Anaerobic Digestion of Cattle Manure Contaminated with an Antibiotic Mixture: A Nature-Based Solution for Environmental Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling of Manure and Digestate
2.2. Set-Up of the Antibiotic-Spiked Tests
- –
- Enrofloxacin + ciprofloxacin + sulfamethoxazole (2.5 mg kg−1 each): Mix_L.
- –
- Enrofloxacin + ciprofloxacin + sulfamethoxazole (7.5 mg kg−1 each): Mix_H.
- –
- Enrofloxacin (7.5 mg kg−1): ENR.
- –
- Control batches (not spiked with ABs): Control.
2.3. Methane Production Determination and Analysis of Process Intermediates
2.4. Analytical Determination of Antibiotics
2.5. Microbial Abundance and Fluorescence In Situ Hybridisation
2.6. DNA Extraction and qPCR Analysis (ARGs)
2.7. Statistical Analysis
3. Results and Discussion
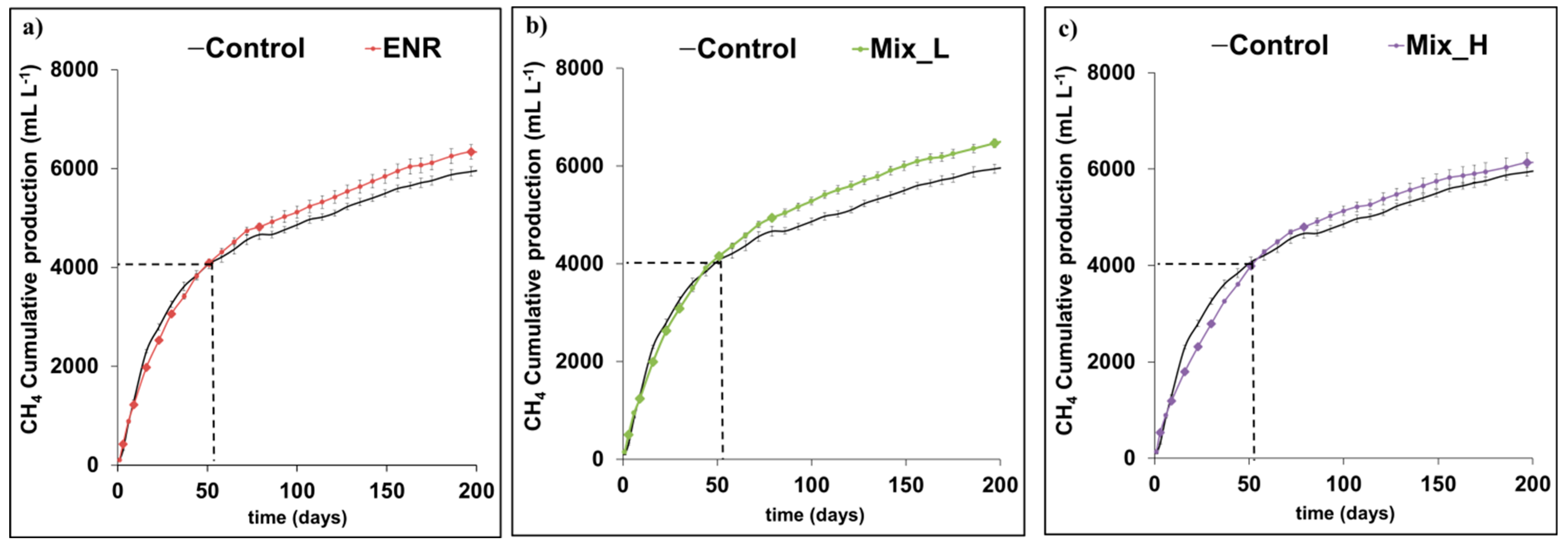
3.1. Biogas Production
3.2. Antibiotic Removal
3.3. Microbial Community Structure
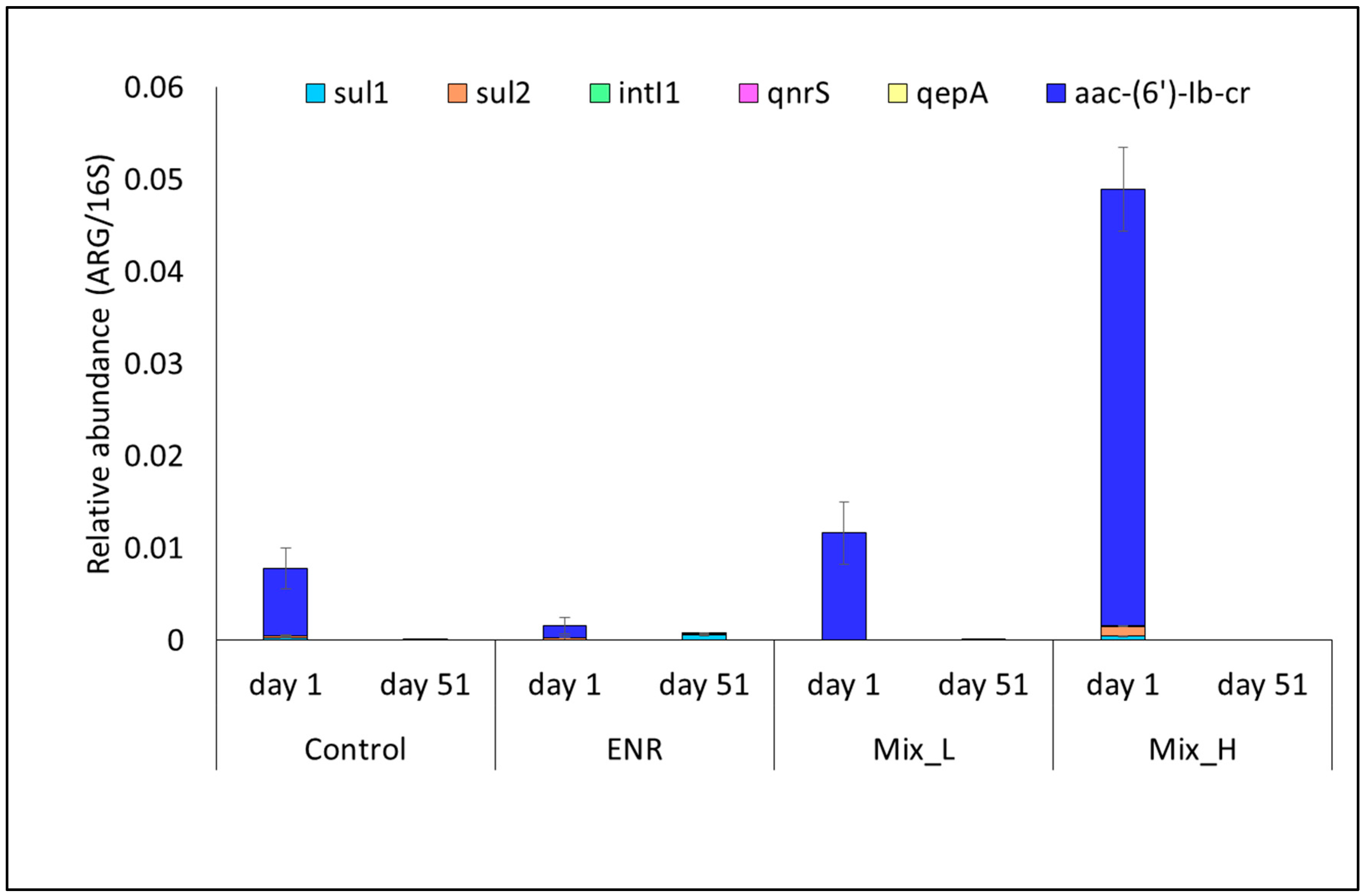
3.4. Antibiotic Resistance Genes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO/WHO. Codex Texts on Foodborne Antimicrobial Resistance; Food and Agriculture Organization of United Nations & World Health Organization: Rome, Italy, 2015; ISBN 978-92-5-008610-1. [Google Scholar]
- Gurmessa, B.; Pedretti, E.F.; Cocco, S.; Cardelli, V.; Corti, G. Manure Anaerobic Digestion Effects and the Role of Pre- and Post-Treatments on Veterinary Antibiotics and Antibiotic Resistance Genes Removal Efficiency. Sci. Total Environ. 2020, 721, 137532. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Q.; Ji, Z.; Andom, O.; Wang, X.; Guo, X.; Li, Z. Current Status and Spatiotemporal Evolution of Antibiotic Residues in Livestock and Poultry Manure in China. Agriculture 2023, 13, 1877. [Google Scholar] [CrossRef]
- Pan, M.; Chu, L.M. Transfer of Antibiotics from Wastewater or Animal Manure to Soil and Edible Crops. Environ. Pollut. 2017, 231, 829–836. [Google Scholar] [CrossRef]
- Visca, A.; Rauseo, J.; Spataro, F.; Patrolecco, L.; Grenni, P.; Massini, G.; Mazzurco Miritana, V.; Barra Caracciolo, A. Antibiotics and Antibiotic Resistance Genes in Anaerobic Digesters and Predicted Concentrations in Agroecosystems. J. Environ. Manag. 2022, 301, 113891. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kim, D.-W.; Lee, D.-H.; Kim, Y.-S.; Bu, J.-H.; Cha, J.-H.; Thawng, C.N.; Hwang, E.-M.; Seong, H.J.; Sul, W.J.; et al. Mobile Resistome of Human Gut and Pathogen Drives Anthropogenic Bloom of Antibiotic Resistance. Microbiome 2020, 8, 2. [Google Scholar] [CrossRef]
- Barra Caracciolo, A.; Visca, A.; Massini, G.; Patrolecco, L.; Mazzurco Miritana, V.; Grenni, P. Environmental Fate of Antibiotics and Resistance Genes in Livestock Waste and Digestate from Biogas Plants. Environ. Sci. Pollut. Res. Manag. 2020, 2020, ESPRM-102. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, Y.; Lichtfouse, E.; Li, Z.; Kumar, P.S.; Liu, J.; Feng, D.; Yang, Q.; Liu, F. Effect of Antibiotics on the Microbial Efficiency of Anaerobic Digestion of Wastewater: A Review. Front. Microbiol. 2021, 11, 611613. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Wang, Z.; Ganeshan, P.; Sar, T.; Xu, S.; Rajendran, K.; Raveendran, S.; Parameswaran, B.; Ashok, P.; Zengqiang, Z.; et al. Anaerobic digestion in global bio-energy production for sustainable bioeconomy: Potential and research challenges. Renew. Sustain. Energy Rev. 2025, 208, 114985. [Google Scholar] [CrossRef]
- Kazemi Shariat Panahi, H.; Dehhaghi, M.; Guillemin, G.J.; Gupta, V.K.; Lam, S.S.; Aghbashlo, M.; Tabatabaei, M. A Comprehensive Review on Anaerobic Fungi Applications in Biofuels Production. Sci. Total Environ. 2022, 829, 154521. [Google Scholar] [CrossRef] [PubMed]
- Lankiewicz, T.S.; Choudhary, H.; Gao, Y.; Amer, B.; Lillington, S.P.; Leggieri, P.A.; Brown, J.L.; Swift, C.L.; Lipzen, A.; Na, H.; et al. Lignin Deconstruction by Anaerobic Fungi. Nat. Microbiol. 2023, 8, 596–610. [Google Scholar] [CrossRef]
- Talavera-Caro, A.G.; Lira, I.O.H.-D.; Cruz, E.R.; Sánchez-Muñoz, M.A.; Balagurusamy, N. The Realm of Microorganisms in Biogas Production: Microbial Diversity, Functional Role, Community Interactions, and Monitoring the Status of Biogas Plant. In Biogas Production; Balagurusamy, N., Chandel, A.K., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 179–212. ISBN 978-3-030-58826-7. [Google Scholar]
- Agneessens, L.M.; Ottosen, L.D.M.; Voigt, N.V.; Nielsen, J.L.; de Jonge, N.; Fischer, C.H.; Kofoed, M.V.W. In-Situ Biogas Upgrading with Pulse H2 Additions: The Relevance of Methanogen Adaption and Inorganic Carbon Level. Bioresour. Technol. 2017, 233, 256–263. [Google Scholar] [CrossRef]
- Yan, X.; Ying, Y.; Li, K.; Zhang, Q.; Wang, K. A Review of Mitigation Technologies and Management Strategies for Greenhouse Gas and Air Pollutant Emissions in Livestock Production. J. Environ. Manage. 2024, 352, 120028. [Google Scholar] [CrossRef]
- Shi, L.; Simplicio, W.S.; Wu, G.; Hu, Z.; Hu, H.; Zhan, X. Nutrient Recovery from Digestate of Anaerobic Digestion of Livestock Manure: A Review. Curr. Pollut. Rep. 2018, 4, 74–83. [Google Scholar] [CrossRef]
- Mitchell, R.; Gu, J.D. Environmental Microbiology, 2nd ed.; Mitchell, R., Gu, J., Eds.; Wiley: Hoboken, NJ, USA, 2009; ISBN 9780470177907. [Google Scholar]
- Iocoli, G.A.; Zabaloy, M.C.; Pasdevicelli, G.; Gómez, M.A. Use of Biogas Digestates Obtained by Anaerobic Digestion and Co-Digestion as Fertilizers: Characterization, Soil Biological Activity and Growth Dynamic of Lactuca sativa L. Sci. Total Environ. 2019, 647, 11–19. [Google Scholar] [CrossRef] [PubMed]
- European Commission. A European Green Deal. Available online: https://ec.europa.eu/info/strategy/priorities-2019-2024/european-green-deal_en (accessed on 5 February 2025).
- Han, S.H.; An, J.Y.; Hwang, J.; Kim, S.B.; Park, B.B. The Effects of Organic Manure and Chemical Fertilizer on the Growth and Nutrient Concentrations of Yellow Poplar (Liriodendron tulipifera Lin.) in a Nursery System. Forest Sci. Technol. 2016, 12, 137–143. [Google Scholar] [CrossRef]
- Ashrafi Esfahani, A.; Niknejad, Y.; Fallah, H.; Dastan, S. Integrated Management of Organic Manures and Chemical Fertilizers for Enhancing Paddy Yield and the Nutrient Content of Rice Cultivars. Commun. Soil Sci. Plant Anal. 2019, 50, 570–585. [Google Scholar] [CrossRef]
- Lerminiaux, N.A.; Cameron, A.D.S. Horizontal Transfer of Antibiotic Resistance Genes in Clinical Environments. Can. J. Microbiol. 2019, 65, 34–44. [Google Scholar] [CrossRef]
- Martinez, J.L.; Baquero, F. Mutation Frequencies and Antibiotic Resistance. Antimicrob. Agents Chemother. 2000, 44, 1771–1777. [Google Scholar] [CrossRef]
- Mazzurco Miritana, V.; Patrolecco, L.; Barra Caracciolo, A.; Visca, A.; Piccinini, F.; Signorini, A.; Rosa, S.; Grenni, P.; Garbini, G.L.; Spataro, F.; et al. Effects of Ciprofloxacin Alone or in Mixture with Sulfamethoxazole on the Efficiency of Anaerobic Digestion and Its Microbial Community. Antibiotics 2022, 11, 1111. [Google Scholar] [CrossRef]
- Visca, A.; Barra Caracciolo, A.; Grenni, P.; Patrolecco, L.; Rauseo, J.; Massini, G.; Mazzurco Miritana, V.; Spataro, F. Anaerobic Digestion and Removal of Sulfamethoxazole, Enrofloxacin, Ciprofloxacin and Their Antibiotic Resistance Genes in a Full-Scale Biogas Plant. Antibiotics 2021, 10, 502. [Google Scholar] [CrossRef] [PubMed]
- Haffiez, N.; Chung, T.H.; Zakaria, B.S.; Shahidi, M.; Mezbahuddin, S.; Hai, F.I.; Dhar, B.R. A Critical Review of Process Parameters Influencing the Fate of Antibiotic Resistance Genes in the Anaerobic Digestion of Organic Waste. Bioresour. Technol. 2022, 354, 127189. [Google Scholar] [CrossRef] [PubMed]
- APHA (American Public Health Association). Standard Methods for the Examination of Water and Waste Waters, 23rd ed.; Rice, E.W., Baird, R.B., Eaton, A.D., Eds.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2017; ISBN 978-0-87553-287-5. [Google Scholar]
- Boshagh, F.; Rostami, K. A Review of Measurement Methods of Biological Hydrogen. Int. J. Hydrogen Energy 2020, 45, 24424–24452. [Google Scholar] [CrossRef]
- Logan, B.E.; Oh, S.-E.; Kim, I.S.; Van Ginkel, S. Biological Hydrogen Production Measured in Batch Anaerobic Respirometers. Environ. Sci. Technol. 2002, 36, 2530–2535. [Google Scholar] [CrossRef]
- Rauseo, J.; Spataro, F.; Pescatore, T.; Patrolecco, L. Multiresidue determination and predicted risk assessment of emerging contaminants in sediments from Kongsfjorden, Svalbard. Sci. Total Environ. 2024, 922, 171156. [Google Scholar] [CrossRef] [PubMed]
- Spataro, F.; Ademollo, N.; Pescatore, T.; Rauseo, J.; Patrolecco, L. Antibiotic Residues and Endocrine Disrupting Compounds in Municipal Wastewater Treatment Plants in Rome, Italy. Microchem. J. 2019, 148, 634–642. [Google Scholar] [CrossRef]
- Ferraro, A.; Dottorini, G.; Massini, G.; Mazzurco Miritana, V.; Signorini, A.; Lembo, G.; Fabbricino, M. Combined Bioaugmentation with Anaerobic Ruminal Fungi and Fermentative Bacteria to Enhance Biogas Production from Wheat Straw and Mushroom Spent Straw. Bioresour. Technol. 2018, 260, 364–373. [Google Scholar] [CrossRef]
- Franklin, R.B.; Campbell, A.H.; Higgins, C.B.; Barker, M.K.; Brown, B.L. Enumerating Bacterial Cells on Bioadhesive Coated Slides. J. Microbiol. Methods 2011, 87, 154–160. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barra Caracciolo, A.; Grenni, P.; Cupo, C.; Rossetti, S. In Situ Analysis of Native Microbial Communities in Complex Samples with High Particulate Loads. FEMS Microbiol. Lett. 2005, 253, 55–58. [Google Scholar] [CrossRef]
- Ancona, V.; Rascio, I.; Aimola, G.; Campanale, C.; Grenni, P.; Lenola, M.D.; Garbini, G.L.; Uricchio, V.F.; Barra Caracciolo, A. Poplar-Assisted Bioremediation for Recovering a PCB and Heavy-Metal-Contaminated Area. Agriculture 2021, 11, 689. [Google Scholar] [CrossRef]
- Greuter, D.; Loy, A.; Horn, M.; Rattei, T. ProbeBase—An Online Resource for RRNA-Targeted Oligonucleotide Probes and Primers: New Features 2016. Nucleic Acids Res. 2016, 44, D586–D589. [Google Scholar] [CrossRef] [PubMed]
- Rasconi, S.; Jobard, M.; Jouve, L.; Sime-Ngando, T. Use of Calcofluor White for Detection, Identification, and Quantification of Phytoplanktonic Fungal Parasites. Appl. Environ. Microbiol. 2009, 75, 2545–2553. [Google Scholar] [CrossRef]
- Vila-Costa, M.; Gioia, R.; Aceña, J.; Pérez, S.; Casamayor, E.O.; Dachs, J. Degradation of Sulfonamides as a Microbial Resistance Mechanism. Water Res. 2017, 115, 309–317. [Google Scholar] [CrossRef]
- Kerrn, M.B. Susceptibility of Danish Escherichia Coli Strains Isolated from Urinary Tract Infections and Bacteraemia, and Distribution of Sul Genes Conferring Sulphonamide Resistance. J. Antimicrob. Chemother. 2002, 50, 513–516. [Google Scholar] [CrossRef]
- Byrne-Bailey, K.G.; Gaze, W.H.; Zhang, L.; Kay, P.; Boxall, A.; Hawkey, P.M.; Wellington, E.M.H. Integron Prevalence and Diversity in Manured Soil. Appl. Environ. Microbiol. 2011, 77, 684–687. [Google Scholar] [CrossRef] [PubMed]
- Mckinney, C.W.; Dungan, R.S.; Moore, A.; Leytem, A.B. Occurrence and Abundance of Antibiotic Resistance Genes in Agricultural Soil Receiving Dairy Manure. FEMS Microbiol. Ecol. 2018, 94, 1–10. [Google Scholar] [CrossRef]
- Marti, E.; Balcázar, J.L. Real-Time PCR Assays for Quantification of Qnr Genes in Environmental Water Samples and Chicken Feces. Appl. Environ. Microbiol. 2013, 79, 1743–1745. [Google Scholar] [CrossRef]
- Yamane, K.; Wachino, J.; Suzuki, S.; Arakawa, Y. Plasmid-Mediated QepA Gene among Escherichia Coli Clinical Isolates from Japan. Antimicrob. Agents Chemother. 2008, 52, 1564–1566. [Google Scholar] [CrossRef]
- Ruiz, E.; Ocampo-Sosa, A.A.; Alcoba-Flórez, J.; Román, E.; Arlet, G.; Torres, C.; Martínez-Martínez, L. Changes in Ciprofloxacin Resistance Levels in Enterobacter Aerogenes Isolates Associated with Variable Expression of the Aac (6 ′) -Ib-Cr Gene. Antimicrob. Agents Chemother. 2012, 56, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Mori, U.; Mendiburu, A.; Lozano, J.A. Distance Measures for Time Series in R: The TSdist Package. R J. 2016, 8, 451. [Google Scholar] [CrossRef]
- Zhi, S.; Li, Q.; Yang, F.; Yang, Z.; Zhang, K. How Methane Yield, Crucial Parameters and Microbial Communities Respond to the Stimulating Effect of Antibiotics during High Solid Anaerobic Digestion. Bioresour. Technol. 2019, 283, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Yang, L.; Chen, L.; Li, S.; Sun, L. Bioaccumulation of Antibiotics in Crops under Long-Term Manure Application: Occurrence, Biomass Response and Human Exposure. Chemosphere 2019, 219, 882–895. [Google Scholar] [CrossRef]
- Tang, T.; Liu, M.; Du, Y.; Chen, Y. Deciphering the Internal Mechanisms of Ciprofloxacin Affected Anaerobic Digestion, Its Degradation and Detoxification Mechanism. Sci. Total Environ. 2022, 842, 156718. [Google Scholar] [CrossRef] [PubMed]
- Cetecioglu, Z.; Ince, B.; Orhon, D.; Ince, O. Acute Inhibitory Impact of Antimicrobials on Acetoclastic Methanogenic Activity. Bioresour. Technol. 2012, 114, 109–116. [Google Scholar] [CrossRef]
- Mazzurco Miritana, V.; Massini, G.; Visca, A.; Grenni, P.; Patrolecco, L.; Spataro, F.; Rauseo, J.; Garbini, G.L.; Signorini, A.; Rosa, S.; et al. Effects of Sulfamethoxazole on the Microbial Community Dynamics During the Anaerobic Digestion Process. Front. Microbiol. 2020, 11, 537783. [Google Scholar] [CrossRef] [PubMed]
- Patrolecco, L.; Rauseo, J.; Ademollo, N.; Grenni, P.; Cardoni, M.; Levantesi, C.; Luprano, M.L.; Barra Caracciolo, A. Persistence of the Antibiotic Sulfamethoxazole in River Water Alone or in the Co-Presence of Ciprofloxacin. Sci. Total Environ. 2018, 640–641, 1438–1446. [Google Scholar] [CrossRef]
- Barra Caracciolo, A.; Grenni, P.; Rauseo, J.; Ademollo, N.; Cardoni, M.; Rolando, L.; Patrolecco, L. Degradation of a Fluoroquinolone Antibiotic in an Urbanized Stretch of the River Tiber. Microchem. J. 2018, 136, 43–48. [Google Scholar] [CrossRef]
- Trouchon, T.; Lefebvre, S. A Review of Enrofloxacin for Veterinary Use. Open J. Vet. Med. 2016, 6, 40–58. [Google Scholar] [CrossRef]
- Rauseo, J.; Barra Caracciolo, A.; Ademollo, N.; Cardoni, M.; Di Lenola, M.; Gaze, W.H.; Stanton, I.C.; Grenni, P.; Pescatore, T.; Spataro, F.; et al. Dissipation of the Antibiotic Sulfamethoxazole in a Soil Amended with Anaerobically Digested Cattle Manure. J. Hazard. Mater. 2019, 378, 120769. [Google Scholar] [CrossRef] [PubMed]
- Demirel, B.; Yenigün, O. Changes in Microbial Ecology in an Anaerobic Reactor. Bioresour. Technol. 2006, 97, 1201–1208. [Google Scholar] [CrossRef]
- Zhang, J.; Qi, C.; Wang, Y.; Li, Y.; Han, T.; Gong, X.; Shan, M.; Li, G.; Luo, W. Enhancing Biogas Production from Livestock Manure in Solid-State Anaerobic Digestion by Sorghum-Vinegar Residues. Environ. Technol. Innov. 2022, 26, 102276. [Google Scholar] [CrossRef]
- Xue, Y.; Shen, R.; Li, Y.; Sun, Z.; Sun, X.; Li, F.; Li, X.; Cheng, Y.; Zhu, W. Anaerobic Fungi Isolated From Bactrian Camel Rumen Contents Have Strong Lignocellulosic Bioconversion Potential. Front. Microbiol. 2022, 13, 888964. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Chen, J.; Fei, Q.; Ma, Y. The Migration Regularity and Removal Mechanism of Antibiotic Resistance Genes During In Situ Enzymatic Hydrolysis and Anaerobic Digestion of Food Waste. Bioresour. Technol. 2023, 385, 129388. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zou, D.; Zheng, X.; Liu, F.; Li, L.; Xiao, Z. Effects of Antibiotics on Anaerobic Digestion of Sewage Sludge: Performance of Anaerobic Digestion and Structure of the Microbial Community. Sci. Total Environ. 2022, 845, 157384. [Google Scholar] [CrossRef]
- Xie, J.; Duan, X.; Feng, L.; Yan, Y.; Wang, F.; Dong, H.; Jia, R.; Zhou, Q. Influence of Sulfadiazine on Anaerobic Fermentation of Waste Activated Sludge for Volatile Fatty Acids Production: Focusing on Microbial Responses. Chemosphere 2019, 219, 305–312. [Google Scholar] [CrossRef]



| Primer Name | Target Gene | Primer Sequence (5′->3′) | Reference |
|---|---|---|---|
| Sul1 fw | sul1 | CGCACCGGAAACATCGCTGCAC | [37] |
| Sul1 rv | TGAAGTTCCGCCGCAAGGCTCG | ||
| Sul2 fw | sul2 | GCGCTCAAGGCAGATGGCATT | [38] |
| Sul2 rv | GCGTTTGATACCGGCACCCGT | ||
| IntI1 fw | intI1 | TCGTGCGTCGCCATACA | [39] |
| IntI1 rv | GCTTGTTCTACGGCCGTTTGA | ||
| 16S fw | 16S rRNA | CGGTGAATACGTTCYCGG | [40] |
| 16S rv | TACCTTGTTACGACTT | ||
| qnrS fw | qnrS | GACGTGCTAACTTGCGTGAT | [41] |
| qnrS rv | TGGCATTGTTGGAAACTT | ||
| qepA fw | qepA | GCAGGTCCAGCAGCGGGTAG | [42] |
| qepA rv | CTTCCTGCCCGAGTATCGTG | ||
| Aac-(6′)-Ib cr fw | aac-(6′)-Ib-cr | TGCATCACAACTGGGCAAAGGCT | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massini, G.; Barra Caracciolo, A.; Rauseo, J.; Spataro, F.; Scordo, G.; Patrolecco, L.; Garbini, G.L.; Visca, A.; Grenni, P.; Rolando, L.; et al. Anaerobic Digestion of Cattle Manure Contaminated with an Antibiotic Mixture: A Nature-Based Solution for Environmental Management. Land 2025, 14, 353. https://doi.org/10.3390/land14020353
Massini G, Barra Caracciolo A, Rauseo J, Spataro F, Scordo G, Patrolecco L, Garbini GL, Visca A, Grenni P, Rolando L, et al. Anaerobic Digestion of Cattle Manure Contaminated with an Antibiotic Mixture: A Nature-Based Solution for Environmental Management. Land. 2025; 14(2):353. https://doi.org/10.3390/land14020353
Chicago/Turabian StyleMassini, Giulia, Anna Barra Caracciolo, Jasmin Rauseo, Francesca Spataro, Giulia Scordo, Luisa Patrolecco, Gian Luigi Garbini, Andrea Visca, Paola Grenni, Ludovica Rolando, and et al. 2025. "Anaerobic Digestion of Cattle Manure Contaminated with an Antibiotic Mixture: A Nature-Based Solution for Environmental Management" Land 14, no. 2: 353. https://doi.org/10.3390/land14020353
APA StyleMassini, G., Barra Caracciolo, A., Rauseo, J., Spataro, F., Scordo, G., Patrolecco, L., Garbini, G. L., Visca, A., Grenni, P., Rolando, L., & Mazzurco Miritana, V. (2025). Anaerobic Digestion of Cattle Manure Contaminated with an Antibiotic Mixture: A Nature-Based Solution for Environmental Management. Land, 14(2), 353. https://doi.org/10.3390/land14020353











