 |
| 29 | NDVI | Normalized Difference Vegetation Index, derived from remote sensing data, measuring vegetation greenness and density. It is calculated as (NIR − Red)/(NIR + Red), where NIR (near-infrared) and Red refer to spectral reflectance values. NDVI helps assess habitat quality and vegetation cover dynamics. |
| Serial Number | Variables |
|---|---|
| 1 | Road |
| 2 | Bio 18 |
| 3 | Bio 14 |
| 4 | Elevation |
| 5 | Aspect |
| 6 | Waterbodies |
| 7 | Landuse |
| 8 | Light |
| 9 | NDVI |
| 10 | Bio 7 |
| 11 | Slope |
| 12 | Treecover |
| 13 | Bio 15 |
| 14 | Villages |
| Variable | Description | Percent Contribution | Permutation Importance |
|---|---|---|---|
| Bio 18 | Precipitation of warmest quarter | 59.6 | 62.6 |
| Road | Distance from road | 29.4 | 37.4 |
| Bio 14 | Precipitation of driest month | 10.9 | 0 |
| Elevation | Digital elevation model (DEM) | 0 | 0 |
| Suitable Habitat (in km2) | ||||
|---|---|---|---|---|
| Habitat Suitability | Baseline | RCP 2.6 | RCP 4.5 | RCP 8.5 |
| Current Time (present) | 23 | |||
| 2070 | 11 (−52.17%) | 20 (−13.04%) | 21 (−8.69%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gnanaolivu, S.D.; Erinjery, J.J.; Campera, M.; Singh, M. Distribution and Habitat Suitability of the Malabar Slender Loris (Loris lydekkerianus malabaricus) in the Aralam Wildlife Sanctuary, India. Land 2025, 14, 872. https://doi.org/10.3390/land14040872
Gnanaolivu SD, Erinjery JJ, Campera M, Singh M. Distribution and Habitat Suitability of the Malabar Slender Loris (Loris lydekkerianus malabaricus) in the Aralam Wildlife Sanctuary, India. Land. 2025; 14(4):872. https://doi.org/10.3390/land14040872
Chicago/Turabian StyleGnanaolivu, Smitha D., Joseph J. Erinjery, Marco Campera, and Mewa Singh. 2025. "Distribution and Habitat Suitability of the Malabar Slender Loris (Loris lydekkerianus malabaricus) in the Aralam Wildlife Sanctuary, India" Land 14, no. 4: 872. https://doi.org/10.3390/land14040872
APA StyleGnanaolivu, S. D., Erinjery, J. J., Campera, M., & Singh, M. (2025). Distribution and Habitat Suitability of the Malabar Slender Loris (Loris lydekkerianus malabaricus) in the Aralam Wildlife Sanctuary, India. Land, 14(4), 872. https://doi.org/10.3390/land14040872







