Influence of Tree Community Characteristics on Carbon Sinks in Urban Parks: A Case Study of Xinyang, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
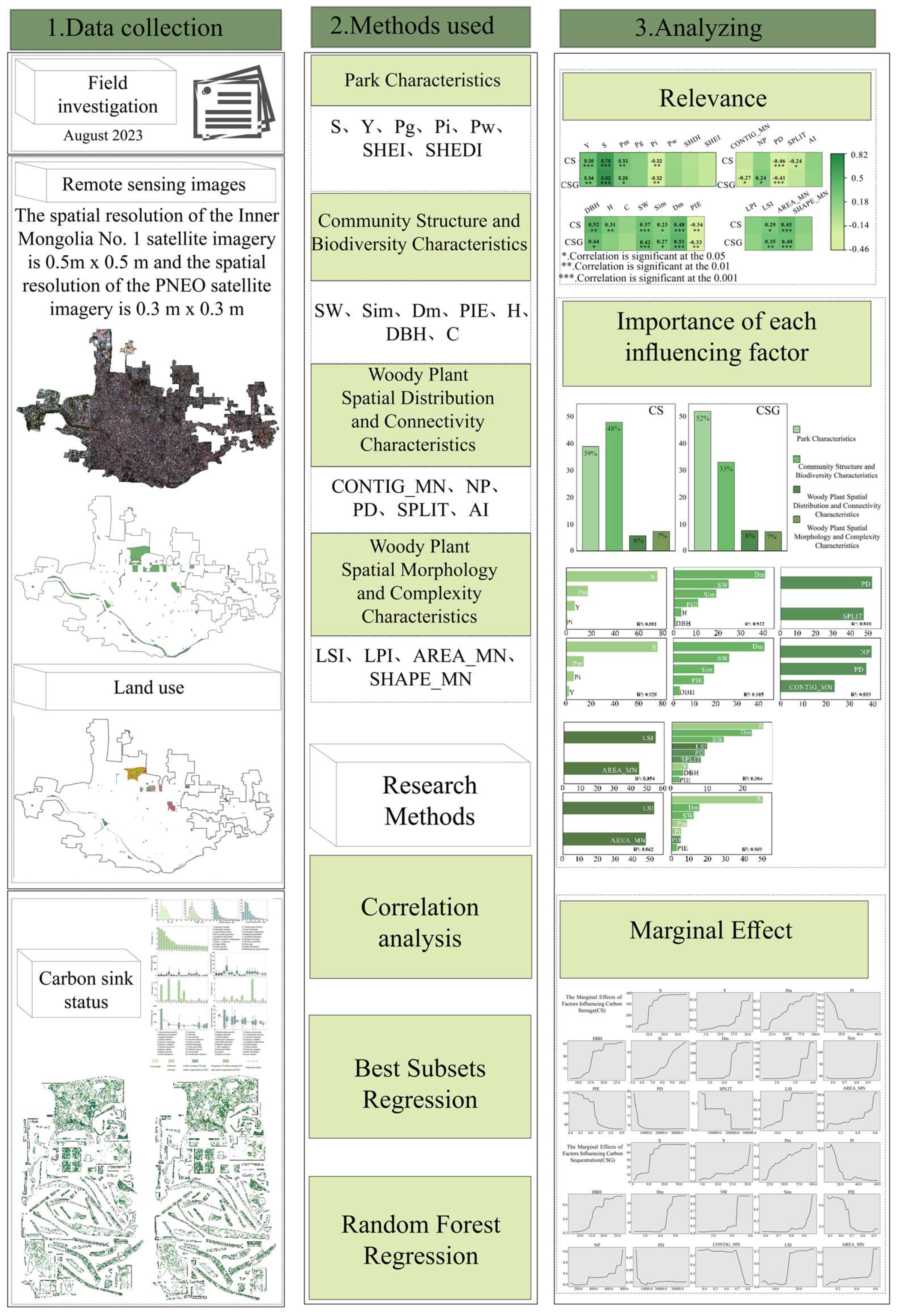
2.2. Research Methods
2.2.1. Field Surveys
2.2.2. Estimation of CS and CSG
2.2.3. Processing of Satellite Images
2.2.4. Selection of Influencing Factors
3. Results
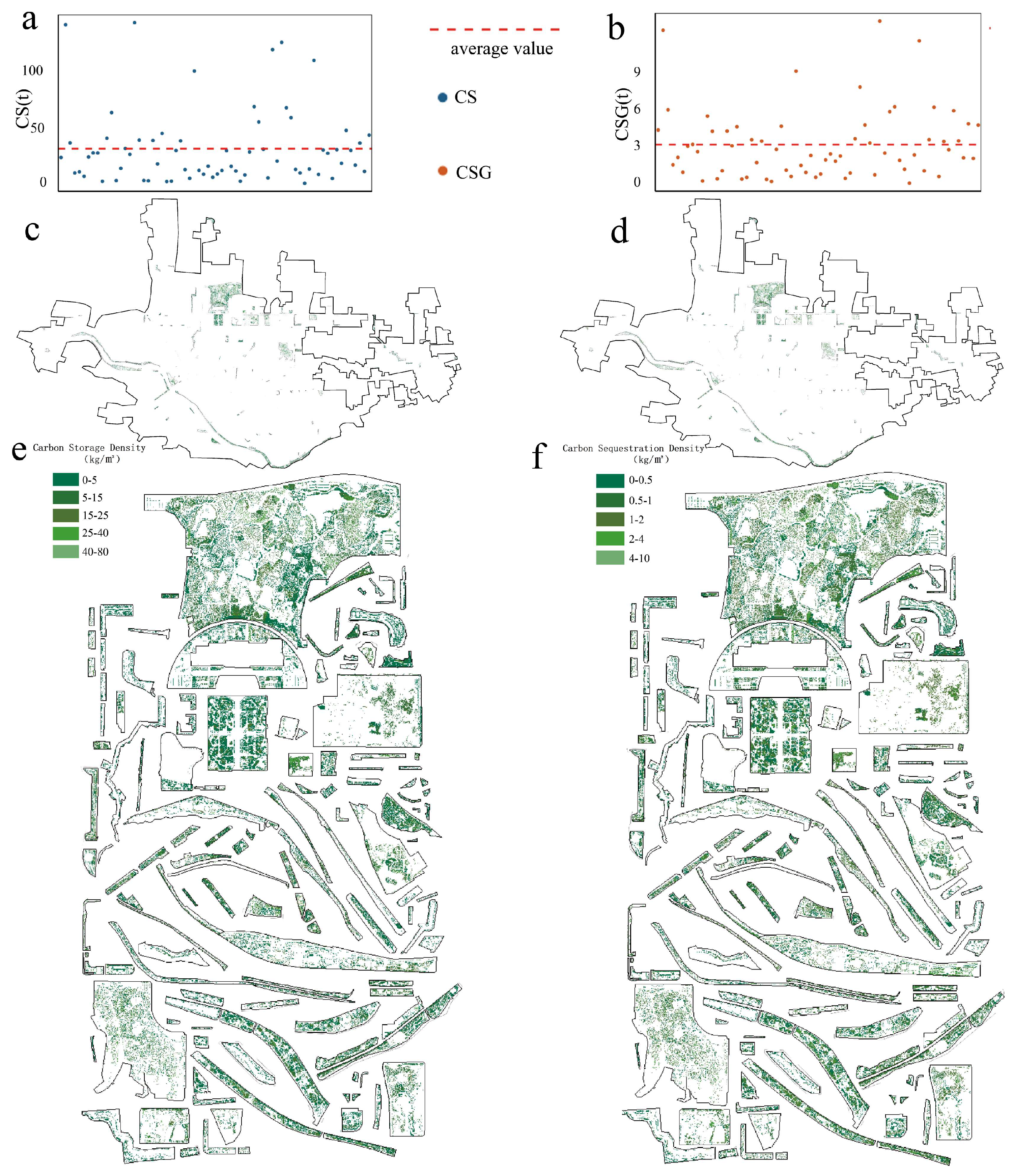
3.1. Overview of CS and CSG in Park Green Spaces
3.2. Characterization of CS and CSG by Woody Plants in Park Green Spaces
3.3. Analysis of Drivers of CS and CSG in Park Green Spaces
3.3.1. Correlation Analysis of Influencing Factors with CS and CSG
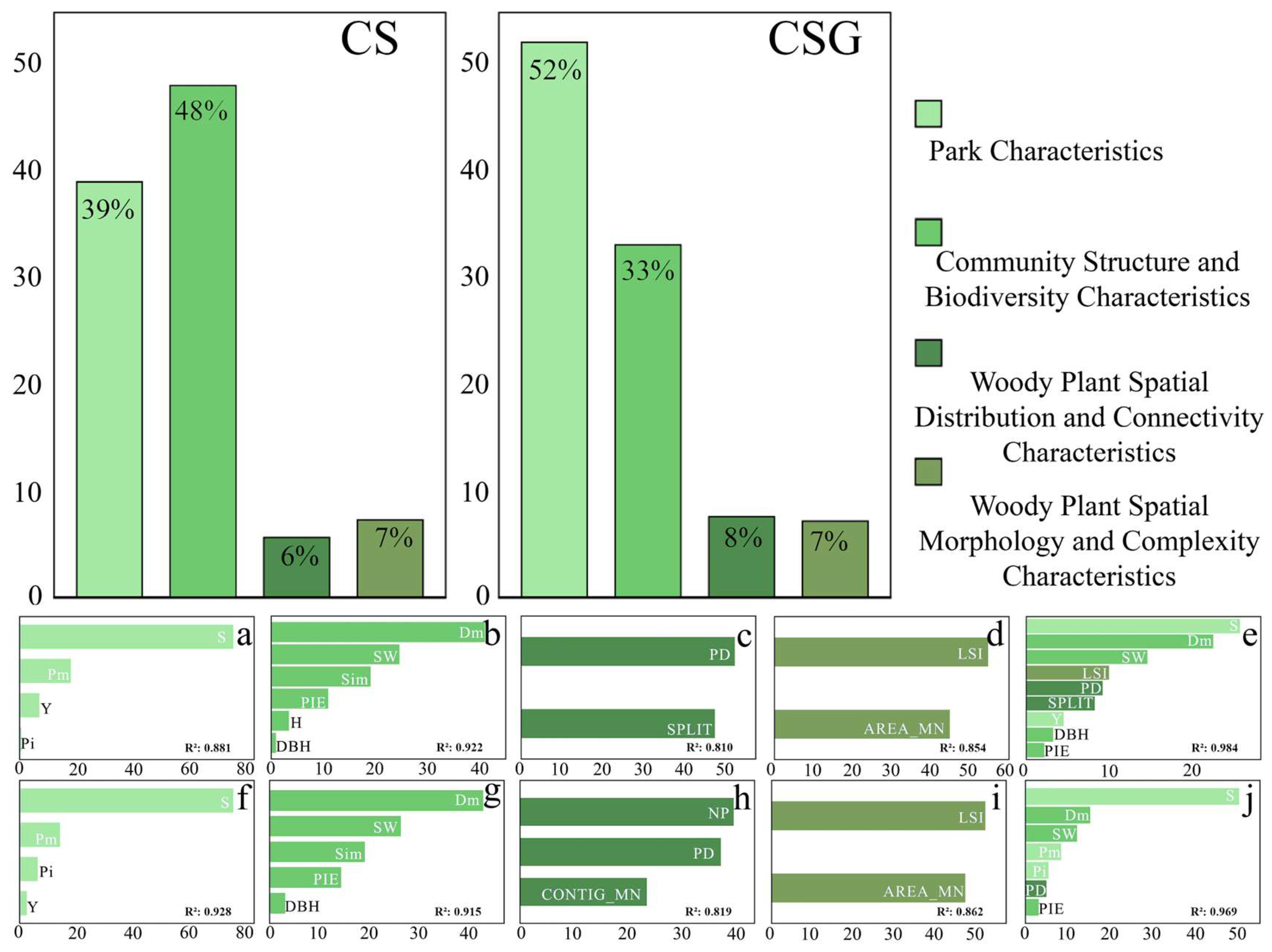
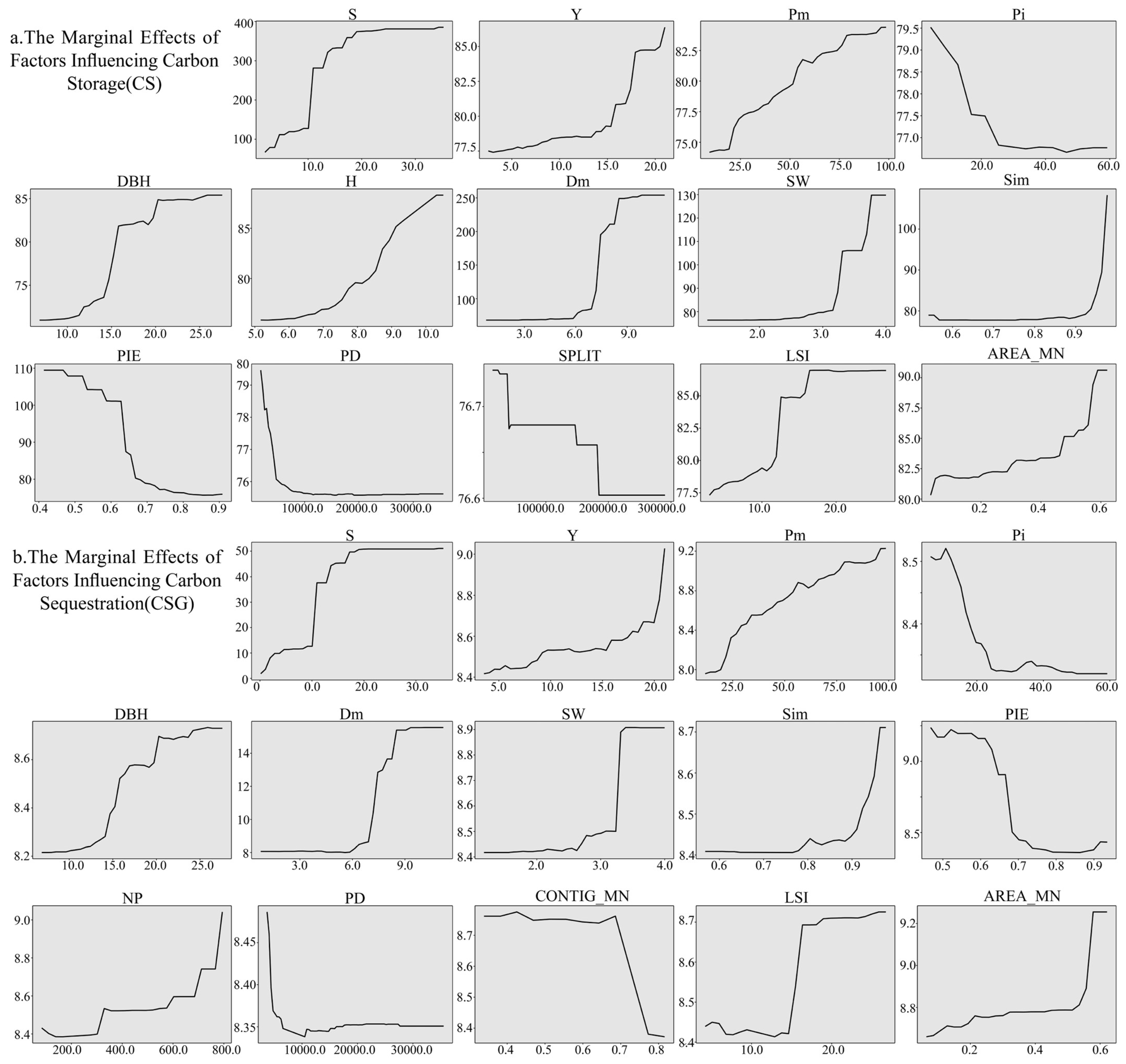
3.3.2. Contribution and Impact of Influencing Factors on CS, CSG
4. Discussion
4.1. Comparison with Other Studies
4.2. Key Influencing Factors for CS and CSG
4.3. Implications and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, G.Y.; Yu, F.F.; Chen, J.; Duan, Z.Y. Research on Urbanization Development of Mountainous Area—Take in Yichang for Example. Adv. Mater. Res. 2011, 250–253, 2723–2728. [Google Scholar] [CrossRef]
- Wang, J.; Wang, R. The Impact of Urbanization on Environmental Quality in Ecologically Fragile Areas: Evidence from Hengduan Mountain, Southwest China. Land 2024, 13, 503. [Google Scholar] [CrossRef]
- Gong, P.; Li, X.; Wang, J.; Bai, Y.; Chen, B.; Hu, T.; Liu, X.; Xu, B.; Yang, J.; Zhang, W.; et al. Annual maps of global artificial impervious area (GAIA) between 1985 and 2018. Remote Sens. Environ. 2020, 236, 111510. [Google Scholar] [CrossRef]
- Seto, K.C.; Güneralp, B.; Hutyra, L.R. Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc. Natl. Acad. Sci. USA 2012, 109, 16083–16088. [Google Scholar] [CrossRef]
- McCarthy, M.P.; Best, M.J.; Betts, R.A. Climate change in cities due to global warming and urban effects. Geophys. Res. Lett. 2010, 37, 2010GL042845. [Google Scholar] [CrossRef]
- Grimm, N.B.; Faeth, S.H.; Golubiewski, N.E.; Redman, C.L.; Wu, J.; Bai, X.; Briggs, J.M. Global Change and the Ecology of Cities. Science 2008, 319, 756–760. [Google Scholar] [CrossRef]
- Ren, H.; Gu, G.; Zhou, H. Assessing the low-carbon city pilot policy on carbon emission from consumption and production in China: How underlying mechanism and spatial spillover effect? Environ. Sci. Pollut. Res. 2022, 29, 71958–71977. [Google Scholar] [CrossRef]
- Zeng, S.; Jin, G.; Tan, K.; Liu, X. Can low-carbon city construction reduce carbon intensity? Empirical evidence from low-carbon city pilot policy in China. J. Environ. Manag. 2023, 332, 117363. [Google Scholar] [CrossRef]
- Nowak, D.J.; Crane, D.E. Carbon storage and sequestration by urban trees in the USA. Environ. Pollut. 2002, 116, 381–389. [Google Scholar] [CrossRef]
- Amoatey, P.; Sulaiman, H.; Kwarteng, A.; Al-Reasi, H.A. Above-ground carbon dynamics in different arid urban green spaces. Environ. Earth Sci. 2018, 77, 431. [Google Scholar] [CrossRef]
- Strohbach, M.W.; Arnold, E.; Haase, D. The carbon footprint of urban green space—A life cycle approach. Landsc. Urban Plan. 2012, 104, 220–229. [Google Scholar] [CrossRef]
- Francesco Agostini, A.S.G.; Richter, G.M. Carbon Sequestration by Perennial Energy Crops: Is the Jury Still Out? Bioenergy Res. 2015, 8, 1057–1080. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Jim, C.-Y.; Gao, J.; Zhao, M. Determinants of Aboveground Carbon Storage of Woody Vegetation in an Urban–Rural Transect in Shanghai, China. Sustainability 2023, 15, 8574. [Google Scholar] [CrossRef]
- Liu, X.; Trogisch, S.; He, J.-S.; Niklaus, P.A.; Bruelheide, H.; Tang, Z.; Erfmeier, A.; Scherer-Lorenzen, M.; Pietsch, K.A.; Yang, B.; et al. Tree species richness increases ecosystem carbon storage in subtropical forests. Proc. R. Soc. B Biol. Sci. 2018, 285, 20181240. [Google Scholar] [CrossRef]
- Tatsumi, S. Tree diversity effects on forest productivity increase through time because of spatial partitioning. BioRxiv 2020, 7, 24. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, H.; Tu, K.; Li, R.; Zhang, X.; Wang, P.; Li, Y.; Yang, Q.; Acerman, A.C.; Guo, N.; et al. Effects of plant community structural characteristics on carbon sequestration in urban green spaces. Sci. Rep. 2023, 14, 7382. [Google Scholar] [CrossRef]
- Lin, J.; Ma, Q.; Ju, Y.; Zhang, H.; Wang, Q.; Huang, B. Relationships between urbanization, tree morphology, and carbon density: An integration of remote sensing, allometric models, and field survey. Urban For. Urban Green. 2022, 76, 127725. [Google Scholar] [CrossRef]
- Kavana, G.B. Miyawaki Forest; University of Agricultural & Horticultural Sciences: Shivamogga, India, 2023. [Google Scholar] [CrossRef]
- Zhao, K.; Suarez, J.C.; Garcia, M.; Hu, T.; Wang, C.; Londo, A. Utility of multitemporal lidar for forest and carbon monitoring: Tree growth, biomass dynamics, and carbon flux. Remote Sens. Environ. 2018, 204, 883–897. [Google Scholar] [CrossRef]
- Gülçin, D.; Van Den Bosch, C.C.K. Assessment of Above-Ground Carbon Storage by Urban Trees Using LiDAR Data: The Case of a University Campus. Forests 2021, 12, 62. [Google Scholar] [CrossRef]
- Choudhury, M.A.M.; Marcheggiani, E.; Galli, A.; Modica, G.; Somers, B. Mapping the Urban Atmospheric Carbon Stock by LiDAR and WorldView-3 Data. Forests 2021, 12, 692. [Google Scholar] [CrossRef]
- Li, N.; Deng, L.; Yan, G.; Cao, M.; Cui, Y. Estimation for Refined Carbon Storage of Urban Green Space and Minimum Spatial Mapping Scale in a Plain City of China. Remote Sens. 2024, 16, 217. [Google Scholar] [CrossRef]
- Gu, X.; Zhang, P.; Zhang, W.; Liu, Y.; Jiang, P.; Wang, S.; Lai, X.; Long, A. A Study of Drought and Flood Cycles in Xinyang, China, Using the Wavelet Transform and M-K Test. Atmosphere 2023, 14, 1196. [Google Scholar] [CrossRef]
- Yao, X.; Zhu, Z.; Zhou, X.; Shen, Y.; Shen, X.; Xu, Z. Investigating the effects of urban morphological factors on seasonal land surface temperature in a “Furnace city” from a block perspective. Sustain. Cities Soc. 2022, 86, 104165. [Google Scholar] [CrossRef]
- Lin, Z.; Xu, H.; Yao, X.; Yang, C.; Yang, L. Exploring the relationship between thermal environmental factors and land surface temperature of a “furnace city” based on local climate zones. Build. Environ. 2023, 243, 110732. [Google Scholar] [CrossRef]
- Ossola, A.; Jenerette, G.D.; McGrath, A.; Chow, W.; Hughes, L.; Leishman, M.R. Small vegetated patches greatly reduce urban surface temperature during a summer heatwave in Adelaide, Australia. Landsc. Urban Plan. 2021, 209, 104046. [Google Scholar] [CrossRef]
- Jia, X.; Han, H.; Feng, Y.; Song, P.; He, R.; Liu, Y.; Wang, P.; Zhang, K.; Du, C.; Ge, S.; et al. Scale-dependent and driving relationships between spatial features and carbon storage and sequestration in an urban park of Zhengzhou, China. Sci. Total Environ. 2023, 894, 164916. [Google Scholar] [CrossRef]
- Escobedo, F.J.; Nowak, D.J. Spatial Heterogeneity and Air Pollution Removal by an Urban Forest. Landsc. Urban Plan. 2008, 90, 102–110. [Google Scholar] [CrossRef]
- Nowak, D.J.; Maco, S.; Binkley, M. i-Tree: Global Tools to Assess Tree Benefits and Risks to Improve Forest Management. Arboric. Consult. 2018, 51, 10–13. [Google Scholar]
- Colgan, M.S.; Asner, G.P.; Swemmer, T. Harvesting tree biomass at the stand level to assess the accuracy of field and airborne biomass estimation in savannas. Ecol. Appl. 2013, 23, 1170–1184. [Google Scholar] [CrossRef]
- West, G.B.; Brown, J.H.; Enquist, B.J. A general model for ontogenetic growth. Nature 2001, 413, 628–631. [Google Scholar] [CrossRef]
- Niklas, K.J.; Enquist, B.J. On the Vegetative Biomass Partitioning of Seed Plant Leaves, Stems, and Roots. Am. Nat. 2002, 159, 482–497. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.E.H.; Kalff, J. Size—Dependent Phosphorus Uptake Kinetics and Cell Quota in Phytoplankton. J. Phycol. 1982, 18, 275–284. [Google Scholar] [CrossRef]
- Kouamé, Y.A.G.; Millan, M.; N’Dri, A.B.; Charles-Dominique, T.; Konan, M.; Bakayoko, A.; Gignoux, J. Multispecies allometric equations for shrubs and trees biomass prediction in a Guinean savanna (West Africa). Silva Fenn. 2022, 56, 10617. [Google Scholar] [CrossRef]
- Conti, G.; Gorné, L.D.; Zeballos, S.R.; Lipoma, M.L.; Gatica, G.; Kowaljow, E.; Whitworth-Hulse, J.I.; Cuchietti, A.; Poca, M.; Pestoni, S.; et al. Developing allometric models to predict the individual aboveground biomass of shrubs worldwide. Glob. Ecol. Biogeogr. 2019, 28, 961–975. [Google Scholar] [CrossRef]
- Mildrexler, D.J.; Berner, L.T.; Law, B.E.; Birdsey, R.A.; Moomaw, W.R. Large Trees Dominate Carbon Storage in Forests East of the Cascade Crest in the United States Pacific Northwest. Front. For. Glob. Chang. 2020, 3, 594274. [Google Scholar] [CrossRef]
- Chen, W.Y. The role of urban green infrastructure in offsetting carbon emissions in 35 major Chinese cities: A nationwide estimate. Cities 2015, 44, 112–120. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, Q.; Li, X. Promoting sustainable carbon sequestration of plants in urban greenspace by planting design: A case study in parks of Beijing. Urban For. Urban Green. 2021, 64, 127291. [Google Scholar] [CrossRef]
- Jo, H.-K.; Park, H.-M.; Kim, J.-Y. Carbon Offset Service of Urban Park Trees and Desirable Planting Strategies for Several Metropolitan Cities in South Korea. Forests 2023, 14, 278. [Google Scholar] [CrossRef]
- Shadman, S.; Ahanaf Khalid, P.; Hanafiah, M.M.; Koyande, A.K.; Islam, M.A.; Bhuiyan, S.A.; Sin Woon, K.; Show, P.-L. The carbon sequestration potential of urban public parks of densely populated cities to improve environmental sustainability. Sustain. Energy Technol. Assess. 2022, 52, 102064. [Google Scholar] [CrossRef]
- Jo, H.-K.; Kim, J.-Y.; Park, H.-M. Carbon reduction and planning strategies for urban parks in Seoul. Urban For. Urban Green. 2019, 41, 48–54. [Google Scholar] [CrossRef]
- Xu, Q.; Dong, Y.; Yang, R. Influence of different geographical factors on carbon sink functions in the Pearl River Delta. Sci. Rep. 2017, 7, 110. [Google Scholar] [CrossRef] [PubMed]
- Nie, Q.; Wu, G.; Li, L.; Man, W.; Ma, J.; Bao, Z.; Luo, L.; Li, H. Exploring scaling differences and spatial heterogeneity in drivers of carbon storage Changes: A comprehensive geographic analysis framework. Ecol. Indic. 2024, 165, 112193. [Google Scholar] [CrossRef]
- Lloyd, G.; Ossola, A.; Burley, H.; Evans, K. Climate change threatens carbon storage in Europe’s urban trees. Urban For. Urban Green. 2024, 101, 128532. [Google Scholar] [CrossRef]
- Chen, Y.; Guerschman, J.P.; Cheng, Z.; Guo, L. Remote sensing for vegetation monitoring in carbon capture storage regions: A review. Appl. Energy 2019, 240, 312–326. [Google Scholar] [CrossRef]
- Suriya Prabhakaran, M.; Rohith, V.; Prabu, T. Carbon Capture and Storage Potential in India. In Proceedings of the First International Conference on Science, Engineering and Technology Practices for Sustainable Development, ICSETPSD 2023, Coimbatore, India, 17–18 November 2023; EAI: Coimbatore, India, 2024. [Google Scholar] [CrossRef]
- Brown, S. Measuring carbon in forests: Current status and future challenges. Environ. Pollut. 2002, 116, 363–372. [Google Scholar] [CrossRef]
- Cavanaugh, K.C.; Bell, T.; Costa, M.; Eddy, N.E.; Gendall, L.; Gleason, M.G.; Hessing-Lewis, M.; Martone, R.; McPherson, M.; Pontier, O.; et al. A Review of the Opportunities and Challenges for Using Remote Sensing for Management of Surface-Canopy Forming Kelps. Front. Mar. Sci. 2021, 8, 753531. [Google Scholar] [CrossRef]
- Dubovik, O.; Schuster, G.L.; Xu, F.; Hu, Y.; Bösch, H.; Landgraf, J.; Li, Z. Grand Challenges in Satellite Remote Sensing. Front. Remote Sens. 2021, 2, 619818. [Google Scholar] [CrossRef]
- Yang, H.; Chen, W. Spatio-temporal pattern of urban vegetation carbon sink and driving mechanisms of human activities in Huaibei, China. Environ. Sci. Pollut. Res. 2022, 29, 31957–31971. [Google Scholar] [CrossRef]
- Chen, J.; Fan, W.; Li, D.; Liu, X.; Song, M. Driving factors of global carbon footprint pressure: Based on vegetation carbon sequestration. Appl. Energy 2020, 267, 114914. [Google Scholar] [CrossRef]
- Ren, Y.; Yan, J.; Wei, X.; Wang, Y.; Yang, Y.; Hua, L.; Xiong, Y.; Niu, X.; Song, X. Effects of rapid urban sprawl on urban forest carbon stocks: Integrating remotely sensed, GIS and forest inventory data. J. Environ. Manag. 2012, 113, 447–455. [Google Scholar] [CrossRef]
- Zhang, Z.; Wan, X.; Sheng, K.; Sun, H.; Jia, L.; Peng, J. Impact of Carbon Sequestration by Terrestrial Vegetation on Economic Growth: Evidence from Chinese County Satellite Data. Sustainability 2023, 15, 1369. [Google Scholar] [CrossRef]
- Chen, G.-s.; Zeng, D.-h.; Chen, F.-s. Concentrations of foliar and surface soil nutrients in Pinus spp. plantations in relation to species and stand age in Zhanggutai sandy land, northeast China. J. For. Res. 2004, 15, 11–18. [Google Scholar] [CrossRef]
- Ghareghiye, Z.N. Management of Carbon Sequestration by Identification of Soil Limitation Factors That Affect Plants Growing. Innov. Educ. Res. 2019, 7, 538–557. [Google Scholar] [CrossRef]
- Hoover, C.M.; Smith, J.E. Aboveground live tree carbon stock and change in forests of conterminous United States: Influence of stand age. Carbon Balance Manag. 2023, 18, 7. [Google Scholar] [CrossRef]
- Stephenson, N.L.; Das, A.J.; Condit, R.; Russo, S.E.; Baker, P.J.; Beckman, N.G.; Coomes, D.A.; Lines, E.R.; Morris, W.K.; Rüger, N.; et al. Rate of tree carbon accumulation increases continuously with tree size. Nature 2014, 507, 90–93. [Google Scholar] [CrossRef]
- Domeignoz-Horta, L.A.; Cappelli, S.L.; Shrestha, R.; Gerin, S.; Lohila, A.K.; Heinonsalo, J.; Nelson, D.B.; Kahmen, A.; Duan, P.; Sebag, D.; et al. Plant diversity drives positive microbial associations in the rhizosphere enhancing carbon use efficiency in agricultural soils. Nat. Commun. 2024, 15, 8065. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Jaen, M.C.; Potvin, C. Can we predict carbon stocks in tropical ecosystems from tree diversity? Comparing species and functional diversity in a plantation and a natural forest. New Phytol. 2011, 189, 978–987. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, J.; Wang, J.; Han, S.; Guo, Z.; Fan, C.; Yu, J. Relationships between Tree Species Diversity and Aboveground Biomass Are Mediated by Site-Dependent Factors in Northeastern China Natural Reserves on a Small Spatial Scale. Sustainability 2024, 16, 8378. [Google Scholar] [CrossRef]
- Marquard, E.; Weigelt, A.; Temperton, V.M.; Roscher, C.; Schumacher, J.; Buchmann, N.; Fischer, M.; Weisser, W.W.; Schmid, B. Plant species richness and functional composition drive overyielding in a six-year grassland experiment. Ecology 2009, 90, 3290–3302. [Google Scholar] [CrossRef]
- Szwagrzyk, J.; Gazda, A. Above-ground standing biomass and tree species diversity in natural stands of Central Europe. J. Veg. Sci. 2007, 18, 555–562. [Google Scholar] [CrossRef]
- Sintayehu, D.W.; Belayneh, A.; Dechassa, N. Aboveground carbon stock is related to land cover and woody species diversity in tropical ecosystems of Eastern Ethiopia. Ecol. Process. 2020, 9, 37. [Google Scholar] [CrossRef]
- Lei, H.; Duan, D.; Chen, Y.; Guo, H.; Li, J.; Li, X. Effects of landscape fragmentation of plantation forests on carbon storage in the Loess Plateau, China. J. Arid Land 2024, 16, 266–281. [Google Scholar] [CrossRef]
- Mesa-Sierra, N.; Laborde, J.; Chaplin-Kramer, R.; Escobar, F. Carbon stocks in a highly fragmented landscape with seasonally dry tropical forest in the Neotropics. For. Ecosyst. 2022, 9, 100016. [Google Scholar] [CrossRef]
- Jha, C.S.; Goparaju, L.; Tripathi, A.; Gharai, B.; Raghubanshi, A.S.; Singh, J.S. Forest fragmentation and its impact on species diversity: An analysis using remote sensing and GIS. Biodivers. Conserv. 2005, 14, 1681–1698. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, L.; Li, Z.; Ouyang, X.; Xiao, T.; Wang, H.; Li, W.; Nie, X. Landscape fragmentation and regularity lead to decreased carbon stocks in basins: Evidence from century-scale research. J. Environ. Manag. 2024, 367, 121937. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, H.; Wang, C.; Zhou, Y. Impact of land use change on regional carbon sink capacity: Evidence from Sanmenxia, China. Ecol. Indic. 2023, 156, 111189. [Google Scholar] [CrossRef]
- Wang, G.; Guan, D.; Xiao, L.; Peart, M.R. Forest biomass-carbon variation affected by the climatic and topographic factors in Pearl River Delta, South China. J. Environ. Manag. 2019, 232, 781–788. [Google Scholar] [CrossRef]
- Dong, Z.; Hou, L.; Ruan, Q. Effect of Elevation Gradient on Carbon Pools in a Juniperus przewalskii Kom. Forest in Qinghai, China. Sustainability 2023, 15, 6163. [Google Scholar] [CrossRef]
- Zhu, K.; Zhang, J.; Niu, S.; Chu, C.; Luo, Y. Limits to growth of forest biomass carbon sink under climate change. Nat. Commun. 2018, 9, 2709. [Google Scholar] [CrossRef]




| Family | Conversion Coefficient | Family | Conversion Coefficient | Family | Conversion Coefficient |
|---|---|---|---|---|---|
| Taxaceae | 3.10 | Malvaceae | 13.02 | Paeoniaceae | 7.81 |
| Euphorbiaceae | 4.23 | Arecaceae | 8.05 | Lamiaceae | 18.82 |
| Aquifoliaceae | 5.54 | Calycanthaceae | 15.86 | Salicaceae | 9.54 |
| Fabaceae | 6.61 | Cupressaceae | 10.23 | Pinaceae | 10.32 |
| Theaceae | 4.39 | Podocarpaceae | 8.01 | Cycadaceae | 6.37 |
| Moraceae | 27.34 | Caprifoliaceae | 3.49 | Myrtaceae | 10.68 |
| Rosaceae | 9.23 | Magnoliaceae | 28.97 | Sapindaceae | 15.08 |
| Lythraceae | 9.56 | Oleaceae | 17.84 | Berberidaceae | 4.00 |
| Celastraceae | 12.76 | Apocynaceae | 5.53 | Pentaphylacaceae | 8.58 |
| Other Shrubs | 10.12 | ||||
| Factor Type | Factor Name | Abbreviation | Ecological Meaning |
|---|---|---|---|
| Park Characteristics | Park Green Space Area | S | Area covered by green space within the park |
| Park Establishment Time | Y | Time since the park was established | |
| Vegetation Area Ratio | Pg | Ratio of green space area to total area within the region | |
| Impervious Surface Ratio | Pi | Ratio of impervious surface area to total area within the region | |
| Water Body Ratio | Pw | Ratio of water body area to total area within the region | |
| Woody Vegetation Ratio | Pm | Ratio of woody vegetation cover area to total area within the region | |
| Shannon Evenness Index | SHEI | Reflects the evenness of different patch types in the landscape | |
| Shannon Diversity Index | SHDI | Indicates the diversity of different patch types in the landscape | |
| Community Structure and Biodiversity Characteristics | Shannon–Wiener Diversity Index | SW | Describes species occurrence disorder and uncertainty, considering species count and unevenness; higher values indicate greater diversity |
| Simpson Diversity Index | Sim | Probability that two randomly selected individuals belong to different species; values closer to 1 indicate higher biodiversity | |
| Margalef Richness Index | Dm | Normalizes richness by total individual count for comparability across sample sizes; higher values indicate greater species richness | |
| Pielou Evenness Index | PIE | Describes the uniformity of individual distribution across species in a community; higher values indicate more even distribution | |
| Average Tree Height | H | Average height of trees within the park | |
| Average Diameter at Breast Height | DBH | Average DBH of trees within the park | |
| Average Crown Width | C | Average crown width of trees within the park | |
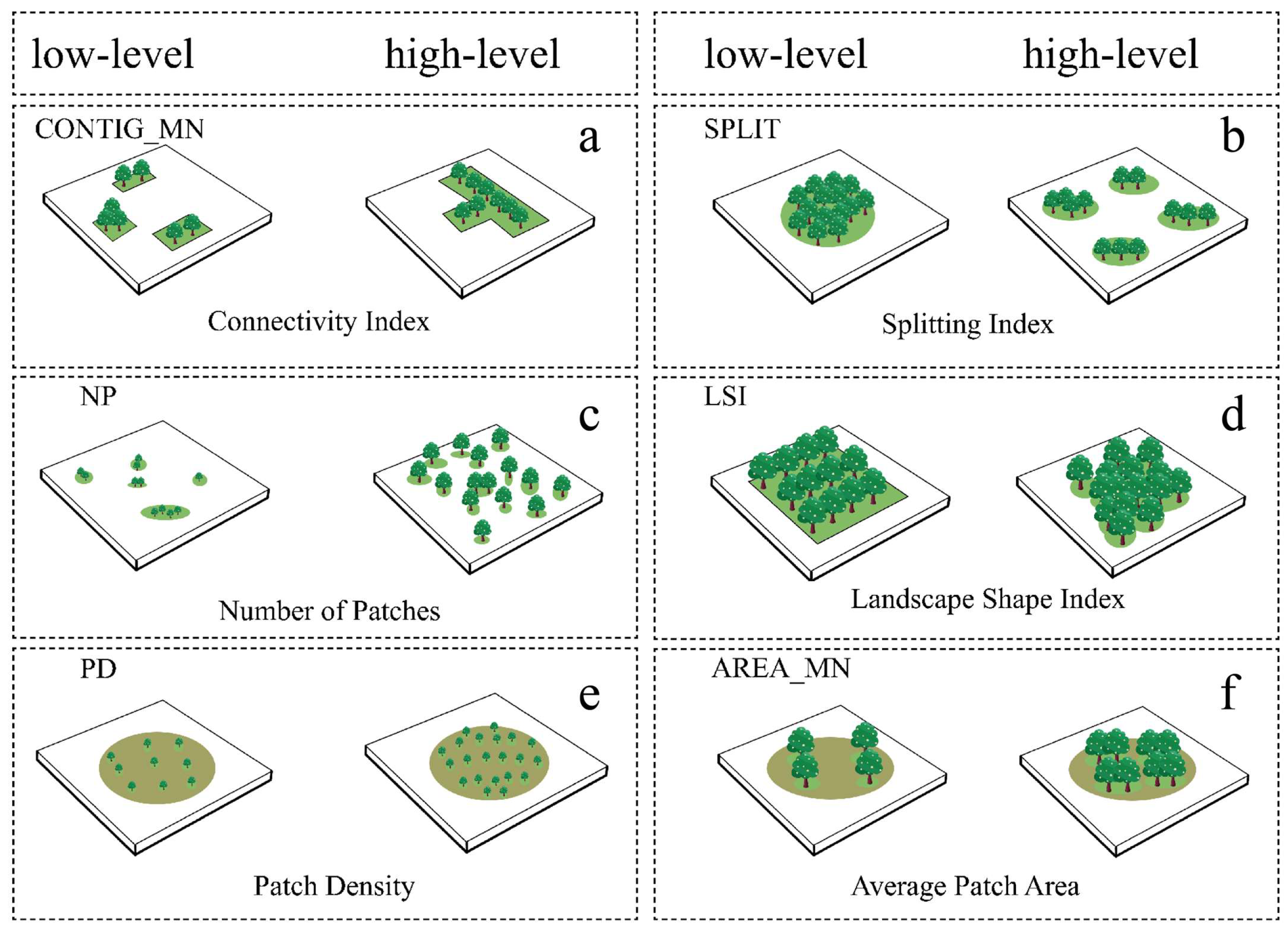
| Woody Plant Spatial Distribution and Connectivity Characteristics | Connectivity Index | CONTIG_MN | Average connectivity between woody plant patches values closer to 1 indicate stronger connectivity |
| Number of Patches | NP | Number of woody plant patches | |
| Patch Density | PD | Density of woody plant patches | |
| Splitting Index | SPLIT | Degree of fragmentation of woody plant patches | |
| Aggregation Index | AI | Degree of aggregation of woody plant patches | |
| Woody Plant Spatial Morphology and Complexity Characteristics | Landscape Shape Index | LSI | Overall woody plant patch shape complexity |
| Largest Patch Index | LPI | Percentage of landscape area occupied by the largest woody plant patches | |
| Average Patch Area | AREA_MN | Average size of woody plant patches | |
| Mean Shape Index | SHAPE_MN | Average complexity of woody plant patches |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Ren, Q.; Zhou, Y.; Dong, N.; Wang, H.; Hu, Y.; Song, P.; He, R.; Tian, G.; Ge, S. Influence of Tree Community Characteristics on Carbon Sinks in Urban Parks: A Case Study of Xinyang, China. Land 2025, 14, 653. https://doi.org/10.3390/land14030653
Zhang H, Ren Q, Zhou Y, Dong N, Wang H, Hu Y, Song P, He R, Tian G, Ge S. Influence of Tree Community Characteristics on Carbon Sinks in Urban Parks: A Case Study of Xinyang, China. Land. 2025; 14(3):653. https://doi.org/10.3390/land14030653
Chicago/Turabian StyleZhang, Honglin, Qiutan Ren, Yuyang Zhou, Nalin Dong, Hua Wang, Yongge Hu, Peihao Song, Ruizhen He, Guohang Tian, and Shidong Ge. 2025. "Influence of Tree Community Characteristics on Carbon Sinks in Urban Parks: A Case Study of Xinyang, China" Land 14, no. 3: 653. https://doi.org/10.3390/land14030653
APA StyleZhang, H., Ren, Q., Zhou, Y., Dong, N., Wang, H., Hu, Y., Song, P., He, R., Tian, G., & Ge, S. (2025). Influence of Tree Community Characteristics on Carbon Sinks in Urban Parks: A Case Study of Xinyang, China. Land, 14(3), 653. https://doi.org/10.3390/land14030653










