A Brave New World: Managing for Biodiversity Conservation under Ecosystem Transformation
Abstract
1. Unprecedented Ecosystem Transformation
2. The Resist–Accept–Direct (RAD) Framework
- Resist, where managers focus on maintaining current or historical ecosystem structure and function;
- Accept, where managers do not intervene and allow the ecosystem structure and function to emerge from ongoing transformations;
- Direct, where managers actively steer the transformation toward a particular ecosystem structure and function.
3. RAD and Biodiversity Conservation
3.1. Mojave Desert Case Study


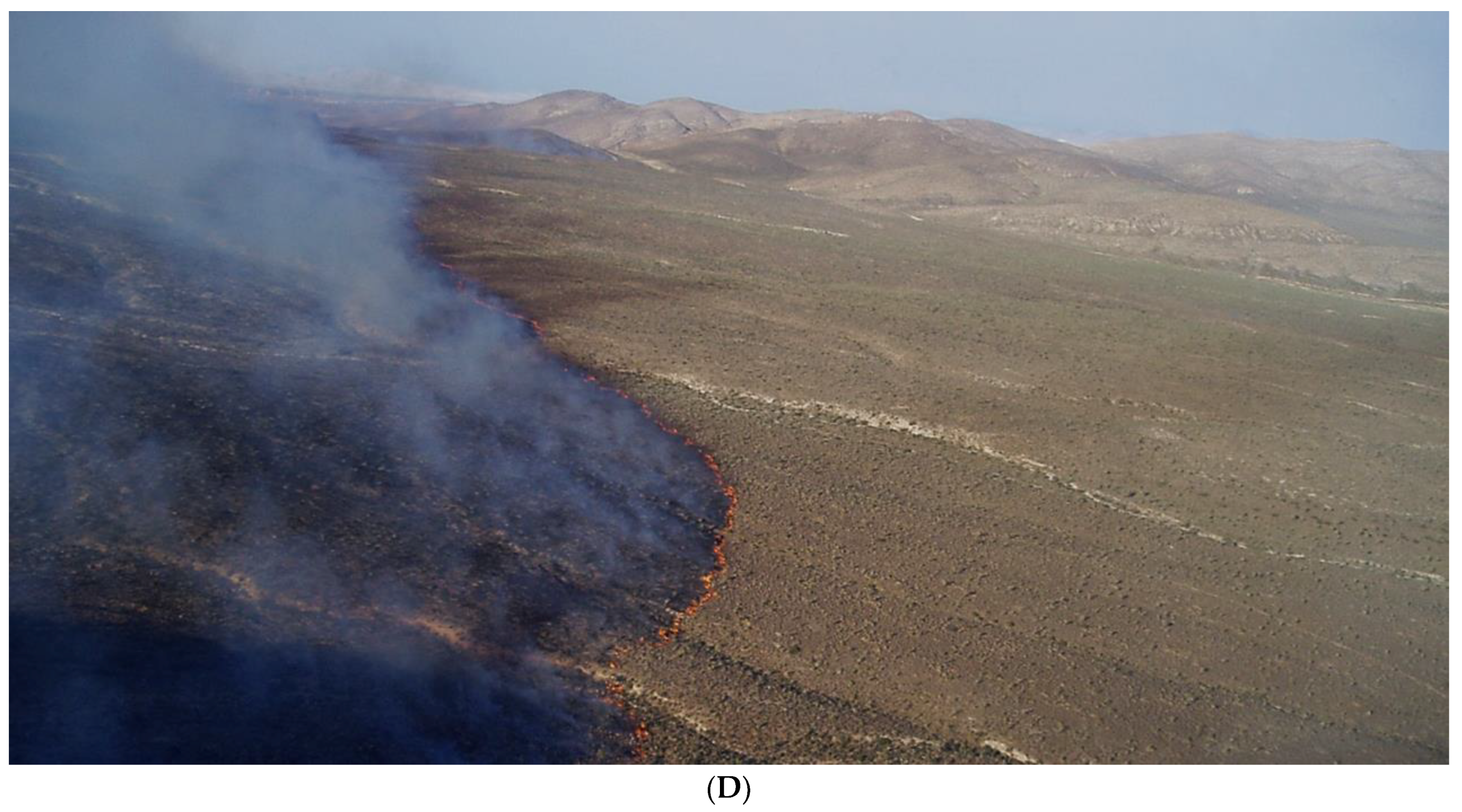
4. Natural Resource Management Guidance for Maximizing Biodiversity under RAD
5. Conclusions
- Recognizing that biological change maintains biodiversity and ecosystems during global upheaval;
- Identifying refugia and relocating at-risk species;
- Promoting evolutionary processes (e.g., speciation) to maintain biological complexity;
- Managing for ecosystem function rather than system components;
- Facilitating adaptation by removing barriers to species movement.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nolan, C.; Overpeck, J.T.; Allen, J.R.M.; Anderson, P.M.; Betancourt, J.L.; Binney, H.A.; Brewer, S.; Bush, M.B.; Chase, B.M.; Cheddadi, R.; et al. Past and future global transformation of terrestrial ecosystems under climate change. Science 2018, 361, 920–923. [Google Scholar] [CrossRef] [PubMed]
- Jaureguiberry, P.; Titeux, N.; Wiemers, M.; Bowler, D.E.; Coscieme, L.; Golden, A.S.; Guerra, C.A.; Jacob, U.; Takahashi, Y.; Settele, J.; et al. The direct drivers of recent global anthropogenic biodiversity loss. Sci. Adv. 2022, 8, eabm9982. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.M.; Lynch, A.J.; Beever, E.A.; Engman, A.C.; Falke, J.A.; Jackson, S.T.; Krabbenhoft, T.J.; Lawrence, D.J.; Limpinsel, D.; Magill, R.T.; et al. Responding to Ecosystem Transformation: Resist, Accept, or Direct? Fisheries 2021, 46, 8–21. [Google Scholar] [CrossRef]
- Jackson, S.T. Conservation and Resource Management in a Changing World: Extending Historical Range of Variation Beyond the Baseline. Hist. Environ. Var. Conserve. Nat. Res. Manag. 2012, 1, 92–109. [Google Scholar] [CrossRef]
- Ellis, E.C.; Kaplan, J.O.; Fuller, D.Q.; Vavrus, S.; Klein Goldewijk, K.; Verburg, P.H. Used planet: A global history. Proc. Natl. Acad. Sci. USA 2013, 110, 7978–7985. [Google Scholar] [CrossRef]
- Vitousek, P.M. Beyond Global Warming: Ecology and Global Change. Ecology 1994, 75, 1861–1876. [Google Scholar] [CrossRef]
- Díaz, S.; Settele, J.; Brondízio, E.S.; Ngo, H.T.; Agard, J.; Arneth, A.; Balvanera, P.; Brauman, K.A.; Butchart, S.H.M.; Chan, K.M.A.; et al. Pervasive human-driven decline of life on Earth points to the need for transformative change. Science 2019, 366, eaax3100. [Google Scholar] [CrossRef]
- Geldmann, J.; Manica, A.; Burgess, N.D.; Coad, L.; Balmford, A. A global-level assessment of the effectiveness of protected areas at resisting anthropogenic pressures. Proc. Natl. Acad. Sci. USA 2019, 116, 23209–23215. [Google Scholar] [CrossRef]
- Seastedt, T.R.; Hobbs, R.J.; Suding, K.N. Management of novel ecosystems: Are novel approaches required? Front. Ecol. Environ. 2008, 6, 547–553. [Google Scholar] [CrossRef]
- Folke, C.; Carpenter, S.; Elmqvist, T.; Gunderson, L.; Holling, C.S.; Walker, B. Resilience and sustainable development: Building adaptive capacity in a world of transformations. AMBIO 2002, 31, 437–440. [Google Scholar] [CrossRef]
- Lynch, A.J.; Thompson, L.M.; Beever, E.A.; Cole, D.N.; Engman, A.C.; Hawkins Hoffman, C.; Jackson, S.T.; Krabbenhoft, T.J.; Lawrence, D.J.; Limpinsel, D.; et al. Managing for RADical ecosystem change: Applying the Resist-Accept-Direct (RAD) framework. Front. Ecol. Environ. 2021, 19, 461–469. [Google Scholar] [CrossRef]
- Schuurman, G.W.; Cole, D.N.; Cravens, A.E.; Covington, S.; Crausbay, S.D.; Hoffman, C.H.; Lawrence, D.J.; Magness, D.R.; Morton, J.M.; Nelson, E.A.; et al. Navigating Ecological Transformation: Resist–Accept–Direct as a Path to a New Resource Management Paradigm. Bioscience 2022, 72, 16–29. [Google Scholar] [CrossRef]
- Lynch, A.J.; Rahel, F.J.; Limpinsel, D.; Sethi, S.A.; Engman, A.C.; Lawrence, D.J.; Mills, K.E.; Morrison, W.; Peterson, J.O.; Porath, M.T. Ecological and social strategies for managing fisheries using the Resist-Accept-Direct (RAD) framework. Fish. Manag. Ecol. 2022, 29, 329–345. [Google Scholar] [CrossRef]
- Lynch, A.J.; Thompson, L.M.; Morton, J.M.; Beever, E.A.; Clifford, M.; Limpinsel, D.; Magill, R.T.; Magness, D.R.; Melvin, T.A.; Newman, R.A.; et al. RAD Adaptive Management for Transforming Ecosystems. Bioscience 2022, 72, 45–56. [Google Scholar] [CrossRef]
- Mace, G.M.; Norris, K.; Fitter, A.H. Biodiversity and ecosystem services: A multilayered relationship. Trends Ecol. Evol. 2012, 27, 19–26. [Google Scholar] [CrossRef]
- Lausch, A.; Bannehr, L.; Beckmann, M.; Boehm, C.; Feilhauer, H.; Hacker, J.; Heurich, M.; Jung, A.; Klenke, R.; Neumann, C.; et al. Linking Earth Observation and taxonomic, structural and functional biodiversity: Local to ecosystem perspectives. Ecol. Indic. 2016, 70, 317–339. [Google Scholar] [CrossRef]
- Marshall, E.; Wintle, B.A.; Southwell, D.; Kujala, H. What are we measuring? A review of metrics used to describe biodiversity in offsets exchanges. Biol. Conserv. 2020, 241, 108250. [Google Scholar] [CrossRef]
- Noss, R.F. Indicators for Monitoring Biodiversity: A Hierarchical Approach. Conserv. Biol. 1990, 4, 355–364. [Google Scholar] [CrossRef]
- Convention on Biological Diversity. Available online: https://www.cbd.int/indicators/intro.shtml (accessed on 7 June 2023).
- Hill, S.L.; Harfoot, M.; Purvis, A.; Purves, D.W.; Collen, B.; Newbold, T.; Burgess, N.D.; Mace, G.M. Reconciling Biodiversity Indicators to Guide Understanding and Action. Conserv. Lett. 2016, 9, 405–412. [Google Scholar] [CrossRef]
- Hoban, S.; Bruford, M.; Jackson, J.D.; Lopes-Fernandes, M.; Heuertz, M.; Hohenlohe, P.A.; Paz-Vinas, I.; Sjögren-Gulve, P.; Segelbacher, G.; Vernesi, C.; et al. Genetic diversity targets and indicators in the CBD post-2020 Global Biodiversity Framework must be improved. Biol. Conserv. 2020, 248, 108654. [Google Scholar] [CrossRef]
- Brooks, T.M.; Mittermeier, R.A.; Da Fonseca, G.A.B.; Gerlach, J.; Hoffmann, M.; Lamoreux, J.F.; Mittermeier, C.G.; Pilgrim, J.D.; Rodrigues, A.S.L. Global Biodiversity Conservation Priorities. Science 2006, 313, 58–61. [Google Scholar] [CrossRef]
- Carrasco, L.; Papeş, M.; Sheldon, K.S.; Giam, X. Global progress in incorporating climate adaptation into land protection for biodiversity since Aichi targets. Glob. Chang. Biol. 2021, 27, 1788–1801. [Google Scholar] [CrossRef]
- Morelli, T.L.; Barrows, C.W.; Ramirez, A.R.; Cartwright, J.M.; Ackerly, D.D.; Eaves, T.D.; Ebersole, J.L.; Krawchuk, M.A.; Letcher, B.H.; Mahalovich, M.F.; et al. Climate-change refugia: Biodiversity in the slow lane. Front. Ecol. Environ. 2020, 18, 228–234. [Google Scholar] [CrossRef]
- Convention on Biological Diversity. Strategic Plan for Biodiversity 2011–2020, Including Aichi Biodiversity Targets. Available online: https://www.cbd.int/sp/targets/ (accessed on 6 June 2023).
- Convention on Biological Diversity. Kunming-Montreal Global Biodiversity Framework. Available online: https://www.cbd.int/gbf/ (accessed on 6 June 2023).
- Harrison, P.; Berry, P.; Simpson, G.; Haslett, J.; Blicharska, M.; Bucur, M.; Dunford, R.; Egoh, B.; Garcia-Llorente, M.; Geamănă, N.; et al. Linkages between biodiversity attributes and ecosystem services: A systematic review. Ecosyst. Serv. 2014, 9, 191–203. [Google Scholar] [CrossRef]
- Simberloff, D. Flagships, umbrellas, and keystones: Is single-species management passé in the landscape era? Biol. Conserv. 1998, 83, 247–257. [Google Scholar] [CrossRef]
- Wesner, J.S.; Belk, M.C. Habitat relationships among biodiversity indicators and co-occurring species in a freshwater fish community. Anim. Conserv. 2012, 15, 445–456. [Google Scholar] [CrossRef]
- Ellis, C.J. Ancient woodland indicators signal the climate change risk for dispersal-limited species. Ecol. Indic. 2015, 53, 106–114. [Google Scholar] [CrossRef]
- Hafner, D.J. Pikas and Permafrost: Post-Wisconsin Historical Zoogeography of Ochotona in the Southern Rocky Mountains, U.S.A. Arct. Alp. Res. 1994, 26, 375. [Google Scholar] [CrossRef]
- Beever, E.A.; Perrine, J.D.; Rickman, T.; Flores, M.; Clark, J.P.; Waters, C.; Weber, S.S.; Yardley, B.; Thoma, D.; Chesley-Preston, T.; et al. Pika (Ochotona princeps) losses from two isolated regions reflect temperature and water balance, but reflect habitat area in a mainland region. J. Mammal. 2016, 97, 1495–1511. [Google Scholar] [CrossRef]
- Hagedorn, M.; Page, C.A.; O’neil, K.L.; Flores, D.M.; Tichy, L.; Conn, T.; Chamberland, V.F.; Lager, C.; Zuchowicz, N.; Lohr, K.; et al. Assisted gene flow using cryopreserved sperm in critically endangered coral. Proc. Natl. Acad. Sci. USA 2021, 118, e2110559118. [Google Scholar] [CrossRef]
- Price, K.; Roburn, A.; MacKinnon, A. Ecosystem-based management in the Great Bear Rainforest. For. Ecol. Manag. 2009, 258, 495–503. [Google Scholar] [CrossRef]
- Wood, S.; Dupras, J. Increasing functional diversity of the urban canopy for climate resilience: Potential tradeoffs with ecosystem services? Urban For. Urban Green. 2021, 58, 126972. [Google Scholar] [CrossRef]
- Sáenz-Romero, C.; O’Neill, G.; Aitken, S.N.; Lindig-Cisneros, R. Assisted Migration Field Tests in Canada and Mexico: Lessons, Limitations, and Challenges. Forests 2020, 12, 9. [Google Scholar] [CrossRef]
- Aplet, G.H.; Mckinley, P.S. A portfolio approach to managing ecological risks of global change. Ecosyst. Health Sustain. 2017, 3, e01261. [Google Scholar] [CrossRef]
- Galatowitsch, S.; Frelich, L.; Phillips-Mao, L. Regional climate change adaptation strategies for biodiversity conservation in a midcontinental region of North America. Biol. Conserv. 2009, 142, 2012–2022. [Google Scholar] [CrossRef]
- Mittermeier, C.G.; Konstant, W.R.; Lovich, R.E.; Lovich, J.E. The Mojave Desert. Wilderness: Earth’s Last Wild Places. USGC 2002, 1, 351–356. [Google Scholar]
- Randall, J.M.; Parker, S.S.; Moore, J.; Cohen, B.; Crane, L.; Christian, B.; Cameron, D.; MacKenzie, J.; Klausmeyer, K.; Mor-rison, S. Mojave Desert Ecoregional Assessment. Unpublished Report. The Nature Conservancy, San Francisco, California. 106 pages + Appendices. 2010. Available online: http://conserveonline.org/workspaces/mojave/documents/mojave-desertecoregional-2010/@@view.html (accessed on 7 June 2023).
- Seager, R.; Ting, M.; Held, I.; Kushnir, Y.; Lu, J.; Vecchi, G.; Huang, H.-P.; Harnik, N.; Leetmaa, A.; Lau, N.-C.; et al. Model projections of an imminent transition to a more arid climate in southwestern North America. Science 2007, 316, 1181–1184. [Google Scholar] [CrossRef]
- Diffenbaugh, N.S.; Giorgi, F.; Pal, J.S. Climate change hotspots in the United States. Geophys. Res. Lett. 2008, 35, 1–5. [Google Scholar] [CrossRef]
- Cook, B.I.; Ault, T.R.; Smerdon, J.E. Unprecedented 21st century drought risk in the American Southwest and Central Plains. Sci. Adv. 2015, 1, e1400082. [Google Scholar] [CrossRef]
- Iknayan, K.J.; Beissinger, S.R. Collapse of a desert bird community over the past century driven by climate change. Proc. Natl. Acad. Sci. USA 2018, 115, 8597–8602. [Google Scholar] [CrossRef]
- Rich, L.N.; Furnas, B.J.; Newton, D.S.; Brashares, J.S. Acoustic and camera surveys inform models of current and future vertebrate distributions in a changing desert ecosystem. Divers. Distrib. 2019, 25, 1441–1456. [Google Scholar] [CrossRef]
- Riddell, E.A.; Iknayan, K.J.; Hargrove, L.; Tremor, S.; Patton, J.L.; Ramirez, R.; Wolf, B.O.; Beissinger, S.R. Exposure to climate change drives stability or collapse of desert mammal and bird communities. Science 2021, 371, 633–636. [Google Scholar] [CrossRef]
- Hernandez, R.R.; Hoffacker, M.K.; Murphy-Mariscal, M.L.; Wu, G.C.; Allen, M.F. Solar energy development impacts on land cover change and protected areas. Proc. Natl. Acad. Sci. USA 2015, 112, 13579–13584. [Google Scholar] [CrossRef]
- Grodsky, S.M.; Hernandez, R.R. Reduced ecosystem services of desert plants from ground-mounted solar energy development. Nat. Sustain. 2020, 3, 1036–1043. [Google Scholar] [CrossRef]
- Brooks, M.L. Alien annual grasses and fire in the Mojave Desert. Madroño 1999, 46, 13–19. [Google Scholar]
- Brooks, M.; Matchett, J. Spatial and temporal patterns of wildfires in the Mojave Desert, 1980–2004. J. Arid. Environ. 2006, 67, 148–164. [Google Scholar] [CrossRef]
- Hereford, R.; Webb, R.; Longpré, C. Precipitation history and ecosystem response to multidecadal precipitation variability in the Mojave Desert region, 1893–2001. J. Arid. Environ. 2006, 67, 13–34. [Google Scholar] [CrossRef]
- Abatzoglou, J.T.; Kolden, C.A. Climate Change in Western US Deserts: Potential for Increased Wildfire and Invasive Annual Grasses. Rangel. Ecol. Manag. 2011, 64, 471–478. [Google Scholar] [CrossRef]
- Tagestad, J.; Brooks, M.; Cullinan, V.; Downs, J.; McKinley, R. Precipitation regime classification for the Mojave Desert: Implications for fire occurrence. J. Arid. Environ. 2016, 124, 388–397. [Google Scholar] [CrossRef]
- Cameron, D.R.; Cohen, B.S.; Morrison, S.A. An Approach to Enhance the Conservation-Compatibility of Solar Energy Development. PLoS ONE 2012, 7, e38437. [Google Scholar] [CrossRef]
- Kreitler, J.; Schloss, C.A.; Soong, O.; Hannah, L.; Davis, F.W. Conservation Planning for Offsetting the Impacts of Development: A Case Study of Biodiversity and Renewable Energy in the Mojave Desert. PLoS ONE 2015, 10, e0140226. [Google Scholar] [CrossRef]
- Hromada, S.J.; Esque, T.C.; Vandergast, A.G.; Dutcher, K.E.; Mitchell, C.I.; Gray, M.E.; Chang, T.; Dickson, B.G.; Nussear, K.E. Using movement to inform conservation corridor design for Mojave desert tortoise. Mov Ecol. 2020, 8, 1–18. [Google Scholar] [CrossRef]
- Harju, S.; Cambrin, S.; Jenkins, K. Mapping Low-Elevation Species Richness and Biodiversity in the Eastern Mojave Desert. Nat. Areas J. 2023, 43, 53–61. [Google Scholar] [CrossRef]
- Shryock, D.F.; DeFalco, L.A.; Esque, T.C. Spatial decision-support tools to guide restoration and seed-sourcing in the Desert Southwest. Ecosphere 2018, 9, e02453. [Google Scholar] [CrossRef]
- Magness, D.R.; Wilkening, J.L.; Smetzer, J.; Guilbeau, K.; Miles, W. Climate change adaptation in action: The U.S. Fish and Wildlife Service can take action to resist, accept, and direct change. Wildl. Prof. 2022, 16, 34–38. [Google Scholar]
- Alexander, J.M.; Chalmandrier, L.; Lenoir, J.; Burgess, T.I.; Essl, F.; Haider, S.; Kueffer, C.; McDougall, K.; Milbau, A.; Nuñez, M.A.; et al. Lags in the response of mountain plant communities to climate change. Glob. Chang. Biol. 2018, 24, 563–579. [Google Scholar] [CrossRef]
- Rumpf, S.B.; Hülber, K.; Zimmermann, N.E.; Dullinger, S. Elevational rear edges shifted at least as much as leading edges over the last century. Glob. Ecol. Biogeogr. 2019, 28, 533–543. [Google Scholar] [CrossRef]
- Rubenstein, M.A.; Weiskopf, S.R.; Bertrand, R.; Carter, S.L.; Comte, L.; Eaton, M.J.; Johnson, C.G.; Lenoir, J.; Lynch, A.J.; Miller, B.W.; et al. Climate change and the global redistribution of biodiversity: Substantial variation in empirical support for expected range shifts. Environ. Evid. 2023, 12, 7. [Google Scholar] [CrossRef]
- Nordberg, E.J.; Caley, M.J.; Schwarzkopf, L. Designing solar farms for synergistic commercial and conservation outcomes. Sol. Energy 2021, 228, 586–593. [Google Scholar] [CrossRef]
- Nordberg, E.J.; Schwarzkopf, L. Developing conservoltaic systems to support biodiversity on solar farms. Austral Ecol. 2023, 48, 643–649. [Google Scholar] [CrossRef]
- Wilkening, J.L.; Rautenstrauch, K. Can solar farms be wildlife friendly? A facility in the southwest hopes to find the answer. Wildl. Prof. 2019, 13, 46–50. [Google Scholar]
- Custer, N.A.; Schwinning, S.; DeFalco, L.A.; Esque, T.C. Local climate adaptations in two ubiquitous Mojave Desert shrub species, Ambrosia dumosa and Larrea tridentata. J. Ecol. 2022, 110, 1072–1089. [Google Scholar] [CrossRef]
- Schwinning, S.; Lortie, C.J.; Esque, T.C.; DeFalco, L.A. What common-garden experiments tell us about climate responses in plants. J. Ecol. 2022, 110, 986–996. [Google Scholar] [CrossRef]
- Abella, S.R.; Engel, E.C.; Lund, C.L.; Spencer, J.E. Early Post-Fire Plant Establishment on a Mojave Desert Burn. BioOne 2009, 56, 137–148. [Google Scholar] [CrossRef]
- Noel, A.R.; Shriver, R.K.; Crausbay, S.D.; Bradford, J.B. Where can managers effectively resist climate-driven ecological transformation in pinyon–juniper woodlands of the US Southwest? Glob. Chang. Biol. 2023, 29, 4327–4341. [Google Scholar] [CrossRef]
- Butt, N.; Chauvenet, A.L.; Adams, V.M.; Beger, M.; Gallagher, R.V.; Shanahan, D.F.; Ward, M.; Watson, J.E.M.; Possingham, H.P. Importance of species translocations under rapid climate change. Conserv. Biol. 2021, 35, 775–783. [Google Scholar] [CrossRef]
- Bell, D.A.; Robinson, Z.L.; Funk, W.C.; Fitzpatrick, S.W.; Allendorf, F.W.; Tallmon, D.A.; Whiteley, A.R. The Exciting Potential and Remaining Uncertainties of Genetic Rescue. Trends Ecol. Evol. 2019, 34, 1070–1079. [Google Scholar] [CrossRef]
- Gardner, C.J.; Bullock, J.M. In the Climate Emergency, Conservation Must Become Survival Ecology. Front. Conserv. Sci. 2021, 2, 659912. [Google Scholar] [CrossRef]
- Thomas, C.D. The development of Anthropocene biotas. Philos. Trans. R. Soc. B 2020, 375, 20190113. [Google Scholar] [CrossRef]
- Pfennig, K.S.; Kelly, A.L.; Pierce, A.A. Hybridization as a facilitator of species range expansion. Proc. R. Soc. B 2016, 283, 20161329. [Google Scholar] [CrossRef]
- Kennedy, P.L.; Fontaine, J.B.; Hobbs, R.J.; Johnson, T.; Boyle, R.; Lueders, A.S. Do novel ecosystems provide habitat value for wildlife? Revisiting the physiognomy vs. floristics debate. Ecosphere 2018, 9, e02172. [Google Scholar] [CrossRef]
- Hendry, A.P.; Gotanda, K.M.; Svensson, E.I. Human influences on evolution, and the ecological and societal con-sequences. Philos. Trans. R. Soc. B 2017, 372, 20160028. [Google Scholar] [CrossRef]
- Evers, C.R.; Wardropper, C.B.; Branoff, B.; Granek, E.F.; Hirsch, S.L.; Link, T.E.; Olivero-Lora, S.; Wilson, C. The ecosystem services and biodiversity of novel ecosystems: A literature review. Glob. Ecol. Conserv. 2018, 13, e00362. [Google Scholar] [CrossRef]
- Planchuelo, G.; von Der Lippe, M.; Kowarik, I. Untangling the role of urban ecosystems as habitats for endangered plant species. Landsc. Urban Plan. 2019, 189, 320–334. [Google Scholar] [CrossRef]
- Vellend, M.; Baeten, L.; Myers-Smith, I.H.; Elmendorf, S.C.; Beauséjour, R.; Brown, C.D.; De Frenne, P.; Verheyen, K.; Wipf, S. Global me-ta-analysis reveals no net change in local-scale plant biodiversity over time. Proc. Natl. Acad. Sci. USA 2013, 110, 19456–19459. [Google Scholar] [CrossRef]
- Steffen, W.; Richardson, K.; Rockström, J.; Cornell, S.E.; Fetzer, I.; Bennett, E.M.; Biggs, R.; Carpenter, S.R.; de Vries, W.; de Wit, C.A.; et al. Planetary boundaries: Guiding human development on a changing planet. Science 2015, 347, 1259855. [Google Scholar] [CrossRef]
- Leclère, D.; Obersteiner, M.; Barrett, M.; Butchart, S.H.M.; Chaudhary, A.; De Palma, A.; DeClerck, F.A.J.; Di Marco, M.; Doelman, J.C.; Dürauer, M.; et al. Bending the curve of terrestrial biodiversity needs an integrated strategy. Nature 2020, 585, 551–556. [Google Scholar] [CrossRef]
- Schlaepfer, M.A.; Lawler, J.J. Conserving biodiversity in the face of rapid climate change requires a shift in priorities. WIREs Clim. Chang. 2023, 14, e798. [Google Scholar] [CrossRef]
- Pascual, U.; Adams, W.; Diaz, S.; Lele, S.; Mace, G.M.; Turnhout, E. Biodiversity and the challenge of pluralism. Nat. Sustain. 2021, 4, 567–572. [Google Scholar] [CrossRef]
- Leopold, A. Round River; Oxford University Press: New York, NY, USA, 1972. [Google Scholar]
- Obura, D.O.; Katerere, Y.; Mayet, M.; Kaelo, D.; Msweli, S.; Mather, K.; Harris, J.; Louis, M.; Kramer, R.; Teferi, T.; et al. Integrate biodiversity targets from local to global levels. Science 2021, 373, 746–748. [Google Scholar] [CrossRef]
- Schlaepfer, M.A. Do non-native species contribute to biodiversity? PLOS Biol. 2018, 16, e2005568. [Google Scholar] [CrossRef]
- NatureServe 2023. Available online: https://www.natureserve.org/biodiversity-in-focus/story (accessed on 7 June 2023).
- Rosenzweig, M.L. Win-Win Ecology How the Earth’s Species Can Survive in the Midst of Human Enterprise, 1st ed.; Oxford University Press: New York, NY, USA, 2003; pp. 1–2. [Google Scholar]
| Management Target | Biodiversity Level or Its Benefits * | ||
|---|---|---|---|
| Genes/Populations/Species | Communities/Ecosystems | Ecosystem Services | |
| Species or indicator (and other surrogate) species | Example metric/indicator: Gene diversity Resist: Improve corridors between populations to increase gene flow and genetic diversity Resist: Improve corridors between populations to increase gene flow and genetic diversity  Accept: Allow decline of gene flow between populations at range edge because the climate is no longer suitable Accept: Allow decline of gene flow between populations at range edge because the climate is no longer suitable  Direct: Conservation introductions to encourage hybridization and increase evolutionary potential Direct: Conservation introductions to encourage hybridization and increase evolutionary potential | ||
| Multiple species or communities | Example metric/indicator: Species richness  Resist: Protect regions with high species richness (e.g., biodiversity hotspots) Resist: Protect regions with high species richness (e.g., biodiversity hotspots)  Accept: Allow loss of biodiverse regions where climate exposure and other land use changes are increasingly intense Accept: Allow loss of biodiverse regions where climate exposure and other land use changes are increasingly intense  Direct: Protect regions that may yield future species richness, such as areas with topographic complexity Direct: Protect regions that may yield future species richness, such as areas with topographic complexity | ||
| Ecosystem services | Example metric/indicator: Area of available wildlife habitat in coastal wetland Resist: Restore coastal forests; manipulate river channels to allow for freshwater spill Resist: Restore coastal forests; manipulate river channels to allow for freshwater spill Accept: Allow loss of habitat where inundation is increasingly frequent Accept: Allow loss of habitat where inundation is increasingly frequent Direct: Develop habitat farther inland to allow for the migration of wildlife populations Direct: Develop habitat farther inland to allow for the migration of wildlife populations | ||
| Landscapes (management portfolio) | Example metric/indicator: Trend in forest species abundance Resist: Minimize invasive species abundance to promote natives Resist: Minimize invasive species abundance to promote natives Accept: Allow decline of keystone species affected by climate exposure Accept: Allow decline of keystone species affected by climate exposure  Direct: Assist migration of potential alternative keystone species into the range Direct: Assist migration of potential alternative keystone species into the range | Example metric/indicator: Forest diversity  Resist: Reforest degraded areas to maintain the diversity of forest types Resist: Reforest degraded areas to maintain the diversity of forest types Accept: Allow vulnerable groups of species to disappear Accept: Allow vulnerable groups of species to disappear  Direct: Promote migration of non-native climate-resilient forest types to increase forest diversity Direct: Promote migration of non-native climate-resilient forest types to increase forest diversity | Example metric/indicator: Freshwater fishing Resist: Restore riparian areas to promote stream cooling for popular game species and aesthetic fishing experiences Resist: Restore riparian areas to promote stream cooling for popular game species and aesthetic fishing experiences Accept: Allow loss of coldwater stream fishes at lower elevations Accept: Allow loss of coldwater stream fishes at lower elevations Direct: Introduce non-native coolwater/warmwater fishes to provide alternative fishing targets Direct: Introduce non-native coolwater/warmwater fishes to provide alternative fishing targets |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilkening, J.L.; Magness, D.R.; Thompson, L.M.; Lynch, A.J. A Brave New World: Managing for Biodiversity Conservation under Ecosystem Transformation. Land 2023, 12, 1556. https://doi.org/10.3390/land12081556
Wilkening JL, Magness DR, Thompson LM, Lynch AJ. A Brave New World: Managing for Biodiversity Conservation under Ecosystem Transformation. Land. 2023; 12(8):1556. https://doi.org/10.3390/land12081556
Chicago/Turabian StyleWilkening, Jennifer L., Dawn Robin Magness, Laura M. Thompson, and Abigail J. Lynch. 2023. "A Brave New World: Managing for Biodiversity Conservation under Ecosystem Transformation" Land 12, no. 8: 1556. https://doi.org/10.3390/land12081556
APA StyleWilkening, J. L., Magness, D. R., Thompson, L. M., & Lynch, A. J. (2023). A Brave New World: Managing for Biodiversity Conservation under Ecosystem Transformation. Land, 12(8), 1556. https://doi.org/10.3390/land12081556






