Abstract
As an important indicator species for ecological environments, birds can effectively reflect the ecological quality of urban parks through their diversity characteristics. This study takes Xuzhou Quanshan Forest Park as an example to systematically investigate avian diversity and habitat variations by using the line transect and direct counting methods. A total of 120 bird species from 16 orders and 40 families were recorded, accounting for 24.89% of the total bird species in Jiangsu Province, 45.28% in Xuzhou City, and 79% in Quanshan District. The results showed that the Shannon-Wiener diversity index (H’) was highest in wetland habitats (H’ = 2.40), while the lowest was found in coniferous forest habitats (H’ = 1.09). Jaccard similarity coefficient analysis revealed the highest similarity of bird communities between broadleaf forests and mixed coniferous-broadleaf forests (Cj = 0.363), and the lowest similarity between wetlands and coniferous forests (Cj = 0.071). From a zoogeographical perspective, widespread species dominated across different habitats. Resident birds were the most abundant, and passerines constituted the highest proportion of all birds recorded. Based on these results, recommendations such as optimizing vegetation structures, expanding wetland areas, and reducing human disturbance are proposed to enhance avian diversity and promote sustainable development of urban ecosystems. This study provides scientific evidence for ecological planning and avian conservation in urban parks.
1. Introduction
In the context of rapid global urbanization, urban ecological environment quality has become a focal issue of public concern. Urban bird diversity serves as a significant indicator and representation of ecological quality changes. However, the protection and development of urban bird biodiversity face considerable challenges during urbanization. Problems such as habitat fragmentation, alterations in land-use patterns, and homogenization of community structure have significantly degraded habitat quality, consequently threatening bird survival and potentially leading to homogenization of urban bird diversity [1,2,3,4,5]. Urban parks, as the most vibrant and open ecological units within urban ecosystems, not only serve as ideal venues for residents’ recreation and leisure but also act as vital refuges for urban biodiversity. Their unique ecological functions are crucial for maintaining urban ecological balance and enhancing ecosystem services. Birds, an essential component of global biodiversity, play significant roles in both ecosystem functioning and the provision of ecological services [6,7,8,9]. Avian community diversity can reflect not only the intrinsic characteristics and ecological functions of bird communities themselves but also the environmental conditions in which they live [10,11]. The habitat preferences of birds directly influence their survival, fitness, and other behavioral responses; conversely, habitat quality directly affects the completion of birds’ life histories and the structure of their populations and communities [12,13]. Studies have shown that the composition and distribution of avian communities in urban parks are influenced by a range of complex factors. Among these, park size determines the extent of available habitats, vegetation diversity offers critical food and shelter for birds with varying dietary and habitat preferences, and levels of human disturbance directly or indirectly affect avian survival and reproduction [4,14,15,16]. Investigations into avian diversity in urban parks have been conducted extensively worldwide, revealing community composition, distribution, and relationships with environmental factors in numerous metropolitan areas. However, each city differs in its geographic setting, climate conditions, vegetation composition, and intensity of human activities. Consequently, the factors influencing avian diversity in different urban parks can vary.
Xuzhou Quanshan Forest Park is an important ecological asset within the city of Xuzhou, boasting unique natural geographical features and abundant vegetation resources. However, research specifically focusing on the park’s avian diversity and the relationships between bird communities and habitat types remains limited. This research gap hinders a more comprehensive and in-depth understanding of the park’s ecosystems and constrains the formulation of evidence-based ecological conservation and management strategies. In light of these considerations, this study focuses on the characteristics of avian biodiversity in Xuzhou Quanshan Forest Park and its relationship with habitat quality, aiming to fill the research gap in this field and provide a scientific basis for the ecological protection and management of the park, thus contributing to the sustainable development of the urban ecosystem in Xuzhou and the enhancement of its ecological resilience. The primary research questions are: (1) What are the species composition and diversity indices of birds in Quanshan Forest Park? (2) How do bird community structures differ among habitat types (e.g., lakes, wetlands, shrublands)? (3) Which habitat factors most critically affect avian diversity in this area? By addressing these questions, this study will provide important reference value and practical significance for the conservation planning of avian biodiversity in urban mountain forest ecosystems. At the same time, as a typical urban “mountain + forest” habitat system, the research results of Xuzhou Quanshan Forest Park also have important reference significance for similar ecosystems that are commonly found worldwide.
2. Materials and Methods
2.1. Study Area
Xuzhou, an important city in Jiangsu Province, is situated in the southeastern part of the North China Plain and the northwestern region of Jiangsu Province. The city serves as a key gateway in eastern China and lies along an essential migratory route for birds. It exhibits a typical temperate monsoon climate characterized by distinct seasonal variations. Historically reliant on coal mining, Xuzhou is now transitioning toward ecological restoration and sustainable urban development due to the gradual depletion of its coal resources. Ecologically, the surrounding landscape is diverse, including plains, mountains, and rivers, which provides significant ecological complexity and biodiversity. Such diverse ecosystems position Xuzhou Quanshan Forest Park as a representative study area within regional ecological research contexts (Figure 1), established on 1 January 1984. In recent years, it has been designated as a provincial environmental protection and science education base, as well as a provincial youth education base [17]. The total area of the reserve spans 370 ha, consisting of low mountain and hilly terrain formed by five peaks: Yunlong shan, Jinshan, Taishan, East Quanshan (the highest at an elevation of 235 m), and West Quanshan. East and West Quanshan are connected in the southern part (34°13′10″–34°11′30″ N, 117°08′21″–117°09′60″ E), forming a “U” shape when viewed from above. A natural channel known as the Longquan Stream lies between these two peaks. Because the other three peaks are over 1 km away and located in the suburban area, the core zone of the reserve—Quanshan Forest Park—has been established mainly as the area between East and West Quanshan. The core area covers 1.85 ha [18,19].
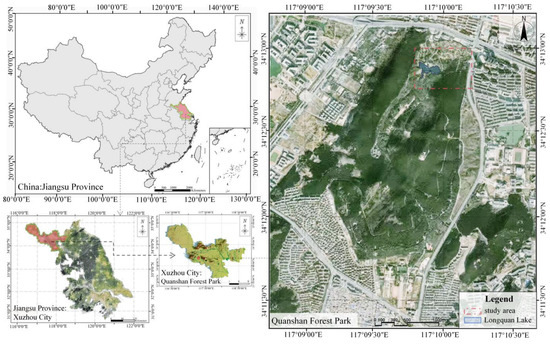
Figure 1.
Location of study area.
The park is situated in a warm temperate monsoon climate zone with an average annual temperature of 14.2 °C, a recorded extreme low of −22.6 °C, and an extreme high of 40.6 °C. Annual precipitation averages around 848.1 mm, with uneven seasonal distribution: spring accounts for 18.6%, summer for 58.6%, autumn for 18.9%, and winter for 5.7% of the total [17,19]. The vegetation is diverse: the region marks a transitional zone between northern temperate plant species extending southward and tropical plant species extending northward. Two main vegetation formations are found in the park: one is the Chinese arborvitae (Platycladus orientalis) forest located in the middle to upper slopes, with an understory of shrubs and herbaceous plants; the other is the black locust (Robinia pseudoacacia) forest in the middle to lower slopes, which also supports a shrub and herb layer.
In the lower slopes and open areas surrounding Longquan Stream, mixed forests contain Chinese arborvitae (Platycladus orientalis), black locust (Robinia pseudoacacia), Chinese juniper (Sabina chinensis), cedar (Cedrus deodara), dawn redwood (Metasequoia glyptostroboides), bamboo (Phyllostachys spp.), camphor tree (Cinnamomum camphora), ginkgo (Ginkgo biloba), glossy privet (Ligustrum lucidum), paper mulberry (Broussonetia papyrifera), white mulberry (Morus alba), Chinese tallow (Sapium sebiferum), and blue sandalwood (Pteroceltis tatarinowii Maxim), among others [18]. Soils in Quanshan Forest Park are primarily coarse-bone brown earth and leached brown earth [17], and these suitable soil and climate conditions support rich plant resources. According to statistics, the Quanshan area and its vicinity host 88 families, 267 genera, and 437 species (including subspecies and cultivated varieties) of vascular plants, among which Pteroceltis tatarinowii and wild soybean are listed as Class III nationally protected plants [20]. Overall, the nature reserve has abundant plant resources and exhibits marked plant diversity. Although Xuzhou cannot fully represent the ecological conditions of China as a whole, the ecological issues and characteristics it exhibits can, to some extent, reflect similar ecological challenges faced by cities located in the temperate monsoon climate zone of eastern China. Thus, studying Xuzhou can provide valuable references and insights for ecological research in other comparable urban areas. Moreover, as a significant part of Xuzhou’s ecological system, the ecological protection and investigation of Quanshan Forest Park hold great importance for ecological development in Xuzhou and other similar regions.
2.2. Research Methods
2.2.1. Community Investigation Methods
Considering the complex topography of Quanshan Forest Park and the diverse activity patterns of birds in varying spatial environments, this study focuses on the park’s bird communities, employing a combined approach of line transect and point count methods to comprehensively cover different habitat types within the study area. The goal is to fully characterize bird community structures and their associations with various habitats. Line transects were utilized in closed and semi-closed habitats, whereas point counts were applied in open and semi-open habitats. This methodological approach, guided by bird activity patterns in different spatial environments, ensured effective recording of avian data across habitat types. The methodological framework for this study is illustrated in Figure 2.

Figure 2.
Flowchart of bird community survey research methods.
2.2.2. Data Collection
(1) Survey Schedule: Bird observations were conducted weekly on Saturdays or Sundays from 07:00 to 11:00, corresponding to the peak period of bird activity and foraging, which enhances comprehensive observation of their behaviors and distributions. Observations were carried out in clear weather with minimal wind, with each selected site surveyed once weekly for a duration of 10–15 min per session. The data collection and analysis period spanned from October 2024 to January 2025, supplemented by publicly available data from the China Bird Watching Record Center http://www.birdreport.cn (accessed on 22 January 2025) collected between January 2022 and January 2025. A total of 16 field surveys were conducted over these four months, ensuring equal observation frequency across all sites.
(2) Line Transects: Two line transects were established, originating from the East Gate of Quanshan Forest Park (Niaoyuetang) and terminating at the Longquan Lake viewing platform. Transect 1 measured approximately 3.2 km, and transect 2 was approximately 3.5 km long, covering diverse habitat types, vegetation variations, levels of disturbance, and elevation gradients [12] (Figure 3). Observers walked at a consistent speed of 1–2 km/h along predetermined routes, carefully noting bird species and their quantities observed along both sides of the transects [21].
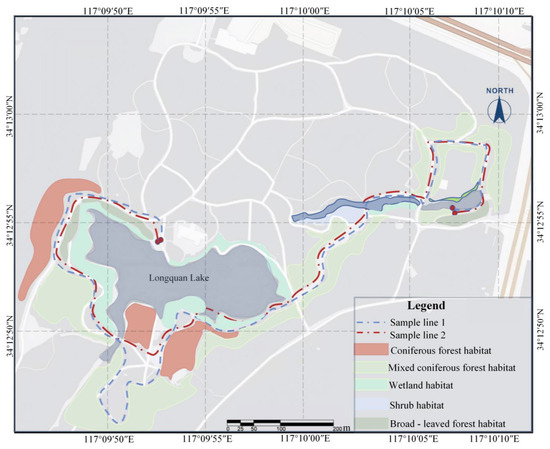
Figure 3.
Sample lines and habitat distribution of birds surveyed in Quan Shan Forest Park.
(3) Point Counts: For open and semi-open habitats, 2–3 fixed observation points were selected based on comprehensive considerations of terrain, vegetation coverage, and historical bird observation records, ensuring visual coverage of entire surveyed areas. To maintain sample independence and mitigate immediate anthropogenic disturbances affecting adjacent habitats, surveys were conducted at sites separated by at least 100 m. Researchers remained stationary upon arrival until the surrounding environment returned to natural conditions, minimizing bird disturbance [21,22]. Bird observations and records were made using telescopes (models: BOSMA Jinghong 20-60 × 80 ED monocular scope (Bosma Enterprises Co., Ltd., Guangzhou, China), Shengtu Senlinren 8 × 42 ED binoculars (Shengtu Optoelectronic Technology Co., Ltd., Shanghai, China)), and were supplemented with photographic evidence (habitat, species, and field activity photos). Movement tracks were recorded using GARMIN GPS (Fenix6 S) and Liangbulu software (V7.9.4).
(4) Unidentified Bird Species: Birds not identifiable in the field were documented with a DSLR camera (Sony R6700 with Sigma 150–600 mm lens, Sony Corporation, Tokyo, Japan; Sigma, Fukushima, Japan), including all visual and auditory occurrences. Species were subsequently identified through comparisons with authoritative bird guides. Researchers maintained fixed observers throughout the study period to control systematic errors [22].
(5) Bird Classification: Based on geographic distribution and ecological habits, avian fauna were classified into Palearctic, Oriental, and widespread species. According to their ecological habits and environments, birds were categorized into six ecological groups: waterfowl, waders, terrestrial birds, climbing birds, passerines, and raptors [23]. Birds were further classified based on seasonal migration and breeding status into resident birds, summer migrants, winter migrants, passage migrants, and vagrants [24]. Identification primarily relied on “A Field Guide to the Birds of China” [21,25] and “Atlas of Birds of China” [26,27]. Taxonomic references were based on the “A Checklist on the Classification and Distribution of the Birds of China (Second)” [24].
2.2.3. Habitat Type Classification
The term “habitat” was first proposed by Grinnell (1917) in the United States, referring to the spatial environment in which individuals, populations, or communities complete their life processes [28]. Habitat type denotes the spatial categories of habitat conditions required by individuals, populations, or communities for distribution, settlement, and succession under different ecological factors [29,30]. Based on the current land use conditions in Quanshan Forest Park and the arrangement of transects, six habitat types were identified for avian surveys: (a) lake habitat, (b) wetland habitat, (c) shrubland habitat, (d) broadleaf forest habitat, (e) coniferous forest habitat, and (f) mixed coniferous-broadleaf forest habitat (Figure 4).
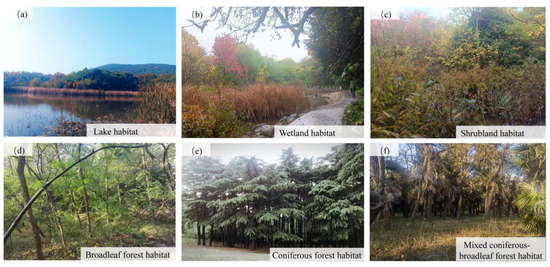
Figure 4.
Habitat types. (a) Lake Habitat: This habitat represents lacustrine ecosystems that serve as resting and breeding grounds for waterfowl, supporting a diverse assemblage of waders and water birds. (b) Wetland Habitat: This habitat illustrates wetland vegetation landscapes, providing vital support for a multitude of waterbirds and wetland-dependent avian species. (c) Shrubland Habitat: Composed of various shrub species, this habitat offers an excellent concealment environment for forest birds and small passerines. (d) Broadleaf Forest Habitat: Dominated by broadleaf trees, this habitat supplies typ-ical forest birds with essential resources for foraging, shelter, and nesting. (e) Coniferous Forest Habitat: This habitat demonstrates coniferous ecosystems where conifer seeds provide food resources for birds and the dense canopy affords necessary protection. (f) Mixed Coniferous–Broadleaf Forest Habitat: Featuring the coexistence of coniferous and broadleaf trees, this habitat meets the diverse roosting and foraging requirements of various forest bird species.
2.2.4. Data Collection and Analysis
- Bird species enumeration.A comprehensive list of bird species was compiled for Quanshan Forest Park. Data were gathered from multiple field surveys conducted between January 2022 and November 2024, supplemented by publicly available records from the China Birdwatching Record Center and the relevant published literature and reports.
- Dominance analysis.Based on the proportion PPP of each bird species in the total observed population, dominance levels were classified as follows: species with P ≥ 10% are considered dominant, those with 1% ≤ P < 10% common, those with 0.1% ≤ P < 1% rare, and those with P < 0.1% very rare [12,13,31].
- Diversity indices.Four diversity indices were used to assess bird species diversity: the Shannon-Wiener index (H′), Pielou’s evenness index (J′), Simpson’s index (D), and Margalef’s index (M). We employed Biodiversity Pro for the calculations. In these formulas, S represents the total number of species, Pi the proportion of individuals of species i in the total sample, Hmax the maximum value of diversity, and N the total number of individuals.
- Community similarity.The Jaccard similarity coefficient was used to assess similarities in bird communities among different habitats, employing the equation: Cj = j/(a + b − j). where a and b are the numbers of species in communities A and B, respectively, and j is the number of species shared by both communities. A higher Cj indicates greater similarity. Bird species were classified according to The Checklist of the Classification and Distribution of the Birds of China (3rd Edition) [12,24]. All statistical analyses and figure preparation were conducted using Biodiversity Pro 2.0, Origin Pro 8, and Microsoft Office Excel 2024.
3. Results and Analysis
3.1. Bird Species Enumeration
From January 2022 to November 2024, we conducted avian diversity surveys in Xuzhou Quanshan Forest Park. A total of 120 bird species were identified, belonging to 16 orders and 40 families (Appendix A). These species represent 24.89% of the 482 bird species recorded in Jiangsu Province (covering 76% of bird families and 50.6% of orders in the province), 45.28% of the 265 species recorded in Xuzhou City (covering 88% of its families and 67.78% of its orders), and 79% of the 151 bird species recorded in Quanshan District (covering 87% of its families and 94% of its orders).
3.2. Rare, Protected, and Endemic Species
Among the 120 recorded species, one is listed as a First-Class National Protected Species: the Oriental Stork (Ciconia boyciana). Twenty-seven species fall under Second-Class National Protection, including the Tundra Swan (Cygnus columbianus), Common Merganser (Mergus merganser), Lesser Cuckoo (Cuculus poliocephalus), Little Egret (Egretta garzetta), Collared Scops Owl (Otus sunia), Black-winged Kite (Elanus caeruleus), Crested Honey Buzzard (Pernis ptilorhynchus), Crested Goshawk (Accipiter trivirgatus), Chinese Sparrowhawk (Accipiter soloensis), Japanese Sparrowhawk (Accipiter gularis), Besra (Accipiter virgatus), Eurasian Sparrowhawk (Accipiter nisus), Northern Goshawk (Accipiter gentilis), Grey-faced Buzzard (Butastur indicus), Eastern Buzzard (Buteo japonicus), Eurasian Hoopoe (Upupa epops), Dollarbird (Eurystomus orientalis), Common Kingfisher (Alcedo atthis), Grey-headed Woodpecker (Picus canus), Great Spotted Woodpecker (Dendrocopos major), Common Kestrel (Falco tinnunculus), Amur Falcon (Falco amurensis), Eurasian Hobby (Falco subbuteo), Black-naped Oriole (Oriolus chinensis), Ashy Minivet (Pericrocotus divaricatus), and Chinese Hwamei (Garrulax canorus). Two species, the Large Hawk-Cuckoo (Hierococcyx sparverioides) and the Grey-capped Pygmy Woodpecker (Picoides canicapillus), are listed as First-Class Protected Species at the provincial level (Figure 5).

Figure 5.
Rare, protected, and endemic Species. (a) Cygnus columbianus (Tundra Swan) reflects the excellent and diverse ecological quality of the park, as it appears during migration. (b) Buteo japonicus (Eastern Buzzard), a nationally protected species, underscores the integrity of the forest ecosystem. (c) Upupa epops (Common Hoopoe), a characteristic bird of the park, enriches the biodiversity of the forest–grassland ecotone. (d) Alcedo atthis (Common Kingfisher), an indicator species of aquatic ecosystems, signifies a healthy water environment in the park. (e) Garrulax canorus (Hwamei), a protected species, indicates high vegetation health within the mountainous forest areas. (f) Picoides canicapillus (Grey-capped Pygmy Woodpecker), a beneficial forest bird, contributes to maintaining ecological balance in the park’s forest ecosystems.
3.3. Community Composition, Residency Status, and Zoogeographical Characteristics
Passeriformes, with 74 species across 23 families, comprises the largest group (61.6% of the total), followed by Accipitriformes, with 10 species in one family. The least represented orders include Ciconiiformes, Suliformes, Strigiformes, and Bucerotiformes, each represented by one family and one species (Figure 6).
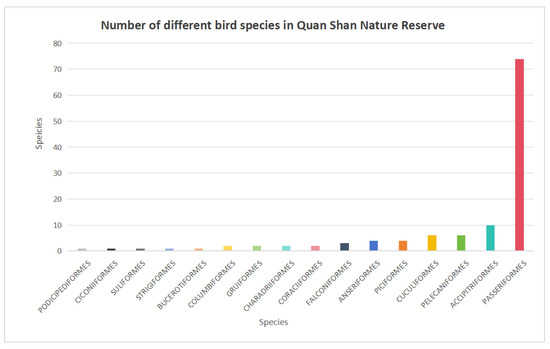
Figure 6.
Number of bird species of different orders in Quanshan Forest Park.
Using the zoogeographic classifications proposed by Zheng Zuoxin, we analyzed 120 breeding bird species in Quanshan Forest Park. Oriental Realm species (62) and Palearctic species (57) dominated, accounting for 51.6% and 47.5% of total breeding species, respectively. Only one species (0.83%) belongs to the Nearctic Realm. Some species have overlapping distributions, all of which have been included in these statistics.
Regarding residency status, winter visitors numbered 32 (17.4%), passage migrants 27 (14.6%), resident birds 74 (40.3%), and summer visitors 51 (27.7%). Overall, this survey establishes a foundational understanding of the avian resources in Quanshan Forest Park, offering valuable data for further conservation and management.
3.4. Dominance Analysis of Bird Species
Based on abundance, species were categorized as dominant, common, or rare [12]. Three species (2.5%) were identified as dominant: the Oriental Turtle Dove (Streptopelia orientalis), Azure-winged Magpie (Cyanopica cyanus), and Masked Laughingthrush (Pterorhinus perspicillatus) (Figure 7). Thirty-two species (26.6%), including the Yellow-bellied Tit (Pardaliparus venustulus), Light-vented Bulbul (Pycnonotus sinensis), Pallas’s Leaf Warbler (Phylloscopus inornatus), Chinese Blackbird (Turdus mandarinus), and Red-flanked Bluetail (Tarsiger cyanurus), were classified as common. Eighty-five species (70.8%) were rare, such as the Hawfinch (Coccothraustes coccothraustes), Goldcrest (Regulus regulus), Rufous-tailed Robin (Larvivora sibilans), Silky Starling (Spodiopsar sericeus), and Carrion Crow (Corvus corone).

Figure 7.
Dominant species of birds in Quan Shan Forest Park. (a) Streptopelia orientalis (Oriental Collared Dove) is widely distributed throughout the forested areas, frequently seen flitting among tree branches. (b) Cyanopica cyanus (Azure-winged Magpie) exhibits highly gregarious behavior and remarkable adaptability. (c) Pterorhinus perspicillatus (Masked Laughingthrush) shows a preference for park areas with abundant vegetation and high canopy cover.
3.5. Avian Diversity and Similarity Across Different Habitats
Bird community diversity is closely related to the specific environments birds inhabit [22]. Analysis of diversity indices across different habitat types (Table 1) showed that wetlands had the highest Shannon-Wiener index (H′ = 2.40), followed by mixed coniferous-broadleaf forests (H′ = 2.10). Coniferous forests had the lowest value (H′ = 1.09). Pielou’s evenness (J′) was highest in coniferous forests (J′ = 0.88), followed by broadleaf forests (J′ = 0.78), and lowest in shrublands (J′ = 0.59). Coniferous forests also had the highest Simpson index (D = 0.33), followed by shrublands (D = 0.31), whereas wetlands had the lowest (D = 0.14). Wetlands exhibited the highest Margalef index (M = 3.70), followed by broadleaf forests (M = 3.26), and coniferous forests had the lowest (M = 1.82).

Table 1.
Bird diversity in different habitats of Quanshan Forest Park.
Jaccard’s similarity coefficients (Table 2) were used to measure the similarity of bird communities among different habitat types. The highest similarities were between broadleaf forest and mixed coniferous-broadleaf forest (Cj = 0.363), and between the coniferous forest and lake habitats (Cj = 0.250). This was followed by coniferous and broadleaf forest (Cj = 0.200). The lowest similarity (Cj = 0.071) was observed between wetlands and coniferous forests.

Table 2.
Similarity of bird communities among different habitats in Quanshan Forest Park.
3.6. Habitat-Based Bird Community Composition
From a zoogeographical perspective, widespread species predominated in all habitat types, followed by Palearctic species. Notably, the proportion of Palearctic species in shrubland habitats reached 46%. The proportion of Oriental species was highest (67%) in coniferous forests. In coniferous forests, wetlands, and lake habitats, Oriental species all accounted for roughly 33%. Additionally, 45% of species in broadleaf forests were Palearctic, while Oriental and widespread species both contributed about 26% (Figure 8a).
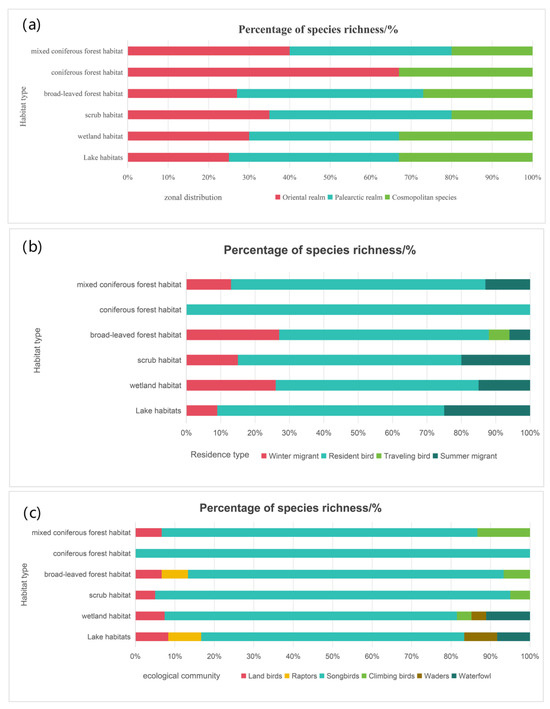
Figure 8.
Characteristics of birds in different habitats in Quan shan Nature Reserve. (a) Map of zonal distribution analysis. (b) Map of ecological community analysis. (c) Map of residence type analysis.
In terms of residency status, resident birds overwhelmingly dominated all habitats (over 60% in each). Summer and winter visitors were also recorded in each habitat type, ranging from 10% to 30%. Moreover, broadleaf forests had a small proportion (approximately 6%) of passage migrants (Figure 8b).
Regarding ecological groups, songbirds made up the largest proportion in all habitat types, exceeding 70% in wetlands, shrublands, coniferous forests, broadleaf forests, and mixed coniferous-broadleaf forests. Climbing birds were found in wetlands, shrublands, and mixed coniferous-broadleaf forests. Raptors appeared only in lake habitats and broadleaf forests. Waders and waterfowl, although fewer in species, were primarily observed in lake and wetland habitats (Figure 8c).
4. Discussion
4.1. Bird Dominance
Based on bird abundance, dominance analysis indicates that among the 120 species recorded, 3 species were dominant, accounting for 2.5% of all recorded species, namely, the Oriental Turtle Dove (Streptopelia orientalis), Azure-winged Magpie (Cyanopica cyanus), and Masked Laughingthrush (Pterorhinus perspicillatus)—all found in six habitat types. Thirty-two species, such as the Yellow-bellied Tit (Pardaliparus venustulus), Light-vented Bulbul (Pycnonotus sinensis), Pallas’s Leaf Warbler (Phylloscopus inornatus), Chinese Blackbird (Turdus mandarinus), and Red-flanked Bluetail (Tarsiger cyanurus), were classified as common (26.6%). The remaining 85 species were rare (70.8%), including the Hawfinch (Coccothraustes coccothraustes), Goldcrest (Regulus regulus), Rufous-tailed Robin (Larvivora sibilans), Silky Starling (Spodiopsar sericeus), and Carrion Crow (Corvus corone). Each of these rare species had fewer than ten individuals recorded, distributed in only one or two habitat types (Table 3). These results suggest a relationship between abundance rank and habitat preference. The breadth of habitat usage indirectly reflects a species’ niche width, providing evidence that species with wider niches generally have broader food sources [12].

Table 3.
Distribution characteristics of birds with different dominance degrees in multiple habitats.
4.2. Avian Diversity and Similarity in Different Habitats
In this survey of Xuzhou Quanshan Forest Park, wetland habitats had the highest Shannon-Wiener diversity index (H′ = 2.40), followed by mixed coniferous-broadleaf forests (H′ = 2.10). Wetlands feature diverse plant communities of varying heights and forms, offering birds abundant foraging opportunities, shelter, and nesting sites. Additionally, wetlands support numerous aquatic organisms, ensuring ample food supply for birds. For migratory birds, wetlands serve as critical stopover sites during long-distance flights. Mixed coniferous-broadleaf forests, meanwhile, represent a transitional vegetation type with a complex vertical structure. This stratification provides varied microhabitats for birds of different sizes and habits. Furthermore, the rich flora harbors numerous insects, supplying birds with both plant-based and animal-based food sources. In contrast, other habitat types exhibited relatively low diversity due to their monocultural characteristics and higher levels of disturbance.
Pielou’s evenness (J′) was highest in broadleaf forests (J′ = 0.78), followed by mixed coniferous-broadleaf forests (J′ = 0.77), and lowest in shrublands (J′ = 0.59). Evenness reflects how evenly individuals are distributed among species in a community, which isan important indicator of biodiversity. Only five species were recorded in coniferous forests, each with one to three individuals, resulting in fewer conspicuous population differences and thus a higher evenness. By contrast, 27 species were recorded in wetlands, with populations ranging from 1 to 347 individuals—a significant gradient that lowered the evenness index for wetlands.
Coniferous forests recorded the highest Simpson diversity index (D = 0.33), whereas wetlands had the lowest (D = 0.14), broadly mirroring the patterns in Shannon-Wiener diversity. The Margalef index, which measures species richness relative to total individuals, was highest in wetlands, followed by broadleaf forests, and lowest in coniferous forests. This metric aligns with fluctuations in total bird abundance across habitats. Differences in ecological factors—such as vegetation cover and height, plant diversity, water resources, and disturbance intensity—lead to variations in niche selection by birds and ultimately shape distinct avian community compositions.
Jaccard similarity coefficients further clarify habitat-based community patterns. Broadleaf forests and mixed coniferous-broadleaf forests were most similar (Cj = 0.363) due to comparable vegetation structures, each featuring multiple tree species that provide similar foraging and nesting resources. Coniferous forests and lake habitats showed the next highest similarity (Cj = 0.250), likely because the proximity of coniferous stands to water edges creates overlapping zones that accommodate certain waterbirds and forest birds. By contrast, wetlands and coniferous forests were least similar (Cj = 0.071), as hydrophyte-dominated wetlands differ markedly from conifer-dominated stands in both vegetation composition and bird community.
4.3. Effects of Landscape Heterogeneity on Bird Communities
Landscape heterogeneity encompasses diversity in landscape composition, spatial configuration, and functional attributes [32,33]. In urban parks, it often appears as small-scale habitat mosaics that play a critical role in urban biodiversity, ecological functions, and visitor experiences. Heterogeneity enriches ecological niches, allowing species to exploit a variety of habitats based on their specific preferences. The vertical stratification in heterogeneous landscapes—created by diverse vegetation and terrain—further partitions resources, enabling multiple bird species to coexist. Additionally, complex terrain, rich plant assemblages, and varied landscape patches improve concealment and perceived security for birds, thereby increasing their breeding and survival rates. While heterogeneous landscapes promote robust bird community structures, practical management must also consider trade-offs among different ecosystem services.
4.4. Conservation Recommendations and Spatial Optimization Strategies
Xuzhou Quanshan Forest Park is a vital component of the urban ecosystem, playing a key role in maintaining bird diversity. This study analyzed avian species composition, diversity indices, and habitat-related community variations, identifying crucial habitat factors influencing bird diversity. Based on our findings, we propose a multi-scale approach, encompassing macro-, meso-, and micro-level spatial planning.
- Macro-Scale (Regional Level)Constructing ecological corridors, connecting more habitats types of species and shaping regional habitats nets are paramount for protecting rare species. Quanshan Forest Park should be functionally integrated with neighboring parks, woodlands, and wetlands. Through vegetation protection and restoration, corridors can be established to facilitate wildlife movement, thereby enhancing regional ecological connectivity and promoting genetic exchange (Figure 9). Additionally, a coordinated plan is needed to clarify the park’s ecological functions relative to surrounding land uses, limiting excessive development to preserve adequate ecological buffers.
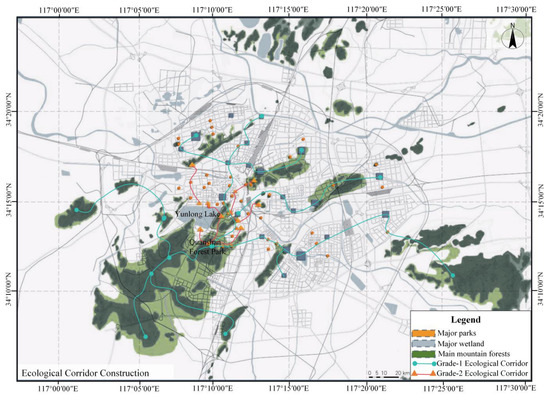 Figure 9. Construction map of the ecological corridor (In the ecological corridor system, the primary corridors consist of mountain forests and wetland ecosystems with key ecological functions, as well as comprehensive parks. The secondary corridors include community parks, square green spaces, and linear green spaces, which play supplementary and connective roles).
Figure 9. Construction map of the ecological corridor (In the ecological corridor system, the primary corridors consist of mountain forests and wetland ecosystems with key ecological functions, as well as comprehensive parks. The secondary corridors include community parks, square green spaces, and linear green spaces, which play supplementary and connective roles). - Meso-Scale (Park Level)Analyzing at the medium-scale park site level, optimizing bird habitat structures and enriching habitat types are critically important for ecological restoration and increasing bird populations. Large-scale plantings of artificial vegetation for aesthetic or landscaping purposes often lack the complexity of naturally regenerated communities [34], reducing ecological resilience and the park’s attractiveness to local wildlife. Overly dense canopy layers in some areas constrain understory illumination, impeding shrub and herb growth and limiting vertical habitat stratification [35]. To remedy this, forest stands should be managed to adjust stand structure and improve understory plant richness. Ecological restoration, such as adding diverse plant species and increasing habitat layers, is particularly necessary in monocultural habitats like shrublands. Enhancing landscape heterogeneity—by introducing different patch types, making use of natural topographical variations, and diversifying vertical spaces—creates more ecological niches for birds.Wetlands should be a priority for protection and proper management. As critical bird habitats, wetlands respond sensitively to environmental changes, making them indicators of ecological balance [36,37]. Efforts should focus on pollution control, maintaining stable water levels and quality, and expanding wetland areas and aquatic plant diversity. Human activities, such as recreation and infrastructure development, can generate noise, interfering with birds’ acoustic communication. This disrupts reproduction, population dynamics, and community structure, ultimately affecting ecosystem stability [35,38]. Park regulations should limit disturbances by designating visitor routes, capping visitor numbers, and emphasizing environmental education to reduce noise pollution at the source.
- Micro-Scale (Site and Infrastructure Level)From a micro-habitat and facility development perspective, improving the quality of bird habitats and preserving natural wilderness conditions hold practical significance for habitat maintenance and bird conservation. Artificial bird nests and feeding stations should be installed strategically at wetland edges and forest clearings, carefully considering material selection and site placement to minimize negative impacts. Vegetation management should adopt refined, wilderness-oriented, and naturalized approaches, selecting plant species based on birds’ dietary habits and habitat requirements, and retaining dead wood and fallen logs. Enhanced water quality management should include debris removal and planting aquatic and wetland vegetation (Figure 10). Infrastructure should employ environmentally friendly materials, include ecological bridges and culverts, and feature greenery strips along roads to reduce disturbances to birds.
 Figure 10. Diagram of Ecological Optimization Measures for Birds in the Park. (a) Install artificial nest boxes to provide breeding sites for birds. (b) Maintain natural vegetation to foster environments conducive to avian habitation. (c) Manage aquatic habitats to establish high-quality waterbird ecosystems.
Figure 10. Diagram of Ecological Optimization Measures for Birds in the Park. (a) Install artificial nest boxes to provide breeding sites for birds. (b) Maintain natural vegetation to foster environments conducive to avian habitation. (c) Manage aquatic habitats to establish high-quality waterbird ecosystems.
In summary, the protection and spatial optimization of Xuzhou Quanshan Forest Park is a systematic project requiring comprehensive advancement at multiple spatial planning levels, incorporating ecological, cultural, and other considerations to effectively protect bird diversity and promote sustainable development of the park’s ecosystem.
5. Conclusions
5.1. Main Findings
Focusing on Xuzhou Quanshan Forest Park, this study investigated avian diversity and habitat relationships within an urban park context. We examined species composition, calculated diversity indices, compared bird communities across various habitat types, and identified critical habitat factors shaping avian diversity. The key findings are:
- Species Richness and Diversity: We recorded 120 bird species across 16 orders and 40 families. The diversity indices suggest that the park exhibits a relatively high level of avian diversity in terms of species richness, evenness, and distribution patterns, implying a moderate degree of complexity and stability in supporting bird nesting and reproduction.
- Habitat Variation: Among the different habitats, wetlands exhibited the highest levels of species richness and diversity, primarily due to their complex vegetation structure and abundant food and shelter. Mixed coniferous-broadleaf forests ranked second. Other habitats showed lower diversity because of monoculture-type vegetation and/or higher disturbance levels.
- Key Habitat Factors: Landscape heterogeneity, vegetation richness, water area, and intensity of human disturbance are pivotal in driving avian diversity within an urban park ecosystem. These factors influence birds’ habitat selection, foraging, breeding, and migratory behaviors through complex ecological processes, thereby shaping the structure and diversity of park bird communities.
5.2. Limitations and Future Prospects
First, our data primarily came from the China Birdwatching Record Center, field observations, earlier survey records, and the relevant literature. Variations in survey timing, protocols, and methodologies across data sources may lead to inconsistencies or errors. Second, habitat classification in this study was based on general categories, yet the park’s ecological environments are highly complex. Certain micro- or transitional habitats were difficult to classify but may significantly affect bird diversity. Third, because urban parks have frequent human activity, capturing and quantifying all dimensions of disturbance in habitat classification is challenging.
Future research will delve deeper into the mechanisms by which habitat preferences and temporal changes shape avian community structures, aiming to uncover the drivers behind shifts in species diversity. Specific directions include: (1) investigating the influences of site-scale habitat characteristics (e.g., vegetation vertical stratification, species composition, soil moisture, and light conditions) on avian diversity to refine habitat design recommendations; (2) conducting long-term dynamic monitoring of bird diversity and habitat conditions in Xuzhou Quanshan Forest Park, thereby clarifying the impacts of ecological changes on bird communities; and (3) utilizing advanced technologies, such as drone surveillance and infrared cameras, to enhance the accuracy and comprehensiveness of bird population monitoring.
This study takes Xuzhou Quanshan Forest Park, a typical urban “mountain + forest” habitat system, as the research object, and deeply reveals the internal relationship between the characteristics of avian biodiversity and habitat quality. By identifying key habitat factors, such as vegetation structure, water area, and the degree of human disturbance, the study provides a scientific basis for park managers, guiding them to take targeted measures, such as optimizing vegetation configuration, expanding the scope of water area protection, and reducing the interference of human activities. These measures can enhance avian biodiversity and promote the sustainable development of the urban ecological environment. The research results show that optimizing habitat quality is the core strategy for the protection of avian biodiversity in urban mountain forest systems. This discovery not only provides practical guidance for the protection practices of Xuzhou Quanshan Forest Park but also offers important theoretical support and practical directions for the protection planning of avian biodiversity in similar ecosystems worldwide. It highlights the crucial role of habitat quality improvement in biodiversity conservation.
Author Contributions
Conceptualization, H.L., Y.K. and P.L.; methodology, H.L., Y.K., S.Z. and Y.D.; software, H.L., S.Z. and Y.D.; validation, P.L., Y.D., Y.K. and H.L.; formal analysis, Y.D. and Y.K.; data curation, H.L., S.Z. and Y.K.; writing—original draft preparation, H.L.; writing—review and editing, P.L., S.Z., Y.D. and Y.K.; visualization, S.Z., H.L. and Y.K.; supervision, P.L., H.L., S.Z., Y.D. and Y.K.; project administration, H.L. and Y.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Yellow Sea Wetland Research Foundation of Yancheng grant number No. HHSDKT202415 (Y.K.); Jiangsu Province Basic Research Special Fund (Soft Science Research) Special Support grant number No. BK20241661 (Y.K.); National Natural Science Foundation of China, grant number 52208091 (S.Z.); the Fundamental Research Funds for the Central Universities grant number No. 2023QN1089 (Y.K.) and China postdoctoral Science Foundation on the 73th grant program grant number No. 2023M732641 (Y.K.).
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to privacy and property rights issues.
Acknowledgments
We thank the Xuzhou Birdwatching Association for their assistance in data collection. Special thanks to Zhu Jintong for taking bird photos, Luhong Teacher, Shuijing Teacher, Zhuque Teacher, Heitu Teacher, Yueyueniao Teacher, Daxiang Teacher, and the volunteers who participated in birdwatching events in Quanshan Forest Park.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A. List of Birds in Quan Shan Forest Park
| Order-Family-Species | Dominance | Residence | Fauna | Protection Grade | Eco-Groups |
| ANSERIFORMES | |||||
| Anatidae | |||||
| 1. (Tundra Swan) Cygnus columbianus | + | W | PR | II | Waterfowl |
| 2. (Common Merganser) Mergus merganser | + | W | PR | LC | Waterfowl |
| 3. (Eastern Spot-billed Duck) Anas zonorhyncha | + | W | ES | LC | Waterfowl |
| 4. (Mallard) Anas platyrhynchos | + | W | PR | II | Waterfowl |
| PODICIPEDIFORMES | Waterfowl | ||||
| Podicipedidae | Waterfowl | ||||
| 1. (Little Grebe) Tachybaptus ruficollis | ++ | R | ES | LC | Waterfowl |
| COLUMBIFORMES | |||||
| Columbidae | |||||
| 1. (Oriental Turtle Dove) Streptopelia orientalis | ++ | R | ES | LC | Terrestrial Bird |
| 2. (Spotted Dove) Spilopelia chinensis | ++ | R | OR | LC | Terrestrial Bird |
| CUCULIFORMES | |||||
| Cuculidae | |||||
| 1. (Chestnut-winged Cuckoo) Clamator coromandus | + | S | ES | LC | Climbing Bird |
| 2. (Asian Koel) Eudynamys scolopaceus | + | S | OR | LC | Climbing Bird |
| 3. (Large Hawk-Cuckoo) Hierococcyx sparverioides | ++ | S | ES | LC | Climbing Bird |
| 4. (Indian Cuckoo) Cuculus micropterus | + | S | ES | LC | Climbing Bird |
| 5. (Common Cuckoo) Cuculus canorus | + | S | ES | LC | Climbing Bird |
| 6. (Lesser Cuckoo) Cuculus poliocephalus | + | S | ES | LC | Climbing Bird |
| GRUIFORMES | |||||
| Rallidae | |||||
| 1. (Common Moorhen) Gallinula chloropus | ++ | R | ES | II | Wader |
| 2. (Eurasian Coot) Fulica atra | + | W | ES | LC | Wader |
| CICONIIFORMES | Wader | ||||
| Ciconiidae | Wader | ||||
| (Oriental Stork) Ciconia boyciana | + | S | OR | I | Wader |
| PELECANIFORMES | Wader | ||||
| Ardeidae | Wader | ||||
| 1. (Yellow Bittern) Ixobrychus sinensis | + | S | ES | LC | Wader |
| 2. (Schrenck’s Bittern) Ixobrychus eurhythmus | + | LC | Wader | ||
| 3. (Black-crowned Night Heron) Nycticorax nycticorax | + | S | ES | LC | Wader |
| 4. (Chinese Pond Heron) Ardeola bacchus | + | S | ES | LC | Wader |
| 5. (Grey Heron) Ardea cinerea | + | S | ES | LC | Wader |
| 6. (Little Egret) Egretta garzetta | + | S | ES | LC | Wader |
| SULIFORMES | |||||
| Phalacrocoracidae | |||||
| 1. (Great Cormorant) Phalacrocorax carbo | + | W | ES | LC | Waterfowl |
| CHARADRIIFORMES | |||||
| Charadrius hiaticula | |||||
| 1. (Northern Lapwing) Vanellus vanellus | + | W | PR | LC | Wader |
| 2. (Whiskered Tern) Chlidonias hybrida | + | S | ES | LC | Wader |
| STRIGIFORMES | |||||
| Strigidae | |||||
| 1. (Collared Scops Owl) Otus sunia | + | W | PR | II, Appendix II, LC | Raptor |
| ACCIPITRIFORMES | Raptor | ||||
| Accipitridae | Raptor | ||||
| 1. (Black-winged Kite) Elanus caeruleus | + | R | ES | II, Appendix II, LC | Raptor |
| 2. (Crested Honey Buzzard) Pernis ptilorhynchus | + | W | PR | II, Appendix II, LC | Raptor |
| 3. (Crested Goshawk) Accipiter trivirgatus | + | W | PR | II, Appendix II, LC | Raptor |
| 4. (Chinese Sparrowhawk) Accipiter soloensis | + | R | OR | II, Appendix II, LC | Raptor |
| 5. (Japanese Sparrowhawk) Accipiter gularis | + | W | PR | II, Appendix II, LC | Raptor |
| 6. (Besra) Accipiter virgatus | + | W | PR | II, Appendix II, LC | Raptor |
| 7. (Eurasian Sparrowhawk) Accipiter nisus | + | R | ES | II, Appendix II, LC | Raptor |
| 8. (Northern Goshawk) Accipiter gentilis | + | R | PR | II, Appendix II, LC | Raptor |
| 9. (Grey-faced Buzzard) Butastur indicus | + | W | PR | II, Appendix II, LC | Raptor |
| 10. (Eastern Buzzard) Buteo japonicus | + | W | PR | II, Appendix II, LC | Raptor |
| BUCEROTIFORMES | |||||
| Upupidae | |||||
| 1. (Eurasian Hoopoe) Upupa epops | + | R | ES | LC | Climbing Bird |
| CORACIIFORMES | Climbing Bird | ||||
| Coraciidae | Climbing Bird | ||||
| 1. (Dollarbird) Eurystomus orientalis | + | R | ES | LC | Climbing Bird |
| Alcedinidae | Climbing Bird | ||||
| 2. (Common Kingfisher) Alcedo atthis | + | R | ES | LC | Climbing Bird |
| PICIFORMES | |||||
| Picidae | |||||
| 1. (Speckled Piculet) Picumnus innominatus | + | R | OR | LC | Climbing Bird |
| 2. (Grey-headed Woodpecker) Picus canus | + | R | ES | LC | Climbing Bird |
| 3. (Grey-capped Pygmy Woodpecker) Picoides canicapillus | ++ | R | PR | LC | Climbing Bird |
| 4. (Great Spotted Woodpecker) Dendrocopos major | + | R | ES | LC | Climbing Bird |
| FALCONIFORMES | |||||
| Falconidae | |||||
| 1. (Common Kestrel) Falco tinnunculus | + | R | ES | II, Appendix II, LC | Raptor |
| 2. (Amur Falcon) Falco amurensis | + | W | PR | II, Appendix II, LC | Raptor |
| 3. (Eurasian Hobby) Falco subbuteo | + | R | ES | II, Appendix II, LC | Raptor |
| PASSERIFORMES | |||||
| Oriolidae | |||||
| 1. (Black-naped Oriole) Oriolus chinensis | + | S | ES | LC | Songbird |
| Campephagidae | Songbird | ||||
| 1. (Grey-throated Minivet) Pericrocotus solaris | + | S | OR | LC | Songbird |
| 2. (Ashy Minivet) Pericrocotus divaricatus | + | R | OR | LC | Songbird |
| 3. (Indochinese Cuckooshrike) Lalage melaschistos | + | S | OR | LC | Songbird |
| Dicruridae | Songbird | ||||
| 1. (Black Drongo) Dicrurus macrocercus | + | S | ES | LC | Songbird |
| Laniidae | Songbird | ||||
| 1. (Brown Shrike) Lanius cristatus | + | R | PR | LC | Songbird |
| 2. (Long-tailed Shrike) Lanius schach | + | R | OR | LC | Songbird |
| Corvidae | Songbird | ||||
| 1. (Azure-winged Magpie) Cyanopica cyanus | ++ | R | ES | LC | Songbird |
| 2. (Red-billed Blue Magpie) Urocissa erythroryncha | ++ | R | ES | LC | Songbird |
| 3. (Grey Treepie) Dendrocitta formosae | ++ | R | OR | LC | Songbird |
| 4. (Oriental Magpie) Pica serica | ++ | R | PR | LC | Songbird |
| 5. (Daurian Jackdaw) Corvus dauuricus | + | LC | Songbird | ||
| 6. (Carrion Crow) Corvus corone | + | W | PR | LC | Songbird |
| Paridae | Songbird | ||||
| 1. (Coal Tit) Periparus ater | + | Songbird | |||
| 2. (Yellow-bellied Tit) Pardaliparus venustulus | ++ | R | OR | LC * | Songbird |
| 3. (Far Eastern Great Tit) Parus minor | ++ | R | PR | LC | Songbird |
| Hirundinidae | Songbird | ||||
| 1. (Barn Swallow) Hirundo rustica | ++ | S | PR | LC | Songbird |
| 2. (Red-rumped Swallow) Cecropis daurica | ++ | R | ES | LC | Songbird |
| Pycnonotidae | Songbird | ||||
| 1. (Collared Finchbill) Spizixos semitorques | + | R | OR | LC | Songbird |
| 2. (Light-vented Bulbul) Pycnonotus sinensis | ++ | R | OR | LC | Songbird |
| 3. (Chestnut Bulbul) Hemixos castanonotus | + | R | OR | LC | Songbird |
| Phylloscopidae | Songbird | ||||
| 1. (Pallas’s Leaf Warbler) Phylloscopus inornatus | ++ | W | PR | LC | Songbird |
| 2. (Lemon-rumped Warbler) Phylloscopus proregulus | + | W | PR | LC | Songbird |
| 3. (Radde’s Warbler) Phylloscopus schwarzi | + | W | PR | LC | Songbird |
| 4. (Dusky Warbler) Phylloscopus fuscatus | + | W | PR | LC | Songbird |
| 5. (Eastern Crowned Warbler) Phylloscopus coronatus | + | W | PR | LC | Songbird |
| 6. (Pale-legged Leaf Warbler) Phylloscopus tenellipes | + | W | PR | LC | Songbird |
| 7. (Arctic Warbler) Phylloscopus borealis | + | W | PR | LC | Songbird |
| Cettiidae | Songbird | ||||
| 1. (Rufous-faced Warbler) Abroscopus albogularis | ++ | R | ES | LC | Songbird |
| 2. (Strong-footed Bush Warbler) Horornis fortipes | ++ | R | OR | LC | Songbird |
| Aegithalidae | Songbird | ||||
| 1. (Silver-throated Bushtit) Aegithalos glaucogularis | ++ | R | ES | LC | Songbird |
| 2. (Red-headed Bushtit) Aegithalos concinnus | ++ | R | OR | LC | Songbird |
| Paradoxornithidae | Songbird | ||||
| 1. (Vinous-throated Parrotbill) Sinosuthora webbiana | ++ | R | ES | LC | Songbird |
| Zosteropidae | Songbird | ||||
| 1. (Chestnut-flanked White-eye) Zosterops erythropleurus | + | P | PR | LC | Songbird |
| 2. (Swinhoe’s White-eye) Zosterops simplex | ++ | R | OR | LC | Songbird |
| Leiothrichidae | Songbird | ||||
| 1. (Chinese Hwamei) Garrulax canorus | + | R | OR | Appendix II, LC | Songbird |
| 2. (Masked Laughingthrush) Pterorhinus perspicillatus | ++ | R | OR | II | Songbird |
| 3. (Black-throated Laughingthrush) Pterorhinus pectoralis | ++ | R | OR | II | Songbird |
| Troglodytidae | Songbird | ||||
| 1. (Eurasian Wren) Troglodytes troglodytes | + | W | PR | LC | Songbird |
| Sturnidae | Songbird | ||||
| 1.(Crested Myna) Acridotheres cristatellus | + | R | OR | LC | Songbird |
| 2. (Silky Starling) Spodiopsar sericeus | + | R | OR | LC | Songbird |
| 3. (White-cheeked Starling) Spodiopsar cineraceus | + | W | PR | LC | Songbird |
| Turdidae | Songbird | ||||
| 1. (Grey-backed Thrush) Turdus hortulorum | + | W | PR | LC | Songbird |
| 2. (Chinese Blackbird) Turdus mandarinus | ++ | R | ES | LC * | Songbird |
| 3. (Pale Thrush) Turdus pallidus | + | W | PR | LC | Songbird |
| 4. (Naumann’s Thrush) Turdus naumanni | ++ | W | PR | LC | Songbird |
| 5. (Dusky Thrush) Turdus eunomus | + | W | PR | LC | Songbird |
| 6. (Sichuan Thrush) Turdus mupinensis | + | R | PR | LC * | Songbird |
| Muscicapidae | Songbird | ||||
| 1. (Grey-streaked Flycatcher) Muscicapa griseisticta | + | P | PR | LC | Songbird |
| 2. (Dark-sided Flycatcher) Muscicapa sibirica | + | P | PR | LC | Songbird |
| 3. (Asian Brown Flycatcher) Muscicapa dauurica | + | P | PR | LC | Songbird |
| 4. (Siberian Blue Robin) Larvivora cyane | + | W | PR | LC | Songbird |
| 5. (Rufous-tailed Robin) Larvivora sibilans | + | P | PR | LC | Songbird |
| 6. (Red-flanked Bluetail) Tarsiger cyanurus | ++ | W | PR | LC | Songbird |
| 7. (Yellow-rumped Flycatcher) Ficedula zanthopygia | + | R | ES | LC | Songbird |
| 8. (Mugimaki Flycatcher) Ficedula mugimaki | + | P | PR | LC | Songbird |
| 9. (Daurian Redstart) Phoenicurus auroreus | + | W | PR | LC | Songbird |
| Regulidae | Songbird | ||||
| 1. (Goldcrest) Regulus regulus | + | LC | Songbird | ||
| Passeridae | Songbird | ||||
| 1(Eurasian Tree Sparrow) Passer montanus | ++ | R | ES | LC | Songbird |
| Motacillidae | Songbird | ||||
| 1. (Olive-backed Pipit) Anthus hodgsoni | + | W | ES | LC | Songbird |
| 2. (White Wagtail) Motacilla alba | + | R | ES | LC | Songbird |
| Fringillidae | Songbird | ||||
| 1. (Brambling) Fringilla montifringilla | ++ | W | PR | LC | Songbird |
| 2. (Hawfinch) Coccothraustes coccothraustes | + | W | PR | LC | Songbird |
| 3. (Japanese Grosbeak) Eophona migratoria | ++ | W | PR | LC | Songbird |
| 4. (Masked Grosbeak) Eophona personata | + | W | PR | LC | Songbird |
| 5. (Pallas’s Rosefinch) Carpodacus roseus | + | W | PR | LC | Songbird |
| 6. (Oriental Greenfinch) Chloris sinica | ++ | R | PR | LC | Songbird |
| 7. (Eurasian Siskin) Spinus spinus | ++ | R | PR | LC | Songbird |
| Emberizidae | |||||
| 1. (Yellow-throated Bunting) Emberiza elegans | + | R | PR | LC | Songbird |
| 2. (Rustic Bunting) Emberiza rustica | + | W | PR | LC | Songbird |
| 3. (Little Bunting) Emberiza pusilla | + | W | PR | LC | Songbird |
| 4. (Grey-headed Bunting) Emberiza spodocephala | + | W | PR | LC | Songbird |
| 5. (Yellow-browed Bunting) Emberiza chrysophrys | ++ | W | PR | LC | Songbird |
| 6. (Tristram’s Bunting) Emberiza tristrami | + | W | PR | LC | Songbird |
| Note: Dominance: “++” indicates common species, and “+” indicates rare species; Residency: “R” means resident bird, “W” means winter visitor, “S” means summer visitor, “P” means passage migrant, and “V” means vagrant; Fauna: “PR” represents the Palearctic Realm, “OR” represents the Oriental Realm, and “ES” represents widespread species. Domestic Protection Classification: “II” denotes National Grade II Protected Wild Animals; Appendix I/II: Listed in CITES (the Convention on International Trade in Endangered Species of Wild Fauna and Flora) Appendices I or II. IUCN Red List Categories: “LC” indicates Least Concern, “NT” indicates Near Threatened. “*” signifies a species endemic to China. | |||||
References
- Yang, Y.; Li, Y.; Lei, G.; Wang, N.; Fang, H.; Wu, L.; Townshend, T.; Huang, G.; Li, Z.; Wang, Y. Characteristics of bird diversity and habitat optimization strategies in Wenyu River Park, Beijing. Res. Environ. Sci. 2025, 38, 49–58. [Google Scholar]
- Zhao, Y.L.; Wang, C.; Bai, Z.T.; Hao, Z.Z. Changes of bird community under urbanization and its relationship with urban vegetation. Acta Ecol. Sin. 2021, 41, 479–489. [Google Scholar]
- Li, X.; Zhang, Z.; Ma, H.; Liu, Y.; Huang, Q.; Zhang, Y. Research Progress in Relationship Between Urban Bird Communities and Green Spaces. World For. Res. 2022, 35, 49–53. [Google Scholar]
- Guo, S.; Saito, K.; Natuhara, Y.; Cao, Y. Impacts of urban built environments on bird diversity. Environ. Sci. 2023, 78, 2850–2863. [Google Scholar]
- Wu, J.; Yang, M.; Zhou, K. Urbanization responses and adaptation of birds in China based on citizen science data. ACTA Geogr. Sin. 2023, 78, 2850–2863. [Google Scholar]
- Sekercioglu, C. Increasing awareness of avian ecological function. Trends Ecol. Evol. 2006, 21, 464–471. [Google Scholar]
- Whelan, C.J.; Wenny, D.G.; Marquis, R.J. Ecosystem Services Provided by Birds. Ann. N. Y. Acad. Sci. 2008, 1134, 25–60. [Google Scholar]
- Kellermann, J.K.; Johnson, M.D.; Stercho, A.M.; Hackett, S.C. Ecological and Economic Services Provided by Birds on Jamaican Blue Mountain Coffee Farms. Conserv. Biol. 2008, 22, 1177–1185. [Google Scholar]
- Whelan, C.J.; Şekercioğlu, Ç.H.; Wenny, D.G. (Eds.) Bird Ecosystem Services: Economic Ornithology for the 21st Century; University of Chicago Press: Chicago, IL, USA, 2016. [Google Scholar]
- Xu, H.; Peng, C.; Zhu, X.; Yong, F.; Yi, J.; Zhang, W.; Li, J.; Tong, W.; Jiang, B.; Cai, L. Progress in Construction of China Bird Diversity Observation Network (China BON-Birds). J. Ecol. Rural. Environ. 2018, 34, 1–11. [Google Scholar]
- Wu, Y.; Hong, M.; Bao, J.; Zhao, G. Summer avian community diversity and interannual dynamics in southern Alukerqin Banner. J. Arid. Land Resour. Environ. 2025, 39, 181–187. [Google Scholar]
- Zhang, H.; Sun, X.; Li, G.; Wu, Z.; Kuang, Z.; Su, H.; Xia, F.; Tan, H. Diversity Analysis of Bird Communities in Aha Lake National Wetland Park, Guiyang, China. Chin. J. Wildl. 2020, 41, 626–640. [Google Scholar]
- Zhang, H.; Su, H.; Liu, W.; Zhang, M.; Li, Z. Relationship of Community Structure of Main Waterfowl with Habitat in Caohai National Nature Reserve in Winter. J. Ecol. Rural. Environ. 2014, 30, 601–607. [Google Scholar]
- Hu, Z.Q.; Dong, L. Effects of urbanization on interspecific interactions involving birds. Biodivers. Sci. 2024, 32, 24048. [Google Scholar] [CrossRef]
- Qiao, S.; Gong, D.; Xu, X.; Huang, S.; Zhang, Y. Structure and diversity of bird communities in urban parks of Lanzhou. J. Arid. Land Resour. Environ. 2021, 35, 191–199. [Google Scholar]
- Li, B.; Ma, S.; Zhang, S.; Tan, L.; Huang, J.; Zhang, W. Effects of urban park characteristics on the diversity of breeding birds in Liuzhou. Biot. Resour. 2024, 46, 359–367. [Google Scholar]
- Ding, L.; Hu, C. Study on Insect Community of Quanshan Natural Reserve in Xuzhou City. Acta Asriculturae Jiangxi 2009, 21, 62–67. [Google Scholar]
- Guo, Y.; Sun, X.; Xu, Y.; Gu, S.; Jin, S.; Feng, Z. Abundance, Distribution and Spatial Niche of Two Laughingthrush Species in Quanshan Forest Park of Xuzhou City, Jiangsu Province. Sichuan J. Zool. 2015, 34, 925–929. [Google Scholar]
- Dal, X.; Fu, W.; Dai, Q.; Zhao, H. Stability Analysis on Plant Community in Quanshan Nature Reserve. J. Anhui Agric. Sci. 2009, 37, 13707–13709. [Google Scholar]
- Yan, C. The Evaluation of Quanshan Nature Reserve, Xuzhou City. Ecol. Sci. 1998, 17, 70–75. [Google Scholar]
- Peng, Z.; Gao, T.; Shi, C.; Chen, Y.; Bi, J.; Qiu, L. The relationships between vegetation structure, habitat characteristics and bird diversity in campus green spaces. Chin. J. Ecol. 2020, 39, 3032–3042. [Google Scholar]
- Yang, G. The Influence of Multiple-Scale Habitat Structure on Bird Community in Urban Parks; East China Normal University: Shanghai, China, 2014. [Google Scholar]
- Zhang, W.; Dong, L. Study of Bird Preference to Plant Habitat and Species in Beiling Urban Park. Chin. Landsc. Archit. 2015, 31, 15–19. [Google Scholar]
- Zheng, G. A Checklist on the Classification and Distribution of the Birds of China, 3rd ed.; Science Press: Beijing, China, 2017. [Google Scholar]
- MacKinnon, J.R.; Phillipps, K. A Field Guide to the Birds of China; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Qian, Y.J. A Field Guide to the Birds of China; Henan Science and Technology Press: Zhengzhou, China, 1995. [Google Scholar]
- Lin, S.; Ye, Y.; Li, J.; Wu, G.; Sun, F. The Study of Bird Diversity in Tiegang Reservoir in the Quickly Urbanization Area. Sichuan J. Zool. 2013, 32, 297–301. [Google Scholar]
- Wang, T.; Jlao, J.; Li, C.; Zhi, X. Comparative Study on Rhizosphere Soil Properties andRoot Physiology of Reed in Different Habitats. Chin. J. Grassl. 2021, 43, 78–86. [Google Scholar]
- Liu, H.; Wu, X.; Li, C. An Experimental ResearchApproach on Habitat-site Design(II): Studyon Habitat-site Types and Zoning in Urban Green Space. Chin. Landsc. Archit. 2017, 33, 46–53. [Google Scholar]
- Tu, X.; Peng, J.; Duan, H.; Song, Y.; Gao, H. Study on Measures of Natural Habitat Restoration in the Mainstream of Liaohe Conservation Area. J. Environ. Eng. Technol. 2013, 3, 503–507. [Google Scholar]
- Wang, X.; Wang, H.; Sun, H.; Chang, Q.; Ding, J. Community structure and diversity of birds in Xuwei New District, Lianyungang City. J. Jiangsu For. Sci. Technol. 2019, 46, 1–7. [Google Scholar]
- Lu, X.L.; Zhao, H.P.; Sun, J.B.; Yang, G. Bird diversity during the breeding period in different habitats in an agricultural landscape in the Huang-Huai plain. Acta Ecol. Sin. 2019, 39, 3133–3143. [Google Scholar]
- Fahrig, L.; Baudry, J.; Brotons, L.; Burel, F.G.; Crist, T.O.; Fuller, R.J.; Sirami, C.; Siriwardena, G.M.; Martin, J. Functional landscape heterogeneity and animal biodiversity in agricultural landscapes. Ecol. Lett. 2011, 14, 101–112. [Google Scholar]
- Chen, J.; Niu, M.; Gong, G.; Zhou, D.; Zhu, Z.; Li, Y.; Zheng, S.; Xie, T.; Mu, C. Transformation of Cypress Monoculture by Cutting in Band and Replanting in Sichuan Central Basin. Southwest China J. Agric. Sci. 2019, 32, 636–644. [Google Scholar]
- Wang, S.; Lei, G.; Zhou, Y.; Guo, X.; Zhou, X.; Zhu, X.; Chen, C.; Zhang, M.; Ding, L. Analysis of Current Status of Avian Diversity and Habitat Restoration StrategiesAnalysis of Current Status of Avian Diversity and Habitat Restoration Strategies of the Yunlong Lake Scenic Areaof the Yunlong Lake Scenic Area. Wetl. Sci. 2024, 22, 236–244. [Google Scholar]
- Wang, Q.; Lu, X. Application of Water Bird to Monitor and Evaluate Wetland Ecosystem. Wetl. Sci. 2007, 5, 274–281. [Google Scholar]
- Miao, L.; Qian, Y.; Wei, L. Habitat Protection and Ecological RestorationStrategies for Waterbirds at Lianhuatan in Xixi National Wetland Park. Chin. Landsc. Archit. 2024, 40, 20–23. [Google Scholar]
- Ji, T.; Zhang, Y. Impacts of ambient noise on bird song and adaptation strategies of birds. Chin. J. Ecol. 2011, 30, 831–836. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).