Soil’s Physical, Chemical, and Biological Responses to Different Post-Harvest Management of Pinus elliottii in Santa Catarina, Brazil
Abstract
1. Introduction
2. Materials and Methods
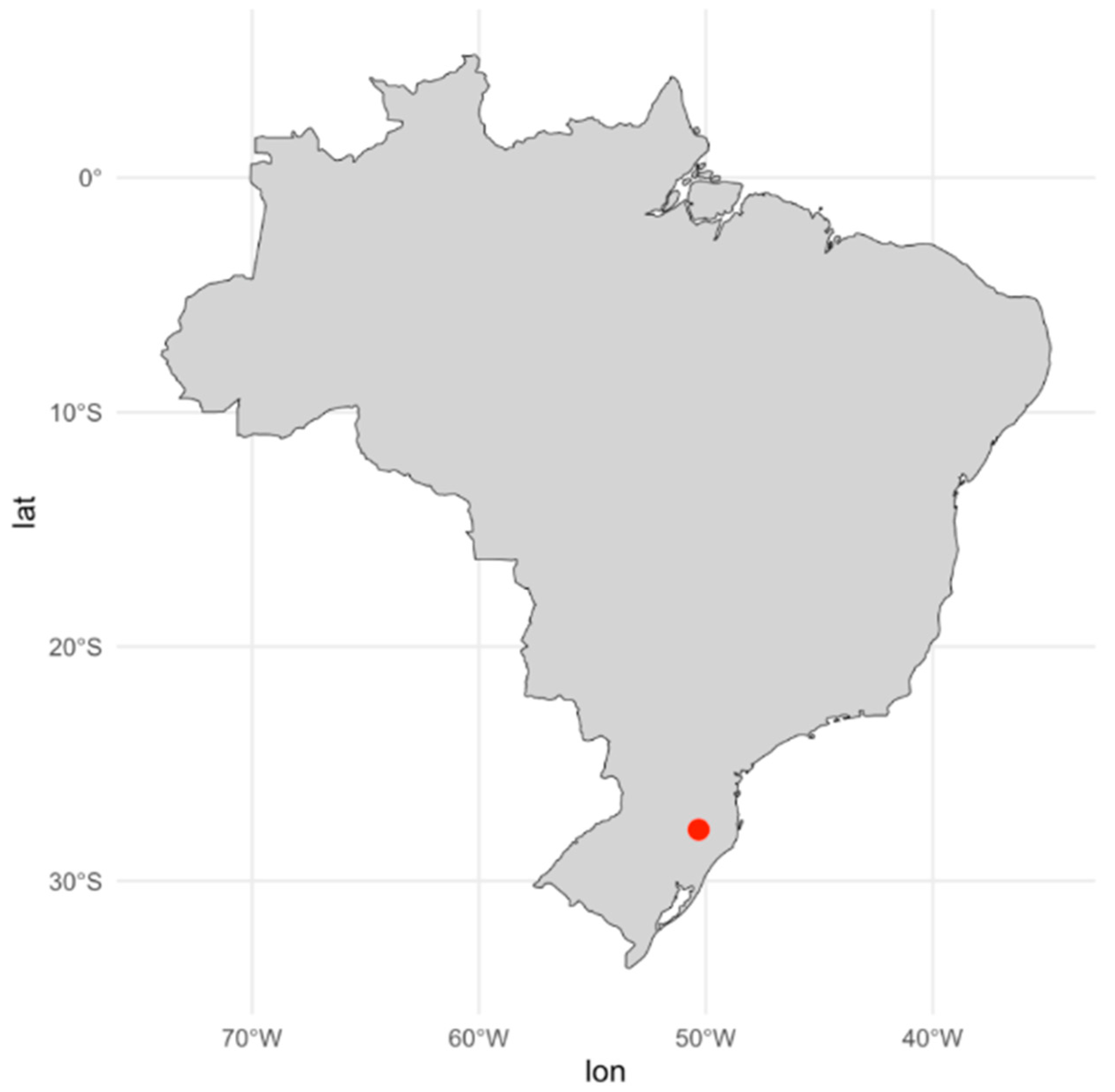
2.1. Experimental Site
2.2. Site Preparation and Treatment Applications
2.3. Soil Sampling and Soil Analysis
2.4. Statistical Procedures
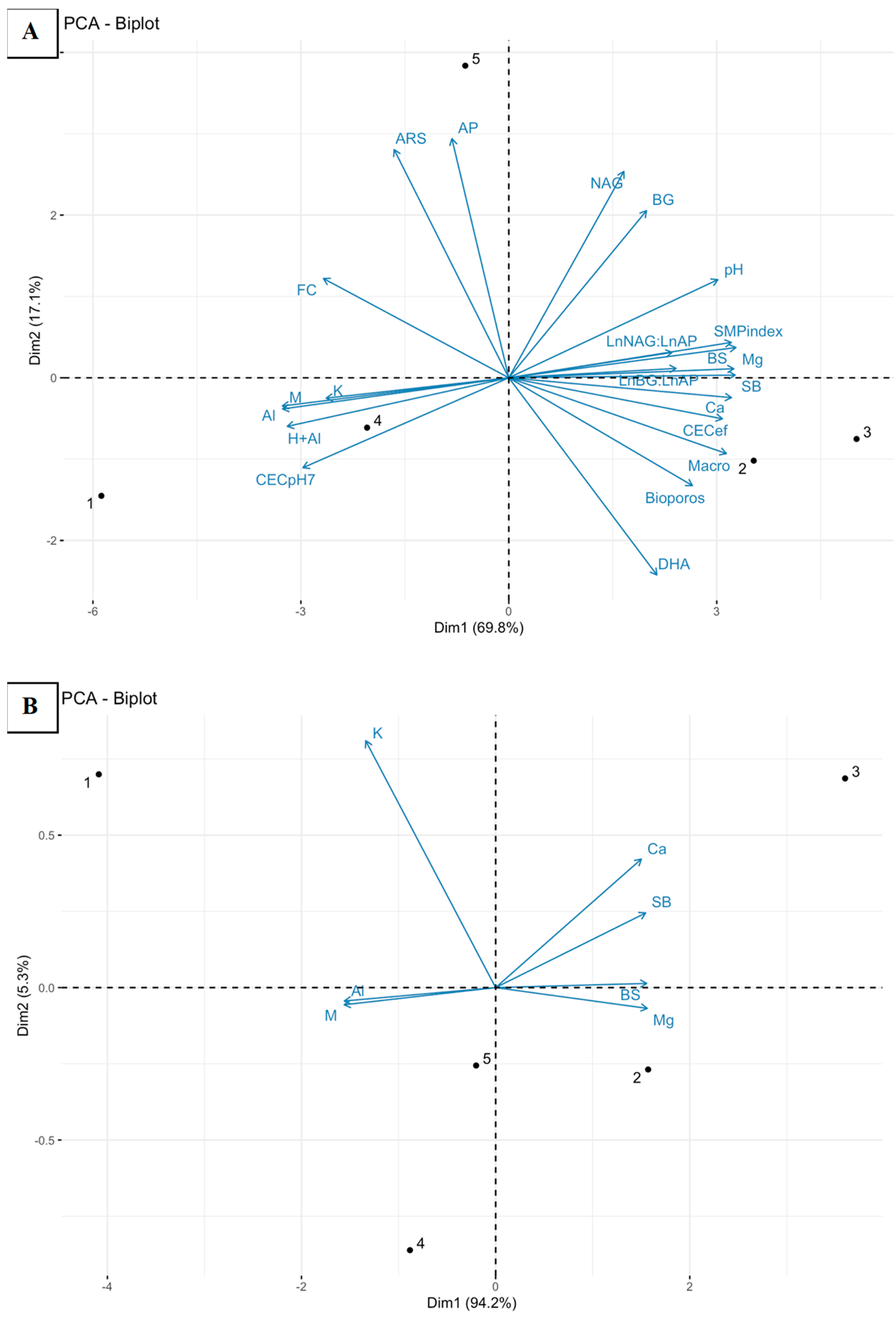
3. Results and Discussion
4. Conclusions
5. Study Limitations and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Henderson, K.A.; Reis, M.; Blanco, C.C.; Pillar, V.D.; Printes, R.C.; Bauch, C.T.; Anand, M. Landowner perceptions of the value of natural forest and natural grassland in a mosaic ecosystem in southern Brazil. Sustain. Sci. 2016, 11, 321–330. [Google Scholar] [CrossRef]
- Schumacher, M.V.; Witschoreck, R.; Calil, F.N.; Lopes, V.G. Biomass and nutrients in a 27 years Pinus taeda L. clear cutting stand in Cambará do Sul, Rio Grande do Sul State. Cienc. Florest. 2013, 22, 321–332. [Google Scholar] [CrossRef]
- Cunha, T.Q.G.; Barbosa, P.V.G.; Lima, P.A.F.; Pimentel, T.S.; Peixoto, L.L.S.; Junior, C.R.S. Characterization of MDF residue and its use in pellet production. Nativa 2018, 6, 300–304. [Google Scholar] [CrossRef]
- Silva, L.B.; Dick, D.P.; Junior, A.V.I. Highland subtropical soils: Chemical attributes, content of organic matter and its resistance to chemical oxidation. Cienc. Rural 2008, 38, 1167–1171. [Google Scholar] [CrossRef]
- Klug, I.; Mafra, A.L.; Friederichs, A.; Rech, C.; Neto, J.F. Soil chemical properties in forestry plantations replacing native vegetation in high-altitude grasslands. Cienc. Florest. 2020, 30, 279–290. [Google Scholar] [CrossRef]
- Wiesmeier, M.; Dick, D.P.; Rumpel, C.; Dalmolin, R.S.D.; Hilscher, A.; Knicker, H. Depletion of soil organic carbon and nitrogen under pine plantations in southern Brazilian grasslands (Campos). Eur. J. Soil Sci. 2009, 60, 347–359. [Google Scholar] [CrossRef]
- Dick, D.P.; Leite, S.B.; Dalmolin, R.S.D.; Almeida, H.C.; Knicker, H. Pine afforestation in South Brazilian highlands: Soil chemical attributes and organic matter composition. Sci. Agric. 2011, 68, 175–181. [Google Scholar] [CrossRef]
- Tomasi, C.A.; Inda, A.V.; Dick, D.P. Humic substances in subtropical altitude Latosol under distinct uses and managements. Cienc. Rural 2012, 42, 2180–2184. [Google Scholar] [CrossRef]
- Denardin, R.B.N.; Mattias, J.L.; Wildner, L.P.; Nesi, C.N.; Sordi, A. Carbon stock in soil under different forest formations, Chapecó, Santa Catarina State. Cienc. Florest. 2014, 24, 59–69. [Google Scholar] [CrossRef]
- Braida, J.A.; Reichert, J.M.; Veiga, M.; Reinert, D.J. Mulch and soil organic carbon content and their relationship with the maximum soil density obtained in the proctor test. Rev. Bras. Cienc. Solo 2006, 30, 605–614. [Google Scholar] [CrossRef]
- Szymczak, D.A.; Brun, E.J.; Reinert, D.J.; Frigotto, T.; Mazzalira, C.C.; Lucio, A.D.; Marafiga, J. Soil compaction caused by forest tractors in the Pinus taeda L. harvesting in the South-Western region of Paraná. Rev. Árvore 2014, 38, 641–648. [Google Scholar] [CrossRef]
- Holthusen, D.; Brandt, A.A.; Reichert, J.M.; Horn, R. Soil porosity, permeability, and static and dynamic strength parameters under native forest/grassland compared to no-tillage cropping. Soil Tillage Res. 2018, 177, 113–124. [Google Scholar] [CrossRef]
- Tonhato, L.; Lopes, E.S.; Rodrigues, C.K.; Sampietro, J.A.; Pelissari, A.L.; Silva, M.K.C. Soil structural quality after forest cutting by tire harvester and adapted hydraulic excavator. Biofix Sci. J. 2022, 7, 74–79. [Google Scholar] [CrossRef]
- Pincelli, A.L.P.S.M.; Seixas, F.; Nunes, R. Compaction and soil fertility after eucalyptus harvest using feller buncher and skidder. Cerne 2014, 20, 191–198. [Google Scholar] [CrossRef]
- Costa, A.; Albuquerque, J.A.; Costa, A.; Warmling, M.T.; Magro, B.A. Pine harvest impact on soil structure of a Dystric Cambisol (Humic). Rev. Bras. Cienc. Solo 2016, 40, e20140643. [Google Scholar] [CrossRef]
- Andrade, M.L.C.; Tassinari, D.; Junior, M.S.D.; Martins, R.P.; Rocha, W.W.; Souza, Z.R. Soil compaction caused by harvest and logging operations in eucalyptus forests in coarse-textured soils from northeastern Brazil. Cienc. Agrotec. 2017, 41, 191–200. [Google Scholar] [CrossRef]
- Andognini, J.; Albuquerque, J.A.; Warmling, M.I.; Teles, J.S.; Silva, G.B. Soil compaction effect on black oat yield in Santa Catarina, Brazil. Rev. Bras. Cienc. Solo 2020, 44, e20190157. [Google Scholar] [CrossRef]
- Bonetti, J.A.; Anghinoni, I.; Moraes, M.T.; Fink, J.R. Resilience of soils with different texture, mineralogy and organic matter under long-term conservation systems. Soil Tillage Res. 2017, 174, 104–112. [Google Scholar] [CrossRef]
- Reichert, J.M.; Cechin, N.F.; Reinert, D.J.; Rodrigues, M.F.; Suzuki, L.E.A.S. Ground-based harvesting operations of pine affects structure and pore functioning of clay and sandy clay soils. Geoderma 2018, 331, 38–49. [Google Scholar] [CrossRef]
- Silva, S.R.; Barros, N.F.; Costa, L.M.; Leite, F.P. Soil compaction and eucalyptus growth in response to forwarder traffic intensity and load. Rev. Bras. Cienc. Solo 2008, 32, 921–932. [Google Scholar] [CrossRef]
- Ansorge, D.; Godwin, R.J. The effect of tires and a rubber track at high axle loads on soil compaction–Part 2: Multi-axle machine studies. Biosyst. Eng. 2008, 99, 338–347. [Google Scholar] [CrossRef]
- Grecenko, A.; Prikner, P. Tire rating based on soil compaction capacity. J. Terramech. 2014, 52, 77–92. [Google Scholar] [CrossRef]
- Malinovski, R. Conversion of forest areas. Forest 2020, 67, 1–5. [Google Scholar]
- Vasconcelos, W.L.F.; Rodrigues, D.M.; Silva, R.O.C.; Alfaia, S.S. Diversity and abundance of soil macrofauna in three land use systems in eastern Amazonia. Rev. Bras. Cienc. Solo 2020, 44, e0190136. [Google Scholar] [CrossRef]
- Silva, L.L.; Ribon, A.A.; Backes, C.; Lopes, L.C.A.; Magalhaes, A.F. Atributos físicos do solo e produtividade da pastagem em sistema de manejo de integração lavoura-pecuária-floresta. Sci. Electron. Arch. 2021, 14, 88–102. [Google Scholar] [CrossRef]
- Cassol, E.A.; Eltz, F.L.F.; Bazzano, M.G.P. Erosivity and hydrological characteristics of rainfalls in Rio Grande (RS, Brazil). Rev. Bras. Cienc. Solo 2010, 34, 235–244. [Google Scholar] [CrossRef]
- Mroz, G.D.; Jurgensen, M.F.; Harvey, A.E.; Larsen, M.J. Effects of fire on nitrogen in forest floor horizons. Soil Sci. Soc. Am. J. 1980, 44, 235–242. [Google Scholar] [CrossRef]
- Bertol, I.; Cogo, N.P.; Levien, R. Erosion in different soil preparations after corn and wheat harvests, in the presence and absence of crop residues. Rev. Bras. Cienc. Solo 1997, 21, 409–418. [Google Scholar] [CrossRef]
- Silva, L.L.; Schneider, P.R.; Eltz, F.L.F. Influence of residues of harvesting of black wattle forest (Acacia mearnsii de Wild) on soil and water losses. Cienc. Florest. 1998, 8, 43–53. [Google Scholar] [CrossRef]
- Fu, B.; Chen, L.; Huang, H.; Qu, P.; Wei, Z. Impacts of crop residues on soil health: A review. Environ. Pollut. Bioavail. 2021, 33, 163–174. [Google Scholar] [CrossRef]
- Redin, M.; Santos, G.D.F.; Miguel, P.; Denega, G.L.; Lupatini, M.; Doneda, A.; Souza, E.L. Impacts of burning on chemical, physical, and biological attributes of soil. Cienc. Florest. 2011, 21, 381–392. [Google Scholar] [CrossRef]
- DeBano, L.F.; Eberlein, G.E.; Dunn, P.H. Effects of burning on chaparral soils: I—Soil nitrogen. Soil Sci. Soc. Am. J. 1979, 43, 504–509. [Google Scholar] [CrossRef]
- Heringer, I.; Jacques, A.V.A. Characteristics of a red Latosol under natural pasture subjected to prolonged fire action and alternative management practices. Cienc. Rural 2002, 32, 309–314. [Google Scholar] [CrossRef]
- Rheinheimer, D.S.; Santos, J.C.P.; Fernandes, V.B.B.; Mafra, A.L.; Almeida, J.A. Changes of chemical attributes of a soil after burning its native permanent pasture. Cienc. Rural 2003, 33, 49–55. [Google Scholar] [CrossRef]
- Pressler, Y.; Moore, J.C.; Cotrufo, M.F. Belowground community responses to fire: Meta-analysis reveals contrasting responses of soil microorganisms and mesofauna. Oikos 2019, 128, 309–327. [Google Scholar] [CrossRef]
- Nelson, A.R.; Narrowe, A.B.; Rhoades, C.C.; Fegel, T.S.; Daly, R.A.; Roth, H.K.; Chu, R.K.; Amundson, K.K.; Young, R.B.; Steindorff, A.S.; et al. Wildfire-dependent changes in soil microbiome diversity and function. Nat. Microbiol. 2022, 7, 1419–1430. [Google Scholar] [CrossRef]
- Caiafa, M.V.; Nelson, A.R.; Borch, T.; Roth, H.K.; Fegel, T.S.; Rhoades, C.C.; Wilkins, M.J.; Glassman, S.I. Distinct fungal and bacterial responses to fire severity and soil depth across a ten-year wildfire chronosequence in beetle-killed lodgepole pine forests. For. Ecol. Manag. 2023, 544, 121160. [Google Scholar] [CrossRef]
- Whitman, T.; Whitman, E.; Woolet, J.; Flannigan, M.D.; Thompson, D.K.; Parisien, M. Soil bacterial and fungal response to wildfires in the Canadian boreal forest across a burn severity gradient. Soil Biol. Biochem. 2019, 138, 107571. [Google Scholar] [CrossRef]
- Woolet, J.; Whitman, T. Pyrogenic organic matter effects on soil bacterial community composition. Soil Biol. Biochem. 2020, 141, 107678. [Google Scholar] [CrossRef]
- Avila, A.C.M.; Albuquerque, J.A.; Kirchhof, G. Pinelands: Impacts of different long-term land uses on soil physical properties in Red Ferrosols. Land 2025, 14, 1471. [Google Scholar] [CrossRef]
- Avila, A.C.M.; Albuquerque, J.A.; Campos, C.G.C. Climate change and its effect on the soil water balance of Lages, Santa Catarina. Rev. Bras. Geogr. Fis. 2022, 15, 2796–2809. [Google Scholar] [CrossRef]
- CQFS RS/SC. Manual de Calagem e Adubação para os Estados do Rio Grande do Sul e de Santa Catarina; Sociedade Brasileira de Ciência do Solo—Núcleo Regional Sul: Porto Alegre, Brazil, 2016. [Google Scholar]
- Gubiani, P.I.; Albuquerque, J.A.; Reinert, D.J.; Reichert, J.M. Water tension and extraction by suction table and sand suction column in two soils with high bulk density. Cienc. Rural 2009, 39, 2535–2538. [Google Scholar] [CrossRef]
- Libardi, P.L. Dynamics of Water in Soil; EdUSP: Sao Paulo, Brazil, 2005. [Google Scholar]
- Embrapa. Manual de Métodos de Análise de Solo; EMBRAPA: Brasília, Brazil, 2017. [Google Scholar]
- Gubiani, P.I.; Reinert, D.J.; Reichert, J.M.; Gelain, N.S.; Minella, J.P.G. Falling head permeameter and software to determine the hydraulic conductivity of saturated soil. Rev. Bras. Cienc. Solo 2010, 34, 993–997. [Google Scholar] [CrossRef]
- Kemper, W.D.; Rosenau, R.C. Aggregate stability and size distribution. In Methods of Soil Analysis: Part 1 Physical and Mineralogical Methods; American Society of Agronomy: Madison, WI, USA, 1986. [Google Scholar] [CrossRef]
- Gee, G.W.; Bauder, J.W. Particle size analysis. In Methods of Soil Analysis: Part 1 Physical and Mineralogical Methods; American Society of Agronomy: Madison, WI, USA, 1986. [Google Scholar] [CrossRef]
- Suzuki, L.E.A.S.; Reichert, J.M.; Albuquerque, J.A.; Reinert, D.J.; Kaiser, D.R. Dispersion and flocculation of Vertisols, Alfisols, and Oxisols in southern Brazil. Geoderma Reg. 2015, 5, 64–70. [Google Scholar] [CrossRef]
- Tedesco, M.J. Soil, Plant, and Other Material Analyses; UFRGS: Porto Alegre, Brazil, 1995. [Google Scholar]
- Jackson, C.R.; Tyler, H.L.; Millar, J.J. Determination of microbial extracellular enzyme activity in waters, soils, and sediments using high throughput microplate assays. J. Vis. Exp. 2013, 80. [Google Scholar] [CrossRef]
- Ramirez, D.; Shaw, L.J.; Collins, C.D. Ecotoxicity of oil sludges and residuals from their washing with surfactants: Soil dehydrogenase and ryegrass germination tests. Environ. Sci. Pollut. Res. Int. 2021, 28, 13312–13322. [Google Scholar] [CrossRef]
- Waring, B.G.; Weintraub, S.R.; Sinsabaugh, R.L. Ecoenzymatic stoichiometry of microbial nutrient acquisition in tropical soils. Biogeochemistry 2014, 117, 101–113. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Ejigu, W.; Selassie, Y.G.; Elias, E.; Molla, E. Effect of lime rates and method of application on soil properties of acidic Luvisols and wheat (Triticum aestivum L.) yields in northwest Ethiopia. Heliyon 2023, 9, e13988. [Google Scholar] [CrossRef]
- Alvarez, E.; Fernandez-Marcos, M.L.; Monterroso, M.J.; Fernandez-Sanjurjo, M.J. Application of aluminium toxicity indices to soils under various forest species. For. Ecol. Manag. 2005, 211, 227–239. [Google Scholar] [CrossRef]
- Gurmu, G.; Beyene, S.; Selassie, Y.; Kidanu, S. Lime requirement determination methods on acid neutralisation efficiency under selected acidic soils of the Ethiopian highlands. Trop. Agric. 2024, 101, 68–89. [Google Scholar]
- Spera, S.T.; Reatto, A.; Correia, J.R.; Silva, J.C.S. Physical characteristics of a dark-red Latosol (Oxisol) of the Brazilian savannas (Cerrados) of Planaltina under fire action. Pesqui. Agropecu. Bras. 2000, 35, 1817–1824. [Google Scholar] [CrossRef]
- Meirelles, M.L. Effect of fire on soil moisture in a Cerrado scrubland area. Sci. Cult. 1990, 42, 359–360. [Google Scholar]
- Knicker, H. How does fire affect the nature and stability of soil organic nitrogen and carbon? A review. Biogeochemistry 2007, 85, 91–118. [Google Scholar] [CrossRef]
- Sousa, C.T.C.; Bastos, A.T. Queimadas no Brasil e o direito ao meio ambiente ecologicamente equilibrado. Rev. Cient. Intracienc. 2020, 1, 1–10. [Google Scholar]
- Ciotta, M.N.; Bayer, C.; Ernani, P.R.; Fontoura, S.M.V.; Wobeto, C.; Albuquerque, J.A. Liming management and its effect on acidity components of an Oxisol under no-tillage. Rev. Bras. Cienc. Solo 2004, 28, 317–326. [Google Scholar] [CrossRef]
- Kaminski, J.; Rheinheimer, D.S.; Santos, E.J.S.; Gatiboni, L.C.; Bortoluzzi, E.C.; Xavier, F.M. Liming superficial and incorporated on soil under native sod and crops yield. Cienc. Rural 2000, 30, 605–609. [Google Scholar] [CrossRef]
- Rheinheimer, D.S.; Santos, E.J.S.; Kaminski, J.; Xavier, F.M. Surface application of lime on no-tillage. Cienc. Rural 2000, 30, 263–268. [Google Scholar] [CrossRef]
| Physical–Chemical Attributes | Unit | Value |
|---|---|---|
| Organic Matter | g kg−1 | 39 |
| pH H2O | 4.5 | |
| Phosphorus | mg kg−1 | 6.5 |
| Potassium | mg kg−1 | 127 |
| Calcium | cmolc dm−3 | 2.6 |
| Magnesium | cmolc dm−3 | 1.3 |
| Aluminum | cmolc dm−3 | 3.4 |
| CECpH7 | cmolc dm−3 | 28.6 |
| Base saturation | % | 15 |
| Al saturation | % | 45 |
| Sampling | E1 | E2 | E1 | E2 | E1 | E2 | E1 | E2 | E1 | E2 | ANOVA | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (0–10 cm) | Control | LimeInc + Burn | LimeInc + Res | LimeSur + Burn | LimeSur + Res | Factor treatment | Factor time | Factor Treatment– Time | |||||
| Bulk density | 1.34 | 1.34 | 1.25 | 1.21 | 1.27 | 1.27 | 1.31 | 1.28 | 1.28 | 1.36 | ns | ns | ns |
| Total porosity | 0.52 | 0.52 | 0.54 | 0.57 | 0.51 | 0.56 | 0.54 | 0.54 | 0.55 | 0.52 | ns | ns | ns |
| Microporosity | 0.42 | 0.43 | 0.40 | 0.43 | 0.36 | 0.43 | 0.41 | 0.43 | 0.41 | 0.44 | ns | * | ns |
| Macroporosity | 0.10 | 0.09 | 0.14 | 0.14 | 0.15 | 0.13 | 0.13 | 0.11 | 0.13 | 0.08 | * | * | ns |
| Biopores | 0.06 | 0.05 | 0.08 | 0.08 | 0.08 | 0.07 | 0.07 | 0.06 | 0.08 | 0.04 | * | ns | ns |
| Field capacity | 0.40 | 0.41 | 0.37 | 0.41 | 0.33 | 0.40 | 0.38 | 0.40 | 0.39 | 0.42 | * | * | ns |
| Permanent wilting point | 0.31 | 0.32 | 0.27 | 0.29 | 0.24 | 0.30 | 0.29 | 0.29 | 0.30 | 0.32 | ns | ns | ns |
| Available water | 0.10 | 0.11 | 0.12 | 0.14 | 0.11 | 0.12 | 0.11 | 0.13 | 0.11 | 0.11 | ns | * | ns |
| MWD | 5.6 | 6.0 | 5.7 | 5.9 | 5.8 | 6.1 | 5.6 | 6.1 | 5.8 | 6.0 | ns | * | ns |
| SHC | 2.0 | 1.9 | 2.2 | 2.5 | 2.4 | 2.3 | 2.1 | 2.3 | 2.3 | 1.7 | ns | ns | ns |
| (10–20 cm) | |||||||||||||
| Bulk density | 1.29 | 1.34 | 1.29 | 1.28 | 1.26 | 1.30 | 1.29 | 1.32 | 1.28 | 1.30 | ns | ns | ns |
| Total porosity | 0.54 | 0.52 | 0.55 | 0.54 | 0.54 | 0.54 | 0.52 | 0.53 | 0.52 | 0.54 | ns | ns | ns |
| Microporosity | 0.43 | 0.44 | 0.42 | 0.44 | 0.40 | 0.43 | 0.41 | 0.43 | 0.40 | 0.44 | ns | * | ns |
| Macroporosity | 0.11 | 0.08 | 0.13 | 0.11 | 0.15 | 0.10 | 0.11 | 0.09 | 0.12 | 0.10 | ns | * | ns |
| Biopores | 0.07 | 0.05 | 0.07 | 0.06 | 0.07 | 0.05 | 0.06 | 0.05 | 0.06 | 0.05 | ns | * | ns |
| Field capacity | 0.40 | 0.41 | 0.39 | 0.41 | 0.37 | 0.40 | 0.38 | 0.41 | 0.37 | 0.41 | ns | * | ns |
| Permanent wilting point | 0.30 | 0.33 | 0.30 | 0.31 | 0.28 | 0.31 | 0.29 | 0.31 | 0.29 | 0.31 | ns | ns | ns |
| Available water | 0.12 | 0.10 | 0.11 | 0.12 | 0.12 | 0.12 | 0.11 | 0.12 | 0.10 | 0.12 | ns | ns | ns |
| MWD | 5.6 | 6.1 | 5.4 | 6.0 | 5.8 | 6.0 | 5.6 | 6.0 | 5.8 | 5.9 | ns | * | ns |
| SHC | 1.7 | 2.2 | 2.1 | 2.3 | 2.3 | 2.3 | 1.9 | 1.9 | 2.3 | 2.5 | ns | ns | ns |
| Sampling | E1 | E2 | E1 | E2 | E1 | E2 | E1 | E2 | E1 | E2 | ANOVA | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (0–10 cm) | Control | LimeInc + Burn | LimeInc + Res | LimeSur + Burn | LimeSur + Res | Factor Treatment | Factor Time | Factor Treatment–Time | |||||
| Clay dispersion | 16 | 17 | 18 | 19 | 20 | 21 | 15 | 16 | 15 | 14 | ns | ns | ns |
| pH | 4.7 | 4.8 | 5.0 | 5.1 | 5.1 | 5.1 | 4.9 | 5.0 | 5.0 | 5.1 | * | ns | ns |
| P | 4.3 | 5.7 | 4.4 | 6.5 | 4.6 | 5.1 | 4.2 | 6.0 | 5.7 | 5.3 | ns | * | ns |
| K | 117 | 110 | 96 | 68 | 86 | 69 | 89 | 68 | 98 | 82 | * | * | ns |
| Organic matter | 4.2 | 4.0 | 4.3 | 3.9 | 4.6 | 4.2 | 4.1 | 3.9 | 4.1 | 3.8 | ns | * | ns |
| Al | 2.8 | 3.6 | 1.2 | 1.2 | 1.3 | 1.1 | 2.1 | 2.6 | 1.7 | 2.1 | * | ns | ns |
| Ca | 3.5 | 2.7 | 5.1 | 4.5 | 5 | 4.7 | 3.7 | 3 | 4.2 | 3.4 | * | * | ns |
| Mg | 1.3 | 1.3 | 2.4 | 3.1 | 2.3 | 3.2 | 1.7 | 2 | 2 | 2.4 | * | * | ns |
| H + Al | 21.1 | 21.2 | 14.2 | 14.8 | 15.5 | 14.5 | 18.8 | 18.5 | 16.9 | 16.5 | * | ns | ns |
| CECef | 8.0 | 7.9 | 9.0 | 9.0 | 8.9 | 9.3 | 7.9 | 7.9 | 8.2 | 8.3 | * | ns | ns |
| CECpH7 | 26 | 25 | 22 | 22 | 23 | 22 | 24 | 23 | 23 | 22 | * | ns | ns |
| Sum bases | 5.1 | 4.3 | 7.8 | 7.8 | 7.6 | 8.1 | 5.7 | 5.2 | 6.5 | 6.1 | * | ns | ns |
| Base saturation | 20 | 17 | 36 | 34 | 34 | 36 | 24 | 22 | 28 | 27 | * | ns | ns |
| Al saturation | 35 | 45 | 14 | 13 | 15 | 13 | 27 | 34 | 21 | 26 | * | ns | ns |
| (10–20 cm) | |||||||||||||
| Clay dispersion | 14 | 14 | 19 | 18 | 18 | 18 | 14 | 15 | 12 | 11 | ns | ns | ns |
| pH | 4.7 | 4.8 | 4.8 | 4.9 | 4.9 | 4.8 | 4.8 | 4.7 | 4.8 | 4.9 | ns | ns | ns |
| P | 3.3 | 4.7 | 4.8 | 5.2 | 4.7 | 4.1 | 4.5 | 4.1 | 3.7 | 3.9 | ns | ns | ns |
| K | 98 | 91 | 83 | 66 | 86 | 66 | 86 | 68 | 89 | 71 | * | * | ns |
| Organic matter | 3.4 | 3.4 | 3.6 | 3.4 | 3.8 | 3.4 | 3.6 | 3.2 | 3.4 | 3.6 | ns | ns | ns |
| Al | 3.4 | 4.3 | 2.8 | 3.3 | 2.4 | 2.9 | 2.9 | 3.9 | 2.8 | 3.6 | * | * | ns |
| Ca | 2.6 | 2.0 | 3.1 | 2.6 | 3.6 | 2.7 | 2.9 | 2.0 | 3.0 | 2.2 | * | * | ns |
| Mg | 0.9 | 1.0 | 1.3 | 1.8 | 1.7 | 1.8 | 1.3 | 1.4 | 1.4 | 1.5 | * | ns | ns |
| H + Al | 23.0 | 22.7 | 19.5 | 22.1 | 19.5 | 20.3 | 17.9 | 22.5 | 20.4 | 22.0 | ns | ns | ns |
| CECef | 7.3 | 7.6 | 7.5 | 7.9 | 8.0 | 7.7 | 7.4 | 7.5 | 7.4 | 7.5 | ns | ns | ns |
| CECpH7 | 26.9 | 26.1 | 24.3 | 26.7 | 25.1 | 25.1 | 22.3 | 26.1 | 25.0 | 25.9 | ns | ns | ns |
| Sum bases | 3.8 | 3.3 | 4.7 | 4.5 | 5.6 | 4.7 | 4.4 | 3.6 | 4.6 | 3.9 | * | * | ns |
| Base saturation | 14 | 13 | 21 | 17 | 24 | 19 | 20 | 14 | 19 | 15 | * | * | ns |
| Al saturation | 47 | 56 | 37 | 42 | 32 | 38 | 40 | 51 | 38 | 48 | * | * | ns |
| Sampling | E1 | E2 | E1 | E2 | E1 | E2 | E1 | E2 | E1 | E2 | ANOVA | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (0–10 cm) | Control | LimeInc + Burn | LimeInc + Res | LimeSur + Burn | LimeSur + Res | Factor Treatment | Factor Time | Factor Treatment–Time | |||||
| NAG | 9 | 14 | 18 | 11 | 24 | 19 | 22 | 5 | 21 | 27 | * | ns | * |
| AP | 70 | 53 | 68 | 72 | 46 | 48 | 100 | 50 | 103 | 118 | * | ns | * |
| BG | 16 | 9 | 19 | 14 | 27 | 24 | 22 | 16 | 24 | 26 | * | * | ns |
| DHA | 40 | 27 | 33 | 54 | 31 | 63 | 39 | 42 | 29 | 24 | * | ns | * |
| ARS | 12 | 20 | 10 | 11 | 6 | 8 | 18 | 7 | 24 | 27 | * | ns | * |
| LnNAG:LnAP | 0.47 | 0.68 | 0.67 | 0.55 | 0.83 | 0.76 | 0.62 | 0.42 | 0.58 | 0.70 | * | ns | * |
| LnBG:LnAP | 0.67 | 0.56 | 0.69 | 0.61 | 0.86 | 0.80 | 0.66 | 0.69 | 0.68 | 0.68 | * | * | ns |
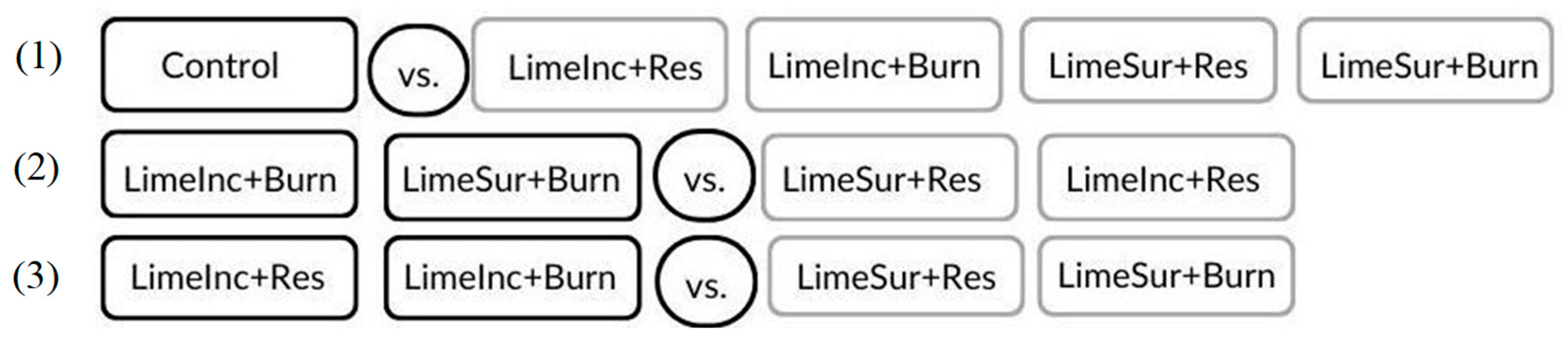
| Contrast 1 | ||||
| Average | ||||
| Control | Others | |||
| Layer 0–10 cm | * | Macroporosity, m3 m−3 | 0.10 | 0.13 |
| * | pH | 4.8 | 5.0 | |
| * | K, mg kg−1 | 114 | 82 | |
| * | Al, cmolc kg−1 | 3.2 | 1.7 | |
| * | Ca, cmolc kg−1 | 3.1 | 4.2 | |
| * | Mg, cmolc kg−1 | 1.3 | 2.4 | |
| * | H + Al, cmolc kg−1 | 21.3 | 16.2 | |
| * | CECef, cmolc kg−1 | 8.0 | 8.6 | |
| * | CECpH7, cmolc kg−1 | 25.9 | 23.1 | |
| * | SB, cmolc kg−1 | 4.7 | 6.9 | |
| * | BS, % | 19 | 30 | |
| * | M, % | 41 | 21 | |
| ns | BD, TP, micro, macro, FC, PWP AW, MWD, SHC, clay dispersion, P, OM. | |||
| Layer 10–20 cm | * | K, mg kg−1 | 95 | 77 |
| * | Al, cmolc kg−1 | 3.8 | 3.1 | |
| * | Mg, cmolc kg−1 | 1.0 | 1.5 | |
| * | SB, cmolc kg−1 | 3.6 | 4.5 | |
| * | BS, % | 14 | 20 | |
| * | M, % | 52 | 41 | |
| ns | BD, TP, micro, macro, biopores, AW FC, PWP, MWD, SHC, clay dispersion, pH, P, OM, Ca, H + Al, CECef, CECpH7. | |||
| Contrast 2 | ||||
| Res (LimeInc or LimeSur) | Burn (LimeInc or LimeSur) | |||
| Layer 0–10 cm | ns | All variables | ||
| Layer 10–20 cm | ns | All variables | ||
| Contrast 3 | ||||
| Average | ||||
| LimeInc (Res or Burn) | LimeSur (Res or Burn) | |||
| Layer 0–10 cm | * | Macroporosity, m3 m−3 | 0.14 | 0.11 |
| * | Biopores, m3 m−3 | 0.08 | 0.06 | |
| * | Field capacity, m3 m−3 | 0.38 | 0.40 | |
| * | Al, cmolc kg−1 | 1.2 | 2.2 | |
| * | Ca, cmolc kg−1 | 4.8 | 3.6 | |
| * | Mg, cmolc kg−1 | 2.8 | 2.0 | |
| * | H + Al, cmolc kg−1 | 1.3 | 1.9 | |
| * | CECef, cmolc kg−1 | 9.1 | 8.1 | |
| * | SB, cmolc kg−1 | 7.8 | 5.9 | |
| * | BS, % | 35 | 25 | |
| * | M, % | 14 | 27 | |
| ns | BD, TP, Micro, PWP, AW MWD, SHC, clay dispersion, pH, P, K, OM, CECpH7. | |||
| Layer 10–20 cm | * | K, mg kg−1 | 76 | 79 |
| * | Al, cmolc kg−1 | 2.9 | 3.3 | |
| * | Mg, cmolc kg−1 | 1.6 | 1.4 | |
| * | SB, cmolc kg−1 | 4.9 | 4.1 | |
| * | BS, % | 20 | 17 | |
| * | M, % | 37 | 44 | |
| ns | BD, TP, micro, macro, biopores, FC PWP, AW, MWD, SHC, clay dispersion, pH, P, K, OM, Al, H + Al, CECef, CECpH7. | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Mattos e Avila, A.C.; Kirchhof, G.; Nara Ciotta, M.; Camargo Mendes, S.D.; Mangrich dos Passos, J.F.; do Nascimento, M.; Adriano Albuquerque, J. Soil’s Physical, Chemical, and Biological Responses to Different Post-Harvest Management of Pinus elliottii in Santa Catarina, Brazil. Land 2025, 14, 2331. https://doi.org/10.3390/land14122331
de Mattos e Avila AC, Kirchhof G, Nara Ciotta M, Camargo Mendes SD, Mangrich dos Passos JF, do Nascimento M, Adriano Albuquerque J. Soil’s Physical, Chemical, and Biological Responses to Different Post-Harvest Management of Pinus elliottii in Santa Catarina, Brazil. Land. 2025; 14(12):2331. https://doi.org/10.3390/land14122331
Chicago/Turabian Stylede Mattos e Avila, Ana Carolina, Gunnar Kirchhof, Marlise Nara Ciotta, Sandra Denise Camargo Mendes, João Frederico Mangrich dos Passos, Marieli do Nascimento, and Jackson Adriano Albuquerque. 2025. "Soil’s Physical, Chemical, and Biological Responses to Different Post-Harvest Management of Pinus elliottii in Santa Catarina, Brazil" Land 14, no. 12: 2331. https://doi.org/10.3390/land14122331
APA Stylede Mattos e Avila, A. C., Kirchhof, G., Nara Ciotta, M., Camargo Mendes, S. D., Mangrich dos Passos, J. F., do Nascimento, M., & Adriano Albuquerque, J. (2025). Soil’s Physical, Chemical, and Biological Responses to Different Post-Harvest Management of Pinus elliottii in Santa Catarina, Brazil. Land, 14(12), 2331. https://doi.org/10.3390/land14122331







