Medium-Term Effect of Livestock Grazing Intensities on the Vegetation Dynamics in Alpine Meadow Ecosystems
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experiment
2.3. Plant Measurement
2.4. Soil Measurement
2.5. Data Analysis
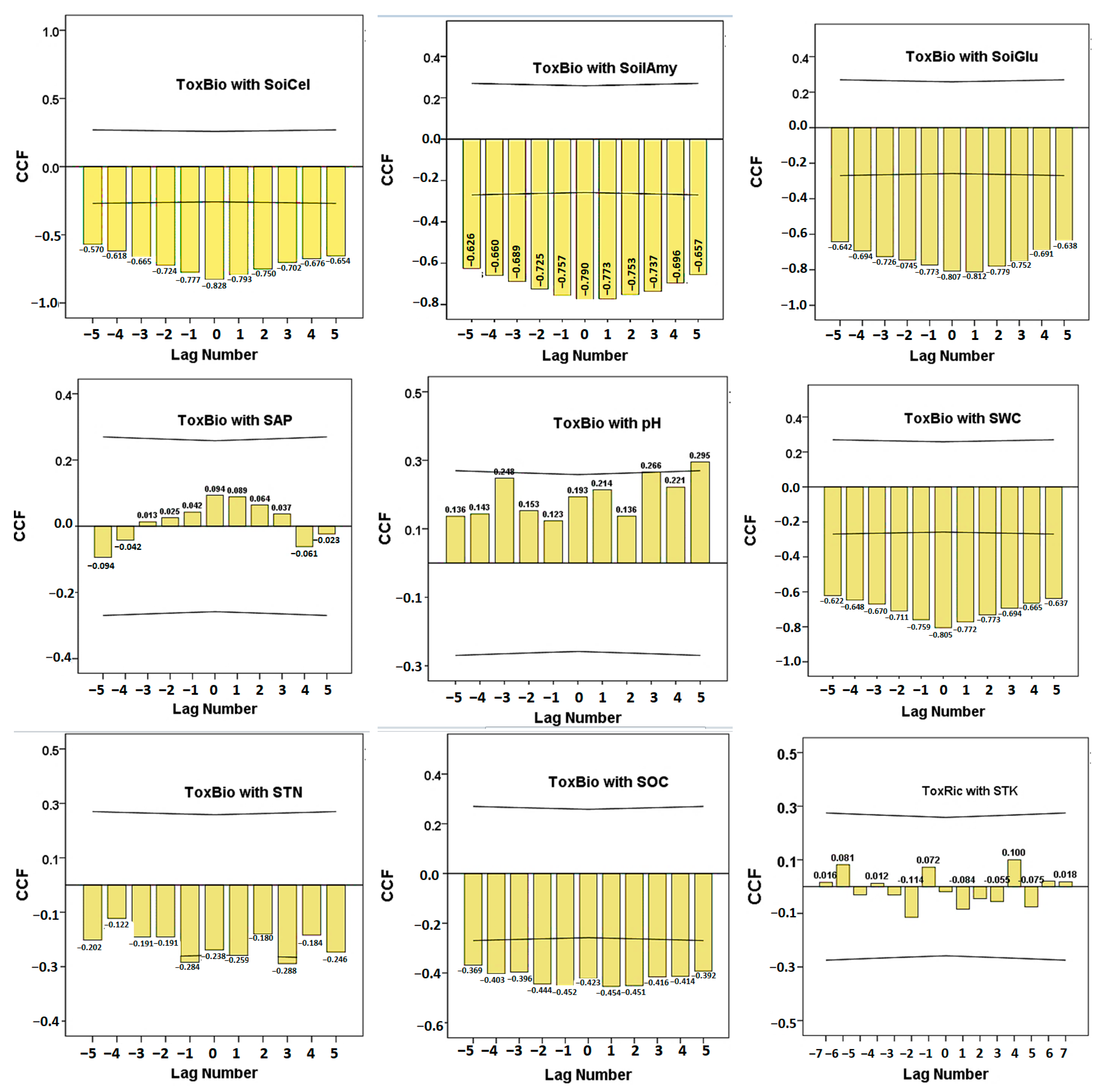
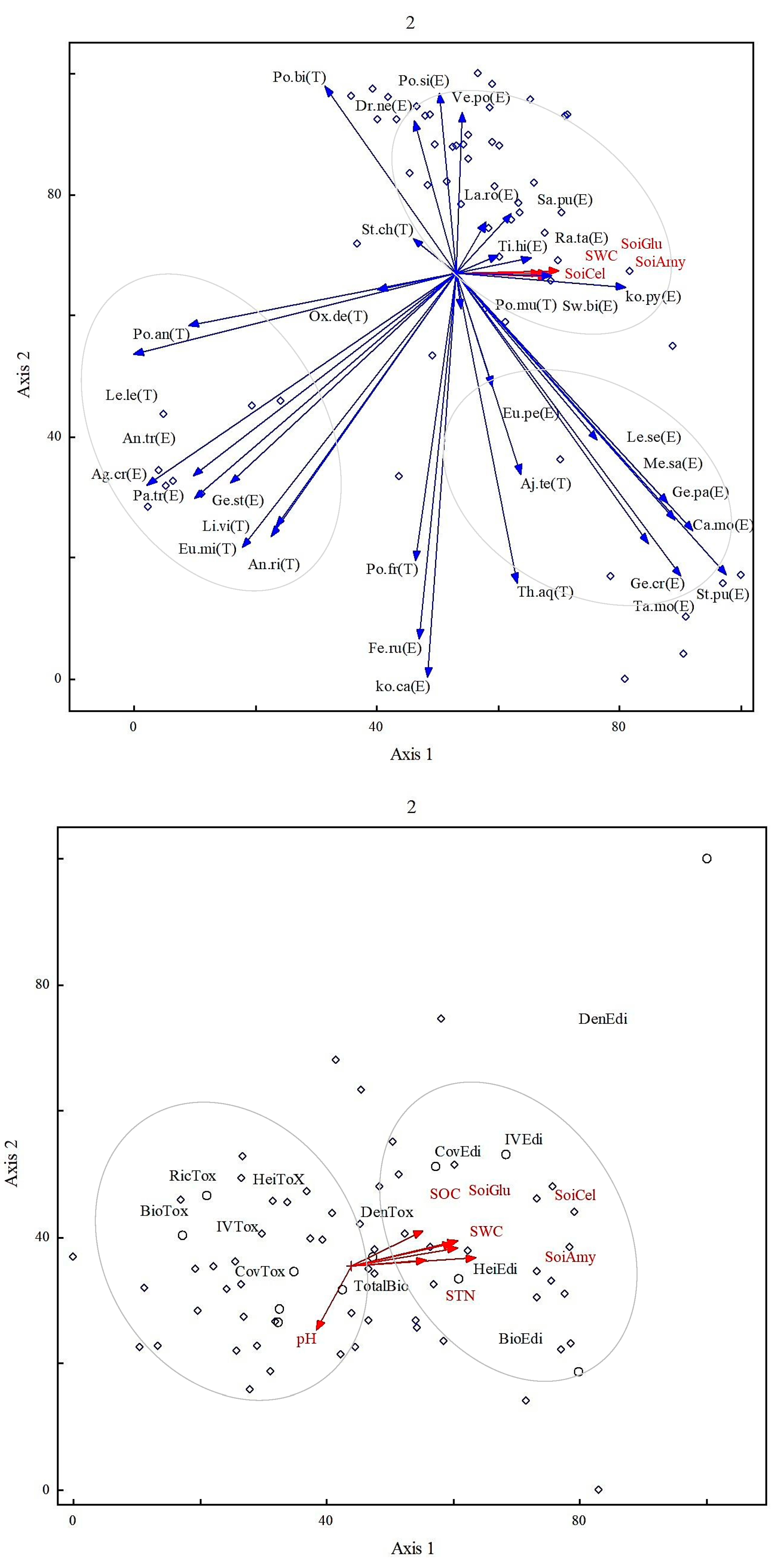
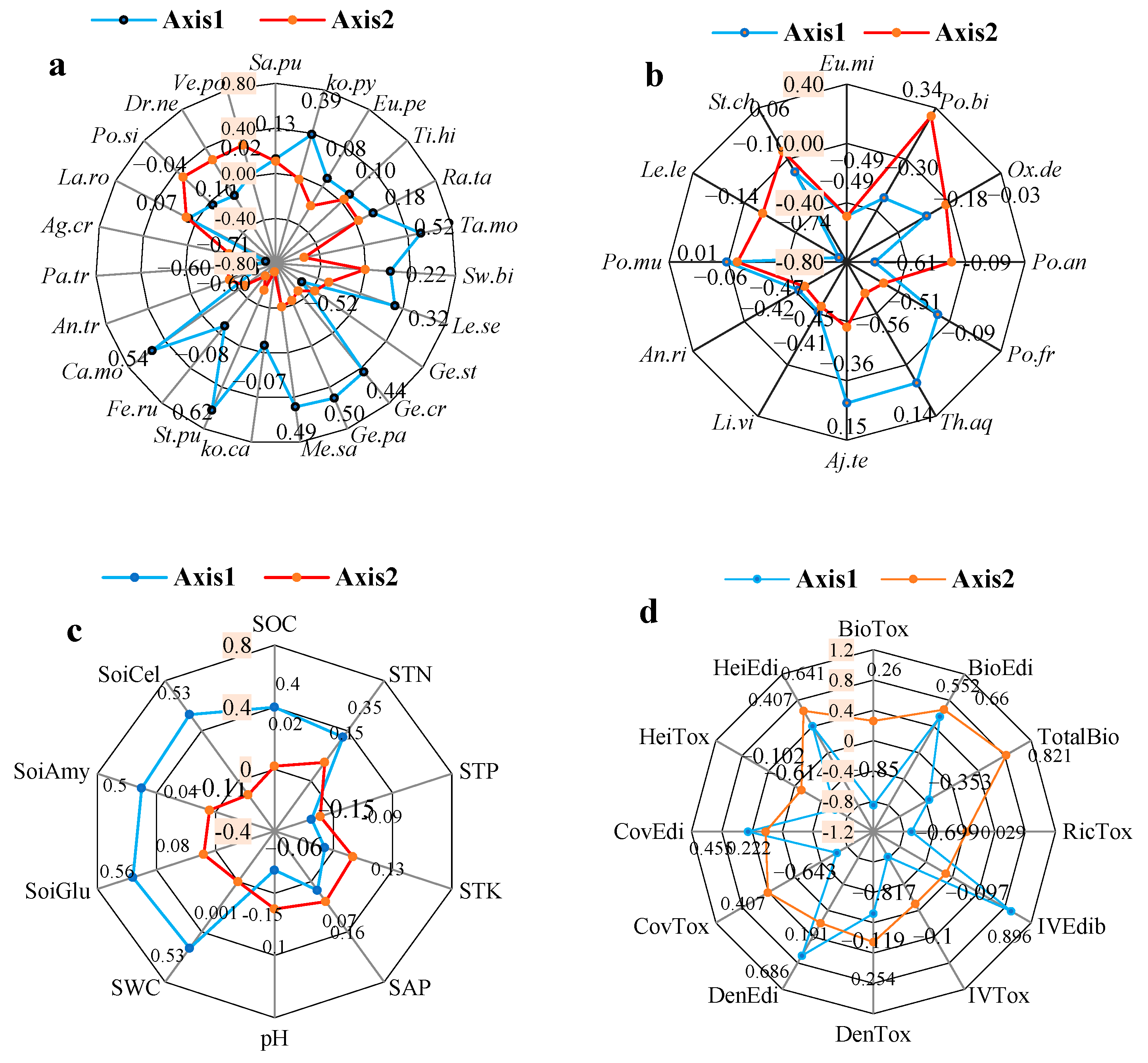
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dai, J.; Roberts, D.A.; Stow, D.A.; An, L.; Hall, S.J.; Yabiku, S.T.; Kyriakidis, P.C. Mapping understory invasive plant species with field and remotely sensed data in Chitwan, Nepal. Remote Sens. Environ. 2020, 250, 112037. [Google Scholar] [CrossRef]
- Gaskin, J.F.; Espeland, E.; Johnson, C.D.; Larson, D.L.; Mangold, J.M.; McGee, R.A.; Milner, C.; Paudel, S.; Pearson, D.E.; Perkins, L.B.; et al. Managing invasive plants on Great Plains grasslands: A discussion of current challenges. Rangel. Ecol. Manag. 2020, 78, 235–249. [Google Scholar] [CrossRef]
- Melo, A.K.P.; Albuquerque, J.A.A.; Siqueira, R.H.S.; da Silva, E.E.; de Medeiros, R.D.; Souza, K.T.S.; Souza, L.T.; Goncalves, A.C.M.; Soares, M.B.B. Occurrence of noxious weeds under different soil management systems. Appl. Ecol. Environ. Res. 2021, 19, 2061–2072. [Google Scholar]
- Hu, Y.; Gou, X.; Tsunekawa, A.; Cheng, Y.; Hou, F. Assessment of the vegetation sensitivity index in alpine meadows with a high coverage and toxic weed invasion under grazing disturbance. Front. Plant Sci. 2022, 13, 1068941. [Google Scholar] [CrossRef]
- Mangold, J.M.; Fuller, K.B.; Davis, S.C.; Rinella, M.J. The Economic Cost of Noxious Weeds on Montana Grazing Lands. Invasive Plant Sci. Manag. 2018, 11, 96–100. [Google Scholar] [CrossRef]
- Lacey, J.R.; Olson, B.E. Environmental and economic impacts of noxious range weeds. In Noxious Range Weeds; CRC Press: Boca Raton, FL, USA, 2021; pp. 5–16. [Google Scholar]
- Ma, Y.; Liu, Y.; Rodrigo-Comino, J.; López-Vicente, M.; Shi, Z.; Wu, G.-L. Artificially cultivated grasslands decrease the activation of soil detachment and soil erodibility on the alpine degraded hillslopes. Soil Tillage Res. 2024, 243, 106176. [Google Scholar] [CrossRef]
- Wang, L.; Jia, Z.; Li, Q.; He, L.; Tian, J.; Ding, W.; Liu, T.; Gao, Y.; Zhang, J.; Han, D.; et al. Grazing Impacts on Soil Enzyme Activities Vary with Vegetation Types in the Forest-Steppe Ecotone of Northeastern China. Forests 2023, 14, 2292. [Google Scholar] [CrossRef]
- Li, P.; Zhu, W.; He, B. Regional differences in the impact paths of climate on aboveground biomass in alpine grasslands across the Qinghai-Tibet Plateau. Sci. Total. Environ. 2024, 947, 174421. [Google Scholar] [CrossRef]
- Scasta, J.D.; Jorns, T.; Derner, J.D.; Stam, B.; McClaren, M.; Calkins, C.; Stewart, W. Technical note: Toxic plants in sheep diets grazing extensive landscapes: Insights from Fecal DNA metabarcoding. Livest. Sci. 2020, 236, 104002. [Google Scholar] [CrossRef]
- Xiang, M.; Wu, J.; Duo, L.; Niu, B.; Zhang, X. The effects of grazing and fencing on grassland productivity and diversity in alpine grassland ecosystem in the Tibetan highland. Glob. Ecol. Conserv. 2023, 44, e02495. [Google Scholar] [CrossRef]
- Watson, B.L.; Lukas, S.B.; Morris, L.R.; DeBano, S.J.; Schmalz, H.J.; Leffler, A.J. Forb community response to prescribed fire, livestock grazing, and an invasive annual grass in the Pacific Northwest Bunchgrass Prairie. Appl. Veg. Sci. 2021, 24, e12619. [Google Scholar] [CrossRef]
- Xiang, M.; Wu, J.; Wu, J.; Guo, Y.; Lha, D.; Pan, Y.; Zhang, X. Heavy Grazing Altered the Biodiversity–Productivity Relationship of Alpine Grasslands in Lhasa River Valley, Tibet. Front. Ecol. Evol. 2021, 9, 698707. [Google Scholar] [CrossRef]
- Lin, L.; Xu, X.L.; Cao, G.M.; Zhang, F.W.; Li, Y.K.; Huang, J.J. Self-stabilizing maintenance process in plant communities of alpine meadows under different grazing intensities. Grassl. Res. 2023, 2, 140–152. [Google Scholar] [CrossRef]
- Sun, J.; Hou, G.; Liu, M.; Fu, G.; Zhan, T.; Zhou, H.; Tsunekawa, A.; Haregeweyn, N. Effects of climatic and grazing changes on desertification of alpine grasslands, Northern Tibet. Ecol. Indic. 2019, 107, 105647. [Google Scholar] [CrossRef]
- Sun, J.; Zhan, T.; Liu, M.; Zhang, Z.; Wang, Y.; Liu, S.; Wu, G.-L.; Liu, G.; Tsunekawa, A. Verification of the biomass transfer hypothesis under moderate grazing across the Tibetan plateau: A meta-analysis. Plant Soil 2019, 458, 139–150. [Google Scholar] [CrossRef]
- Song, Z.; Li, X.; Su, X.; Li, C. Analyzing the recovery mechanisms of patchy degradation and its response to mowing and plateau pika disturbances in alpine meadow. Ecol. Indic. 2023, 154, 110565. [Google Scholar] [CrossRef]
- Li, W.; Hooper, D.U.; Wu, L.; Bakker, J.D.; Gianuca, A.T.; Wu, X.B.; Taube, F.; Wang, C.; Bai, Y. Grazing regime alters plant community structure via patch-scale diversity in semiarid grasslands. Ecosphere 2021, 12, e03547. [Google Scholar] [CrossRef]
- Cronk, Q.C.; Fuller, J.L. Plant Invaders: The Threat to Natural Ecosystems; Routledge: London, UK, 2014. [Google Scholar]
- Demeter, L.; Molnár, Á.P.; Bede-Fazekas, Á.; Öllerer, K.; Varga, A.; Szabados, K.; Tucakov, M.; Kiš, A.; Biró, M.; Marinkov, J.; et al. Controlling invasive alien shrub species, enhancing biodiversity and mitigating flood risk: A win–win–win situation in grazed floodplain plantations. J. Environ. Manag. 2021, 295, 113053. [Google Scholar] [CrossRef]
- García-Llorente, M.; Martín-López, B.; González, J.A.; Alcorlo, P.; Montes, C. Social perceptions of the impacts and benefits of invasive alien species: Implications for management. Biol. Conserv. 2008, 141, 2969–2983. [Google Scholar] [CrossRef]
- Lorenz, R.J.; Dewey, S.A. Noxious weeds that are poisonous. In The Ecology and Economic Impact of Poisonous Plants on Livestock Production; CRC Press: Boca Raton, FL, USA, 2019; pp. 309–336. [Google Scholar]
- Belgeri, A.; Bajwa, A.A.; Shabbir, A.; Navie, S.; Vivian-Smith, G.; Adkins, S. Managing an Invasive Weed Species, Parthenium hysterophorus, with Suppressive Plant Species in Australian Grasslands. Plants 2020, 9, 1587. [Google Scholar] [CrossRef]
- Xing, F.; An, R.; Wang, B.; Miao, J.; Jiang, T.; Huang, X.; Hu, Y. Mapping the occurrence and spatial distribution of noxious weed species with multisource data in degraded grasslands in the Three-River Headwaters Region, China. Sci. Total. Environ. 2021, 801, 149714. [Google Scholar] [CrossRef]
- Rocchini, D.; Hernández-Stefanoni, J.L.; He, K.S. Advancing species diversity estimate by remotely sensed proxies: A conceptual review. Ecol. Inform. 2015, 25, 22–28. [Google Scholar] [CrossRef]
- Zeynivand, F.; Ajorlo, M.; Ariapour, A. Effect of Livestock Grazing Intensity on Diversity of Invasive Plant Species in Kabirkuh Mountainous area, Darrehshar Town. Iran. J. Plant Res. 2020, 32, 1–12. [Google Scholar]
- Sun, W.; Li, S.; Wang, J.; Fu, G. Effects of grazing on plant species and phylogenetic diversity in alpine grasslands, Northern Tibet. Ecol. Eng. 2021, 170, 106331. [Google Scholar] [CrossRef]
- Lyseng, M.P.; Bork, E.W.; Hewins, D.B.; Alexander, M.J.; Carlyle, C.N.; Chang, S.X.; Willms, W.D. Long-term grazing impacts on vegetation diversity, composition, and exotic species presence across an aridity gradient in northern temperate grasslands. Plant Ecol. 2018, 219, 649–663. [Google Scholar] [CrossRef]
- Dornbusch, M.J.; Limb, R.F.; Sedivec, K.K. Alternative Alternative grazing management strategies combat invasive grass dom-inance. Nat. Areas J. 2020, 40, 86–95. [Google Scholar]
- Dai, L.; Fu, R.; Guo, X.; Ke, X.; Du, Y.; Zhang, F.; Cao, G. Effect of grazing management strategies on alpine grassland on the northeastern Qinghai-Tibet Plateau. Ecol. Eng. 2021, 173, 106418. [Google Scholar] [CrossRef]
- Sun, J.J.; Wang, P.B.; Wang, H.B.; Yu, X.J. Changes in plant communities, soil characteristics, and microbial communities in alpine meadows degraded to different degrees by pika on the Qinghai–Tibetan Plateau. Glob. Ecol. Conserv. 2021, 27, e01621. [Google Scholar] [CrossRef]
- Porensky, L.M.; McGee, R.; Pellatz, D.W. Long-term grazing removal increased invasion and reduced native plant abundance and diversity in a sagebrush grassland. Glob. Ecol. Conserv. 2020, 24, e01267. [Google Scholar] [CrossRef]
- Xu, H.W.; You, C.M.; Tan, B.; Xu, L.; Liu, Y.L.; Wang, M.G.; Xu, Z.F.; Sardans, J.; Peñuelas, J. Effects of livestock grazing on the relationships between soil microbial community and soil carbon in grassland ecosystems. Sci. Total Environ. 2023, 881, 163416. [Google Scholar] [CrossRef]
- Beyene, D.; Berhanu, Y.; Angassa, A. Effects of livestock grazing on rangeland condition, plant species richness and wild ungulate population in a semi-arid savannah. Afr. J. Ecol. 2024, 62, e13295. [Google Scholar] [CrossRef]
- Qi, X.-L.; Xu, H.-J.; Teng, R.-Y.; Chen, T.; Wang, X.-D. Ecological restoration largely alleviates livestock grazing pressure in a montane grassland. J. Clean. Prod. 2024, 467, 143044. [Google Scholar] [CrossRef]
- Chen, S.; Sun, Y.; Wang, Y.; Luo, G.; Ran, J.; Zeng, T.; Zhang, P. Grazing weakens the linkages between plants and soil biotic communities in the alpine grassland. Sci. Total Environ. 2024, 913, 169417. [Google Scholar] [CrossRef]
- Zhang, C.; Dong, Q.; Chu, H.; Shi, J.; Li, S.; Wang, Y.; Yang, X. Grassland Community Composition Response to Grazing Intensity Under Different Grazing Regimes. Rangel. Ecol. Manag. 2018, 71, 196–204. [Google Scholar] [CrossRef]
- Yu, L.; Sun, W.; Zhang, H.; Cong, N.; Chen, Y.; Hu, J.; Jing, X. Grazing exclusion jeopardizes plant biodiversity effect but enhances dryness effect on multifunctionality in arid grasslands. Agric. Ecosyst. Environ. 2024, 363, 108883. [Google Scholar] [CrossRef]
- Nie, X.Q.; Yang, L.C.; Li, F.; Xiong, F.; Li, C.B.; Zhou, G.Y. Storage, patterns and controls of soil organic carbon in the alpine shrubland in the Three Rivers Source Region on the Qinghai-Tibetan Plateau. CATENA 2019, 178, 154–162. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.H.; Deng, L.; Wu, G.L. Species diversity and functional groups responses to different seasonal grazing in alpine grassland. Pratacultural Sci. 2016, 33, 1403–1409. [Google Scholar]
- Li, X.; Li, G.J.; Kang, X.H.; Pu, W.J.; Huang, W.L.; Zhang, J.Y. Livestock carrying capacity analysis and coping strategy of overgrazing in natural grassland of Maqu. Gansu Sci. Technol. 2023, 39, 4–8. [Google Scholar]
- Zhou, X.L.; Yan, Y.E.; Zhang, J.; Zhou, X.J.; Yan, Y.Q.; Yang, F.Q.; Cao, X.P.; Zhao, A.; Zhao, Y.L.; Su, J.Y. Vegetation community structure and diversity in a burned area of Picea asperata-Abies fabri forest on different aspects on the northeastern margin of the Qinghai-Tibetan Plateau. Acta Prataculture Sin. 2022, 31, 144–155. [Google Scholar]
- Li, M.; Zhang, K.R.; Yan, Z.Q.; Liu, L.; Kang, E.Z.; Kang, X.M. Soil Water Content Shapes Microbial Community Along Gradients of Wetland Degradation on the Tibetan Plateau. Front. Microbiol. 2022, 13, 824267. [Google Scholar] [CrossRef]
- Ebregt, A.; Boldewijn, J.M.A.M. Influence of heavy metals in spruce forest soil on amylase activity, CO2 evolution from starch and soil respiration. Plant Soil 1977, 47, 137–148. [Google Scholar] [CrossRef]
- Guan, S.Y.; Zhang, D.; Zhang, Z. Soil Enzyme and Its Research Methods; Agriculturre Press: Beijing, China, 1986; pp. 274–297. [Google Scholar]
- Pei, L.; Wu, Z.; Qian, Y.; Li, X.; Zhang, J.; Sun, J.; Wang, Y. Plant community stability, indicator species and their driving factors at a gradient of grazing intensity in an alpine meadow. Ecol. Indic. 2024, 162, 112012. [Google Scholar] [CrossRef]
- Usman, M.; Li, L.; Wang, M.; Wang, Z.; Hu, A.; Shi, L.; Hou, F. Response of microbial communities to the changes in grazing intensity and season in a typical steppe. Environ. Res. 2024, 246, 118126. [Google Scholar] [CrossRef] [PubMed]
- Aranda, M.J.; Tognetti, P.M.; Mochi, L.S.; Mazía, N. Intensive rotational grazing in pastures reduces the early establishment of an invasive tree species. Biol. Invasions 2023, 25, 3137–3150. [Google Scholar] [CrossRef]
- Li, Q.; Sun, Y.N.; Lin, L.; Li, Y.K.; Du, Y.G.; Guo, X.W.; Yang, Y.S.; Cao, G.M. Changes of soil enzyme activities and nutrients across different succession stages of grazing alpine Kobresia grassland. J. Appl. Ecol. 2019, 30, 2267–2274. [Google Scholar]
- dos Santos, J.V.; Bento, L.R.; Bresolin, J.D.; Mitsuyuki, M.C.; Oliveira, P.P.A.; Pezzopane, J.R.M.; de Campos Bernardi, A.C.; Mendes, I.C.; Martin-Neto, L. The long-term effects of intensive grazing and silvopastoral systems on soil physicochemical properties, enzymatic activity, and microbial biomass. CATENA 2022, 219, 106619. [Google Scholar] [CrossRef]
- Garbuz, S.; Mackay, A.; Camps-Arbestain, M.; DeVantier, B.; Minor, M. Biochar increases soil enzyme activities in two contrasting pastoral soils under different grazing management. Crop Pasture Sci. 2022, 74, 101–111. [Google Scholar] [CrossRef]
- Wang, X.; Zi, H.B.; Wang, J.B.; Guo, X.W.; Zhang, Z.H.; Yan, T.; Wang, Q.; He, J.S. Grazing-induced changes in soil microclimate and aboveground biomass modulate freeze–thaw processes in a Tibetan alpine meadow. Agric. Ecosyst. Environ. 2023, 357, 108659. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.; Sai, X.; Chun, F.; Li, X.; Lu, X.; Wang, H. Grazing altered soil aggregates, nutrients and enzyme activities in a Stipa kirschnii steppe of Inner Mongolia. Soil Tillage Res. 2022, 219, 105327. [Google Scholar] [CrossRef]
- Li, C.; de Jong, R.; Schmid, B.; Wulf, H.; Schaepman, M.E. Changes in grassland cover and in its spatial heterogeneity indicate degradation on the Qinghai-Tibetan Plateau. Ecol. Indic. 2020, 119, 106641. [Google Scholar] [CrossRef]
- Filazzola, A.; Brown, C.; Dettlaff, M.A.; Batbaatar, A.; Grenke, J.; Bao, T.; Peetoom Heida, I.; Cahill, J.F., Jr. The effects of livestock grazing on biodiversity are multi-trophic: A meta-analysis. Ecol. Lett. 2020, 23, 1298–1309. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.; García, C.C.; Vergara-Tabares, D.L. Alliance between invasive plants management and farming: Cutting and livestock browsing reduce resprout and fruit production in an invasive shrub. For. Ecol. Manag. 2024, 559, 121809. [Google Scholar] [CrossRef]
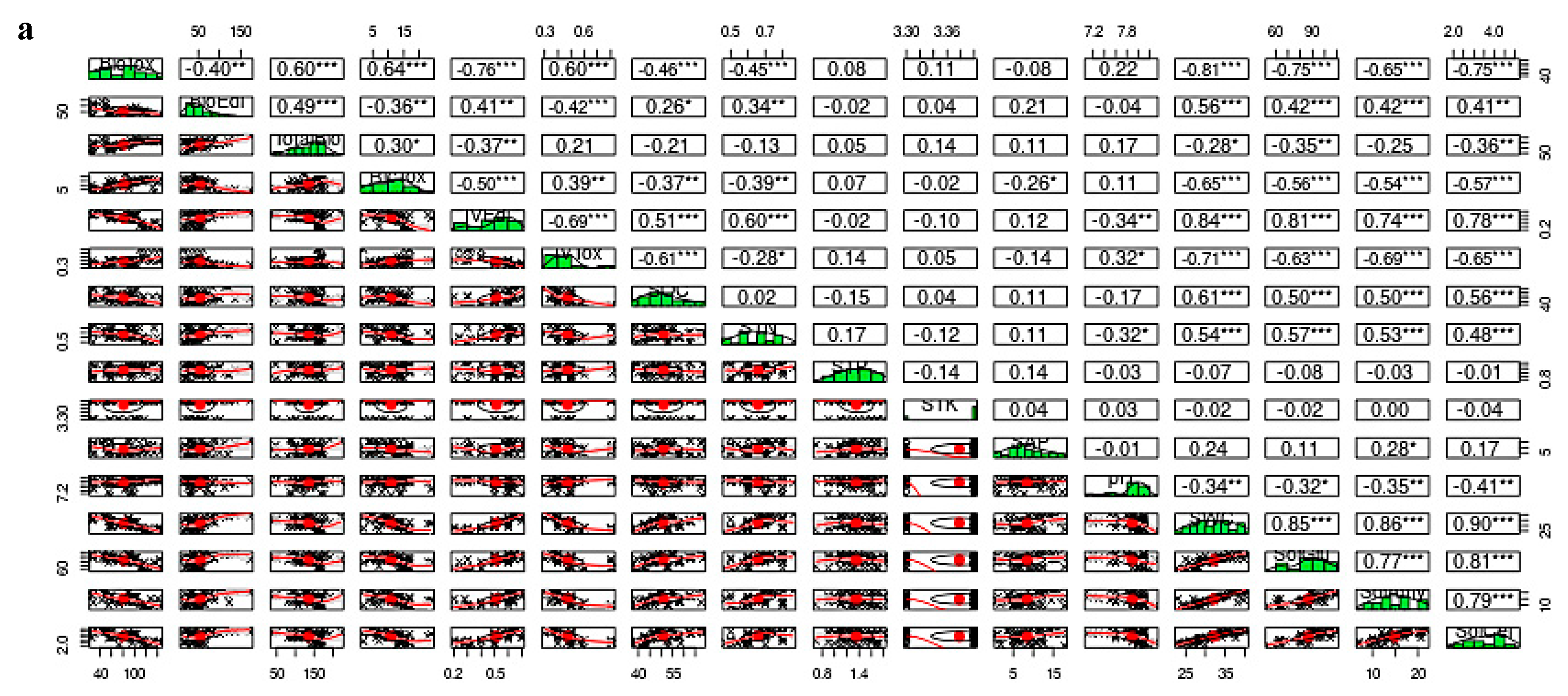
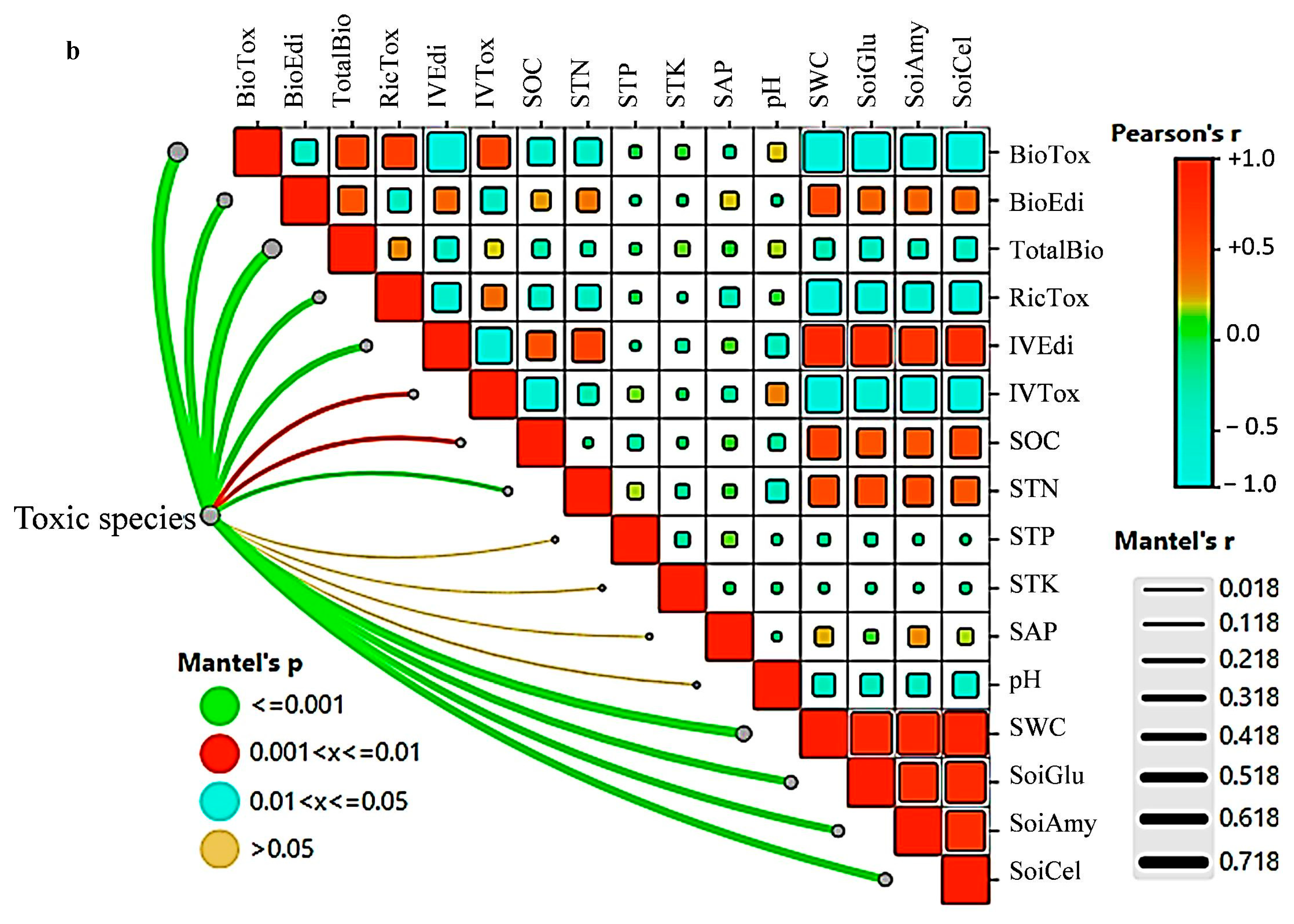
| Plant Treatment/Grazing Intensity | Control (Non-Grazing) | Light Grazing | Moderate Grazing | Heavy Grazing |
|---|---|---|---|---|
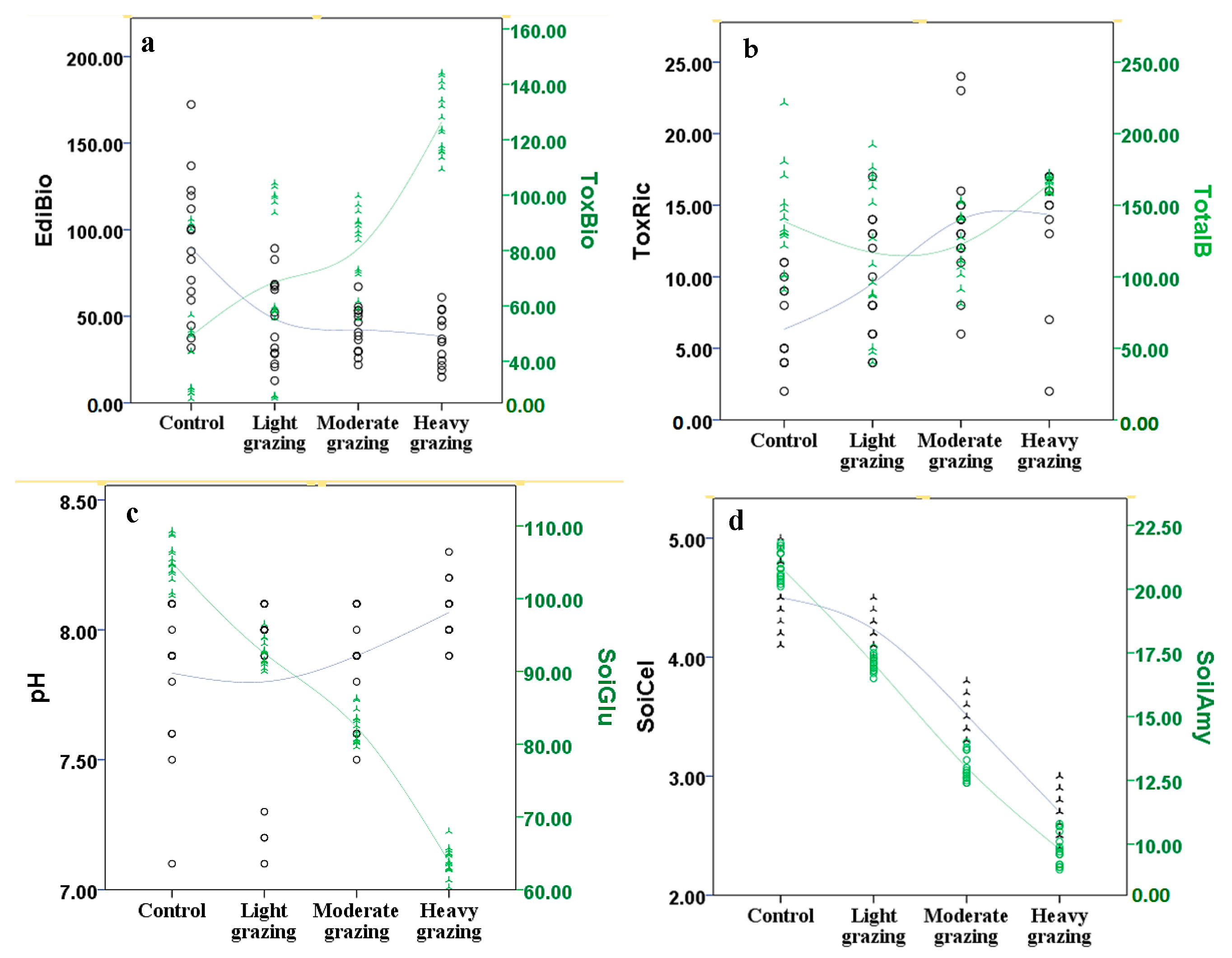
| Toxic weed biomass (g/m2) | 49.1 ± 8.3 d | 68.5 ± 11.4 c | 80.6 ± 10.8 b | 126.4 ± 13.8 a |
| Edible grass biomass (g/m2) | 89.5 ± 10.7 a | 48.4 ± 8.9 b | 42.0 ± 7.4 bc | 38.5 ± 5.4 c |
| Toxic weed richness | 6.3 ± 1.3 c | 9.5 ± 2.7 b | 14.0 ± 3.4 a | 14.3 ± 3.3 a |
| Importance value of edible species | 0.62 ± 0.09 a | 0.55 ± 0.07 b | 0.51 ± 0.07 b | 0.29 ± 0.04 c |
| Importance value of toxic weeds | 0.38 ± 0.04 b | 0.45 ± 0.07 b | 0.49 ± 0.05 ab | 0.71 ± 0.07 a |
| Soil Factor/Grazing Intensity | Non-Grazing | Light Grazing | Moderate Grazing | Heavy Grazing |
|---|---|---|---|---|
| Soil organic carbon (g/kg) | 57.3 ± 11.7 a | 53.3 ± 8.9 ab | 49.2 ± 6.4 bc | 45.0 ± 7.1 c |
| Soil total nitrogen (g/kg) | 0.70 ± 0.1 a | 0.68 ± 0.05 ab | 0.65 ± 0.08 ab | 0.61 ± 0.07 b |
| Soil total phosphorous (g/kg) | 1.3 ± 0.2 a | 1.4 ± 0.08 a | 1.3 ± 0.2 a | 1.4 ± 0.1 a |
| Soil total potassium (g/kg) | 3.37 ± 0.01 a | 3.36 ± 0.02 a | 3.36 ± 0.02 a | 3.36 ± 0.02 a |
| Soil available P (mg/kg) | 12.3 ± 3.2 a | 7.8 ± 1.1 b | 4.9 ± 0.4 c | 11.8 ± 1.0 a |
| pH | 7.8 ± 0.3 b | 7.8 ± 0.3 b | 7.9 ± 0.3 ab | 8.1 ± 0.4 a |
| Soil water content (%) | 38.5 + 6.2 a | 34.0 ± 3.5 ab | 30.1 ± 5.2 bc | 26.4 ± 4.8 c |
| Soil glucosidase (umol/g·soil·d) | 104.9 ± 10.9 a | 92.5 ± 10.3 b | 82.3 ± 8.5 c | 63.9 ± 7.6 d |
| Soil amylase (umol/g·soil·d) | 20.9 ± 5.8 a | 17.1 ± 3.8 b | 13.0 ± 3.2 c | 9.8 ± 2.7 d |
| Soil cellulose (mg/g·soil·72 h) | 4.5 ± 0.8 a | 4.2 ± 0.6 a | 3.5 ± 0.6 ab | 2.7 ± 0.3 bc |
| Axis | Eigenvalue | % of Variance | Cum.% of Var. | Broken-Stick Eigenvalue |
|---|---|---|---|---|
| 1 | 5.5 | 31.90 | 31.90 | 3.84 |
| 2 | 4.3 | 22.92 | 54.82 | 2.36 |
| 3 | 3.25 | 11.23 | 66.05 | 2.06 |
| 4 | 2.34 | 7.72 | 73.77 | 1.82 |
| 5 | 1.7 | 5.36 | 79.13 | 1.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.; Ma, X.; Zhou, X.; Zhang, X.; Wang, X.; Li, Z.; Yang, X.; Lu, S.; Du, W. Medium-Term Effect of Livestock Grazing Intensities on the Vegetation Dynamics in Alpine Meadow Ecosystems. Land 2025, 14, 591. https://doi.org/10.3390/land14030591
Chen B, Ma X, Zhou X, Zhang X, Wang X, Li Z, Yang X, Lu S, Du W. Medium-Term Effect of Livestock Grazing Intensities on the Vegetation Dynamics in Alpine Meadow Ecosystems. Land. 2025; 14(3):591. https://doi.org/10.3390/land14030591
Chicago/Turabian StyleChen, Bo, Xujun Ma, Xiaolei Zhou, Xiaowei Zhang, Xuhu Wang, Zizhen Li, Xinyi Yang, Songsong Lu, and Weibo Du. 2025. "Medium-Term Effect of Livestock Grazing Intensities on the Vegetation Dynamics in Alpine Meadow Ecosystems" Land 14, no. 3: 591. https://doi.org/10.3390/land14030591
APA StyleChen, B., Ma, X., Zhou, X., Zhang, X., Wang, X., Li, Z., Yang, X., Lu, S., & Du, W. (2025). Medium-Term Effect of Livestock Grazing Intensities on the Vegetation Dynamics in Alpine Meadow Ecosystems. Land, 14(3), 591. https://doi.org/10.3390/land14030591







