Combining Fuzzy, Multicriteria and Mapping Techniques to Assess Soil Fertility for Agricultural Development: A Case Study of Firozabad District, Uttar Pradesh, India
Abstract
1. Introduction
2. Materials and Methods
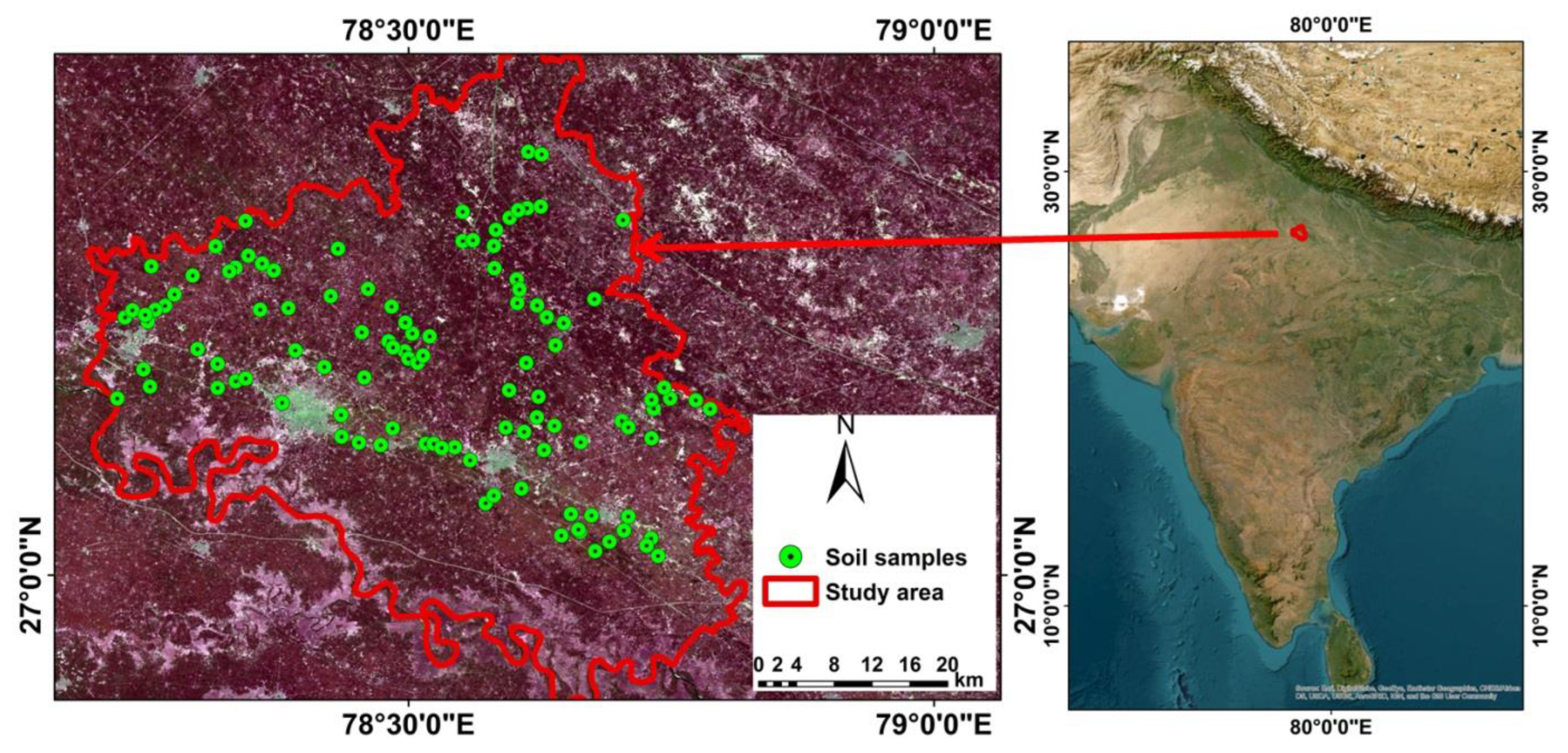
2.1. Study Area
2.2. Soil Sampling and Analysis
2.3. Geostatistical Modelling
2.4. Computation of Soil Fertility Index
2.5. Validation of SFI
3. Results and Discussion
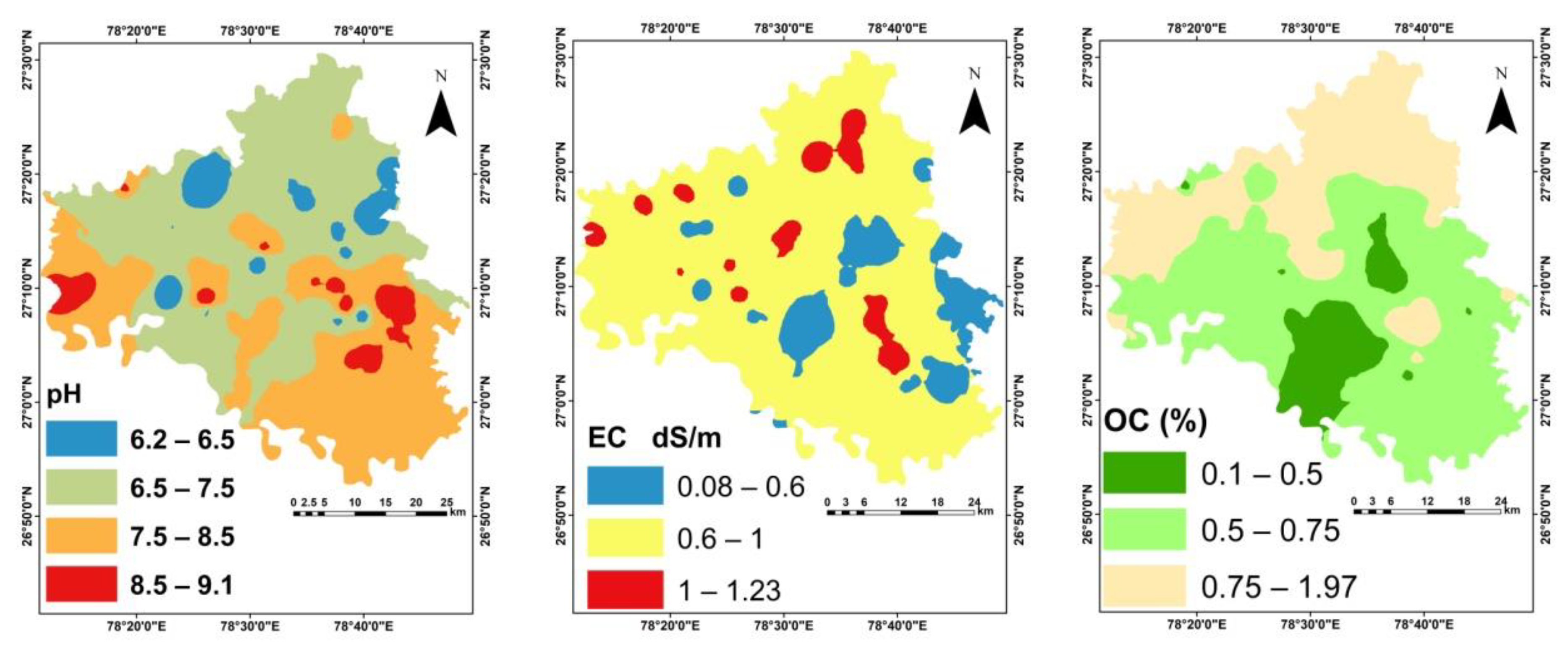
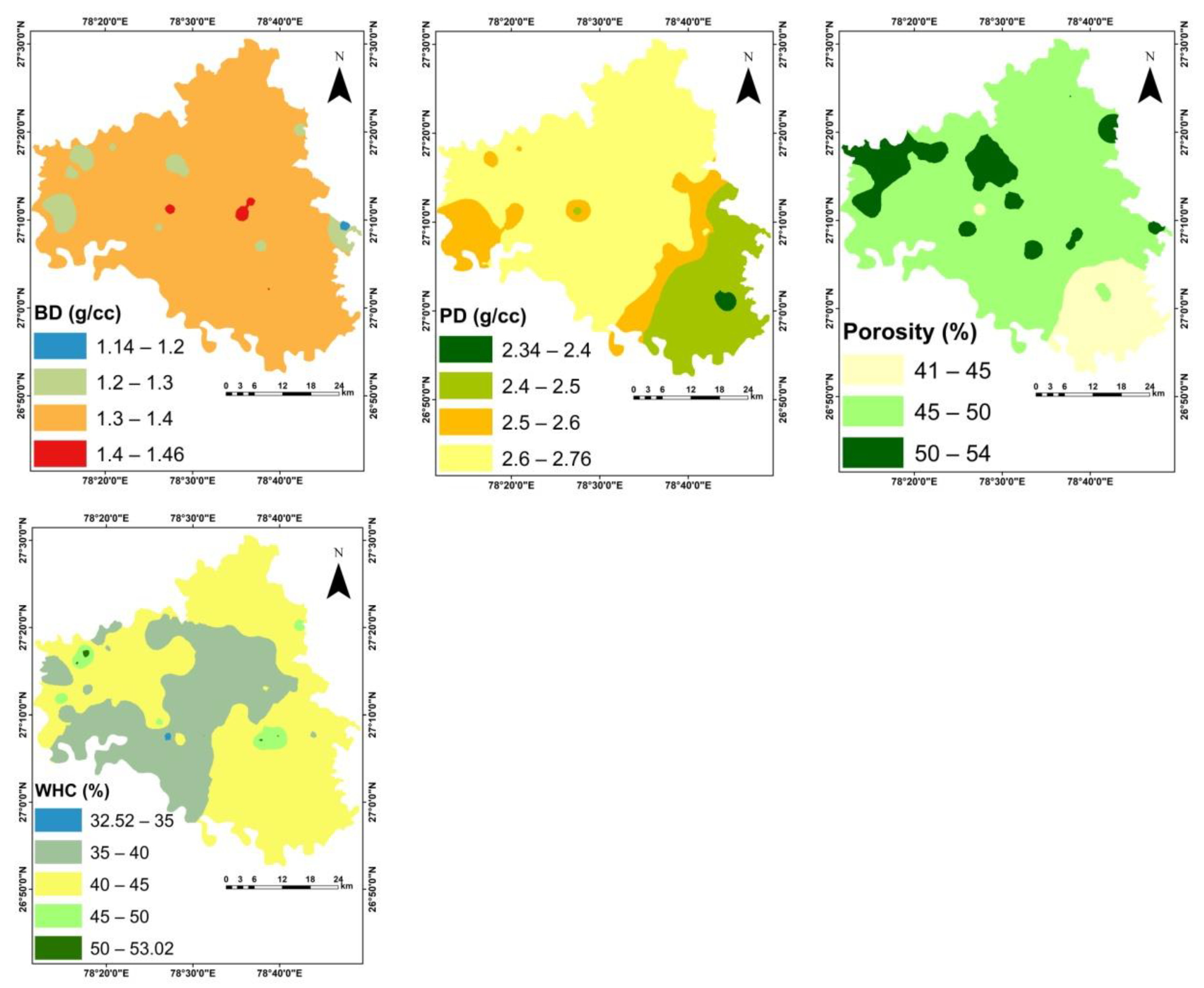
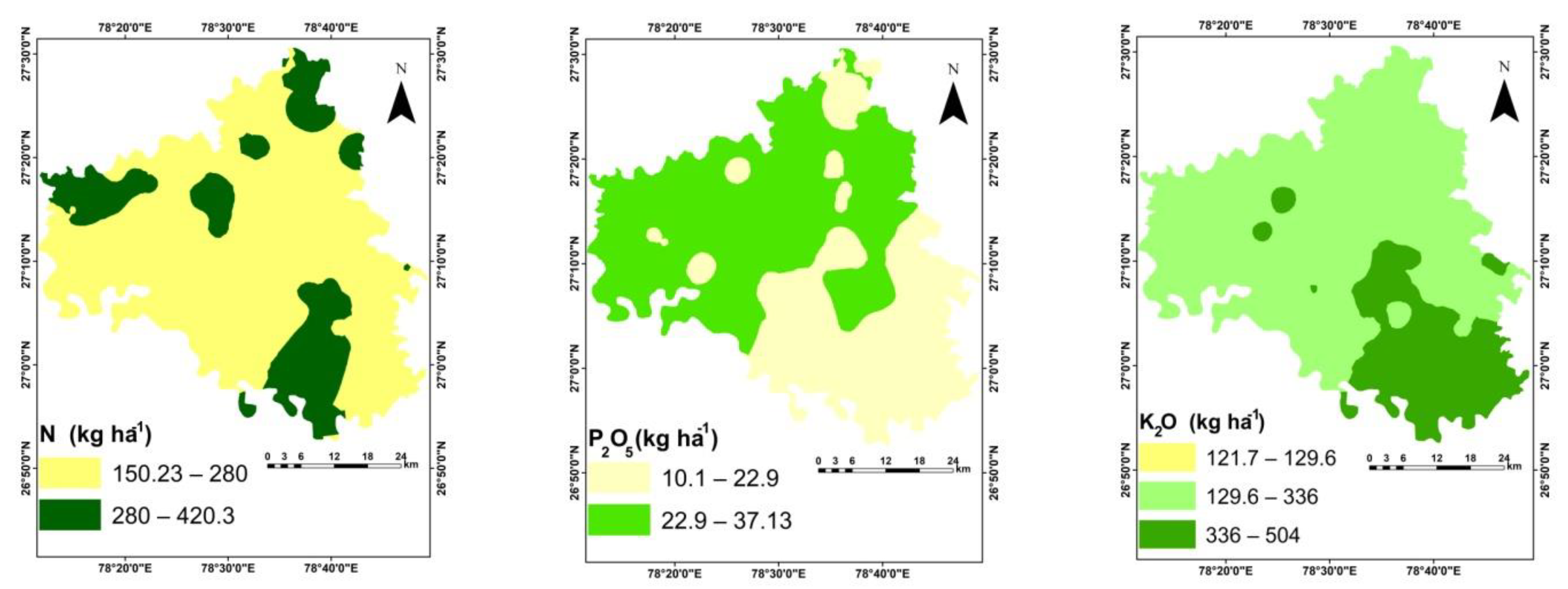
3.1. Physico-Chemical Properties of Soil
3.2. The Semivariograms of Soil Fertility Indicators
3.3. Spatial Distribution of Soil Fertility Indicators and SFI
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Status of the World’s Soil Resources (SWSR)—Main Report; Food and Agriculture Organization of the United Nations and Intergovernmental Technical Panel on Soils: Rome, Italy, 2015; p. 650. [Google Scholar]
- Majhi, P.K.; Raza, B.; Behera, P.P.; Singh, S.K.; Shiv, A.; Mogali, S.C.; Bhoi, T.K.; Patra, B.; Behera, B. Future-Proofing Plants Against Climate Change: A Path to Ensure Sustainable Food Systems. In Biodiversity, Functional Ecosystems and Sustainable Food Production; Springer: Berlin/Heidelberg, Germany, 2022; pp. 73–116. [Google Scholar]
- Verma, R.K.; Singh, A.; Hasanain, M.; Raza, M.B.; Singh, R.K. Nitrogen Management Practices for Improving Yield and Nitrogen Use Efficiency in Rice (Oryza Sativa). Indian J. Agric. Sci. 2020, 90, 1342–1344. [Google Scholar] [CrossRef]
- Megahed, H.A. GIS-Based Assessment of Groundwater Quality and Suitability for Drinking and Irrigation Purposes in the Outlet and Central Parts of Wadi El-Assiuti, Assiut Governorate, Egypt. Bull. Natl. Res. Cent. 2020, 44, 187. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, Y.V.; Saraswat, A.; Prajapat, V.; Kashiwar, S.R. Groundwater Quality Assessment of Different Villages of Sanganer Block in Jaipur District of Rajasthan. J. Soil Salin. Water Qual. 2021, 13, 248–254. [Google Scholar]
- Mujiyo, M.; Suntoro, S.; Akbar, R.R.; Rahayu, R. Mapping of Agricultural Land Use Change and Effect on Land Capability as a Basis for Land Use Direction in Nguntoronadi-Indonesia. Int. J. Agric. Syst. 2022, 10, 13–25. [Google Scholar] [CrossRef]
- De Feudis, M.; Falsone, G.; Gherardi, M.; Speranza, M.; Vianello, G.; Antisari, L.V. GIS-Based Soil Maps as Tools to Evaluate Land Capability and Suitability in a Coastal Reclaimed Area (Ravenna, Northern Italy). Int. Soil Water Conserv. Res. 2021, 9, 167–179. [Google Scholar] [CrossRef]
- Priori, S.; Pellegrini, S.; Vignozzi, N.; Costantini, E.A.C. Soil Physical-Hydrological Degradation in the Root-Zone of Tree Crops: Problems and Solutions. Agronomy 2020, 11, 68. [Google Scholar] [CrossRef]
- AbdelRahman, M.A.E.; Metwaly, M.M.; Afifi, A.A.; D’Antonio, P.; Scopa, A. Assessment of Soil Fertility Status under Soil Degradation Rate Using Geomatics in West Nile Delta. Land 2022, 11, 1256. [Google Scholar] [CrossRef]
- AbdelRahman, M.A.E.; Saleh, A.M.; Arafat, S.M. Assessment of Land Suitability Using a Soil-Indicator-Based Approach in a Geomatics Environment. Sci. Rep. 2022, 12, 18113. [Google Scholar] [CrossRef]
- AbdelRahman, M.A.E.; Engel, B.; SM Eid, M.; Aboelsoud, M.H. A New Index to Assess Soil Sustainability Based on Temporal Changes of Soil Measurements Using Geomatics–An Example from El-Sharkia, Egypt. All Earth 2022, 34, 147–166. [Google Scholar] [CrossRef]
- AbdelRahman, M.A.E.; Natarajan, A.; Hegde, R. Assessment of Land Suitability and Capability by Integrating Remote Sensing and GIS for Agriculture in Chamarajanagar District, Karnataka, India. Egypt. J. Remote Sens. Space Sci. 2016, 19, 125–141. [Google Scholar] [CrossRef]
- Tiwari, K.R.; Sitaula, B.K.; Borresen, T.; Bajracharya, R.M. An Assessment of Soil Quality in Pokhare Khola Watershed of the Middle Mountains in Nepal. J. Food Agric. Environ. 2006, 4, 276. [Google Scholar]
- Hombegowda, H.C.; Adhikary, P.P.; Jakhar, P.; Madhu, M. Alley Cropping Agroforestry System for Improvement of Soil Health. In Soil Health and Environmental Sustainability: Application of Geospatial Technology; Springer: Berlin/Heidelberg, Germany, 2022; pp. 529–549. [Google Scholar]
- Guo, L.; Sun, Z.; Ouyang, Z.; Han, D.; Li, F. A Comparison of Soil Quality Evaluation Methods for Fluvisol along the Lower Yellow River. Catena 2017, 152, 135–143. [Google Scholar] [CrossRef]
- Armenise, E.; Redmile-Gordon, M.A.; Stellacci, A.M.; Ciccarese, A.; Rubino, P. Developing a Soil Quality Index to Compare Soil Fitness for Agricultural Use under Different Managements in the Mediterranean Environment. Soil Tillage Res. 2013, 130, 91–98. [Google Scholar] [CrossRef]
- Amorim, H.C.S.; Ashworth, A.J.; Brye, K.R.; Wienhold, B.J.; Savin, M.C.; Owens, P.R.; Silva, S.H.G. Soil Quality Indices as Affected by Long-term Burning, Irrigation, Tillage, and Fertility Management. Soil Sci. Soc. Am. J. 2021, 85, 379–395. [Google Scholar] [CrossRef]
- Amorim, H.C.S.; Ashworth, A.J.; Wienhold, B.J.; Savin, M.C.; Allen, F.L.; Saxton, A.M.; Owens, P.R.; Curi, N. Soil Quality Indices Based on Long-term Conservation Cropping Systems Management. Agrosyst. Geosci. Environ. 2020, 3, e20036. [Google Scholar] [CrossRef]
- Manivannan, S.; Khola, O.P.S.; Kannan, K.; Hombegowda, H.C.; Singh, D.V.; Sundarambal, P.; Thilagam, V.K. Comprehensive Impact Assessment of Watershed Development Projects in Lower Bhavani Catchments of Tamil Nadu. J. Soil Water Conserv. 2021, 20, 66–73. [Google Scholar] [CrossRef]
- Oladipo, J.O.; Aboyeji, O.S.; Akinwumiju, A.S.; Adelodun, A.A. Fuzzy Logic Interference for Characterization of Surface Water Potability in Ikare Rural Community, Nigeria. J. Geovis. Spat. Anal. 2020, 4, 1. [Google Scholar] [CrossRef]
- Mora-Herrera, D.Y.; Guillaume, S.; Snoeck, D.; Escobar, O.Z. A Fuzzy Logic Based Soil Chemical Quality Index for Cacao. Comput. Electron. Agric. 2020, 177, 105624. [Google Scholar] [CrossRef]
- Ogunleye, G.O.; Fashoto, S.G.; Mashwama, P.; Arekete, S.A.; Olaniyan, O.M.; Omodunbi, B.A. Fuzzy Logic Tool to Forecast Soil Fertility in Nigeria. Sci. World J. 2018, 2018, 3170816. [Google Scholar] [CrossRef]
- Prabakaran, G.; Vaithiyanathan, D.; Ganesan, M. Fuzzy Decision Support System for Improving the Crop Productivity and Efficient Use of Fertilizers. Comput. Electron. Agric. 2018, 150, 88–97. [Google Scholar] [CrossRef]
- Prabakaran, G.; Vaithiyanathan, D.; Ganesan, M. Soil Fertility Review using Fuzzy Logic. J. Eng. Res. 2021, 192, 202. [Google Scholar] [CrossRef]
- Aliyu, K.T.; Kamara, A.Y.; Jibrin, J.M.; Huising, J.E.; Shehu, B.M.; Adewopo, J.B.; Mohammed, I.B.; Solomon, R.; Adam, A.M.; Samndi, A.M. Delineation of Soil Fertility Management Zones for Site-Specific Nutrient Management in the Maize Belt Region of Nigeria. Sustainability 2020, 12, 9010. [Google Scholar] [CrossRef]
- El Behairy, R.A.; El Baroudy, A.A.; Ibrahim, M.M.; Mohamed, E.S.; Kucher, D.E.; Shokr, M.S. Assessment of Soil Capability and Crop Suitability Using Integrated Multivariate and GIS Approaches toward Agricultural Sustainability. Land 2022, 11, 1027. [Google Scholar] [CrossRef]
- Metwally, M.S.; Shaddad, S.M.; Liu, M.; Yao, R.-J.; Abdo, A.I.; Li, P.; Jiao, J.; Chen, X. Soil Properties Spatial Variability and Delineation of Site-Specific Management Zones Based on Soil Fertility Using Fuzzy Clustering in a Hilly Field in Jianyang, Sichuan, China. Sustainability 2019, 11, 7084. [Google Scholar] [CrossRef]
- Saaty, T.L. The Analytical Hierarchy Process: Planning, Priority Setting, Resource Allocation; McGraw-Hill: New York, NY, USA, 1980. [Google Scholar]
- Datta, S.P.; Rao, A.S.; Ganeshamurthy, A.N. Effect of Electrolytes Coupled with Variable Stirring on Soil PH. J. Indian Soc. Soil Sci. 1997, 45, 185–187. [Google Scholar]
- Walkley, A.; Black, I.A. An Examination of the Degtjareff Method for Determining Soil Organic Matter, and a Proposed Modification of the Chromic Acid Titration Method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Subbiah, B.V.; Asija, G.L. A Rapid Method for the Estimation of Nitrogen in Soil. Curr. Sci. 1956, 26, 259–260. [Google Scholar]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; US Department of Agriculture: Washington, DC, USA, 1954.
- Watanabe, F.S.; Olsen, S.R. Test of an Ascorbic Acid Method for Determining Phosphorus in Water and NaHCO3 Extracts from Soil. Soil Sci. Soc. Am. J. 1965, 29, 677–678. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis: Advanced Course; UW-Madison Libraries Parallel Press: Madison, WI, USA, 2005. [Google Scholar]
- Singh, D.; Chhonkar, P.K.; Dwivedi, B.S. Manual on Soil, Plant and Water Analysis; Westville Publishing House: Delhi, India, 2005. [Google Scholar]
- Lindsay, W.L.; Norvell, W. Development of a DTPA Soil Test for Zinc, Iron, Manganese, and Copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Teegavarapu, R.S.V.; Chandramouli, V. Improved Weighting Methods, Deterministic and Stochastic Data-Driven Models for Estimation of Missing Precipitation Records. J. Hydrol. 2005, 312, 191–206. [Google Scholar] [CrossRef]
- Isaaks, E.H.; Srivastava, R.M. Applied Geostatistics; Oxford University Press: New York, NY, USA, 1989; Volume 561. [Google Scholar]
- Eldeiry, A.A.; Garcia, L.A. Comparison of Ordinary Kriging, Regression Kriging, and Cokriging Techniques to Estimate Soil Salinity Using LANDSAT Images. J. Irrig. Drain. Eng. 2010, 136, 355–364. [Google Scholar] [CrossRef]
- Parker, F.W.; Nelson, W.L.; Winters, E.; Miles, I.E. The Broad Interpretation and Application of Soil Test Information. Agron. J. 1951, 43, 105–112. [Google Scholar] [CrossRef]
- Kellogg, C.E. Soil Survey Division Staff: Soil Survey Manual; United States Department of Agriculture: Washington, DC, USA, 1993.
- Machado, R.M.A.; Serralheiro, R.P. Soil Salinity: Effect on Vegetable Crop Growth. Management Practices to Prevent and Mitigate Soil Salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Snapp, S.S.; Shennan, C.; Van Bruggen, A.H.C. Effects of Salinity on Severity of Infection by Phytophthora Parasitica Dast., Ion Concentrations and Growth of Tomato, Lycopersicon Esculentum Mill. New Phytol. 1991, 119, 275–284. [Google Scholar] [CrossRef]
- Kirschbaum, M.U.F. The Temperature Dependence of Organic-Matter Decomposition—Still a Topic of Debate. Soil Biol. Biochem. 2006, 38, 2510–2518. [Google Scholar] [CrossRef]
- Pawar, A.B.; Kumawat, C.; Verma, A.K.; Meena, R.K.; BasitRaza, M.; Anil, A.S.; Trivedi, V.K. Threshold Limits of Soil in Relation to Various Soil Functions and Crop Productivity. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2293–2302. [Google Scholar] [CrossRef]
- Chen, S.; Lin, B.; Li, Y.; Zhou, S. Spatial and Temporal Changes of Soil Properties and Soil Fertility Evaluation in a Large Grain-Production Area of Subtropical Plain, China. Geoderma 2020, 357, 113937. [Google Scholar] [CrossRef]
- Sahoo, J.; Dinesh, B.M.A.; Anil, A.S.; Raza, M. Nutrient Distribution and Relationship with Soil Properties in Different Watersheds of Haryana. Indian J. Agric. Sci. 2020, 90, 172–177. [Google Scholar] [CrossRef]
- Das, D.; Sahoo, J.; Raza, M.B.; Barman, M.; Das, R. Ongoing Soil Potassium Depletion under Intensive Cropping in India and Probable Mitigation Strategies. A Review. Agron. Sustain. Dev. 2022, 42, 4. [Google Scholar] [CrossRef]
- Saraswat, A.; Nath, T.; Omeka, M.; Unigwe, C.O.; Anyanwu, I.E.; Ugar, S.I.; Latare, A.; Raza, M.B.; Behera, B.; Adhikary, P.P. Irrigation Suitability and Health Risk Assessment of Groundwater Resources in the Firozabad Industrial Area of North-Central India: An Integrated Indexical, Statistical, and Geospatial Approach. Front. Environ. Sci. 2023, 11, 296. [Google Scholar] [CrossRef]
- Du, H.; Lu, X. Spatial Distribution and Source Apportionment of Heavy Metal (Loid) s in Urban Topsoil in Mianyang, Southwest China. Sci. Rep. 2022, 12, 10407. [Google Scholar] [CrossRef]
- Behera, B.; Das, T.K.; Raj, R.; Ghosh, S.; Raza, M.B.; Sen, S. Microbial Consortia for Sustaining Productivity of Non-Legume Crops: Prospects and Challenges. Agric. Res. 2021, 10, 1–14. [Google Scholar] [CrossRef]
- AbdelRahman, M.A.E.; Zakarya, Y.M.; Metwaly, M.M.; Koubouris, G. Deciphering Soil Spatial Variability through Geostatistics and Interpolation Techniques. Sustainability 2021, 13, 194. [Google Scholar] [CrossRef]
- AbdelRahman, M.A.E.; Rehab, H.H.; Yossif, T.M.H. Soil fertility assessment for optimal agricultural use using remote sensing and GIS technologies. Appl. Geomat. 2021, 13, 605–618. [Google Scholar] [CrossRef]
- AbdelRahman, M.A.E.; Arafat, S.M. An Approach of Agricultural Courses for Soil Conservation Based on Crop Soil Suitability Using Geomatics. Earth Syst. Environ. 2020, 4, 273–285. [Google Scholar] [CrossRef]
- Rabia, A.H.; Neupane, J.; Lin, Z.; Lewis, K.; Cao, G.; Guo, W. Principles and Applications of Topography in Precision Agriculture. Adv. Agron. 2022, 171, 143–189. [Google Scholar]
- Ofori, E.; Atakora, E.T.; Kyei-Baffour, N.; Antwi, B. Relationship between Landscape Positions and Selected Soil Properties at a Sawah Site in Ghana. Afr. J. Agric. Res. 2013, 8, 3646–3652. [Google Scholar]
- Rengel, Z. Handbook of Plant Growth pH as the Master Variable; CRC Press: Boca Raton, FL, USA, 2002; Volume 88. [Google Scholar]
- Fu, W.; Tunney, H.; Zhang, C. Spatial Variation of Soil Nutrients in a Dairy Farm and Its Implications for Site-Specific Fertilizer Application. Soil Tillage Res. 2010, 106, 185–193. [Google Scholar] [CrossRef]
- Gorai, T.; Ahmed, N.; Sahoo, R.N.; Pradhan, S.; Datta, S.C.; Sharma, R.K. Spatial Variability Mapping of Soil Properties at Farm Scale. J. Agric. Phys. 2015, 15, 29–37. [Google Scholar]
- Walker, D.J.; Clemente, R.; Bernal, M.P. Contrasting Effects of Manure and Compost on Soil PH, Heavy Metal Availability and Growth of Chenopodium Album L. in a Soil Contaminated by Pyritic Mine Waste. Chemosphere 2004, 57, 215–224. [Google Scholar] [CrossRef]
- Trivedi, V.K.; Raza, M.D.B.; Dimree, S.; Verma, A.K.; Pawar, A.B.; Upadhyay, D.P. Effect of Balanced Use of Nutrients on Yield Attributes, Yield and Protein Content of Wheat (Triticum Aestivum). Indian J. Agric. Sci. 2021, 90, 1369–1372. [Google Scholar] [CrossRef]
- Gavrilescu, M. Water, Soil, and Plants Interactions in a Threatened Environment. Water 2021, 13, 2746. [Google Scholar] [CrossRef]
- Goss, M.J.; Tubeileh, A.; Goorahoo, D. A Review of the Use of Organic Amendments and the Risk to Human Health. Adv. Agron. 2013, 120, 275–379. [Google Scholar]
- Francaviglia, R.; Almagro, M.; Vicente-Vicente, J.L. Conservation Agriculture and Soil Organic Carbon: Principles, Processes, Practices and Policy Options. Soil Syst. 2023, 7, 17. [Google Scholar] [CrossRef]


| Hydraulic Properties | Physical Properties | Chemical Properties | Pollution Properties | Slope/Land Cover/Rainfall | Row Total | % of Ratio Scale | Average | Sum | Weighted Rating | Lambda (λmax) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydraulic properties | 0.4 | 0.4 | 0.3 | 0.4 | 0.4 | 2.1 | 0.4 | 0.4 | 2.1 | 2.1 | 5.2 |
| Physical properties | 0.2 | 0.2 | 0.3 | 0.2 | 0.2 | 1.1 | 0.2 | 0.2 | 1.1 | 1.2 | 5.2 |
| Chemical properties | 0.1 | 0.1 | 0.1 | 0.0 | 0.2 | 0.4 | 0.1 | 0.1 | 0.4 | 0.4 | 5.1 |
| Pollution properties | 0.2 | 0.2 | 0.3 | 0.2 | 0.2 | 1.2 | 0.2 | 0.2 | 1.2 | 1.3 | 5.3 |
| Slope/Land cover/Rainfall | 0.1 | 0.1 | 0.0 | 0.1 | 0.1 | 0.2 | 0.0 | 0.0 | 0.2 | 0.2 | 5.1 |
| Column total | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 5.0 | 1.0 | 1.0 | 5.2 (Average) |
| Sl. No. | Criteria/Factors | Weight |
|---|---|---|
| 1 | Temperature | 0.41 |
| 2 | Rainfall | 0.24 |
| 3 | Altitude | 0.22 |
| 4 | Land cover type | 0.08 |
| 5 | Slope | 0.05 |
| Total | 1.00 |
| Parameters | Min | Max | Mean | Median | SD | CV | Skewness | Kurtosis |
|---|---|---|---|---|---|---|---|---|
| pH | 6.2 | 9.1 | 7.61 | 7.5 | 0.55 | 7.26 | 0.5 | 0.22 |
| EC (dS m−1) | 0.08 | 1.23 | 0.41 | 0.35 | 0.26 | 61.7 | 1 | 0.39 |
| BD (g cc−1) | 1.14 | 1.46 | 1.34 | 1.34 | 0.05 | 3.81 | −0.41 | 1.37 |
| PD (g cc−1) | 2.34 | 2.76 | 2.6 | 2.63 | 0.1 | 3.93 | −0.96 | −0.04 |
| Porosity (%) | 40.7 | 54.0 | 48.5 | 49.2 | 2.74 | 5.64 | −1.1 | 0.9 |
| WHC (%) | 32.5 | 53.0 | 40.5 | 40.3 | 4.34 | 10.7 | 0.69 | 0.38 |
| SOC (%) | 0.1 | 1.97 | 0.71 | 0.65 | 0.33 | 46.5 | 1.49 | 2.98 |
| N (kg ha−1) | 150.2 | 490.3 | 263.0 | 267.7 | 59.3 | 22.6 | 0.63 | 1.52 |
| P (kg ha−1) | 10.1 | 37.1 | 24.3 | 24.7 | 7.1 | 29.3 | −0.21 | −1.01 |
| K (kg ha−1) | 121.7 | 504 | 285.0 | 284.3 | 80.1 | 28.1 | 0.27 | −0.32 |
| Ca (cmol(p+) kg−1) | 0.8 | 40.2 | 13.0 | 12.4 | 7.88 | 60.5 | 0.77 | 1.12 |
| Mg (cmol(p+) kg−1) | 1.2 | 91.1 | 30.8 | 25.9 | 24.5 | 79.6 | 1.01 | 0.13 |
| S (mg kg−1) | 0.97 | 22.2 | 5.56 | 4.8 | 3.62 | 65.2 | 1.81 | 5.12 |
| Fe (mg kg−1) | 1.2 | 20.9 | 4.74 | 3.22 | 3.77 | 79.6 | 2.14 | 4.24 |
| Mn (mg kg−1) | 0.14 | 12.3 | 2.66 | 0.55 | 3.84 | 144.3 | 1.31 | 0.05 |
| Cu (mg kg−1) | 0.6 | 4.88 | 2.86 | 3.72 | 1.42 | 49.5 | −0.36 | −1.64 |
| Zn (mg kg−1) | 0.21 | 3.0 | 0.63 | 0.31 | 0.62 | 99.4 | 1.87 | 2.91 |
| Criteria | Inverse Distance Weighing-IDW | |||||||
|---|---|---|---|---|---|---|---|---|
| IDW-1 | IDW-2 | IDW-3 | IDW-4 | |||||
| SFI | 0.5397 | 0.5397 | 0.5453 | 0.5546 | ||||
| Ordinary Kriging | Simple Kriging | |||||||
| Gaussian | Exponential | Spherical | Linear | Gaussian | Exponential | Spherical | Linear | |
| SFI | 0.5291 | 0.5454 | 0.5523 | 0.5552 | 0.5323 | 0.5201 | 0.5333 | 0.5260 |
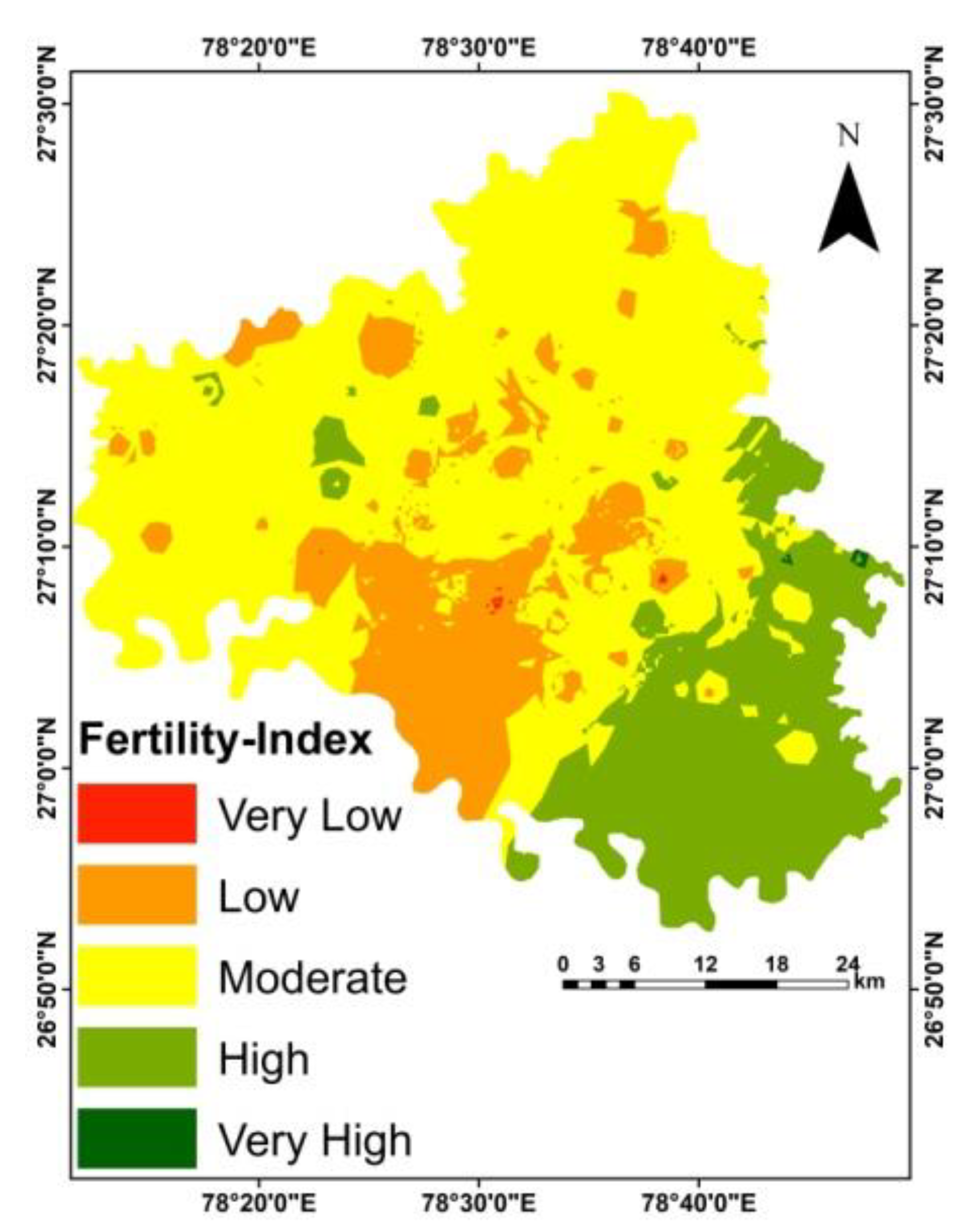
| Class | Definition | SFI Index Value | Area (ha) | Area (%) |
|---|---|---|---|---|
| F1 | Very highly fertile | >8.953 | 136.58 | 0.05 |
| F2 | Highly fertile | 7.695–8.953 | 41,274.54 | 16.59 |
| F3 | Moderately fertile | 6.208–7.695 | 151,616.08 | 60.94 |
| N1 | Low fertile | 4.455–6.208 | 55,581.93 | 22.34 |
| N2 | Very low fertile | <4.455 | 168.76 | 0.07 |
| Total | 248,777.89 | 100.0 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saraswat, A.; Ram, S.; AbdelRahman, M.A.E.; Raza, M.B.; Golui, D.; HC, H.; Lawate, P.; Sharma, S.; Dash, A.K.; Scopa, A.; et al. Combining Fuzzy, Multicriteria and Mapping Techniques to Assess Soil Fertility for Agricultural Development: A Case Study of Firozabad District, Uttar Pradesh, India. Land 2023, 12, 860. https://doi.org/10.3390/land12040860
Saraswat A, Ram S, AbdelRahman MAE, Raza MB, Golui D, HC H, Lawate P, Sharma S, Dash AK, Scopa A, et al. Combining Fuzzy, Multicriteria and Mapping Techniques to Assess Soil Fertility for Agricultural Development: A Case Study of Firozabad District, Uttar Pradesh, India. Land. 2023; 12(4):860. https://doi.org/10.3390/land12040860
Chicago/Turabian StyleSaraswat, Anuj, Shri Ram, Mohamed A. E. AbdelRahman, Md Basit Raza, Debasis Golui, Hombegowda HC, Pramod Lawate, Sonal Sharma, Amit Kumar Dash, Antonio Scopa, and et al. 2023. "Combining Fuzzy, Multicriteria and Mapping Techniques to Assess Soil Fertility for Agricultural Development: A Case Study of Firozabad District, Uttar Pradesh, India" Land 12, no. 4: 860. https://doi.org/10.3390/land12040860
APA StyleSaraswat, A., Ram, S., AbdelRahman, M. A. E., Raza, M. B., Golui, D., HC, H., Lawate, P., Sharma, S., Dash, A. K., Scopa, A., & Rahman, M. M. (2023). Combining Fuzzy, Multicriteria and Mapping Techniques to Assess Soil Fertility for Agricultural Development: A Case Study of Firozabad District, Uttar Pradesh, India. Land, 12(4), 860. https://doi.org/10.3390/land12040860












