Beyond the Surface: Understanding Salt Crusts’ Impact on Water Loss in Arid Regions
Abstract
1. Introduction
2. Materials and Methods
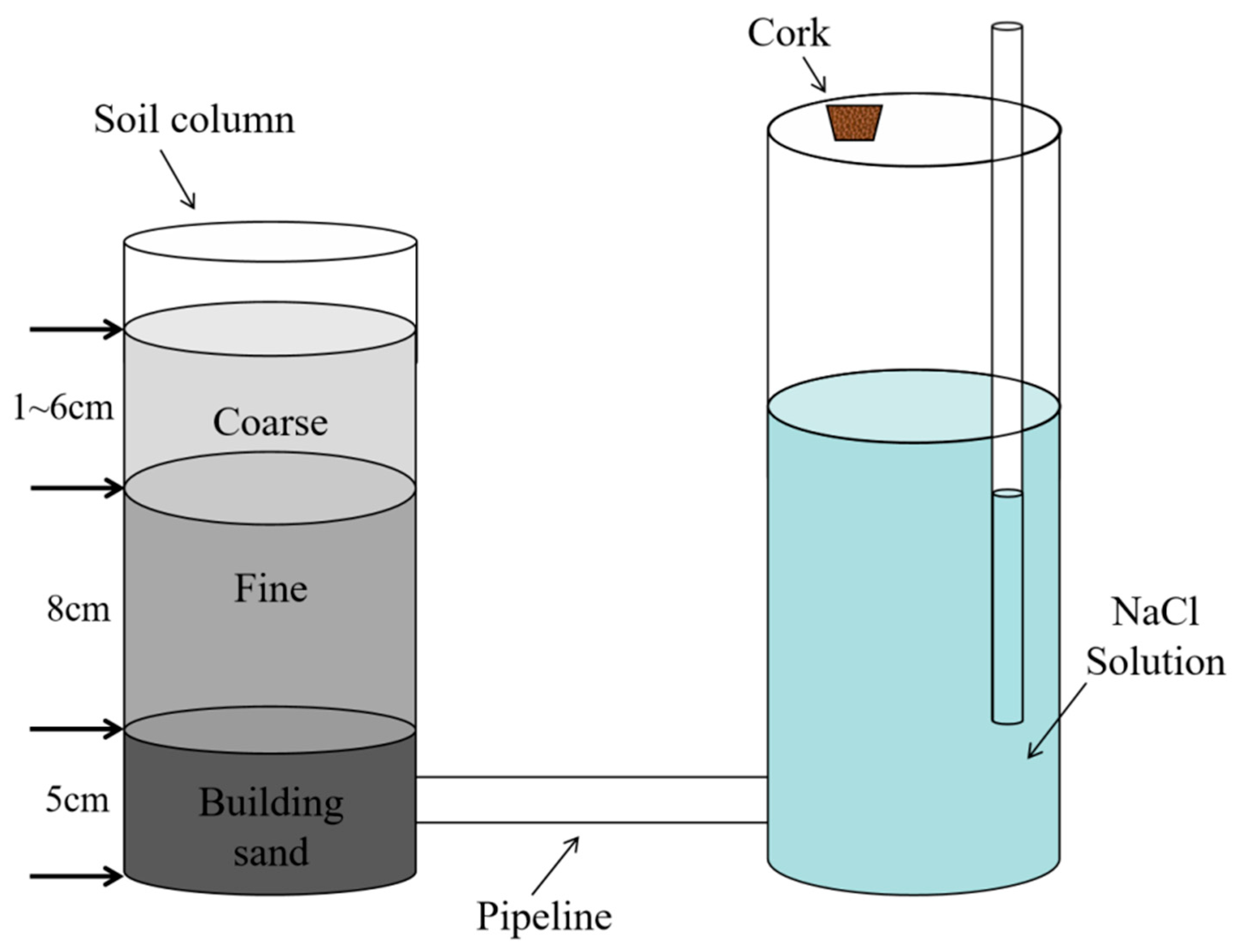
2.1. Experimental Materials
2.2. Experimental Design and Equipment
2.3. Data Acquisition and Processing
3. Results
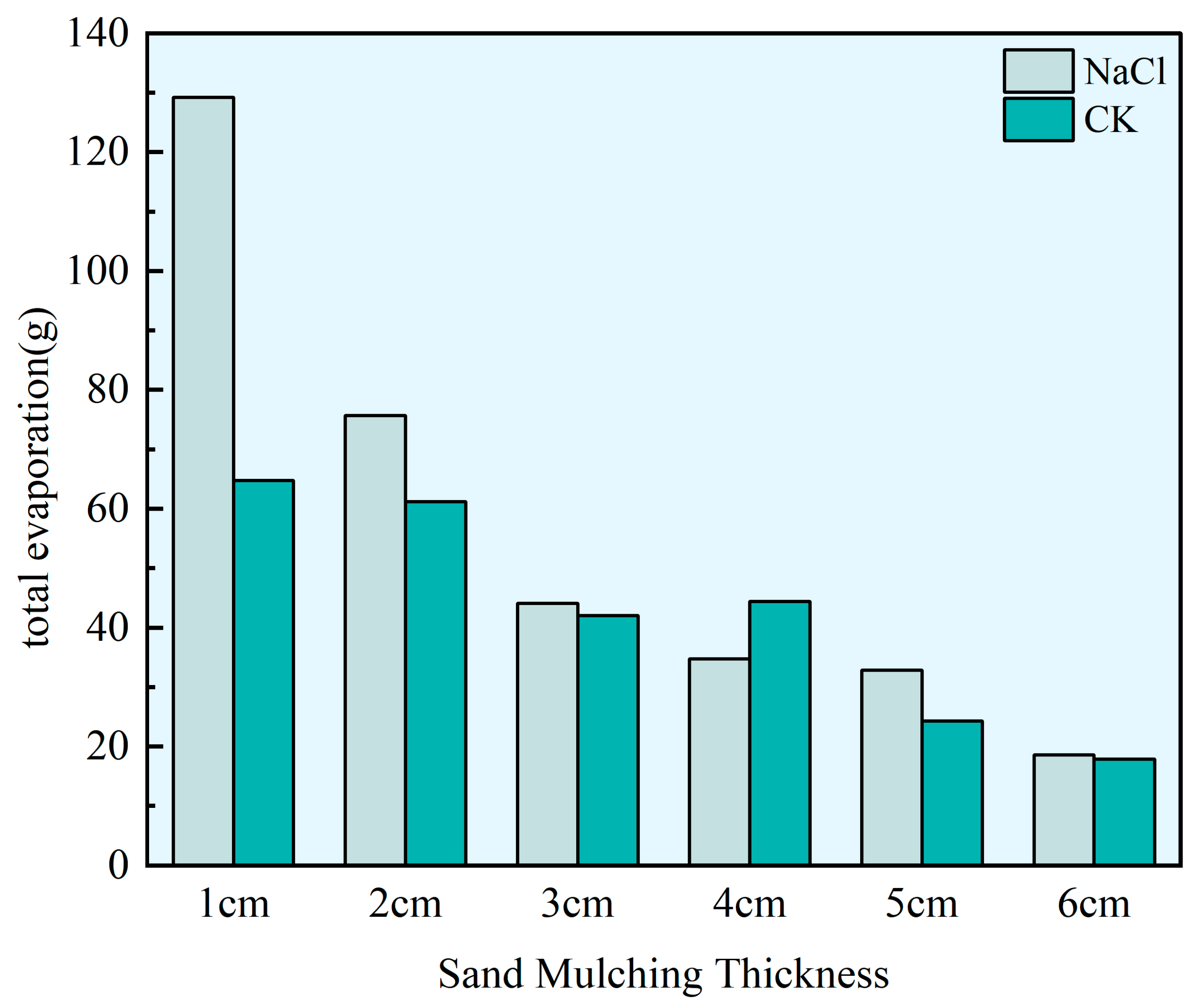
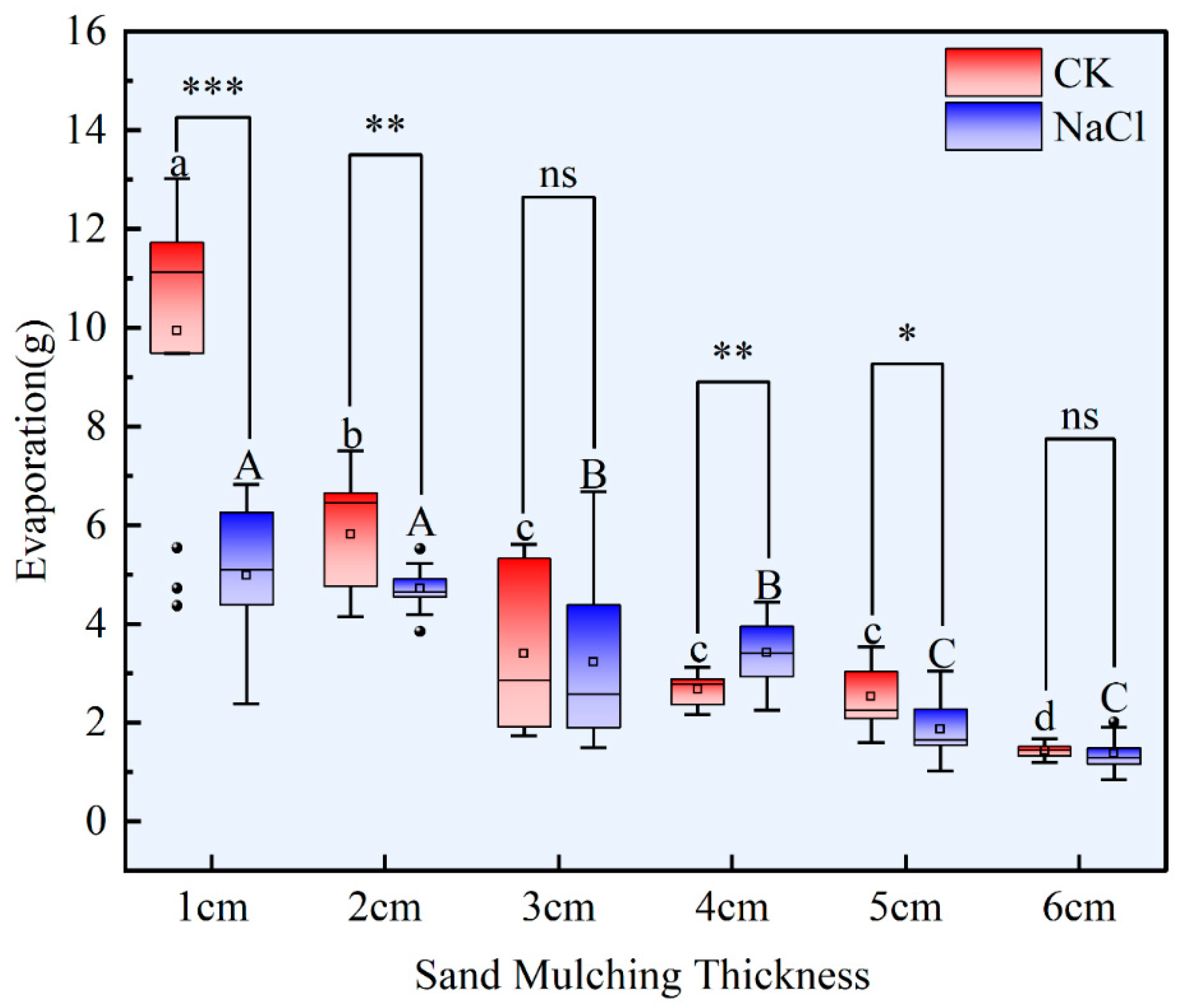
3.1. Soil Moisture Evaporation
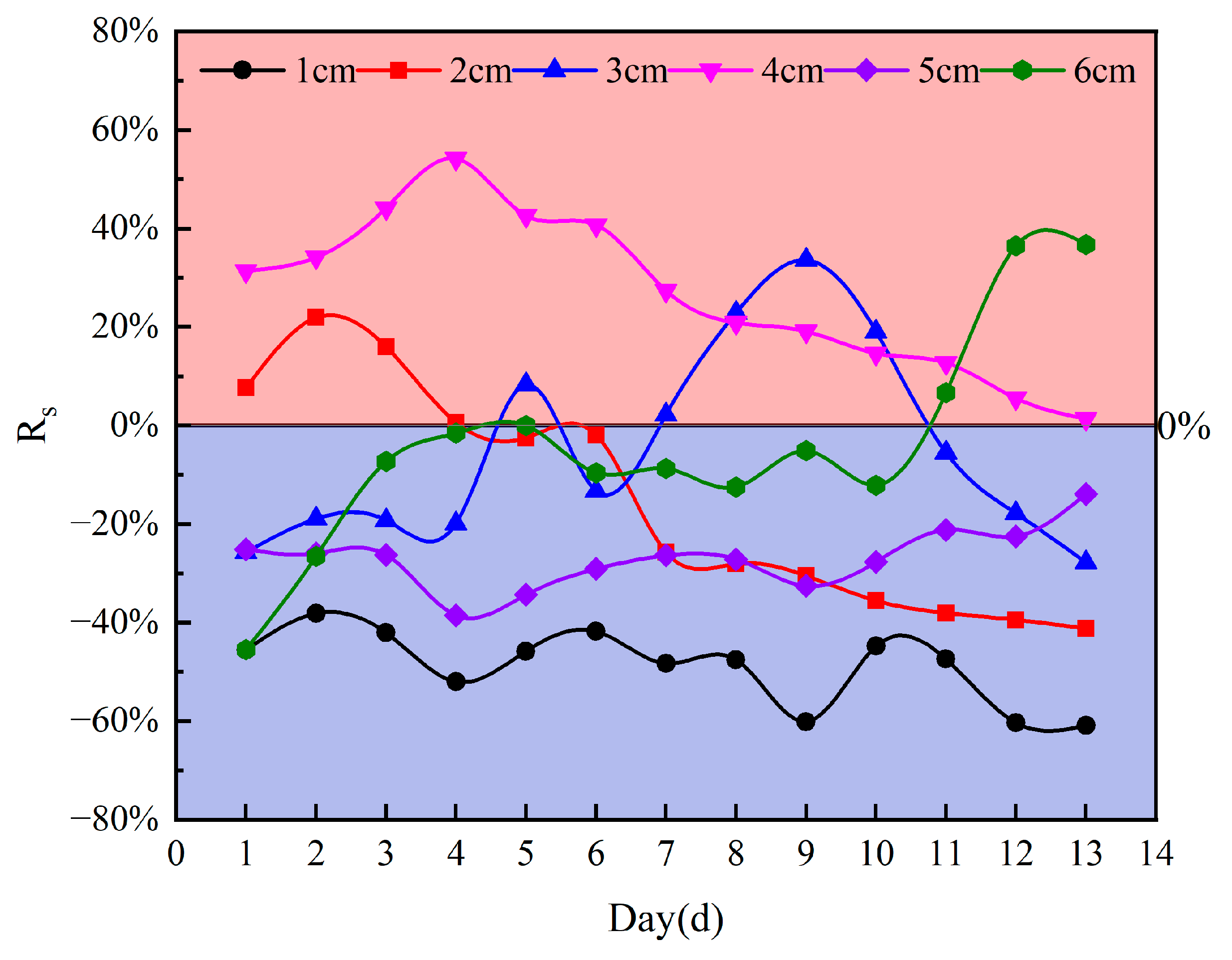
3.2. The Effect of Salinity on Soil Water Evaporation Rate
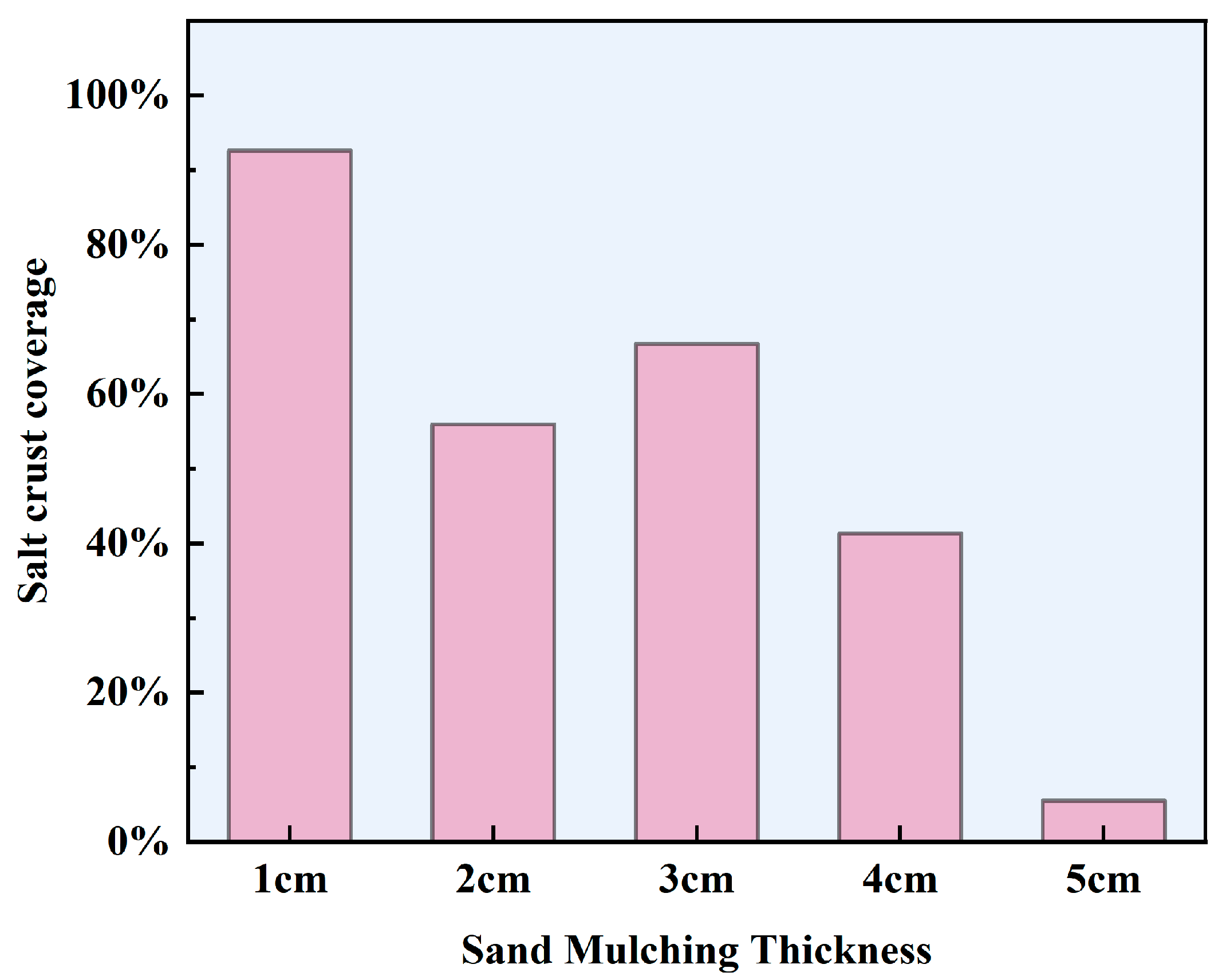
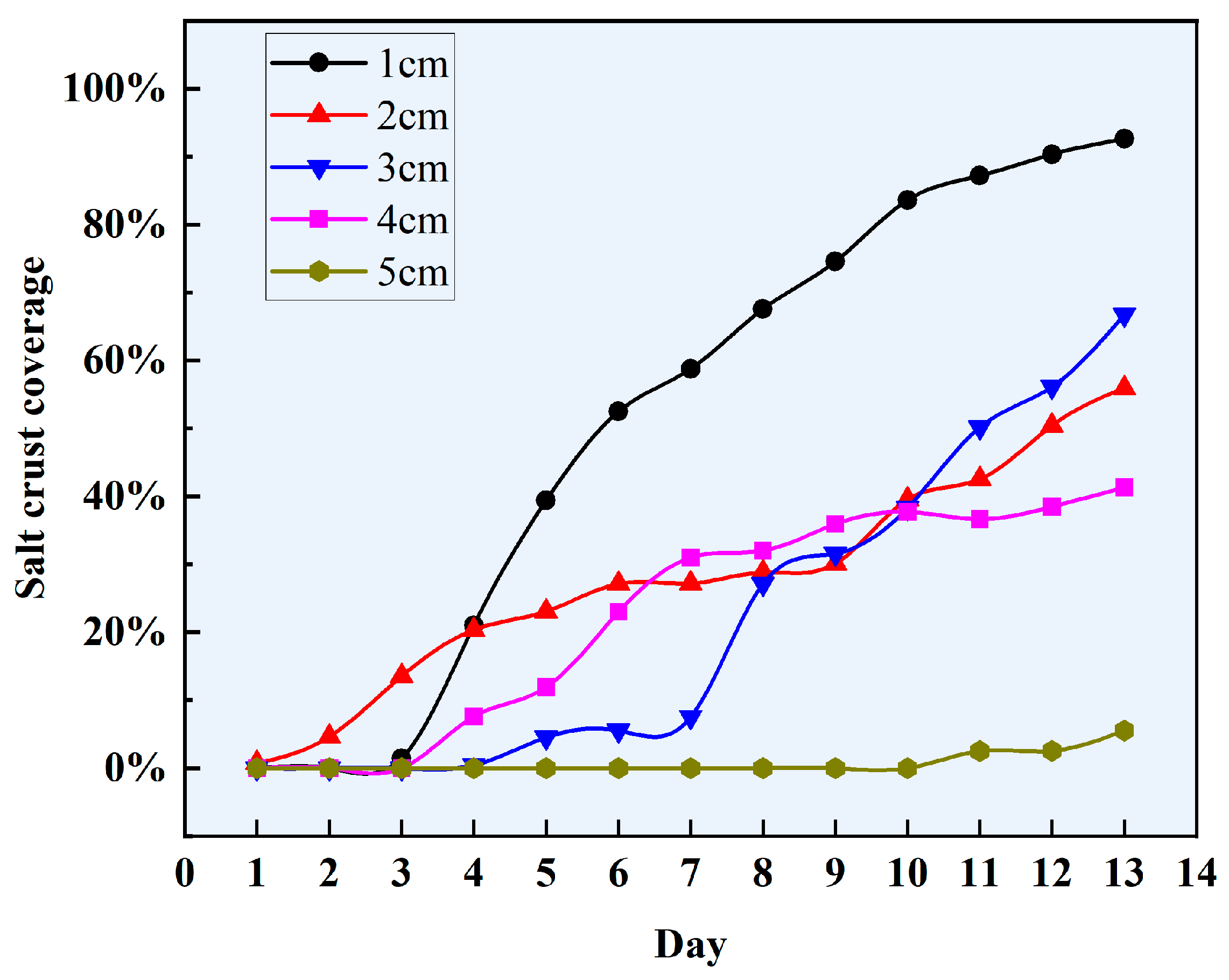
3.3. Salt Crust on the Surface of a Brine Soil Column
4. Discussion
4.1. The Effect of Sand Mulching on Soil Moisture Evaporation
4.2. Effect of SCC on Soil Water Evaporation
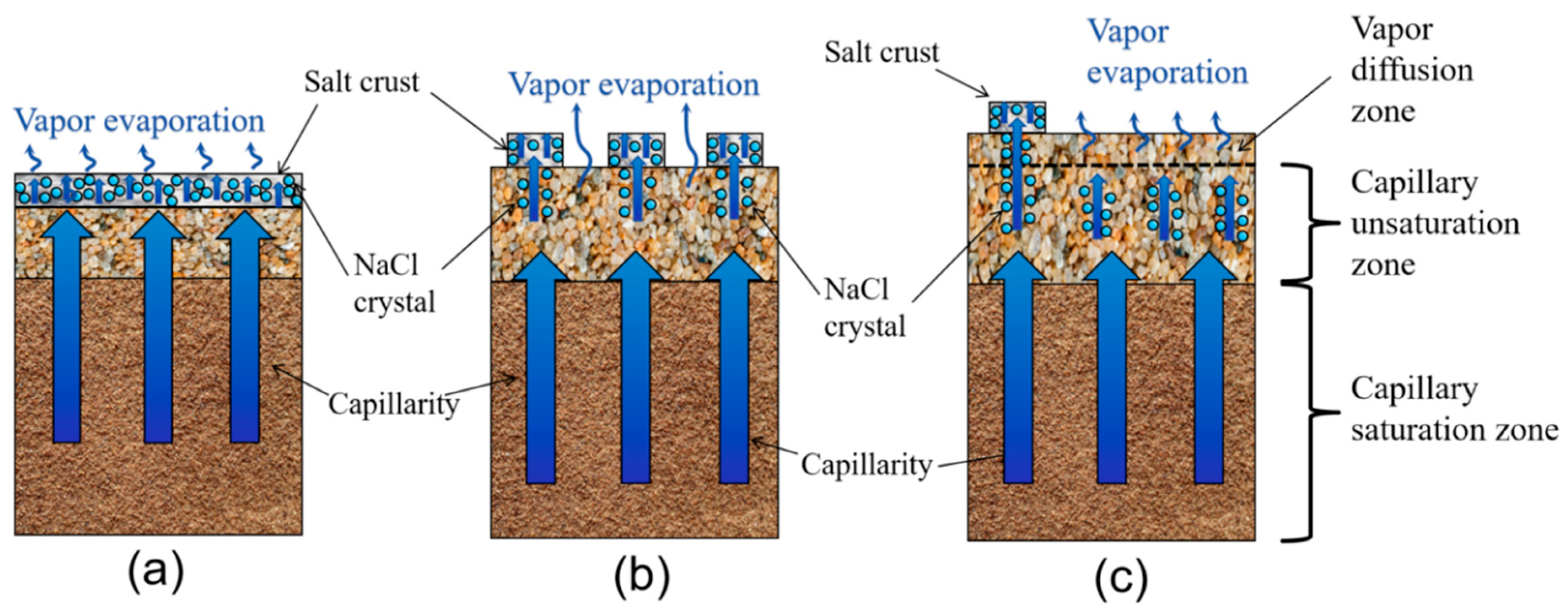
4.2.1. Mechanism of High-Coverage Crusty Crusts Suppresses Evaporation
4.2.2. Mechanism of Medium-Coverage Patchy Crusts Promotes Evaporation
4.2.3. Mechanism of Weakened Effect of Thick Sand Mulching Without Crusts
4.3. Implications and Future Perspectives
5. Conclusions
- Sand mulching can suppress evaporation overall, but salinity regulates the evaporation process. The soil evaporation process in NaCl brine differs significantly from that in deionized water conditions, with the evaporation rate depending on the morphology and coverage of the surface NaCl salt crust. When a high-coverage crusty crust forms, evaporation is markedly inhibited; conversely, when a medium-coverage patchy crust forms, evaporation is enhanced.
- The regulatory mechanisms of salt crust morphology and coverage (SCC) on evaporation can be categorized into the following three types:
- (1)
- Mechanism of high-coverage crusty crusts suppresses soil evaporation (sand mulching 1–2 cm). High SCC (92.62–55.93%) suppresses evaporation by blocking pores and increasing diffusion resistance, with a maximum inhibition rate of 60.91% (1 cm sand mulching);
- (2)
- Mechanism of medium-coverage patchy crusts promotes soil evaporation (sand mulching 3–4 cm). At 4 cm sand mulching, the salt crust morphology transformed into a medium-coverage patchy crust, significantly enhancing water evaporation with a maximum promotion rate of 54.15%. At 3 cm sand mulching, the salt crust exhibited a medium-coverage patchy crust, also showing significant promotion of water evaporation with a maximum promotion rate of 33.61%. However, excessively low (SCC = 0.42%) or high (SCC = 50.14%) coverage may inhibit water evaporation.
- (3)
- Mechanism of non-crusting weak influence (sand mulching 5–6 cm). Salt crystals form ”salt tree” structures within the soil, with no salt crust on the surface. During the late evaporation phase (days 11–13), the ”salt tree” structures shorten the distance for water vapor diffusion, resulting in promoted soil evaporation (promotion rate: 36.73%).
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharafatmandrad, M.; Khosravi Mashizi, A. Temporal and Spatial Assessment of Supply and Demand of the Water-yield Ecosystem Service for Water Scarcity Management in Arid to Semi-arid Ecosystems. Water Resour. Manag. 2021, 35, 63–82. [Google Scholar] [CrossRef]
- Jiao, L.; An, W.; Li, Z.; Gao, G.; Wang, C. Regional variation in soil water and vegetation characteristics in the Chinese Loess Plateau. Ecol. Indic. 2020, 115, 106399. [Google Scholar] [CrossRef]
- Hamarash, H.; Hamad, R.; Rasul, A. Meteorological drought in semi-arid regions: A case study of Iran. J. Arid Land 2022, 14, 1212–1233. [Google Scholar] [CrossRef]
- Feng, Y.; Sun, F.; Deng, X. Attributing the divergent changes of drought from humid to dry regions across China. J. Hydrol. 2025, 660, 133363. [Google Scholar] [CrossRef]
- Guo, J.; Sun, M.; Zhang, C.; Lienhart, W.; Jiang, H.; Shi, B. Water Vapor Transport in Dry Sand During Evaporation Monitored by Quasi-Distributed Fiber-Optic Sensing Technology. Water Resour. Res. 2025, 61, e2024WR038719. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Xiao, B.; Wang, F. Evaporation Characteristics of Soil Covered with Moss Crust in the Wind-water Erosion Crisscross Region of the Loess Plateau. J. Soil Water Conserv. 2020, 34, 208–215. [Google Scholar]
- Jia, H.; Li, W.; Wang, Z.; Ding, H.; Xu, H. Effects of biodegradable mulching film on soil water evaporation characteristics. Agric. Res. Arid Areas 2020, 38, 1–9. [Google Scholar]
- Zhang, X.; Ye, P.; Wu, Y.; Zhai, E. Experimental study on simultaneous heat-water-salt migration of bare soil subjected to evaporation. J. Hydrol. 2022, 609, 127710. [Google Scholar] [CrossRef]
- Christiansen, F.W. Polygonal Fracture and Fold Systems in the Salt Crust, Great Salt Lake Desert, Utah. Science 1963, 139, 607–609. [Google Scholar] [CrossRef]
- Veran-Tissoires, S.; Prat, M. Evaporation of a sodium chloride solution from a saturated porous medium with efflorescence formation. J. Fluid Mech. 2014, 749, 701–749. [Google Scholar] [CrossRef]
- Roy, R.; Weibel, J.A.; Garimella, S.V. Modeling the formation of efflorescence and subflorescence caused by salt solution evaporation from porous media. Int. J. Heat Mass Transf. 2022, 189, 122645. [Google Scholar] [CrossRef]
- Rengasamy, P. World salinization with emphasis on Australia. J. Exp. Bot. 2006, 57, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wallach, R.; Mao, X. Understanding evaporation from salinized soils in Xinjiang: Impact of sodium adsorption ratio, salt type, and concentrations. Soil Sci. Soc. Am. J. 2025, 89, e20796. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Li, J.; Cui, M.; Ren, X.; Jin, H. Impact of salt precipitation on evaporation resistance under different soil textures. Environ. Earth Sci. 2025, 84, 12. [Google Scholar] [CrossRef]
- Nachshon, U.; Weisbrod, N.; Dragila, M.I.; Grader, A. Combined evaporation and salt precipitation in homogeneous and heterogeneous porous media. Water Resour. Res. 2011, 47. [Google Scholar] [CrossRef]
- Nachshon, U.; Weisbrod, N. Beyond the Salt Crust: On Combined Evaporation and Subflorescent Salt Precipitation in Porous Media. Transp. Porous Media 2015, 110, 295–310. [Google Scholar] [CrossRef]
- Guo, M.; Li, X.; Wang, H.; Li, J. Effect of salt crust thickness on distribution characteristics of soil water and salt. Arid Land Geogr. 2023, 46, 1303–1313. [Google Scholar]
- Fu, X.; Li, C.; Li, S. Effects of NaCl Content on Salt Crust Properties and Wind-erosion Resistance of Aeolian Sand Soil. Soils 2021, 53, 1064–1071. [Google Scholar]
- Eloukabi, H.; Sghaier, N.; Ben Nasrallah, S.; Prat, M. Experimental study of the effect of sodium chloride on drying of porous media: The crusty-patchy efflorescence transition. Int. J. Heat Mass Transf. 2013, 56, 80–93. [Google Scholar] [CrossRef]
- Gale, W.J.; McColl, R.; Fang, X. Sandy fields traditional farming for water conservation in China. J. Soil Water Conserv. 1993, 48, 474–477. [Google Scholar] [CrossRef]
- Kang, W.R.; Zhang, Y.Y.; Zhao, W.Z.; Wu, S.X. The Effect of Gravel Mulch on soil Evaporation and Resistance: Experimental Findings and Modeling. J. Soil Sci. Plant Nutr. 2025, 25, 1135–1148. [Google Scholar] [CrossRef]
- Zhao, Z.; Luo, Z.; Sun, H.; Li, H.; Liu, Q.; Liu, H. Capillary Rise in Layered Soils. Appl. Sci. 2023, 13, 3374. [Google Scholar] [CrossRef]
- Cai, Y.; Li, Y.; Feng, H. Effects of Gravel-Sand Mulching Degree and Size on Soil Moisture Evaporation. J. Soil Water Conserv. 2014, 28, 273–277, 297. [Google Scholar]
- Liu, C.; Peng, Y.; Zhao, X. Flower-inspired bionic sodium alginate hydrogel evaporator enhancing solar desalination performance. Carbohydr. Polym. 2021, 273, 118536. [Google Scholar] [CrossRef] [PubMed]
- Oroud, I.M. Temperature and evaporation dynamics of saline solutions. J. Hydrol. 1999, 226, 1–10. [Google Scholar] [CrossRef]
- Al-Shammiri, M. Evaporation rate as a function of water salinity. Desalination 2002, 150, 189–203. [Google Scholar] [CrossRef]
- Nassar, I.N.; Horton, R. Salinity and compaction effects on soil water evaporation and water and solute distributions. Soil Sci. Soc. Am. J. 1999, 63, 752–758. [Google Scholar] [CrossRef]
- Yu, C.; Sun, C.; Zhang, Q.; Sun, Y.; Zhu, Z.; Li, B.; Lyu, P.; Yu, S. Response characteristics of saline soil evaporation in Yellow River Delta under different salinity levels. J. Drain. Irrig. Mach. Eng. 2023, 41, 89–95. [Google Scholar]
- Wang, S.; Zhai, S.; Fu, Y.; Fei, L. Study on the Variation Characteristics of Rising CapillaryWater of Layered Soil under Variation of Sand Layer and Thickness. J. Irrig. Drain. 2019, 38, 49–57. [Google Scholar]
- Wen, Y. Influence of Texture and Bulk Density on the Transport’s Law of Cl~- in Soils. Res. Soil Water Conserv. 2002, 9, 73–75. [Google Scholar]
- Li, X.; Guo, M.; Wang, H. Impact of soil texture and salt type on salt precipitation and evaporation under different hydraulic conditions. Hydrol. Process. 2022, 36, e14763. [Google Scholar] [CrossRef]
- Fujimaki, H.; Shimano, T.; Inoue, M.; Nakane, K. Effect of a salt crust on evaporation from a bare saline soil. Vadose Zone J. 2006, 5, 1246–1256. [Google Scholar] [CrossRef]
- Or, D.; Lehmann, P.; Shahraeeni, E.; Shokri, N. Advances in Soil Evaporation Physics-A Review. Vadose Zone J. 2013, 12, 1–16. [Google Scholar] [CrossRef]
- Nachshon, U.; Weisbrod, N.; Katzir, R.; Nasser, A. NaCl Crust Architecture and Its Impact on Evaporation: Three-Dimensional Insights. Geophys. Res. Lett. 2018, 45, 6100–6108. [Google Scholar] [CrossRef]
- Shokri-Kuehni, S.M.S.; Vetter, T.; Webb, C.; Shokri, N. New insights into saline water evaporation from porous media: Complex interaction between evaporation rates, precipitation, and surface temperature. Geophys. Res. Lett. 2017, 44, 5504–5510. [Google Scholar] [CrossRef]
- Veran-Tissoires, S.; Marcoux, M.; Prat, M. Discrete Salt Crystallization at the Surface of a Porous Medium. Phys. Rev. Lett. 2012, 108, 054502. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, A.M.; Zhao, B. Brine Drying and Salt Precipitation in Sandy Soil and Its Impact on Thermal Conductivity. Water Resour. Res. 2025, 61, e2024WR038956. [Google Scholar] [CrossRef]
- Li, X.; Wang, H. Effect of salt crust on the soil temperature of wet sandy soils. Agric. For. Meteorol. 2025, 362, 110346. [Google Scholar] [CrossRef]
- Gou, L.Y.; Zhang, C.; Lu, N.; Hu, S.J. A Soil Hydraulic Conductivity Equation Incorporating Adsorption and Capillarity. J. Geotech. Geoenviron. Eng. 2023, 149, 04023056. [Google Scholar] [CrossRef]
- Yu, X.; Qi, D.; Lu, S. Pore structure alteration in a reclaimed saline-sodic soil identified by multiscale X-ray tomography. Soil Sci. Soc. Am. J. 2022, 86, 1015–1027. [Google Scholar] [CrossRef]
- Hird, R.; Bolton, M.D. Migration of sodium chloride in dry porous materials. Proc. R. Soc. A—Math. Phys. Eng. Sci. 2016, 472, 20150710. [Google Scholar] [CrossRef]
- Jin, K.; Lu, Y.; Zhou, H.; Zhang, Q.; Hu, Y.; Wan, D.; Yan, J. Research Progress on the Hydrology in the Gurbantunggut Desert. J. China Hydrol. 2022, 42, 1–10. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, Z.; Wang, S.; Li, C. Beyond the Surface: Understanding Salt Crusts’ Impact on Water Loss in Arid Regions. Land 2025, 14, 2028. https://doi.org/10.3390/land14102028
Wang Y, Li Z, Wang S, Li C. Beyond the Surface: Understanding Salt Crusts’ Impact on Water Loss in Arid Regions. Land. 2025; 14(10):2028. https://doi.org/10.3390/land14102028
Chicago/Turabian StyleWang, Younian, Zhiwei Li, Shuaiyu Wang, and Chengzhi Li. 2025. "Beyond the Surface: Understanding Salt Crusts’ Impact on Water Loss in Arid Regions" Land 14, no. 10: 2028. https://doi.org/10.3390/land14102028
APA StyleWang, Y., Li, Z., Wang, S., & Li, C. (2025). Beyond the Surface: Understanding Salt Crusts’ Impact on Water Loss in Arid Regions. Land, 14(10), 2028. https://doi.org/10.3390/land14102028





