Abstract
Forests provide essential ecosystem services but face increasing threats from invasive species like Toumeyella parvicornis (pine tortoise scale). Since its introduction to Italy in 2014, this pest has severely impacted Pinus pinea forests, with a major outbreak in 2019 affecting an urban forest in the Rome municipality area. This study aims to develop a tool for detecting forest dieback symptoms caused by the scale and assess the role of prevailing winds in its dispersal by integrating multispectral and hyperspectral earth observation systems, including Sentinel-2 and the Hyperspectral Precursor of the Application Mission (PRISMA). At a 6000-hectare protected area with diverse vegetation, a binary Random Forest classifier, trained on near-infrared and short-wave infrared reflectance data, identified symptomatic stands. A generalized linear mixed model compared uniform and wind-influenced probabilistic dispersal models, assessing the pest spread relative to the initial infestation hotspot. The results confirmed a sharp decline in near-infrared reflectance in 2019, indicating severe defoliation and a shift from evergreen to deciduous canopy phenology by 2021. The classifier achieved 82% accuracy, effectively detecting symptomatic pine forests (91% precision). The scale spread to 51% of the pine forest area by 2021, with no strong correlation to prevailing winds, suggesting other augmenting dispersal drivers, such as vehicles along congested routes, wind tunnels, pest-resistant forests, and the potential mitigating role of alternating coastal wind patterns that are effective in the study area.
1. Introduction
Forests play a key role in providing ecosystem services and mitigating climate change effects [1]. However, several threats have hit forests at an unprecedented scale, including extensive wind throws [2,3,4], repeated frosts [5,6,7], droughts [8,9], and pest outbreaks [10,11], leading to higher tree mortality rates [12], decreases in productivity, and CO2 sink potential [13,14]. Specifically, the well-established intercontinental commercial routes [15,16] are key factors contributing to the repeated emergence of pest outbreaks in agricultural systems, forests, and urban forest ecosystems [1,17]. Such conditions are increasingly facilitating the accidental introduction of alien species, including insects.
The establishment, spread, and survival of alien insect species in new areas are enhanced by the disruption of biological control as well as by human activities and extreme climate events that can create favorable ecological niches [8,9,12]. Pine tortoise scale, Toumeyella parvicornis (Cockerell) (Hemiptera: Coccidae, hereafter “PTS”), is a recent example of an invasive species in the Mediterranean area and is seriously endangering the urban forests and public green areas of several cities. PTS is native to North America, where it is widespread from the Southern USA (i.e., Florida and Georgia) to Canada, and lives and reproduces on Pinus spp. [18]. The first PTS record in Italy dates from 2014 when it was identified in Campania [19]. It then moved gradually northward and southward, becoming established in forests and urban areas [20]. More recently, it was found in France [20] and Albania [21].
In Italy, the infestations of PTS were recorded mainly on Pinus pinea L. (stone pine) trees located in urban areas. The disease symptoms were at times severe, including a reduction in shoot development, desiccation and yellowing of the needles, lack of vegetative renewal, and release of a considerable amount of honeydew that, in turn, favors the development of sooty molds [22]. This damage phenology is similar to that caused by other scale species affecting pine trees in Italy, mainly Matsucoccus feytaudi (Ducasse), which is responsible for infestations on Pinus pinaster (maritime pine), and Crisococcus pini (Kuwana) that has, so far, been recorded only in the northern part of the country [23,24].
The impact of such infestations in the invaded areas is of particular importance due to the widespread presence of stone pine. Indeed, this host tree is an important species in the Italian landscape and is included in several Natura 2000 Network areas and in the European Red List of Habitats for its significant naturalistic, historical, and social interest [13]. Stone pine plantations are described as “Mediterranean coniferous coastal dune woodland” and cover a considerable part of coastal areas in the Mediterranean basin [25].
Urban forests that feature stone pine as the dominant species are present in major Italian cities along the Tyrrhenian coast, where they provide ecosystem services or socio-ecological services for citizens [26]. Since the 19th century, extensive reforestation programs have predominantly resorted to Pinus ssp. seedlings. Pine forests in the Mediterranean area yield resources that are still the backbone of a number of economic activities [27], including tourism and leisure pastimes [28].
Monitoring, eradication, and the setup of containment measures in buffer zones against PTS infestations have been mandatory at the national level since 2021. The boundaries of the PTS-infested areas and the buffer zones surrounding the symptomatic area, at least 5 km wide, have been established in Lazio and were last updated in 2023.
Patterns of insect dispersion are little understood and difficult to predict. The wind is one of the most important factors that can influence the local as well as long-distance dispersion of insects, for example, between agricultural fields as well as between forest stands and plantations, particularly in those ecosystems where the wind is a pervasive climatic factor of the environment [29] and is aided by orography or man-made structures that can channel the wind more effectively. For wingless or very weak-flying insects, such as scales, the main direction of dispersion is downwind up to a few meters away or, more rarely, a few hundred kilometers [30]. PTS was recorded as being carried up to 5 km in [31,32], although its downwind dispersion in relation to prevailing winds has never been investigated.
Large-scale detection of pest infestation, including the use of proximal- [33] and remote-sensing [34], can help assess the local dispersion patterns [35] and contribute to the development of mitigation and containment systems. Multispectral remote sensing has become widely established as a means of monitoring biotic and abiotic anomalies in forests by detecting breakpoints or anomalous trends in phenological patterns. Phenology is one of the most sensitive biological indicators of changes in underlying growing conditions, whether due to a changing climate or any occurring anomaly, such as pest outbreaks [36,37].
There has been much focus on reflectance measurements in the visible (VIS, 450–700 nm), near-infrared (NIR, 700–1400 nm), and short-wave infrared (SWIR, 1400–2500 nm) wavelengths. Changes in these spectral regions are associated with several physiological and biochemical traits [38], including absorption of solar radiation by chlorophyll a, b, and carotenoids (VIS); the scattering of individual leaves and whole plant canopies driven by the internal leaf structure and canopy structure (NIR); and the SWIR, which can be indicative of the water content and the biochemical composition of leaves [39,40,41]. Recent developments in the cloud computing approach to elaborate long-time series of optical images enable the assessment of anomalous spectral changes and the automatic assessment of forest disturbances [42].
There is still uncertainty regarding the use of hyperspectral remote sensing, as such sensors have only recently been deployed in orbit, granting freely available products [43,44,45,46]. PRISMA (Hyperspectral Precursor of the Application Mission) offers global coverage, with a spectral resolution of 12 nm and a spatial resolution of 30 × 30 m, and has a repeat cycle of approximately 29 days. The VIS-NIR and SWIR sensors are integrated with a panchromatic camera [46].
In this context, we assessed the effects of the PTS outbreak in a stone pine urban forest located along the Tyrrhenian coast in Central Italy using hyperspectral PRISMA imaging. Since the first outbreak in Rome in 2018, PTS has spread toward the coastal forests, seriously affecting stone pines. This research aimed to (i) spatially detect the symptoms of defoliation on stone pines infested by PTS in an extensive protected area, (ii) evaluate how the prevailing winds had affected the spread pattern of the pest since its onset, and (iii) assess the potential of using PRISMA hyperspectral imaging to detect pest outbreaks in an urban forest in terms of accuracy and sensitivity.
2. Material and Methods
2.1. Study Site
The Presidential Estate of Castelporziano is a natural vegetation area of approximately 6000 hectares under legal protection status, located 25 km southwest of Rome in the region of Lazio (Figure 1A).
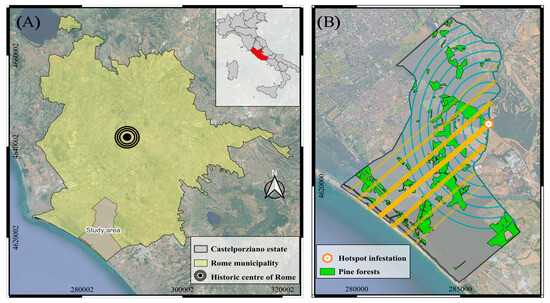
Figure 1.
(A) Geographical location of the Castelporziano Presidential Estate in the Rome municipality area (In red the Lazio region, Italy). (B) Map of Castelporziano Estate with the scale pest infestation hotspot, the concentric circles representing a uniform dispersion model (cyan color), and the linear spread along the line of the prevailing wind passing through the infestation hotspot (yellow color). Coordinate Reference System: WGS84/UTM zone 33N.
Lazio lies within a Thermo-Mediterranean zone, marked by extended aridity in summer and moderate cold snaps in winter. Low-pressure episodes steer the synoptic-scale air mass movements, while high-pressure periods predominantly dictate circulation patterns, fostering a local sea–land breeze regime [47]. Wind data were collected from the meteorological station in Castelporziano. Wind patterns were aggregated in a study period from April to June (i.e., when new generations of PTS arise after overwintering) for the years 2019 and 2020.
The Estate features a wide range of vegetation types, including sand dune vegetation, Mediterranean maquis, high maquis, Quercus ilex evergreen forests, Quercus suber forests, broadleaf mixed forests, Eucalyptus globulus plantations, and cropland [48]. Pine plantations account for 678 ha and are mainly formed by Pinus pinea, with a minor presence of P. halepensis, P. brutia, and P. pinaster [27,49].
The onset of the PTS pest outbreak on the stone pine forests in the study site was reported by the Estate’s personnel during visual ground checks in 2019. The first infestation hotspot (Figure 1B) was pinpointed by high-resolution multispectral imagery in the northeastern part of the Estate and dated as early as August 2018 [50].
2.2. Phenological Dataset
A dataset of long-term near-infrared (NIR) and visible red reflectance data on a randomly chosen subset of locations in the Estate was used to drive the choice of the month and year with which to estimate the PTS symptom detection map and the threshold reflectance value to separate the binary classes of the presence/absence of symptoms.
Forty locations were randomly selected in stone pine stands and labeled as presence/absence of symptoms according to visual ground checks and historical reconstruction of the PTS infestation by the Estate’s personnel. A visual ground inspection included the sight of sooty mold, needle yellowing, and branch desiccation. Such symptoms are strictly associated with PTS infestations, whereas the other stone pine pests that are known to be present in that area, mainly coleopterans belonging to the families Curculionidae and Cerambicidae, as well as lepidopterans belonging to the families Tortricidae and Notodontidae, are responsible for unequivocally different damage to the canopy and trunk. On the other hand, the other Mediterranean scale species producing similar symptoms on stone pines (i.e., Crisicoccus pini (Kuwana) and Marchalina hellenica (Gennadius)) are known to be absent in the surveyed area.
The Copernicus Sentinel-2 mission satellites aim to provide multispectral data products with a 5-day revisit frequency and a 10 × 10 m spatial resolution [51]. Cloud and cirrus formations were detected and removed through the product quality assurance metadata. Near-infrared (NIR) and visible red reflectance data were collected on the sampling points from all images in the timeframe 2017–2022 and aggregated as median value composites using Google Earth Engine [52] (Figure 2).
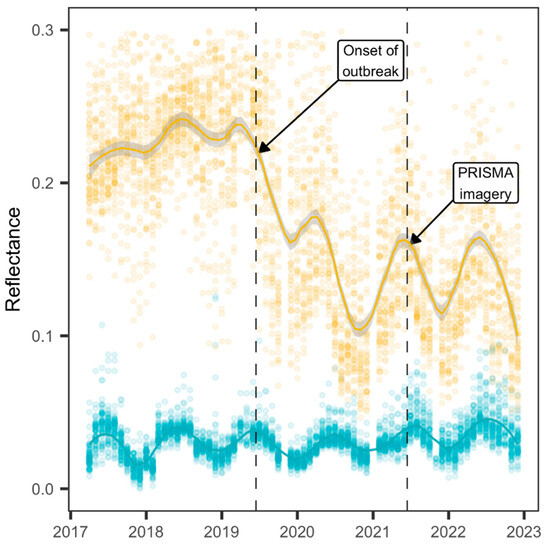
Figure 2.
The monthly median and composite values of near-infrared (yellow color) and visible red (cyan color) reflectance were sampled at 40 random points in the forest stands and loess-smoothed curves. PRISMA is an acronym for “Hyperspectral Precursor of the Application Mission” earth observation system used to detect the symptoms of infestation of Toumeyella parvicornis in forest stands.
2.3. Detection Dataset and Model
PRISMA imagery was used to detect the presence of PTS symptoms on the Estate stone pine stands. Among all the levels provided by the PRISMA mission, level 2D is the most comprehensive, offering reflectance information, atmospheric corrections, and geographical co-registration. Other levels provide raw radiance without radiometric, atmospheric, and geographic corrections.
The imagery used had a maximum of 2.7% cloud coverage, which never affected the scene over the Estate. The downloaded he5 file also contained all the metadata related to the platform required for geometric processing. The original he5 file was opened in the Python (version 3.12) environment using the h5py library (https://pypi.org/project/h5py/ (accessed on 1 June 2024)), and GeoTIFF images containing all the 239 hyperspectral bands were created. From this set of bands, 27 were removed due to many values tending toward zero, representing the spectral region between the VIS-NIR and SWIR, as well as intervals within different parts of the SWIR.
The detection dataset that was used to train, validate, and test a binary Random Forest (RF) classifier was formed by random sampling points geographically located in pine forest stands, with a minimum neighbor distance of 30 m, and including the 40 locations of the phenological dataset. Stands were buffered inward by the same distance to mitigate the potential edge effect caused by the coarse spatial resolution of the PRISMA image. As a result of the buffer process, the total pine forest area decreased to 530 ha.
Reflectance thresholds from the phenological dataset were used to integrate ground validation data to correctly label the binary class (the presence/absence of symptoms in pine forests). For this reason, all PRISMA bands in the visible region were excluded from the detection dataset to avoid any dependency issues in the training of the RF classifier.
The final detection dataset featured 384 locations and 167 features. Train, validation, and test sub-datasets were randomly sampled from the detection dataset with 65%, 10%, and 25% proportions, respectively.
To obtain the highest model accuracy, before applying the Random Forest algorithm for the final classification, the model was fine-tuned using the testing dataset. Different combinations of hyperparameters were used to train the Random Forest model on the training dataset. The sequences of parameters were defined as follows: number of trees ranging from 1 to 100 with a step of 10, variables per split ranging from 1 to 20 with a step of 2, and leaf population ranging from 1 to 10 with a step of 1. The accuracy of each trial was then evaluated on the independent testing dataset that was never used during training, ensuring an unbiased assessment of the model performance for each parameter configuration. Finally, the model was trained and validated with training and validation datasets. A classification accuracy assessment was carried out using the overall accuracy (OA) and Kappa coefficient of agreement (), one of the most common and reliable indicators for reporting the accuracy of thematic maps [53].
Furthermore, RF provides an important score for each variable used for classification (PRISMA image bands), which was used to determine what wavelengths were most significant for infestation diagnosis. A threshold of importance was established to select the most diagnostic bands. The reflectance values were used for labeling each pixel according to the presence/absence of symptoms.
2.4. Wind Dispersion Dataset and Model
Wind dispersion of PTS in the study site was evaluated as the probability of a pine forest location being labeled as symptomatic as a function of its distance from an infestation hotspot, under the assumption that the likelihood of a pine tree being infested decreases with increasing distance.
A balanced binary dataset of pine forest locations was randomly sampled from the detection map by including 525 points for each binary class (total: 1050 points) and fitted to a binomial response generalized linear mixed model (GLMM [54]) that was used to link a binary (presence/absence of PTS symptoms) response variable to its physical distance to the infestation hotspot. A GLMM is an extension of the ordinary linear regression to non-normal data in which the linear predictor contains random effects in addition to fixed effects [55].
The random component of the GLMM included two random intercepts: the forest stands each pixel was sampled in and a spatial term. The expectation that closer observations, in terms of latitude and longitude, should be more correlated than those further away was accounted for by the spatial term using a Matérn covariance function.
The effect of the dominant wind on PTS dispersion was evaluated by comparing two GLMMs differing only by how the distance explanatory variable was computed.
A uniform dispersion model (UDM) included straight distances between observations and the initial infestation hotspot as the explanatory variable. This model assumes a random uniform PTS dispersion direction and a likelihood of infestation decreasing with distance from the infestation hotspot (Figure 1B).
A prevailing wind dispersion model (PWDM) included straight distances between the observations and the dominant wind direction as the explanatory variable. This model assumes that PTS predominantly disperses along the dominant wind direction in the study site (Figure 1B).
The magnitude of the error affecting the estimate of the distance coefficient and the proportion of variance explained by each model (R-squared based on the likelihood ratios) contributed to defining which model could better predict the diffusion of PTS [56]. A lower standard error of the distance coefficient led to more precise estimates, whereas a greater R-squared resulted in a higher contribution of distance toward explaining the variability of the presence/absence of infestation in each model.
3. Results
The onset of the PTS pest outbreak in 2019 on the stone pine forests in the study site, reported by visual inspection by the Estate’s personnel, was confirmed by analyzing the long-term phenology reflectance data in the wavelength ranges of near-infrared (NIR) and visible red, most commonly used to derive common vegetation indexes (Figure 3).
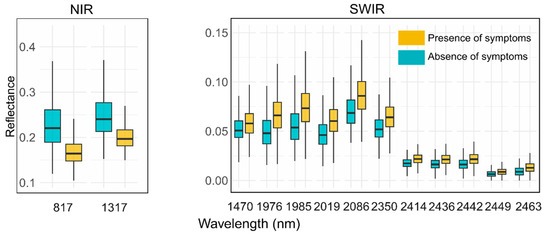
Figure 3.
Boxplot of reflectance value distributions per class in each of the relevant bands for symptom detection.
On the one hand, the steady NIR phenology of evergreen canopy cover [18] clearly showed an abrupt drop in June 2019 and a further decline in 2020, whereas the visible red phenology could not mirror any similar change pattern. By 2021, the NIR phenology changed to a yearly cycle where the original evergreen canopy died or was harvested by alternating photosynthetically active (from spring to autumn) to nonactive seasons (winter) typical of a deciduous broadleaf forest land cover. As a result, to perform detection of the cumulative dispersal of PTS symptoms in a stone pine forest, archive-available PRISMA imagery for 15 June 2021, 10:07 CET, was retrieved.
The map of detected infestation (Figure 4) confirms that, by 2021, most of the pine forests in the study site were irreversibly attacked by PTS. The total outbreak area was estimated to be 270 hectares, or approximately 51% of the entire pine forest area.
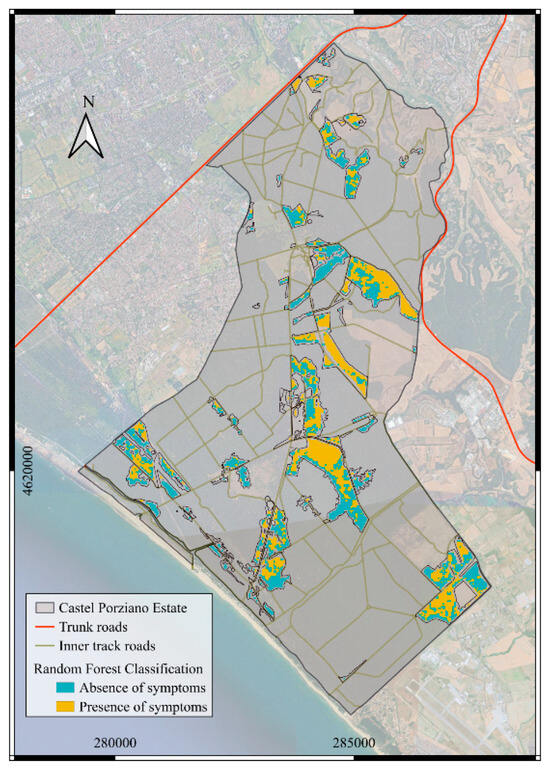
Figure 4.
Binary detection map of PTS infestation in Castelporziano during June 2021 (yellow: presence of symptoms; cyan: absence of symptoms). Coordinate Reference System: WGS84/UTM zone 33N.
The RF classifier revealed an overall good accuracy (82%), specifically in correctly identifying the pine forest pixels affected by PTS (94% user accuracy in the “symptomatic” class, -UA- in Table 1). Despite that, a certain amount of variability affects the estimated predictions (80% producer accuracy in the “symptomatic” class, -PA- in Table 1). Thus, the classifier metric shows the potential for monitoring and correctly detecting PTS outbreaks in pine forests at the cost of misclassifying asymptomatic forest patches as being attacked by PTS once every five predictions of infestation presence.

Table 1.
Random Forest classification confusion matrix.
The stone pine vegetative status (presence/absence of symptoms), picked up by the reflectance spectra (Figure 5), showed higher reflectance for healthy vegetation in the NIR region where leaf internal scattering, foliar biochemical components, and water absorption contribute to the shape of the spectra, and lower reflectance in the SWIR region, where mostly proteins and cell wall components such as lignin and starch share reflective potential.
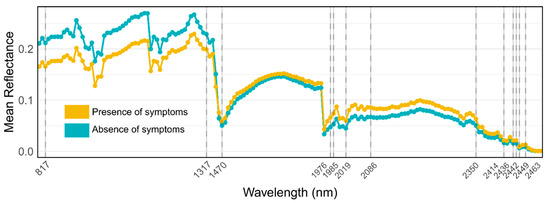
Figure 5.
Average reflectance spectrum graph for the presence (yellow)/absence (cyan) of symptom classes, highlighting important bands selected by RF with vertical segments.
The most useful diagnostic wavelengths used by the RF classifier (Table A1) were found chiefly in the SWIR region (specifically in the 1970–2080 nm and 2350–2500 nm windows) and two more bands in the NIR region (817 nm and 1317 nm). Consistent with previous studies, these results showed that asymptomatic stone pines can be differentiated from symptomatic and stressed vegetation according to their higher reflectance in the NIR regions and lower reflectance in the SWIR region (Figure 5).
Figure 6 shows that the prevailing wind direction during the study period was NE-SW. Such a dynamic confirms the local sea–land breeze regime with mild winds from the sea to the land during daytime hours and from the land to sea at night.
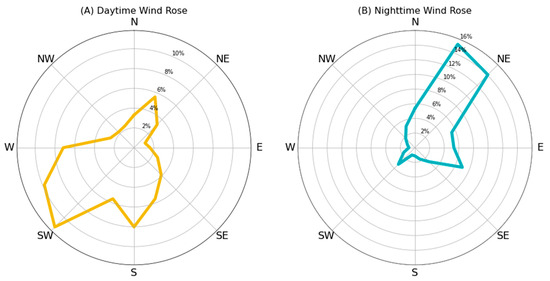
Figure 6.
Wind rose charts of daytime prevailing wind recorded from 08:00 to 21:00 (A) and nighttime prevailing wind recorded from 21:01 to 07:59; (B) The percentages represent the frequency of the wind observations coming from a specific direction, measured in degrees from the North.
The GLMM of the binary detection of infestation by PTS (Table 2) revealed that distance, as the sole explanatory variable, captures only about 22% of its spatial variability, regardless of the dispersion model.

Table 2.
Estimate of the fixed effect distance coefficient for the uniform dispersion model (UDM) and prevailing wind dispersion model (PWDM). The distance coefficient is linked to the decrease in the probability of finding symptoms of PTS infestation per unit change in distance (km) from the origin of dispersal (i.e., hotspot infestation in the case of the UDM or distance from the direction of the prevailing wind in the case of the PWDM).
Although the analysis of variance revealed no significant differences in capturing spatial variability of the symptoms among the UDM and PWDM models (p-value: 0.7661), the probability of PTS presence in stone pines decreases with an increasing distance from the infestation hotspot or the prevailing wind direction (Figure 7).
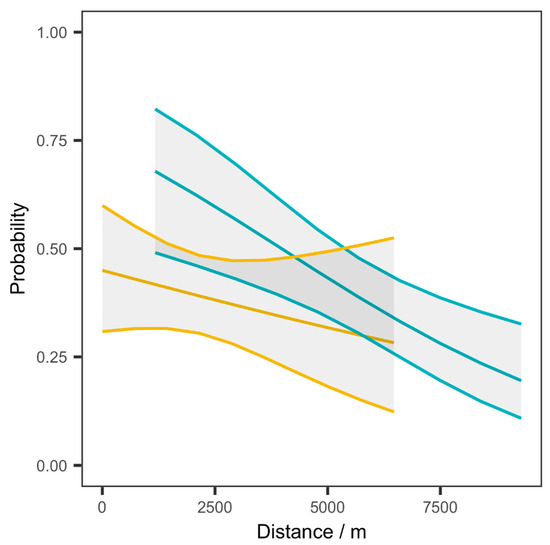
Figure 7.
Probability of the presence of Toumeyella parvicornis symptoms in pine forest pixels in the study site according to the distance from the hotspot infestation (uniform dispersal model, cyan curve) and from the prevailing wind direction (prevailing wind dispersion model, yellow curve).
In UDM, the pine forest pixels that are closest to the infestation hotspot are shown to bear about a 60% probability of being symptomatic, whereas those furthest away have the greatest chances of escaping infestation (less than 25% infestation probability when 8 km away). In the PWDM, the probability of a pine forest pixel being labeled as symptomatic is not significantly affected by the distance from the dominant wind direction due to a wide confidence interval (Figure 7) and a very low t-value (three times lower than the UDM t-value).
4. Discussion
We reported on the detection (by hyperspectral remote sensing) of defoliation in a stone pine urban forest caused by an invasive pest and the modeling of its dispersion due to wind in a study site in Central Italy.
Four years after the first reports of T. parvicornis’ arrival in Europe and Italy [19] in 2014, the pest was detected 150 km further north, in the Castelporziano Presidential Estate, close to Rome. As occurred for the other outbreaks in Italy, PTS reached high levels of population density in a short time thanks to several phenological and ecological factors. Among such factors are the intrinsic high reproduction rate and adaptability to the warm Mediterranean climate that allows several generations per year, as well as the difficulty in implementing sustainable control strategies in an urban environment and the absence of efficient natural enemies [22].
The wind represents one of the drivers for the natural dispersal of wingless insects and very weak fliers whose movement frequently occurs downwind [30]. Airborne dispersal of the first-nymphal instars (crawlers) has been reported in several species of scale insects, and it has been suggested that specific morphological characters allow them to drift longer on air currents. In addition, crawlers of some species display behaviors that seem to be adapted for take-off in wind streams: they accumulate at the tips of leaves, where they are more exposed to air currents, and orient themselves downwind, increasing frictional drag and enhancing removal by air currents [57]. Nymphs of the scale Parthenolecanium corni (Bouché) were found in the prevailing wind sector and were mostly collected on the trap side exposed downwind within and among vineyard plots [58]. Concerning PTS, no information on its wind dispersal mode is currently available.
Most of the pine forests affected by PTS in Castelporziano have been replaced by natural understory deciduous vegetation, which grew intensively thanks to the increased solar radiation penetrating the damaged canopy. The shift from forest canopy to understory vegetation is mirrored by a considerable decrease in mean near-infrared (NIR) reflectance and its shift from a steady inter-annual phenology to a seasonal cycle recorded by both multispectral and hyperspectral satellite sensors. This is in open contrast to the phenology of the reflectance in the photosynthetically active region of the spectrum (visible red), which did not record any symptoms of canopy dieback due to PTS. Thus, the magnitude and seasonal phenology of NIR may be a strong signal of forest canopy dieback. On the other hand, a strong consensus has grown on using vegetation indexes (VIs) for assessing canopy structure, photosynthesis, and LAI [59], which are favored by the convenient interpretation provided by an adimensional indicator that overrides the complexities behind the measurement of radiance and its conversion to reflectance specific to each sensor [60]. VIs usually combine visible and NIR channels, and each VI is suitable for specific uses and is affected by some limiting factors [61], so the choice of a specific VI should be made with caution. A set of recent papers [62,63] mentions that reflectance at NIR wavelengths can be directly used as a proxy for photosynthetic activity assessment. Our results highlight that the red channel carries little or no useful information over the direct usage of the NIR channel for the detection of forest dieback while not being affected by the idiosyncrasies of VIs, such as saturation at high reflectance values or sensitivity to atmospheric effects [64]. This method shows promise for rapidly detecting the onset of pest outbreaks in coniferous forests, providing an alternative to approaches based on VIs often employed in similar studies [65,66].
The full reflectance spectra of the pine canopy from the PRISMA earth observation system proved useful in clearly differentiating the presence/absence of PTS symptoms, probably due to the very high spectral resolution and spectrum, as previously reported in other studies [67,68]. The availability of the SWIR channel, sensitive to the reflectance of the water content and the biochemical composition of leaves, can be considered PRISMA’s main asset over other techniques, such as proximal sensing [69]. The latter technique, conversely, may feature frequent revisit times and higher spatial resolutions that, in turn, may enable multi-temporal studies [33]. This confirms its usefulness in large-scale forest vegetation studies where spatial resolution or temporal resolution are not limiting factors for the detection of canopy stress [70]. It should be noted that although a specific hyperspectral reflectance signature for the PTS infestation on stone pine is potentially feasible, it was not among the objectives of this study.
In the future, numerous satellites for Earth Observation will be launched, such as the IRIDE constellation [71] and CHIME [72], which will include hyperspectral sensors with characteristics and sensitivities similar to those of PRISMA but with shorter revisit times. This will make any pest detection model developed for PRISMA reusable for the remote sensing monitoring of forests, as well as for early warning detection systems such as those that are available today based on multispectral sensors or synthetic aperture radar sensors [73,74].
Airborne dispersal in the study site, originating from the PTS onset infestation hotspot detected in 2018, was found to account for 22% of the spatial variability of its detected presence in pine forests, cumulating years from 2019 to 2021, signaling that other dispersal media like animals, other insects, human-induced transport, or any combination of them in addition to wind and air currents, might be responsible [31]. Although the dispersal direction is downwind for wingless insects and very weak fliers [29], we could not find strong evidence that PTS’s spatial distribution was mediated by the direction of the prevailing winds [49]. This is possibly due to a mitigation effect of sea breezes alternating with land breezes that dominate coastal wind systems during the spring months in the study site. The net effect of an alternating dispersal of PTS could slow down its net forward motion potential from the infestation hotspot and enable the public administration to design effective pest-management control strategies or identify potential predators and parasites.
Limitations of the wind models developed in this study include the coarse spatial resolution of the PTS detection map (30 × 30 m) that did not allow for the inclusion of microclimate, terrain, or canopy effects. Permanent windbreaks on land or artificial wind tunnels, such as wide, straight, and coast-inland roads, if present, could also modify the wind profiles and the distribution and dispersal of PTS [29]. The extent to which these confounding drivers could add to the alternating sea/land breeze wind system effect in slowing down PTS dispersion through the prevailing winds is not yet known. Nonetheless, we suggest monitoring the spread of PTS in coastal areas in all directions from the onset hotspot, regardless of the local wind systems, due to the currently increasing probability of the occurrence of windstorms [75].
5. Conclusions
Multispectral remote sensing confirmed the onset of the Toumeyella parvicornis pest outbreak in 2019 in a coastal urban forest, which led to an abrupt decline in near-infrared reflectance, indicating severe defoliation and a shift from evergreen to deciduous canopy phenology by 2021. Hyperspectral remote sensing, based on PRISMA sensors, enabled the accurate detection of stone pine forest stands bearing symptoms of infestations by picking up a change in reflectance in the near-infrared and short-wave infrared regions of the spectrum. The detection model will be reusable for remote sensing monitoring of forests by hyperspectral satellite constellations that are planned to be operative in the coming years by taking advantage of their high spectral resolution and spectrum.
The prevailing winds were not effective drivers of the cumulative airborne dispersal (2019–2021) of the pest that led to the complete dieback of the stone pine stands in the urban forest. Despite a previous study highlighting the role of the wind as a driver of scale dispersal in a vineyard at the plot level, terrain, canopy, and microclimate conditions could interact to varying degrees in different study sites at a site or regional level. Our results clearly call for the need for large-scale monitoring of the dispersal of pests regardless of the prevailing winds, even in the case of weak fliers such as the pine tortoise scale. This is particularly relevant, especially under climate change scenarios, with an increasing windstorm probability that could spread the pest randomly at unprecedented distances.
The effect and magnitude of confounding factors on the role of the prevailing winds in scale pest dispersion are still unknown. Such factors may include augmenting drivers on pest dispersal, such as the presence of wind tunnels, flat terrains, and intensively trafficked routes, or hindering windbreak drivers, such as the presence of pest-resistant forests, specific terrains, or microclimates. Future work should factor in such effects to provide more effective dispersal models to be included in early warning monitoring systems.
Author Contributions
Conceptualization: S.B., A.T. and M.B.; Formal analysis: A.P. and M.B.; Investigation: S.B., A.T., E.M. and G.C.; Methodology: A.P. and M.B.; Project administration: M.B.; Software: A.P., L.O. and E.M.; Visualization: L.O., E.M. and A.P.; Writing—original draft: M.B., A.P., E.M., L.O., S.B. and A.T.; Writing—review and editing: M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been funded by Comando Unità Forestali, Ambientali e Agroalimentari, Carabinieri (grant CREA PRJ4994m 2.99.99.92.00) “Smart Forest Monitoring”.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
This research was made possible thanks to the personnel of the Presidential Estate of Castelporziano, who assisted the authors with the ground surveys and in the historical reconstruction of the Toumeyella parvicornis infestation and who carried out using the ORIGINAL PRISMA Products—© Italian Space Agency (ASI); the products have been delivered under an ASI License to Use.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PTS | Pine tortoise scale Toumeyella parvicornis (Cockerell) (Hemiptera: Coccidae, hereafter “PTS”) |
| PRISMA | Hyperspectral Precursor of the Application Mission |
| RF | Random Forest |
| VIS | Visible |
| NIR | Near-Infrared Reflectance |
| SWIR | Short-Wave Infrared Reflectance |
| GLMM | Generalized Linear Mixed Model |
| UDM | Uniform Dispersion Model |
| PWDM | Prevailing Wind Dispersion Model |
Appendix A

Table A1.
Average reflectance values for symptomatic and asymptomatic pine forests at relevant wavelengths according to the RF classifier in the 2021 detection map. The NIR spectral region includes bands in the wavelength region of 730–1400 nm, and the SWIR region range is 1400–2500 nm. All reflectance distributions were tested as significantly different by the Kolmogorov–Smirnov non-parametric test of the equality of continuous distributions.
Table A1.
Average reflectance values for symptomatic and asymptomatic pine forests at relevant wavelengths according to the RF classifier in the 2021 detection map. The NIR spectral region includes bands in the wavelength region of 730–1400 nm, and the SWIR region range is 1400–2500 nm. All reflectance distributions were tested as significantly different by the Kolmogorov–Smirnov non-parametric test of the equality of continuous distributions.
| PRISMA Band | Wavelength (nm) | Spectral Region | Average Reflectance Data | |
|---|---|---|---|---|
| Symptomatic Forest | Asymptomatic Forest | |||
| 48 | 817 | NIR | 0.161 | 0.234 |
| 105 | 1317 | NIR | 0.198 | 0.251 |
| 119 | 1470 | SWIR | 0.062 | 0.053 |
| 171 | 1976 | SWIR | 0.070 | 0.049 |
| 172 | 1985 | SWIR | 0.079 | 0.055 |
| 176 | 2019 | SWIR | 0.064 | 0.047 |
| 184 | 2086 | SWIR | 0.091 | 0.071 |
| 218 | 2350 | SWIR | 0.067 | 0.053 |
| 227 | 2414 | SWIR | 0.023 | 0.018 |
| 230 | 2436 | SWIR | 0.022 | 0.016 |
| 231 | 2442 | SWIR | 0.023 | 0.016 |
| 232 | 2449 | SWIR | 0.009 | 0.007 |
| 234 | 2463 | SWIR | 0.014 | 0.009 |
References
- Bellard, C.; Jeschke, J.M.; Leroy, B.; Mace, G.M. Insights from Modeling Studies on How Climate Change Affects Invasive Alien Species Geography. Ecol. Evol. 2018, 8, 5688–5700. [Google Scholar] [CrossRef] [PubMed]
- Pilli, R.; Vizzarri, M.; Chirici, G. Combined Effects of Natural Disturbances and Management on Forest Carbon Sequestration: The Case of Vaia Storm in Italy. Ann. For. Sci. 2021, 78, 46. [Google Scholar] [CrossRef]
- Patacca, M.; Lindner, M.; Lucas-Borja, M.E.; Cordonnier, T.; Fidej, G.; Gardiner, B.; Hauf, Y.; Jasinevičius, G.; Labonne, S.; Linkevičius, E.; et al. Significant Increase in Natural Disturbance Impacts on European Forests since 1950. Glob. Change Biol. 2023, 29, 1359–1376. [Google Scholar] [CrossRef] [PubMed]
- Usbeck, T.; Wohlgemuth, T.; Dobbertin, M.; Pfister, C.; Bürgi, A.; Rebetez, M. Increasing Storm Damage to Forests in Switzerland from 1858 to 2007. Agric. For. Meteorol. 2010, 150, 47–55. [Google Scholar] [CrossRef]
- Bascietto, M.; Bajocco, S.; Ferrara, C.; Alivernini, A.; Santangelo, E. Estimating Late Spring Frost-Induced Growth Anomalies in European Beech Forests in Italy. Int. J. Biometeorol. 2019, 63, 1039–1049. [Google Scholar] [CrossRef]
- Hentschel, K.; Borken, W.; Matzner, E. Repeated Freeze–Thaw Events Affect Leaching Losses of Nitrogen and Dissolved Organic Matter in a Forest Soil. J. Plant Nutr. Soil Sci. 2008, 171, 699–706. [Google Scholar] [CrossRef]
- Sangüesa-Barreda, G.; Di Filippo, A.; Piovesan, G.; Rozas, V.; Di Fiore, L.; García-Hidalgo, M.; García-Cervigón, A.I.; Muñoz-Garachana, D.; Baliva, M.; Olano, J.M. Warmer Springs Have Increased the Frequency and Extension of Late-Frost Defoliations in Southern European Beech Forests. Sci. Total Environ. 2021, 775, 145860. [Google Scholar] [CrossRef]
- Schuldt, B.; Buras, A.; Arend, M.; Vitasse, Y.; Beierkuhnlein, C.; Damm, A.; Gharun, M.; Grams, T.E.E.; Hauck, M.; Hajek, P.; et al. A First Assessment of the Impact of the Extreme 2018 Summer Drought on Central European Forests. Basic Appl. Ecol. 2020, 45, 86–103. [Google Scholar] [CrossRef]
- Senf, C.; Buras, A.; Zang, C.S.; Rammig, A.; Seidl, R. Excess Forest Mortality Is Consistently Linked to Drought across Europe. Nat. Commun. 2020, 11, 6200. [Google Scholar] [CrossRef]
- Haynes, K.J.; Allstadt, A.J.; Klimetzek, D. Forest Defoliator Outbreaks under Climate Change: Effects on the Frequency and Severity of Outbreaks of Five Pine Insect Pests. Glob. Change Biol. 2014, 20, 2004–2018. [Google Scholar] [CrossRef]
- Crossley, M.S.; Smith, O.M.; Barman, A.K.; Croy, J.R.; Schmidt, J.M.; Toews, M.D.; Snyder, W.E. Warmer Temperatures Trigger Insecticide-associated Pest Outbreaks. Pest Manag. Sci. 2024, 80, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.D.; Zeppel, M.J.B.; Anderegg, W.R.L.; Hartmann, H.; Landhäusser, S.M.; Tissue, D.T.; Huxman, T.E.; Hudson, P.J.; Franz, T.E.; Allen, C.D.; et al. A Multi-Species Synthesis of Physiological Mechanisms in Drought-Induced Tree Mortality. Nat. Ecol. Evol. 2017, 1, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Hui, D.; Deng, Q.; Tian, H.; Luo, Y. Climate Change and Carbon Sequestration in Forest Ecosystems. In Handbook of Climate Change Mitigation and Adaptation; Chen, W.-Y., Suzuki, T., Lackner, M., Eds.; Springer: New York, NY, USA, 2015; pp. 1–40. ISBN 978-1-4614-6431-0. [Google Scholar]
- Lorenz, K.; Lal, R. Carbon Sequestration in Forest Ecosystems; Springer: Dordrecht, The Netherlands, 2010; ISBN 978-90-481-3265-2. [Google Scholar]
- Hulme, P.E.; Bacher, S.; Kenis, M.; Klotz, S.; Kühn, I.; Minchin, D.; Nentwig, W.; Olenin, S.; Panov, V.; Pergl, J.; et al. Grasping at the Routes of Biological Invasions: A Framework for Integrating Pathways into Policy. J. Appl. Ecol. 2008, 45, 403–414. [Google Scholar] [CrossRef]
- Hulme, P.E. Trade, Transport and Trouble: Managing Invasive Species Pathways in an Era of Globalization. J. Appl. Ecol. 2009, 46, 10–18. [Google Scholar] [CrossRef]
- Altieri, M.A.; Letourneau, D.K.; Risch, S.J. Vegetation Diversity and Insect Pest Outbreaks. Crit. Rev. Plant Sci. 1984, 2, 131–169. [Google Scholar] [CrossRef]
- Clarke, S.R. Pine Tortoise Scale; US Department of Agriculture, Forest Service, Pacific Northwest Region (R6): Portland, OR, USA, 2013.
- Garonna, A.P.; Scarpato, S.; Vicinanza, F.; Espinosa, B. First Report of Toumeyella parvicornis (Cockerell) in Europe (Hemiptera: Coccidae). Zootaxa 2015, 3949, 142. [Google Scholar] [CrossRef]
- EPPO Global Database. Toumeyella parvicornis (TOUMPA) Reporting Service Articles. Available online: https://gd.eppo.int/taxon/TOUMPA/reporting (accessed on 1 August 2024).
- Di Sora, N.; Contarini, M.; Rossini, L.; Turco, S.; Brugneti, F.; Metaliaj, R.; Vejsiu, I.; Peri, L.; Speranza, S. First Report of Toumeyella parvicornis (Cockerell) (Hemiptera: Coccidae) in Albania and Its Potential Spread in the Coastal Area of the Balkans. EPPO Bull. 2024, 54, 160–165. [Google Scholar] [CrossRef]
- Garonna, A.; Foscari, A.; Russo, E.; Jesu, G.; Somma, S.; Cascone, P.; Guerrieri, E. The Spread of the Non-Native Pine Tortoise Scale Toumeyella parvicornis (Hemiptera: Coccidae) in Europe: A Major Threat to Pinus Pinea in Southern Italy. iForest-Biogeosci. For. 2018, 11, 628–634. [Google Scholar] [CrossRef]
- Sciarretta, A.; Marziali, L.; Squarcini, M.; Marianelli, L.; Benassai, D.; Logli, F.; Roversi, P.F. Adaptive Management of Invasive Pests in Natural Protected Areas: The Case of Matsucoccus feytaudi in Central Italy. Bull. Entomol. Res. 2016, 106, 9–18. [Google Scholar] [CrossRef]
- Boselli, M.; Vai, N.; Mirotti, A.; Mazzini, F.; Mazzoni, F.; Mosti, M.; Foschi, S.; Scapini, C. Crisococcus Pini (Homoptera, Pseudococcidae) in Emilia Romagna: Delimitazione Dell’area Infestata e Piano Di Controllo. In Proceedings of the Atti Giornate Fitopatologiche. ATTI Giornate Fitopatol. 2018, 1, 265–272. [Google Scholar]
- Bonari, G.; Acosta, A.T.R.; Angiolini, C. EU Priority Habitats: Rethinking Mediterranean Coastal Pine Forests. Rendiconti Lincei Sci. Fis. E Nat. 2018, 29, 295–307. [Google Scholar] [CrossRef]
- Chazdon, R.L.; Brancalion, P.H.S.; Laestadius, L.; Bennett-Curry, A.; Buckingham, K.; Kumar, C.; Moll-Rocek, J.; Vieira, I.C.G.; Wilson, S.J. When Is a Forest a Forest? Forest Concepts and Definitions in the Era of Forest and Landscape Restoration. Ambio 2016, 45, 538–550. [Google Scholar] [CrossRef]
- Leone, V.; Lovreglio, R. Conservation of Mediterranean Pine Woodlands: Scenarios and Legislative Tools. Plant Ecol. 2004, 171, 221–235. [Google Scholar] [CrossRef]
- Orenstein, D.E. The Cultural Ecosystem Services of Mediterranean Pine Forests. In Pines and Their Mixed Forest Ecosystems in the Mediterranean Basin; Ne’eman, G., Osem, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 631–655. ISBN 978-3-030-63625-8. [Google Scholar]
- Pasek, J.E. Influence of Wind and Windbreaks on Local Dispersal of Insects. Agric. Ecosyst. Environ. 1988, 22–23, 539–554. [Google Scholar] [CrossRef]
- Gullan, P.J.; Kosztarab, M. ADAPTATIONS IN SCALE INSECTS. Annu. Rev. Entomol. 1997, 42, 23–50. [Google Scholar] [CrossRef]
- Rabkin, F.B.; Lejeune, R.R. Some Aspects of the Biology and Dispersal of the Pine Tortoise Scale, Toumeyella numismaticum (Pettit and McDaniel) (Homoptera: Coccidae). Can. Entomol. 1954, 86, 570–575. [Google Scholar] [CrossRef]
- Malumphy, C.; Hamilton, M.A.; Sanchez, M.D.; Green, P.W.C. Trapping Confirms Aerial Recruitment of Pine Tortoise Scale (Toumeyella parvicornis (Cockerell)) (Hemiptera: Coccidae) in the Turks and Caicos Islands. Entomol. Mon. Mag. 2016, 152, 193–200. [Google Scholar]
- Duarte, A.; Borralho, N.; Cabral, P.; Caetano, M. Recent Advances in Forest Insect Pests and Diseases Monitoring Using UAV-Based Data: A Systematic Review. Forests 2022, 13, 911. [Google Scholar] [CrossRef]
- Mngadi, M.; Germishuizen, I.; Mutanga, O.; Naicker, R.; Maes, W.H.; Odebiri, O.; Schroder, M. A Systematic Review of the Application of Remote Sensing Technologies in Mapping Forest Insect Pests and Diseases at a Tree-Level. Remote Sens. Appl. Soc. Environ. 2024, 36, 101341. [Google Scholar] [CrossRef]
- Kautz, M.; Dworschak, K.; Gruppe, A.; Schopf, R. Quantifying Spatio-Temporal Dispersion of Bark Beetle Infestations in Epidemic and Non-Epidemic Conditions. For. Ecol. Manag. 2011, 262, 598–608. [Google Scholar] [CrossRef]
- Gordo, O.; Sanz, J.J. Long-Term Temporal Changes of Plant Phenology in the Western Mediterranean. Glob. Change Biol. 2009, 15, 1930–1948. [Google Scholar] [CrossRef]
- Fischbein, D.; Corley, J.C. Population Ecology and Classical Biological Control of Forest Insect Pests in a Changing World. For. Ecol. Manag. 2022, 520, 120400. [Google Scholar] [CrossRef]
- Ge, Y.; Atefi, A.; Zhang, H.; Miao, C.; Ramamurthy, R.K.; Sigmon, B.; Yang, J.; Schnable, J.C. High-Throughput Analysis of Leaf Physiological and Chemical Traits with VIS–NIR–SWIR Spectroscopy: A Case Study with a Maize Diversity Panel. Plant Methods 2019, 15, 66. [Google Scholar] [CrossRef]
- Homolová, L.; Malenovský, Z.; Clevers, J.G.P.W.; García-Santos, G.; Schaepman, M.E. Review of Optical-Based Remote Sensing for Plant Trait Mapping. Ecol. Complex. 2013, 15, 1–16. [Google Scholar] [CrossRef]
- Ensminger, I. Fast Track Diagnostics: Hyperspectral Reflectance Differentiates Disease from Drought Stress in Trees. Tree Physiol. 2020, 40, 1143–1146. [Google Scholar] [CrossRef] [PubMed]
- Ollinger, S.V. Sources of Variability in Canopy Reflectance and the Convergent Properties of Plants. New Phytol. 2011, 189, 375–394. [Google Scholar] [CrossRef]
- Francini, S.; Chirici, G. A Sentinel-2 Derived Dataset of Forest Disturbances Occurred in Italy between 2017 and 2020. Data Brief 2022, 42, 108297. [Google Scholar] [CrossRef]
- Guarini, R.; Loizzo, R.; Longo, F.; Mari, S.; Scopa, T.; Varacalli, G. Overview of the Prisma Space and Ground Segment and Its Hyperspectral Products. In Proceedings of the 2017 IEEE International Geoscience and Remote Sensing Symposium (IGARSS), Fort Worth, TX, USA, 23–28 July 2017; pp. 431–434. [Google Scholar]
- Storch, T.; Honold, H.-P.; Chabrillat, S.; Habermeyer, M.; Tucker, P.; Brell, M.; Ohndorf, A.; Wirth, K.; Betz, M.; Kuchler, M.; et al. The EnMAP Imaging Spectroscopy Mission towards Operations. Remote Sens. Environ. 2023, 294, 113632. [Google Scholar] [CrossRef]
- Guanter, L.; Kaufmann, H.; Segl, K.; Foerster, S.; Rogass, C.; Chabrillat, S.; Kuester, T.; Hollstein, A.; Rossner, G.; Chlebek, C.; et al. The EnMAP Spaceborne Imaging Spectroscopy Mission for Earth Observation. Remote Sens. 2015, 7, 8830–8857. [Google Scholar] [CrossRef]
- Coppo, P.; Brandani, F.; Faraci, M.; Sarti, F.; Dami, M.; Chiarantini, L.; Ponticelli, B.; Giunti, L.; Fossati, E.; Cosi, M. Leonardo Spaceborne Infrared Payloads for Earth Observation: SLSTRs for Copernicus Sentinel 3 and PRISMA Hyperspectral Camera for PRISMA Satellite. Appl. Opt. 2020, 59, 6888. [Google Scholar] [CrossRef]
- Fares, S.; Mereu, S.; Mugnozza, G.S.; Vitale, M.; Manes, F.; Frattoni, M.; Ciccioli, P.; Gerosa, G.; Loreto, F. The ACCENT-VOCBAS Field Campaign on Biosphere-Atmosphere Interactions in a Mediterranean Ecosystem of Castelporziano (Rome): Site Characteristics, Climatic and Meteorological Conditions, and Eco-Physiology of Vegetation. Biogeosciences 2009, 6, 1043–1058. [Google Scholar] [CrossRef]
- Gratani, L.; Bonito, A.; Crescente, M.F.; Catoni, R.; Varone, L.; Tinelli, A. The Use of Maps as a Monitoring Tool of Protected Area Management. Rend. Lincei 2015, 26, 325–335. [Google Scholar] [CrossRef]
- Bonari, G.; Těšitel, J.; Migliorini, M.; Angiolini, C.; Protano, G.; Nannoni, F.; Schlaghamerský, J.; Chytrý, M. Conservation of the Mediterranean Coastal Pine Woodlands: How Can Management Support Biodiversity? For. Ecol. Manag. 2019, 443, 28–35. [Google Scholar] [CrossRef]
- Chirici, G. Detection of Toumeyella parvicornis Infestation Hotspot by High-Resolution Remote Sensing in the Casteporziano Presidential Estate. Unpublished Data. 2024. [Google Scholar]
- Drusch, M.; Del Bello, U.; Carlier, S.; Colin, O.; Fernandez, V.; Gascon, F.; Hoersch, B.; Isola, C.; Laberinti, P.; Martimort, P.; et al. Sentinel-2: ESA’s Optical High-Resolution Mission for GMES Operational Services. Remote Sens. Environ. 2012, 120, 25–36. [Google Scholar] [CrossRef]
- Gorelick, N.; Hancher, M.; Dixon, M.; Ilyushchenko, S.; Thau, D.; Moore, R. Google Earth Engine: Planetary-Scale Geospatial Analysis for Everyone. Remote Sens. Environ. 2017, 202, 18–27. [Google Scholar] [CrossRef]
- Sheykhmousa, M.; Mahdianpari, M.; Ghanbari, H.; Mohammadimanesh, F.; Ghamisi, P.; Homayouni, S. Support Vector Machine Versus Random Forest for Remote Sensing Image Classification: A Meta-Analysis and Systematic Review. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2020, 13, 6308–6325. [Google Scholar] [CrossRef]
- Rousset, F.; Ferdy, J. Testing Environmental and Genetic Effects in the Presence of Spatial Autocorrelation. Ecography 2014, 37, 781–790. [Google Scholar] [CrossRef]
- Breslow, N.E.; Clayton, D.G. Approximate Inference in Generalized Linear Mixed Models. J. Am. Stat. Assoc. 1993, 88, 9–25. [Google Scholar] [CrossRef]
- Magee, L. R2 Measures Based on Wald and Likelihood Ratio Joint Significance Tests. Am. Stat. 1990, 44, 250–253. [Google Scholar] [CrossRef]
- EFSA Panel on Plant Health (PLH); Bragard, C.; Baptista, P.; Chatzivassiliou, E.; Di Serio, F.; Gonthier, P.; Jaques Miret, J.A.; Fejer Justesen, A.; Magnusson, C.S.; Milonas, P.; et al. Pest Categorisation of Toumeyella parvaicornis. EFSA J. 2022, 20, e07146. [Google Scholar] [CrossRef]
- Hommay, G.; Wiss, L.; Chadoeuf, J.; Le Maguet, J.; Beuve, M.; Herrbach, E. Gone with the Wind: Aerial Dispersal of Parthenolecanium Corni Crawlers in a Newly Planted Grapevine Plot. Ann. Appl. Biol. 2019, 174, 372–387. [Google Scholar] [CrossRef]
- Grace, J.; Nichol, C.; Disney, M.; Lewis, P.; Quaife, T.; Bowyer, P. Can We Measure Terrestrial Photosynthesis from Space Directly, Using Spectral Reflectance and Fluorescence? Glob. Change Biol. 2007, 13, 1484–1497. [Google Scholar] [CrossRef]
- Peddle, D.R.; Peter White, H.; Soffer, R.J.; Miller, J.R.; LeDrew, E.F. Reflectance Processing of Remote Sensing Spectroradiometer Data. Comput. Geosci. 2001, 27, 203–213. [Google Scholar] [CrossRef]
- Xue, J.; Su, B. Significant Remote Sensing Vegetation Indices: A Review of Developments and Applications. J. Sens. 2017, 2017, 1353691. [Google Scholar] [CrossRef]
- Badgley, G.; Anderegg, L.D.L.; Berry, J.A.; Field, C.B. Terrestrial Gross Primary Production: Using NIRV to Scale from Site to Globe. Glob. Change Biol. 2019, 25, 3731–3740. [Google Scholar] [CrossRef]
- Badgley, G.; Field, C.B.; Berry, J.A. Canopy Near-Infrared Reflectance and Terrestrial Photosynthesis. Sci. Adv. 2017, 3, e1602244. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, Y.J.; Tanre, D. Atmospherically Resistant Vegetation Index (ARVI) for EOS-MODIS. IEEE Trans. Geosci. Remote Sens. 1992, 30, 261–270. [Google Scholar] [CrossRef]
- Niccoli, F.; Kabala, J.P.; Altieri, S.; Faugno, S.; Battipaglia, G. Impact of Toumeyella parvicornis Outbreak in Pinus Pinea L. Forest of Southern Italy: First Detection Using a Dendrochronological, Isotopic and Remote Sensing Analysis. For. Ecol. Manag. 2024, 566, 122086. [Google Scholar] [CrossRef]
- Nicoletti, R.; De Masi, L.; Migliozzi, A.; Calandrelli, M.M. Analysis of Dieback in a Coastal Pinewood in Campania, Southern Italy, through High-Resolution Remote Sensing. Plants 2024, 13, 182. [Google Scholar] [CrossRef]
- Vangi, E.; D’Amico, G.; Francini, S.; Giannetti, F.; Lasserre, B.; Marchetti, M.; Chirici, G. The New Hyperspectral Satellite PRISMA: Imagery for Forest Types Discrimination. Sensors 2021, 21, 1182. [Google Scholar] [CrossRef]
- Lazzeri, G.; Frodella, W.; Rossi, G.; Moretti, S. Multitemporal Mapping of Post-Fire Land Cover Using Multiplatform PRISMA Hyperspectral and Sentinel-UAV Multispectral Data: Insights from Case Studies in Portugal and Italy. Sensors 2021, 21, 3982. [Google Scholar] [CrossRef]
- Bozzini, A.; Brugnaro, S.; Morgante, G.; Santoiemma, G.; Deganutti, L.; Finozzi, V.; Battisti, A.; Faccoli, M. Drone-Based Early Detection of Bark Beetle Infested Spruce Trees Differs in Endemic and Epidemic Populations. Front. For. Glob. Change 2024, 7, 1385687. [Google Scholar] [CrossRef]
- Shaik, R.U.; Periasamy, S.; Zeng, W. Potential Assessment of PRISMA Hyperspectral Imagery for Remote Sensing Applications. Remote Sens. 2023, 15, 1378. [Google Scholar] [CrossRef]
- Orusa, T.; Viani, A.; Borgogno-Mondino, E. IRIDE, the Euro-Italian Earth Observation Program: Overview, Current Progress, Global Expectations, and Recommendations. Environ. Sci. Proc. 2024, 29, 74. [Google Scholar] [CrossRef]
- Celesti, M.; Rast, M.; Adams, J.; Boccia, V.; Gascon, F.; Isola, C.; Nieke, J. The Copernicus Hyperspectral Imaging Mission for the Environment (Chime): Status and Planning. In Proceedings of the IGARSS 2022—2022 IEEE International Geoscience and Remote Sensing Symposium, Kuala Lumpur, Malaysia, 17 July 2022; IEEE: Piscataway, NJ, USA; pp. 5011–5014. [Google Scholar]
- Watanabe, M.; Koyama, C.N.; Hayashi, M.; Nagatani, I.; Tadono, T.; Shimada, M. Refined Algorithm for Forest Early Warning System with ALOS-2/PALSAR-2 ScanSAR Data in Tropical Forest Regions. Remote Sens. Environ. 2021, 265, 112643. [Google Scholar] [CrossRef]
- Minařík, R.; Langhammer, J.; Lendzioch, T. Detection of Bark Beetle Disturbance at Tree Level Using UAS Multispectral Imagery and Deep Learning. Remote Sens. 2021, 13, 4768. [Google Scholar] [CrossRef]
- Ranson, M.; Kousky, C.; Ruth, M.; Jantarasami, L.; Crimmins, A.; Tarquinio, L. Tropical and Extratropical Cyclone Damages under Climate Change. Clim. Change 2014, 127, 227–241. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).