Drought Stress Affects the Reproductive Biology of Avena sterilis ssp. ludoviciana
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biotypes Used
2.2. Experimental Design and Treatment Setup of Pot Trial
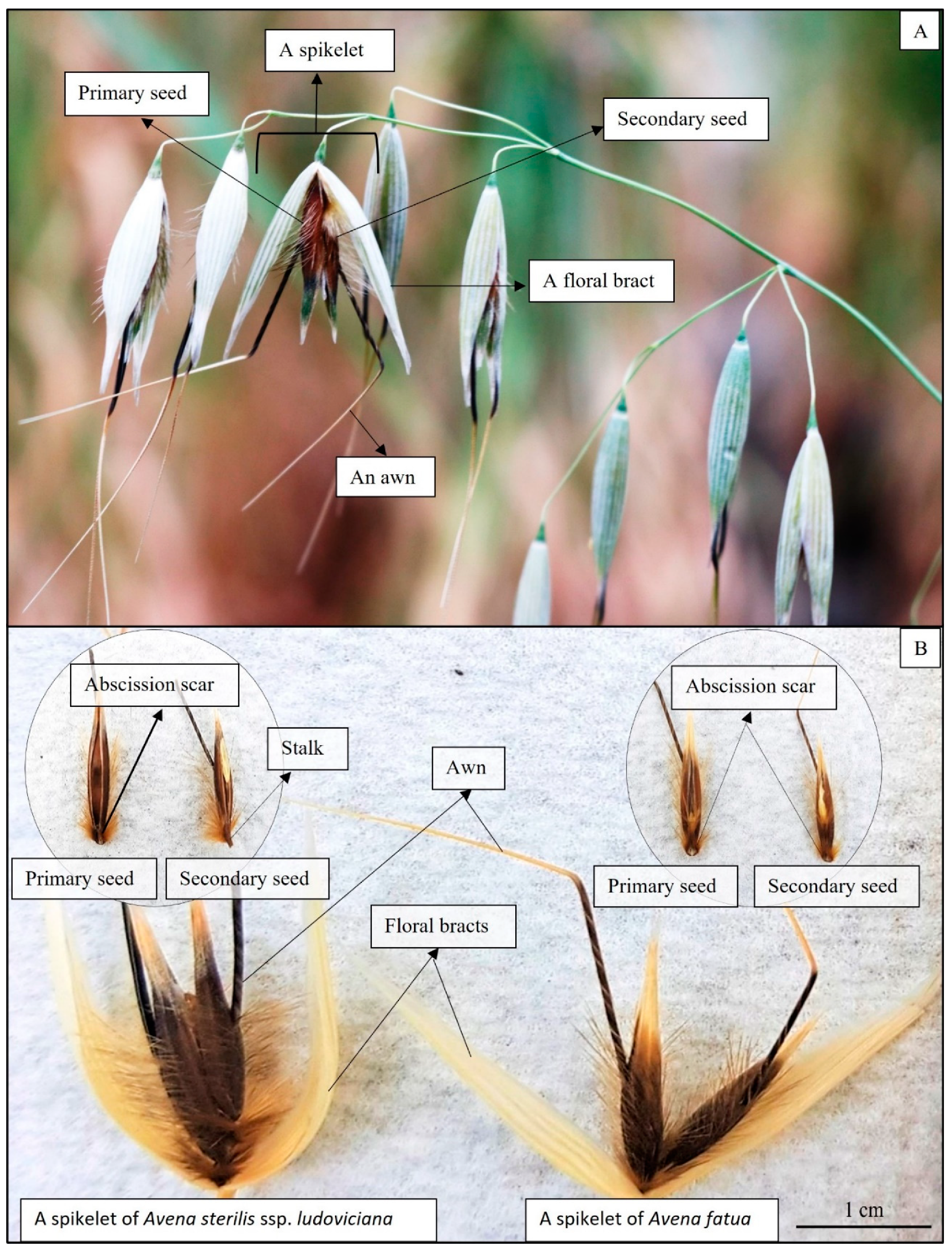
2.3. Data Collection and Spikelet Storage
2.4. Dormancy Tests
2.5. Seed Longevity Determined by Controlled Ageing Test
2.6. Thermal Time Calculation
2.7. Statistical Analysis
3. Results
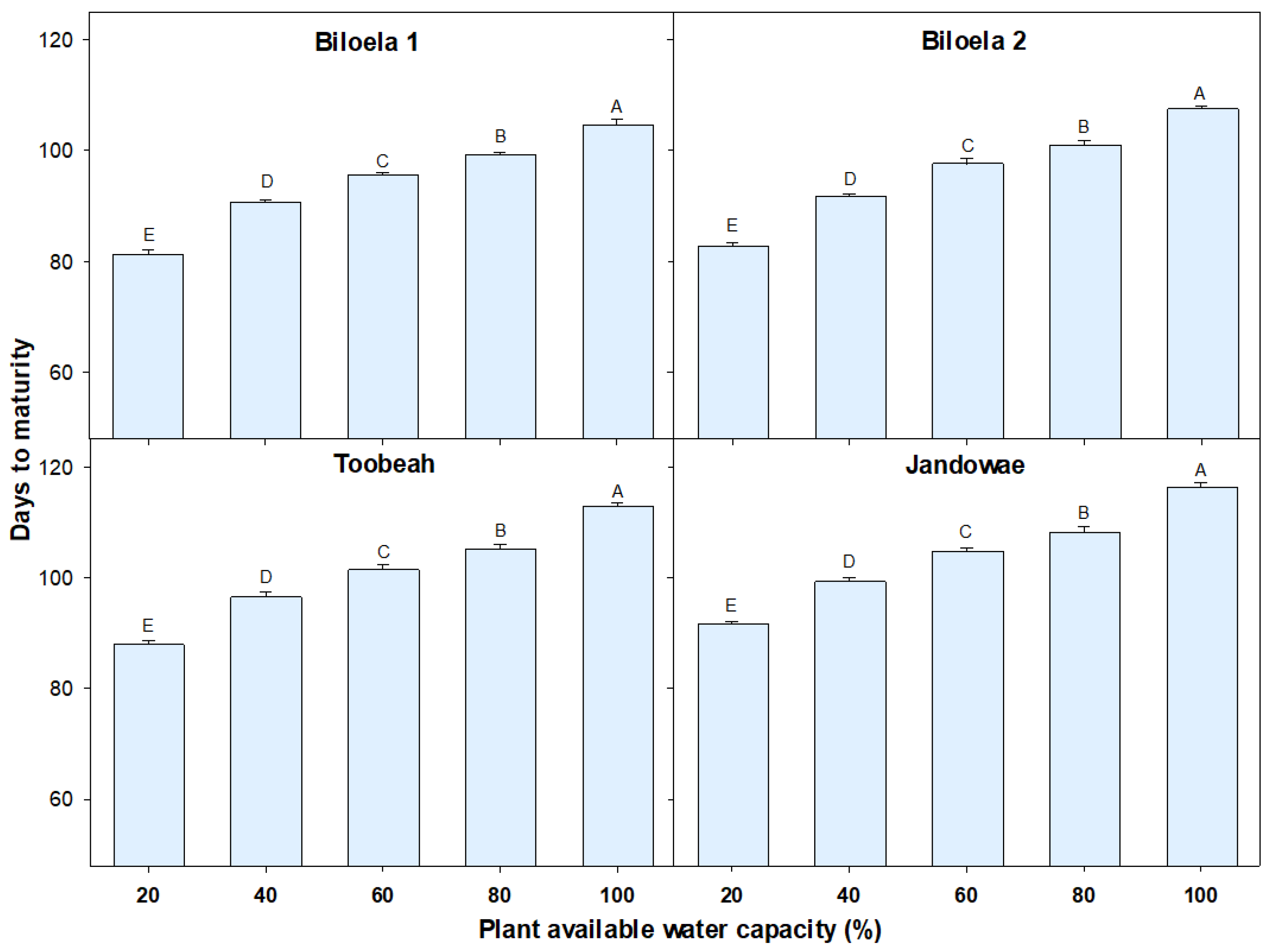
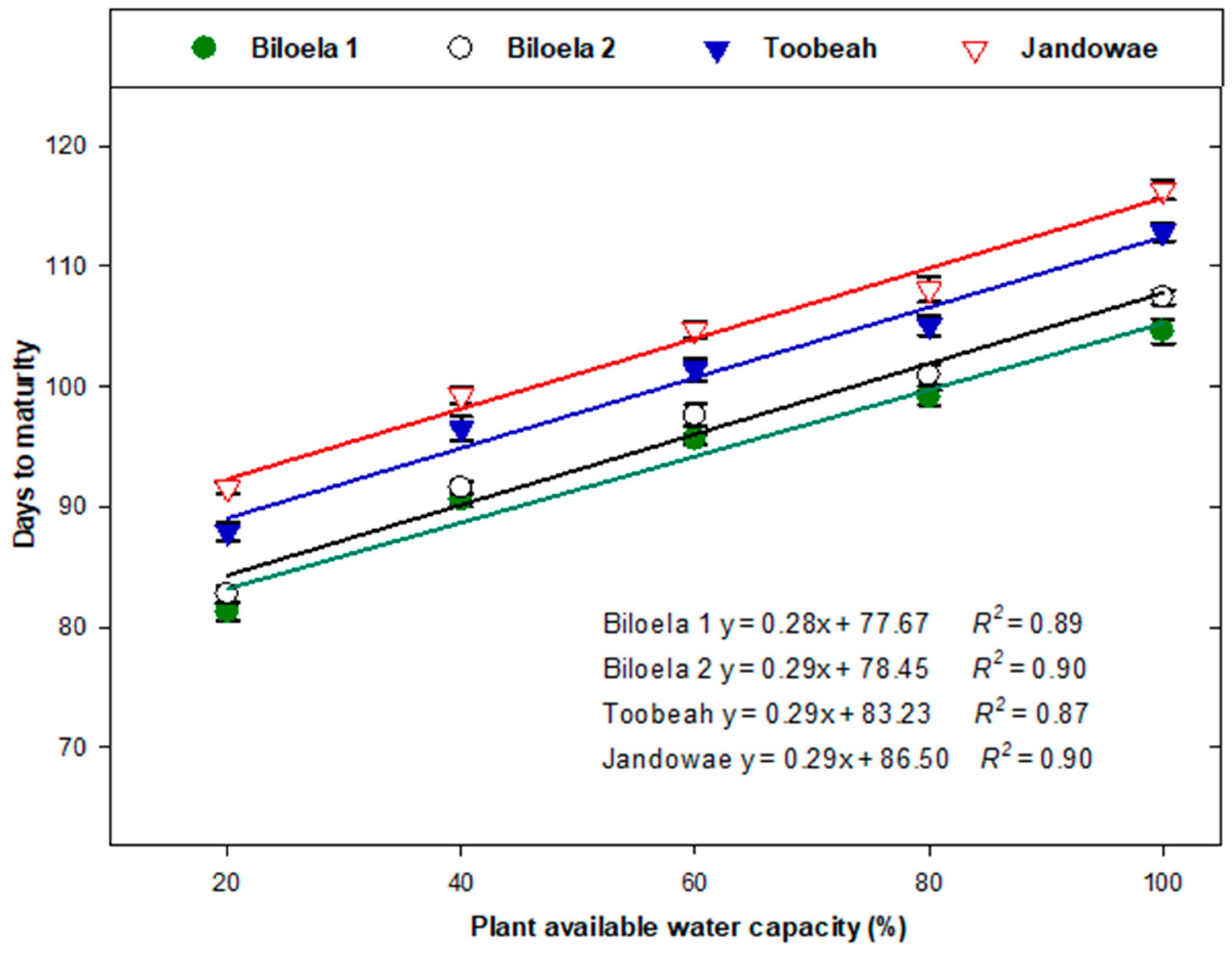
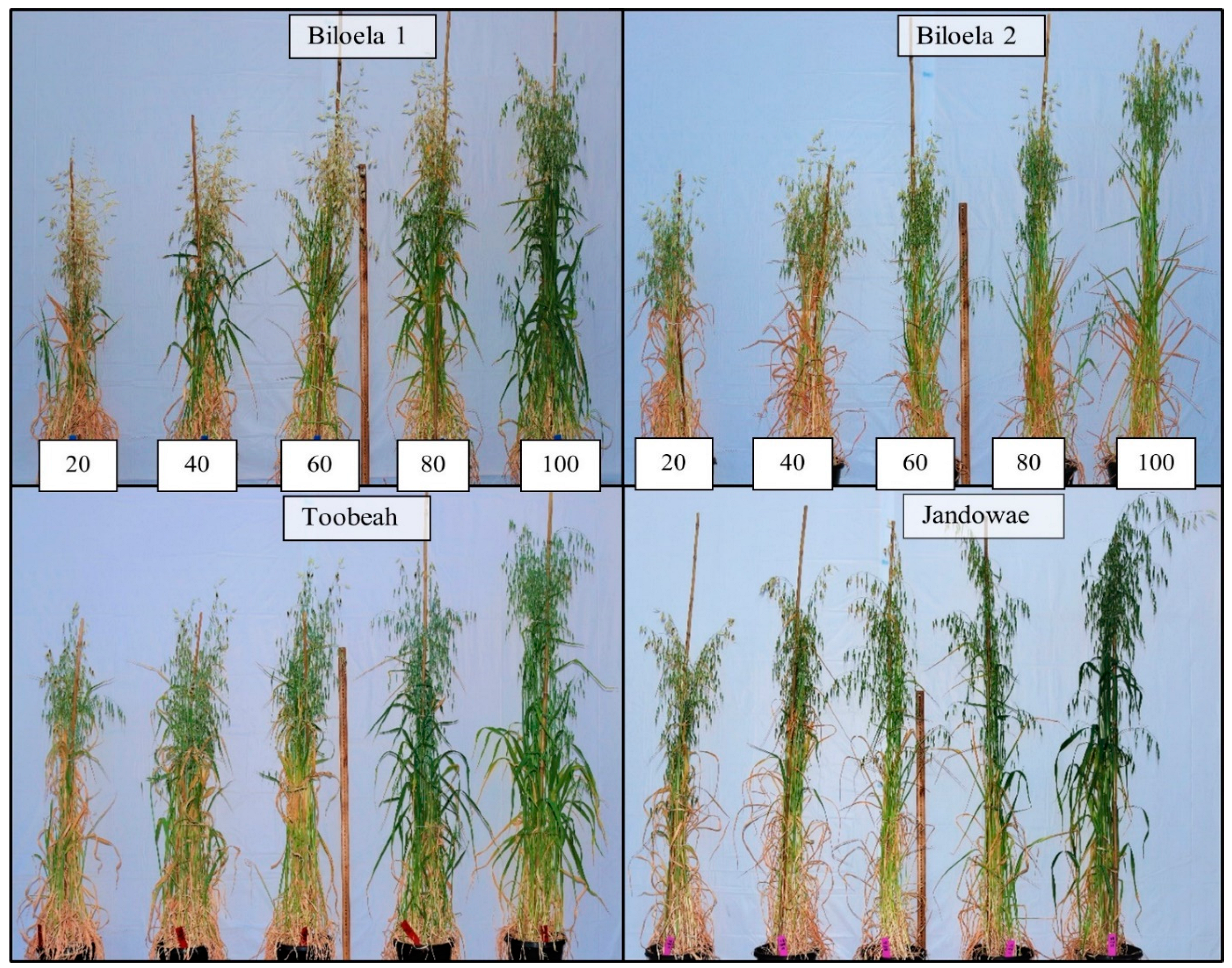
3.1. Time to Plant Maturity
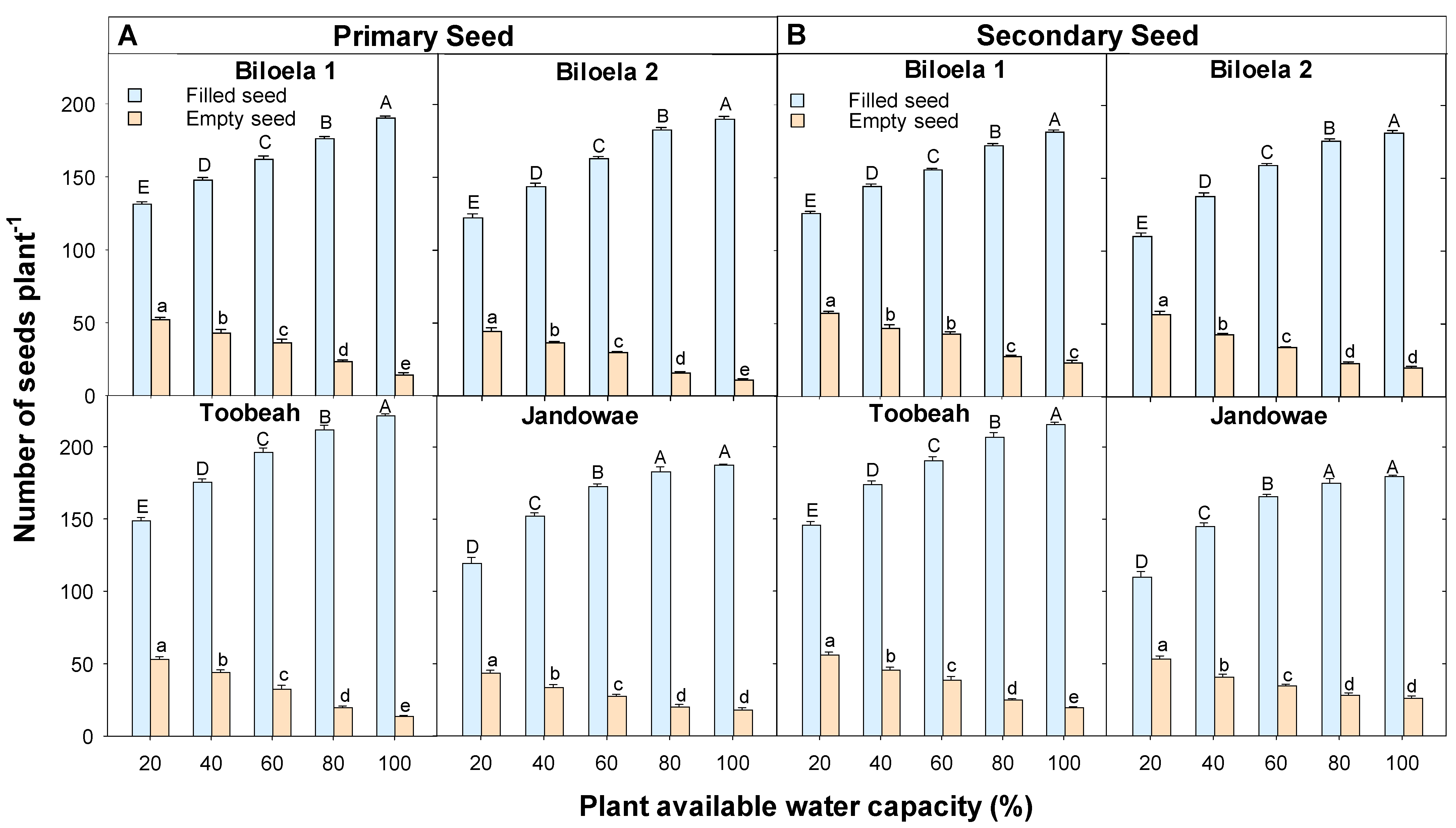
3.2. Seeds Produced
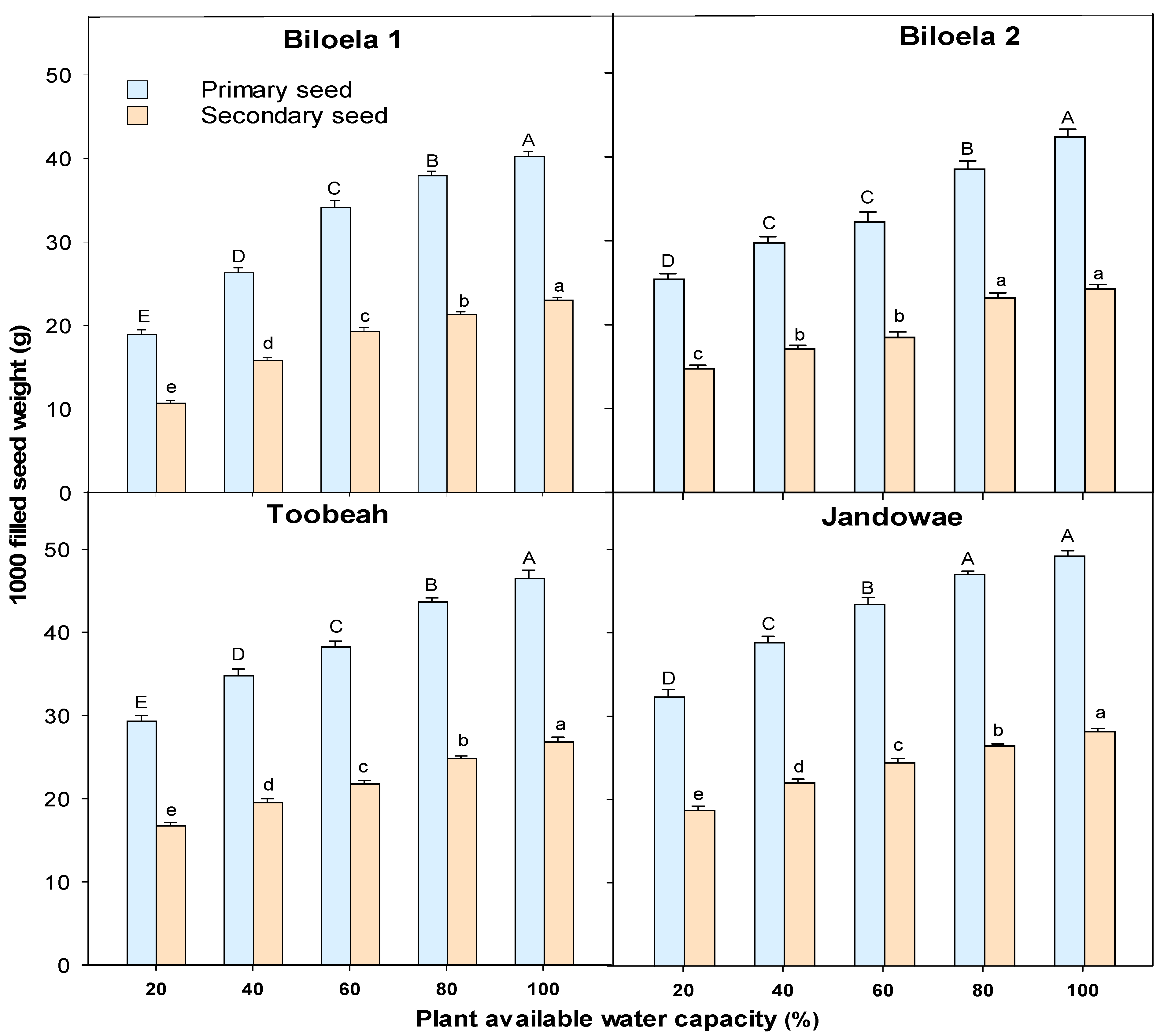
3.3. The 1000 Primary and Secondary Seed Weight
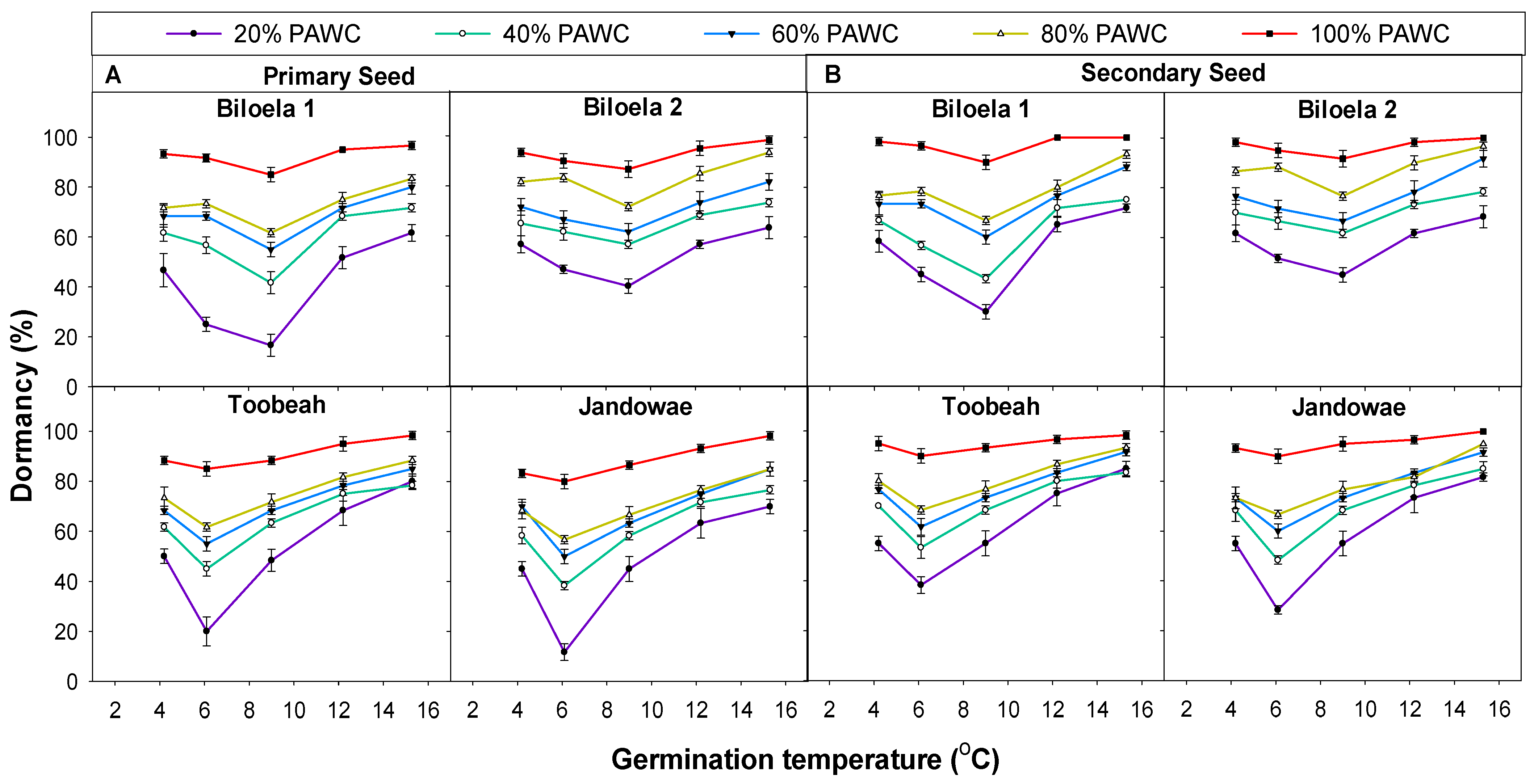
3.4. Dormancy Test of Seeds in the T-Bar
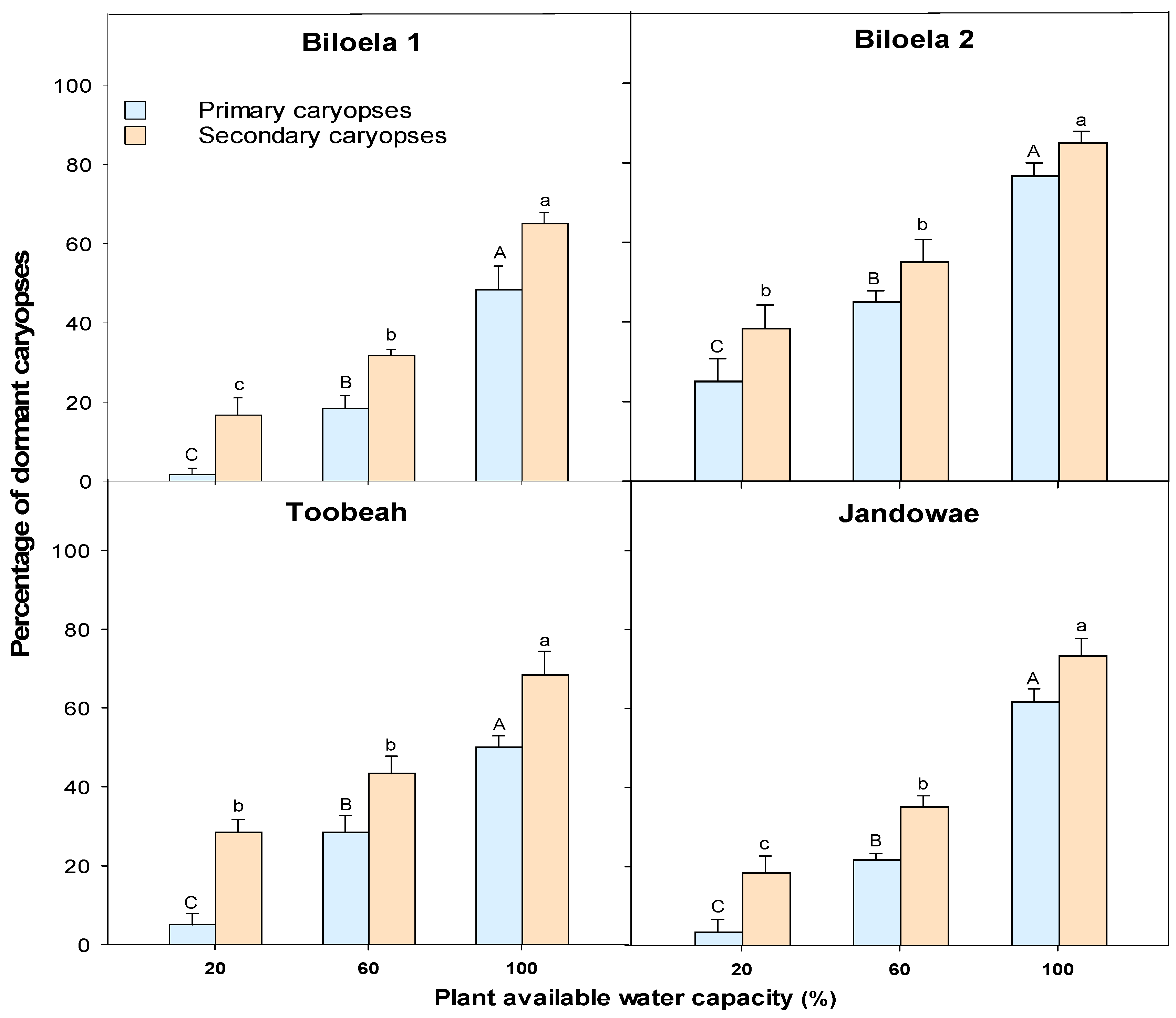
3.5. Dormancy Test of Caryopses in the Germination Incubator
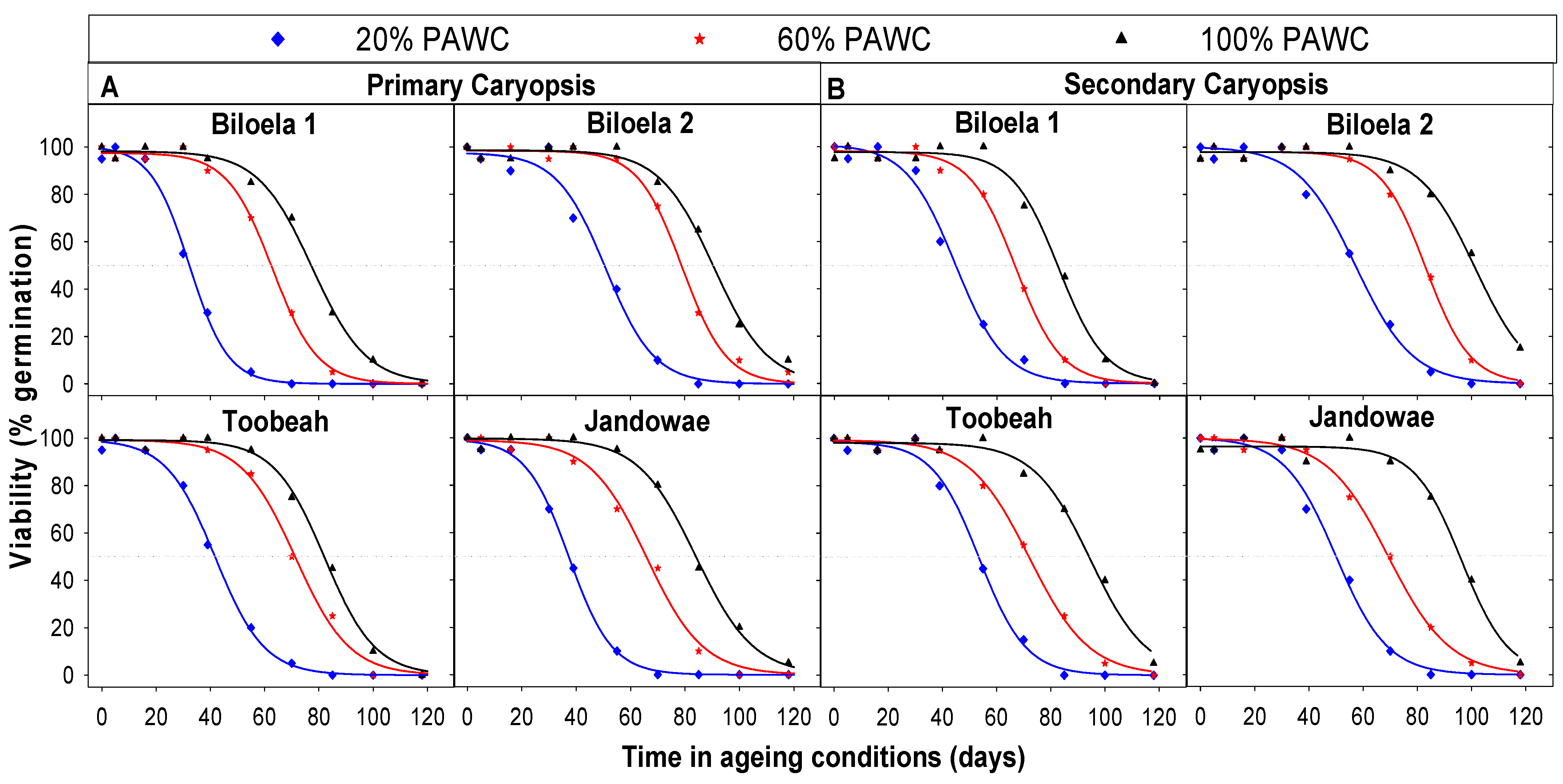
3.6. Seed Longevity Determined by CAT
4. Discussion
4.1. Phenology
4.2. Reproductive Biology
4.3. Dormancy Status
4.4. Longevity Status
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, W.K.; Stephens, D.; Siddique, K.H.M. Dryland Agriculture in Australia: Experiences and Innovations. In Innovations in Dryland Agriculture; Farooq, M., Siddique, K.H.M., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 299–320. [Google Scholar]
- GRDC (Grains Research and Development Corporation). Wheat Grow Notes. 2016. Available online: https://grdc.com.au/__data/assets/pdf_file/0033/364875/grdc-grownotes-wheat-southern.pdf (accessed on 8 April 2019).
- Llewellyn, R.; Ouzman, J. Conservation agriculture in Australia: 30 years. In Australian Agriculture in 2020: From Conservation to Automation; Pratley, J., Kirkegaard, J., Eds.; Agronomy Australia and Charles Sturt University: Wagga, Australia, 2019; pp. 21–32. [Google Scholar]
- Bellotti, B.; Rochecouste, J.F. The development of Conservation Agriculture in Australia-Farmers as innovators. Inter. Soil Water Conserv. Res. 2014, 2, 21–34. [Google Scholar]
- Dang, Y.P.; Moody, P.W.; Bell, M.J.; Seymour, N.P.; Dalal, R.C.; Freebairn, D.M.; Walker, S.R. Strategic tillage in no-till farming systems in Australia’s north-eastern grains-growing regions: II Implications for agronomy, soil and environment. Soil Tillage Res. 2015, 152, 115–123. [Google Scholar]
- Dang, Y.P.; Balzer, A.; Crawford, M.; Rincon-Florez, V.; Liu, H.; Melland, A.R.; Antille, D.; Kodur, S.; Bell, M.J.; Whish, J.P.M.; et al. Strategic tillage in conservation agricultural systems of north-eastern Australia: Why, where, when and how? Environ. Sci. Pollut. Res. 2018, 25, 1000–1015. [Google Scholar]
- Mansfield, T.J.; Atkinson, C.J. Stomatal behaviour in water stressed plants. In Stress Responses in Plants: Adaptation and Acclimation Mechanisms; Alscher, R.G., Cumming, J.R., Eds.; Wiley-Liss: New York, NY, USA, 1990; pp. 241–264. [Google Scholar]
- Cornic, G.; Massacci, A. Leaf photosynthesis under drought stress. In Photosynthesis and the Environment; Baker, N.R., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996. [Google Scholar]
- Yokota, A.; Kawasaki, S.; Iwano, M.; Nakamura, C.; Miyake, C.; Akashi, K. Citrulline and DRIP-1 Protein (ArgE Homologue) in drought tolerance of wild watermelon. Ann. Bot. 2002, 89, 825–832. [Google Scholar] [PubMed]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar]
- Kim, J.Y.; Mahé, A.; Brangeon, J.; Prioul, J.L. A maize vacuolur invertase, IVR2, is induced by water stress. Organ/tissue specificity and diurnal modulation of expression. Plant Physiol. 2000, 124, 71–84. [Google Scholar] [CrossRef]
- Komor, E. Source physiology and assimilate transport: The interaction of sucrose metabolism, starch storage and phloem export in source leaves and the effects on sugar status in phloem. Aust. J. Plant Physiol. 2000, 27, 497–505. [Google Scholar] [CrossRef]
- De Souza, J.G.; Da Silv, J.V. Partitioning of carbohydrates in annual and perennial cotton (Gossypium hirsutum L.). J. Exp. Bot. 1987, 38, 1211–1218. [Google Scholar] [CrossRef]
- Leport, L.; Turner, N.C.; French, R.J.; Barr, M.D.; Duda, R.; Davies, S.L. Physiological responses of chickpea genotypes to terminal drought in a Mediterranean-type environment. Eur. J. Agron. 2006, 11, 279–291. [Google Scholar] [CrossRef]
- Diaz-Espejo, A.; Buckley, T.N.; Sperry, J.S.; Cuevas, M.V.; de Cires Elsayed-Farag, A.S.; Martin-Palomo, M.J.; Muriel, J.L.; Perez-Martin, A.; Rodriguez-Dominguez, C.M.; Rubio-Casal, A.E.; et al. Steps toward an improvement in process-based models of water use by fruit trees: A case study in olive. Agric. Water Manag. 2012, 114, 37–49. [Google Scholar]
- Rich, S.M.; Watt, M. Soil conditions and cereal root system architecture: Review and considerations for linking Darwin and Weaver. J. Exp. Bot. 2013, 64, 1193–1208. [Google Scholar] [CrossRef] [PubMed]
- Asch, F.; Dingkuhn, M.; Sow, A.; Audebert, A. Drought-induced changes in rooting patterns and assimilate partitioning between root and shoot in upland rice. Field Crop Res. 2005, 93, 223–236. [Google Scholar] [CrossRef]
- O’Donnell, C.; Adkins, S.W. Wild oat and climate change: The effect of CO2 concentration, temperature, and water deficit on the growth and development of wild oat in monoculture. Weed Sci. 2001, 49, 694–702. [Google Scholar] [CrossRef]
- Peters, N.C.B. Production and dormancy of wild oat (Avena fatua) seed from plants grown under soil water stress. Ann. Appl. Biol. 1982, 100, 189–196. [Google Scholar] [CrossRef]
- Sawhney, R.; Naylor, J.M. Dormancy studies in seed of Avena fatua. 13. Influence of drought stress during seed development on duration of seed dormancy. Can. J. Bot. 1982, 60, 1016–1020. [Google Scholar] [CrossRef]
- Gallagher, R.S.; Kristen, L.G.; Lidewij, H.K.; Jairus, R.; Dennis, P.; Sebastian, R.; Burnham, M.; Fuerst, E.P. Shade and drought stress-induced changes in phenolic content of wild oat (Avena fatua L.) seeds. J. Stress Physiol. Biochem. 2010, 6, 90–107. [Google Scholar]
- BOM and CSIRO (Bureau of Meteorology; Centre for Scientific and Industrial Research Organization). State of the Climate. 2020. Available online: http://www.bom.gov.au/state-of-the-climate/ (accessed on 15 July 2021).
- Quail, P.H.; Carter, O.G. Survival and seasonal germination of seeds of Avena fatua and A. ludoviciana. Aust. J. Agric. Res. 1968, 19, 721–729. [Google Scholar] [CrossRef]
- Ali, M.; Williams, A.; Widderick, M.; Adkins, S. Elevated temperature affects Avena sterilis ssp. ludoviciana reproductive biology. Agronomy 2023, 13, 474. [Google Scholar]
- Ali, M.; Suthar, P.C.; Williams, A.; Widderick, M.; Adkins, S.W. Germination behaviour of Avena sterilis ssp. ludoviciana under a range of light and temperature. Crop Pasture Sci. 2022, 73, 1395–1405. [Google Scholar] [CrossRef]
- Soil Survey Staff. Kellogg Soil Survey Laboratory Methods Manual. In Soil Survey Investigations; Report No. 42, Version 5.0; Burt, R., Ed.; Soil Survey Staff, United States Department of Agriculture, Natural Resources Conservation Service: Washington, DC, USA, 2014; pp. 140–144. [Google Scholar]
- Long, R.L.; Panetta, F.D.; Steadman, K.J.; Probert, R.; Bekker, R.M.; Brooks, S.; Adkins, S.W. Seed Persistence in the field may be predicted by Laboratory-Controlled Aging. Weed Sci. 2008, 56, 523–528. [Google Scholar] [CrossRef]
- McMaster, G.S.; Wilhelm, W.W. Growing degree-days: One equation, two interpretations. Agric. For. Meteor. 1997, 87, 291–300. [Google Scholar] [CrossRef]
- Richards, R.A.; Hunt, J.R.; Kirkegaard, J.A.; Passioura, J.B. Yield improvement and adaptation of wheat to water-limited environments in Australia—A case study. Crop Pasture Sci. 2014, 65, 676–689. [Google Scholar] [CrossRef]
- Adkins, S.W.; Loewen, M.; Symons, S.J. Variation within pure lines of wild oats (Avena fatua) in relation to degree of primary dormancy. Weed Sci. 1986, 34, 859–864. [Google Scholar] [CrossRef]
- Turner, N.C.; Wright, G.C.; Siddique, K.H.M. Adaptation of grain legumes (pulses) to water-limited environments. Adv. Agron. 2001, 71, 193–231. [Google Scholar]
- Wahid, A.; Rasul, E. Photosynthesis in leaf, stem, flower and fruit. In Handbook of Photosynthesis, 2nd ed.; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 479–497. [Google Scholar]
- Peters, N.C.B. The dormancy of wild oat seed (Avena fatua L.) from plants grown under various temperature and soil moisture conditions. Weed Res. 1982, 22, 205–212. [Google Scholar] [CrossRef]
- Aisthorpe, D.; McCosker, E. Optimising the phenology and grain yield of wheat genotypes-Emerald. In Queensland Grains Research 2018-19; Regional Research Agronomy, Queensland Department of Agriculture and Fisheries, and Grains Research and Development Corporation: Brisbane City, Australia, 2019; pp. 7–14. [Google Scholar]
- Samarah, N.H. Effects of drought stress on growth and yield of barley. Agron. Sustain. Dev. 2005, 25, 145–149. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Yadav, R.S.; Hash, C.T.; Bidinger, F.R.; Devos, K.M.; Howarth, C.J. Genomic regions associated with grain yield and aspects of post-flowering drought tolerance in pearl millet across environments and tester background. Euphytica 2004, 136, 265–277. [Google Scholar] [CrossRef]
- Wellington, P.S. Studies on the germination of cereals. 2. Factors determining the germination behavior of wheat grains during maturation. Ann. Bot. 1956, 20, 481–500. [Google Scholar] [CrossRef]
- King, R.W. Abscisic acid in developing wheat grains and its relationship to grain growth and maturation. Planta 1976, 132, 43–51. [Google Scholar] [CrossRef]
- Ko, J.H.; Yang, S.H.; Han, K.H. Upregulation of an Arabidopsis RING-H2 gene, XERICO, confers drought tolerance through increased abscisic acid biosynthesis. Plant J. 2006, 47, 343–355. [Google Scholar] [CrossRef]
- Thurston, J.M. Biology and Control of Wild Oats; Report for 1962; Rothamsted Experimental Station: Harpenden, UK, 1963; pp. 236–253. [Google Scholar]
- Chancellor, R.J. Growth and development of wild oats plants. In Wild Oats in World Agriculture; Jones, D.P., Ed.; Agricultural Research Council: London, UK, 1976; pp. 89–98. [Google Scholar]
- Peters, N.C.B. Factors affecting seedling emergence of different strains of Avena fatua L. Weed Res. 1986, 26, 29–38. [Google Scholar] [CrossRef]
- Naylor, J.M.; Jana, S. Genetic adaptation for seed dormancy in Avena fatua. Can. J. Bot. 1976, 54, 306–312. [Google Scholar] [CrossRef]
- Jana, S.; Naylor, J.M. Dormancy studies in seed of Avena fatua. 11. Heritability for seed dormancy. Can. J. Bot. 1980, 58, 91–93. [Google Scholar] [CrossRef]
- Fernandez-Quintanilla, C.; Andujar, J.L.G.; Appleby, A.P. Characterization of the germination and emergence response to temperature and soil moisture of Avena fatua and A. sterilis. Weed Res. 1990, 30, 289–295. [Google Scholar] [CrossRef]
- Uremis, I.; Uygur, F. Minimum, optimum and maximum germination temperatures of some important weed species in the Çukurova Region of Turkey. Türk. Herbol. Derg. 1999, 2, 1–12. [Google Scholar]
- Long, R.L. Predicting Weed Seed Persistence: Towards a Technique for Rapid and Reliable Assessment. Ph.D. Thesis, The University of Queensland, Brisbane, Australia, 2007. [Google Scholar]
- Bekker, R.M.; Oomes, M.; Bakker, J.P. The impact of groundwater level on soil seed bank survival. Seed Sci. Res. 1998, 8, 399–404. [Google Scholar] [CrossRef]
- Gonzalez-Zertuche, L.; Vazquez-Yanes, C.; Gamboa, A.; Sanchez-Coronado, M.E.; Aguilera, P.; Orozco-Sergovia, A. Natural priming of Wigandia urens seeds during burial: Effects on germination, growth and protein expression. Seed Sci. Res. 2001, 11, 27–34. [Google Scholar] [CrossRef]
- Taylor, I.N.; Walker, S.R.; Adkins, S.W. Burial depth and cultivation influence emergence and persistence of Phalaris paradoxa seed in an Australian sub-tropical environment. Weed Res. 2005, 45, 33–40. [Google Scholar] [CrossRef]
- Burnside, O.C.; Wilson, R.G.; Weisbert, S.; Hubbard, K.G. Seed longevity of 41 weed species buried 17 years in eastern and western Nebraska. Weed Sci. 1996, 44, 74–86. [Google Scholar] [CrossRef]
- Mahajan, G.; Chauhan, B.S. Seed longevity and seedling emergence behaviour of wild oat (Avena fatua) and sterile oat (Avena sterilis ssp. ludoviciana) in response to burial depth in eastern Australia. Weed Sci. 2021, 69, 362–371. [Google Scholar]

| Location | Year | Monthly Average Rainfall (mm) | 3-Month Average Rainfall (mm) | |||
|---|---|---|---|---|---|---|
| August | September | October | ||||
| Northern NGR | Biloela | 2015 | 43.0 | 26.2 | 79.6 | 49.6 |
| 2016 | 9.8 | 28.2 | 16.6 | 18.2 | ||
| 2017 | 14.0 | 0.0 | 87.8 | 33.9 | ||
| Northern NGR 3-year average | 22.3 | 18.1 | 61.3 | 33.9 | ||
| Southern NGR | Toobeah | 2015 | 30.0 | 3.0 | 12.0 | 15.0 |
| 2016 | 73.0 | 114.0 | 33.0 | 73.3 | ||
| 2017 | 9.0 | 0.0 | 118.0 | 42.3 | ||
| 3-year average | 37.3 | 39.0 | 54.3 | 43.6 | ||
| Jandowae | 2015 | 39.6 | 23.6 | 65.8 | 43.0 | |
| 2016 | 48.0 | 113.6 | 23.2 | 61.6 | ||
| 2017 | 4.2 | 1.2 | 137.4 | 47.6 | ||
| 3-year average | 30.6 | 46.1 | 75.5 | 50.7 | ||
| Southern NGR 3-year average | 33.8 | 42.6 | 64.9 | 47.2 | ||
| Soil Water Stress Treatments (% PAWC) | Soil Water Content (g H2O kg−1 Soil) | Classification of Drought Stress Created |
|---|---|---|
| 100 (Control) | 166 | No stress |
| 80 | 133 | Very mild |
| 60 | 100 | Mild |
| 40 | 66 | Moderate |
| 20 | 33 | Severe |
| Treatment | Biotype | Caryopsis Type | a | b | P50 |
|---|---|---|---|---|---|
| 20% PAWC | Biloela 1 | Primary | 100 | 7 | 32 |
| Secondary | 101 | 9 | 45 | ||
| Biloela 2 | Primary | 98 | 9 | 51 | |
| Secondary | 100 | 11 | 57 | ||
| Toobeah | Primary | 99 | 9 | 42 | |
| Secondary | 99 | 9 | 54 | ||
| Jandowae | Primary | 99 | 8 | 37 | |
| Secondary | 100 | 10 | 50 | ||
| 60% PAWC | Biloela 1 | Primary | 98 | 8 | 63 |
| Secondary | 98 | 8 | 67 | ||
| Biloela 2 | Primary | 98 | 8 | 79 | |
| Secondary | 98 | 8 | 83 | ||
| Toobeah | Primary | 99 | 10 | 71 | |
| Secondary | 99 | 11 | 72 | ||
| Jandowae | Primary | 99 | 10 | 66 | |
| Secondary | 100 | 11 | 69 | ||
| 100% PAWC | Biloela 1 | Primary | 98 | 10 | 78 |
| Secondary | 98 | 9 | 83 | ||
| Biloela 2 | Primary | 99 | 10 | 91 | |
| Secondary | 98 | 11 | 102 | ||
| Toobeah | Primary | 99 | 9 | 82 | |
| Secondary | 98 | 11 | 95 | ||
| Jandowae | Primary | 100 | 11 | 84 | |
| Secondary | 97 | 9 | 97 |
| Biotype | 20% PAWC | 60% PAWC | 100% PAWC | |||
|---|---|---|---|---|---|---|
| P50 Value (Days) | Predicted Longevity in the Seedbank (Years) | P50 Value (Days) | Predicted Longevity in the Seedbank (Years) | P50 Value (Days) | Predicted Longevity in the Seedbank (Years) | |
| Primary caryopses/seed | ||||||
| Biloela 1 | 32 | <1 | 63 | >2 to 4 | 78 | >2 to 4 |
| Biloela 2 | 51 | 1 to 2 | 79 | >2 to 4 | 91 | >4 |
| Toobeah | 42 | 1 to 2 | 71 | >2 to 4 | 82 | >4 |
| Jandowae | 37 | <1 | 66 | >2 to 4 | 84 | >4 |
| Secondary caryopses/seed | ||||||
| Biloela 1 | 45 | 1 to 2 | 67 | >2 to 4 | 83 | >4 |
| Biloela 2 | 57 | 1 to 2 | 83 | >4 | 102 | >4 |
| Toobeah | 54 | 1 to 2 | 72 | >2 to 4 | 95 | >4 |
| Jandowae | 50 | 1 to 2 | 69 | >2 to 4 | 97 | >4 |
| Treatment (% PAWC) | Days to Panicle Initiation | Thermal Time (Degree-Days) | Days to Maturity | Thermal Time until Maturity (Degree-Days) | ||||
|---|---|---|---|---|---|---|---|---|
| 2018–2019 | 2019–2020 | 2-Year Average | 2018–2019 | 2019–2020 | 2-Year Average | |||
| Biloela 1 | ||||||||
| 20% | 58 | 852 | 884 | 868 | 84 | 1335 | 1352 | 1344 |
| 40% | 91 | 1455 | 1481 | 1468 | ||||
| 60% | 96 | 1585 | 1603 | 1594 | ||||
| 80% | 99 | 1651 | 1675 | 1663 | ||||
| 100% | 103 | 1748 | 1761 | 1755 | ||||
| Biloela 2 | ||||||||
| 20% | 58 | 852 | 884 | 868 | 86 | 1376 | 1393 | 1385 |
| 40% | 92 | 1494 | 1525 | 1510 | ||||
| 60% | 98 | 1630 | 1648 | 1639 | ||||
| 80% | 101 | 1699 | 1725 | 1712 | ||||
| 100% | 104 | 1772 | 1778 | 1775 | ||||
| Toobeah | ||||||||
| 20% | 63 | 946 | 965 | 956 | 91 | 1474 | 1503 | 1489 |
| 40% | 97 | 1607 | 1626 | 1617 | ||||
| 60% | 101 | 1699 | 1725 | 1712 | ||||
| 80% | 105 | 1795 | 1795 | 1795 | ||||
| 100% | 108 | 1858 | 1864 | 1861 | ||||
| Jandowae | ||||||||
| 20% | 65 | 978 | 1002 | 990 | 95 | 1561 | 1583 | 1572 |
| 40% | 99 | 1651 | 1675 | 1663 | ||||
| 60% | 105 | 1795 | 1795 | 1795 | ||||
| 80% | 108 | 1858 | 1864 | 1861 | ||||
| 100% | 111 | 1924 | 1939 | 1932 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, M.; Williams, A.; Widderick, M.; Haque, M.A.; Adkins, S. Drought Stress Affects the Reproductive Biology of Avena sterilis ssp. ludoviciana. Land 2023, 12, 1745. https://doi.org/10.3390/land12091745
Ali M, Williams A, Widderick M, Haque MA, Adkins S. Drought Stress Affects the Reproductive Biology of Avena sterilis ssp. ludoviciana. Land. 2023; 12(9):1745. https://doi.org/10.3390/land12091745
Chicago/Turabian StyleAli, Mohammad, Alwyn Williams, Michael Widderick, Mohammad Anamul Haque, and Steve Adkins. 2023. "Drought Stress Affects the Reproductive Biology of Avena sterilis ssp. ludoviciana" Land 12, no. 9: 1745. https://doi.org/10.3390/land12091745
APA StyleAli, M., Williams, A., Widderick, M., Haque, M. A., & Adkins, S. (2023). Drought Stress Affects the Reproductive Biology of Avena sterilis ssp. ludoviciana. Land, 12(9), 1745. https://doi.org/10.3390/land12091745







